Abstract
Human hepatitis viruses (HHVs) include hepatitis A virus, hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis delta virus, and hepatitis E virus and can cause liver inflammation in their common human host. Usually, HHV is rapidly cleared by the immune system, following acute HHV invasion. The morbidities associated with hepatitis A virus and hepatitis E virus infection occur shortly after their intrusion, in the acute stage. Nevertheless, the viral infectious process can persist for a long period of time, especially in HBV and HCV infection, leading to chronic hepatitis and further progressing to hepatic cirrhosis and liver cancer. HHV infection brings about complications in other organs, and both acute and chronic hepatitis have been associated with clinical presentations outside the liver. Vascular involvement with cutaneous and systemic vasculitis is a well-known extrahepatic presentation; moreover, there is growing evidence for a possible causal relationship between viral pathogens and vasculitis. Except for hepatitis delta virus, other HHVs have participated in the etiopathogenesis of cutaneous and systemic vasculitis via different mechanisms, including direct viral invasion of vascular endothelial cells, immune complex-mediated vessel wall damage, and autoimmune responses with stimulation of autoreactive B-cells and impaired regulatory T-cells. Cryoglobulinemic vasculitis and polyarteritis nodosa are recognized for their association with chronic HHV infection. Although therapeutic guidelines for HHV-associated vasculitis have not yet been established, antiviral therapy should be initiated in HBV and HCV-related systemic vasculitis in addition to the use of corticosteroids. Plasma exchange and/or combined cyclophosphamide and corticosteroid therapy can be considered in patients with severe life-threatening vasculitis manifestations.
Keywords: Human hepatitis viruses, Hepatitis B virus, Hepatitis C virus, Cryogo-bulinemic vasculitis, Polyarteritis nodosa, Antiviral therapy
Core Tip: The human hepatitis viruses (HHVs) hepatitis A virus, hepatitis B virus, hepatitis C virus, hepatitis delta virus, and hepatitis E virus can cause liver inflammation in their common human host. With the exception of hepatitis delta virus, all other HHVs can participate in the pathogenesis of cutaneous and systemic vasculitis via different mechanisms like direct viral invasion of vascular endothelial cells and immune complex-mediated vessel wall damage. Cryoglobulinemic vasculitis and polyarteritis nodosa are recognized for their association with chronic HHV infection. Antiviral therapy should be initiated in hepatitis B virus and hepatitis C virus-related systemic vasculitis.
INTRODUCTION
Hepatocellular injury caused by acute or chronic inflammation of the liver is derived from adverse factors, including alcohol consumption, autoimmune response, drug use, steatosis, and viruses[1]. Hepatotropic viruses are known for their role in the etiology of viral hepatitis, and, from the public health perspective, the disease is associated with heavy health burden and higher annual mortality[2]. In addition to uncommon herpes viruses-related hepatitis due to Epstein-Barr virus, cytomegalovirus, and herpes simplex virus infection[3], human hepatitis viruses (HHVs) are the most common causes of acute and chronic hepatitis in their human host[4]. The discovery of HHV starts with clinical description of disease, antigen/antibody reaction establishment, virus-like particles visualization, and finally viral genomes sequencing[5]. Early experiments illustrated two types, infectious hepatitis and serum hepatitis[6]. Australia antigens in serum hepatitis yielded the detection of 42-nm Dane particles[7,8]. Subsequently, deoxyribonucleic acid (DNA) sequencing was performed and hepatitis B virus (HBV) was found[9]. Later, virus-like particles were observed in infectious hepatitis, and complementary DNA (cDNA) from hepatitis A virus (HAV) was sequenced[10,11]. Hepatitis C virus (HCV) was identified in non-A, non-B hepatitis patients receiving multiple transfusions[12], transmission to chimpanzees[13], and cDNA library screens[14]. Hepatitis delta virus (HDV), a HBV-associated virus[15], is a separate defective virus requiring the help of HBV for its infection[16]. After elucidating the hepatitis E virus (HEV) cDNA[17], an endemic non-A, non-B hepatitis[18], there are five members of HHVs[5].
Typically following HHV invasion into humans, there is rapid clearance of viruses by the host defense system, with a self-limiting disease course[4]. The morbidities associated with HAV and HEV infection, commonly transmitted via the fecal-oral route, occur shortly after their intrusion in the acute stage[19-21]. The infectious processes can persist for a long period of time in HBV, HCV, and HDV infection, progressing to chronic hepatitis and leading to liver fibrosis, irreversible cirrhosis, and hepatocellular carcinoma[22-24]. Table 1 summarizes and compares the common characteristics of the five HHV members[4,5,19-29].
Table 1.
Common characteristics and comparative features in five members of human hepatitis viruses
|
|
HAV
|
HBV
|
HCV
|
HDV1
|
HEV
|
| Family; Genus | Picornaviridae Hepatovirus | Hepadnavirida Orthohepadnavirus | Flaviviridae Hepacivirus | Deltaviridae Deltavirus | Hepeviridae Orthohepevirus |
| Discovery2; Frequency | 1983 Highest in AH | 1979 Highest in CH | 1989 Second in CH | 1986 Less in CH | 1990 Less in AH |
| Genome3; Length; Diameter4; Envelope5; Receptor | Linear ssRNA; 7500 nt; 27-32 nm; Quasi-envelope; Unknown | Circular dsDNA; 3200 nt; 42 nm; Envelope; NTCP, HSP | Linear ssRNA; 9600 nt; 55-65 nm; Envelope; CD81, SR-BI | Circular ssRNA; 1700 nt; 36-43 nm; Envelope; NTCP, HSP | Linear ssRNA; 7200 nt; 30-34 nm; Quasi-envelope; Unknown |
| Incubation; Age6; Course | 14-30 d; Children; Acute | 30-180 d; Any; Acute/chronic | 14-180 d; Any; Acute/chronic | 14-160 d; Any; Acute/chronic7 | 14-70 d; Adults; Acute |
| Route of spread | Enteric, sexual | Parental, sexual, vertical | Parental, sexual | Parental, sexual, vertical | Enteric, vertical, parenteral |
| Hepatitis diagnosis | Anti-HAV IgM | Anti-HBc IgM, HBs antigen, DNA by PCR | Anti-HCV IgG, RNA by PCR | Anti-HDV IgM, RNA by PCR | Anti-HEV IgM, RNA by PCR |
| Vaccine; Post-exp; Av agent | Available; Ig/vaccine; Not available | Available; Ig/vaccine; NA, IFN-α | Not available; Not effective; DAA agent | HBV vaccine; Not available; IFN-α | Available in China; Not available; Not available |
| FH8; LC/HCC; Prognosis9 | Very rare; Nil; Full recovery | Very rare; Yes; Chronic carrier | Extremely rare; Yes; High carrier rate | Yes; Yes; Chronic carrier | Yes; Nil; Full recovery |
Co-infection/superinfection with hepatitis B virus.
By cloning and sequencing.
Partial dsRNA in hepatitis B virus.
Diameter of virion.
Quasi-envelop with internal protein rather than viral glycoprotein.
Commonly affected age.
Progress to chronicity in 80% superinfection/5% co-infection hepatitis B virus.
Up to 20% in superinfection with hepatitis B virus and pregnant women.
Poorer in hepatitis B virus superinfection with hepatitis delta virus and pregnant woman with hepatitis E virus. AH: Acute hepatitis; Av: Antiviral; CH: Chronic hepatitis; DAA: Direct-acting antiviral; DNA: Deoxyribonucleic acid; ds: Double-stranded; Post-exp: Post-exposure; FH: Fulminant hepatitis; HBc: Hepatitis B virus core antigen; HBs: Hepatitis B virus surface antigen; HCC: Hepatocellular carcinoma; HSP: Heparan sulfate proteoglycans; IFN: Interferon; Ig: Immunoglobulins; LC: Liver cirrhosis; NA: Nucleoside/nucleotide analogues; nt: Nucleotide; NTCP: Sodium taurocholate co-transporting polypeptide, PCR: Polymerase chain reaction; RNA: Ribonucleic acid; SR-BI: Scavenger receptor class B type I; ss: Single-stranded.
EXTRAHEPATIC MANIFESTATIONS OF HUMAN HEPATITIS VIRUSES INFECTION
Although HHVs primarily affect hepatocytes, they can cause extrahepatic manifestations, with damage to other organs[30-34]. Both acute and chronic forms of viral hepatitis are associated with various clinical presentations outside of the liver, which may precede or follow hepatic dysfunction. The crucial pathogenic mechanism is caused by the immune responses against viral pathogens, with the deposition of immune complexes (IC) in targeted tissues.
Extrahepatic manifestations with evanescent rash and transient arthralgia that develop in acute HAV infection are rare[35]. In the protracted clinical course with cholestasis or relapsing disease, cutaneous vasculitis and arthritis occur with a predilection for lower extremities[36-38], and usually there is spontaneous recovery after the clearance of HAV. Other infrequently observed presentations include glome-rulonephritis, myocarditis, thrombocytopenia, and neurological complications like Guillain-Barre syndrome[20,32,39-41].
Acute HEV infection can be asymptomatic or manifest as fulminant hepatitis[21]. Neurological involvement is the most frequently encountered extrahepatic disorder, followed by hematological and gastrointestinal manifestations, possibly caused by autoimmune responses related to molecular mimicry[42,43]. Neuralgic amyotrophy and Guillain-Barre syndrome are two common neuromuscular disorders[44,45], and other uncommon presentations include mononeuritis multiplex, meningoradiculitis, and encephalitis[46-48]. Thrombocytopenia is a frequently identified hematological disorder[49,50], and pancreatitis is the most common gastrointestinal abnormality[51,52]. Myocarditis and glomerulonephritis are rarely observed complications[53,54]. Although there is evidence to support the treatment of HEV-related neurological manifestations with corticosteroids[42,55], the currently recommended therapy is plasma exchange and immune modulation with intravenous immunoglobulin[42,56].
Although extrahepatic manifestations exist with acute infection of all HHVs, such presentations are more commonly identified in HBV, with up to 20% occurrence[57]. Acute HBV infection is often subclinical and asymptomatic in around two-thirds of cases[58]. In the pre-icteric prodromal phase, there is serum sickness-like illness with arthritis and dermatitis due to the deposition of circulating IC composed of HBV surface (HBs) antigen and further activation of complements[59]. These manifestations are usually transient and are resolved after the onset of jaundice. Guillain-Barre syndrome has been reported to be associated with acute HBV infection, and both plasma exchange and intravenous immunoglobulin are effective treatments[60], implying that there is autoimmune-mediated damage to the myelin sheath of peripheral nerves.
Owing to the availability of viral markers and our understanding of pathogenic pathways, extrahepatic manifestations of chronic HBV infection have been well-elucidated for a while[33,57]. The etiopathogenesis outside the liver in the chronic phase involves the deposition of IC comprised of HBs and/or HBe antigens, followed by the local activation of complement cascades and the recruitment of inflammatory cells[61]. Notably, higher viral load or persistent infection can promote the production of IC, leading to deposition at small or medium-sized arteries[62]. In addition, viral replication has been demonstrated in the endothelium of targeted vessels[63]. Taken together, both mechanisms suggest that inhibition of viral replication, either spontaneously or under antiviral therapy, can reduce extrahepatic manifestations. Up to one-fifth of victims with chronic HBV infection have morbidities outside the liver[61], and these are comprised of arthritis, glomerulonephritis, uveitis, peripheral neuropathy, Raynaud phenomenon, Sjögren syndrome, cutaneous vasculitis, and systemic vasculitis, including polyarteritis nodosa and cryogobulinemic vasculitis[57,62,64,65]. Since the administration of immunosuppressive agents increases the risk of additional hepatic HBV replication with worsening liver disease[61], the treatment of HBV-associated glomerulonephritis and vasculitis is mainly based on antiviral agents, interferon (IFN)- α or nucleoside/nucleotide analogues (NAs)[66-70].
In most cases, symptomatic manifestations are uncommon during acute HCV infection[71]. Extrahepatic presentations include arthralgia, myalgia, and rash[72]. Patients with HCV infection usually progress to chronic stage without recovery[71,72]. Nevertheless, antiviral therapy is highly effective for the acute infection, resulting in the clearance of HCV with sustained virological response (SVR)[72,73].
Persistent HCV infection is a leading cause of chronic liver disease[74]. Although essentially curable with direct-acting antiviral (DAA) therapy, only a portion of patients are diagnosed. Notably, extrahepatic manifestations occur in up to three-quarter of victims with chronic HCV infection[75], and cryoglobulinemia is the most frequently encountered presentation (in 40% to 60% of infected patients)[76]. Direct viral invasion and deposits of soluble IC are two pathogenic processes involved in the development of disease outside the liver[75,76]. The clinical presentations include arthralgia, myalgia, glomerulonephritis, Raynaud phenomenon, Sjögren’s syndrome, Hashimoto’s thyroiditis, Graves’ disease, ulcerative keratitis, peripheral neuropathy, and cryoglobulinemia vasculitis[74-78]. Occasionally, extrahepatic-related autoimmune comorbidities such as cryoglobulinemia vasculitis can lead to the diagnosis of HCV infection[76]. Sustained eradication of HCV by IFN-α or DAA agents has shown to be beneficial for outcomes following these manifestations[78,79]. Notably, a prospective study with 9895 HCV-infected cases receiving DAA medications revealed that viral clearance is responsible for a significant decrease in HCV extrahepatic mortality[80].
VASCULITIS MANIFESTATION IN HUMAN HEPATITIS VIRUSES INFECTION
There is growing evidence demonstrating a causal relationship between viral pathogens and vasculitis. Except for HDV[24,25], other HHVs have been shown to participate in the etiopathogenesis of cutaneous and systemic vasculitis via different mechanisms, including direct viral invasion of vascular endothelium, IC-mediated vessel wall damage, and autoimmune responses with stimulation of autoreactive B-cells and impaired regulatory T cells[81-83].
Cutaneous leukocytoclastic vasculitis (CLV) rarely occurs during acute HAV infection, and it may resolve after the regression of hepatitis[36-38,84]. In chronic HBV infection, CLV is rarely observed, with an 1% incidence[62]. HBs antigen has been identified in affected skin lesions[85]. Notably, IFN-α therapy is effective against HBV-associated CLV[86]. The histopathological picture of HHV-triggered CLV shows relatively less eosinophils and lymphocytes compared with those in drug-induced CLV[81].
Acute HAV or HEV infection can induce Henoch-Schönlein purpura (referred to as HSP)[87-89], a systemic vasculitis caused by the widespread deposition of circulating immunoglobulin (Ig)A IC in small-sized blood vessels of skin, joint, lung, kidney, testis, gastrointestinal tract, and nervous system[90]. HAV- and HEV-related HSP usually have a spontaneous recovery. Owing to the defective liver catabolism of IgA IC with further tissue deposits, HSP occurs in HBV or HCV chronic liver diseases, usually requiring antiviral and corticosteroid therapy[91-94].
Vascular involvement with systemic vasculitis in HHV infection is a recognized extrahepatic morbidity with potential life-threatening organ dysfunction[31,95]. Among them, cryoglobulinemic vasculitis and polyarteritis nodosa are well-known vascular comorbidities in HHV infection[96,97].
HUMAN HEPATITIS VIRUSES-ASSOCIATED CRYOGLOBULINEMIC VASCULITIS
Cryoglobulinemic vasculitis is caused by IC-mediated inflammation of small-sized blood vessels and is accompanied by the activation of complements[96]. Cryoglobulins are circulating antibodies that precipitate in vitro at temperatures less than 37 °C and dissolve after rewarming, and these are either Ig or a mixture of Ig and complement components[98]. Individuals with cryoglobulinemia may remain asymptomatic without clinical abnormalities[96,98]. A typical triad of purpura, arthralgia, and weakness associated with organic dysfunction and elevated levels of rheumatoid factor (RF) was defined as cryoglobulinemic disease in 1966 by Meltzer et al[99]. Based on the composition of Ig, cryoglobulinemia can be classified into type I with monoclonal Ig (usually IgM), type II with polyclonal IgG and monoclonal IgM RF, and type III with polyclonal IgG and polyclonal IgM RF[100]. Type I is an infrequently encountered subgroup and is only found in hematological malignancies[101], whereas mixed types II and III can be associated with viral hepatitis caused by HBV, most commonly observed with HCV, and rarely identified with HAV infection[96,102-104]. Despite the presence of cryoglobulins in up to 60% of chronic HCV patients, cryoglobulinemic vasculitis only appears in around 10% of cases[76]. In chronic HHV infection, envelope glycoproteins can help the virus enter intrahepatic and circulating B lymphocytes to produce Ig with RF activity, resulting in the generation of cryoglobulins and formation of IC with vascular wall deposition[105,106].
Recurrent palpable purpura with the characteristic histopathological finding of leukocytoclastic vasculitis is the most common clinical manifestation[107]. Other cutaneous presentations are ischemic ulcers, digital gangrene, and Raynaud’s phenomenon. In Figure 1, the presence of leukocytoclastic vasculitis in biopsied skin lesions from a HCV-infected patient with cryoglobulinemia-associated purpura is shown. In addition, the presence of Meltzer triad was identified in most patients at the onset of disease[108], and skin, joint, kidney, and peripheral nerve are frequently affected organs in cryoglobulinemic vasculitis[96,108]. Renal involvement with membranoproliferative glomerulonephritis as the most common finding is associated with significant morbidity and even mortality[109]. HCV core protein and Ig are major components of IC and are distributed along the capillary walls of glomeruli[110]. Peripheral neuropathy with mononeuritis multiplex is commonly observed, which can be the first clinical manifestation[111]. Other rarely involved organs include the central nervous system, gastrointestinal tract, myocardium, and lung, and their involvement leads to the clinical presentations of impaired cognitive function, intestinal ischemia, hypertrophic cardiomyopathy, and diffuse alveolar hemorrhage[96,108,112-115].
Figure 1.
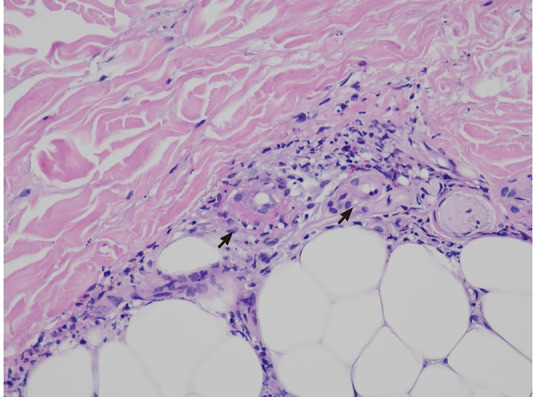
Cryoglobulinemic vasculitis. The small vessels show neutrophilic inflammation, with fibrinoid necrosis and fragmented neutrophil nuclei (black arrows). Hematoxylin and eosin staining, 400 ×.
Sporadic cases with cryoglobulinemic vasculitis, either in adults or children, have been observed during acute HAV infection[36,84,104,116,117]. These patients had biopsy-proven CLV plus arthritis or glomerulonephritis, and the use of corticosteroids were required to control these complications.
Based on clinical observations, the Meltzer triad had been regarded as an IC-mediated disease secondary to the HBV infection[118]. Since then, HBs antigen in biopsied vessel wall and HBs antigen/anti-HBs in circulating cryoprecipitates have been identified in cryoglobulinemic vasculitis[119]. A retrospective study from 913 cases with cryoglobulinemia demonstrated a HBs antigen positivity of 5.8%[120], whereas analyzed data from 231 patients with mixed cryoglobulinemia only revealed an HBV infection rate of 1.8%[107]. Nevertheless, HBV is among three common infectious etiologies in patients with cryoglobulinemia[108]. This virus is predominantly associated with type II cryoglobulinemia, and its common extrahepatic manifestations are purpura, arthralgia, and neuropathy[121]. Furthermore, compared to adults, children with symptomatic cryogobulinemia have a lower occurrence of HBV infection but higher frequencies of articular and cutaneous involvement[122]. Notably, the presence of HBs antigen represents one of the main independent predictors of mortality in cryogobulinemic vasculitis[70].
Accumulated evidence indicates that the use of NAs for viral suppression in HBV-related cryogobulinemic vasculitis can cause the disappearance of cryoglobulins, normalize liver function, and significantly clinically improve most patients with mild disease, indicating a role for HBV replication in the pathogenesis[70,123]. Antiviral therapy with NAs should be a life-long prescription, and discontinuation is considered only after persistent HBs antigen loss with the seroconversion and undetectable HBV DNA[70,124]. Despite an observation with ineffective responses[70], IFN-α can be an alternative therapeutic modality[123]. The use of corticosteroids without NA therapy is ineffective for suppression of HBV viremia, resulting in refractory or relapsing disease[121]. Nevertheless, only a portion of patients with severe disease, including peripheral neuropathy and renal involvement, can achieve clinical regression under NA therapy[70,123,125]. CD20-positive B-cells are expanded and activated in mixed cryogobulinemia, and they participate in the production of cryoglobulins[126]. Clinical remission has been reported in patients with glomerulonephritis treated with NA in combination with rituximab, a monoclonal antibody against CD20 on the surface of B-cells[127,128]. Considering the risk of viral reactivation in patients with positive HBs or an occult infection with negative HBs and positive anti-HBc, prophylaxis with NAs should be initiated before the rituximab treatment[70,124,129]. Because of a potentially fatal complication, its use should be avoided during an active flare of HBV infection[70,130]. Prescription of this biologic as a second-line agent can be considered in patients with severe disease refractory to NA therapy[70,123].
Among the underlying diseases in cryoglobulinemia, HCV infection is the most common cause, with a 73% positive frequency of anti-HCV and a 86% occurrence of HCV ribonucleic acid[102,108,131]. Nevertheless, only a small portion of cryoglobulinemia with HCV infection develops significant vasculitis manifestations[96]. Similar to HBV infection, HCV is mainly associated with type II cryoglobulinemia[108]. The interaction of HCV envelope proteins with CD81 surface receptor can stimulate B-cells to expand clonally and produce monoclonal IgM RF that binds polyclonal anti-HCV core antigen, which suggests that cryoglobulinemia is due to host immune responses against HCV infection[132-134]. Although the HCV-related mixed cryoglobulinemia is a benign B-cell lymphoproliferative condition, chronic viral stimulation on the immune system leads to the selection of abnormal clones[135]. An epidemiological survey elucidated a link between HCV infection and B-cell non-Hodgkin’s lymphoma (NHL)[135]. A large-scale cohort with 146394 HCV-infected patients demonstrated a more than 20% increased risk of this malignancy[136]. Furthermore, the overall risk of B-cell NHL in mixed cryoglobulinemia patients was estimated to be 35 times higher than that in the general population[137]. Collectively, these observations have indicated a pathogenic role of HCV in B-cell NHL.
Since HCV-related cryoglobulinemic vasculitis is an antigen-driven process and its activity usually correlates with viremia, the most effective treatment is the eradication of underlying viral infection[96]. Anti-HCV therapy can follow the existing guideline as cryoglobulinemia does not specifically influence the choice of antiviral drug[138]. DAA agents alone induce SVR with less adverse events and are more effective than the combined IFN-α and ribavirin regimen in cryoglobulinemic vasculitis[79]. In a prospective multicenter study carried out in these patients, all achieved SVR with a 90% complete clinical response after DAA therapy for 12 wk or more[139]. Altogether, patients with HCV-associated cryoglobulinemic vasculitis had higher SVR (74%-100%) and clinical remission (61%-100%) rates after receiving DAA medications[79]. Higher complete response rates (75%-100%) were observed in cutaneous and musculoskeletal presentations, while lower responses (30%-70%) were identified in peripheral nerve and renal involvement[139]. In addition, DAA therapy is beneficial for HCV-associated B-cell malignancy, resulting in higher SVR and lymphoproliferative disease response rates in indolent NHL patients[140].
In addition to optimized DAA agents, corticosteroids in combination with cyclophosphamide are also considered as first-line therapy in severe fulminant manifestations, such as intestinal necrotizing vasculitis, rapid progressive glomerulonephritis, and diffuse alveolar hemorrhage[108,141]. Plasma exchange with warm apheresis solution to avoid cryoglobulin precipitation is an adjunct treatment that is useful in life-threatening disease by removing circulating cryoglobulins to interrupt the IC-mediated pathogenesis[141]. An earlier investigation showed that the addition of rituximab to the combined IFN-α and ribavirin regimen can enhance the clearance of cryoglobulins and shorten the time to clinical remission[142]. A subsequent randomized controlled trial in patients with severe disease revealed that rituximab monotherapy is as effective as conventional immunosuppressive treatment[143]. Despite the promise of rituximab as a therapeutic agent[144], there is a substantial risk for IC formation between this biologic and RF-positive IgM to exacerbate the vasculitis activity in HCV-associated type II cryoglobulinemic vasculitis[145].
HUMAN HEPATITIS VIRUSES-ASSOCIATED POLYARTERITIS NODOSA
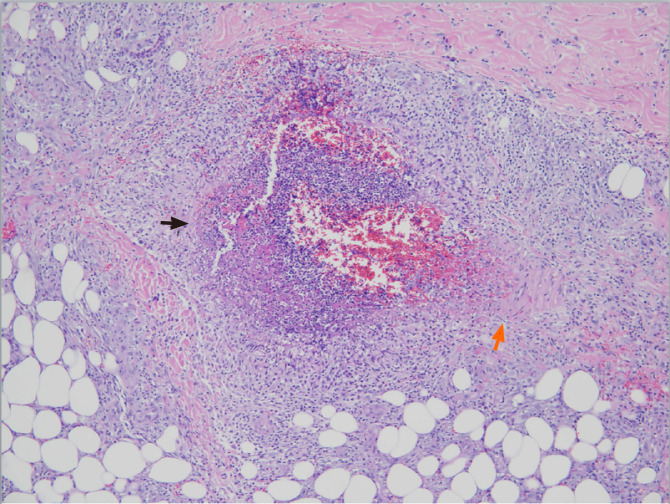
Polyarteritis nodosa is a rare disease, with an annual incidence ranging from 0 to 2 cases per million population[97]. It is a necrotizing vasculitis affecting small- and medium-sized arteries, with systemic involvement but usually sparing the lungs. A skin-restricted form involving the area below the knees can progress to the systemic form, suggesting the same entity for both forms[146,147]. Most patients with polyarteritis nodosa belong to the idiopathic type with autoimmune-mediated mechanisms[97,148]. The secondary type is often observed in viral diseases, like cytomegalovirus, human immunodeficiency virus, and HHV infections. The association between HBV and polyarteritis nodosa was first recognized in 1970[149,150], and since then a substantial portion of cases have been identified after HBV infection. Later on, owing to the introduction of the vaccination protocol, the occurrence of HBV-related polyarteritis nodosa has gradually disappeared from clinical practice[151]. Although HCV positivity has been observed in this disorder, it is not a dominant etiological factor[152]. The occlusion and rupture of inflamed arteries can produce ischemia and hemorrhage in various organs and tissues, including skin, joint, kidney, testis, gastrointestinal tract, and peripheral nerve[97,148]. The most frequently involved organ system is the skin, presenting manifestations of palpable purpura, livedoid lesions, subcutaneous nodules, and necrotic ulcers[153]. Figure 2 demonstrates the histopathological findings of transmural necrotizing arteritis with neutrophilic infiltration and fibrinoid necrosis in a cutaneous biopsy specimen from a patient with polyarteritis nodosa-associated nodules (Case No. 2 in Table 2). Mononeuritis multiplex and symmetrical polyneuropathy are common neurological complications[154]. Gastrointestinal manifestations are usually associated with significant morbidity and can manifest as an acute surgical abdomen[155]. Renal involvement without glomerulonephritis is related to infarction or hemorrhage caused by the rupture of renal micro-aneurysms[97]. Although rare as an initial presentation, orchitis due to testicular ischemia is a characteristic finding of polyarteritis nodosa[156].
Figure 2.
Polyarteritis nodosa. The vascular wall shows transmural necrotizing inflammation, with intense neutrophilic infiltration and fibrinoid necrosis (black arrow). There is residual muscular wall of the vessel (orange arrow). Hematoxylin and eosin staining, 100 ×.
Table 2.
Clinical and medication profiles in five polyarteritis nodosa patients naïve to human hepatitis virus infection1
|
No
|
Age/sex
|
Initial symptom
|
Diagnosisperiod2
|
BVAS/FFS3
|
HHV status4
|
Follow-up manifestation
|
Therapy
|
Outcome
|
| 1 | 23/F | Arthralgia, rash5 | 2 mo | 6/0 | Negative | Joint, PN, skin | Az, Cs | Survival, remission |
| 2 | 29/F | Arthralgia, fever, rash5 | 1 yr | 11/0 | Negative | FN, GI, joint, PN, skin | Az, Cs | Survival, remission |
| 3 | 38/F | Fever, rash5 | 1 yr 5 mo | 14/0 | Negative | Joint, PN, skin | Az, Cs, Cy | Survival, remission |
| 4 | 39/M | Rash | 3 yr 10 mo | 18/1 | Negative | Kidney, skin | Az, Cs, RTX | Survival, chronic renal insufficiency |
| 5 | 42/M | Arthralgia, rash | 6 mo | 7/0 | Negative | Joint, PN, skin, kidney, testis | ADA, Az, Cs, Cy | Survival, remission |
Enrollment from 2012 to 2019.
Time period from initial symptoms to the established diagnosis.
Calculation at the disease diagnosis.
Human hepatitis virus status, including examinations for hepatitis A virus, hepatitis B virus, hepatitis C virus, and hepatitis delta virus.
Erythematous nodosum-like skin lesions. ADA: Adalimumab; Az: Azathioprine; BVAS: Birmingham vasculitis activity score; Cs: Corticosteroids, Cy: Cyclophosphamide; F: Female; FFS: Four-factor score, including age above 65 years, cardiac symptoms, gastrointestinal involvement, and renal insufficiency; FN: Foot necrosis; GI: Gastrointestinal; HHV: Human hepatitis virus; M: Male; PAN: Polyarteritis nodosa; PN: Peripheral neuropathy; RTX: Rituximab.
As supported by clinical evidence, the idiopathic type benefits from combined corticosteroids and cyclophosphamide therapy, which induce remission in severe disease with organ dysfunction; whereas, mild manifestations can be treated with corticosteroids alone[97,148]. After the completion of cyclophosphamide therapy, azathioprine or methotrexate can be prescribed as a remission-maintenance agent[97]. Currently, small-molecule targeted drugs and biologics have been used in refractory patients naïve to HBV infection with successful outcomes, including Janus kinase inhibitor, B-cell targeted agent, tumor necrosis factor blocker, and interleukin-6 blockade[157-161]. The clinical data and medication profiles with biologics in polyarteritis nodosa patients recently diagnosed by authors are shown in Table 2. Although poor prognosis in earlier years is due to a delayed diagnosis[97], the overall outcome has improved to a 5-year survival rate of 80%. Outcomes are significantly worsened in association with HBV infection, age above 65 years, new-onset hypertension, renal impairment with high creatinine levels, gastrointestinal involvement requiring surgery, and peripheral nerve involvement[153].
There are no available reports related to the association of polyarteritis nodosa with HAV. Nevertheless, published cases with cutaneous or renal manifestations have shown histopathological evidence of medium-sized blood vessels’ involvement compatible with the diagnosis of polyarteritis nodosa[162,163].
Simultaneous development of mixed cryoglobulinemia and polyarteritis nodosa has been documented in patients with coinfection of HCV and HBV[164,165]. In a clinical survey on polyarteritis nodosa, the positive frequency of anti-HCV was 20% (5% with detectable HCV ribonucleic acid and they were more likely to have cutaneous manifestation)[166]. In a large cohort with 161 cases of HCV-related vasculitis (19% polyarteritis nodosa and 81% cryoglobulinemic vasculitis), there were more acute and severe clinical presentations in the former group, including constitutional symptoms, new-onset hypertension, gastrointestinal tract involvement, and mononeuritis multiplex[167]. Despite no differences in the 5-year survival rates, there was a higher complete remission rate for polyarteritis nodosa than for cryoglobulinemic vasculitis. The therapeutic guidelines for HCV-related polyarteritis nodosa have not been established yet. Reported cases have received various combinations of corticosteroids, cyclophosphamide, and antiviral agents. B-cell targeted therapy has been applied to patients with HCV-related polyarteritis nodosa[167,168]. A higher clinical relapse rate was observed for rituximab treatment than for a combined regimen with IFN-α and ribavirin in this disease[167].
During the 1970s to 1980s, HBV was a major cause of polyarteritis nodosa, with nearly half of cases having this infection, but the frequency has decreased due to improved blood safety and a viral vaccine campaign since early the 1990s, indicating HBV as a causal etiology[169]. Although histopathological studies have rarely confirmed the presence of HBV antigens in the vessel wall[151], the pathogenesis of HBV-related type is related to the deposits of IC, different from the idiopathic disease[169]. When comparing HBV- with HCV-associated polyarteritis nodosa[167,169], the former group has more general symptoms and orchitis, less cutaneous manifestation and new-onset hypertension, a lower survival rate, and a much shorter average period from viral infection to vasculitis development, 7 mo vs 20 years. In the HBV-related type, most cases have higher viral replication and HBV DNA levels, leading to the persistent presence of circulating IC[169].
The prevalence of HBs antigen in the general Taiwan population used to be near 20%, but the HBV seropositive rate in children has decreased dramatically from 11% to less than 1% after the initiation of the national vaccination program in 1984[170]. Despite a high inpatient prevalence with more than 10 cases per 100000 discharges in the United States[171], polyarteritis nodosa has been very rarely encountered by practicing physicians in Taiwan. In particular, even with more than 2 million infected patients, there is no reported association between HBV and polyarteritis nodosa[159,172-177]. Perinatal mother-to-infant HBV transmission is the most important factor responsible for a high carriage rate of HBs antigen in Taiwan[178]. Vertical transmission in infants is asymptomatic until adulthood and associated with a greater risk of chronic infection, suggesting that HBV infection causes an immunotolerant status in children who are less prone to develop full immune responses[179]. Parenteral HBV infection during adult life can induce polyarteritis nodosa within 1 year after viral infection, indicating an early post-infectious disease via this transmission route[169]. In Table 3, polyarteritis nodosa from various larger-number case series with different HBV-associated frequencies is compared[153,172-177,180-184]. Patients reported from Taiwan have no HBV infection, younger female predominance, and more cutaneous or testicular manifestation than those from other series with a HBV association.
Table 3.
Comparisons of clinical and outcome profiles in polyarteritis nodosa from various case series with different hepatitis B virus-infected frequencies
|
Source of cases
|
No/age1 F%
|
HBV status
|
Fever
|
AM
|
Skin
|
PN
|
GI
|
Renal
|
TestisM%
|
Therapy
|
Death
|
| Taiwan | 12/31, 67% | 0% | 67% | 58% | 75% | 42% | 50% | 75% | 50% | Az, Cs, Cy, Bio | 33% |
| United Kingdom | 17/49, 24% | 31% | 76% | 77% | 65% | 59% | 65% | 77% | NA | Cs, Is | 38% |
| United States | 53/54, 34% | 11% | 31% | 55% | 58% | 60% | 25% | 66% | NA | Cs, Cy | 42% |
| Canada | 45/54, 47% | 19% | 63% | 51% | 44% | 51% | 53% | 44% | 4% | Cs, Cy | 53% |
| South Korea | 27/47, 37% | 56% | 52% | 30% | 44% | 63% | 48% | 48% | 24% | Az, Cs, Cy, Is | 15% |
| France | 348/51,37% | 35% | 64% | 59% | 50% | 74% | 38% | 51% | 17% | Av, Cs, Cy, Is | 25% |
| India | 27/38, 26% | 26% | 52% | 37% | 37% | 82% | 30% | 59% | 30% | Av, Az, Cs, Cy | 11% |
Mean age at disease diagnosis. AM: Articulomuscular; Az: Azathioprine; Av: Antiviral agent; Bio: Biologics; Cs: Corticosteroids; Cy: Cyclophosphamide; F: Female; GI: Gastrointestinal; Is: Immunosuppressive agent, M: Male; NA: Not available; PN: Peripheral neuropathy.
After initial therapy with corticosteroids to reduce acute vascular inflammation, the therapeutic approach in HBV-associated polyarteritis nodosa is to clear circulating IC and suppress HBV replication by plasma exchange and antiviral agents with NAs or IFN-α[169]. Since the addition of cyclophosphamide to corticosteroids has shown to benefit patients presenting with poor prognostic factors[185], this regimen can be considered in patients with severe disease. Notably, plasma exchange has not been shown to be beneficial in polyarteritis nodosa patients without HBV infection[186]. Furthermore, antiviral agents can be used concurrently with corticosteroids or combined cyclophosphamide and corticosteroids therapy[97,148]. Contradictory to the idiopathic disease, relapses have rarely been observed in HBV-related type, especially when viral replication has ceased and seroconversion from HBe antigen to antibody has been achieved after antiviral therapy[148,169].
CONCLUSION
Although HHVs primarily affect hepatocytes, they can also cause complications in other organs, and both acute and chronic viral hepatitis are associated with clinical presentations outside the liver. Vascular involvement with cutaneous and systemic vasculitis is a well-known extrahepatic morbidity. There is growing evidence suggesting a causal relationship between viral pathogens and vasculitis. Except for HDV, other HHVs have participated in the etiopathogenesis of cutaneous and systemic vasculitis via different mechanisms, including direct viral invasion of vascular endothelial cells, IC-mediated vessel wall damage, and autoimmune responses with stimulation of autoreactive B-cells and impaired regulatory T cells. Cryoglobulinemic vasculitis and polyarteritis nodosa are recognized for their association with chronic HHV infection. Therapeutic guidelines for HHV-associated vasculitis have not been established yet. Antiviral therapy should be initiated in HBV and HCV-related systemic vasculitis in addition to the use of corticosteroids. Plasma exchange and/or combined cyclophosphamide and corticosteroid therapy can be considered in patients with severe life-threatening vasculitis manifestations.
ACKNOWLEDGEMENTS
The authors are indebted to Dr. Wu IC, Division of Gastroenterology and Hepatology, for his valuable comments on HHV-related clinical manifestations, and to other physicians at the National Cheng Kung University Hospital involved in the diagnosis and management of reported patients. The Institutional Review Board of National Cheng Kung University Hospital approved this study (No. B-ER-105-108).
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest.
Manuscript source: Invited manuscript
Peer-review started: November 21, 2020
First decision: December 17, 2020
Article in press: December 27, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liang XS, Wang K S-Editor: Zhang L L-Editor: A P-Editor: Ma YJ
Contributor Information
Chrong-Reen Wang, Department of Internal Medicine, National Cheng Kung University Hospital, Tainan 70403, Taiwan. wangcr@mail.ncku.edu.tw.
Hung-Wen Tsai, Department of Pathology, National Cheng Kung University Hospital, Tainan 70403, Taiwan.
References
- 1.Arroyo V, Moreau R, Jalan R. Acute-on-Chronic Liver Failure. N Engl J Med. 2020;382:2137–2145. doi: 10.1056/NEJMra1914900. [DOI] [PubMed] [Google Scholar]
- 2.Razavi H. Global Epidemiology of Viral Hepatitis. Gastroenterol Clin North Am. 2020;49:179–189. doi: 10.1016/j.gtc.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Noor A, Panwala A, Forouhar F, Wu GY. Hepatitis caused by herpes viruses: A review. J Dig Dis. 2018;19:446–455. doi: 10.1111/1751-2980.12640. [DOI] [PubMed] [Google Scholar]
- 4.Rasche A, Sander AL, Corman VM, Drexler JF. Evolutionary biology of human hepatitis viruses. J Hepatol. 2019;70:501–520. doi: 10.1016/j.jhep.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khuroo MS, Sofi AA. The Discovery of Hepatitis Viruses: Agents and Disease. J Clin Exp Hepatol. 2020;10:391–401. doi: 10.1016/j.jceh.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krugman S, Giles JP, Hammond J. Infectious hepatitis. Evidence for two distinctive clinical, epidemiological, and immunological types of infection. JAMA. 1967;200:365–373. doi: 10.1001/jama.200.5.365. [DOI] [PubMed] [Google Scholar]
- 7.BLUMBERG BS, ALTER HJ, VISNICH S. A "NEW" ANTIGEN IN LEUKEMIA SERA. JAMA. 1965;191:541–546. doi: 10.1001/jama.1965.03080070025007. [DOI] [PubMed] [Google Scholar]
- 8.Dane DS, Cameron CH, Briggs M. Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet. 1970;1:695–698. doi: 10.1016/s0140-6736(70)90926-8. [DOI] [PubMed] [Google Scholar]
- 9.Burrell CJ, Mackay P, Greenaway PJ, Hofschneider PH, Murray K. Expression in Escherichia coli of hepatitis B virus DNA sequences cloned in plasmid pBR322. Nature. 1979;279:43–47. doi: 10.1038/279043a0. [DOI] [PubMed] [Google Scholar]
- 10.Koff RS. Feinstone SM, Kapikian AZ, Purcell RH. Hepatitis A: detection by immune electron microscopy of a virus like antigen associated with acute illness [Science 1973;182:1026-1028] J Hepatol. 2002;37:2–6. doi: 10.1016/s0168-8278(02)00169-1. [DOI] [PubMed] [Google Scholar]
- 11.Ticehurst JR, Racaniello VR, Baroudy BM, Baltimore D, Purcell RH, Feinstone SM. Molecular cloning and characterization of hepatitis A virus cDNA. Proc Natl Acad Sci USA. 1983;80:5885–5889. doi: 10.1073/pnas.80.19.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alter HJ, Holland PV, Morrow AG, Purcell RH, Feinstone SM, Moritsugu Y. Clinical and serological analysis of transfusion-associated hepatitis. Lancet. 1975;2:838–841. doi: 10.1016/s0140-6736(75)90234-2. [DOI] [PubMed] [Google Scholar]
- 13.Alter HJ, Purcell RH, Holland PV, Popper H. Transmissible agent in non-A, non-B hepatitis. Lancet. 1978;1:459–463. doi: 10.1016/s0140-6736(78)90131-9. [DOI] [PubMed] [Google Scholar]
- 14.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 15.Rizzetto M, Canese MG, Aricò S, Crivelli O, Trepo C, Bonino F, Verme G. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut. 1977;18:997–1003. doi: 10.1136/gut.18.12.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizzetto M, Hoyer B, Canese MG, Shih JW, Purcell RH, Gerin JL. delta Agent: association of delta antigen with hepatitis B surface antigen and RNA in serum of delta-infected chimpanzees. Proc Natl Acad Sci USA. 1980;77:6124–6128. doi: 10.1073/pnas.77.10.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khuroo MS. Study of an epidemic of non-A, non-B hepatitis. Possibility of another human hepatitis virus distinct from post-transfusion non-A, non-B type. Am J Med. 1980;68:818–824. doi: 10.1016/0002-9343(80)90200-4. [DOI] [PubMed] [Google Scholar]
- 18.Reyes GR, Purdy MA, Kim JP, Luk KC, Young LM, Fry KE, Bradley DW. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science. 1990;247:1335–1339. doi: 10.1126/science.2107574. [DOI] [PubMed] [Google Scholar]
- 19.Hofmeister MG, Foster MA, Teshale EH. Epidemiology and Transmission of Hepatitis A Virus and Hepatitis E Virus Infections in the United States. Cold Spring Harb Perspect Med. 2019;9 doi: 10.1101/cshperspect.a033431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abutaleb A, Kottilil S. Hepatitis A: Epidemiology, Natural History, Unusual Clinical Manifestations, and Prevention. Gastroenterol Clin North Am. 2020;49:191–199. doi: 10.1016/j.gtc.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aslan AT, Balaban HY. Hepatitis E virus: Epidemiology, diagnosis, clinical manifestations, and treatment. World J Gastroenterol. 2020;26:5543–5560. doi: 10.3748/wjg.v26.i37.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuen MF, Chen DS, Dusheiko GM, Janssen HLA, Lau DTY, Locarnini SA, Peters MG, Lai CL. Hepatitis B virus infection. Nat Rev Dis Primers. 2018;4:18035. doi: 10.1038/nrdp.2018.35. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan DE. Hepatitis C Virus. Ann Intern Med. 2020;173:ITC33–ITC48. doi: 10.7326/AITC202009010. [DOI] [PubMed] [Google Scholar]
- 24.Gilman C, Heller T, Koh C. Chronic hepatitis delta: A state-of-the-art review and new therapies. World J Gastroenterol. 2019;25:4580–4597. doi: 10.3748/wjg.v25.i32.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor JM. Infection by Hepatitis Delta Virus. Viruses. 2020;12 doi: 10.3390/v12060648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng Z, Hirai-Yuki A, McKnight KL, Lemon SM. Naked Viruses That Aren't Always Naked: Quasi-Enveloped Agents of Acute Hepatitis. Annu Rev Virol. 2014;1:539–560. doi: 10.1146/annurev-virology-031413-085359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung A, Kwo P. Viral Hepatitis Other than A, B, and C: Evaluation and Management. Clin Liver Dis. 2020;24:405–419. doi: 10.1016/j.cld.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao Y, Bing Z, Guan S, Zhang Z, Wang X. Development of new hepatitis E vaccines. Hum Vaccin Immunother. 2018;14:2254–2262. doi: 10.1080/21645515.2018.1469591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. Hepatitis 2020. https://www.who.int/health-topics/hepatitis#tab=tab_1 . [Google Scholar]
- 30.Schiff ER. Atypical clinical manifestations of hepatitis A. Vaccine. 1992;10 Suppl 1:S18–S20. doi: 10.1016/0264-410x(92)90534-q. [DOI] [PubMed] [Google Scholar]
- 31.Pyrsopoulos NT, Reddy KR. Extrahepatic manifestations of chronic viral hepatitis. Curr Gastroenterol Rep. 2001;3:71–78. doi: 10.1007/s11894-001-0044-1. [DOI] [PubMed] [Google Scholar]
- 32.Amarapurkar DN, Amarapurkar AD. Extrahepatic manifestations of viral hepatitis. Ann Hepatol. 2002;1:192–195. [PubMed] [Google Scholar]
- 33.Han SH. Extrahepatic manifestations of chronic hepatitis B. Clin Liver Dis. 2004;8:403–418. doi: 10.1016/j.cld.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Romano C, Cuomo G, Ferrara R, Del Mastro A, Esposito S, Sellitto A, Adinolfi LE. Uncommon immune-mediated extrahepatic manifestations of HCV infection. Expert Rev Clin Immunol. 2018;14:1089–1099. doi: 10.1080/1744666X.2018.1538790. [DOI] [PubMed] [Google Scholar]
- 35.Shin EC, Jeong SH. Natural History, Clinical Manifestations, and Pathogenesis of Hepatitis A. Cold Spring Harb Perspect Med. 2018;8 doi: 10.1101/cshperspect.a031708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inman RD, Hodge M, Johnston ME, Wright J, Heathcote J. Arthritis, vasculitis, and cryoglobulinemia associated with relapsing hepatitis A virus infection. Ann Intern Med. 1986;105:700–703. doi: 10.7326/0003-4819-105-5-700. [DOI] [PubMed] [Google Scholar]
- 37.Ilan Y, Hillman M, Oren R, Zlotogorski A, Shouval D. Vasculitis and cryoglobulinemia associated with persisting cholestatic hepatitis A virus infection. Am J Gastroenterol. 1990;85:586–587. [PubMed] [Google Scholar]
- 38.Dan M, Yaniv R. Cholestatic hepatitis, cutaneous vasculitis, and vascular deposits of immunoglobulin M and complement associated with hepatitis A virus infection. Am J Med. 1990;89:103–104. doi: 10.1016/0002-9343(90)90107-o. [DOI] [PubMed] [Google Scholar]
- 39.Shenoy R, Nair S, Kamath N. Thrombocytopenia in hepatitis A--an atypical presentation. J Trop Pediatr. 2004;50:241–242. doi: 10.1093/tropej/50.4.241. [DOI] [PubMed] [Google Scholar]
- 40.Allen O, Edhi A, Hafeez A, Halalau A. A Very Rare Complication of Hepatitis A Infection: Acute Myocarditis-A Case Report with Literature Review. Case Rep Med. 2018;2018:3625139. doi: 10.1155/2018/3625139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stübgen JP. Neuromuscular complications of hepatitis A virus infection and vaccines. J Neurol Sci. 2011;300:2–8. doi: 10.1016/j.jns.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 42.Dalton HR, Kamar N, van Eijk JJ, Mclean BN, Cintas P, Bendall RP, Jacobs BC. Hepatitis E virus and neurological injury. Nat Rev Neurol. 2016;12:77–85. doi: 10.1038/nrneurol.2015.234. [DOI] [PubMed] [Google Scholar]
- 43.Rawla P, Raj JP, Kannemkuzhiyil AJ, Aluru JS, Thandra KC, Gajendran M. A Systematic Review of the Extra-Hepatic Manifestations of Hepatitis E Virus Infection. Med Sci (Basel) 2020;8 doi: 10.3390/medsci8010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Avila JD, Lacomis D, Lam EM. Neuralgic Amyotrophy Associated With Hepatitis E Virus Infection: First Case in the United States. J Clin Neuromuscul Dis. 2016;18:96–100. doi: 10.1097/CND.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 45.Loly JP, Rikir E, Seivert M, Legros E, Defrance P, Belaiche J, Moonen G, Delwaide J. Guillain-Barré syndrome following hepatitis E. World J Gastroenterol. 2009;15:1645–1647. doi: 10.3748/wjg.15.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perrin HB, Cintas P, Abravanel F, Gérolami R, d'Alteroche L, Raynal JN, Alric L, Dupuis E, Prudhomme L, Vaucher E, Couzigou P, Liversain JM, Bureau C, Vinel JP, Kamar N, Izopet J, Peron JM. Neurologic Disorders in Immunocompetent Patients with Autochthonous Acute Hepatitis E. Emerg Infect Dis. 2015;21:1928–1934. doi: 10.3201/eid2111.141789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dalton HR, van Eijk JJJ, Cintas P, Madden RG, Jones C, Webb GW, Norton B, Pique J, Lutgens S, Devooght-Johnson N, Woolson K, Baker J, Saunders M, Househam L, Griffiths J, Abravanel F, Izopet J, Kamar N, van Alfen N, van Engelen BGM, Hunter JG, van der Eijk AA, Bendall RP, Mclean BN, Jacobs BC. Hepatitis E virus infection and acute non-traumatic neurological injury: A prospective multicentre study. J Hepatol. 2017;67:925–932. doi: 10.1016/j.jhep.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 48.Despierres LA, Kaphan E, Attarian S, Cohen-Bacrie S, Pelletier J, Pouget J, Motte A, Charrel R, Gerolami R, Colson P. Neurologic disorders and hepatitis E, France, 2010. Emerg Infect Dis. 2011;17:1510–1512. doi: 10.3201/eid1708.102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marion O, Abravanel F, Del Bello A, Esposito L, Lhomme S, Puissant-Lubrano B, Alric L, Faguer S, Izopet J, Kamar N. Hepatitis E virus-associated cryoglobulinemia in solid-organ-transplant recipients. Liver Int. 2018;38:2178–2189. doi: 10.1111/liv.13894. [DOI] [PubMed] [Google Scholar]
- 50.Woolson KL, Forbes A, Vine L, Beynon L, McElhinney L, Panayi V, Hunter JG, Madden RG, Glasgow T, Kotecha A, Dalton HC, Mihailescu L, Warshow U, Hussaini HS, Palmer J, Mclean BN, Haywood B, Bendall RP, Dalton HR. Extra-hepatic manifestations of autochthonous hepatitis E infection. Aliment Pharmacol Ther. 2014;40:1282–1291. doi: 10.1111/apt.12986. [DOI] [PubMed] [Google Scholar]
- 51.Nayak HK, Kamble NL, Raizada N, Garg S, Daga MK. Acute pancreatitis complicating acute hepatitis e virus infection: a case report and review. Case Reports Hepatol. 2013;2013:531235. doi: 10.1155/2013/531235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raj M, Kumar K, Ghoshal UC, Saraswat VA, Aggarwal R, Mohindra S. Acute Hepatitis E-Associated Acute Pancreatitis: A Single Center Experience and Literature Review. Pancreas. 2015;44:1320–1322. doi: 10.1097/MPA.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 53.Sengupta P, Biswas S, Roy T. Hepatitis E-Induced Acute Myocarditis in an Elderly Woman. Case Rep Gastroenterol. 2019;13:342–349. doi: 10.1159/000501998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Del Bello A, Guilbeau-Frugier C, Josse AG, Rostaing L, Izopet J, Kamar N. Successful treatment of hepatitis E virus-associated cryoglobulinemic membranoproliferative glomerulonephritis with ribavirin. Transpl Infect Dis. 2015;17:279–283. doi: 10.1111/tid.12353. [DOI] [PubMed] [Google Scholar]
- 55.van Eijk JJ, van Alfen N, Berrevoets M, van der Wilt GJ, Pillen S, van Engelen BG. Evaluation of prednisolone treatment in the acute phase of neuralgic amyotrophy: an observational study. J Neurol Neurosurg Psychiatry. 2009;80:1120–1124. doi: 10.1136/jnnp.2008.163386. [DOI] [PubMed] [Google Scholar]
- 56.Osman C, Jennings R, El-Ghariani K, Pinto A. Plasma exchange in neurological disease. Pract Neurol. 2020;20:92–99. doi: 10.1136/practneurol-2019-002336. [DOI] [PubMed] [Google Scholar]
- 57.Trepo C, Guillevin L. Polyarteritis nodosa and extrahepatic manifestations of HBV infection: the case against autoimmune intervention in pathogenesis. J Autoimmun. 2001;16:269–274. doi: 10.1006/jaut.2000.0502. [DOI] [PubMed] [Google Scholar]
- 58.McMahon BJ, Alward WL, Hall DB, Heyward WL, Bender TR, Francis DP, Maynard JE. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis. 1985;151:599–603. doi: 10.1093/infdis/151.4.599. [DOI] [PubMed] [Google Scholar]
- 59.Kappus MR, Sterling RK. Extrahepatic manifestations of acute hepatitis B virus infection. Gastroenterol Hepatol (N Y) 2013;9:123–126. [PMC free article] [PubMed] [Google Scholar]
- 60.Yimam KK, Merriman RB, Todd Frederick R. A rare case of acute hepatitis B virus infection causing guillain-barré syndrome. Gastroenterol Hepatol (N Y) 2013;9:121–123. [PMC free article] [PubMed] [Google Scholar]
- 61.Cacoub P, Terrier B. Hepatitis B-related autoimmune manifestations. Rheum Dis Clin North Am. 2009;35:125–137. doi: 10.1016/j.rdc.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 62.Cacoub P, Saadoun D, Bourlière M, Khiri H, Martineau A, Benhamou Y, Varastet M, Pol S, Thibault V, Rotily M, Halfon P. Hepatitis B virus genotypes and extrahepatic manifestations. J Hepatol. 2005;43:764–770. doi: 10.1016/j.jhep.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 63.Mason A, Theal J, Bain V, Adams E, Perrillo R. Hepatitis B virus replication in damaged endothelial tissues of patients with extrahepatic disease. Am J Gastroenterol. 2005;100:972–976. doi: 10.1111/j.1572-0241.2005.41308.x. [DOI] [PubMed] [Google Scholar]
- 64.Levo Y, Gorevic PD, Kassab HJ, Zucker-Franklin D, Franklin EC. Association between hepatitis B virus and essential mixed cryoglobulinemia. N Engl J Med. 1977;296:1501–1504. doi: 10.1056/NEJM197706302962605. [DOI] [PubMed] [Google Scholar]
- 65.Stübgen JP. Neuromuscular disorders associated with hepatitis B virus infection. J Clin Neuromuscul Dis. 2011;13:26–37. doi: 10.1097/CND.0b013e3181df2b2b. [DOI] [PubMed] [Google Scholar]
- 66.Guillevin L, Lhote F, Sauvaget F, Deblois P, Rossi F, Levallois D, Pourrat J, Christoforov B, Trépo C. Treatment of polyarteritis nodosa related to hepatitis B virus with interferon-alpha and plasma exchanges. Ann Rheum Dis. 1994;53:334–337. doi: 10.1136/ard.53.5.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guillevin L, Mahr A, Cohen P, Larroche C, Queyrel V, Loustaud-Ratti V, Imbert B, Hausfater P, Roudier J, Bielefeld P, Petitjean P, Smadja D French Vasculitis Study Group. Short-term corticosteroids then lamivudine and plasma exchanges to treat hepatitis B virus-related polyarteritis nodosa. Arthritis Rheum. 2004;51:482–487. doi: 10.1002/art.20401. [DOI] [PubMed] [Google Scholar]
- 68.Tang S, Lai FM, Lui YH, Tang CS, Kung NN, Ho YW, Chan KW, Leung JC, Lai KN. Lamivudine in hepatitis B-associated membranous nephropathy. Kidney Int. 2005;68:1750–1758. doi: 10.1111/j.1523-1755.2005.00591.x. [DOI] [PubMed] [Google Scholar]
- 69.Chung DR, Yang WS, Kim SB, Yu E, Chung YH, Lee Y, Park JS. Treatment of hepatitis B virus associated glomerulonephritis with recombinant human alpha interferon. Am J Nephrol. 1997;17:112–117. doi: 10.1159/000169083. [DOI] [PubMed] [Google Scholar]
- 70.Mazzaro C, Dal Maso L, Visentini M, Gitto S, Andreone P, Toffolutti F, Gattei V. Hepatitis B virus-related cryogobulinemic vasculitis. The role of antiviral nucleot(s)ide analogues: a review. J Intern Med. 2019;286:290–298. doi: 10.1111/joim.12913. [DOI] [PubMed] [Google Scholar]
- 71.Martinello M, Hajarizadeh B, Grebely J, Dore GJ, Matthews GV. Management of acute HCV infection in the era of direct-acting antiviral therapy. Nat Rev Gastroenterol Hepatol. 2018;15:412–424. doi: 10.1038/s41575-018-0026-5. [DOI] [PubMed] [Google Scholar]
- 72.Loomba R, Rivera MM, McBurney R, Park Y, Haynes-Williams V, Rehermann B, Alter HJ, Herrine SK, Liang TJ, Hoofnagle JH, Heller T. The natural history of acute hepatitis C: clinical presentation, laboratory findings and treatment outcomes. Aliment Pharmacol Ther. 2011;33:559–565. doi: 10.1111/j.1365-2036.2010.04549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morin T, Pariente A, Lahmek P, Rabaud C, Silvain C, Cadranel JF Association Nationale des Hépato-gastroentérologues des Hôpitaux généraux (ANGH) Société de Pathologie Infectieuse de Langue Française (SPILF) Fédération des Pôles de Référence et Réseaux Hépatites (FPRRH) Acute hepatitis C: analysis of a 126-case prospective, multicenter cohort. Eur J Gastroenterol Hepatol. 2010;22:157–166. doi: 10.1097/MEG.0b013e328330a8e8. [DOI] [PubMed] [Google Scholar]
- 74.Spearman CW, Dusheiko GM, Hellard M, Sonderup M. Hepatitis C. Lancet. 2019;394:1451–1466. doi: 10.1016/S0140-6736(19)32320-7. [DOI] [PubMed] [Google Scholar]
- 75.Cacoub P, Poynard T, Ghillani P, Charlotte F, Olivi M, Piette JC, Opolon P. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum. 1999;42:2204–2212. doi: 10.1002/1529-0131(199910)42:10<2204::AID-ANR24>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 76.Kuna L, Jakab J, Smolic R, Wu GY, Smolic M. HCV Extrahepatic Manifestations. J Clin Transl Hepatol. 2019;7:172–182. doi: 10.14218/JCTH.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zegans ME, Anninger W, Chapman C, Gordon SR. Ocular manifestations of hepatitis C virus infection. Curr Opin Ophthalmol. 2002;13:423–427. doi: 10.1097/00055735-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 78.Petta S, Craxì A. Extrahepatic Manifestations of Chronic Viral C Hepatitis. Gastroenterol Clin North Am. 2020;49:347–360. doi: 10.1016/j.gtc.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 79.Comarmond C, Cacoub P, Saadoun D. Treatment of chronic hepatitis C-associated cryoglobulinemia vasculitis at the era of direct-acting antivirals. Therap Adv Gastroenterol. 2020;13:1756284820942617. doi: 10.1177/1756284820942617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, De Ledinghen V, Larrey D, Haour G, Bronowicki JP, Zoulim F, Asselah T, Marcellin P, Thabut D, Leroy V, Tran A, Habersetzer F, Samuel D, Guyader D, Chazouilleres O, Mathurin P, Metivier S, Alric L, Riachi G, Gournay J, Abergel A, Cales P, Ganne N, Loustaud-Ratti V, D'Alteroche L, Causse X, Geist C, Minello A, Rosa I, Gelu-Simeon M, Portal I, Raffi F, Bourliere M, Pol S French ANRS CO22 Hepather cohort. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019;393:1453–1464. doi: 10.1016/S0140-6736(18)32111-1. [DOI] [PubMed] [Google Scholar]
- 81.Thomas K, Vassilopoulos D. Infections and vasculitis. Curr Opin Rheumatol. 2017;29:17–23. doi: 10.1097/BOR.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 82.Lidar M, Lipschitz N, Langevitz P, Shoenfeld Y. The infectious etiology of vasculitis. Autoimmunity. 2009;42:432–438. doi: 10.1080/08916930802613210. [DOI] [PubMed] [Google Scholar]
- 83.Vergani D, Mieli-Vergani G. Autoimmune manifestations in viral hepatitis. Semin Immunopathol. 2013;35:73–85. doi: 10.1007/s00281-012-0328-6. [DOI] [PubMed] [Google Scholar]
- 84.Cozzani E, Herzum A, Burlando M, Parodi A. Cutaneous manifestations of HAV, HBV, HCV. G Ital Dermatol Venereol. 2019 doi: 10.23736/S2784-8671.19.06488-5. [DOI] [PubMed] [Google Scholar]
- 85.Grigorescu I, Dumitrascu DL. Spontaneous and antiviral-induced cutaneous lesions in chronic hepatitis B virus infection. World J Gastroenterol. 2014;20:15860–15866. doi: 10.3748/wjg.v20.i42.15860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Glück T, Weber P, Wiedmann KH. [Hepatitis-B-associated vasculitis. Clinical course with glucocorticoid and alpha-interferon therapy] Dtsch Med Wochenschr. 1994;119:1388–1392. doi: 10.1055/s-2008-1058850. [DOI] [PubMed] [Google Scholar]
- 87.Chemli J, Zouari N, Belkadhi A, Abroug S, Harbi A. [Hepatitis A infection and Henoch-Schonlein purpura: a rare association] Arch Pediatr. 2004;11:1202–1204. doi: 10.1016/j.arcped.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 88.Altinkaynak S, Ertekin V, Selimoglu MA. Association of Henoch-Schonlein purpura and hepatitis A. J Emerg Med. 2006;30:219–220. doi: 10.1016/j.jemermed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 89.Thapa R, Biswas B, Mallick D. Henoch-Schönlein purpura triggered by acute hepatitis E virus infection. J Emerg Med. 2010;39:218–219. doi: 10.1016/j.jemermed.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 90.Heineke MH, Ballering AV, Jamin A, Ben Mkaddem S, Monteiro RC, Van Egmond M. New insights in the pathogenesis of immunoglobulin A vasculitis (Henoch-Schönlein purpura) Autoimmun Rev. 2017;16:1246–1253. doi: 10.1016/j.autrev.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 91.Maggiore G, Martini A, Grifeo S, De Giacomo C, Scotta MS. Hepatitis B virus infection and Schönlein-Henoch purpura. Am J Dis Child. 1984;138:681–682. doi: 10.1001/archpedi.1984.02140450063019. [DOI] [PubMed] [Google Scholar]
- 92.Ergin S, Sanli Erdoğan B, Turgut H, Evliyaoğlu D, Yalçin AN. Relapsing Henoch-Schönlein purpura in an adult patient associated with hepatitis B virus infection. J Dermatol. 2005;32:839–842. doi: 10.1111/j.1346-8138.2005.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 93.Ogawa M, Makino Y, Ueda S, Ohto M, Akikusa B. Rapidly progressive glomerulonephritis in association with Henoch-Schönlein purpura in a patient with advanced liver cirrhosis. Nephron. 1995;71:365–366. doi: 10.1159/000188750. [DOI] [PubMed] [Google Scholar]
- 94.Madison DL, Allen E, Deodhar A, Morrison L. Henoch-Schönlein purpura: a possible complication of hepatitis C related liver cirrhosis. Ann Rheum Dis. 2002;61:281–282. doi: 10.1136/ard.61.3.281-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nishida N, Kudo M. Clinical features of vascular disorders associated with chronic hepatitis virus infection. Dig Dis. 2014;32:786–790. doi: 10.1159/000368023. [DOI] [PubMed] [Google Scholar]
- 96.Silva F, Pinto C, Barbosa A, Borges T, Dias C, Almeida J. New insights in cryoglobulinemic vasculitis. J Autoimmun. 2019;105:102313. doi: 10.1016/j.jaut.2019.102313. [DOI] [PubMed] [Google Scholar]
- 97.De Virgilio A, Greco A, Magliulo G, Gallo A, Ruoppolo G, Conte M, Martellucci S, de Vincentiis M. Polyarteritis nodosa: A contemporary overview. Autoimmun Rev. 2016;15:564–570. doi: 10.1016/j.autrev.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 98.Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: An update in 2019. Joint Bone Spine. 2019;86:707–713. doi: 10.1016/j.jbspin.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 99.Meltzer M, Franklin EC. Cryoglobulinemia--a study of twenty-nine patients. I. IgG and IgM cryoglobulins and factors affecting cryoprecipitability. Am J Med. 1966;40:828–836. doi: 10.1016/0002-9343(66)90199-9. [DOI] [PubMed] [Google Scholar]
- 100.Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med. 1974;57:775–788. doi: 10.1016/0002-9343(74)90852-3. [DOI] [PubMed] [Google Scholar]
- 101.Harel S, Mohr M, Jahn I, Aucouturier F, Galicier L, Asli B, Malphettes M, Szalat R, Brouet JC, Lipsker D, Fermand JP. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br J Haematol. 2015;168:671–678. doi: 10.1111/bjh.13196. [DOI] [PubMed] [Google Scholar]
- 102.Trejo O, Ramos-Casals M, García-Carrasco M, Yagüe J, Jiménez S, de la Red G, Cervera R, Font J, Ingelmo M. Cryoglobulinemia: study of etiologic factors and clinical and immunologic features in 443 patients from a single center. Medicine (Baltimore) 2001;80:252–262. doi: 10.1097/00005792-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 103.Charles ED, Dustin LB. Hepatitis C virus-induced cryoglobulinemia. Kidney Int. 2009;76:818–824. doi: 10.1038/ki.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cheema SR, Arif F, Charney D, Meisels IS. IgA-dominant glomerulonephritis associated with hepatitis A. Clin Nephrol. 2004;62:138–143. doi: 10.5414/cnp62138. [DOI] [PubMed] [Google Scholar]
- 105.Chen PP, Fong S, Goni F, Silverman GJ, Fox RI, Liu MF, Frangione B, Carson DA. Cross-reacting idiotypes on cryoprecipitating rheumatoid factor. Springer Semin Immunopathol. 1988;10:35–55. doi: 10.1007/BF02054022. [DOI] [PubMed] [Google Scholar]
- 106.Quartuccio L, Fabris M, Salvin S, Isola M, Soldano F, Falleti E, Beltrami CA, De Re V, De Vita S. Bone marrow B-cell clonal expansion in type II mixed cryoglobulinaemia: association with nephritis. Rheumatology (Oxford) 2007;46:1657–1661. doi: 10.1093/rheumatology/kem209. [DOI] [PubMed] [Google Scholar]
- 107.Ferri C, Sebastiani M, Giuggioli D, Cazzato M, Longombardo G, Antonelli A, Puccini R, Michelassi C, Zignego AL. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum. 2004;33:355–374. doi: 10.1016/j.semarthrit.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 108.Ramos-Casals M, Stone JH, Cid MC, Bosch X. The cryoglobulinaemias. Lancet. 2012;379:348–360. doi: 10.1016/S0140-6736(11)60242-0. [DOI] [PubMed] [Google Scholar]
- 109.Roccatello D, Fornasieri A, Giachino O, Rossi D, Beltrame A, Banfi G, Confalonieri R, Tarantino A, Pasquali S, Amoroso A, Savoldi S, Colombo V, Manno C, Ponzetto A, Moriconi L, Pani A, Rustichelli R, Di Belgiojoso GB, Comotti C, Quarenghi MI. Multicenter study on hepatitis C virus-related cryoglobulinemic glomerulonephritis. Am J Kidney Dis. 2007;49:69–82. doi: 10.1053/j.ajkd.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 110.Johnson RJ, Gretch DR, Yamabe H, Hart J, Bacchi CE, Hartwell P, Couser WG, Corey L, Wener MH, Alpers CE. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med. 1993;328:465–470. doi: 10.1056/NEJM199302183280703. [DOI] [PubMed] [Google Scholar]
- 111.Ammendola A, Sampaolo S, Ambrosone L, Ammendola E, Ciccone G, Migliaresi S, Di Iorio G. Peripheral neuropathy in hepatitis-related mixed cryoglobulinemia: electrophysiologic follow-up study. Muscle Nerve. 2005;31:382–385. doi: 10.1002/mus.20184. [DOI] [PubMed] [Google Scholar]
- 112.Casato M, Saadoun D, Marchetti A, Limal N, Picq C, Pantano P, Galanaud D, Cianci R, Duhaut P, Piette JC, Fiorilli M, Cacoub P. Central nervous system involvement in hepatitis C virus cryoglobulinemia vasculitis: a multicenter case-control study using magnetic resonance imaging and neuropsychological tests. J Rheumatol. 2005;32:484–488. [PubMed] [Google Scholar]
- 113.Retamozo S, Díaz-Lagares C, Bosch X, Bové A, Brito-Zerón P, Gómez ME, Yagüe J, Forns X, Cid MC, Ramos-Casals M. Life-Threatening Cryoglobulinemic Patients With Hepatitis C: Clinical Description and Outcome of 279 Patients. Medicine (Baltimore) 2013;92:273–284. doi: 10.1097/MD.0b013e3182a5cf71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cavalli G, Berti A, Fragasso G, De Cobelli F. Hypertrophic cardiomyopathy secondary to hepatitis C virus-related vasculitis. J Cardiovasc Med (Hagerstown) 2016;17 Suppl 2:e156–e157. doi: 10.2459/JCM.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 115.Terrier B, Saadoun D, Sène D, Scerra S, Musset L, Cacoub P. Presentation and outcome of gastrointestinal involvement in hepatitis C virus-related systemic vasculitis: a case-control study from a single-centre cohort of 163 patients. Gut. 2010;59:1709–1715. doi: 10.1136/gut.2010.218123. [DOI] [PubMed] [Google Scholar]
- 116.Muñoz-Martínez SG, Díaz-Hernández HA, Suárez-Flores D, Sánchez-Ávila JF, Gamboa-Domínguez A, García-Juárez I, Torre A. Atypical manifestations of hepatitis A virus infection. Rev Gastroenterol Mex. 2018;83:134–143. doi: 10.1016/j.rgmx.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 117.Nassih H, Bourrahouat A, Sab IA. Hepatitis A Virus Infection Associated with Cryoglobulinemic Vasculitis. Indian Pediatr. 2020;57:71–72. [PubMed] [Google Scholar]
- 118.Levo Y, Gorevic PD, Kassab HJ, Tobias H, Franklin EC. Liver involvement in the syndrome of mixed cryoglobulinemia. Ann Intern Med. 1977;87:287–292. doi: 10.7326/0003-4819-87-3-287. [DOI] [PubMed] [Google Scholar]
- 119.Gower RG, Sausker WF, Kohler PF, Thorne GE, McIntosh RM. Small vessel vasculitis caused by hepatitis B virus immune complexes. Small vessel vasculitis and HBsAG. J Allergy Clin Immunol. 1978;62:222–228. doi: 10.1016/0091-6749(78)90211-7. [DOI] [PubMed] [Google Scholar]
- 120.Monti G, Galli M, Invernizzi F, Pioltelli P, Saccardo F, Monteverde A, Pietrogrande M, Renoldi P, Bombardieri S, Bordin G. Cryoglobulinaemias: a multi-centre study of the early clinical and laboratory manifestations of primary and secondary disease. GISC. Italian Group for the Study of Cryoglobulinaemias. QJM. 1995;88:115–126. [PubMed] [Google Scholar]
- 121.Mazzaro C, Dal Maso L, Urraro T, Mauro E, Castelnovo L, Casarin P, Monti G, Gattei V, Zignego AL, Pozzato G. Hepatitis B virus related cryoglobulinemic vasculitis: A multicentre open label study from the Gruppo Italiano di Studio delle Crioglobulinemie - GISC. Dig Liver Dis. 2016;48:780–784. doi: 10.1016/j.dld.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 122.Liou YT, Huang JL, Ou LS, Lin YH, Yu KH, Luo SF, Ho HH, Liou LB, Yeh KW. Comparison of cryoglobulinemia in children and adults. J Microbiol Immunol Infect. 2013;46:59–64. doi: 10.1016/j.jmii.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 123.Mazzaro C, Dal Maso L, Visentini M, Ermacora A, Tonizzo M, Gattei V, Andreone P. Recent news in the treatment of hepatitis B virus-related cryogobulinemic vasculitis. Minerva Med. 2020;111:566–572. doi: 10.23736/S0026-4806.20.06771-3. [DOI] [PubMed] [Google Scholar]
- 124.European Association for the Study of the Liver ; European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 125.Kamimura H, Setsu T, Kimura N, Yokoo T, Sakamaki A, Kamimura K, Tsuchiya A, Takamura M, Yamagiwa S, Terai S. Renal Impairment in Chronic Hepatitis B: A Review. Diseases. 2018;6 doi: 10.3390/diseases6020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ferri C, Antonelli A, Mascia MT, Sebastiani M, Fallahi P, Ferrari D, Giunti M, Pileri SA, Zignego AL. B-cells and mixed cryoglobulinemia. Autoimmun Rev. 2007;7:114–120. doi: 10.1016/j.autrev.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 127.Pasquet F, Combarnous F, Macgregor B, Coppere B, Mausservey C, Ninet J, Hot A. Safety and efficacy of rituximab treatment for vasculitis in hepatitis B virus-associated type II cryoglobulinemia: a case report. J Med Case Rep. 2012;6:39. doi: 10.1186/1752-1947-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Terrier B, Marie I, Lacraz A, Belenotti P, Bonnet F, Chiche L, Graffin B, Hot A, Kahn JE, Michel C, Quemeneur T, de Saint-Martin L, Hermine O, Léger JM, Mariette X, Senet P, Plaisier E, Cacoub P. Non HCV-related infectious cryoglobulinemia vasculitis: Results from the French nationwide CryoVas survey and systematic review of the literature. J Autoimmun. 2015;65:74–81. doi: 10.1016/j.jaut.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 129.Tsutsumi Y, Yamamoto Y, Ito S, Ohigashi H, Shiratori S, Naruse H, Teshima T. Hepatitis B virus reactivation with a rituximab-containing regimen. World J Hepatol. 2015;7:2344–2351. doi: 10.4254/wjh.v7.i21.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Khan ZH, Ilyas K, Ghazanfar H, Khan HH, Hussain Q, Hammad S, Munir A, Asim R. Fatal Fulminant Hepatitis from Rituximab-induced Hepatitis B Reactivation in a Patient with Follicular Lymphoma: A Case Report and a Brief Review of Literature. Cureus. 2018;10:e2257. doi: 10.7759/cureus.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ferri C, Greco F, Longombardo G, Palla P, Moretti A, Marzo E, Mazzoni A, Pasero G, Bombardieri S, Highfield P. Association between hepatitis C virus and mixed cryoglobulinemia [see comment] Clin Exp Rheumatol. 1991;9:621–624. [PubMed] [Google Scholar]
- 132.Rosa D, Saletti G, De Gregorio E, Zorat F, Comar C, D'Oro U, Nuti S, Houghton M, Barnaba V, Pozzato G, Abrignani S. Activation of naïve B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus-associated B lymphocyte disorders. Proc Natl Acad Sci . 2005;102:18544–18549. doi: 10.1073/pnas.0509402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Charles ED, Green RM, Marukian S, Talal AH, Lake-Bakaar GV, Jacobson IM, Rice CM, Dustin LB. Clonal expansion of immunoglobulin M+CD27+ B cells in HCV-associated mixed cryoglobulinemia. Blood. 2008;111:1344–1356. doi: 10.1182/blood-2007-07-101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ferri C, La Civita L, Zignego AL, Pasero G. Viruses and cancers: possible role of hepatitis C virus. Eur J Clin Invest. 1997;27:711–718. doi: 10.1046/j.1365-2362.1997.1790728.x. [DOI] [PubMed] [Google Scholar]
- 135.Mele A, Pulsoni A, Bianco E, Musto P, Szklo A, Sanpaolo MG, Iannitto E, De Renzo A, Martino B, Liso V, Andrizzi C, Pusterla S, Dore F, Maresca M, Rapicetta M, Marcucci F, Mandelli F, Franceschi S. Hepatitis C virus and B-cell non-Hodgkin lymphomas: an Italian multicenter case-control study. Blood. 2003;102:996–999. doi: 10.1182/blood-2002-10-3230. [DOI] [PubMed] [Google Scholar]
- 136.Giordano TP, Henderson L, Landgren O, Chiao EY, Kramer JR, El-Serag H, Engels EA. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA. 2007;297:2010–2017. doi: 10.1001/jama.297.18.2010. [DOI] [PubMed] [Google Scholar]
- 137.Monti G, Pioltelli P, Saccardo F, Campanini M, Candela M, Cavallero G, De Vita S, Ferri C, Mazzaro C, Migliaresi S, Ossi E, Pietrogrande M, Gabrielli A, Galli M, Invernizzi F. Incidence and characteristics of non-Hodgkin lymphomas in a multicenter case file of patients with hepatitis C virus-related symptomatic mixed cryoglobulinemias. Arch Intern Med. 2005;165:101–105. doi: 10.1001/archinte.165.1.101. [DOI] [PubMed] [Google Scholar]
- 138.Bunchorntavakul C, Mitrani R, Reddy KR. Advances in HCV and Cryoglobulinemic Vasculitis in the Era of DAAs: Are We at the End of the Road? J Clin Exp Hepatol. 2018;8:81–94. doi: 10.1016/j.jceh.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Saadoun D, Pol S, Ferfar Y, Alric L, Hezode C, Si Ahmed SN, de Saint Martin L, Comarmond C, Bouyer AS, Musset L, Poynard T, Resche Rigon M, Cacoub P. Efficacy and Safety of Sofosbuvir Plus Daclatasvir for Treatment of HCV-Associated Cryoglobulinemia Vasculitis. Gastroenterology 2017; 153: 49-52. :e5. doi: 10.1053/j.gastro.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 140.Arcaini L, Besson C, Frigeni M, Fontaine H, Goldaniga M, Casato M, Visentini M, Torres HA, Loustaud-Ratti V, Peveling-Oberhag J, Fabris P, Rossotti R, Zaja F, Rigacci L, Rattotti S, Bruno R, Merli M, Dorival C, Alric L, Jaccard A, Pol S, Carrat F, Ferretti VV, Visco C, Hermine O. Interferon-free antiviral treatment in B-cell lymphoproliferative disorders associated with hepatitis C virus infection. Blood. 2016;128:2527–2532. doi: 10.1182/blood-2016-05-714667. [DOI] [PubMed] [Google Scholar]
- 141.Ramos-Casals M, Zignego AL, Ferri C, Brito-Zerón P, Retamozo S, Casato M, Lamprecht P, Mangia A, Saadoun D, Tzioufas AG, Younossi ZM, Cacoub P International Study Group of Extrahepatic Manifestations related to HCV (ISG-EHCV) Evidence-based recommendations on the management of extrahepatic manifestations of chronic hepatitis C virus infection. J Hepatol. 2017;66:1282–1299. doi: 10.1016/j.jhep.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 142.Saadoun D, Resche Rigon M, Sene D, Terrier B, Karras A, Perard L, Schoindre Y, Coppéré B, Blanc F, Musset L, Piette JC, Rosenzwajg M, Cacoub P. Rituximab plus Peg-interferon-alpha/ribavirin compared with Peg-interferon-alpha/ribavirin in hepatitis C-related mixed cryoglobulinemia. Blood. 2010;116:326–34; quiz 504. doi: 10.1182/blood-2009-10-248518. [DOI] [PubMed] [Google Scholar]
- 143.De Vita S, Quartuccio L, Isola M, Mazzaro C, Scaini P, Lenzi M, Campanini M, Naclerio C, Tavoni A, Pietrogrande M, Ferri C, Mascia MT, Masolini P, Zabotti A, Maset M, Roccatello D, Zignego AL, Pioltelli P, Gabrielli A, Filippini D, Perrella O, Migliaresi S, Galli M, Bombardieri S, Monti G. A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis Rheum. 2012;64:843–853. doi: 10.1002/art.34331. [DOI] [PubMed] [Google Scholar]
- 144.Desbois AC, Comarmond C, Saadoun D, Cacoub P. Cryoglobulinemia vasculitis: how to handle. Curr Opin Rheumatol. 2017;29:343–347. doi: 10.1097/BOR.0000000000000390. [DOI] [PubMed] [Google Scholar]
- 145.Sène D, Ghillani-Dalbin P, Amoura Z, Musset L, Cacoub P. Rituximab may form a complex with IgMkappa mixed cryoglobulin and induce severe systemic reactions in patients with hepatitis C virus-induced vasculitis. Arthritis Rheum. 2009;60:3848–3855. doi: 10.1002/art.25000. [DOI] [PubMed] [Google Scholar]
- 146.Ishiguro N, Kawashima M. Cutaneous polyarteritis nodosa: a report of 16 cases with clinical and histopathological analysis and a review of the published work. J Dermatol. 2010;37:85–93. doi: 10.1111/j.1346-8138.2009.00752.x. [DOI] [PubMed] [Google Scholar]
- 147.Morgan AJ, Schwartz RA. Cutaneous polyarteritis nodosa: a comprehensive review. Int J Dermatol. 2010;49:750–756. doi: 10.1111/j.1365-4632.2010.04522.x. [DOI] [PubMed] [Google Scholar]
- 148.Hernández-Rodríguez J, Alba MA, Prieto-González S, Cid MC. Diagnosis and classification of polyarteritis nodosa. J Autoimmun. 2014;48-49:84–89. doi: 10.1016/j.jaut.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 149.Gocke DJ, Hsu K, Morgan C, Bombardieri S, Lockshin M, Christian CL. Association between polyarteritis and Australia antigen. Lancet. 1970;2:1149–1153. doi: 10.1016/s0140-6736(70)90339-9. [DOI] [PubMed] [Google Scholar]
- 150.Trepo C, Thivolet J. Hepatitis associated antigen and periarteritis nodosa (PAN) Vox Sang. 1970;19:410–411. [PubMed] [Google Scholar]
- 151.Ozen S. The changing face of polyarteritis nodosa and necrotizing vasculitis. Nat Rev Rheumatol. 2017;13:381–386. doi: 10.1038/nrrheum.2017.68. [DOI] [PubMed] [Google Scholar]
- 152.Sharma A, Sharma K. Hepatotropic viral infection associated systemic vasculitides-hepatitis B virus associated polyarteritis nodosa and hepatitis C virus associated cryoglobulinemic vasculitis. J Clin Exp Hepatol. 2013;3:204–212. doi: 10.1016/j.jceh.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pagnoux C, Seror R, Henegar C, Mahr A, Cohen P, Le Guern V, Bienvenu B, Mouthon L, Guillevin L French Vasculitis Study Group. Clinical features and outcomes in 348 patients with polyarteritis nodosa: a systematic retrospective study of patients diagnosed between 1963 and 2005 and entered into the French Vasculitis Study Group Database. Arthritis Rheum. 2010;62:616–626. doi: 10.1002/art.27240. [DOI] [PubMed] [Google Scholar]
- 154.Imboden JB. Involvement of the Peripheral Nervous System in Polyarteritis Nodosa and Antineutrophil Cytoplasmic Antibodies-Associated Vasculitis. Rheum Dis Clin North Am. 2017;43:633–639. doi: 10.1016/j.rdc.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 155.Pagnoux C, Mahr A, Cohen P, Guillevin L. Presentation and outcome of gastrointestinal involvement in systemic necrotizing vasculitides: analysis of 62 patients with polyarteritis nodosa, microscopic polyangiitis, Wegener granulomatosis, Churg-Strauss syndrome, or rheumatoid arthritis-associated vasculitis. Medicine (Baltimore) 2005;84:115–128. doi: 10.1097/01.md.0000158825.87055.0b. [DOI] [PubMed] [Google Scholar]
- 156.Brimo F, Lachapelle J, Epstein JI. Testicular vasculitis: a series of 19 cases. Urology. 2011;77:1043–1048. doi: 10.1016/j.urology.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 157.Rimar D, Alpert A, Starosvetsky E, Rosner I, Slobodin G, Rozenbaum M, Kaly L, Boulman N, Awisat A, Ginsberg S, Zilber K, Shen-Orr SS. Tofacitinib for polyarteritis nodosa: a tailored therapy. Ann Rheum Dis. 2016;75:2214–2216. doi: 10.1136/annrheumdis-2016-209330. [DOI] [PubMed] [Google Scholar]
- 158.Seri Y, Shoda H, Hanata N, Nagafuchi Y, Sumitomo S, Fujio K, Yamamoto K. A case of refractory polyarteritis nodosa successfully treated with rituximab. Mod Rheumatol. 2017;27:696–698. doi: 10.3109/14397595.2015.1014153. [DOI] [PubMed] [Google Scholar]
- 159.Wang CR, Yang CC. Adalimumab therapy in hepatitis B virus-negative polyarteritis nodosa: A case report. Medicine (Baltimore) 2018;97:e11053. doi: 10.1097/MD.0000000000011053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ginsberg S, Rosner I, Slobodin G, Rozenbaum M, Kaly L, Jiries N, Boulman N, Awisat A, Hussein H, Novofastovski I, Silawy A, Rimar D. Infliximab for the treatment of refractory polyarteritis nodosa. Clin Rheumatol. 2019;38:2825–2833. doi: 10.1007/s10067-019-04474-9. [DOI] [PubMed] [Google Scholar]
- 161.Krusche M, Ruffer N, Kötter I. Tocilizumab treatment in refractory polyarteritis nodosa: a case report and review of the literature. Rheumatol Int. 2019;39:337–344. doi: 10.1007/s00296-018-4210-2. [DOI] [PubMed] [Google Scholar]
- 162.Press J, Maslovitz S, Avinoach I. Cutaneous necrotizing vasculitis associated with hepatitis A virus infection. J Rheumatol. 1997;24:965–967. [PubMed] [Google Scholar]
- 163.Candan F, Ayan S, Tas F, Gökce G, Elagoz S. Spontaneous renal laceration as the presenting feature of polyarteritis nodosa in a patient with familial Mediterranean fever after hepatitis A infection. Rheumatol Int. 2005;25:475–477. doi: 10.1007/s00296-005-0597-7. [DOI] [PubMed] [Google Scholar]
- 164.Della Rossa A, Tavoni A, Lorefice P, Casula F, Bombardieri S. HBV and HCV infection, polyarteritis nodosa and mixed cryoglobulinaemia: a case report. Clin Rheumatol. 2000;19:502–504. doi: 10.1007/s100670070018. [DOI] [PubMed] [Google Scholar]
- 165.Garcia de La Peña Lefebvre P, Mouthon L, Cohen P, Lhote F, Guillevin L. Polyarteritis nodosa and mixed cryoglobulinaemia related to hepatitis B and C virus coinfection. Ann Rheum Dis. 2001;60:1068–1069. doi: 10.1136/ard.60.11.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Carson CW, Conn DL, Czaja AJ, Wright TL, Brecher ME. Frequency and significance of antibodies to hepatitis C virus in polyarteritis nodosa. J Rheumatol. 1993;20:304–309. [PubMed] [Google Scholar]
- 167.Saadoun D, Terrier B, Semoun O, Sene D, Maisonobe T, Musset L, Amoura Z, Rigon MR, Cacoub P. Hepatitis C virus-associated polyarteritis nodosa. Arthritis Care Res (Hoboken) 2011;63:427–435. doi: 10.1002/acr.20381. [DOI] [PubMed] [Google Scholar]
- 168.Néel A, Masseau A, Hervier B, Bossard C, Cacoub P, Pagnoux C, Hamidou MA. Life-threatening hepatitis C virus-associated polyarteritis nodosa successfully treated by rituximab. J Clin Rheumatol. 2011;17:439–441. doi: 10.1097/RHU.0b013e31823a58d7. [DOI] [PubMed] [Google Scholar]
- 169.Guillevin L, Mahr A, Callard P, Godmer P, Pagnoux C, Leray E, Cohen P French Vasculitis Study Group. Hepatitis B virus-associated polyarteritis nodosa: clinical characteristics, outcome, and impact of treatment in 115 patients. Medicine (Baltimore) 2005;84:313–322. doi: 10.1097/01.md.0000180792.80212.5e. [DOI] [PubMed] [Google Scholar]
- 170.Lin CL, Kao JH. Perspectives and control of hepatitis B virus infection in Taiwan. J Formos Med Assoc. 2015;114:901–909. doi: 10.1016/j.jfma.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 171.Ungprasert P, Koster MJ, Cheungpasitporn W, Wijarnpreecha K, Thongprayoon C, Kroner PT. Inpatient burden and association with comorbidities of polyarteritis nodosa: National Inpatient Sample 2014. Semin Arthritis Rheum. 2020;50:66–70. doi: 10.1016/j.semarthrit.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 172.Chen WY, Lin KT, Chuang CY, Chen CY. Clinical studies of polyarteritis nodosa. Taiwan Yi Xue Hui Za Zhi. 1977;76:982–989. [PubMed] [Google Scholar]
- 173.Wang CR, Liu MF, Tsai RT, Chuang CY, Chen CY. Circulating intercellular adhesion molecules-1 and autoantibodies including anti-endothelial cell, anti-cardiolipin, and anti-neutrophil cytoplasma antibodies in patients with vasculitis. Clin Rheumatol. 1993;12:375–380. doi: 10.1007/BF02231583. [DOI] [PubMed] [Google Scholar]
- 174.Tsai WL, Tsai IC, Lee T, Hsieh CW. Polyarteritis nodosa: MDCT as a "one-stop shop" modality for whole-body arterial evaluation. Cardiovasc Intervent Radiol. 2008;31 Suppl 2:S26–S29. doi: 10.1007/s00270-007-9059-9. [DOI] [PubMed] [Google Scholar]
- 175.Huang MN, Wu CH. Polyarteritis nodosa and antiphospholipid syndrome causing bilateral renal infarction. J Rheumatol. 2009;36:197. doi: 10.3899/jrheum.080601. [DOI] [PubMed] [Google Scholar]
- 176.Li KJ, Hsieh SC, Toh YC, Yu CL. Clinical images: non-hepatitis B virus-related polyarteritis nodosa presenting with fever and diffuse intra-abdominal microaneurysms. Arthritis Rheum. 2011;63:3597. doi: 10.1002/art.30579. [DOI] [PubMed] [Google Scholar]
- 177.Tsai HC, Liao HT, Tsai CY. Polyarteritis nodosa with intra-hepatic arterial haemorrhage. Liver Int. 2020;40:2858–2859. doi: 10.1111/liv.14641. [DOI] [PubMed] [Google Scholar]
- 178.Lu FT, Ni YH. Elimination of Mother-to-Infant Transmission of Hepatitis B Virus: 35 Years of Experience. Pediatr Gastroenterol Hepatol Nutr. 2020;23:311–318. doi: 10.5223/pghn.2020.23.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hong M, Bertoletti A. Tolerance and immunity to pathogens in early life: insights from HBV infection. Semin Immunopathol. 2017;39:643–652. doi: 10.1007/s00281-017-0641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Travers RL, Allison DJ, Brettle RP, Hughes GR. Polyarteritis nodosa: a clinical and angiographic analysis of 17 cases. Semin Arthritis Rheum. 1979;8:184–199. doi: 10.1016/s0049-0172(79)80007-4. [DOI] [PubMed] [Google Scholar]
- 181.Cohen RD, Conn DL, Ilstrup DM. Clinical features, prognosis, and response to treatment in polyarteritis. Mayo Clin Proc. 1980;55:146–155. [PubMed] [Google Scholar]
- 182.Fortin PR, Larson MG, Watters AK, Yeadon CA, Choquette D, Esdaile JM. Prognostic factors in systemic necrotizing vasculitis of the polyarteritis nodosa group--a review of 45 cases. J Rheumatol. 1995;22:78–84. [PubMed] [Google Scholar]
- 183.Bae YD, Choi HJ, Lee JC, Park JJ, Lee YJ, Lee EB, Song YW. Clinical features of polyarteritis nodosa in Korea. J Korean Med Sci. 2006;21:591–595. doi: 10.3346/jkms.2006.21.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sharma A, Pinto B, Dhooria A, Rathi M, Singhal M, Dhir V, Sharma K, Parkash M, Modi M, Vijayvergiya R, Sinha SK, Nada R, Minz RW, Singh S. Polyarteritis nodosa in north India: clinical manifestations and outcomes. Int J Rheum Dis. 2017;20:390–397. doi: 10.1111/1756-185X.12954. [DOI] [PubMed] [Google Scholar]
- 185.Guillevin L, Lhote F, Gayraud M, Cohen P, Jarrousse B, Lortholary O, Thibult N, Casassus P. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome. A prospective study in 342 patients. Medicine (Baltimore) 1996;75:17–28. doi: 10.1097/00005792-199601000-00003. [DOI] [PubMed] [Google Scholar]
- 186.Guillevin L, Lhote F, Cohen P, Jarrousse B, Lortholary O, Généreau T, Léon A, Bussel A. Corticosteroids plus pulse cyclophosphamide and plasma exchanges versus corticosteroids plus pulse cyclophosphamide alone in the treatment of polyarteritis nodosa and Churg-Strauss syndrome patients with factors predicting poor prognosis. A prospective, randomized trial in sixty-two patients. Arthritis Rheum. 1995;38:1638–1645. doi: 10.1002/art.1780381116. [DOI] [PubMed] [Google Scholar]