Abstract
BACKGROUND
Hepatic steatosis commonly occurs in some chronic liver diseases and may affect disease progression.
AIM
To investigate the performance of controlled attenuation parameter (CAP) for the diagnosis of hepatic steatosis in patients with autoimmune liver diseases (AILDs).
METHODS
Patients who were suspected of having AILDs and underwent liver biopsy were consistently enrolled. Liver stiffness measurement (LSM) and CAP were performed by transient elastography. The area under the receiver operating characteristic (AUROC) curve was used to evaluate the performance of CAP for diagnosing hepatic steatosis compared with biopsy.
RESULTS
Among 190 patients with biopsy-proven hepatic steatosis, 69 were diagnosed with autoimmune hepatitis (AIH), 18 with primary biliary cholangitis (PBC), and 27 with AIH-PBC overlap syndrome. The AUROCs of CAP for the diagnosis of steatosis in AILDS were 0.878 (0.791-0.965) for S1, 0.764 (0.676-0.853) for S2, and 0.821 (0.716-0.926) for S3. The CAP value was significantly related to hepatic steatosis grade (P < 0.001). Among 69 patients with AIH, the median CAP score was 205.63 ± 47.36 dB/m for S0, 258.41 ± 42.83 dB/m for S1, 293.00 ± 37.18 dB/m for S2, and 313.60 ± 27.89 dB/m for S3. Compared with patients with nonalcoholic fatty liver disease (NAFLD) presenting with autoimmune markers, patients with AIH concomitant with NAFLD were much older and had higher serum IgG levels and LSM values.
CONCLUSION
CAP can be used as a noninvasive diagnostic method to evaluate hepatic steatosis in patients with AILDs. Determination of LSM combined with CAP may help to identify patients with AIH concomitant with NAFLD from those with NAFLD with autoimmune phenomena.
Keywords: Controlled attenuation parameter, Hepatic steatosis, Autoimmune liver diseases, Nonalcoholic fatty liver disease, Liver stiffness measurement, Autoimmune hepatitis
Core Tip: This retrospective study determined that controlled attenuation parameter (CAP) could be used as a noninvasive diagnostic tool to evaluate hepatic steatosis effectively and accurately in patients with autoimmune liver diseases. Patients with autoimmune hepatitis concomitant with nonalcoholic fatty liver disease (NAFLD) had higher IgG levels and liver stiffness measurement values, while patients with NAFLD with autoimmune phenomena had higher gamma-glutamyl transferase levels and CAP values, which benefits the identification of these two kinds of patients.
INTRODUCTION
Hepatic steatosis is the accumulation of lipids within hepatocytes and is considered pathologic when it affects more than 5% of hepatocytes[1]. The most common cause of steatosis is insulin resistance associated with nonalcoholic fatty liver disease (NAFLD)[2]. It also occurs in alcoholic liver disease and chronic viral hepatitis, defined as a “cofactor” capable of affecting disease progression and treatment perspectives[3]. Autoimmune liver diseases (AILDs) are a group of autoimmune diseases associated with the liver and are characterized by dysregulation of immune cell homeostasis and inflammation, including autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), primary sclerosing cholangitis, and their overlapping subtypes[4,5]. When hepatic steatosis is present in subjects with AILDs, this coexisting scenario may cause a synergistic combination of steatosis, cellular adaptation, and oxidative damage that aggravates liver injury and affects the treatment effect[6]. Moreover, the standard regimen for AIH involves glucocorticoids[7], the long-term administration of which will further aggravate hepatic steatosis. However, the incidence of AILDs combined with hepatic steatosis has not been reported. Therefore, the evaluation of hepatic steatosis is a component that cannot be ignored in the management of patients with AILDs.
Liver biopsy is considered the standard method for staging hepatic inflammation, steatosis, and fibrosis. Thus, it plays a vital role in the diagnosis and follow-up of AILDs[7,8]. However, liver biopsy is an invasive procedure, which bears a potential risk of severe complications and is of limited acceptance among patients. Furthermore, the severity of hepatic steatosis may change within weeks after treatment that cannot be sufficiently monitored by repetitive invasive procedures. Accordingly, noninvasive assessments are urgently needed[9].
The controlled attenuation parameter (CAP) measured by transient elastography (TE) is an easy and rapid noninvasive examination method for the detection of hepatic steatosis. It is based on the physical phenomenon that the amplitude of ultrasound waves is attenuated more quickly when they traverse across a steatotic liver[10,11]. TE can also quantify the speed of a mechanically induced shear wave in liver tissue and hence generate a parameter called liver stiffness measurement (LSM) to estimate liver fibrosis[12]. CAP is measured simultaneously with LSM, making it possible to assess hepatic steatosis and fibrosis at the same time. Studies with CAP have been performed in NAFLD, alcoholic liver disease, and viral hepatitis, but very few data are available on AILDs[13-15]. It has been showed that in patients with chronic viral hepatitis and advanced liver fibrosis, CAP performed better than ultrasound for assessing liver steatosis[16]. Besides, a recent meta-analysis showed that CAP diagnosed moderate and severe hepatic steatosis with diagnostic accuracies above 0.85 in patients with liver disease of mixed etiology. However, the analysis did not include patients with AILDs[17].
In this study, we assessed the performance of CAP for evaluating hepatic steatosis in AILDs to determine whether it could be regarded as a reliable tool to monitor disease course.
MATERIALS AND METHODS
Patients
Patients who were suspected of having AILDs and eventually underwent liver biopsy at Shanghai Jiao Tong University Renji Hospital were consistently enrolled from January 2016 to November 2018. A total of 800 patients were analyzed for steatosis in liver histology.
Diagnostic criteria
Diagnosis was made according to the diagnostic criteria described in the clinical practice guidelines for AIH, PBC, AIH-PBC overlap syndrome, and NAFLD. AIH[18] was diagnosed according to a simple score based on four measurements: Liver histology, autoantibody titers, gamma-globulin/IgG levels, and the absence of viral hepatitis. The diagnosis of PBC[19] required fulfilment of two or more of the following criteria: (1) Biochemical evidence of cholestasis based mainly on alkaline phosphatase (AKP) elevation; (2) Detection of anti-mitochondrial autoantibodies (AMA); and (3) Typical histologic evidence of nonsuppurative destructive cholangitis and destruction of interlobular bile ducts. AIH-PBC overlap syndrome[20] was diagnosed based on clinical, biochemical, serological, and histological features overlapping those of PBC and AIH. The diagnosis of NAFLD[21] required imaging or histological evidence of diffuse hepatic steatosis and ruling out other causes of hepatic steatosis, such as excessive alcohol consumption.
Histological examination
Percutaneous liver biopsy guided by ultrasound was performed under local anesthesia using a 16G disposable needle. Liver specimens at least 1 cm in length with eight complete portal tracts were included. The specimens were immediately fixed in 10% neutral formalin and embedded in paraffin. Hematoxylin and eosin staining was used to observe the morphology of the liver, and Masson’s trichrome and reticulin staining was performed to detect fibrosis. One single experienced pathologist who was blinded to the patients’ clinical data assessed liver histology using a METAVIR-derived scoring system. Hepatic steatosis was scored as S0: < 5%, S1: 5%-33%, S2: > 33%-66%, and S3: > 66%. Fibrosis was staged as follows: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal and periportal fibrosis with few septa; F3, portal and periportal fibrosis with numerous septa without cirrhosis; and F4, cirrhosis. Hepatic inflammatory activity was graded as follows: A0, none; A1, mild; A2, moderate; and A3, severe.
Clinical measurements
Medical records of the patients who were finally included were reviewed, and clinical data and laboratory findings were collected and analyzed. Body mass index (BMI) was calculated. Laboratory evaluations included liver biochemistry [i.e., alanine aminotransferase (ALT), aspartate aminotransferase, AKP, gamma-glutamyl transferase (GGT), total bilirubin, direct bilirubin, globulin, and albumin], serum immunoglobulins (IgG, IgM, and IgA), routine blood tests (white blood cell count and platelet count), and prothrombin time. Serum autoantibodies, including anti-nuclear antibody (ANA), AMA, and anti-smooth muscle actin antibody (ASMA), were detected by indirect immunofluorescence (Euroimmun AG, Hangzhou, China).
CAP and LSM by TE
TE measured with a FibroScan device and an M probe ultrasound transducer (Echosens, Paris, France) was performed in all patients who underwent liver biopsy on the same day. Subjects were placed in the supine position with the right arm in maximal abduction, and measurements were taken over the right hepatic lobe through an intercostal space. We obtained ten valid CAP and LSM measurements from each participant and considered LSM with an interquartile range ≤ 30% and a success rate ≥ 60% as reliable. The median CAP and LSM were taken as the estimates for hepatic steatosis and fibrosis, expressed in dB/m and kilopascals (kPa), respectively.
Statistical analysis
Data were analyzed using SPSS software version 22.0 (SPSS Inc, Chicago, IL, United States). Summary data are reported as the mean ± SD or median (interquartile range) according to distribution. Quantitative variables were compared using independent samples Student’s t-test or one-way analysis of variance when appropriate. Spearman’s rank correlation test was used to explore the correlation between CAP and hepatic steatosis grade. The diagnostic accuracy of CAP for the prediction of hepatic steatosis grade was calculated using a receiver operator characteristic (ROC) curve. Optimal CAP cut-off values for each steatosis stage were determined based on the highest combined sensitivity and specificity (Youden index). The area under the ROC curve (AUROC), sensitivity, specificity, positive predictive value, and negative predictive value for the predefined cut-off values were calculated. A P value < 0.05 was considered statistically significant.
RESULTS
Characteristics of the patients
In 800 patients with liver biopsy, a total of 190 patients were finally included in the study according to the existence of various grades of hepatic steatosis in liver histology, with a mean age of 46.36 ± 12.82 years, including 45 males (23.68%) and 145 females (76.32%). Among these patients, 69 were diagnosed with AIH, 18 with PBC, 27 with AIH-PBC overlap syndrome, 66 with NAFLD, and 10 with other liver diseases. In all patients, the prevalence of autoantibodies, including ANA, AMA, and ASMA, was 70.53%. The average BMI was 23.97 ± 2.69 kg/m2.
Diagnostic accuracy of CAP to grade hepatic steatosis in patients with mixed etiology liver disease
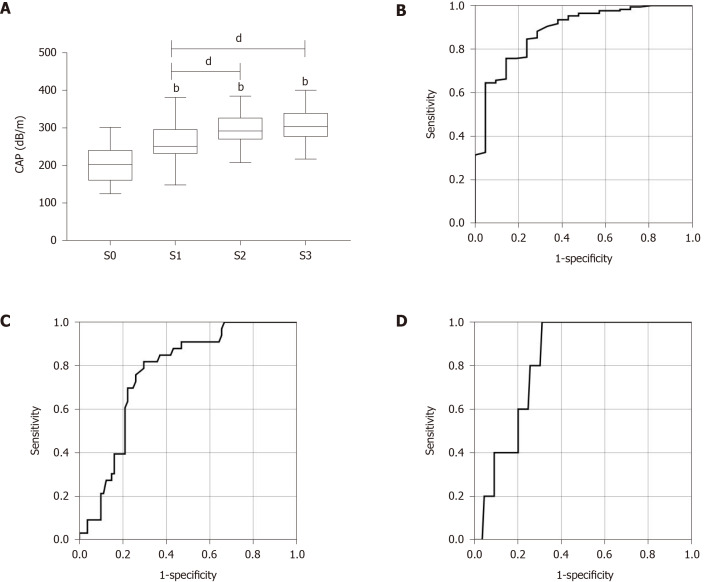
In all 190 patients, the median CAP score was 270.17 ± 54.52 dB/m, and the median LSM score was 7.66 ± 5.57 kPa (Table 1). The distribution of the CAP value for each steatosis grade in all patients with mixed etiology liver disease is as follows. The median CAP score was 201.6 ± 46.78 dB/m for S0, 260.5 ± 47.92 dB/m for S1, 293.6 ± 40.13 dB/m for S2, and 307.4 ± 45.31 dB/m for S3. The CAP value was significantly related to hepatic steatosis grade (ρ = 0.549, P < 0.001) (Figure 1A).
Table 1.
Baseline patient characteristics
|
Variable
|
All (n = 190)
|
AILDs (n = 114)
|
NAFLD (n = 66)
|
| Age, yr | 46.36 ± 12.82 | 48.72 ± 11.57 | 42.15 ± 13.80 |
| Male sex, n (%) | 45 (23.68) | 23 (20.18) | 18 (27.27) |
| BMI, kg/m2 | 23.97 ± 2.69 | 24.33 ± 1.80 | 23.63 ± 2.34 |
| Prevalence of autoantibodies, n (%) | 134 (70.53) | 89 (78.07) | 37 (56.06) |
| Laboratory | |||
| AST, U/L | 63.96 ± 86.42 | 72.54 ± 102.23 | 54.6 ± 59.10 |
| ALT, U/L | 83.86 ± 86.42 | 81.98 ± 92.78 | 92.81 ± 78.67 |
| LDH, U/L | 182.70 ± 35.43 | 183.02 ± 37.39 | 182.64 ± 32.86 |
| AKP, U/L | 80.00 (63.00, 108.50) | 85.00 (62.25, 129.5) | 75.00 (64.00, 94.00) |
| GGT, U/L | 103.67 ± 126.83 | 126.00 ± 151.92 | 76.82 ± 73.15 |
| Total bilirubin, mg/dL | 10.90 (8.10, 15.30) | 11.20 (8.30, 16.70) | 9.80 (6.95, 14.15) |
| Direct bilirubin, mg/dL | 3.60 (2.80, 4.90) | 3.70 (2.90, 5.70) | 3.45 (2.52, 4.50) |
| Histological steatosis stage, n (%) | |||
| S0 (< 5%) | 22 (11.6) | 20 (11.1) | 0 (0.0) |
| S1 (5%-33%) | 85 (44.7) | 61 (33.9) | 16 (24.2) |
| S2 (> 33%-66%) | 55 (28.9) | 28 (15.6) | 27 (40.9) |
| S3 (> 66%) | 28 (14.7) | 5 (2.8) | 23 (34.8) |
| Histological fibrosis stage, n (%) | |||
| F0 (no fibrosis) | 12 (6.3) | 1 (0.9) | 10 (15.2) |
| F1 (portal fibrosis without septa) | 70 (36.8) | 38 (33.3) | 30 (45.5) |
| F2 (portal and periportal fibrosis with few septa) | 64 (33.7) | 40 (35.1) | 18 (27.3) |
| F3 (portal and periportal fibrosis with numerous septa without cirrhosis) | 35 (18.4) | 26 (22.8) | 8 (12.1) |
| F4 (cirrhosis) | 9 (4.7) | 9 (7.9) | 0 (0.0) |
| CAP, dB/m | 270.17 ± 54.52 | 261.73 ± 53.80 | 290.97 ± 50.68 |
| LSM, kPa | 7.66 ± 5.57 | 8.48 ± 6.50 | 6.61 ± 3.66 |
Distributions are expressed as the mean ± SD or median (interquartile range) or number (percentage). AIH: Autoimmune hepatitis; NAFLD: Nonalcoholic fatty liver disease; BMI: Body mass index; AKP: Alkaline phosphatase; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; CAP: Controlled attenuation parameter; GGT: Gamma-glutamyl transferase; LDH: Lactate dehydrogenase; LSM: Liver stiffness measurement.
Figure 1.
The receiver operator characteristic curve of controlled attenuation parameter for diagnosis of hepatic steatosis grade in patients with mixed etiology liver disease. A: Correlation between the controlled attenuation parameter (CAP) and the grade of hepatic steatosis (ρ = 0.549, P < 0.001); B-D: The receiver operator characteristic curve of CAP for diagnosis of (B) steatosis grade ≥ S1, (C) steatosis grade ≥ S2, and (D) steatosis grade ≥ S3 in patients with mixed etiology liver disease. bP < 0.01 vs S0; dP < 0.01. CAP: Controlled attenuation parameter.
The AUROCs of CAP for the diagnosis of steatosis were 0.883 (0.807-0.958) for S1, 0.772 (0.705-0.838) for S2, and 0.732 (0.640-0.824) for S3 (Figure 1B-D and Table 2). The optimal cut-off values of CAP for steatosis grades were 229 dB/m for S ≥ 1, 259 dB/m for S ≥ 2, and 283.5 dB/m for S3 with the highest combined sensitivity and specificity (Table 2).
Table 2.
Diagnostic performance of controlled attenuation parameter for assessment of hepatic steatosis in patients with mixed etiology liver disease and in patients with autoimmune liver diseases
|
Etiology
|
Grade
|
AUROC (95%CI)
|
Cut-off, dB/m (kPa)
|
Sensitivity
|
Specificity
|
PPV
|
NPV
|
| Mixed etiology | S ≥ 1 | 0.883 (0.807-0.958) | 229 | 0.852 | 0.714 | 0.960 | 0.375 |
| S ≥ 2 | 0.772 (0.705-0.838) | 259 | 0.875 | 0.636 | 0.636 | 0.875 | |
| S = 3 | 0.732 (0.640-0.824) | 283.5 | 0.750 | 0.654 | 0.273 | 0.938 | |
| AILDs | S ≥ 1 | 0.878 (0.791-0.965) | 220.5 | 0.874 | 0.737 | 0.937 | 0.538 |
| S ≥ 2 | 0.764 (0.676-0.853) | 271.5 | 0.818 | 0.704 | 0.510 | 0.937 | |
| S = 3 | 0.821 (0.716-0.926) | 283.5 | 1.000 | 0.688 | 0.128 | 1.000 |
AUROC: Area under receiver operating characteristic; CAP: Controlled attenuation parameter; NPV: Negative predictive value; PPV: Positive predictive value.
Diagnostic accuracy of CAP for grading hepatic steatosis in AILDs
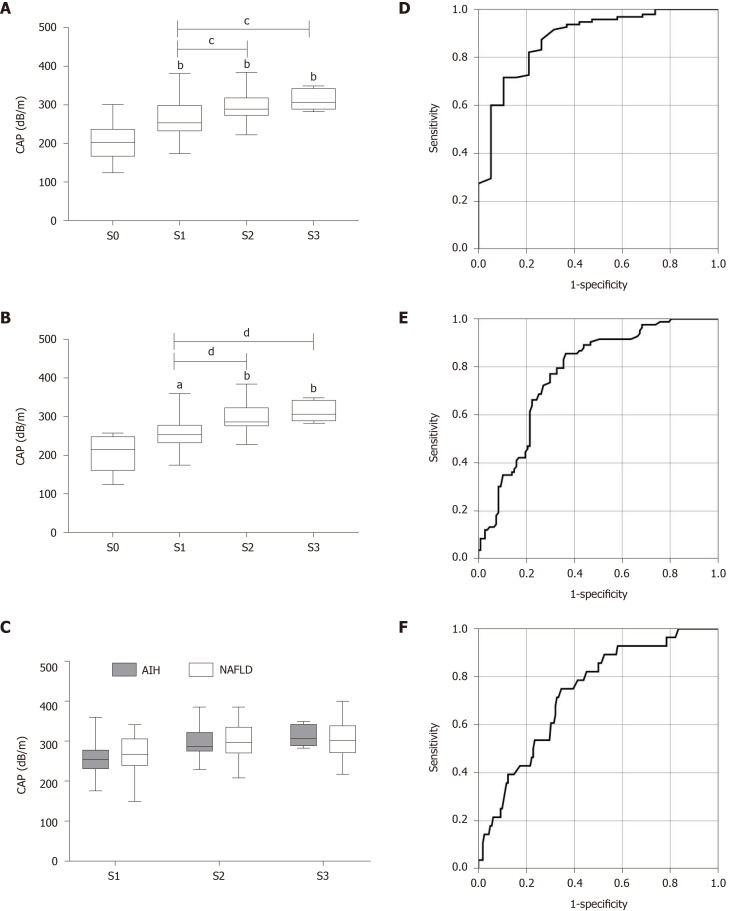
Next, we further assessed the performance of CAP for evaluating hepatic steatosis in AILDs. The median CAP score for each steatosis grade in AILDs was very similar to that in all patients. The CAP value was also significantly related to hepatic steatosis grade (ρ = 0.553, P < 0.001) (Figure 2A). The AUROCs of CAP for the diagnosis of steatosis in AILDs were 0.878 (0.791-0.965) for S1, 0.764 (0.676-0.853) for S2, and 0.821 (0.716-0.926) for S3 (Figure 2D-F and Table 2). The optimal cut-off values of CAP for steatosis grades were 220.5 dB/m for S ≥ 1, 271.5 dB/m for S ≥ 2, and 283.5 dB/m for S3 (Table 2).
Figure 2.
Controlled attenuation parameter value in different hepatic steatosis grades and etiologies and the receiver operator characteristic curve of controlled attenuation parameter for diagnosis of hepatic steatosis grade in patients with autoimmune liver diseases. A and B: Correlation between the controlled attenuation parameter (CAP) and the grade of hepatic steatosis in patients with (A) autoimmune liver diseases (AILDs) or (B) autoimmune hepatitis (AIH); C: Comparison of the CAP value in patients with AIH and nonalcoholic fatty liver disease in different hepatic steatosis grades; D-F: The receiver operator characteristic curve of CAP for diagnosis of (D) steatosis grade ≥ S1, (E) steatosis grade ≥ S2, and (F) steatosis grade ≥ S3 in patients with AILDs. aP < 0.05; bP < 0.01 vs S0; cP < 0.05; dP < 0.01. CAP: Controlled attenuation parameter; AIH: Autoimmune hepatitis; NAFLD: Nonalcoholic fatty liver disease.
Considering the relatively high incidence and clinical significance of AIH, we focused on calculating the median CAP value for patients with AIH. Among 69 patients with AIH, the median CAP score was 205.63 ± 47.36 dB/m for S0, 258.41 ± 42.83 dB/m for S1, 293.00 ± 37.18 dB/m for S2, and 313.60 ± 27.89 dB/m for S3 (Figure 2B). Furthermore, there was no significant difference in the median CAP value between patients with AIH and NAFLD in each hepatic steatosis grade (Figure 2C).
Factors affecting the performance of CAP
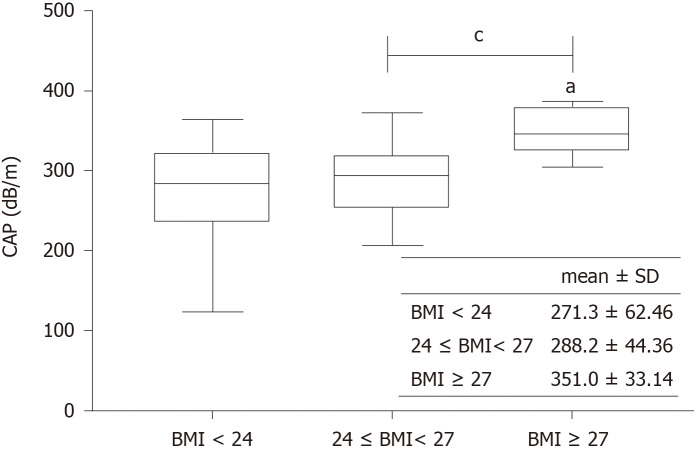
Univariate and multivariate analyses showed that the CAP value was significantly correlated with BMI and hepatic steatosis (P < 0.001, P < 0.001). When patients were divided into three subgroups according to BMI < 24 kg/m2, 24-27 kg/m2, and BMI ≥ 27 kg/m2, patients with BMI ≥ 27 kg/m2 had a significantly higher CAP value than the other two groups (Figure 3). The performance of CAP was stable across fibrosis stages. In addition, traditional factors affecting the performance of LSM, such as bilirubin and serum ALT level, did not affect the performance of CAP (data not shown).
Figure 3.
Controlled attenuation parameter in patients with different body mass indexes. a P < 0.05 vs S0; cP < 0.05. CAP: Controlled attenuation parameter; BMI: Body mass index.
Comparative analysis of patients with AIH concomitant with NAFLD and patients with NAFLD with autoimmune phenomena
In this study, we defined patients with histologic evidence of both AIH and NAFLD as patients with AIH concomitant with NAFLD and patients with histologic evidence of NAFLD and positive autoantibodies or elevated IgG or IgM as patients with NAFLD with autoimmune phenomena.
The results showed that patients with AIH concomitant with NAFLD were older and had higher IgG levels and LSM values than patients with NAFLD with autoimmune phenomena. In contrast, the GGT level and CAP value were higher in patients with NAFLD with autoimmune phenomena (Table 3). Interestingly, when we compared the LSM value in each steatosis grade of the two groups, patients with AIH concomitant with NAFLD were higher in both S1 and S3 grades, suggesting a potential diagnostic marker for patients with AIH concomitant with NAFLD.
Table 3.
Comparative analysis of patients with autoimmune hepatitis concomitant with nonalcoholic fatty liver disease and patients with nonalcoholic fatty liver disease with autoimmune phenomena
|
Variable
|
Patients with AIH concomitant with NAFLD (n = 61)
|
Patients with NAFLD with autoimmune phenomena (n = 34)
|
P
value
|
| Age, yr | 52.05 ± 11.11 | 43.68 ± 13.71 | < 0.001 |
| Male sex, n (%) | 13 (21.3) | 6 (17.6) | |
| BMI, kg/m2 | 24.21 ± 3.10 | 24.65 ± 2.02 | |
| Laboratory | |||
| AST, U/L | 55.03 ± 47.31 | 50.03 ± 29.26 | |
| ALT, U/L | 66.62 ± 74.94 | 86.01 ± 62.59 | |
| AKP, U/L | 63.00 (45.00, 84.00) | 75.00 (54.00, 87.00) | |
| GGT, U/L | 49.27 ± 32.24 | 69.26 ± 44.07 | < 0.05 |
| Total bilirubin, mg/dL | 10.20 (8.60, 14.10) | 10.25 (7.40, 13.67) | |
| Direct bilirubin, mg/dL | 4.20 (3.20, 5.00) | 3.70 (2.92, 4.65) | |
| IgG | 15.60 ± 4.57 | 13.81 ± 2.67 | < 0.05 |
| IgM | 1.18 ± 0.45 | 1.28 ± 0.57 | |
| Histological steatosis stage, n (%) | |||
| S1 | 39 (63.9) | 8 (23.5) | |
| S2 | 17 (27.9) | 10 (29.4) | |
| S3 | 5 (8.2) | 16 (47.1) | |
| Histological fibrosis stage, n (%) | |||
| F1 | 17 (27.9) | 6 (17.6) | |
| F2 | 22 (36.1) | 14 (41.2) | |
| F3 | 16 (26.2) | 11 (32.4) | |
| F4 | 6 (9.8) | 3 (8.8) | |
| CAP, dB/m | 272.57 ± 44.39 | 293.41 ± 51.04 | < 0.05 |
| LSM in total, kPa | 9.34 ± 7.14 | 6.49 ± 2.44 | < 0.05 |
| In steatosis S1 | 9.19 ± 8.11 | 5.28 ± 1.88 | < 0.05 |
| In steatosis S2 | 7.80 ± 3.47 | 6.31 ± 2.18 | |
| In steatosis S3 | 15.76 ± 6.25 | 7.20 ± 2.69 | < 0.01 |
Distributions are expressed as the mean ± SD or median [interquartile range] or number (percentage). AKP: Alkaline phosphatase; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; BMI: Body mass index; CAP: Controlled attenuation parameter; GGT: Gamma-glutamyl transferase; LSM: Liver stiffness measurement; AIH: Autoimmune hepatitis; NAFLD: Nonalcoholic fatty liver.
DISCUSSION
The prevalence of hepatic steatosis is rising in association with the global increase in obesity. The overall prevalence of NAFLD over the past two decades is 29.6%, up to 46.4% in heavy drinkers and 50%-80% in the obese population[22]. Close attention was paid to the potential interactions between chronic liver disease and hepatic steatosis with the growing NAFLD prevalence. However, the particular role of hepatic steatosis has been less of a focus of attention in AILDs than in other chronic liver diseases, partly due to the lack of proper evaluation tools. Herein, we demonstrated that CAP measured by TE could be used as a noninvasive diagnostic tool to evaluate hepatic steatosis effectively and accurately in patients with AILDs.
In this study, we confirmed that CAP correlated well with hepatic steatosis on histology, and we were able to establish cut-off values with high diagnostic accuracies. The cut-off values for each steatosis grade in patients with mixed etiology liver disease were similar to the previously proposed cut-off values in an individual patient data meta-analysis (229 dB/m for S ≥ 1, 258 dB/m for S ≥ 2)[17]. However, the accuracy of CAP in separating steatosis grades 2 and 3 was suboptimal, which was similar to prior reports[13]. Nevertheless, in clinical practice, the identification of moderate steatosis is of greater utility than distinctions between S2 and S3, and thus, the Youden cut-off for S2 of 258 dB/m is sufficient.
Given the high risk of glycolipid metabolism disorder and increased possibility of hepatic steatosis in patients with AIH due to the long-term administration of immunosuppressive drugs such as glucocorticoids, the noninvasive method may be particularly suited for monitoring current hepatic histological changes and therapeutic effects[7,23]. Here, we found that CAP was closely related to hepatic steatosis in patients with AILDs, as well as patients with AIH. Surprisingly, the performance of CAP for the diagnosis of hepatic steatosis grade in patients with AIH remained stable, which supported that CAP can be used as a noninvasive and reliable diagnostic method to monitor steatosis and disease course.
Notably, we found a strong correlation between CAP and BMI, in agreement with previous studies[24,25]. The skin capsular distance (SCD) has also been shown to be associated with increased CAP values[26]. Since BMI and SCD are both surrogate markers of adiposity, it is difficult to determine the mechanism underlying the association. However, we did not find a correlation between CAP and LSM as reported for patients with NAFLD[27].
It is found in the clinic that elevated immunoglobulin and/or positive autoantibodies may occur during the progression of NAFLD, which we defined as “patients with NAFLD with autoimmune phenomena” here. On the other hand, patients with AIH can also have NAFLD, thereby affecting the diagnosis and treatment. In this study, we found that patients with AIH concomitant with NAFLD were older and had higher IgG levels and LSM values than patients with NAFLD with autoimmune phenomena, which could be related to the repeated and long-term course of AILDs. In contrast, the GGT level was higher in patients with NAFLD with autoimmune phenomena. Therefore, serum IgG and GGT levels and the LSM value can benefit the identification of these two kinds of patients.
There are a few limitations in this study. First, our study is retrospective in nature due to the data collection despite the “blinded” analysis of histology. Second, the sample size is limited due to the low prevalence of AILDs, especially PBC and AIH-PBC overlap syndrome. Third, for historical reasons, CAP was measured only using the M probe. However, a recent multicenter prospective study has shown that there was no significant difference in diagnostic accuracy between the two probes to diagnose liver fibrosis and steatosis in patients with NAFLD[28].
CONCLUSION
In summary, this study revealed that CAP could be used as a noninvasive diagnostic method to evaluate hepatic steatosis in patients with AILDs. Determination of LSM combined with CAP may help to identify patients with AIH concomitant with NAFLD from those with NAFLD with autoimmune phenomena.
ARTICLE HIGHLIGHTS
Research background
The controlled attenuation parameter (CAP) assesses hepatic steatosis with high diagnostic accuracies among several chronic liver diseases. However, it has not been studied in patients with autoimmune liver diseases (AILDs).
Research motivation
This study aimed to investigate the performance of CAP for the diagnosis of hepatic steatosis in patients with AILDs.
Research objectives
We evaluated the performance and usefulness of CAP for detection of hepatic steatosis in patients with AILDs.
Research methods
The area under the receiver operating characteristic curve was used to evaluate the performance of CAP for diagnosing hepatic steatosis compared with biopsy. Optimal CAP cut-off values were determined based on the highest combined sensitivity and specificity.
Research results
CAP can accurately detect hepatic steatosis as a noninvasive method in patients with AILDs. Compared with patients with nonalcoholic fatty liver disease (NAFLD) presenting with autoimmune markers, patients with autoimmune hepatitis (AIH) concomitant with NAFLD were much older and had higher serum IgG levels and liver stiffness measurement (LSM) values.
Research conclusions
CAP can be used as a noninvasive diagnostic method to evaluate hepatic steatosis in patients with AILDs. Determination of LSM combined with CAP may help to identify patients with AIH concomitant with NAFLD from patients with NAFLD with autoimmune phenomena.
Research perspectives
Larger multicenter studies using both M and XL probes are needed to confirm our results.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of Renji Hospital.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: The authors have no financial relationships to disclose.
Manuscript source: Unsolicited manuscript
Peer-review started: September 11, 2020
First decision: November 23, 2020
Article in press: December 11, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kita K S-Editor: Huang P L-Editor: Wang TQ P-Editor: Li JH
Contributor Information
Xi-Xi Ni, Department of Gastroenterology and Hepatology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai Institute of Digestive Disease, Shanghai 200127, China.
Min Lian, Department of Gastroenterology and Hepatology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China.
Hui-Min Wu, Department of Gastroenterology and Hepatology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai Institute of Digestive Disease, Shanghai 200127, China.
Xiao-Yun Li, Department of Gastroenterology and Hepatology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai Institute of Digestive Disease, Shanghai 200127, China.
Li Sheng, School of Medicine, Shanghai Jiao Tong University, Shanghai Cancer Institute, Shanghai Institute of Digestive Disease, Shanghai 200127, China.
Han Bao, Department of Gastroenterology and Hepatology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China.
Qi Miao, School of Medicine, Shanghai Jiao Tong University, Shanghai Institute of Digestive Disease, Shanghai 200127, China.
Xiao Xiao, Department of Gastroenterology and Hepatology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai Institute of Digestive Disease, Shanghai 200127, China.
Can-Jie Guo, Department of Gastroenterology and Hepatology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China.
Hai Li, Department of Gastroenterology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200127, China.
Xiong Ma, School of Medicine, Shanghai Jiao Tong University, Shanghai Cancer Institute, Shanghai Institute of Digestive Disease, Shanghai 200127, China; Department of Gastroenterology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai Institute of Digestive Disease, Shanghai 200001, China.
Jing Hua, Department of Gastroenterology and Hepatology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai Institute of Digestive Disease, Shanghai 200127, China. hua_jing88@163.com.
Data sharing statement
No additional data are available.
References
- 1.Yeh MM, Brunt EM. Pathological features of fatty liver disease. Gastroenterology. 2014;147:754–764. doi: 10.1053/j.gastro.2014.07.056. [DOI] [PubMed] [Google Scholar]
- 2.Sasso M, Miette V, Sandrin L, Beaugrand M. The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin Res Hepatol Gastroenterol. 2012;36:13–20. doi: 10.1016/j.clinre.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Persico M, Iolascon A. Steatosis as a co-factor in chronic liver diseases. World J Gastroenterol. 2010;16:1171–1176. doi: 10.3748/wjg.v16.i10.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka A, Mori M, Matsumoto K, Ohira H, Tazuma S, Takikawa H. Increase trend in the prevalence and male-to-female ratio of primary biliary cholangitis, autoimmune hepatitis, and primary sclerosing cholangitis in Japan. Hepatol Res. 2019;49:881–889. doi: 10.1111/hepr.13342. [DOI] [PubMed] [Google Scholar]
- 5.Beringer A, Miossec P. IL-17 and IL-17-producing cells and liver diseases, with focus on autoimmune liver diseases. Autoimmun Rev. 2018;17:1176–1185. doi: 10.1016/j.autrev.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Powell EE, Jonsson JR, Clouston AD. Steatosis: co-factor in other liver diseases. Hepatology. 2005;42:5–13. doi: 10.1002/hep.20750. [DOI] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63:971–1004. doi: 10.1016/j.jhep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 8.European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145–172. doi: 10.1016/j.jhep.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Castera L, Vilgrain V, Angulo P. Noninvasive evaluation of NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:666–675. doi: 10.1038/nrgastro.2013.175. [DOI] [PubMed] [Google Scholar]
- 10.Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, Sandrin L, Miette V. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–1835. doi: 10.1016/j.ultrasmedbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Sasso M, Audière S, Kemgang A, Gaouar F, Corpechot C, Chazouillères O, Fournier C, Golsztejn O, Prince S, Menu Y, Sandrin L, Miette V. Liver Steatosis Assessed by Controlled Attenuation Parameter (CAP) Measured with the XL Probe of the FibroScan: A Pilot Study Assessing Diagnostic Accuracy. Ultrasound Med Biol. 2016;42:92–103. doi: 10.1016/j.ultrasmedbio.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, Beaugrand M, Palau R. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, Guha IN, Cobbold JF, Deeks JJ, Paradis V, Bedossa P, Newsome PN. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1717–1730. doi: 10.1053/j.gastro.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 14.Seto WK, Hui RWH, Mak LY, Fung J, Cheung KS, Liu KSH, Wong DK, Lai CL, Yuen MF. Association Between Hepatic Steatosis, Measured by Controlled Attenuation Parameter, and Fibrosis Burden in Chronic Hepatitis B. Clin Gastroenterol Hepatol 2018; 16: 575-583. :e2. doi: 10.1016/j.cgh.2017.09.044. [DOI] [PubMed] [Google Scholar]
- 15.Thiele M, Rausch V, Fluhr G, Kjærgaard M, Piecha F, Mueller J, Straub BK, Lupșor-Platon M, De-Ledinghen V, Seitz HK, Detlefsen S, Madsen B, Krag A, Mueller S. Controlled attenuation parameter and alcoholic hepatic steatosis: Diagnostic accuracy and role of alcohol detoxification. J Hepatol. 2018;68:1025–1032. doi: 10.1016/j.jhep.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 16.Ferraioli G, Tinelli C, De Silvestri A, Lissandrin R, Above E, Dellafiore C, Poma G, Di Gregorio M, Maiocchi L, Maserati R, Filice C. The clinical value of controlled attenuation parameter for the noninvasive assessment of liver steatosis. Liver Int. 2016;36:1860–1866. doi: 10.1111/liv.13207. [DOI] [PubMed] [Google Scholar]
- 17.Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, Kumar M, Lupsor-Platon M, Han KH, Cardoso AC, Ferraioli G, Chan WK, Wong VW, Myers RP, Chayama K, Friedrich-Rust M, Beaugrand M, Shen F, Hiriart JB, Sarin SK, Badea R, Jung KS, Marcellin P, Filice C, Mahadeva S, Wong GL, Crotty P, Masaki K, Bojunga J, Bedossa P, Keim V, Wiegand J. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022–1030. doi: 10.1016/j.jhep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H, Bianchi FB, Shibata M, Schramm C, Eisenmann de Torres B, Galle PR, McFarlane I, Dienes HP, Lohse AW International Autoimmune Hepatitis Group. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 19.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology. 2009;50:291–308. doi: 10.1002/hep.22906. [DOI] [PubMed] [Google Scholar]
- 20.Chazouillères O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology. 1998;28:296–301. doi: 10.1002/hep.510280203. [DOI] [PubMed] [Google Scholar]
- 21.National Workshop on Fatty Liver and Alcoholic Liver Disease CSoH; Chinese Medical Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: a 2018 update. Linchuang Gandan Bing Zazhi. 2018;34:947–957. doi: 10.3760/cma.j.issn.1007-3418.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Zhou J, Zhou F, Wang W, Zhang XJ, Ji YX, Zhang P, She ZG, Zhu L, Cai J, Li H. Epidemiological Features of NAFLD From 1999 to 2018 in China. Hepatology. 2020;71:1851–1864. doi: 10.1002/hep.31150. [DOI] [PubMed] [Google Scholar]
- 23.Mieli-Vergani G, Vergani D, Czaja AJ, Manns MP, Krawitt EL, Vierling JM, Lohse AW, Montano-Loza AJ. Autoimmune hepatitis. Nat Rev Dis Primers. 2018;4:18017. doi: 10.1038/nrdp.2018.17. [DOI] [PubMed] [Google Scholar]
- 24.Wong VW, Petta S, Hiriart JB, Cammà C, Wong GL, Marra F, Vergniol J, Chan AW, Tuttolomondo A, Merrouche W, Chan HL, Le Bail B, Arena U, Craxì A, de Lédinghen V. Validity criteria for the diagnosis of fatty liver by M probe-based controlled attenuation parameter. J Hepatol. 2017;67:577–584. doi: 10.1016/j.jhep.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 25.de Lédinghen V, Vergniol J, Capdepont M, Chermak F, Hiriart JB, Cassinotto C, Merrouche W, Foucher J, Brigitte le B. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60:1026–1031. doi: 10.1016/j.jhep.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Shen F, Zheng RD, Shi JP, Mi YQ, Chen GF, Hu X, Liu YG, Wang XY, Pan Q, Chen GY, Chen JN, Xu L, Zhang RN, Xu LM, Fan JG. Impact of skin capsular distance on the performance of controlled attenuation parameter in patients with chronic liver disease. Liver Int. 2015;35:2392–2400. doi: 10.1111/liv.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petta S, Wong VW, Cammà C, Hiriart JB, Wong GL, Marra F, Vergniol J, Chan AW, Di Marco V, Merrouche W, Chan HL, Barbara M, Le-Bail B, Arena U, Craxì A, de Ledinghen V. Improved noninvasive prediction of liver fibrosis by liver stiffness measurement in patients with nonalcoholic fatty liver disease accounting for controlled attenuation parameter values. Hepatology. 2017;65:1145–1155. doi: 10.1002/hep.28843. [DOI] [PubMed] [Google Scholar]
- 28.Oeda S, Takahashi H, Imajo K, Seko Y, Ogawa Y, Moriguchi M, Yoneda M, Anzai K, Aishima S, Kage M, Itoh Y, Nakajima A, Eguchi Y. Accuracy of liver stiffness measurement and controlled attenuation parameter using FibroScan® M/XL probes to diagnose liver fibrosis and steatosis in patients with nonalcoholic fatty liver disease: a multicenter prospective study. J Gastroenterol. 2020;55:428–440. doi: 10.1007/s00535-019-01635-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.