Abstract
MicroRNA-126 (miR-126) has been reported to be implicated in the pathogenesis of cerebral ischemia/reperfusion (I/R) injury; however, its role is still unclear and requires further investigation. The objective of the present study was to determine the neuroprotective effect of miR-126 overexpression against oxygen-glucose deprivation/reoxygenation (OGD/R)-induced human umbilical vein endothelial cell (HUVEC) injury, an in vitro model of cerebral I/R injury, and to further explore the role of the NAD-dependent protein deacetylase sirtuin-1 (SIRT1)/nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway in this process. The results of the present study revealed that miR-126 expression was markedly reduced in HUVECs subjected to OGD/R treatment. Functional experiments demonstrated that transfection with miR-126 mimics attenuated OGD/R-induced down-regulation of cell viability, and reversed OGD/R-induced up-regulation of lactate dehydrogenase release, apoptosis and caspase-3 activity in HUVECs. Notably, OGD/R reduced SIRT1 and heme oxygenase-1 expression, and induced the nuclear translocation of Nrf2, as demonstrated by the increase in cytoplasmic Nrf2 expression and the decrease in nuclear Nrf2 expression. Following transfection with miR-126 mimics, these effects of OGD/R were reversed, indicating that miR-126 overexpression promoted the SIRT1/Nrf2 signaling pathway. Additionally, miR-126 mimics attenuated OGD/R-induced cytotoxicity and apoptosis, which was blocked by inhibition of the SIRT1/Nrf2 signaling pathway followed by transfection with SIRT1-small interfering RNA (siRNA). Furthermore, miR-126 mimics decreased ROS generation and malondialdehyde content, and increased superoxide dismutase and glutathione peroxidase activity in HUVECs exposed to OGD/R, and these effects of miR-126 mimics were also blocked by SIRT1-siRNA. Additionally, the miR-126 mimics-induced the decreases in the levels of pro-inflammatory cytokines, including tumor necrosis factor-α, interleukin (IL)-1β and IL-6, and the miR-126 mimics-induced increase in anti-inflammatory cytokines, including IL-10, were reversed by SIRT1-siRNA. Overall, these results suggested that miR-126 overexpression attenuated OGD/R-induced neurotoxicity to HUVECs by alleviating oxidative stress and the inflammatory response via promotion of the SIRT1/Nrf2 signaling pathway.
Keywords: microRNA-126, oxygen-glucose deprivation/reperfusion injury, oxidative stress, inflammatory response, NAD-dependent protein deacetylase sirtuin-1/nuclear factor erythroid 2-related factor 2 signaling pathway, human umbilical vein endothelial cells
Introduction
Cerebral ischemia is considered to be a leading cause of morbidity and mortality in the developed world (1). Although reperfusion is the recommended therapy for effective treatment of cerebral ischemia, it may also exacerbate brain injury and functional damage in a process termed cerebral ischemia/reperfusion (I/R) injury (2). In recent years, numerous studies have demonstrated that multiple mechanisms involving a variety of signaling pathways and biological processes are involved in the process of cerebral I/R injury (3–5); however, the mechanism is complex and our knowledge on the subject is unsatisfactory at present. A number of studies have revealed that oxidative stress and inflammation are the primary pathological causes of neuronal loss following cerebral I/R (6,7). Therefore, a focus on understanding the molecular mechanisms related to oxidative stress and the inflammatory response behind cerebral I/R injury may promote the development of more efficient therapeutic agents.
MicroRNAs (miRs/miRNAs) are a class of small non-coding RNAs that are 18–23 nucleotides in length that can regulate a wide range of biological processes in cerebral ischemia, including oxidative stress and the inflammatory reaction. It has been proposed that they could be potential diagnostic markers and promising therapeutic agents in cerebral ischemia (8,9). Since miR-126 has been reported to regulate vascular integrity and angiogenesis, numerous studies have demonstrated that miR-126 serves an important role in the pathogenesis and complications of cerebral ischemic stroke (10,11). Pan et al (12) revealed that miR-126 overexpression may promote the functions of endothelial progenitor cells (EPCs) under hypoxic conditions, resulting in the enhanced therapeutic efficacy of EPCs in ischemic stroke. Furthermore, the promotion of the miR-126-related signaling pathway induced by long non-coding RNAs attenuates ischemic neuronal death and apoptosis (10). However, the underlying protective mechanisms of miR-126 in cerebral I/R injury remain to be elucidated.
NAD-dependent protein deacetylase sirtuin-1(SIRT1), a nicotinamide adenine dinucleotide-dependent histone deacetylase, regulates numerous physiological and pathological processes, including cell survival, apoptosis, oxidative stress, inflammation and neuroprotection (13–15). Additionally, increasing evidence has suggested that SIRT1 may have beneficial effects in cerebral I/R injury (16). Nuclear factor erythroid 2-related factor 2 (Nrf2) is capable of multiple biological effects, and the activation of this protein, which promotes Nrf2 translocation from the cytoplasm to the nucleus and activates the transcription of antioxidant, cytoprotective and anti-inflammatory genes, such as heme oxygenase-1 (HO-1), is crucial for cellular defense mechanisms (17). Furthermore, it has been reported that SIRT1 is associated with the activation of Nrf2 (18). Several studies have demonstrated that activation of the SIRT1/Nrf2 signaling pathway increases resistance to oxidative stress injury and has protective effects against inflammation (19,20). Recent studies have demonstrated that the dysregulation of the SIRT1/Nrf2 signaling pathway is involved in the pathogenesis of a number of neurodegenerative diseases (20,21). Furthermore, Chan et al (22) has demonstrated that the SIRT1/Nrf2 signaling pathway mediates the protective effects against diabetic hyperglycemia-exacerbated cerebral I/R injury. Notably, a study by Xu et al (23) revealed that SIRT1 can be regulated by miR-126. However, at present, the role of the SIRT1/Nrf2 signaling pathway in the development of cerebral I/R injury and the protective effects of miR-126 on cerebral I/R injury remains largely unknown.
The present study aimed to investigate the protective effects of miR-126 overexpression on oxidative stress and the inflammatory response in human umbilical vein endothelial cells (HUVECs) subjected to oxygen-glucose deprivation/reoxygenation (OGD/R), and the role of the SIRT1/Nrf2 signaling pathway in this process. The present study demonstrated that miR-126 overexpression attenuated OGD/R-induced cell injury of HUVECs by inhibiting oxidative stress and inflammatory response via the activation of SIRT1/Nrf2 signaling pathway.
Materials and methods
Chemicals and reagents
Dulbecco's modified Eagle's medium (DMEM; cat. no. 11965092) and fetal bovine serum (FBS; cat. no. 16140071) were purchased from Gibco (Thermo Fisher Scientific, Inc.). The Cell Counting Kit-8 (CCK-8) assay (cat. no. C0038) and Reactive Oxygen Species (ROS) assay kit (cat. no. S0033M) were obtained from Beyotime Institute of Biotechnology. The Annexin V-FITC/propidium iodide (PI) apoptosis kit (cat. no. 556547) was purchased from BD Biosciences. The lactate dehydrogenase (LDH) assay kit (cat. no. A020-2-2), cell malondialdehyde (MDA) assay kit (cat. no. A003-4-1), superoxide dismutase (SOD) assay kit (cat. no. A001-3-2), glutathione peroxidase (GSH-Px) assay kit (cat. no. A005-1-2), interleukin-6 (IL-6) assay kit (cat. no. H007), interleukin-1β (IL-1β) assay kit (cat. no. H002), tumor necrosis factor-α (TNF-α) assay kit (cat. no. H052) and interleukin-10 (IL-10) assay kit (cat. no. H009) were obtained from Nanjing Jiancheng Bioengineering Institute. Antibodies against SIRT1 (cat. no. 8469), Nrf2 (cat. no. 12721), HO-1 (cat. no. 82206), histone H3 (cat. no. 4499) and GAPDH (cat. no. 5174) were purchased from Cell Signaling Technology, Inc. The antibody against GAPDH (cat. no. ab181602) was obtained from Abcam.
Cell culture and OGD/R treatment
HUVECs were provided by The Cell Bank of Type Culture Collection of the Chinese Academy of Sciences, and cultured in DMEM containing 10% FBS, 100 U/ml penicillin and 100 U/ml streptomycin in a humidified incubator with 5% CO2 at 37°C. Culture media were exchanged every 2 days. To induce OGD/R injury, HUVECs were incubated with glucose-free DMEM in a humidified chamber filled with 95% N2 and 5% CO2 at 37°C for 4 h. Subsequently, cells were transferred to complete culture medium and maintained in normoxic conditions for 6, 12 or 24 h to induce reperfusion.
Cell transfection
miR-126 mimics (cat. no. miR10000444-1-5; 5′-UCGUACCGUGAGUAAUAAUGCG-3′) and miR-negative control (miR-NC; cat. no. miR1N0000002-1-5; 5′-UUCUCCGAACGUGUCACGUTT-3′) were synthesized by Guangzhou RiboBio Co, Ltd. SIRT1-small interfering RNA (SIRT1-siRNA; forward, 5′-ACUUUGCUGUAACCCUGUA-3′ and reverse, 5′-UACAGGGUUACAGCAAAGU-3′) or negative control siRNA sequence (Con-siRNA; 5′-CUAGCUUAUGUGGACCUCG-3′) were synthesized by Sangon Biotech Co., Ltd. Transfection of miR-126 mimics, miR-NC, SIRT1-siRNA or Con-siRNA at a final concentration of 50 nM were performed using Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocols. At 48 h after transfection, transfection efficiency was determined by reverse transcription-quantitative PCR (RT-qPCR) or western blotting.
RT-qPCR
Total RNA was extracted from HUVECs using TRIzol® reagent (cat. no. 15596018; Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocols. cDNA was synthesized with the PrimeScript™ One Step RT-PCR kit (cat. no. RR055B; Takara Biotechnology Co., Ltd.) at a temperature of 45°C for 60 min and 72°C for 5 min using the extracted total RNA (30 µg) as the template. miR-126 was amplified using a Power SYBR-Green PCR Master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) with an Applied Biosystems 7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The primers were as follows: miR-126 forward, 5′-GCAATGCTAGATTTAGTAATT-3′ and reverse, 3′-GCGCAUGGUUUCAUUAUUAC-5′; and U6 forward, 5′-TGACACGCAAATTCGTGAAGCGTTC-3′ and reverse, 5′-CCAGTCTCAGGGTCCGAGGTATTC-3′. U6 was used as the endogenous control. The RT-qPCR conditions were as follows: 94°C for 4 min, followed by 94°C for 20 sec, 60°C for 30 sec and 72°C for 30 sec (35 cycles). Relative gene expression was calculated according to the 2−ΔΔCq method (24).
CCK-8 assay
The viability of HUVECs was detected using the CCK-8 assay kit according to the manufacturer's protocol. Briefly, cells in 96-well plates were incubated with CCK-8 solution (10 µl/well) for 3 h at 37°C, and then the optical density (OD) at an emission wavelength of 450 nm was measured on a microplate reader (Spectra Max M5; Molecular Devices, LLC).
Lactate dehydrogenase (LDH) releases assay
HUVECs injury was determined by the release of LDH using a LDH assay kit (cat. no. C0016; Beyotime Institute of Biotechnology) according to manufacturer's protocols. After treatment as detailed above, the culture supernatant was collected and then incubated with LDH reagent for 30 min at room temperature in the dark. The absorbance at 490 nm was assessed by a microplate reader (Spectra Max M5; Molecular Devices, LLC).
Measurement of intracellular ROS generation
Intracellular ROS production was determined using the non-fluorescent probe 2′,7′-dichlorofluorescein diacetate (DCFH-DA; cat. no. S0033M; Beyotime Institute of Biotechnology) according to the manufacturer's protocol. In brief, HUVECs cultured in 6-well plates were incubated with DCFH-DA (10 µM) in FBS-free DMEM at 37°C for 20 min. After three washes with FBS-free DMEM, the cells were collected and the fluorescent density of each group was quantified using a FACSCanto II flow cytometer (BD Biosciences) and the data were analyzed by the FlowJo software v10.2 (FlowJo LLC).
MDA content, and SOD and GSH-Px activity assays
HUVECs were lysed in RIPA buffer (Beyotime Institute of Biotechnology) on ice for 30 min. Following centrifugation at 13,400 × g at 4°C for 10 min, the MDA content, and SOD and GSH-Px activity in the supernatant were evaluated using the appropriate kits (Nanjing Jiancheng Bioengineering Institute), according to the manufacturer's protocols.
Evaluation of IL-1β, IL-6, TNF-α and IL-10 levels via ELISA
HUVECs were collected and homogenized in ice-cold PBS. Following centrifugation at 13,400 × g for 10 min at 4°C, the supernatant was assayed for IL-1β, IL-6, TNF-α and IL-10 levels in accordance with the manufacturer's protocols. The OD was measured at a wavelength of 450 nm on a microplate reader (Infinite® 200 PRO NanoQuant; Tecan Group, Ltd.).
Caspase-3 activity assay
Caspase-3 activity was measured using the Apo-ONE® Homogeneous Caspase-3 assay kit (Promega Corporation), according to the manufacturer's protocols. The OD at an emission wavelength of 570 nm was measured on a microplate reader (Spectra Max M5; Molecular Devices, LLC).
Flow cytometry
HUVEC apoptosis was detected using the Annexin V-FITC/PI Apoptosis Detection kit according to the manufacturer's protocols. Following the aforementioned treatment, cells were harvested, washed twice with PBS and then resuspended in 1X buffer solution. Subsequently, cells were stained successively with Annexin V-FITC (50 µl) and PI (10 µl) at 37°C for 15 min. The apoptotic cells were evaluated using a flow cytometer (FACScan; BD Biosciences) and data were analyzed using CellQuest software (version 3.1; BD Biosciences). The experiment was performed independently three times. The percentage of apoptotic cells was calculated by the ratio of cells with FITC-Annexin V+PI− (early apoptosis) and FITC-Annexin V+PI+ (late apoptosis) to the total number of cells using a FACSCanto II flow cytometer (BD Biosciences) and the data were analyzed by the FlowJo software v10.2 (FlowJo LLC).
Western blotting
HUVECs were lysed with RIPA buffer containing 1% (v/v) protease and phosphatase inhibitors (Beyotime Institute of Biotechnology). Cytoplasmic and nuclear proteins were extracted using a Nuclear and Cytoplasmic Protein Extraction Kit (cat. no. P0028; Beyotime Institute of Biotechnology), according to the manufacturer's instructions. Protein concentration was determined by a BCA Protein Assay Kit (cat. no. P0012; Beyotime Institute of Biotechnology). Equal amounts of protein (30 µg) were separated via SDS-PAGE on a 12% gel, and then transferred to PVDF membranes (EMD Millipore). Following blocking with 5% non-fat dry milk at room temperature for 2 h, the membranes were incubated with the following primary antibodies at 4°C overnight: SIRT1, Nrf2, HO-1, histone H3 and GAPDH (1:2,000). Histone H3 was used as an internal reference for nuclear protein and GAPDH was used as an internal reference for total protein. Subsequently, the membranes were washed three times. Afterwards, the membranes were incubated with anti-rabbit HRP-conjugated secondary antibody (cat. no. 7074; 1:5,000; Cell Signaling Technology, Inc.) and anti-rat HRP-conjugated secondary antibody (cat. no. 7077; 1:5,000; Cell Signaling Technology, Inc.) at room temperature for 2 h, and then the bands were visualized using enhanced chemiluminescence (Amersham; Cytiva). The band densities were quantified using ImageJ software (version 1.41; National Institutes of Health).
Statistical analysis
All data were analyzed using GraphPad Prism 5.0 (GraphPad Software, Inc.) and presented as the mean ± SD. One-way ANOVA followed by the Tukey's post hoc test was used to compare data from different groups. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-126 mimics reverse OGD/R-induced HUVEC injury
As shown in Fig. 1, compared with the control group, cell activity was significantly decreased with increasing hypoxia duration in the OGD/R group (Fig. 1A). Furthermore, LDH release assay results revealed that OGD/R increased LDH release in the culture supernatant (Fig. 1B). Additionally, the expression of miR-126 in the OGD/R group was significantly reduced compared with those in the control group (Fig. 1C). OGD treatment for 4 h and R for 24 h were selected for subsequent experiments.
Figure 1.
Effect of OGD/R on cell survival and apoptosis in the presence or absence of miR-126 mimics in HUVECs. Cells were exposed to OGD for 4 h, followed by reoxygenation for 6, 12 and 24 h. (A) The cell viability of each group was determined by CCK-8 assay. (B) LDH release from HUVECs was measured using a LDH Cytotoxicity assay kit. (C) miR-126 expression was determined via RT-qPCR. HUVECs were transfected with miR-126 m or miR-NC, and (D) miR-126 expression was determined via RT-qPCR. HUVECs were transfected with miR-126 m or miR-NC before OGD (4 h)/R (24 h) exposure. (E) The cell viability of each group was determined by CCK-8 assay. (F) LDH release from HUVECs was measured using a LDH Cytotoxicity assay kit. (G) The rate of apoptosis was detected by Annexin V/PI double staining followed by flow cytometry. Data are presented as the mean ± SD of three independent experiments. *P<0.05 and **P<0.01 vs. control group; ##P<0.01 vs. miR-NC or miR-NC + OGD/R group. miR-126 m, miR-126 mimics; miR-NC, miR-negative control; miR, microRNA; OGD, oxygen-glucose deprivation; R, reoxygenation; HUVEC, human umbilical vein endothelial cell; LDH, lactate dehydrogenase; RT-qPCR, reverse transcription-quantitative PCR.
Based on the decrease in miR-126 expression and the protective effects of miR-126 in nervous system diseases (10–12), it was speculated that the gain-of-function of miR-126 may protect against neurological deficits induced by OGD/R injury. To verify this assumption, HUVECs were transfected with miR-126 mimics to promote the expression of miR-126. The results demonstrated that miR-126 mimics successfully increased the level of miR-126 compared with miR-NC transfection (Fig. 1D). Based on this, it was found that transfection with miR-126 mimics resulted in an increase in cell viability (Fig. 1E) and a decrease in LDH release (Fig. 1F) compared with miR-NC transfection under OGD/R conditions. Additionally, Annexin V/PI double staining followed by flow cytometry revealed that, compared with the control group, OGD/R increased the apoptotic rate of HUVECs. However, this effect of OGD/R was reversed by transfection with miR-126 mimics (Fig. 1G). These results indicated that miR-126 overexpression protected HUVECs against OGD/R injury and apoptosis.
miR-126 mimics attenuate OGD/R-induced inhibition of the SIRT1/Nrf2 signaling pathway in HUVECs
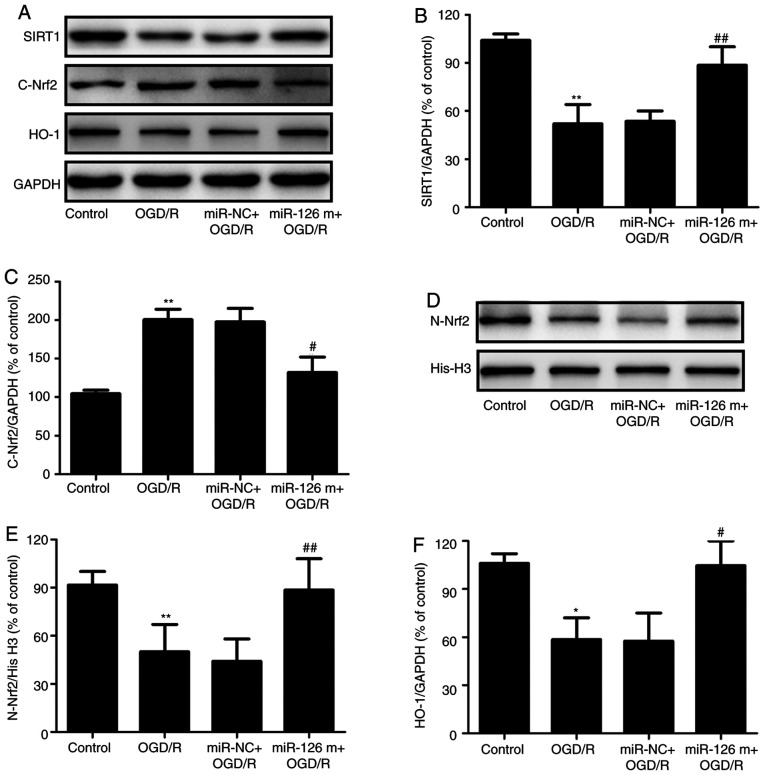
SIRT1 and Nrf2 are pivotal regulators of I/R injury in the brain (16,17). To investigate whether the SIRT1/Nrf2 signaling pathway was involved in the neuroprotective effects of miR-126, the expression levels of proteins related to the SIRT1/Nrf2 signaling pathway were detected in OGD/R-treated HUVECs in the presence of miR-126 mimics. Western blotting (Fig. 2A) revealed that transfection with miR-126 mimics increased the expression level of SIRT1 in OGD/R-treated HUVECs compared with miR-NC transfection (Fig. 2B). In addition, transfection with miR-126 mimics significantly decreased Nrf2 expression in the cytoplasm (Fig. 2C), with a corresponding increase in the nucleus (Fig. 2D and E), compared with miR-NC transfection in HUVECs exposed to OGD/R, indicating that miR-126 promoted the nuclear translocation of Nrf2. Additionally, miR-126 mimics reversed the OGD/R-induced decrease in the expression of HO-1 (Fig. 2F). Overall, these results suggested that miR-126 overexpression enhanced the activation of the SIRT1/Nrf2 signaling pathway.
Figure 2.
Effects of miR-126 overexpression on SIRT1 expression, Nrf2 protein distribution and HO-1 protein expression in OGD/R-exposed HUVECs. HUVECs were transfected with miR-126 m or miR-NC prior to OGD (4 h)/R (24 h) exposure. (A) Protein expression was determined via western blotting. Semi-quantification of (B) cytosolic SIRT1 and (C) Nrf2 normalized to GAPDH. (D) Representative western blots of nuclear Nrf2. (E) Quantification of nuclear Nrf2 normalized to histone H3. (F) Quantification of HO-1 normalized to GAPDH. Data are presented as the mean ± SD of at least three independent experiments. *P<0.05 and **P<0.01 vs. control group; #P<0.05 and ##P<0.01 vs. miR-NC + OGD/R group. miR-126 m, miR-126 mimics; miR-NC, miR-negative control; miR, microRNA; OGD, oxygen-glucose deprivation; R, reoxygenation; HUVEC, human umbilical vein endothelial cell; SIRT1, NAD-dependent protein deacetylase sirtuin-1; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase 1.
Inhibition of the SIRT1/Nrf2 signaling pathway reverses the protective effect of miR-126 against OGD/R injury in HUVECs
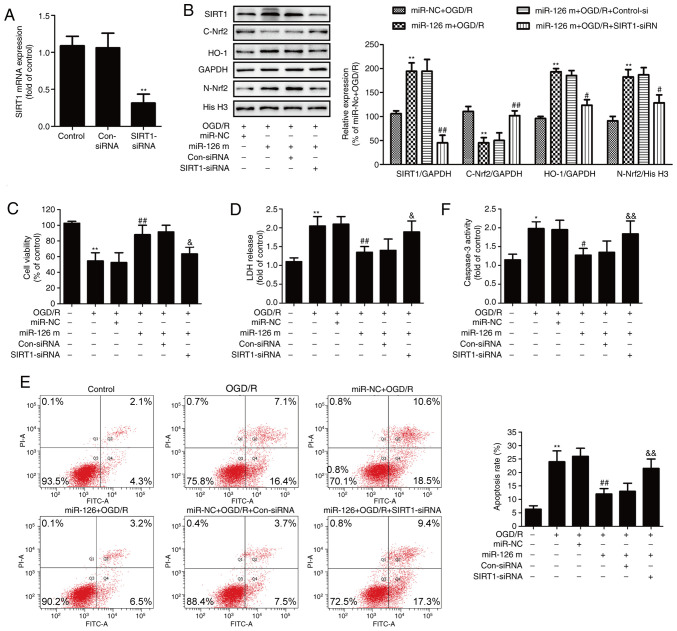
To determine the exact role of the SIRT1/Nrf2 signaling pathway in the protective effects of miR-126 on OGD/R injury, HUVECs were pre-transfected with SIRT1-siRNA to inhibit the SIRT1/Nrf2 signaling pathway. RT-qPCR results showed that SIRT1-siRNA transfection significantly reduced the expression of SIRT1 mRNA compared with Con-siRNA transfection (Fig. 3A). In addition, western blotting results (Fig. 3B) demonstrated that SIRT1-siRNA significantly reduced the miR-126 mimics-induced upregulation of SIRT1 in OGD/R-treated HUVECs. Furthermore, SIRT1-siRNA significantly reversed the miR-126 mimics-induced nuclear translocation of Nrf2, as demonstrated by the increase in Nrf2 expression in the cytoplasm (Fig. 3B) and the decrease in Nrf2 expression in the nucleus (Fig. 3B). Additionally, SIRT1-siRNA blocked the miR-126 mimics-induced up-regulation of HO-1 expression (Fig. 3B). These results indicated that transfection with SIRT1-siRNA attenuated the miR-126 mimics-induced activation of the SIRT1/Nrf2 signaling pathway.
Figure 3.
Effects of SIRT1 siRNA on the SIRT1/Nrf2 signaling pathway, cell injury and apoptosis in the presence of miR-126 overexpression in OGD/R-exposed HUVECs. HUVECs were pre-transfected with SIRT1-siRNA or Con-siRNA for 48 h, and (A) the expression of SIRT1 mRNA was determined via reverse transcription-quantitative PCR. HUVECs were pre-transfected with SIRT1-siRNA or Con-siRNA for 30 min and then transfected with miR-126 m or miR-NC before OGD (4 h)/R (24 h) exposure. (B) Protein expression levels of SIRT1, nuclear Nrf2, cytosolic Nrf2 and HO-1 were determined via western blotting and bands were semi-quantified. (C) Cell viability of each group was determined by CCK-8 assay. (D) LDH release from HUVECs was measured using a LDH Cytotoxicity assay kit. (E) The rate of apoptosis was detected by Annexin V/PI double staining followed by flow cytometry. (F) Caspase-3 activity was measured by Apo-ONE® Homogeneous Caspase-3 assay kit. Data are presented as the mean ± SD of at least three independent experiments. *P<0.05 and **P<0.01 vs. control group; #P<0.05 and ##P<0.01 vs. miR-NC + OGD/R group; &P<0.05 and &&P<0.01 vs. miR-126 m + OGD/R + Con-siRNA group. miR-126 m, miR-126 mimics; miR-NC, miR-negative control; miR, microRNA; OGD, oxygen-glucose deprivation; R, reoxygenation; HUVEC, human umbilical vein endothelial cell; SIRT1, NAD-dependent protein deacetylase sirtuin-1; Nrf2, nuclear factor erythroid 2-related factor 2; siRNA, small interfering RNA; Con-siRNA, negative control siRNA; LDH, lactate dehydrogenase.
The results further revealed that SIRT1-siRNA reversed the miR-126 mimics-induced increase in cell viability (Fig. 3C) and the miR-126 mimics-induced decrease in LDH release in OGD/R-treated HUVECs (Fig. 3D). Flow cytometry analysis showed that SIRT1-siRNA also inhibited the miR-126 mimics-induced reduction of apoptosis (Fig. 3E). In addition, transfection with miR-126 mimics resulted in a decrease in caspase-3 activity in OGD/R-treated HUVECs, whereas this effect was reversed by SIRT1-siRNA (Fig. 3F). Overall, these results indicated that the SIRT1/Nrf2 signaling pathway mediated the neuroprotective effect of miR-126 on OGD/R injury.
miR-126 mimics mitigate OGD/R-induced oxidative stress by potentiating the SIRT1/Nrf2 signaling pathway in HUVECs
Increasing evidence has revealed that oxidative stress is critical for I/R-induced neuronal injury, which eventually results in apoptosis (3). Therefore, the effects of miR-126 mimics on oxidative stress in HUVECs exposed to OGD/R and the role of the SIRT1/Nrf2 signaling pathway in these processes were further investigated. DCFH-DA staining revealed that miR-126 mimics inhibited the OGD/R-induced up-regulation of ROS generation in HUVECs (Fig. 4A and B). However, SIRT1-siRNA pretreatment reversed the effects of miR-126 mimic transfection on ROS production compared with Con-siRNA transfection in OGD/R-treated HUVECs. Similarly, compared with miR-NC transfection, transfection with miR-126 mimics reduced the level of intracellular MDA, a marker of lipid peroxidation, whereas this effect of miR-126 mimics was attenuated by SIRT1-siRNA (Fig. 4C). To investigate the mechanisms that may be involved in the protective effects of miR-126, the activities of the antioxidant enzymes SOD and GSH-Px were measured. Following transfection of HUVECs with miR-126 mimics, intracellular SOD (Fig. 4D) and GSH-Px (Fig. 4E) activities were increased. However, this antioxidant ability of miR-126 was reversed by transfection with SIRT1-siRNA. These results suggested that miR-126 overexpression attenuated oxidative stress induced by OGD/R via the up-regulation of the SIRT1/Nrf2 signaling pathway in HUVECs.
Figure 4.
Effects of miR-126 on oxidative stress in HUVECs exposed to OGD/R in the presence or absence of SIRT1 siRNA or Con-siRNA. HUVECs were pre-transfected with SIRT1-siRNA or Con-siRNA for 30 min, and then transfected with miR-126 m or miR-NC prior to OGD (4 h)/R (24 h) exposure. (A) Intracellular ROS generation was assayed by dichlorohydrofluorescein diacetate staining followed by flow cytometry. (B) Quantitative analysis of ROS level. (C) MDA content was measured using a Lipid Peroxidation MDA assay kit. (D) SOD activity was determined by a Superoxide Dismutase assay kit. (E) GSH-PX activity was detected using a Cellular Glutathione Peroxidase assay kit. Data are presented as the mean ± SD of at least three independent experiments. *P<0.05 and **P<0.01 vs. control group; #P<0.05 and ##P<0.01 vs. miR-NC + OGD/R group; &P<0.05 and &&P<0.01 vs. miR-126 m + OGD/R + Con-siRNA group. miR-126 m, miR-126 mimics; miR-NC, miR-negative control; miR, microRNA; OGD, oxygen-glucose deprivation; R, reoxygenation; HUVEC, human umbilical vein endothelial cell; SIRT1, NAD-dependent protein deacetylase sirtuin-1; siRNA, small interfering RNA; Con-siRNA, negative control siRNA; ROS, reactive oxygen species; MDA, cell malondialdehyde; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase.
miR-126 mimics suppress the OGD/R-induced inflammatory response in a SIRT1-dependent manner
To gain insight into the potential intracellular mechanisms involved in the inhibitory effects of miR-126 overexpression on OGD/R injury, the levels of inflammatory-related factors were investigated. As shown in Fig. 5, exposure to OGD/R resulted in the up-regulation of pro-inflammatory cytokines, including TNF-α (Fig. 5A), IL-1β (Fig. 5B) and IL-6 (Fig. 5C). However, transfection with miR-126 mimics prevented the OGD/R-induced elevation of these pro-inflammatory cytokines compared with miR-NC transfection. Notably, the effect of transfection with miR-126 mimics was reversed by transfection with SIRT1-siRNA compared with Con-siRNA transfection. Additionally, transfection with miR-126 mimics led to a marked inhibitory effect on the OGD/R-induced down-regulation of anti-inflammatory cytokine IL-10 in HUVECs compared with miR-NC, whereas this effect was attenuated by transfection with SIRT1-siRNA (Fig. 5D). These results demonstrated that miR-126 overexpression had anti-inflammatory effects under OGD/R conditions via the promotion of the SIRT1/Nrf2 signaling pathway in HUVECs.
Figure 5.
Effects of miR-126 on the levels of inflammatory-related cytokines in the presence of SIRT1-siRNA or Con-siRNA in OGD/R-exposed HUVECs. HUVECs were pre-transfected with SIRT1-siRNA or Con-siRNA for 30 min and then transfected with miR-126 m or miR-NC prior to OGD (4 h)/R (24 h) exposure. The levels of (A) TNF-α, (B) IL-1β, (C) IL-6 and (D) IL-10 were measured using ELISA kits and the concentrations (µg/mg protein) were calculated using a standard calibration curve. Data are presented as the mean ± SD of at least three independent experiments. *P<0.05 and **P<0.01 vs. control group; #P<0.05 and ##P<0.01 vs. miR-NC + OGD/R group; &P<0.05 and &&P<0.01 vs. miR-126 m + OGD/R + Con-siRNA group. miR-126 m, miR-126 mimics; miR-NC, miR-negative control; miR, microRNA; OGD, oxygen-glucose deprivation; R, reoxygenation; HUVEC, human umbilical vein endothelial cell; SIRT1, NAD-dependent protein deacetylase sirtuin-1; siRNA, small interfering RNA; Con-siRNA, negative control siRNA; TNF-α, tumor necrosis factor-α; IL, interleukin.
Discussion
The results of the present study demonstrated that miR-126 expression was reduced in HUVECs exposed to OGD/R, and miR-126 mimics reduced OGD/R-induced cytotoxicity and apoptosis. Notably, miR-126 mimics activated the SIRT1/Nrf2 signaling pathway under OGD/R conditions. Furthermore, the present study demonstrated the protective effect of miR-126 overexpression against OGD/R injury via promotion of the SIRT1/Nrf2 signaling pathway. Furthermore, miR-126 attenuated OGD/R-induced oxidative stress and inflammation by promoting the SIRT1/Nrf2 signaling pathway. Therefore, these results indicated that overexpression of miR-126 had protective effects against OGD/R injury by inhibiting oxidative stress and the inflammatory response by promoting the activation of the SIRT1/Nrf2 signaling pathway in HUVECs.
According to previous studies, miR-126 is emerging as a key player in the pathogenesis of ischemic stroke (11,25). For instance, miR-126 expression is down-regulated following cerebral ischemia, and miR-126 overexpression contributes to the neurorestorative effects after stroke (26,27). This was also observed in the present study. The results revealed that OGD/R markedly reduced the expression levels of miR-126, and overexpression of miR-126 induced by transfection with miR-126 mimics attenuated OGD/R-induced cytotoxicity and apoptosis in HUVECs. Although, the present study did not investigate the effects of miR-126 alone on cytotoxicity and apoptosis under normal conditions, previous reports revealed that both miR-126 mimics and miR-126 inhibitor have no effect on the activity and apoptosis of HUVECs under normal conditions (28,29). Taken together, these results indicated the protective effect of miR-126 overexpression against I/R injury in the brain.
Previous studies have demonstrated that SIRT1 is involved in the regulation of multiple cellular processes, including cell survival, apoptosis, oxidative stress and inflammation, and exerts important functions in ameliorating cerebral I/R injury (30,31). As an important transcription factor, Nrf2 serves as a downstream target of the SIRT1 signaling pathway in increasing resistance to nerve damage (20,32). A previous study revealed that SIRT1 served an important physiological role during cerebral ischemia by activating Nrf2 (32). Notably, it was reported that miR-126 regulated SIRT1 expression in a glucose intolerance and peripheral artery disease mouse model (33). Consistent with these studies, the present study revealed that miR-126 mimics increased the expression of SIRT1 in OGD/R-treated HUVECs. However, it needs to be further verified whether SIRT1 is a direct or indirect target of miR-126. It is commonly known that once activated, Nrf2 translocates from the cytoplasm to the nucleus, where this process can up-regulate several antioxidant enzymes, particularly HO-1, resulting in enhanced cell survival in numerous types of tissues (17). The results of the present study further demonstrated that miR-126 mimics increased Nrf2 expression in the nucleus, promoted HO-1 expression and decreased Nrf2 expression in the cytoplasm in OGD/R-treated HUVECs. These results indicated that miR-126 overexpression promoted the activation of the SIRT1/Nrf2 signaling pathway under I/R injury. Additionally, the present results further demonstrated that inhibition of the SIRT1/Nrf2 signaling pathway induced by transfection with SIRT1-siRNA inhibited the protective effects of miR-126 mimics against OGD/R injury in HUVECs. This was consistent with a previous study that demonstrated that SIRT1 overexpression reduced OGD/R-induced apoptosis, whereas SIRT1 knockdown increased OGD/R-induced apoptosis (34). Taken together, these results indicated that the SIRT1/Nrf2 signaling pathway served a significant role in the neuroprotective effects of miR-126 against I/R injury.
Oxidative stress, which is primarily caused by excess ROS, is widely involved in multiple pathophysiological processes, including apoptosis, inflammation and nerve injury, and is the core mechanism of neuronal damage following cerebral I/R (35). Defensive antioxidants, such as SOD and GSH-Px, can ameliorate the elevation of oxidants and therefore protect brain tissues against ROS cytotoxicity (35). miR-126 has been demonstrated to reduce oxidative stress and apoptosis in I/R injury (36). Similarly, the present study revealed that miR-126 mimics reversed the OGD/R-induced increase in ROS generation and MDA content (a major marker of lipid peroxidation caused by oxidative stress), and the OGD/R-induced decrease in SOD and GSH-Px activities in HUVECs. Thus, these results indicated that miR-126 overexpression effectively prevented vascular I/R injury caused by oxidative damage induced by ROS and the lipid peroxidative effect. Notably, the SIRT1/Nrf2 signaling pathway was revealed to serve a critical role in cellular antioxidant defense mechanisms (20,37). It has been reported that up-regulation of the SIRT and Nrf2 signaling pathway leads to cellular defense mechanisms against oxidative stress injury triggered by cerebral ischemia (32,38). Consistent with these studies, the present results demonstrated that SIRT1-siRNA-induced inhibition of the SIRT1/Nrf2 signaling pathway blocked the anti-oxidative ability of miR-126 in OGD/R-treated HUVECs. These effects indicated that miR-126 overexpression attenuated oxidative stress, resulting in protection against vascular I/R injury in a SIRT1/Nrf2 signaling pathway-dependent manner.
The inflammatory response is important in the progression of cerebral ischemia (39). A number of studies have demonstrated that, during I/R, there is a marked elevation in the levels of pro-inflammatory mediators, including TNF-α, IL-1β and IL-6, and a marked reduction in the levels of anti-inflammatory cytokines, including IL-4 and IL-10 in the brain (40,41). The present results also revealed that OGD/R treatment increased the levels of TNF-α, IL-1β and IL-6, and decreased the level of IL-10 in HUVECs. Growing evidence has suggested that, in addition to promoting the neuroprotective effect, miR-126 also has anti-inflammatory effects (42,43). However, to the best of our knowledge, the anti-inflammatory effects of miR-126 in I/R injury in HUVECs have not yet been investigated. The results of the present study found that miR-126 mimics reduced the OGD/R-induced inflammatory response. In addition, numerous studies have reported that the SIRT1/Nrf2 signaling pathway is a promising anti-inflammatory pathway target to reduce nerve injury (21,44). Furthermore, the present results indicated that inhibition of the SIRT1/Nrf2 signaling pathway reversed the inhibitory effect of miR-126 mimics on the OGD/R-induced inflammatory response. These results suggested that the SIRT1/Nrf2 signaling pathway mediated the anti-inflammatory action of miR-126 under I/R conditions in HUVECs.
However, there are a few limitations of the present study. It is unclear whether miR-126 directly targets SIRT1/Nrf2 signaling molecules, which requires further investigation. In addition, in vivo experiments and studies using cell lines derived from the brain are required to elucidate the specific mechanisms involved in the neuroprotection of miR-126 against cerebral I/R injury.
In conclusion, the present findings demonstrated that the amelioration of I/R injury induced by miR-126 overexpression could be the result of its anti-oxidative and anti-inflammatory effects in HUVECs. Notably, the SIRT1/Nrf2 signaling pathway was identified to contribute to the neuroprotective effects of miR-126 overexpression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JL designed and performed the experiments and wrote and revised the manuscript. CY performed the experiments and analyzed data. YW conceived the study and corrected the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Broussalis E, Killer M, McCoy M, Harrer A, Trinka E, Kraus J. Current therapies in ischemic stroke. Part A. Recent developments in acute stroke treatment and in stroke prevention. Drug Discov Today. 2012;17:296–309. doi: 10.1016/j.drudis.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Correction to: Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2019;50:e440–e441. doi: 10.1161/STR.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 3.Galkin A. Brain ischemia/reperfusion injury and mitochondrial complex I damage. Biochemistry (Mosc) 2019;84:1411–1423. doi: 10.1134/S0006297919110154. [DOI] [PubMed] [Google Scholar]
- 4.LeBaron TW, Kura B, Kalocayova B, Tribulova N, Slezak J. A new approach for the prevention and treatment of cardiovascular disorders. Molecular hydrogen significantly reduces the effects of oxidative stress. Molecules. 2019;24:2076. doi: 10.3390/molecules24112076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enzmann G, Kargaran S, Engelhardt B. Ischemia-reperfusion injury in stroke: Impact of the brain barriers and brain immune privilege on neutrophil function. Ther Adv Neurol Disord. 2018 Aug 18; doi: 10.1177/1756286418794184. (Epub ahead of print). doi: 10.1177/1756286418794184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen Z, Hou W, Wu W, Zhao Y, Dong X, Bai X, Peng L, Song L. 6′-O-Galloylpaeoniflorin attenuates cerebral ischemia reperfusion-induced neuroinflammation and oxidative stress via PI3K/Akt/Nrf2 activation. Oxid Med Cell Longev. 2018;2018:8678267. doi: 10.1155/2018/8678267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamorro A, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15:869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Chen M, Cao RY, Li Q, Zhu F. The role of circular RNAs in cerebral ischemic diseases: Ischemic stroke and cerebral ischemia/reperfusion injury. Adv Exp Med Biol. 2018;1087:309–325. doi: 10.1007/978-981-13-1426-1_25. [DOI] [PubMed] [Google Scholar]
- 9.Dewdney B, Trollope A, Moxon J, Thomas Manapurathe D, Biros E, Golledge J. Circulating MicroRNAs as biomarkers for acute ischemic stroke: A systematic review. J Stroke Cerebrovasc Dis. 2018;27:522–530. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.058. [DOI] [PubMed] [Google Scholar]
- 10.Gai HY, Wu C, Zhang Y, Wang D. Long non-coding RNA CHRF modulates the progression of cerebral ischemia/reperfusion injury via miR-126/SOX6 signaling pathway. Biochem Biophys Res Commun. 2019;514:550–557. doi: 10.1016/j.bbrc.2019.04.161. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Cui C, Yang X, Xu J, Venkat P, Zacharek A, Yu P, Chopp M. MiR-126 affects brain-heart interaction after cerebral ischemic stroke. Transl Stroke Res. 2017;8:374–385. doi: 10.1007/s12975-017-0520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan Q, Zheng J, Du D, Liao X, Ma C, Yang Y, Chen Y, Zhong W, Ma X. MicroRNA-126 priming enhances functions of endothelial progenitor cells under physiological and hypoxic conditions and their therapeutic efficacy in cerebral ischemic damage. Stem Cells Int. 2018;2018:2912347. doi: 10.1155/2018/2912347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren Z, He H, Zuo Z, Xu Z, Wei Z, Deng J. The role of different SIRT1-mediated signaling pathways in toxic injury. Cell Mol Biol Lett. 2019;24:36. doi: 10.1186/s11658-019-0158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitada M, Ogura Y, Monno I, Koya D. Sirtuins and type 2 diabetes: Role in inflammation, oxidative stress, and mitochondrial function. Front Endocrinol (Lausanne) 2019;10:187. doi: 10.3389/fendo.2019.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hattori Y, Ihara M. Sirt1. Nihon Rinsho. 2016;74:589–594. (In Japanese) [PubMed] [Google Scholar]
- 16.Meng X, Tan J, Li M, Song S, Miao Y, Zhang Q. Sirt1: Role under the condition of ischemia/hypoxia. Cell Mol Neurobiol. 2017;37:17–28. doi: 10.1007/s10571-016-0355-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma R, Liang W, Sun Q, Qiu X, Lin Y, Ge X, Jueraitetibaike K, Xie M, Zhou J, Huang X, et al. Sirt1/Nrf2 pathway is involved in oocyte aging by regulating Cyclin B1. Aging (Albany NY) 2018;10:2991–3004. doi: 10.18632/aging.101609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arioz BI, Tastan B, Tarakcioglu E, Tufekci KU, Olcum M, Ersoy N, Bagriyanik A, Genc K, Genc S. Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Front Immunol. 2019;10:1511. doi: 10.3389/fimmu.2019.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah SA, Khan M, Jo MH, Jo MG, Amin FU, Kim MO. Melatonin stimulates the SIRT1/Nrf2 signaling pathway counteracting lipopolysaccharide (LPS)-induced oxidative stress to rescue postnatal rat brain. CNS Neurosci Ther. 2017;23:33–44. doi: 10.1111/cns.12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagib MM, Tadros MG, Al-Khalek HAA, Rahmo RM, Sabri NA, Khalifa AE, Masoud SI. Molecular mechanisms of neuroprotective effect of adjuvant therapy with phenytoin in pentylenetetrazole-induced seizures: Impact on Sirt1/NRF2 signaling pathways. Neurotoxicology. 2018;68:47–65. doi: 10.1016/j.neuro.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Chan YH, Liu TC, Liao CK, Cheng YF, Tsai CH, Lu YC, Hu CJ, Lin HJ, Lee YL, Wu CC, Hsu CJ. Consumption of betel quid contributes to sensorineural hearing impairment through arecoline-induced oxidative stress. Sci Rep. 2019;9:14554. doi: 10.1038/s41598-019-49815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu JQ, Liu P, Si MJ, Ding XY. MicroRNA-126 inhibits osteosarcoma cells proliferation by targeting Sirt1. Tumour Biol. 2013;34:3871–3877. doi: 10.1007/s13277-013-0974-x. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Wang JQ, Gu WP, Deng QQ, Huang Q, Wang AM, Liu SX, Tang HY, Liang Y, Yan JH, Ouyang S. Endothelial progenitor cell miR-126 promotes homing of endothelial progenitor cells within arterial thrombus in patients with cerebral infarction and its molecular mechanism. Eur Rev Med Pharmacol Sci. 2018;22:1078–1083. doi: 10.26355/eurrev_201802_14394. [DOI] [PubMed] [Google Scholar]
- 26.Geng W, Tang H, Luo S, Lv Y, Liang D, Kang X, Hong W. Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am J Transl Res. 2019;11:780–792. [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Ning R, Zacharek A, Cui C, Cui X, Yan T, Venkat P, Zhang Y, Chopp M. MiR-126 contributes to human umbilical cord blood cell-induced neurorestorative effects after stroke in type-2 diabetic mice. Stem Cells. 2016;34:102–113. doi: 10.1002/stem.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ge HY, Han ZJ, Tian P, Sun WJ, Xue DX, Bi Y, Yang ZH, Liu P. VEGFA expression is inhibited by arsenic trioxide in HUVECs through the upregulation of Ets-2 and miRNA-126. PLoS One. 2015;10:e0135795. doi: 10.1371/journal.pone.0135795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang F, Yang TL. MicroRNA-126 alleviates endothelial cells injury in atherosclerosis by restoring autophagic flux via inhibiting of PI3K/Akt/mTOR pathway. Biochem Biophys Res Commun. 2018;495:1482–1489. doi: 10.1016/j.bbrc.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 30.He Q, Li Z, Wang Y, Hou Y, Li L, Zhao J. Resveratrol alleviates cerebral ischemia/reperfusion injury in rats by inhibiting NLRP3 inflammasome activation through Sirt1-dependent autophagy induction. Int Immunopharmacol. 2017;50:208–215. doi: 10.1016/j.intimp.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 31.Koronowski KB, Perez-Pinzon MA. Sirt1 in cerebral ischemia. Brain Circ. 2015;1:69–78. doi: 10.4103/2394-8108.162532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue F, Huang JW, Ding PY, Zang HG, Kou ZJ, Li T, Fan J, Peng ZW, Yan WJ. Nrf2/antioxidant defense pathway is involved in the neuroprotective effects of Sirt1 against focal cerebral ischemia in rats after hyperbaric oxygen preconditioning. Behav Brain Res. 2016;309:1–8. doi: 10.1016/j.bbr.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 33.Togliatto G, Trombetta A, Dentelli P, Gallo S, Rosso A, Cotogni P, Granata R, Falcioni R, Delale T, Ghigo E, Brizzi MF. Unacylated ghrelin induces oxidative stress resistance in a glucose intolerance and peripheral artery disease mouse model by restoring endothelial cell miR-126 expression. Diabetes. 2015;64:1370–1382. doi: 10.2337/db14-0991. [DOI] [PubMed] [Google Scholar]
- 34.Ren Q, Hu Z, Jiang Y, Tan X, Botchway BOA, Amin N, Lin G, Geng Y, Fang M. SIRT1 protects against apoptosis by promoting autophagy in the oxygen glucose deprivation/reperfusion-induced injury. Front Neurol. 2019;10:1289. doi: 10.3389/fneur.2019.01289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun MS, Jin H, Sun X, Huang S, Zhang FL, Guo ZN, Yang Y. Free radical damage in ischemia-reperfusion injury: An obstacle in acute ischemic stroke after revascularization therapy. Oxid Med Cell Longev. 2018;2018:3804979. doi: 10.1155/2018/3804979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W, Zheng Y, Wang M, Yan M, Jiang J, Li Z. Exosomes derived miR-126 attenuates oxidative stress and apoptosis from ischemia and reperfusion injury by targeting ERRFI1. Gene. 2019;690:75–80. doi: 10.1016/j.gene.2018.12.044. [DOI] [PubMed] [Google Scholar]
- 37.Han J, Liu X, Li Y, Zhang J, Yu H. Sirt1/Nrf2 signalling pathway prevents cognitive impairment in diabetic rats through anti-oxidative stress induced by miRNA-23b-3p expression. Mol Med Rep. 2018;17:8414–8422. doi: 10.3892/mmr.2018.8876. [DOI] [PubMed] [Google Scholar]
- 38.Tao X, Sun X, Xu L, Yin L, Han X, Qi Y, Xu Y, Zhao Y, Wang C, Peng J. Total flavonoids from rosa laevigata michx fruit ameliorates hepatic ischemia/reperfusion injury through inhibition of oxidative stress and inflammation in rats. Nutrients. 2016;8:418. doi: 10.3390/nu8070418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anrather J, Iadecola C. Inflammation and stroke: An overview. Neurotherapeutics. 2016;13:661–670. doi: 10.1007/s13311-016-0483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao XL, Du J, Zhang Y, Yan JT, Hu XM. Hyperlipidemia exacerbates cerebral injury through oxidative stress, inflammation and neuronal apoptosis in MCAO/reperfusion rats. Exp Brain Res. 2015;233:2753–2765. doi: 10.1007/s00221-015-4269-x. [DOI] [PubMed] [Google Scholar]
- 41.Tobin MK, Bonds JA, Minshall RD, Pelligrino DA, Testai FD, Lazarov O. Neurogenesis and inflammation after ischemic stroke: What is known and where we go from here. J Cereb Blood Flow Metab. 2014;34:1573–1584. doi: 10.1038/jcbfm.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Q, Zeng S, Quan C, Lin X. Induction function of miR-126 in survival and proliferation in neural stem cells. Med Sci Monit. 2015;21:3023–3027. doi: 10.12659/MSM.894672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu J, Zeng L, Huang J, Wang G, Lu H. miR-126 promotes angiogenesis and attenuates inflammation after contusion spinal cord injury in rats. Brain Res. 2015;1608:191–202. doi: 10.1016/j.brainres.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 44.Lee JY, Song H, Dash O, Park M, Shin NE, McLane MW, Lei J, Hwang JY, Burd I. Administration of melatonin for prevention of preterm birth and fetal brain injury associated with premature birth in a mouse model. Am J Reprod Immunol. 2019;82:e13151. doi: 10.1111/aji.13151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.