Abstract
Background
A structure called the pleurogram makes up a large part of the seed coat of some species in subfamilies Caesalpinioideae and Mimosoideae of Fabaceae, but little is known about its function. It has been hypothesized that this structure acts as a hygroscopic valve during the maturation drying of seeds. However, a new hypothesis has recently emerged that proposes a distinct function for the pleurogram.
Scope
Here, we provide an overview of the structure and function of the pleurogram, which is diverse and complex. This large structure can be dislodged, thereby creating a pathway for water entry into water-impermeable seeds. However, the pleurogram is non-functional as a pathway of water into the seed of some species. Thus, the evolutionary history of species with a pleurogram may be related to a loss/gain in its function. A complete model for the function of the pleurogram is proposed.
Conclusions
The pleurogram may act on several stages of the seed, from maturation to germination. As a hygroscopic valve, it regulates dehydration of the seed during maturation. As a pathway for water entry into the seed, the pleurogram acts as a water gap in seeds with physical dormancy, thereby regulating dormancy break/germination. The occurrence of a pleurogram in several genera of legumes and Cucurbitaceae is confirmed. Single or multiple pleurograms can serve as (the) point(s) of water entry into seeds that do not otherwise have a hilar water gap.
Keywords: Functional seed trait, legumes, physical dormancy break, seed drying, seed germination, water gap
LEGUMES, SEEDS AND PLEUROGRAMS
Fabaceae (Leguminosae) is the third-largest angiosperm family with an estimated 770 genera and 19 500 species (Lewis et al., 2005), and it is the second most important plant family for humankind (LPWG, 2017). There is a mark or depression on the seed coat of some legumes called the pleurogram (Fig. 1A–H and Corner, 1951). This structure has been given several names: ‘lignes sutures’ (Capitaine, 1912), ‘linea fissural’ (Boelcke, 1946) and ‘face line’ (Isely, 1955). Werker (1997) described the pleurogram as a ‘specialized structure’ on seeds. However, the literature on the pleurogram published before 2019 does not clarify what the word ‘specialized’ means. There were no published studies on the function of the pleurogram until Rodrigues-Junior et al. (2019).
Fig. 1.
Pleurograms of seeds. (A–D) Closed and open (E–H) types of pleurograms in seeds. Seeds of the genera Tamarindus (A) and Senna (B, C) are examples of the closed type (left) and seeds of the genera Leucaena (F), Parkia (G) and Albizia (H) of the open type (right). Arrows indicate the pleurogram, and asterisks indicate the ‘opening’ in open-type pleurograms. (I–K) An unusual pleurogram in seeds. Due to differential compression of seeds during maturation, the wide greenish pleurogram in Senna alata seems to be located in a different position than usual. In this seed, the pleurogram is near the hilar region. ar, areola; hr, hilar region; ra, raphe. Scale bar = 1 mm.
The pleurogram is present on seeds of Fabaceae subfamilies Caesalpinioideae and Mimosoideae (Corner, 1976; Werker, 1997). However, in the most recent classification of the legume family, subfamily Mimosoideae is recognized as a distinct clade inserted in the recircumscribed subfamily Caesalpinioideae (LPWG, 2017). Nevertheless, the pleurogram is more common on mimosoid than on caesalpinioid seeds (Gunn, 1981). Thus, the structure was described in detail only for mimosoid seeds by Corner (1951). Recently, Rodrigues-Junior et al. (2019) showed that the pleurogram occurs in at least 31 genera in Fabaceae. However, a pleurogram is said to occur in Cucurbitaceae (Corner, 1976; Werker, 1997), which is a ‘complicated’ structure as described by Corner (1976). Even in detailed taxonomic reviews of the Cucurbitaceae such as the one by Schaefer and Renner (2011), the pleurogram is not included as a seed structure. Since there is no detailed investigation of the pleurogram for Cucurbitaceae, the presence (or absence) of a pleurogram in this family needs to be confirmed. Also, Kasem et al. (2011) reported that a pleurogram was present on seeds of Sinapis arvensis (Brassicaceae). However, from the microscopic images in the paper by Kasem et al. (2011) it is evident that a pleurogram is not present on seeds of Brassicaceae, since there is no delimitation mark of a pleurogram in the lateral region of the seeds. There seems to be only a simple compression in this region [Fig. 25 in Kasem et al. (2011)].
DEFINITION AND STRUCTURAL CHARACTERIZATION OF THE PLEUROGRAM
By definition, the pleurogram is a mark on both sides (seed faces) of the seed coat (Fig. 1A–H). However, due to an unusual compression [peripheral compression (typical in Senna alata)] of the seed during maturation the pleurogram may seem to be located in a different region of the seed (Fig. 1I–K). This mark is caused by variation in height of (or a break in) the palisade cells in the exotestal part of the seed coat (Corner, 1951) (Fig. 2). Cross-sections of the seed coat of species in legume subfamilies Mimosoideae and Caesalpinioideae and in Cucurbitaceae are shown in Fig. 2. According to Gunn (1981), there are two distinct types of pleurograms: closed and open (Fig. 1D, E). The latter type is reported as being U-shaped or horseshoe-shaped (Isely, 1955; Werker, 1997), and the ‘opening’ always occurs at the hilar end of the seed (Corner, 1951). If the line demarcating the pleurogram on the seed coat is complete, the pleurogram type is characterized as ‘closed’ and if not as ‘open’. The closed type occurs only in Caesalpinioideae and the open type only in Mimosoideae (Manning and Van Staden, 1987). The area inside the line that demarcates the pleurogram is called an ‘areola’ (Fig. 1D, E). Indeed, the distinction between closed and open pleurograms is clear. However, Lima (1985) found that mimosoid species in the genus Plathymenia have seeds with an ‘almost’ closed pleurogram, i.e. without a clear opening. This condition possibly demonstrates the evolutionary proximity between the genus Plathymenia (Mimoisoideae) and Caesalpinioideae [see phylogenetic tree in Daibes et al. (2019)].
Fig. 2.
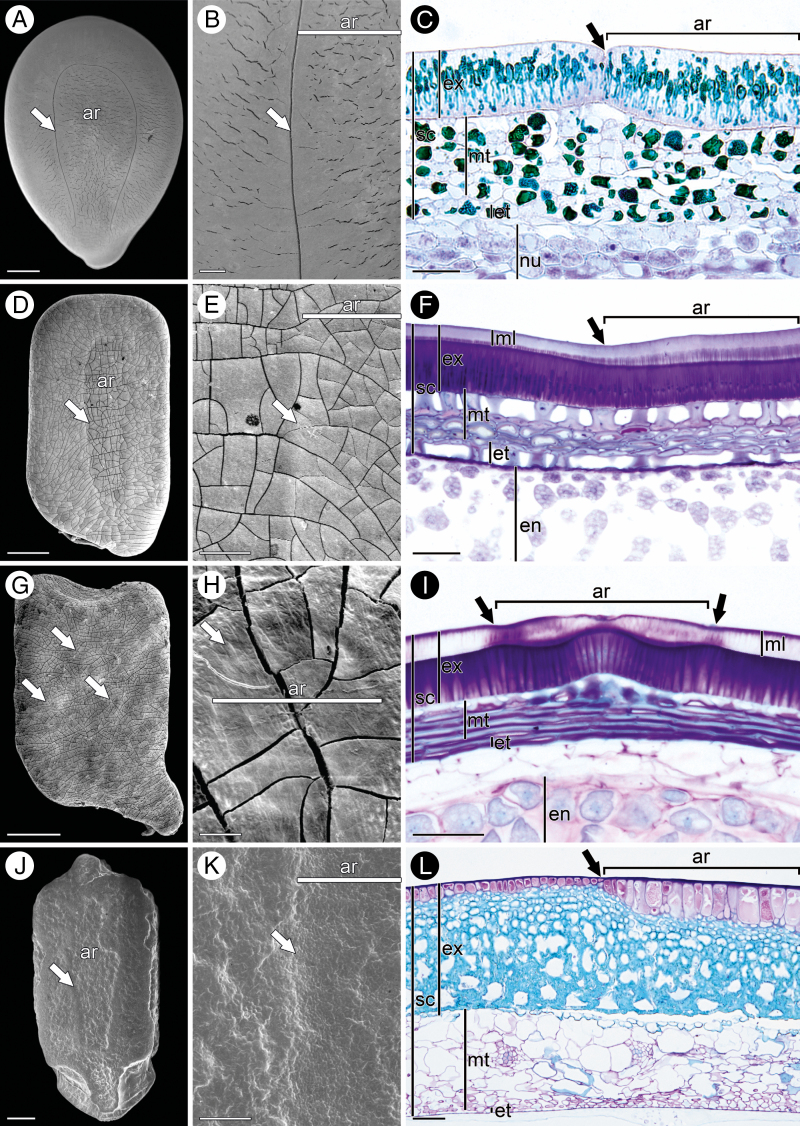
Scanning electron micrographs (A, B, D, E, G, H, J, K) and cross-sections (C, F, I, L) of pleurogramic seeds. (A–C) Leucaena leucocephala. (A) Whole seed showing open type or horseshoe-shaped pleurogram. (B) Lateral view showing details of pleurogram, areola and non-pleurogramic area. (C) Pleurogram formed by a constriction in the palisade layer (exotesta). (D–F) Senna reniformis. (D) Whole seed showing closed or circular pleurogram type. (E) Lateral view showing details of pleurogram, areola and non-pleurogramic area. (F) Pleurogram formed by a difference in height of mucilage and palisade layers. (G–I) Chamaecrista rotundifolia. (G) Whole seed showing small closed multiple pleurograms (multiple type). (H) Lateral view showing details of pleurogram, areola and non-pleurogramic area. (I) Pleurogram formed by a difference in height of the mucilaginous layer accompanied by a ripple on the palisade and larger hourglass cells. (J–L) Momordica charantia. (J) Whole seed showing closed or circular type of pleurogram. (K) Lateral view showing details of pleurogram, areola and non-pleurogramic area. (L) Pleurogram formed by a difference in height of outer exotestal cells. Arrows in all images point to the line demarcating the pleurogram. ar, areola; en, endosperm; et, endotesta; ex, exotesta; ml, mucilaginous layer; mt, mesotesta; nu, nucellus; sc, seed coat. Scale bar (A, D, J) = 1 mm, (G) = 500 μm, (B, E, K) = 200 μm, (C, F, I, L) = 50 μm, (H) = 20 μm.
In addition to the morphological aspect of pleurograms in caesalpinioid and mimosoid seeds, Corner (1976) stated that there is a structural difference between the two groups. The mimosoid pleurogram is delimited by a linea fissura, whereas the caesalpinioid pleurogram does not have a linea fissura. Our work corroborates this statement, as shown in Fig. 2. Thus, according to Corner (1976) the mark that delimits the pleurogram in caesalpinioid seeds is not formed by a break in the palisade layer (the linea fissura mentioned above) of the testa but by a difference in height of the mucilage and palisade layers (Fig. 2D–F). All described features of pleurogramic seeds are detailed in Fig. 2, which confirms the presence of a pleurogram in seeds of Cucurbitaceae (Fig. 2J–L). All changes occurring in the structural conformation of pleurograms are related to exotestal variations in both Fabaceae and Cucurbitaceae (Fig. 2)
During the last 100 years, few studies have investigated the pleurogram but none this trait exclusively; thus, our knowledge about the pleurogram has increased very little. However, recently there have been some studies on the pleurogram in legume seeds, and thus we are beginning to understand its structure and function. Two examples illustrate the lack of knowledge about this seed trait. In a morphological study by Fawzi (2011), seeds of Senna alata and Chamaecrista nigricans were characterized as having no pleurogram. Also, Isely (1955) and Irwin and Barneby (1982) indicated that the pleurogram is absent in seeds of the genus Chamaecrista. For S. alata, this misinterpretation occurred because an unusual compression (but typical for the species) of the seed during maturation arranges the pleurogram close to the hilar region (Fig. 1I–K). In this case, it is common for the pleurogram to go unnoticed. In the case of Chamaecrista, the pleurogram is distinct from all other kinds of pleurograms, differing in number and size (De-Paula and Oliveira, 2008).
A distinctive kind of pleurogram has been reported to occur on seeds of Chamaecrista (Caesalpinioideae) (De-Paula and Oliveira, 2008, 2012) (Fig. 2), which is a group of randomly arranged pleurograms. Chamaecrista seeds were formerly classified as non-pleurogramic (or ‘exareolate’, referring to the absence of an areola) by Irwin and Barneby (1982). Since multiple pleurograms are distributed over the entire seed face, Isely (1955) identified them as being ‘pits’ on the seed coat of Chamaecrista species. These recently discovered pleurograms do not appear as a single and wide structure on each face of the seeds; instead, they are small and scattered over the seed coat (De-Paula and Oliveira, 2008, 2012). These multiple pleurograms are difficult to see in detail without magnification, but when magnified it can be seen that the morphology of each of them is similar to that of the single pleurogram in other caesalpinioid seeds. The difference in height of the palisade cells creates the well-defined line delimiting the pleurogram. However, the slight variation in cell height does not form an evident depression on the seed coat (e.g. Chamaecrista desvauxii) (De-Paula and Oliveira, 2008). The range of variation in morphology in the pleurogram among species demonstrates its structural complexity, which could affect its function in seeds.
WHAT DO WE KNOW ABOUT THE FUNCTION OF THE PLEUROGRAM?
Most information on pleurogram function is about its role as a hygroscopic valve (Gunn, 1981, 1984, 1991; Kelly et al., 1992; Morrison et al., 1998). This suggested function of the pleurogram is based on the proposed role of the hilum in seeds of subfamily Faboideae by Hyde (1954). However, the anatomy of the pleurogram differs from that of the hilum. The pleurogram lacks the tracheid bar and the single groove that is present in the hilum of Faboideae. The main anatomical feature responsible for hilar movements is likely the tracheid bar. This specialized vascular structure may control opening of the hilum according to the relative humidity of the air surrounding the seed (Hyde, 1954; Lersten, 1982). The structure of the pleurogram does not seem to match the hygroscopic valve hypothesis. However, for most species with a pleurogram, it seems to be a fragile region of the seed coat visible as a large depression with distinct colour and texture that differ from the rest of the seed coat. The pleurogram sometimes suggests a connection between the external and internal environment of the seed due to its apparent fragility caused by a slight separation of the cells (Fig. 2A, B). Although it has been hypothesized that the pleurogram acts as a hygroscopic valve, a mechanism for its opening and closing has not been proposed.
Additionally, several cracks occur in the areola (Fig. 2). Gunn (1984, 1991) called these cracks as fracture lines, and Isely (1955) said that they develop during long periods of dry storage. Rodrigues-Junior et al. (2014) demonstrated that these cracks are not deep enough to make impermeable seeds water-permeable; however, the water-impermeable (palisade) layer of the seeds is complex. To determine the role(s) of the pleurogram in water loss by the seed requires understanding of the structure of the seed coat in legumes with water-impermeable seeds.
For a water-impermeable seed to become permeable, the palisade layer must be disrupted (at least to a certain depth), thus breaching the impermeable layer and allowing water to reach the embryo (Werker et al., 1973; Serrato-Valenti et al., 1986; Mello-Pina et al., 1999; Janská et al., 2019). Impermeability of the seed coat is conferred by a specific part of the palisade layer, not by this entire layer (Serrato-Valenti et al., 1986; Mello-Pina et al., 1999; Janská et al., 2019). Serrato-Valenti et al. (1986) subdivided the palisade layer into three zones. The middle zone (or ‘cylindrical part’) is considered to be water-impermeable, and the upper and lower zones water-permeable [see the schematic drawing in Fig. 1 in Serrato-Valenti et al. (1986)]. If the upper zone of the palisade layer is not impermeable, water flux into it may occur, but water will not cross the impermeable middle zone. Additionally, cracks may occur in the cuticle/mucilaginous layer external to the palisade layer of cells (in legume seeds, a thick mucilage stratum covers the coat) (Fig. 2F, I and Corner, 1951), being more noticeable in the areola than the rest of the coat. Hence, cracks are superficial and do not represent a path by which water can cross the middle impermeable zone of the palisade layer of the seed coat.
The mucilaginous layer that usually covers the seed coat is hydrophobic, but cracks in it allow the passage of water (Serrato-Valenti et al., 1986). Thus, the mucilaginous layer (or even the cuticle) does not make the seed coat impermeable, unlike the middle zone of the palisade layer, which is called the ‘cylindrical part’. The cylindrical part of the palisade layer efficiently prevents water uptake by the seed, even after a long period of immersion in water (Serrato-Valenti et al., 1986). These findings support the conclusions of Janská et al. (2019) that impermeability in the pea seed coat is caused by a specific zone of the palisade layer. Gunn (1981) suggested that the fracture lines (or cracks) in the areola also may be related to seed desiccation. However, based on the seed coat structure, it seems that water loss through the cracks in the areola may be possible (to a limited extent) only before the seed becomes water-impermeable.
Many species of legumes, especially those in temperate/arctic regions, have water-impermeable seeds, i.e. physical dormancy (PY; Baskin and Baskin, 2004, 2014; Rubio de Casas et al., 2017) caused by the palisade layer in the seed coat (Baskin et al., 2000). In seeds with PY, the pleurogram may be present on the coat, and it has been referred to as a ‘break’ or ‘gaping break’ in the exotestal palisade layer of the seed coat of these species (sensuCorner, 1951, 1976; Gunn, 1984, 1991), at least for mimosoid seeds. The palisade layer is a set of linearly juxtaposed cells that confers water impermeability on seeds, as described above. In seeds with PY, (an) opening(s) in the seed coat allow(s) water to reach the inner tissues, after which the seed can germinate (Baskin, 2003; Baskin et al., 2000). These openings via specialized seed structures in the seed coat have been called ‘water gaps’ by Baskin et al. (2000). Structures like the lens, hilum and micropyle can open, after which legume seeds become permeable (Table 3.14 in Baskin and Baskin, 2014). The opening of water gaps is controlled by specific parts of these seed structures, which cause structural weakness in this region of the seed. Details of the seed dormancy break mechanism are provided by Jayasuriya et al. (2009) and Rodrigues-Junior et al. (2018). Thus, if structures on the seed coat serve as a pathway of water entry into the seed, the pleurogram, which was previously called a ‘gaping break’, also could function as a water gap.
The descriptions of the pleurogram by Corner (1951, 1976) and Gunn (1984, 1991) indicate that it is a weakness in the seed coat. If so, could the seed coat be disrupted in the pleurogram region? Rodrigues-Junior et al. (2014) investigated the pleurogram with fracture lines in the areola in Senna multijuga and asked if this is the site of water entry into water-impermeable seeds after they become water-permeable. However, for S. multijuga the pleurogram is entirely sealed (closed), and the breaks (or cracks) in the areola are superficial and do not reach the permeable tissues below the palisade layer. Thus, when the hilar region was blocked after breaking PY by immersion in hot water (i.e. opening the water gap), the seeds did not absorb water through the pleurogram (Rodrigues-Junior et al., 2014). Gunn (1984, 1991) defined the fracture lines as cracks in the cuticle only, which do not make the seeds water-permeable. However, Manning and Van Staden (1987) described the fracture lines in legume seeds in detail, and they concluded that the fracture lines ‘penetrate the epidermis [palisade layer] completely’ in caesalpinioid seeds but that the gap does not penetrate the palisade layer completely in mimosoid seeds. According to these authors, the fracture lines are breaks in the seed coat that extend entirely across the palisade (impermeable) layer. Manning and Van Staden (1987) provide evidence for these deep fracture lines in seeds from genera in which seeds have PY, i.e. Caesalpinia, Delonix, Peltophorum and Senna (as Cassia) (Figs 4, 8, 16 and 20 in Manning and Van Staden, 1987).
However, the main question is how a water-impermeable seed has such a deep gap in it and continues to be water-impermeable. A possible answer to this question is that prior to the time seeds were examined, they were exposed to conditions that could disrupt the pleurogram [i.e. frozen in liquid nitrogen (Manning and Van Staden, 1987)]. Thus, the fracture lines are not distinct between caesalpinioid and mimosoid seeds, i.e. they do not penetrate the palisade layer, as described by Manning and Van Staden (1987); if they did, these seeds would not be water-impermeable. However, this result suggests that the pleurogram might be susceptible to rupture. In support of this, Supplementary Data Fig. S1 provides a bit of evidence that seeds malformed during development can form a pleurogram with distinct levels of disruption (Supplementary Data Fig. S1A). A pleurogram is evident even at the early stages of seed development (Supplementary Data Fig. S1B), and its disruption can occur in different species with malformed seeds (Supplementary Data Fig. S1A, C, D), providing evidence that this dysfunction occasionally occurs in pleurogramic seeds.
A TEST OF THE HYPOTHESIS
The hypothesis that the pleurogram in mature seeds is a pathway for water entry into seeds with PY was formulated and tested by Rodrigues-Junior et al. (2019). They showed that, indeed, the pleurogram in seeds of some species of Senna (Caesalpinioideae) acts as a water gap. Disruption of the pleurogram during PY break creates an opening through the palisade layer, thereby allowing water to enter the seed, as shown in Supplementary Data Fig. S1E. These results confirm the role of the pleurogram as a functional trait in seeds.
Opening of the pleurogram is irreversible due to physical disruption of the structure. This opening interrupts the continuity of the water-impermeable layer, thus making the seed coat water-permeable. Although opening of the pleurogram is caused by creation of a linear fissure in the seed coat (Supplementary Data Fig. S1F–I), its disruption results in a morpho-anatomically distinct water gap, e.g. a lid-like structure that is dislodged from the palisade layer [see the classification scheme for water gaps in Gama-Arachchige et al. (2013) and Geneve et al. (2018)].
Interestingly, the pleurogram is not a functional water gap in all species. It seems to be non-functional in seeds of Prosopis tamarugo (Fabaceae, subfamily Caesalpinioideae) (Serrato-Valenti et al., 1986). These authors found that the pleurogram is important in the taxonomy of the genus Prosopis but does not seem to have a function in water uptake by seeds, since there was no specific disruption in this structure. However, they cited studies by Trivedi et al. (1979) which suggest that cracks in the pleurogram region of mimosoid species (including the genera Acacia and Prosopis) fail to maintain impermeability of the seed coat. This implies a possible loss/gain in function of the pleurogram during the evolution of plants, since it can be present or absent in seeds.
To summarize, we propose a model for the functions described for the pleurogram during seed development (Fig. 3). The pleurogram could act in different stages, from seed maturation drying (steps A–F) to seed germination (steps G–M) (Fig. 3). As a hygroscopic valve, the pleurogram might function in dehydrating the seed when the relative humidity (RH) of the environment is low; if RH is high, the pleurogram prevents an increase in seed moisture content – this condition may allow seeds to dehydrate. Along with maturation drying, PY develops, i.e. the seed becomes water-impermeable. The pleurogram can detect environmental cues that break PY. Thus, water can enter the seed through this water gap, after which the seed resumes metabolic activity – this condition is not cyclical, i.e. after the pleurogram is physically disrupted it cannot be reclosed. If the environmental cues to break PY are not met, the pleurogram remains closed. In this proposed model, the pleurogram acts in both acquisition and release of seed dormancy (Fig. 3).
Fig. 3.
Model proposed for function of pleurogram during seed maturation and drying (A–F) and dormancy release and germination (G–M). Arrows with continuous lines indicate steps with experimental confirmation and dashed arrows steps that lack experimental confirmation. During the final stages of seed maturation, the pleurogram functions as a hygroscopic valve, thus dehydrating the seed. (A) Cross-section of immature seed. (B) During this stage, seeds may pass through two conditions. (C) One, air relative humidity is high and the pleurogram is closed, preventing entry of water and hydration of the seed. (D) Two, humidity is low; thus, the pleurogram opens and the seed loses water. (E) During maturation of seeds, the pleurogram may open and close several times. (F) Seeds become impermeable at the end of maturation. (G) Cross-section of mature/dormant seed. (H) Seeds may pass through two conditions. (I) One, humidity and/or temperature is/are not the same as when they act as environmental cues for opening the pleurogram. (J) Two, humidity and/or temperature signal(s) for the pleurogram to open, and water enters the seed. (K) When the pleurogram opens in mature seeds, it cannot be reclosed. (L) The hydrated seed resumes its former metabolic rate. (M) If environmental factors are favourable, the seed can germinate.
CONCLUDING REMARKS AND FUTURE RESEARCH DIRECTIONS
Variation in pleurogram morphology, such as multiple small pleurograms in Chamaecrista versus a single large one in Senna, is evidence that the pleurogram does not represent a simple physical contact between seed and fruit tissue but a genetically programmed trait formed during seed development. Thus, an accurate characterization of the pleurogram, including its development, is needed to fully understand the significance of this arcane structure in the evolution of seeds with PY. Indeed, the pleurogram is not functional as a water gap in all species in which it occurs, and the presence of a functional versus non-functional pleurogram may be used to investigate gain/loss in its function within specific groups of legumes. Thus, we could evaluate how the pleurogram evolved in plants by testing its functionality as a water pathway into seeds with PY, as described in Rodrigues-Junior et al. (2019). Then we could map the occurrence of the pleurogram onto phylogenetic diagrams for a group such as the genus Senna, whose phylogeny is well known and where PY is common.
In genera with functional and non-functional pleurograms, several questions arise. (1) Does a functional pleurogram confer a selective advantage over the non-functional state? (2) On a per unit of seed mass basis, is imbibition faster if a pleurogram opens or if a lens opens? (3) Is there an association between the presence of a functional pleurogram versus a lens in arid and/or seasonally unpredictable habitats in terms of amount of precipitation in the habitat? (4) In species with PY and a functional pleurogram, is the timing of germination as tightly controlled via responses of the pleurogram to environmental cues as it is for species with PY and a water gap such as a lens? (5) Do seeds with a pleurogram dehydrate faster than those with no pleurogram? These questions may stimulate new advances in the knowledge of this complex functional trait in seeds, which is involved in several aspects of the seed.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: disruption of pleurogram in legume seeds.
ACKNOWLEDGEMENTS
We thank Dra HM Torezan-Silingardi for providing the stereomicroscope for seed images in this work and the staff of the Laboratório Multiusuário de Microscopia Eletrônica of the Faculdade de Engenharia Química (UFU) for assistance in making the SEM images. A.G.R.J. thanks CAPES for the PNPD scholarship.
FUNDING
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
LITERATURE CITED
- Baskin CC. 2003. Breaking physical dormancy in seeds – focusing on the lens. New Phytologist 158: 229–232. [Google Scholar]
- Baskin CC, Baskin JM. 2014. Seeds: ecology, biogeography and evolution of dormancy and germination, 2nd edn. San Diego: Elsevier/Academic Press. [Google Scholar]
- Baskin JM, Baskin CC. 2004. A classification system for seed dormancy. Seed Science Research 14: 1–16. [Google Scholar]
- Baskin JM, Baskin CC, Li X. 2000. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biology 15: 139–152. [Google Scholar]
- Boelcke O. 1946. Estudio morfológico de las semillas de Leguminosas Mimosoideas y Caesalpinioideas de interés agronómico en la Argentina. Darwiniana 7: 240–321. [Google Scholar]
- Capitaine L. 1912. Les graines dês légumineuses. Paris: Emile Larose and Paul Lechevalier. [Google Scholar]
- Corner EJH. 1951. The leguminous seed. Phytomorphology 1: 117–150. [Google Scholar]
- Corner EJH. 1976. The seeds of dicotyledons (two volumes). Cambridge: Cambridge University Press. [Google Scholar]
- Daibes LF, Pausas JG, Bonani N, Nunes J, Silveira FAO, Fidelis A. 2019. Fire and legume germination in a tropical savanna: ecological and historical factors. Annals of Botany 123: 1219–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Paula OC, Oliveira DMT. 2008. Multiple pleurograms in Chamaecrista Moench (Leguminosae, Caesalpinioideae). Botanical Journal of the Linnean Society 157: 487–492. [Google Scholar]
- De-Paula OC, Oliveira DMT. 2012. Seed ontogeny of Chamaecrista and its systematic implications in Cassinae (Leguminosae, Caesalpinioideae). Plant Systematics and Evolution 298: 1659–1669. [Google Scholar]
- Fawzi NM. 2011. Macro-and micromorphological seed characteristics of some selected species of Caesalpinioideae-Leguminosae. Research Journal of Botany 6: 68–77. [Google Scholar]
- Gama-Arachchige NS, Baskin JM, Geneve RL, Baskin CC. 2013. Identification and characterization of ten new water gaps in seeds and fruits with physical dormancy and classification of water-gap complexes. Annals of Botany 112: 69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geneve RL, Baskin CC, Baskin JM, Jayasuriya KMG, Gama-Arachchige NS. 2018. Functional morpho-anatomy of the water-gap complexes in physically dormant seed. Seed Science Research 28: 186–191. [Google Scholar]
- Gunn CR. 1981. Seeds of the Leguminosae. In: Polhill RM, Raven PH, eds. Advances in Legume systematics, Part 2. Kew: Royal Botanic Gardens, 913–926. [Google Scholar]
- Gunn CR. 1984. Fruits and seeds of genera in the subfamily Mimosoideae (Fabaceae). Technical Bulletin no. 1681. Springfield: United States Department of Agriculture. [Google Scholar]
- Gunn CR. 1991. Fruits and seeds of genera in the subfamily Caesalpinioideae (Fabaceae). Technical Bulletin no. 1755. Springfield: United States Department of Agriculture. [Google Scholar]
- Hyde EOC. 1954. The function of the hilum in some Papilionaceae in relation to the ripening of the seed and the permeability of the testa. Annals of Botany 18: 241–256. [Google Scholar]
- Isely D. 1955. Observations on seed of the Leguminosae: Mimosoideae and Caesalpinioideae. Proceedings of the Iowa Academy of Science 62: 142–145. [Google Scholar]
- Irwin HS, Barneby RC. 1982. The American Cassiinae. Memoirs of the New York Botanical Garden 35: 1–918. [Google Scholar]
- Janská A, Pecková E, Sczepaniak B, Smýkal P, Soukup A. 2019. The role of the testa during the establishment of physical dormancy in the pea seed. Annals of Botany 123: 815–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasuriya KMGG, Baskin JM, Geneve RL, Baskin CC. 2009. A proposed mechanism for physical dormancy break in seeds of Ipomoea lacunosa (Convolvulaceae). Annals of Botany 103: 433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasem WT, Ghareeb A, Marwa E. 2011. Seed morphology and seed coat sculpturing of 32 taxa of family Brassicaceae. Journal of American Science 7: 166–178. [Google Scholar]
- Kelly KM, Van Staden J, Bell WE. 1992. Seed coat structure and dormancy. Plant Growth Regulation 11: 201–209. [Google Scholar]
- Lersten NR. 1982. Tracheid bar and vestured pits in legume seeds (Leguminosae: Papilionoideae). American Journal of Botany 69: 98–107. [Google Scholar]
- Lewis GP, Schrire B, Mackinder B, Lock M. 2005. Legumes of the world. Richmond: Royal Botanic Gardens, Kew. [Google Scholar]
- Lima MPM. 1985. Morfologia dos frutos e sementes dos gêneros da tribo Mimoseae (Leguminosae-Mimosoideae) aplicada à sistemática. Rodriguésia 37: 53–78. [Google Scholar]
- LPWG (Legume Phylogeny Working Group) 2017. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 66: 44–77. [Google Scholar]
- Manning JC, Van Staden J. 1987. The systematic significance of testa anatomy in the Leguminosae – an illustrated survey. South African Journal of Botany 53: 210–230. [Google Scholar]
- Mello-Pinna GFA, Neiva MSM, Barbosa DCA. 1999. Estrutura do tegumento seminal de quatro espécies de Leguminosae (Caesalpinioideae), ocorrentes numa área de caatinga (PE - Brasil). Revista Brasileira de Botânica 22: 375–379. [Google Scholar]
- Morrison DA, McClay K, Porter C, Rish S. 1998. The role of the lens in controlling heat-induced breakdown of testa-imposed dormancy in native Australian legumes. Annals of Botany 82: 35–40. [Google Scholar]
- Rodrigues-Junior AG, Faria JMR, Vaz TAA, Nakamura AT, José AC. 2014. Physical dormancy in Senna multijuga (Fabaceae: Caesalpinioideae) seeds: the role of seed structures in water uptake. Seed Science Research 24: 147–157. [Google Scholar]
- Rodrigues-Junior AG, Mello ACMP, Baskin CC, Baskin JM, Oliveira DMT, Garcia QS. 2018. Why large seeds with physical dormancy become nondormant earlier than small ones. PLoS One 13: e0202038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Junior AG, Mello ACMP, Baskin CC, Baskin JM, Oliveira DMT, Garcia QS. 2019. A function for the pleurogram in physically dormant seeds. Annals of Botany 123: 867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio de Casas R, Willis C, Pearse W, Baskin CC, Baskin JM, Cavender-Bares J. 2017. Global biogeography of seed dormancy is determined by seasonality and seed size: a case study in the legumes. New Phytologist 214: 1527–1536. [DOI] [PubMed] [Google Scholar]
- Serrato-Valenti G, Modenesi P, Roti-Michelozzi G, Bevilacqua L. 1986. Structural and histochemical characters of the Prosopis tamarugo Phil. seed coat, in relation to its hardness. Acta Botanica Neerlandica 35: 475–487. [Google Scholar]
- Schaefer H, Renner SS. 2011. Cucurbitaceae. In: Kutbitzki K, ed. The families and genera of vascular plants. Berlin: Springer. [Google Scholar]
- Trivedi BS, Bagchi GD, Bajpai U. 1979. Scanning electron microscopic studies on the spermoderm of some Mimosoideae (Leguminosae). Phytomorphology 29: 211–218. [Google Scholar]
- Werker E. 1997. Seed anatomy. Berlin: Gebrüder Borntraeger. [Google Scholar]
- Werker E, Dafni A, Negbi M. 1973. Variability in Prosopis farcata in Israel: anatomical features of the seed. Botanical Journal of the Linnean Society 66: 223–232. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.