Abstract
BACKGROUND
Synaptophysin plays a key role in synaptic development and plasticity of neurons and is closely related to the cognitive process of Alzheimer’s disease (AD) patients. Exogenous neural stem cells (NSCs) improve the damaged nerve function. The effects of Sanjiao acupuncture on cognitive impairment may be related to the regulation of the NSC microenvironment.
AIM
To explore the anti-dementia mechanism of acupuncture by regulating the NSC microenvironment.
METHODS
NSCs were isolated from pregnant senescence-accelerated mouse resistant 1 (SAMR1) mice, labeled with BrdU, and injected into the hippocampus of senescence-accelerated mouse prone 8 (SAMP8) mice. Eight-month-old senescence-accelerated mice (SAM) were randomly divided into six groups: SAMR1 (RC), SAMP8 (PC), sham transplantation (PS), NSC transplantation (PT), NSC transplantation with acupuncture (PTA), and NSC transplantation with non-acupoint acupuncture (PTN). Morris water maze test was used to study the learning and memory ability of mice after NSC transplantation. Hematoxylin-eosin staining and immunofluorescence were used to observe the his-topathological changes and NSC proliferation in mice. A co-culture model of hippocampal slices and NSCs was established in vitro, and the synaptophysin expression in the hippocampal microenvironment of mice was observed by flow cytometry after acupuncture treatment.
RESULTS
Morris water maze test showed significant cognitive impairment of learning and memory in 8-mo-old SAMP8, which improved in all the NSC transplantation groups. The behavioral change in the PTA group was stronger than those in the other two groups (P < 0.05). Histopathologically, the hippocampal structure was clear, the cell arrangement was dense and orderly, and the necrosis of cells in CA1 and CA3 areas was significantly reduced in the PTA group when compared with the PC group. The BrdU-positive proliferating cells were found in NSC hippocampal transplantation groups, and the number increased significantly in the PTA group than in the PT and PTN groups (P < 0.05). Flow cytometry showed that after co-culture of NSCs with hippocampal slices in vitro, the synaptophysin expression in the PC group decreased in comparison to the RC group, that in PT, PTA, and PTN groups increased as compared to the PC group, and that in the PTA group increased significantly as compared to the PTN group with acupoint-related specificity (P < 0.05).
CONCLUSION
Acupuncture may promote nerve regeneration and synaptogenesis in SAMP8 mice by regulating the microenvironment of NSC transplantation to improve the nerve activity and promote the recovery of AD-damaged cells.
Keywords: Neurodegeneration, Alzheimer's disease, Neural stem cells, Micro-environment, Synaptophysin, Acupuncture
Core Tip: Neural stem cells (NSCs) transplantation offers high hopes for clinical therapy of Alzheimer’s disease. Our previous studies suggested that Sanjiao acupuncture might affect some materials in the NSCs microenvironment. In order to study whether acupuncture can play a positive role in neuron regeneration by acting on the microenvironment of exogenous neural stem cells, in this study, we observed the effects of Sanjiao acupuncture on neural regeneration and synaptophysin production in senescence-accelerated mouse prone 8 mice with grafted exogenous NSCs.
INTRODUCTION
According to the World Alzheimer Report 2018, 50 million people worldwide are living with dementia in 2018, and this number is likely to escalate to triple to 152 million by 2050. A new case of dementia arises every 3 s globally. The total estimated worldwide cost of dementia in 2018 is 1 trillion dollars, and this figure will rise to 2 trillion dollars by 2030. The recent statistics show that there are more than 950 million Alzheimer’s disease (AD) patients in China, which account for more than a quarter of the world’s total cases[1]. If there is no effective early prevention and treatment measure, it will have a great impact on the social economy and the medical security system. Exogenous neural stem cells (NSCs) can compensate, replace, or regulate the degenerative neurons in the brain and improve the damaged nerve function[2-4]. Senescence-accelerated mouse prone 8 (SAMP8) mice have been used as an AD model of significantly low learning and memory ability. Previous studies have shown that the improvement effectuated by acupuncture on the cognitive impairment in SAMP8 is related to the regulation of the NSC microenvironment. The present study aimed to observe the effect of acupuncture on nerve regeneration and synapse production after regulating the microenvironment of NSCs and further explore the anti-dementia mechanism of acupuncture through regulating NSCs. These findings would provide an experimental basis for the treatment of nerve regeneration in AD.
MATERIALS AND METHODS
NSC transplantation
Senescence-accelerated mice (SAM) are characterized by a variety of functional disorders with aging and divided into SAM/prone (SAMP) and SAM/resistant (SAMR) mice. Among them, SAMP8 mice show significant age-related deficits of learning and memory. While the physiological index of SAMR1 mice is similar to that in the normal animals, they have been used as homologous controls. The uterus was taken out of 12–16-day-old pregnant SAMR1 mice, and the placenta was removed under sterile conditions. After obtaining the embryo, the hippocampus was separated by opening the cranial cavity. The tissue was cut into pieces of 0.5 mm3 and then digested for 20 min with 0.25% trypsin at 37 °C to obtain a single-cell suspension. After centrifugation for 10 min at 60 g/min, the cells were cultured in DMEM/F12 medium (supplemented with 2% B27, 20 ng/mL EGF, 20 ng/mL bFGF, 100 U/mL penicillin, and 100 μg/mL streptomycin) at 37 °C with 5% CO2. The positive rate of Nestin staining was evaluated by indirect immu-nofluorescence with a rabbit monoclonal anti-nestin (1:50, Sigma Aldrich, United States), and normal rabbit IgG (1:50, Santa Cruz Biotechnology, United States) was used as a negative control. The proliferation of NSCs was observed by methyl thiazolyl tetrazolium (MTT) assay, and the differentiation into neurons or neurogliocytes was observed by indirect immu-nofluorescence with a rabbit monoclonal anti-neuronal nuclei (NeuN) antibody (1:500, Cell Signaling Technology, United States) or a rabbit monoclonal anti-glial fbrillary acidic protein (GFAP) antibody (1:200, Cell Signaling Technology, United States), respectively, and normal rabbit IgG (1:50, Santa Cruz Biotechnology, United States) was used as a negative control[5]. Adequately proliferating NSCs were labeled with BrdU and injected into the hippocampus at a density of 5 × 105 cells/μL with a small animal stereotaxic device. The coordinates of the NSCs relative to the anterior fontanel were as follows: AP: -2.06, ML: ± 1.75, DV: -1.75. In the sparse area of the dorsal dentate gyrus of the hippocampus, 1 μL of NSCs was injected slowly and evenly into each hippocampus for a sustained injection time of 1 min, and then retained in situ for another 1 min. In the sham transplantation group, 0.9% saline solution was injected in the same position. Acupuncture was administered after 24 h.
Experimental animals and groups
The experimental procedures were carried out according to the National Institute of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 1985). The study protocol was approved by the Ethics Committee of Tianjin University of Traditional Chinese Medicine (approval number: TCM-LAEC2019036). Healthy 8-mo-old male SAMP8 and SAMR1 mice were randomly divided into six groups (n = 9 each): (1) SAMR1 control group (RC): Catching-grasping stimulation; (2) SAMP8 control group (PC): Catching-grasping stimulation; (3) SAMP8 sham operation group (PS): 15 d of catching-grasping stimulation before sham transplantation surgery, and another 15 d of catching-grasping stimulation post-surgery; (4) SAMP8 NSC transplantation group (PT): 15 d of catching-grasping stimulation before NSC transplantation surgery, and another 15 d of catching-grasping stimulation post-surgery; (5) SAMP8 NSC transplantation with acupuncture group (PTA): 15 d of acupuncture intervention before NSC transplantation surgery, and another 15 d of acupuncture intervention post-surgery; and (6) SAMP8 NSC transplantation with non-acupoint acupuncture group (PTN): 15 d of non-acupoint needling intervention before NSC transplantation surgery, and another 15 d of non-acupoint needling intervention post-surgery. The mice were raised under exactly the same conditions and fed freely at 24 ± 2 °C.
Acupuncture method
Sanjiao acupuncture was administered. The acupoints were Danzhong (CV17), Zhongwan (CV12), Qihai (CV6), bilateral Xuehai (SP10), and bilateral Zusanli (ST36), and the location was identified using the Laboratory Acupuncture and Atlas of Animal Acupoints enacted by Experimental Acupuncture-Moxibustion Research Association of China Academy of Acupuncture and Moxibustion. For mice in the PTA group, locations CV17, CV12, CV6, and bilateral ST36 were needled by twisting reinforcing manipulation method, while bilateral SP10 was needled by twisting reducing manipulation method for 30 s. For mice in the PTN group, two fixed non-acupoints located at the bilateral subcostal area were needled with moderate reinforcing-reducing manipulation method for 210 s. Mice in the other four groups were caught and grasped with the same intensity for the same duration. All intervention protocols were implemented once a day for 15 d and suspended only on day 7.
Morris water maze test
After 15-d Sanjiao acupuncture treatments, behavioral changes were assessed by the Morris water maze test, which consisted of a circular pool, a platform, and a recording system. All equipment for the test was provided by the Chinese Academy of Medical Sciences (Beijing, China). The pool was 90 cm in diameter, with a height of 50 cm. On the day before the trial, mice were allowed to swim freely for 90 s in the pool once in the morning and once in the afternoon to acclimatize to the maze environment. Then, a cylindrical platform, 9 cm in diameter and 28 cm high, was placed in the center of any quadrant with 2 cm under the water. The pool was divided into four quadrants (northeast, southeast, southwest, and northwest). On the offside of the platform, two points were selected at the same distance from the point of entry. After the animals were placed in the water, the time elapsed from entering the water to finding the platform was recorded as the escape latency. The swimming data of the animals were automatically recorded with Morris 1.0.2 software using an automatic image acquisition and analysis system (Beijing, China). If a mouse could not find the platform within 90 s, the escape latency was recorded as 90 s. Each mouse was tested twice daily from two different water entering points, and the average escape latency was calculated. The hidden platform trial was performed for 5 consecutive days.
Hematoxylin-eosin and immunofluorescence staining
Three mice in each experimental group were sacrificed after cardiac perfusion. Coronal paraffin sections of the brain tissue were prepared. Brain sections of mice were embedded in paraffin, cut into 5 µm slices under a stereology microscope (Olympus BX-51TF, Japan), and placed onto hydrophilic adhesive slides. Slides were allowed to air dry overnight and then baked for an hour at 60 °C. After BrdU-positive staining was confirmed under a microscope, adjacent sections were dewaxed and hematoxylin-eosin (HE)-stained. Following deparaffinization and gradient ethanol dehydration, slices were stained with Harris hematoxylin, differentiated with hydrochloric acid ethanol, and washed with warm water to turn blue. After eosin staining, slices were dehydrated, cleared, and sealed. The pathological changes in the brain tissue were observed under a microscope after NSC transplantation. Then the paraffin sections of the hippocampus tissue of the mice were stained by immunofluorescence. The positive rate of BrdU staining was evaluated by indirect immunofluorescence and diaminobenzidine (DAB) staining with a rabbit polyclonal anti-BrdU antibody (1:100, Abcam, United Kingdom), and normal rabbit IgG (1:50, Santa Cruz Biotechnology, United States) was used as a negative control. After nuclei were counterstained with hematoxylin, positive BrdU labeled cells were observed and counted.
Preparation of brain slices and flow cytometry assay
Three mice in each group were killed by dislocation, and the whole brain was immediately removed and placed in artificial cerebrospinal fluid (ACSF) (NaCl 124 mmol/L, KCl 3.5 mmol/L, NaH2PO4·2H2O 1.2 mmol/L, MgCl2·6H2O 1.3 mmol/L, CaCl2 2 mmol/L, NaHCO3 25 mmol/L, D-Glucose 10 mmol/L). After cooling, the hippocampus was rapidly dissected and sliced into 400-μm sections parallel to the hippocampal groove fibers. These slices were placed in the upper layer of the Transwell co-culture system. Then, the well-developed BrdU-labeled NSCs (2 × 105/well) were inoculated into the lower layer of the Transwell co-culture system, and NSCs were collected on the 4th, 7th, and 10th d after inoculation. Cells were washed one time with phosphate buffer saline (PBS), digested with 0.02% ethylene diamine tetraacetic acid (EDTA), and resuspended into single-cell suspension. After methanol fixation, penetration, and serum blocking, the rabbit synaptophysin antibody (#5461, Cell Signaling) and PE-conjugated donkey anti-rabbit IgG (DantiR-PE, Saierbio) were added on to the sections. Finally, 2000 cells of each sample were collected, and synaptophysin expression was detected by flow cytometry (BD FACS Calibur).
Statistical analysis
All data are expressed as the mean ± standard deviation (SD) and were analyzed using SPSS16. Data of multiple groups were compared using one-way ANOVA, and comparison between two groups was conducted by Student-Newman-Keuls (SNK) test. P < 0.05 was considered statistically significant.
RESULTS
Effect of Sanjiao acupuncture on cognitive impairment in SAMP8 mice after NSC hippocampal transplantation
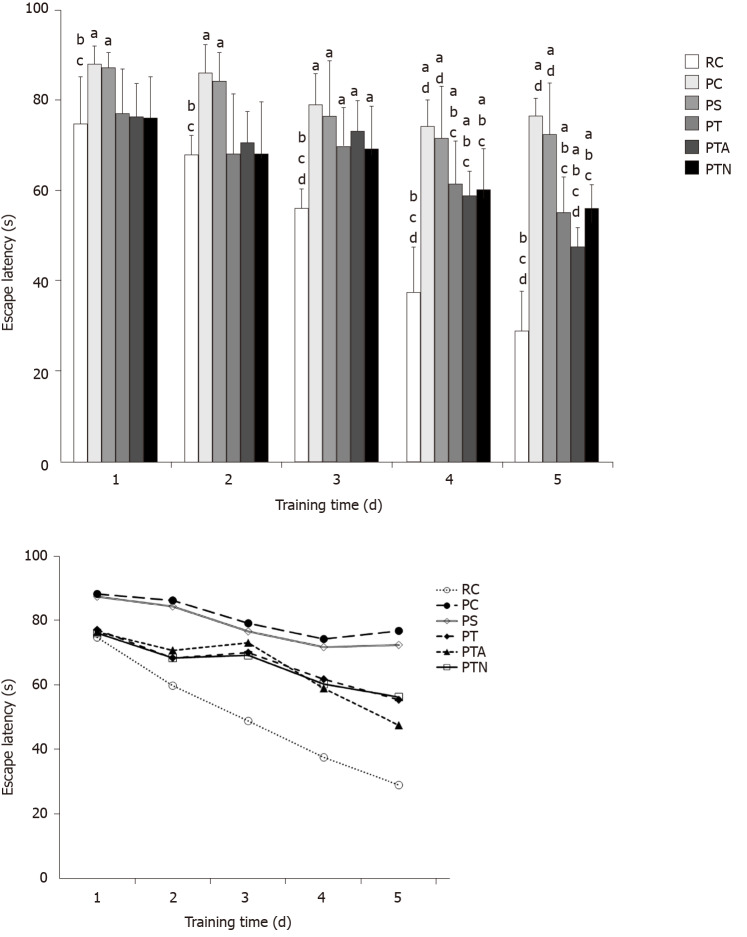
The Morris water maze test was utilized to study the learning ability to find a fixed position of the hidden platform and form a stable spatial cognitive and memory ability in brain dysfunction animals, which reflects the acquisition of spatial memory by the animals after multiple training. The results showed that the average escape latency of mice in each group decreased with the passage of training days. In comparison to the RC group, the average escape latency of the PC or PS group was significantly longer (P < 0.05). When compared with the PC group, the escape latency of the PT, PTA, or PTN group was significantly shorter from the 4th d (P < 0.05). In comparison to the PS group, the escape latency of the PT, PTA, or PTN group was significantly shorter from the 4th day (P< 0.05). The escape latency of the PTA group was significantly shorter on the 5th day as compared to that of the PTN group (P < 0.05). The results suggested that the 8-mo-old SAMP8 mice had significant cognitive impairment in learning and memory. Herein, the cognitive impairment ability of mice was significantly improved in all the NSC transplantation groups, among which, the behavioral changes of dementia mice in the PTA group were stronger than those in the other two groups with acupoint-related specificity (Figure 1).
Figure 1.
Morris water maze test for behavior of senescence-accelerated mouse. The time elapsed from entering the water to finding the platform was recorded as escape latency. If the mouse could not find the platform within 90 s, the escape latency was recorded as 90 s. aP < 0.05 when compared to senescence-accelerated mouse resistant 1 control group, bP < 0.05 when compared to senescence-accelerated mouse prone 8 (SAMP8) control group, cP < 0.05 when compared to SAMP8 sham operation group, dP < 0.05 when compared to SAMP8 neural stem cells transplantation with non-acupoint group. RC: Senescence-accelerated mouse resistant 1 control group; PC: Senescence-accelerated mouse prone 8 control group; PS: Senescence-accelerated mouse prone 8 sham operation group; PT: Senescence-accelerated mouse prone 8 neural stem cells transplantation group; PTA: Senescence-accelerated mouse prone 8 neural stem cells transplantation with acupuncture group; PTN: Senescence-accelerated mouse prone 8 neural stem cells transplantation with non-acupoint group.
Histopathological changes by HE staining
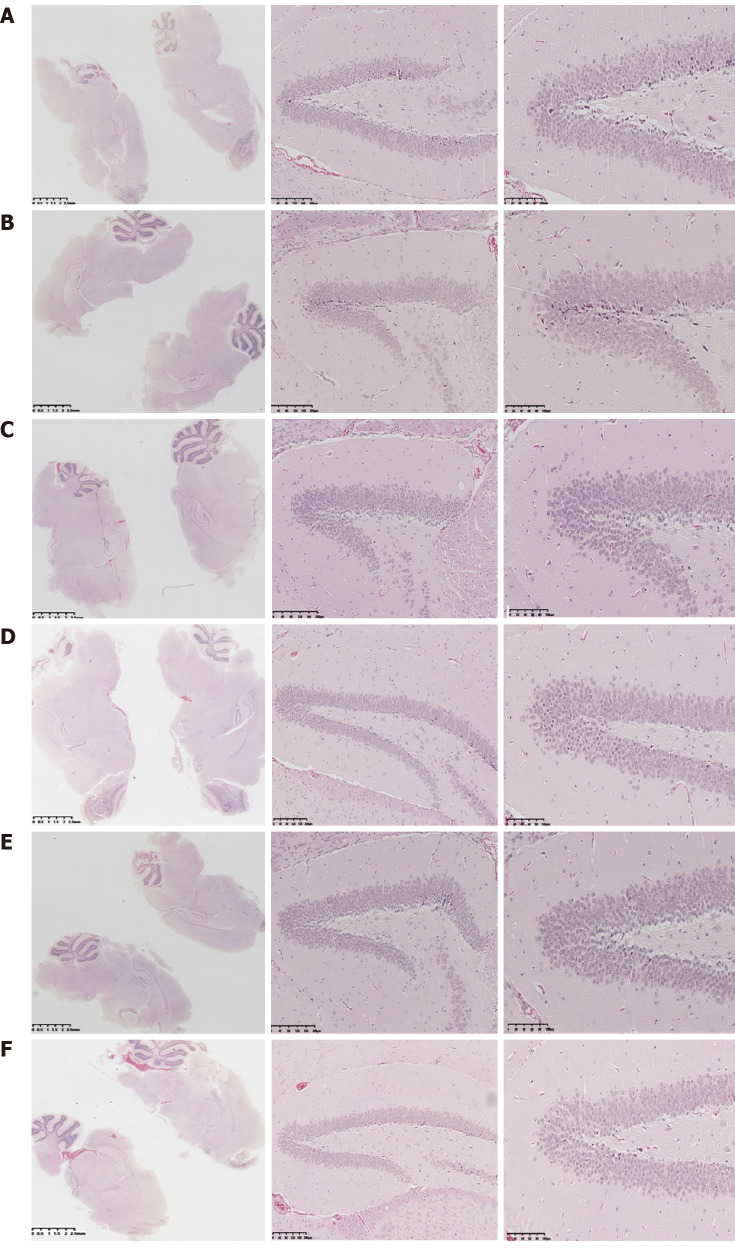
Mice in the RC group showed a clear and complete hippocampal structure, dense and orderly nerve cells, abundant cytoplasm, light staining, nucleus in the middle, and complete and compact arrangement of normal glial cells, while no edema was detected in the pericellular space. In the PC group, hippocampal tissue structure was not clear and the arrangement of the nerve cells was irregular. The cells in the CA1 and CA3 areas were solidified and atrophied and deeply stained, and the intercellular structure was loose. In the PT, PTA, and PTN groups, the hippocampal structure was clear, the cell arrangement was dense and orderly, and the necrosis of cells in the CA1 and CA3 areas was reduced (Figure 2).
Figure 2.
Histopathological changes by hematoxylin-eosin staining. Coronal paraffin sections of the brain tissue of three mice in each experimental group were prepared. After BrdU-positive staining was confirmed under a microscope, adjacent sections were dewaxed and subjected to hematoxylin-eosin staining. A: Senescence-accelerated mouse resistant 1 control group; B: Senescence-accelerated mouse prone 8 control group; C: Senescence-accelerated mouse prone 8 sham operation group; D: Senescence-accelerated mouse prone 8 neural stem cells transplantation group; E: Senescence-accelerated mouse prone 8 neural stem cells transplantation with acupuncture group; F: Senescence-accelerated mouse prone 8 neural stem cells transplantation with non-acupoint group.
Proliferating NSCs labeled by BrdU
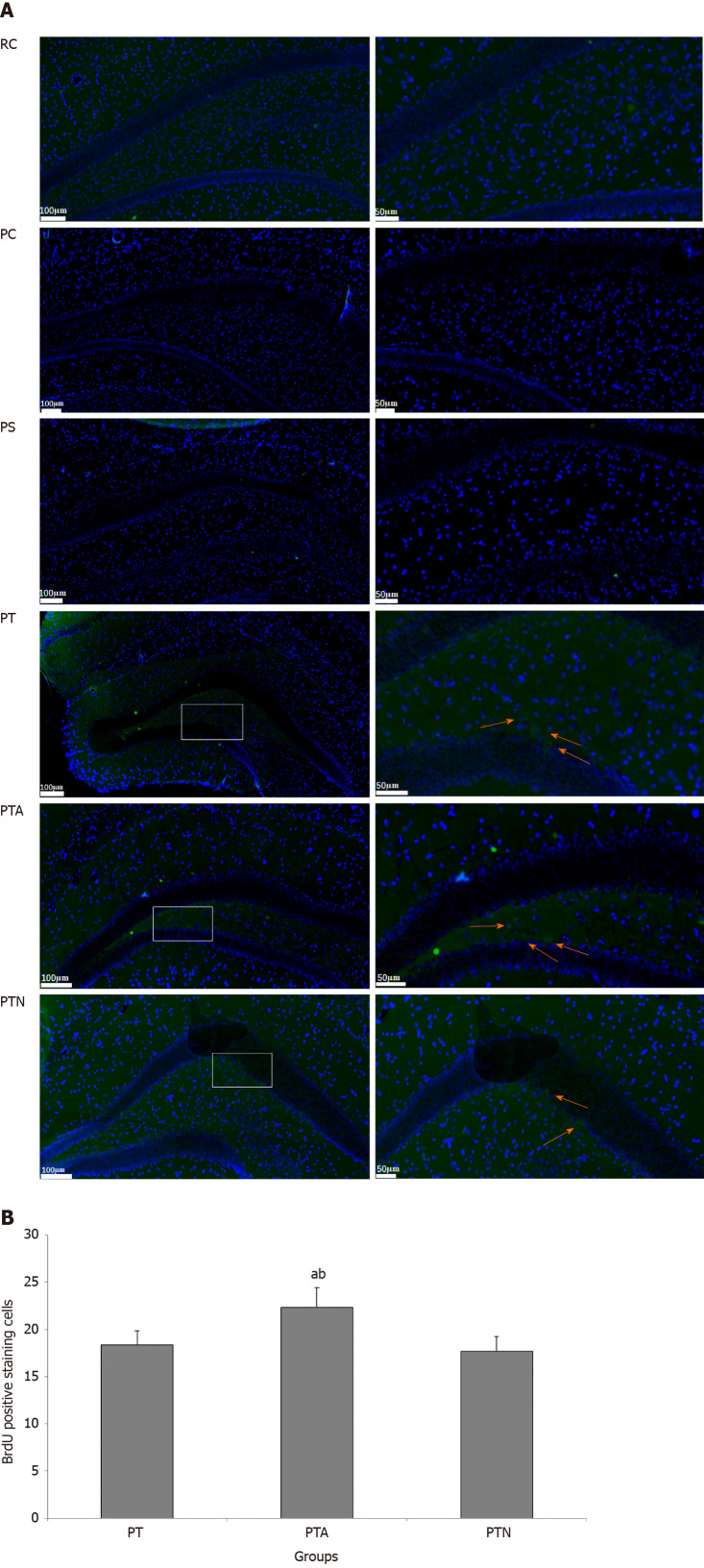
BrdU, a cell proliferation marker, is a derivative of thymine. It replaces thymine in the DNA synthesis phase (S phase)[6]. After BrdU is injected in vivo or added to cell culture, the proliferating cells can be shown by anti-BrdU monoclonal antibody and immunofluorescence staining. Consequently, the proliferation of the cells was found in NSC hippocampal transplantation groups, and BrdU-positive cells were more in the PTA group than the PT and PTN groups (P < 0.05) (Figure 3).
Figure 3.
Neural stem cell proliferation by immunofluorescence staining. Paraffin section of hippocampus tissue of mice was evaluated by indirect immunofluorescence and diaminobenzidine staining. After nuclei were counterstained with hematoxylin, positive BrdU-labeled cells were observed and counted. Arrows indicate positive cells marked by BrdU expression. A: Immunofluorescence staining; B: BrdU positive cell counting. aP < 0.05 when compared to senescence-accelerated mouse prone 8 (SAMP8) neural stem cells (NSCs) transplantation group, bP < 0.05 when compared to SAMP8 NSCs transplantation with non-acupoint group. RC: Senescence-accelerated mouse resistant 1 control group; PC: Senescence-accelerated mouse prone 8 control group; PS: Senescence-accelerated mouse prone 8 sham operation group; PT: Senescence-accelerated mouse prone 8 neural stem cells transplantation group; PTA: Senescence-accelerated mouse prone 8 neural stem cells transplantation with acupuncture group; PTN: Senescence-accelerated mouse prone 8 neural stem cells transplantation with non-acupoint group.
Effect of microenvironment on synaptophysin expression of NSCs in hippocampal slices after acupuncture
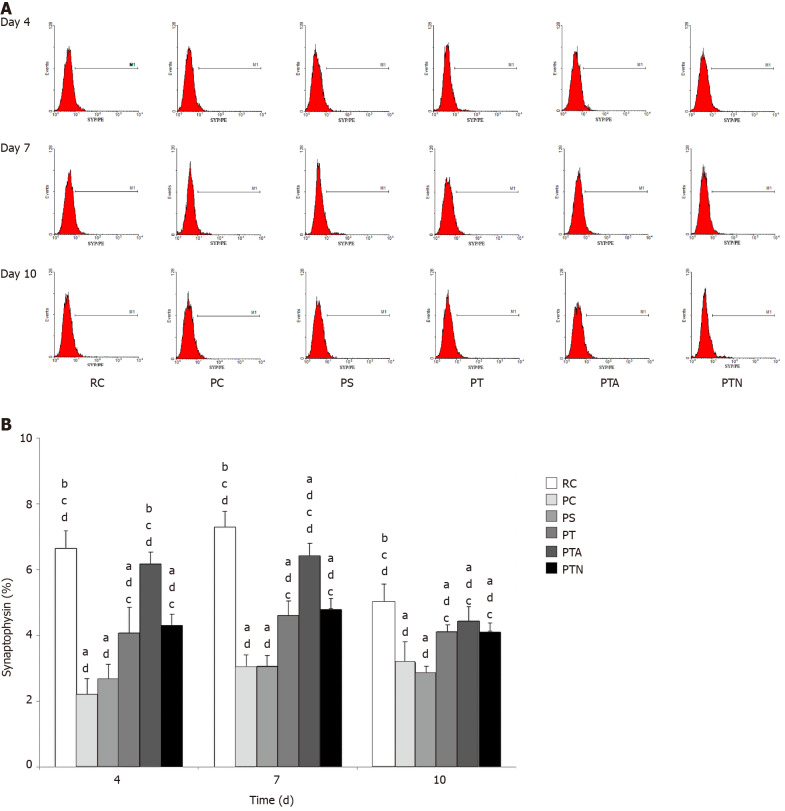
Flow cytometry detection showed that on the 4th d after co-culture of NSCs with hippocampal slices in vitro, the expression of synaptophysin in the PC, PS, PT, and PTN groups decreased significantly as compared to the RC group (P < 0.05). When compared to the PC or PS group, the expression of synaptophysin in the PT, PTA, and PTN groups increased significantly (P < 0.05); when compared to the PTN group, the expression of synaptophysin in the PTA group increased significantly (P < 0.05). On the 7th d, the expression of synaptophysin in the PC, PS, PTA, and PTN groups decreased significantly as compared to that in the RC group (P < 0.05); the expression of synaptophysin in the PT, PTA, and PTN groups increased significantly as compared to the PC and PS groups (P < 0.05), and that in the PTA group increased significantly as compared to the PTN group (P < 0.05). On the 10th d, the expression of synaptophysin decreased significantly in the PC, PS, PT, PTA, and PTN groups as compared to that of the RC group (P < 0.05), while that the PT, PTA, and PTN groups increased significantly as compared to that in the PC or PS group (P < 0.05), and when compared to the PTN group, the expression in the PTA group did not change significantly than that in the other groups (P > 0.05) (Figure 4).
Figure 4.
Synaptophysin expression by flow cytometry assay. Hippocampal brain specimens and neural stem cells were inoculated in the Transwell co-culture system. On the 4th, 7th, and 10th d, flow cytometry was used to detect the expression of synaptophysin-positive cells. A: Synaptophysin expression; B: Proportion of cells with synaptophysin expression. aP < 0.05 when compared to senescence-accelerated mouse resistant 1 control group, bP < 0.05 when compared to senescence-accelerated mouse prone 8 (SAMP8) control group, cP < 0.05 when compared to SAMP8 sham operation group, dP < 0.05 when compared to SAMP8 neural stem cells transplantation with non-acupoint group. RC: Senescence-accelerated mouse resistant 1 control group; PC: Senescence-accelerated mouse prone 8 control group; PS: Senescence-accelerated mouse prone 8 sham operation group; PT: Senescence-accelerated mouse prone 8 neural stem cells transplantation group; PTA: Senescence-accelerated mouse prone 8 neural stem cells transplantation with acupuncture group; PTN: Senescence-accelerated mouse prone 8 neural stem cells transplantation with non-acupoint group.
DISCUSSION
NSCs are the source of cell replacement therapy for neurodegenerative diseases[7-11]. The microenvironment affects the survival, proliferation, differentiation, and migration of NSCs[9,12-14]. The hippocampus is a crucial neurogenic area and the most vulnerable for dementia in the brain[15]. The microenvironment of the hippocampus is closely related to learning and memory function (especially spatial cognitive process)[16-19]. A human behavioral study on bilateral hippocampal damage showed that hippocampal damage is associated with spatial memory deficits that are manifested in the event of loss of correct spatial navigation in daily life[20]. The extensive study of hippocampal damage in rodents included spatial working memory impairment[21] and discriminated impairment for similar environments[22]. SAM mice are a rapidly aging dementia mouse line developed from AKR/J mice by Takeda et al[23]. SAMP8 mice began to show a decline in learning and memory function from an early stage, and particulate matter similar to senile plaque appeared[24]. At the age of 8–10 mo, SAMP8 mice showed significantly low learning and memory ability, as well as multi-system senescence. SAMP8 mice showed characteristics similar to those of AD, such as lifespan shortening, spine kyphosis, alopecia, lack of gloss, rough skin, periocular inflammation, and decreased physical activity[25-28]. Among these, the most important characteristics of SAMP8 are progressive cognitive decline and neurodegenerative changes[29]. Thus, SAMP8 mice are considered an ideal AD model for the basic research of AD. Previous studies showed that acupuncture significantly improves the cognitive impairment of SAMP8 mice[30,31]. Thus, the present study used SAMP8 mice as an AD animal model. Local transplantation to the hippocampus significantly improved the dementia status of mice, and the cognitive improved after acupuncture.
The synapse is a special cell adhesion site where neurons bind to target cells or other neurons. It is the main structure of information transmission between neurons. Plasticity is the biological basis of learning and memory formation. Synaptic changes are early pathological changes of AD[32,33] and closely related to cognitive impairment[34-38]. The decrease in the number of synapses and the expression of synapse-related proteins leads to synaptic dysfunction, which interrupts the connection among multiple functional pathways in the brain. This phenomenon leads to dysfunction, which is manifested as the deterioration of cognitive and memory abilities[39-41]. Synaptophysin is a polysaccharide membrane structural protein located on the synaptic vesicle membrane and closely related to synaptic function[42-44]. It participates in synaptic vesicle fusion and mediates neurotransmitter release and synaptic vesicle recycling[45-47]. In addition, it plays a key role in synaptic development and plasticity of neurons and is related to cognitive process[48]. Synaptogenesis and the expression of synaptophysin are synchronous during the development process. Synaptophysin can promote synapse formation and participate in nerve growth, repair, and regeneration, and synaptic remodeling. The expression of the molecule reflects the number, density, and distribution of synapses and affects learning and memory ability[49,50]. The decrease in the synaptophysin expression in the brain tissue has been seen in many neurodegenerative diseases[51,52]. A clinical autopsy study confirmed the decrease in synaptophysin expression in the prefrontal lobe and the hippocampus of patients with AD, and that the expression was negatively correlated with clinical symptoms[53]. Moreover, a strong correlation was established between AD patients’ cognitive ability and synaptic density of the hippocampus and cortex[54]. The current results showed that when compared to SAMR1 mice, the hippocampal microenvironment of all the SAMP8 groups showed decreased expression of NSC synaptophysin, indicating a decrease in the number and density of synapses during neuronal development induced by brain microenvironment in dementia mice. In comparison to the sham transplantation group, the expression of synaptophysin in all the transplantation groups increased, indicating that the microenvironment of NSCs is regulated after hippocampal transplantation, which promotes synaptic development and structural reconstruction in exogenous NSCs. When compared to the non-acupoint group, the expression of NSC synaptophysin in the acupuncture group was further increased, indicating that the brain microenvironment of dementia mice was improved after acupuncture, and the adjusted microenvironment enhanced the transportability of the synaptic vesicle and promoted the transmission efficiency of NSCs. The results suggested that Sanjiao acupuncture increases the exogenous expression of synaptophysin in implanted NSCs by improving the hippocampal microenvironment in dementia mice, increasing the number and efficiency of synapses, enhancing the synaptic plasticity, and generating new axons and dendrites to participate in the establishment of new neural networks that regulate the nerve activity, promote the recovery of injured cells, and improve the learning and memory function of dementia mice.
CONCLUSION
In conclusion, the number and density of synapses in the brain microenvironment of SAMP8 mice are reduced, and acupuncture may promote nerve regeneration and synaptogenesis by improving the microenvironment of NSC transplantation in dementia mice to increase the nerve activity and promote the recovery of AD-damaged cells.
ARTICLE HIGHLIGHTS
Research background
Alzheimer's disease (AD) is a chronic, progressive, age-related degenerative disease of the central nervous system, which seriously affects the quality of life of the elderly. Synaptic changes are closely related to cognitive impairment of AD. The effects of acupuncture on cognitive impairment may be related to the regulation of the neural stem cell (NSC) microenvironment.
Research motivation
Previous studies have shown that the improvement of senescence-accelerated mouse prone 8 (SAMP8) cognitive impairment by acupuncture is related to the regulation of NSCs microenvironment. This study aimed to observe the effects of acupuncture on nerve regeneration and synapse after regulating the microenvironment of NSCs, and further explore the anti-dementia mechanism of acupuncture.
Research objectives
To observe the mechanism of acupuncture promoting nerve regeneration and synaptophysin expression in dementia mice after NSC transplantation.
Research methods
Senescence-accelerated mice (SAM) were divided into six groups: SAMR1 (RC), SAMP8 (PC), sham transplantation (PS), NSC transplantation (PT), NSC transplantation with acupuncture (PTA), and NSC transplantation with non-acupoint acupuncture (PTN). The behavior changes of mice after NSC transplantation were observed by the Morris water maze test. The histological changes of the hippocampus, NSC proliferation, as well as synaptophysin production in the hippocampal microenvironment were studied by hematoxylin-eosin staining, immunofluorescence staining, and flow cytometry.
Research results
Escape latency of all the NSC transplantation groups increased obviously. The behavioral change in the PTA group was stronger than those of the other two groups (P < 0.05). After acupuncture, the hippocampal structure in the PTA group was clear, the cell arrangement was orderly, and the necrosis of cells in CA1 and CA3 areas was significantly reduced. The number of BrdU-positive proliferating cells increased significantly in the PTA group compared to those in the PT and PTN groups (P < 0.05). The synaptophysin expression in the PC group decreased in comparison to the RC group, that in PT, PTA, and PTN groups increased as compared to the PC group, and that in the PTA group increased significantly as compared to the PTN group with acupoint-related specificity (P < 0.05).
Research conclusions
Acupuncture accelerates nerve regeneration and synaptophysin production in SAMP8 mice by regulating the hippocampal microenvironment after NSCs transplantation.
Research perspectives
These findings provide an experimental basis for the treatment of nerve regeneration in AD.
ACKNOWLEDGEMENTS
The authors would like to thank all members of the Tianjin Institution of Acupuncture and Moxibustion who provided us with critical comments and assistance.
Footnotes
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Ethics Committee of Tianjin University of Traditional Chinese Medicine (approval number: TCM-LAEC2019036).
Conflict-of-interest statement: The authors declare that they have no conflict of interest to disclose.
ARRIVE guidelines statement: The authors have read the ARRIVE Guidelines, and the manuscript was prepared and revised according to the ARRIVE Guidelines.
Manuscript source: Invited manuscript
Peer-review started: February 27, 2020
First decision: September 11, 2020
Article in press: October 13, 2020
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Bolshakova GB, Exbrayat JM, Radenovic L, Schmidt N S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Ma YJ
Contributor Information
Lan Zhao, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin 300381, China; Tianjin Key Laboratory of Acupuncture and Moxibustion, Tianjin 300381, China; National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin 300381, China. lanzhao69@163.com.
Jian-Wei Liu, Tianjin University of Traditional Chinese Medicine, Tianjin 300193, China.
Bo-Hong Kan, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin 300381, China; Tianjin Key Laboratory of Acupuncture and Moxibustion, Tianjin 300381, China; National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin 300381, China.
Hui-Yan Shi, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin 300381, China; Tianjin Key Laboratory of Acupuncture and Moxibustion, Tianjin 300381, China; National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin 300381, China.
Lin-Po Yang, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin 300381, China.
Xin-Yu Liu, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin 300381, China.
Data sharing statement
Technical appendix, statistical code, and dataset available from the corresponding author at lanzhao69@163.com. Participants gave informed consent for data sharing.
References
- 1.Jia J, Wei C, Chen S, Li F, Tang Y, Qin W, Zhao L, Jin H, Xu H, Wang F, Zhou A, Zuo X, Wu L, Han Y, Han Y, Huang L, Wang Q, Li D, Chu C, Shi L, Gong M, Du Y, Zhang J, Zhang J, Zhou C, Lv J, Lv Y, Xie H, Ji Y, Li F, Yu E, Luo B, Wang Y, Yang S, Qu Q, Guo Q, Liang F, Zhang J, Tan L, Shen L, Zhang K, Zhang J, Peng D, Tang M, Lv P, Fang B, Chu L, Jia L, Gauthier S. The cost of Alzheimer's disease in China and re-estimation of costs worldwide. Alzheimers Dement. 2018;14:483–491. doi: 10.1016/j.jalz.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Huang L, Zhang L. Neural stem cell therapies and hypoxic-ischemic brain injury. Prog Neurobiol. 2019;173:1–17. doi: 10.1016/j.pneurobio.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholls FJ, Liu JR, Modo M. A Comparison of Exogenous Labels for the Histological Identification of Transplanted Neural Stem Cells. Cell Transplant. 2017;26:625–645. doi: 10.3727/096368916X693680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hou B, Ma J, Guo X, Ju F, Gao J, Wang D, Liu J, Li X, Zhang S, Ren H. Exogenous Neural Stem Cells Transplantation as a Potential Therapy for Photothrombotic Ischemia Stroke in Kunming Mice Model. Mol Neurobiol. 2017;54:1254–1262. doi: 10.1007/s12035-016-9740-6. [DOI] [PubMed] [Google Scholar]
- 5.Borhani-Haghighi M, Kashani IR, Mohamadi Y, Pasbakhsh P. Embryonic intraventricular transplantation of neural stem cells augments inflammation-induced prenatal brain injury. J Chem Neuroanat. 2018;94:54–62. doi: 10.1016/j.jchemneu.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez CN, Nguyen H. Identifying Quiescent Stem Cells in Hair Follicles. Methods Mol Biol. 2018;1686:137–147. doi: 10.1007/978-1-4939-7371-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiaoying L, Li T, Yu S, Jiusheng J, Jilin Z, Jiayi W, Dongxin L, Wengang F, Xinyue Z, Hao Y, Yuhua C, Deshu S. Resistin-Inhibited Neural Stem Cell-Derived Astrocyte Differentiation Contributes to Permeability Destruction of the Blood-Brain Barrier. Neurochem Res. 2019;44:905–916. doi: 10.1007/s11064-019-02726-3. [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro MF, Genebra T, Rego AC, Rodrigues CMP, Solá S. Amyloid β Peptide Compromises Neural Stem Cell Fate by Irreversibly Disturbing Mitochondrial Oxidative State and Blocking Mitochondrial Biogenesis and Dynamics. Mol Neurobiol. 2019;56:3922–3936. doi: 10.1007/s12035-018-1342-z. [DOI] [PubMed] [Google Scholar]
- 9.Vogel A, Upadhya R, Shetty AK. Neural stem cell derived extracellular vesicles: Attributes and prospects for treating neurodegenerative disorders. EBioMedicine. 2018;38:273–282. doi: 10.1016/j.ebiom.2018.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muckom R, McFarland S, Yang C, Perea B, Gentes M, Murugappan A, Tran E, Dordick JS, Clark DS, Schaffer DV. High-throughput combinatorial screening reveals interactions between signaling molecules that regulate adult neural stem cell fate. Biotechnol Bioeng. 2019;116:193–205. doi: 10.1002/bit.26815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marsh SE, Blurton-Jones M. Neural stem cell therapy for neurodegenerative disorders: The role of neurotrophic support. Neurochem Int. 2017;106:94–100. doi: 10.1016/j.neuint.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Díaz-Moreno M, Armenteros T, Gradari S, Hortigüela R, García-Corzo L, Fontán-Lozano Á, Trejo JL, Mira H. Noggin rescues age-related stem cell loss in the brain of senescent mice with neurodegenerative pathology. Proc Natl Acad Sci USA. 2018;115:11625–11630. doi: 10.1073/pnas.1813205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.L'Episcopo F, Tirolo C, Peruzzotti-Jametti L, Serapide MF, Testa N, Caniglia S, Balzarotti B, Pluchino S, Marchetti B. Neural Stem Cell Grafts Promote Astroglia-Driven Neurorestoration in the Aged Parkinsonian Brain via Wnt/β-Catenin Signaling. Stem Cells. 2018;36:1179–1197. doi: 10.1002/stem.2827. [DOI] [PubMed] [Google Scholar]
- 14.Navarro Quiroz E, Navarro Quiroz R, Ahmad M, Gomez Escorcia L, Villarreal JL, Fernandez Ponce C, Aroca Martinez G. Cell Signaling in Neuronal Stem Cells. Cells. 2018;7 doi: 10.3390/cells7070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gatt A, Lee H, Williams G, Thuret S, Ballard C. Expression of neurogenic markers in Alzheimer's disease: a systematic review and metatranscriptional analysis. Neurobiol Aging. 2019;76:166–180. doi: 10.1016/j.neurobiolaging.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Forcelli PA, Palchik G, Leath T, DesJardin JT, Gale K, Malkova L. Memory loss in a nonnavigational spatial task after hippocampal inactivation in monkeys. Proc Natl Acad Sci USA. 2014;111:4315–4320. doi: 10.1073/pnas.1320562111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Téglás T, Németh Z, Koller Á, Van der Zee EA, Luiten PGM, Nyakas C. Effects of Long-Term Moderate Intensity Exercise on Cognitive Behaviors and Cholinergic Forebrain in the Aging Rat. Neuroscience. 2019;411:65–75. doi: 10.1016/j.neuroscience.2019.05.037. [DOI] [PubMed] [Google Scholar]
- 18.Wang B, Wu Q, Lei L, Sun H, Michael N, Zhang X, Wang Y, Zhang Y, Ge B, Wu X, Wang Y, Xin Y, Zhao J, Li S. Long-term social isolation inhibits autophagy activation, induces postsynaptic dysfunctions and impairs spatial memory. Exp Neurol. 2019;311:213–224. doi: 10.1016/j.expneurol.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Xu B, Sun A, He Y, Qian F, Xi S, Long D, Chen Y. Loss of thin spines and small synapses contributes to defective hippocampal function in aged mice. Neurobiol Aging. 2018;71:91–104. doi: 10.1016/j.neurobiolaging.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Maguire EA, Nannery R, Spiers HJ. Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain. 2006;129:2894–2907. doi: 10.1093/brain/awl286. [DOI] [PubMed] [Google Scholar]
- 21.Voikar V, Krackow S, Lipp HP, Rau A, Colacicco G, Wolfer DP. Automated dissection of permanent effects of hippocampal or prefrontal lesions on performance at spatial, working memory and circadian timing tasks of C57BL/6 mice in IntelliCage. Behav Brain Res. 2018;352:8–22. doi: 10.1016/j.bbr.2017.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ólafsdóttir HF, Bush D, Barry C. The Role of Hippocampal Replay in Memory and Planning. Curr Biol. 2018;28:R37–R50. doi: 10.1016/j.cub.2017.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeda T, Hosokawa M, Higuchi K, Hosono M, Akiguchi I, Katoh H. A novel murine model of aging, Senescence-Accelerated Mouse (SAM) Arch Gerontol Geriatr. 1994;19:185–192. doi: 10.1016/0167-4943(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 24.Chikamoto A, Sekizawa SI, Tochinai R, Kuwahara M. Early attenuation of autonomic nervous function in senescence accelerated mouse-prone 8 (SAMP8) Exp Anim. 2019;68:511–517. doi: 10.1538/expanim.19-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng CZ, Cao L, Luo D, Ju LS, Yang JJ, Xu XY, Yu YP. Dendrobium polysaccharides attenuate cognitive impairment in senescence-accelerated mouse prone 8 mice via modulation of microglial activation. Brain Res. 2019;1704:1–10. doi: 10.1016/j.brainres.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 26.Farr SA, Roesler E, Niehoff ML, Roby DA, McKee A, Morley JE. Metformin Improves Learning and Memory in the SAMP8 Mouse Model of Alzheimer's Disease. J Alzheimers Dis. 2019;68:1699–1710. doi: 10.3233/JAD-181240. [DOI] [PubMed] [Google Scholar]
- 27.Vela S, Sainz N, Moreno-Aliaga MJ, Solas M, Ramirez MJ. DHA Selectively Protects SAMP-8-Associated Cognitive Deficits Through Inhibition of JNK. Mol Neurobiol. 2019;56:1618–1627. doi: 10.1007/s12035-018-1185-7. [DOI] [PubMed] [Google Scholar]
- 28.Griñán-Ferré C, Corpas R, Puigoriol-Illamola D, Palomera-Ávalos V, Sanfeliu C, Pallàs M. Understanding Epigenetics in the Neurodegeneration of Alzheimer's Disease: SAMP8 Mouse Model. J Alzheimers Dis. 2018;62:943–963. doi: 10.3233/JAD-170664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puigoriol-Illamola D, Griñán-Ferré C, Vasilopoulou F, Leiva R, Vázquez S, Pallàs M. 11β-HSD1 Inhibition by RL-118 Promotes Autophagy and Correlates with Reduced Oxidative Stress and Inflammation, Enhancing Cognitive Performance in SAMP8 Mouse Model. Mol Neurobiol. 2018;55:8904–8915. doi: 10.1007/s12035-018-1026-8. [DOI] [PubMed] [Google Scholar]
- 30.Ding N, Jiang J, Xu A, Tang Y, Li Z. Manual Acupuncture Regulates Behavior and Cerebral Blood Flow in the SAMP8 Mouse Model of Alzheimer's Disease. Front Neurosci. 2019;13:37. doi: 10.3389/fnins.2019.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding N, Jiang J, Lu M, Hu J, Xu Y, Liu X, Li Z. Manual Acupuncture Suppresses the Expression of Proinflammatory Proteins Associated with the NLRP3 Inflammasome in the Hippocampus of SAMP8 Mice. Evid Based Complement Alternat Med. 2017;2017:3435891. doi: 10.1155/2017/3435891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith LA, McMahon LL. Deficits in synaptic function occur at medial perforant path-dentate granule cell synapses prior to Schaffer collateral-CA1 pyramidal cell synapses in the novel TgF344-Alzheimer's Disease Rat Model. Neurobiol Dis. 2018;110:166–179. doi: 10.1016/j.nbd.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen YL, Wang LM, Chen Y, Gao JY, Marshall C, Cai ZY, Hu G, Xiao M. Changes in astrocyte functional markers and β-amyloid metabolism-related proteins in the early stages of hypercholesterolemia. Neuroscience. 2016;316:178–191. doi: 10.1016/j.neuroscience.2015.12.039. [DOI] [PubMed] [Google Scholar]
- 34.Gejl M, Gjedde A, Egefjord L, Møller A, Hansen SB, Vang K, Rodell A, Brændgaard H, Gottrup H, Schacht A, Møller N, Brock B, Rungby J. In Alzheimer's Disease, 6-Month Treatment with GLP-1 Analog Prevents Decline of Brain Glucose Metabolism: Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Front Aging Neurosci. 2016;8:108. doi: 10.3389/fnagi.2016.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo C, Long B, Hu Y, Yuan J, Gong H, Li X. Early-stage reduction of the dendritic complexity in basolateral amygdala of a transgenic mouse model of Alzheimer's disease. Biochem Biophys Res Commun. 2017;486:679–685. doi: 10.1016/j.bbrc.2017.03.094. [DOI] [PubMed] [Google Scholar]
- 36.Völgyi K, Gulyássy P, Todorov MI, Puska G, Badics K, Hlatky D, Kékesi KA, Nyitrai G, Czurkó A, Drahos L, Dobolyi A. Chronic Cerebral Hypoperfusion Induced Synaptic Proteome Changes in the rat Cerebral Cortex. Mol Neurobiol. 2018;55:4253–4266. doi: 10.1007/s12035-017-0641-0. [DOI] [PubMed] [Google Scholar]
- 37.Eslami M, Sadeghi B, Goshadrou F. Chronic ghrelin administration restores hippocampal long-term potentiation and ameliorates memory impairment in rat model of Alzheimer's disease. Hippocampus. 2018;28:724–734. doi: 10.1002/hipo.23002. [DOI] [PubMed] [Google Scholar]
- 38.Bos I, Vos S, Verhey F, Scheltens P, Teunissen C, Engelborghs S, Sleegers K, Frisoni G, Blin O, Richardson JC, Bordet R, Tsolaki M, Popp J, Peyratout G, Martinez-Lage P, Tainta M, Lleó A, Johannsen P, Freund-Levi Y, Frölich L, Vandenberghe R, Westwood S, Dobricic V, Barkhof F, Legido-Quigley C, Bertram L, Lovestone S, Streffer J, Andreasson U, Blennow K, Zetterberg H, Visser PJ. Cerebrospinal fluid biomarkers of neurodegeneration, synaptic integrity, and astroglial activation across the clinical Alzheimer's disease spectrum. Alzheimers Dement. 2019;15:644–654. doi: 10.1016/j.jalz.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Li S, Jin M, Liu L, Dang Y, Ostaszewski BL, Selkoe DJ. Decoding the synaptic dysfunction of bioactive human AD brain soluble Aβ to inspire novel therapeutic avenues for Alzheimer's disease. Acta Neuropathol Commun. 2018;6:121. doi: 10.1186/s40478-018-0626-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee E, Chung WS. Glial Control of Synapse Number in Healthy and Diseased Brain. Front Cell Neurosci. 2019;13:42. doi: 10.3389/fncel.2019.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez B, Peplow PV. Amelioration of Alzheimer's disease pathology and cognitive deficits by immunomodulatory agents in animal models of Alzheimer's disease. Neural Regen Res. 2019;14:1158–1176. doi: 10.4103/1673-5374.251192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park HL, Kim SW, Kim JH, Park CK. Increased levels of synaptic proteins involved in synaptic plasticity after chronic intraocular pressure elevation and modulation by brain-derived neurotrophic factor in a glaucoma animal model. Dis Model Mech. 2019;12 doi: 10.1242/dmm.037184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu X, Liu J, Huang S, Zhu W, Wang Y, Chen O, Xue J. Neuroprotective effects of isoliquiritigenin against cognitive impairment via suppression of synaptic dysfunction, neuronal injury, and neuroinflammation in rats with kainic acid-induced seizures. Int Immunopharmacol. 2019;72:358–366. doi: 10.1016/j.intimp.2019.04.028. [DOI] [PubMed] [Google Scholar]
- 44.Li Q, Wu X, Na X, Ge B, Wu Q, Guo X, Ntim M, Zhang Y, Sun Y, Yang J, Xiao Z, Zhao J, Li S. Impaired Cognitive Function and Altered Hippocampal Synaptic Plasticity in Mice Lacking Dermatan Sulfotransferase Chst14/D4st1. Front Mol Neurosci. 2019;12:26. doi: 10.3389/fnmol.2019.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon SL, Harper CB, Smillie KJ, Cousin MA. A Fine Balance of Synaptophysin Levels Underlies Efficient Retrieval of Synaptobrevin II to Synaptic Vesicles. PLoS One. 2016;11:e0149457. doi: 10.1371/journal.pone.0149457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magalhães RC, Pimenta LP, Barbosa IG, Moreira JM, de Barros JLVM, Teixeira AL, Simões E Silva AC. Inflammatory molecules and neurotrophic factors as biomarkers of neuropsychomotor development in preterm neonates: A Systematic Review. Int J Dev Neurosci. 2018;65:29–37. doi: 10.1016/j.ijdevneu.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Kwak M, Yum MS, Yeh HR, Kim HJ, Ko TS. Brain Magnetic Resonance Imaging Findings of Congenital Cytomegalovirus Infection as a Prognostic Factor for Neurological Outcome. Pediatr Neurol. 2018;83:14–18. doi: 10.1016/j.pediatrneurol.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 48.Ren QG, Gong WG, Zhou H, Shu H, Wang YJ, Zhang ZJ. Spatial Training Ameliorates Long-Term Alzheimer's Disease-Like Pathological Deficits by Reducing NLRP3 Inflammasomes in PR5 Mice. Neurotherapeutics. 2019;16:450–464. doi: 10.1007/s13311-018-00698-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gondard E, Teves L, Wang L, McKinnon C, Hamani C, Kalia SK, Carlen PL, Tymianski M, Lozano AM. Deep Brain Stimulation Rescues Memory and Synaptic Activity in a Rat Model of Global Ischemia. J Neurosci. 2019;39:2430–2440. doi: 10.1523/JNEUROSCI.1222-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao F, Liao Y, Tang H, Piao J, Wang G, Jin Y. Effects of developmental arsenite exposure on hippocampal synapses in mouse offspring. Metallomics. 2017;9:1394–1412. doi: 10.1039/c7mt00053g. [DOI] [PubMed] [Google Scholar]
- 51.Qin Y, Zhang Y, Tomic I, Hao W, Menger MD, Liu C, Fassbender K, Liu Y. Ginkgo biloba Extract EGb 761 and Its Specific Components Elicit Protective Protein Clearance Through the Autophagy-Lysosomal Pathway in Tau-Transgenic Mice and Cultured Neurons. J Alzheimers Dis. 2018;65:243–263. doi: 10.3233/JAD-180426. [DOI] [PubMed] [Google Scholar]
- 52.Liu Y, Zhang Y, Zheng X, Fang T, Yang X, Luo X, Guo A, Newell KA, Huang XF, Yu Y. Galantamine improves cognition, hippocampal inflammation, and synaptic plasticity impairments induced by lipopolysaccharide in mice. J Neuroinflammation. 2018;15:112. doi: 10.1186/s12974-018-1141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin SB, Dowling AL, Lianekhammy J, Lott IT, Doran E, Murphy MP, Beckett TL, Schmitt FA, Head E. Synaptophysin and synaptojanin-1 in Down syndrome are differentially affected by Alzheimer's disease. J Alzheimers Dis. 2014;42:767–775. doi: 10.3233/JAD-140795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen MK, Mecca AP, Naganawa M, Finnema SJ, Toyonaga T, Lin SF, Najafzadeh S, Ropchan J, Lu Y, McDonald JW, Michalak HR, Nabulsi NB, Arnsten AFT, Huang Y, Carson RE, van Dyck CH. Assessing Synaptic Density in Alzheimer Disease With Synaptic Vesicle Glycoprotein 2A Positron Emission Tomographic Imaging. JAMA Neurol. 2018;75:1215–1224. doi: 10.1001/jamaneurol.2018.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Technical appendix, statistical code, and dataset available from the corresponding author at lanzhao69@163.com. Participants gave informed consent for data sharing.