Abstract
Background:
Breast cancer mortality is higher for black and younger women. This study evaluated two possible contributors to disparities -- time to treatment and treatment duration -- by race and age.
Methods:
Among 2,841 participants with stage I-III disease in the Carolina Breast Cancer Study, we identified groups of women with similar patterns of socioeconomic status (SES), access to care, and tumor characteristics using latent class analysis. We then evaluated latent classes in association with treatment delay (initiation >60 days after diagnosis) and treatment duration (in quartiles by treatment modality).
Results:
Thirty-two percent of younger black women were in the highest quartile of treatment duration (versus 22% of younger whites). Black women experienced a higher frequency of delayed treatment [adjusted relative frequency difference (RFD) = 5.5%; 95% confidence interval (CI): 3.2%, 7.8%)] and prolonged treatment duration (RFD = 8.8%; 95% CI: 5.7%, 12.0%). Low SES was significantly associated with treatment delay among white women (RFD 3.5%; 95% CI: 1.1, 5.9), but treatment delay was high at all levels of SES in black women (e.g. 11.7% in high SES black women compared to 10.6% and 6.7% among low and high SES whites, respectively). Neither SES nor access to care classes were significantly associated with delayed initiation among black women, but both low SES and more barriers were associated with treatment duration across both races.
Conclusions:
Factors that influence treatment timeliness persist throughout the care continuum, with prolonged treatment duration being a sensitive indicator of differences by race, SES, and care barriers.
Keywords: Breast cancer, treatment delay, treatment duration, latent class analysis, racial disparities, health care disparities
Precis:
Economic and other barriers to care appear to compound across the continuum, with treatment duration representing a sensitive indicator of barriers to care. By developing an integrated view of multiple patient factors that contribute to duration, appropriate multidimensional interventions can be conceptualized to reduce racial mortality disparities.
Introduction
Black women have a 42% higher breast cancer mortality rate than white women despite similar incidence rates, and among women under the age of 45, black women have a breast cancer mortality rate more than twice as high as white women [1, 2]. These mortality differences, overall and at younger ages, have been attributed to a variety of factors including screening guidelines and screening use, later stage at diagnosis, and more adverse tumor biology [3–8]. After diagnosis, timely initiation of treatment improves survival [9]. Black women experience delays in time from diagnosis to surgery [10–13], and delays in initiating chemotherapy [14–17] and radiation [18, 19]. Greater adverse reactions and lower adherence to endocrine therapy has also been described for black women [20, 21].
Previous research on white-black differences in treatment delay has been limited because many studies used area-level socioeconomic status (SES) proxy variables, are single institution or hospital-based studies, and most studies that use individual level data, use a single variable to assess SES [22–26]. Moreover, many focused on delayed treatment initiation without considering other delays on the care continuum [27]. We previously published work with a more comprehensive approach to the definition and measurement of breast cancer treatment delays [27], and here we seek to apply this conceptualization of treatment delays in combination with latent class variables for SES and access to care. As reviewed in Palumbo et al. [28], latent class analysis (LCA) has distinct advantages compared to area-level SES and continuous measurements of SES. First, continuous SES combines a multidimensional construct into a single unit-less measure, which can be difficult to interpret. Second, SES indicators (i.e., education and income) can be highly correlated and difficult to model simultaneously, and may not have similar impact in other settings. Indeed, Palumbo et al. showed that a single SES index did not perform as well as multiple latent class variables at identifying women in high risk neighborhoods.
Our objective in this analysis was to assess the role of tumor biology and access factors in both treatment delay and treatment duration, using LCA, a multivariate data dimensionally reduction approach. Using data from the Carolina Breast Cancer Study Phase 3 (CBCS3), a population-based study initiated to disentangle tumor biological factors and the role of health services in breast cancer disparities, we identified distinct SES, access to care, and tumor factor latent classes [29]. We then estimate the associations of these classes with treatment initiation and duration by race and age.
Methods
Data Source and Study Population
CBCS3 is a population-based cohort study of women diagnosed with invasive breast cancer. All cases were identified within two months of diagnosis by rapid case ascertainment via the North Carolina Central Cancer Registry. Younger (<50 years in age) cases and cases of black patients were oversampled by randomized recruitment so that half of the population was younger (<50 years in age) and half was black. Patient characteristics, including SES, are ascertained from in-home structured interviews administered by a nurse, who also gathered anthropometric information. Comorbidities, tumor characteristics, and treatment data were abstracted from medical record and pathology report documentation. Tumor grading was assigned by a single breast cancer pathologist using the Nottingham breast cancer grading system [30]. Informed consent was obtained from all participants. All study protocols in CBCS3 were in accordance with the ethical standards of the Institutional Review Board of the University of North Carolina at Chapel Hill.
Because management of metastatic disease occurs on a distinct clinical pathway compared to localized disease, we restricted this analysis to stage I-III breast cancers. We also restricted to cases that received surgical treatment within 18 months of diagnosis. The study sample included 2,841 women, between the ages of 20 and 74 at the time of diagnosis, receiving a first, primary diagnosis of breast cancer between May 1, 2008 and October 21, 2013 and living within the 44-county study area.
Covariate assessment
Interviewer-administered questionnaire and follow-up survey.
Patients self-reported income (USD > $50K, $15K to $50K and < $15K), education (college degree or higher, some college, high school graduate/GED, and less than high school education), marital status (married vs. not married), family history of breast cancer (yes vs. no), current smoker status (not current vs. current), insurance status (yes vs. no), and rural residence (yes vs. no) at the baseline interview. We defined rural residence as >10K vs. ≤10K population, in accordance with definitions from the Office of Management and Budget [31]. A nurse measured height and weight collected in-home during the baseline interview, was used to define body mass index (BMI) as ≤25, 25-30, and >30 kg/m2. Job loss because of breast cancer diagnosis and inability to see a doctor because of financial and transportation issues were assessed via a telephone survey 18 months post-diagnosis.
Medical record review and pathology reports.
Diabetes, heart disease, tumor size, nodal status, and grade data were abstracted from patients’ medical records. Hormone receptor (HR), human epidermal growth factor receptor 2 (HER2), and triple negative breast cancer (TNBC) positivity data were ascertained from pathology reports.
Treatment modalities.
Information on treatment type included surgery, radiation, and chemotherapy and the dates of each were abstracted from the medical record. Within each treatment type, the time to first and last treatment was collected. Patients were sorted into four treatment groups: surgery only, surgery and radiation, surgery and chemotherapy, and all three modalities.
Patterns of SES, comorbidity, access and tumor factors.
SES is typically measured as a single variable or as an area-level proxy variable, which limits multidimensional understanding of the role of SES in cancer outcomes. Therefore, we created person-centered groups of SES, access to care, and tumor characteristics using individual-level data through the use of latent class analysis (LCA). LCA identifies unobservable, or latent, groups of individuals within a population based on numerical responses to observed set of factors [32]. These subgroups are mutually exclusive and exhaustive. An iterative approach to parameter estimation using expectation-maximization (EM) for maximum-likelihood (ML) estimation generated estimates of all model parameters and item-response probabilities of class assignment. We consider these three latent class groups in association with treatment delay (Table 1). We previously described identification of latent classes [33]. Briefly, we a priori identified three separate latent class domains: SES and comorbidity factors (henceforth, SES), access to care factors, and tumor characteristics using latent class analysis in SAS PROC LCA, as described in [34]. We combined comorbidities and SES in a single LCA variable, because a previous systemic review has shown that multimorbidity is associated with healthcare deprivation [35]. Latent classes of SES (high vs. low) were defined by categorical variables describing income, education, BMI, marital status, family history of breast cancer, insurance, urban/rural residence, diabetes, heart disease, and smoking status. The high SES latent class was characterized by a high probability of the highest categories of income, education, and married status, and low probability of comorbidities. Latent classes of access to care (fewer vs. more barriers) were defined by insurance, urban/rural residence, job loss, and having financial or transportation issues. The fewer barriers class had lower probabilities of uninsured status, financial or transportation issues, and job loss. Latent classes of tumor characteristics (HR+/HER2−/node negative vs. HER2+/higher grade, HR+/HER2−/larger tumor/node positive, and TNBC/higher grade) were defined by tumor size, grade, nodal, HR and HER2 status. We used several criteria to determine the number of classes from the ML solution using many sets of starting values. We examined the G2 likelihood ratio test statistic produced using 100,000 sets of starting values, Akaike’s information criterion (AIC) and Bayesian Information Criterion (BIC), a goodness-of-fit measure to find more parsimonious models. Once the optimal model for parsimony and model fit was determined, individuals were assigned to the classes based on their highest probability of membership.
Table 1.
Characterization of Latent Classes
| Class | Class labels | Characterization |
|---|---|---|
| SES | High SES/low comorbidity | Highest levels of income and education, being married, less diabetes, heart disease, and current smoking status |
| Low SES/high comorbidity | ||
| Access to care | Fewer barriers | Lower probabilities of being uninsured, job loss, and financial and transportation issues |
| More barriers | ||
| Tumor characteristics | HR+/HER2−/node negative | Highest probability of HR+ |
| HER2+/higher grade | Highest probabilities of high grade and HER2+ | |
| HR+/HER2−/larger tumor/node positive | Highest probabilities of large tumor size and HR+ | |
| TNBC/higher grade | Highest probabilities of high grade and TNBC |
Abbreviations: HER2, human epidermal growth factor receptor 2; HR, hormone receptor; TNBC, triple negative breast cancer; SES, socioeconomic status
Outcome assessment
We evaluated two outcomes reflecting treatment delay using dates from the medical record: delay in treatment initiation and prolonged treatment duration. Treatment initiation (in days) is the time between breast cancer diagnosis date and the first treatment (definitive surgery, chemotherapy [either adjuvant or neoadjuvant] or radiation). Treatment initiation was dichotomized as >60 days, based on clinical guidelines and previous literature [26]. Treatment duration (in days) is the time interval between the date of first treatment and the date of last treatment (definitive surgery, chemotherapy or radiation). Treatment duration was stratified by modality, and within each group the upper quartile of duration was defined (radiation, >56 days; definitive surgery, >74 days; chemotherapy, >119 days). Then, the “prolonged treatment duration” category represented women in this fourth quartile of treatment duration, and these women were compared to all other patients.
Statistical analysis
Generalized linear models were used to estimate prevalence differences, relative frequency differences (RFDs) and 95% confidence intervals (95% CIs) as measures of association between treatment delay measures and race, age, and latent classes of individual-level SES, access to care, and tumor characteristics. Race-stratified generalized linear models were age-adjusted; age-stratified models were race-adjusted. We further stratified latent class-treatment delay models by treatment modality. Lastly, we estimated the association between treatment duration and single factors, overall and according to treatment modality. All analyses were done in SAS version 9.4 (SAS Institute, Cary, NC). P-values were produced for a two-sided test with an alpha of 0.05.
Results
In the CBCS3 population, comprising roughly equal numbers of black and white women, median time to treatment initiation was 34 days (interquartile range: 19, 43). Overall, 10.6% of participants had treatment initiation >60 days after diagnosis. Figure 1 illustrates the study population’s flow into categories of treatment initiation and duration by stage. The diagram illustrates that individuals who experience one form of delay are more likely to experience additional types of delays.
Figure 1. Flow diagram of stage, delayed initiation, and prolonged treatment duration among 2,841 cases in the Carolina Breast Cancer Study Phase 3 (2008-2013).
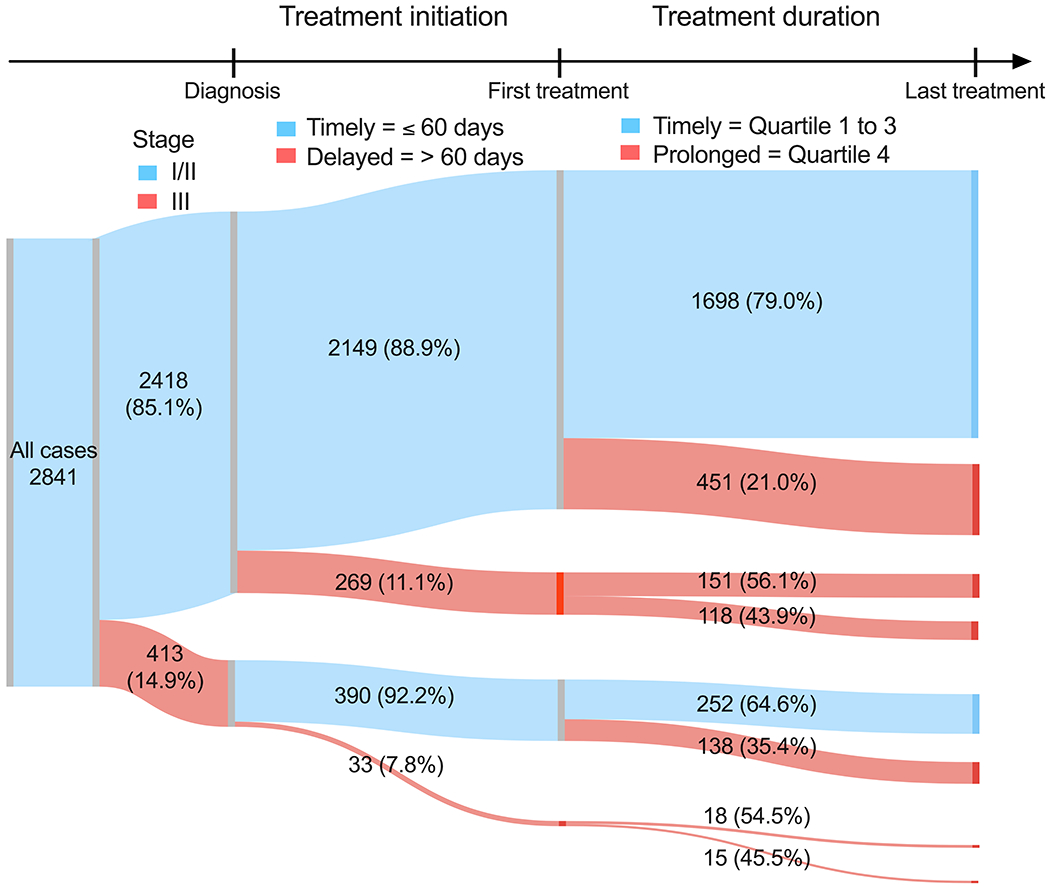
All cases included first, primary breast cancers stage I to III, who received surgical treatment within 18-months of diagnosis. Treatment initiation is defined by days since diagnosis and split by timely and delayed treatment initiation, where timely indicates ≤60 days (delayed = >60 days) from diagnosis to first treatment (definitive surgery, chemotherapy [adjuvant or neoadjuvant], or radiation). Treatment duration is defined based on quartiles of patients with the same treatment modality (definitive surgery, chemotherapy, or radiation) and split by timely and prolonged treatment duration, were timely indicates quartiles 1 to 3 (prolonged = quartile 4) of time interval, in days, between the date of first treatment and the date of the last treatment (definitive surgery, chemotherapy, or radiation).
To quantitatively estimate associations between latent classes of SES, access to care, and tumor characteristics, Table 2 shows delayed initiation and prolonged duration frequency by race, age, and latent classes. Black women experienced greater frequency of delayed initiation (13.4% vs. 7.9%) and prolonged duration (29.9% vs. 21.1%) compared to white women. After adjusting for age, black women were more likely to experience both delayed treatment initiation and prolonged treatment duration; compared to white women, the RFD among black women was 5.5% (95% CI: 3.2%, 7.8%) for delayed treatment initiation and was 8.8% (95% CI: 5.7%, 12.0%) for prolonged treatment duration. Compared to older women, younger women had similar rates of delayed initiation, but experienced more prolonged duration (27.0% vs. 23.8%). Lower SES was modestly associated with delayed initiation, but lower SES, more barriers to care and tumor aggressiveness latent classes were more strongly associated with prolonged treatment duration (Supplemental Table 1). Some associations may vary by race and age, so all four groups were examined separately (young black, young white, older black, older white). Thirty-two percent of younger black women experienced prolonged treatment duration compared to 22.3% of younger white women; similarly, 27.9% of older black women experienced prolonged treatment duration compared to 19.9% of older white women (Figure 2).
Table 2.
Frequency of delayed treatment initiation and prolonged treatment duration by race, age, and latent classes of SES and access to care in Carolina Breast Cancer Study 3 (2008-2013)
| Treatment initiation | Treatment durationa | |||||||
|---|---|---|---|---|---|---|---|---|
| ≤60 days | >60 days | Q1-3 | Q4 | |||||
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | |
| Raceb | ||||||||
| White | 1333 | (92.1) | 115 | (7.9) | 1143 | (78.9) | 305 | (21.1) |
| Black | 1206 | (86.6) | 187 | (13.4) | 976 | (70.1) | 417 | (29.9) |
| Black vs. white, RFD (95% CI) | ref | 5.5% (3.2, 7.8) | ref | 8.8% (5.7, 12.0) | ||||
| Agec | ||||||||
| Older | 1272 | (89.3) | 153 | (10.7) | 1086 | (76.2) | 339 | (23.8) |
| Younger | 1267 | (89.5) | 149 | (10.5) | 1033 | (73.0) | 383 | (27.0) |
| Younger vs. older, RFD (95% CI) | ref | 0.3% (−1.9, 2.5) | ref | 3.2% (0.1, 6.3) | ||||
| SES latent classesd | ||||||||
| High SES | 1378 | (91.6) | 127 | (8.4) | 1192 | (79.2) | 313 | (20.8) |
| Low SES | 1161 | (86.9) | 175 | (13.1) | 927 | (69.4) | 409 | (30.6) |
| Low vs. High, RFD (95% CI) | ref | 3.5% (1.2, 5.9) | ref | 8.1% (4.7, 11.5) | ||||
| Access to care latent classesd | ||||||||
| Fewer Barriers to Care | 2311 | (89.8) | 264 | (10.2) | 1954 | (75.9) | 621 | (24.1) |
| More Barriers to Care | 227 | (85.7) | 38 | (14.3) | 164 | (61.9) | 101 | (38.1) |
| Low vs. high, RFD (95% CI) | ref | 2.8% (−1.6, 7.2) | ref | 11.7% (5.5, 17.8) | ||||
Quartiles were separately defined by modality and Q4 was equal to 56, 74, 119 days for radiation, definitive surgery, and chemotherapy modalities, respectively
Multivariate models adjusting for age
Multivariate models adjusting for race
Multivariate models adjusting for race and age
Abbreviations: CI, confidence interval; RFD, relative frequency difference; SES, socioeconomic status
Figure 2. Proportions of delayed initiation and prolonged treatment duration by race and age in the Carolina Breast Cancer Study Phase 3 (2008-2013).
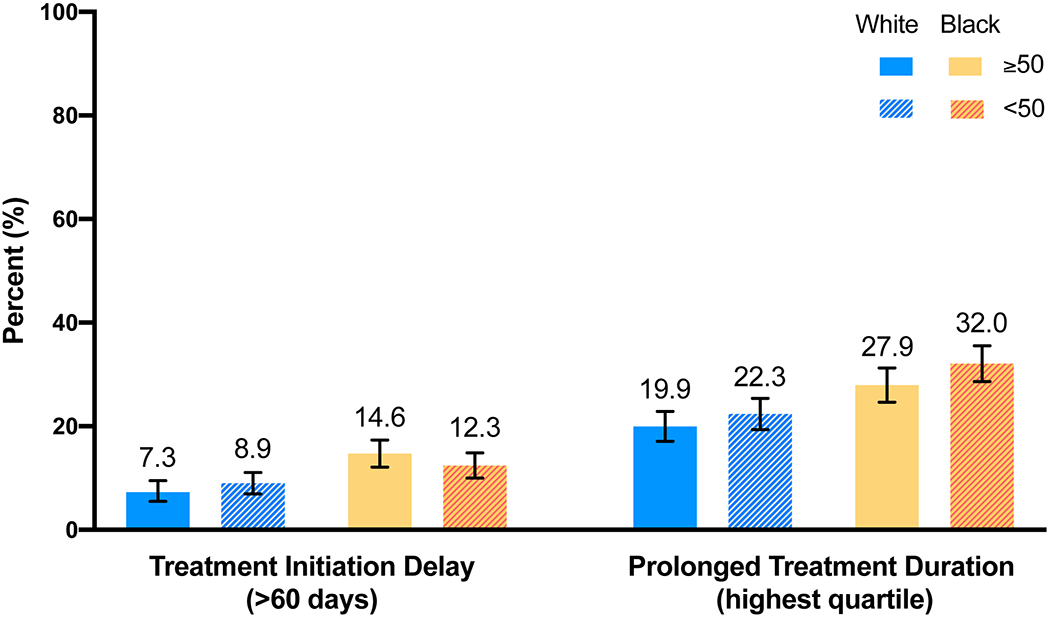
Each percentage represents a proportion of delayed initiation and prolonged treatment duration within each race and age category. Treatment initiation is defined by days since diagnosis. Treatment duration is defined based on quartiles of patients with the same treatment modality.
While latent classes had effects on treatment delay in the population as a whole, higher rates by race suggest that it is important to study treatment timeliness within race strata. To evaluate how these latent class variables impact treatment in the context of race, we conducted analyses stratified on race. Table 3 shows the associations between latent classes and treatment delay separately for black and white women. Among white women there was a modest, statistically significant increase in delayed initiation associated with low SES (3.5%). However, the effect of SES class on treatment initiation was not as large in black women (2.4%). In fact, the prevalence of delayed initiation among black women was high in both the low SES and high SES groups (14.4% and 11.7%, respectively), whereas delayed initiation was less prevalent in white women (10.6% and 6.7% in higher and lower SES). Thus, the impact of SES groups on treatment initiation did not appear to be additive among black women. In contrast, treatment duration did appear to be additively associated with both SES and race. The estimated RFDs for low SES and treatment duration were statistically significant and elevated in both races (RFD for white and black women: 8.1%; 95% CI: 4.7%, 11.5% and 8.4%; 95% CI: 3.5%, 13.3% low vs. high SES), although the baseline frequency of prolonged duration was higher among black women. We also observed a substantially higher magnitude of prolonged treatment duration among black and white women with more barriers to care (compared to fewer barriers); the RFD for prolonged treatment duration for black women with more barriers was 14.1% (95% CI: 6.5%, 21.8%) and for white women was 11.7% (95% CI: 5.5%, 17.8%).
Table 3.
Frequency of delayed treatment initiation and prolonged treatment duration by race, and latent classes of SES and access to care in Carolina Breast Cancer Study 3 (2008-2013)a
| Treatment initiation | Treatment durationb | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White |
Black |
White |
Black |
|||||||||||||
| ≤60 days | >60 days | ≤60 days | >60 days | Q1-Q3 | Q4 | Q1-Q3 | Q4 | |||||||||
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | |
| SES latent classes | ||||||||||||||||
| High SES | 927 | (93.3) | 67 | (6.7) | 451 | (88.3) | 60 | (11.7) | 808 | (81.3) | 186 | (18.7) | 384 | (75.1) | 127 | (24.9) |
| Low SES | 406 | (89.4) | 48 | (10.6) | 755 | (85.6) | 127 | (14.4) | 335 | (73.8) | 119 | (26.2) | 592 | (67.1) | 290 | (32.9) |
| Low vs. high, RFD (95% CI) | ref | 3.5% (1.1, 5.9) | ref | 2.4% (−1.3, 6.0) | ref | 8.1% (4.7, 11.5) | ref | 8.4% (3.5, 13.3) | ||||||||
| Access to care latent classesc | ||||||||||||||||
| Fewer Barriers to Care | 1259 | (92.2) | 107 | (7.8) | 1052 | (87.0) | 157 | (13.0) | 1084 | (79.4) | 263 | (20.6) | 870 | (72.0) | 339 | (28.0) |
| More Barriers to Care | 74 | (90.2) | 8 | (9.8) | 153 | (83.6) | 30 | (16.4) | 59 | (71.9) | 28 | (28.1) | 105 | (57.4) | 78 | (42.6) |
| More vs. fewer, RFD (95% CI) | ref | 2.8% (−1.6, 7.2) | ref | 3.6% (−2.1, 9.3) | ref | 11.7% (5.5, 17.8) | ref | 14.1% (6.5, 21.8) | ||||||||
Multivariate models adjusting for age
Quartiles were separately defined by modality and Q4 was equal to 56, 74, 119 days for radiation, definitive surgery, and chemotherapy modalities, respectively
n=2840
Abbreviations: CI, confidence interval; RFD, relative frequency difference; SES, socioeconomic status
Given the variation in treatment duration by race, age and SES and other barriers, we performed sensitivity analyses to assess whether these differences varied by treatment modality (Table 4). Across all modalities, black women more frequently experienced prolonged treatment duration than white women. Across most modalities, low SES women also experienced more frequent prolonged treatment duration. Fewer barriers to care were statistically significantly associated with prolonged treatment duration among treatment modalities that included radiation and/or chemotherapy; the relative frequency of prolonged treatment duration among women with more barriers to care was 21.2% if they had surgery and radiation (95% CI: 5.1%, 37.2%) and was 16.0% higher (95% CI: 7.9%, 24.0%) if they had surgery, radiation and chemotherapy.
Table 4.
Association of race, age, SES and access to care latent classes with treatment duration by modality in Carolina Breast Cancer Study 3 (2008-2013)
| Treatment durationa |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery only |
Surgery & radiation |
Surgery & chemotherapy |
Surgery, rad. & chemo. |
|||||||||||||
| Q1-3 (74.7%) | Q4 (25.3%) | Q1-3 (73.8%) | Q4 (26.2%) | Q1-3 (74.8%) | Q4 (25.2%) | Q1-3 (74.9%) | Q4 (25.1%) | |||||||||
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | |
| Race | ||||||||||||||||
| White | 171 | (78.8) | 46 | (21.2) | 302 | (77.4) | 88 | (22.6) | 302 | (77.4) | 88 | (22.6) | 175 | (81.8) | 39 | (18.2) |
| Black | 104 | (68.9) | 47 | (31.1) | 184 | (68.4) | 85 | (31.6) | 184 | (68.4) | 85 | (31.6) | 122 | (66.7) | 61 | (33.3) |
| Black vs. white, RFD (95% CI) | ref | 9.9% (0.8, 19.1) | ref | 9.0% (2.1, 16.0) | ref | 15.1% (6.5, 23.7) | ref | 7.3% (2.8, 11.8) | ||||||||
| Age | ||||||||||||||||
| Older | 166 | (79.4) | 43 | (20.6) | 354 | (74.8) | 119 | (25.2) | 108 | (69.7) | 47 | (30.3) | 458 | (77.9) | 130 | (22.1) |
| Younger | 109 | (68.6) | 50 | (31.5) | 132 | (71.0) | 54 | (29.0) | 189 | (78.1) | 53 | (21.9) | 603 | (72.7) | 226 | (27.3) |
| Younger vs. older, RFD (95% CI) | ref | 10.9% (1.8, 19.9) | ref | 3.9% (−3.7, 11.5) | ref | −8.4% (−17.3, 0.1) | ref | 5.2% (0.6, 9.7) | ||||||||
| SES latent classes | ||||||||||||||||
| High SES | 144 | (77.4) | 42 | (22.6) | 290 | (78.2) | 81 | (21.8) | 180 | (79.7) | 46 | (20.3) | 578 | (80.1) | 144 | (19.9) |
| Low SES | 131 | (72.0) | 51 | (28.0) | 196 | (68.1) | 92 | (31.9) | 117 | (68.4) | 54 | (31.6) | 483 | (69.5) | 212 | (30.5) |
| Low vs. High, RFD (95% CI) | ref | 5.4% (−3.4, 14.3) | ref | 10.1% (3.3, 16.9) | ref | 11.2% (2.5, 19.9) | ref | 10.6% (6.1, 15.1) | ||||||||
| Access to care latent classes | ||||||||||||||||
| Fewer Barriers to Care | 259 | (75.7) | 83 | (24.3) | 465 | (75.0) | 155 | (25.0) | 263 | (74.9) | 88 | (25.1) | 967 | (76.6) | 295 | (23.4) |
| More Barriers to Care | 16 | (61.5) | 10 | (38.5) | 21 | (53.9) | 18 | (46.2) | 33 | (73.3) | 12 | (26.7) | 94 | (60.7) | 61 | (39.4) |
| Low vs. high, RFD (95% CI) | ref | 14.2% (−5.1, 33.4) | ref | 21.2% (5.1, 37.2) | ref | 1.6% (−12.1, 15.3) | ref | 16.0% (7.9, 24.0) | ||||||||
n = 368, 659, 397, and 1417 for surgery only, surgery + radiation, surgery + chemotherapy, and surgery + radiation + chemotherapy, respectively
Abbreviations: CI, confidence interval; RFD, relative frequency difference; SES, socioeconomic status
To evaluate possible specific points of intervention, some of the individual factors that make up SES and access to care latent classes were examined (Table 5). Considering all treatment paths, prolonged treatment duration was associated with uninsured, financial and transportation issues. These individual factors persisted in different treatment modalities. Financial issues were statistically significantly associated with delay among treatment modalities that included radiation and/or chemotherapy; the relative frequency of prolonged treatment duration among women with financial issues was 14.8% higher than the frequency among women without financial issues who had surgery and radiation (95% CI: 2.4%, 27.1%) and was also 9.3% higher (95% CI: 3.1%, 15.5%) among those who had surgery, radiation and chemotherapy. Transportation issues were also statistically significant predictors of prolonged treatment duration among those undergoing radiation therapy.
Table 5.
Individual variables for SES and access to care in association with treatment duration by treatment modality in Carolina Breast Cancer Study 3 (2008-2013)
| Treatment durationa |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery & radiation |
Surgery & chemotherapy |
Surgery, radiation & chemo. |
||||||||||
| Q1-3 (73.5%) | Q4 (26.5%) | Q1-3 (73.5%) | Q4 (26.5%) | Q1-3 (73.5%) | Q4 (26.5%) | |||||||
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | |
| Uninsured | ||||||||||||
| No | 467 | (74.3) | 161 | (25.6) | 276 | (74.2) | 96 | (25.8) | 1000 | (75.6) | 323 | (24.4) |
| Yes | 19 | (61.3) | 12 | (38.7) | 20 | (83.3) | 4 | (16.7) | 61 | (65.6) | 32 | (34.4) |
| RFD (95% CI) | ref | 13.1% (−4.4, 30.6) | ref | −9.1% (−24.7, 6.4) | ref | 10.0% (0.1, 19.9) | ||||||
| Financial issues | ||||||||||||
| No | 425 | (75.4) | 139 | (24.7) | 229 | (75.1) | 76 | (24.9) | 812 | (77.3) | 239 | (22.7) |
| Yes | 40 | (60.6) | 26 | (39.4) | 49 | (74.2) | 17 | (25.1) | 176 | (68.0) | 83 | (32.0) |
| RFD (95% CI) | ref | 14.8% (2.4, 27.1) | ref | 0.8% (−10.8, 12.5) | ref | 9.3% (3.1, 15.5) | ||||||
| Transportation issues | ||||||||||||
| No | 448 | (74.8) | 151 | (25.2) | 250 | (76.0) | 79 | (24.0) | 1124 | (77.0) | 381 | (23.0) |
| Yes | 15 | (50.0) | 15 | (50.0) | 26 | (65.0) | 14 | (35.0) | 109 | (61.2) | 60 | (38.8) |
| RFD (95% CI) | ref | 24.8% (6.6, 43.0) | ref | 11.0% (−4.5, 26.5) | ref | 15.7% (7.0, 24.5) | ||||||
| Job loss due to diagnosis | ||||||||||||
| No | 472 | (74.2) | 164 | (25.8) | 284 | (75.1) | 94 | (24.9) | 1012 | (75.2) | 333 | (24.8) |
| Yes | 10 | (76.9) | 3 | (23.1) | 11 | (68.8) | 5 | (31.2) | 42 | (67.7) | 20 | (32.3) |
| RFD (95% CI) | ref | −2.7% (−25.9, 20.4) | ref | 6.4% (−16.7, 29.5) | ref | 7.5% (−4.4, 19.4) | ||||||
n = 368, 659, 397, and 1417 for surgery only, surgery + radiation, surgery + chemotherapy, and surgery + radiation + chemotherapy, respectively
Abbreviations: CI, confidence interval; RFD, relative frequency difference
Discussion
In this population-based cohort of 2,841 women with stage I-III breast cancer, we found that black women experienced both delayed treatment initiation and prolonged treatment duration more often than whites. Associations of latent classes (SES, access to care, tumor biology) were stronger for prolonged treatment duration than for treatment initiation, suggesting that treatment duration may be a more sensitive marker for access to care disparities and that delays may compound across the care continuum. Black women with low SES and more barriers to care had substantial proportions (32.9% and 42.6%, respectively) of prolonged treatment duration, especially when radiation therapy was part of treatment.
Our results for treatment initiation are consistent with previous findings that race [11, 13, 14, 36, 37], SES factors, and insurance coverage [38–40] are associated with delays. Some of these previous studies have shown that black patients had longer times to follow-up or incomplete follow-up after an abnormal screening result [41, 42], delays in surgery, delayed radiation, and delayed and incomplete chemotherapy [11, 14, 24, 43, 44]. However, our findings added to this literature, by also evaluating treatment duration. Radiation therapy emerged as a pathway that may be sensitive to treatment duration. Radiation therapy typically requires treatment for 5 days a week for several weeks, which may add complications such as travel distance, longer clinical visits, and additional patient burdens.
A strength of LCA is data dimensionality reduction which allows for a more comprehensive understanding of patient groups; however, latent classes and aggregated SES factors may not be readily intervenable. Conversely, associations between treatment delay and individual factors may suggest more specific interventions. For example, previous CBCS results have shown that financial factors impact treatment compliance by race [45]. Gallups et al. found that employment status and number of comorbidities predicted treatment delays among black women [46]. Previous interventions have targeted these factors (e.g., cancer navigator programs [47, 48] and multidisciplinary clinics [37]). However, single factor analyses suggest interventions, but may not represent a holistic view of the complex barriers to care. Including both approaches, however, provides some insights about overall patterns and suggests possible interventions meriting further consideration.
It is particularly important to understand how treatment differences compound across the care continuum. Our results are also consistent with a previous body of work showing that treatment differences compound for disadvantaged groups [49–51]. A recent paper has highlighted a mechanism for this compounding effect, by measuring the ‘workload’ associated with cancer treatment; this work demonstrated that cancer treatment requires between 29 and 81 hours of treatment related work, with a greater workload for later stage cancers [52]. We hypothesize that the workload associated with breast cancer care is experienced differently by different populations, depending on SES and other factors, and that treatment paths that include radiation are particularly sensitive to these differences because radiation requires multiple visits within a short time period. Future efforts will be aimed at developing measures of workload in this population to directly test this hypothesis.
Our findings should be interpreted with some limitations in mind. We could not evaluate the long-term impact of prolonged treatment duration on survival or recurrence. CBCS3 recruitment ended in 2013, with median follow-up currently at 7 years. Thus, the study is not mature or adequately powered to evaluate long-term mortality in association with treatment timeliness. Greater resolution is also needed on specific financial and transportation issues, as self-reported ‘yes/no’ responses to financial and transportation barriers do not detail specific concerns. Additionally, we were unable to assess some biological factors that may affect treatment duration by race. Black women may have lower white blood cell counts compared to white women [53], which has been hypothesized to impact bone marrow reserve or white blood cell count recovery in response to cytotoxic chemotherapy [53, 54]. Such underlying biological differences could result in longer duration of adjuvant chemotherapy treatment in black women. System-level factors, such as institutional/academic affiliations and facility type, size and location, which may affect quality and timeliness of care, interact with patient characteristics and vary by race [55], but were not evaluated in the current analysis. In fact, few studies have examined how characteristics of the health system affect racial differences in treatment [51]. We previously took initial steps toward addressing health care factors by assessing treatment delay by Area Health Education Center (AHEC) region [27], and future work should capture more complex care-coordination variables such as distance to care, type of care center, and work load associated with treatment [52].
Conclusion
There are many dimensions of treatment delay, from diagnosis to treatment completion. Economic and other barriers to care appear to compound across the continuum, with treatment duration representing a sensitive indicator of barriers to care. By developing an integrated view of multiple patient factors that contribute to duration, appropriate multidimensional interventions can be conceptualized to reduce racial mortality disparities.
Supplementary Material
Acknowledgments
Funding support
This work was supported by a grant from the UNC Lineberger Comprehensive Cancer Center funded by the University Cancer Research Fund (LCCC2017T204), Susan G. Komen Graduate Training in Disparities Research (GTDR16381071), the National Cancer Institute of the National Institutes of Health (P50-CA58223, U01-CA179715, T32-CA057726), and the National Institute of Environmental Health Sciences of the National Institutes of Health (P30-ES010126).
Footnotes
Conflict of interest statement
We have no conflicts of interest to disclose.
References
- 1.DeSantis CE, Fedewa SA, Goding Sauer A, et al. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66(1):31–42. [DOI] [PubMed] [Google Scholar]
- 2.Williams DR, Mohammed SA, Shields AE. Understanding and effectively addressing breast cancer in African American women: Unpacking the social context. Cancer. 2016;122(14):2138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dean L, Subramanian SV, Williams DR, et al. The role of social capital in African-American women's use of mammography. Soc Sci Med. 2014;104:148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shavers VL, Harlan LC, Stevens JL. Racial/ethnic variation in clinical presentation, treatment, and survival among breast cancer patients under age 35. Cancer. 2003;97(1):134–47. [DOI] [PubMed] [Google Scholar]
- 5.Jatoi I, Becher H, Leake CR. Widening disparity in survival between white and African-American patients with breast carcinoma treated in the U. S. Department of Defense Healthcare system. Cancer. 2003;98(5):894–9. [DOI] [PubMed] [Google Scholar]
- 6.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. Jama-Journal of the American Medical Association. 2006;295(21):2492–2502. [DOI] [PubMed] [Google Scholar]
- 7.DeSantis C, Jemal A, Ward E. Disparities in breast cancer prognostic factors by race, insurance status, and education. Cancer Causes Control. 2010;21(9):1445–50. [DOI] [PubMed] [Google Scholar]
- 8.Press R, Carrasquillo O, Sciacca RR, et al. Racial/ethnic disparities in time to follow-up after an abnormal mammogram. J Womens Health (Larchmt). 2008;17(6):923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards MA, Westcombe AM, Love SB, et al. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353(9159):1119–26. [DOI] [PubMed] [Google Scholar]
- 10.Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg. 2013;148(6):516–23. [DOI] [PubMed] [Google Scholar]
- 11.Sheppard VB, Oppong BA, Hampton R, et al. Disparities in breast cancer surgery delay: the lingering effect of race. Ann Surg Oncol. 2015;22(9):2902–11. [DOI] [PubMed] [Google Scholar]
- 12.George P, Chandwani S, Gabel M, et al. Diagnosis and surgical delays in African American and white women with early-stage breast cancer. J Womens Health (Larchmt). 2015;24(3):209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fedewa SA, Edge SB, Stewart AK, et al. Race and ethnicity are associated with delays in breast cancer treatment (2003–2006). J Health Care Poor Underserved. 2011;22(1):128–41. [DOI] [PubMed] [Google Scholar]
- 14.Green AK, Aviki EM, Matsoukas K, et al. Racial disparities in chemotherapy administration for early-stage breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2018;172(2):247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, et al. Delayed Initiation of Adjuvant Chemotherapy Among Patients With Breast Cancer. JAMA Oncol. 2016;2(3):322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fedewa SA, Ward EM, Stewart AK, et al. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004-2006. J Clin Oncol. 2010;28(27):4135–41. [DOI] [PubMed] [Google Scholar]
- 17.Vandergrift JL, Niland JC, Theriault RL, et al. Time to adjuvant chemotherapy for breast cancer in National Comprehensive Cancer Network institutions. J Natl Cancer Inst. 2013;105(2):104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357–62. [DOI] [PubMed] [Google Scholar]
- 19.Freedman RA, He Y, Winer EP, et al. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27(5):713–9. [DOI] [PubMed] [Google Scholar]
- 20.Spencer JC, Reeve BB, Troester MA, et al. Factors Associated with Endocrine Therapy Non-Adherence in Breast Cancer Survivors. Psychooncology. 2020; 10.1002/pon.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wheeler SB, Spencer J, Pinheiro LC, et al. Endocrine Therapy Nonadherence and Discontinuation in Black and White Women. J Natl Cancer Inst. 2019;111(5):498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert SA, Strombom I, Trentham-Dietz A, et al. Socioeconomic risk factors for breast cancer: distinguishing individual- and community-level effects. Epidemiology. 2004;15(4):442–50. [DOI] [PubMed] [Google Scholar]
- 23.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490–6. [DOI] [PubMed] [Google Scholar]
- 24.Freedman RA, Virgo KS, He Y, et al. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer. 2011;117(1):180–9. [DOI] [PubMed] [Google Scholar]
- 25.Gorin SS, Heck JE, Cheng B, et al. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med. 2006;166(20):2244–52. [DOI] [PubMed] [Google Scholar]
- 26.Bleicher RJ, Ruth K, Sigurdson ER, et al. Time to Surgery and Breast Cancer Survival in the United States. JAMA Oncol. 2016;2(3):330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reeder-Hayes KE, Mayer SE, Olshan AF, et al. Race and delays in breast cancer treatment across the care continuum in the Carolina Breast Cancer Study. Cancer. 2019; 10.1002/cncr.32378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palumbo A, Michael Y, Hyslop T. Latent class model characterization of neighborhood socioeconomic status. Cancer Causes Control. 2016;27(3):445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hair BY, Hayes S, Tse CK, et al. Racial differences in physical activity among breast cancer survivors: implications for breast cancer care. Cancer. 2014;120(14):2174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–10. [DOI] [PubMed] [Google Scholar]
- 31.Office of Management and Budget. Standards for Delineating Metropolitan and Micropolitan Statistical Areas. 2010:75 FR 37245. [Google Scholar]
- 32.Lanza ST, Collins LM, Lemmon DR, et al. PROC LCA: A SAS Procedure for Latent Class Analysis. Struct Equ Modeling. 2007;14(4):671–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emerson MA, Golightly YM, Tan X, et al. Integrating access to care and tumor patterns by race and age in the Carolina Breast Cancer Study, 2008-2013. Cancer Causes Control. 2020; 10.1007/s10552-019-01265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.PROC LCA & PROC LTA, (Version 1.3.2), [Software]. University Park: The Methodology Center Penn State; Retrieved from http://methodology.psu.edu/. In; 2015. [Google Scholar]
- 35.Pathirana TI, Jackson CA. Socioeconomic status and multimorbidity: a systematic review and meta-analysis. Aust N Z J Public Health. 2018;42(2):186–194. [DOI] [PubMed] [Google Scholar]
- 36.Hoppe EJ, Hussain LR, Grannan KJ, et al. Racial disparities in breast cancer persist despite early detection: analysis of treatment of stage 1 breast cancer and effect of insurance status on disparities. Breast Cancer Res Treat. 2019;173(3):597–602. [DOI] [PubMed] [Google Scholar]
- 37.Bilimoria KY, Ko CY, Tomlinson JS, et al. Wait times for cancer surgery in the United States: trends and predictors of delays. Ann Surg. 2011;253(4):779–85. [DOI] [PubMed] [Google Scholar]
- 38.Gwyn K, Bondy ML, Cohen DS, et al. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004;100(8):1595–604. [DOI] [PubMed] [Google Scholar]
- 39.Kerlikowske K Timeliness of follow-up after abnormal screening mammography. Breast Cancer Res Treat. 1996;40(1):53–64. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan CP, Crane LA, Stewart S, et al. Factors affecting follow-up among low-income women with breast abnormalities. J Womens Health (Larchmt). 2004;13(2):195–206. [DOI] [PubMed] [Google Scholar]
- 41.Polverini AC, Nelson RA, Marcinkowski E, et al. Time to Treatment: Measuring Quality Breast Cancer Care. Ann Surg Oncol. 2016;23(10):3392–402. [DOI] [PubMed] [Google Scholar]
- 42.Caplan LS, May DS, Richardson LC. Time to diagnosis and treatment of breast cancer: results from the National Breast and Cervical Cancer Early Detection Program, 1991-1995. Am J Public Health. 2000;90(1):130–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akinyemiju T, Moore JX, Ojesina AI, et al. Racial disparities in individual breast cancer outcomes by hormone-receptor subtype, area-level socio-economic status and healthcare resources. Breast Cancer Res Treat. 2016;157(3):575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parekh A, Fu W, Hu C, et al. Impact of race, ethnicity, and socioeconomic factors on receipt of radiation after breast conservation surgery: analysis of the national cancer database. Breast Cancer Res Treat. 2018;172(1):201–208. [DOI] [PubMed] [Google Scholar]
- 45.Wheeler SB, Spencer JC, Pinheiro LC, et al. Financial Impact of Breast Cancer in Black Versus White Women. J Clin Oncol. 2018;36(17):1695–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gallups SF, Connolly MC, Bender CM, et al. Predictors of Adherence and Treatment Delays among African American Women Recommended to Receive Breast Cancer Chemotherapy. Womens Health Issues. 2018;28(6):553–558. [DOI] [PubMed] [Google Scholar]
- 47.Dohan D, Schrag D. Using navigators to improve care of underserved patients: current practices and approaches. Cancer. 2005;104(4):848–55. [DOI] [PubMed] [Google Scholar]
- 48.Wells KJ, Battaglia TA, Dudley DJ, et al. Patient navigation: state of the art or is it science? Cancer. 2008;113(8):1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gold HT, Thwin SS, Buist DS, et al. Delayed radiotherapy for breast cancer patients in integrated delivery systems. Am J Manag Care. 2009;15(11):785–9. [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng AC, Levy MA. Determining Burden of Commuting for Treatment Using Online Mapping Services - A Study of Breast Cancer Patients. AMIA Annu Symp Proc. 2017;2017:555–564. [PMC free article] [PubMed] [Google Scholar]
- 51.Wheeler SB, Carpenter WR, Peppercorn J, et al. Structural/organizational characteristics of health services partly explain racial variation in timeliness of radiation therapy among elderly breast cancer patients. Breast Cancer Res Treat. 2012;133(1):333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng AC, Levy MA. Measures of Treatment Workload for Patients With Breast Cancer. JCO Clin Cancer Inform. 2019;3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hershman D, Weinberg M, Rosner Z, et al. Ethnic neutropenia and treatment delay in African American women undergoing chemotherapy for early-stage breast cancer. J Natl Cancer Inst. 2003;95(20):1545–8. [DOI] [PubMed] [Google Scholar]
- 54.Atallah-Yunes SA, Ready A, Newburger PE. Benign ethnic neutropenia. Blood Rev. 2019;37:100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bickell NA, Mendez J, Guth AA. The quality of early-stage breast cancer treatment: what can we do to improve? Surg Oncol Clin N Am. 2005;14(1):103–17, vi. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


