Abstract
Studies have examined sex differences in emotion processing in health and illness. However, it remains unclear how these neural processes may relate to individual differences in affective traits. We addressed this issue with a dataset of 970 subjects (508 women) curated from the Human Connectome Project. Participants were assessed with the NIH Toolbox Emotion Measures and fMRI while identifying negative facial emotion and neutral shape targets in alternating blocks. Imaging data were analyzed with published routines and the results were reported at a corrected threshold. Men scored similarly in Anger- but lower in Fear-Affect, as compared to women. Men as compared with women engaged the occipital-temporal visual cortex, retrosplenial cortex (RSC), and both anterior and posterior cingulate cortex to a greater extent during face versus shape identification. Women relative to men engaged higher activation of bilateral middle frontal cortex. In regional brain responses to face versus shape identification, men relative to women showed more significant modulations by both Anger- and Fear- Affect traits. The left RSC and right RSC/precuneus each demonstrated activities during face vs. shape identification in negative correlation with Anger- and Fear- Affect scores in men only. Anger affect was positively correlated with prolonged RT in identifying face vs. shape target in men but not women. In contrast, women relative to men showed higher Fear-Affect score and higher activation in the right middle frontal cortex, which was more strongly correlated with prolonged RT during face vs. shape identification. Together, men and women with higher Fear-Affect demonstrated lower accuracy in identifying negative facial emotion versus neutral shape target, a relationship mediated by activity of the RSC. These findings add to the literature of sex and trait individual differences in emotion processing and may help research of sex-shared and sex-specific behavioral and neural markers of emotional disorders.
Keywords: Fear, Anger, Gender, HCP, Retrosplenial, Posterior cingulate
1. Introduction
Numerous studies have demonstrated sex differences in cognitive and affective processing (Hirnstein et al., 2019; Kogler et al., 2015; Li and Singh, 2014; McCarrey et al., 2016; Miller and Halpern, 2014). For instance, men appeared to perform better than women on spatial and working memory (Callicott et al., 1998; Chai and Jacobs, 2009; Cohen et al., 1997; Gevins et al., 1987; Mantyla, 2013; Peters et al., 1995; Reed et al., 2017; Sternberg, 1966; Vandenberg and Kuse, 1978). In contrast, women demonstrated advantages in object recognition and verbal memory (Heinzel et al., 2013; McGivern et al., 2012; Munro et al., 2012). Men and women could also show differences in brain activation despite equitable performance during cognitive and affective challenges. For instance, in an earlier study combining the stop-signal task with functional magnetic resonance imaging (fMRI), women relative to men showed greater error-related activations in bilateral thalamus and dorsal anterior cingulate cortex, despite being indistinguishable in all performance measures (Li et al., 2009). In another study where healthy subjects were provoked by money taken by an opponent and given the opportunity to retaliate, men showed higher left amygdala activation during provocation, and the amygdala activation correlated with trait anger scores in men, but not in women, absent differences in behavioral performance (Repple et al., 2018). These findings suggest brain imaging as a more sensitive measure of sex differences as compared to behavioral observations; men and women may employ different neural mechanisms to support seemingly similar behavior. In accord, studies of large public-domain data set have provided robust evidence for sex differences in structural morphometric features and functional connectomes (Ritchie et al., 2018).
Sex differences in emotion processing have been noted in numerous studies. Overall, women were more sensitive to fearful and sad stimuli whereas men were more sensitive to anger-provoking stimuli (Brody et al., 1995; Deng et al., 2016; He et al., 2018), although there was discrepancy between subjective reports and physiological responses (Kring and Gordon, 1998). Previous studies have also examined how men and women differed in performance during identification of emotional stimuli. In an emotional expression-morphing task, in which a neutral face morphed into an expressive face showing anger, disgust, fear, happiness, sadness or surprise, subjects were to label the emotion as soon as it was perceived (Montagne et al., 2005). The results showed that men were significantly less accurate than women at identifying sadness and surprise and slower than women at identifying anger and disgust. During emotional face recognition with six different emotions varying in intensity from 100% to 25%, women were faster than men at recognizing emotions (Saylik et al., 2018). A study of 1063 participants to identify facial (static pictures) and bodily (dynamic video clips) emotions from picture stimuli with people varying in sex and age for each emotion, women performed significantly better at recognizing facial expressions of disgust and sadness, whereas men performed better at bodily expressions of happiness (Le et al., 2019). In an fMRI study, women completed emotional face matching during the mid-follicular and late-luteal phase (Dan et al., 2019). Reaction time and accuracy showed no significant differences among men, women in mid-follicular phase, and women in late-luteal phase for matching either negative or positive emotional faces. However, men showed higher activation in response to negative emotional faces in the right hippocampus and parahippocampal gyrus, when compared to women in the mid-follicular phase. These and many other studies have highlighted important sex differences in the processing of emotional stimuli (Canli et al., 2002; Colich et al., 2017; Kinner et al., 2014).
Sex differences in emotion processing dysfunction have long been a focus in biomarker research of neuropsychiatric disorders (Bangasser and Valentino, 2014; Kret and De Gelder, 2012; Merz and Wolf, 2017; Rubinow and Schmidt, 2019; Whittle et al., 2011). Behavioral traits, which in the extremes may dispose individuals to mental illnesses, also demonstrate sex differences in neurotypical populations. For instance, men showed higher reward sensitivity than women (Li et al., 2007; Torrubia et al., 2001), which may explain male vulnerability to risk taking behavior. In contrast, showing higher anxiety and tendency in behavioral avoidance, women are more prone to mood disorders (Albert, 2015; Kelly et al., 2008). Fear and anger represent important emotional traits and may exert opposite effects on motivated behavior (Habib et al., 2015; Lerner and Keltner, 2001; Yang et al., 2018). An earlier study evaluated how exposure to fearful, angry and neutral faces influenced decision making in a gambling task (Habib et al., 2015). Following exposure to angry and fearful, relative to neutral, emotions, participants engage more and less frequently in risky options, respectively. These findings were confirmed by others with different behavioral paradigms (Yang et al., 2018). In a longitudinal study adolescents were assessed for temperament, including anger, fear and attention control, at 9 and 11 years, and risk-taking behavior at 11 and 15 years (Kim-Spoon et al., 2015). Higher anger trait was related to more frequent risk-taking behaviors in adolescents with low attention control; in contrast, fear trait tended to be associated with less frequent risk-taking behaviors in those with high attention control. Thus, these studies demonstrate the importance of individual traits in real-life behavioral response to emotional exposures. However, it remains unclear whether men and women may respond differently to emotional contingencies in association with these affective traits and engage distinct neural processes to support the differences.
In the current study, we addressed sex difference in the neural correlates of emotion target identification and examined sex-specific correlates of individual anger and fear traits. To this end we used the imaging data collected from the Human Connectome Project (HCP), where the Negative Affect scores were available from assessment with the NIH Toolbox Emotion Measures (Salsman et al., 2013). This study aimed to be exploratory and we broadly hypothesized that men and women would demonstrate significant differences in regional responses to emotion target identification and in regional correlates of anger and fear traits during emotion target identification. Further, as discussed earlier, men and women appeared to be more responsive to anger and fear provoking stimuli, respectively. We would also test the hypothesis that men and women may each show more significant modulation in neural activities during emotion target identification in relation to individual differences in anger and fear traits. To this end, we identified these correlates separately in men and women, compute the regional activities (β estimates) for all subjects, and followed up with slope tests to confirm or refute the sex differences.
2. Materials and methods
2.1. Dataset
For the present study, we have obtained permission from the HCP to use both the Open and Restricted Access data. The data of a total of 970 adults (508 women; age = 28.7 ± 3.7 years, mean ± SD) were obtained from the HCP (Table 1). All subjects were physically healthy with no severe neurodevelopmental, neuropsychiatric or neurologic disorders. All subject recruitment procedures and informed consents, including consent to share de-identified data, were approved by the Washington University Institutional Review Board.
Table 1.
Demographics and emotional measures of participants.
| Characteristic | Men (n = 462) | Women (n = 508) | p value* |
|---|---|---|---|
| Age, Years | 27.8 ± 3.6 | 29.5 ± 3.6 | 0.000 |
| Education, Years | 14.9 ± 1.7 | 15.0 ± 1.8 | 0.169 |
| Anger Affect | 47.9 ± 8.4 | 47.6 ± 7.7 | 0.408 |
| Fear Affect | 49.2 ± 7.8 | 51.0 ± 7.9 | 0.000 |
Two-sample t test for age and education; two-sample t test with age and years of education as covariates for Anger and Fear Affect score.
All participants were assessed with the NIH-Toolbox Emotion Measures – 18+ (i.e., > 18 years old) battery – which has been widely used to examine emotion function and dysfunction. The battery is a computer-adaptive test comprised of items from the PROMIS Anger Item Bank (22 items) and Fear Item Bank (29 items). Participants responded to each item with Never, Rarely, Sometimes, Often, Always, with a higher total score representing greater intensity of the emotion trait.
2.2. Behavioral, task for FMRI
Each participant completed two runs of an emotion processing task each with 6 blocks – 3 of negative face and 3 of neutral shape pictures – in a fixed order: neutral – negative – neutral – negative – neutral – negative. During the emotion blocks, a target face was presented at the top of the screen and subjects were instructed to select one of two faces presented at the bottom that matched the emotion of the target face. During the neutral blocks, a target ellipse was presented at the top and subjects were to select one of two ellipses at the bottom with matching orientation. Negative faces showed angry or fearful expressions. Each block started with a cue (3 s) to indicate the current task (face or shape), followed by the stimulus (2 s) and an inter-trial interval (1 s) and, with a total of 6 trials, lasting 21 s in duration. However, as noted earlier by the HCP, the last 3 trials were missing from the emotion task because of an E-prime script bug (Barch et al., 2013). The emotion task data were missing for 162 subjects. Subjects with head movements exceeding 2 mm in translation or 2° in rotation were removed. Further, we inspected each individual’s normalized image and removed those visually deemed of poor quality (e.g., odd brain shape). As a result, a total of 970 out of 1206 subjects were included in the current study.
2.3. Imaging protocol and data preprocessing
MRI was done using a customized 3 T Siemens Connectome Skyra with a standard 32-channel Siemens receiver head coil and a body transmission coil. T1-weighted high-resolution structural images were acquired using a 3D MPRAGE sequence with 0.7 mm isotropic resolution (FOV = 224 mm, matrix = 320, 256 sagittal slices, TR = 2400 ms, TE = 2.14 ms, TI = 1000 ms, FA = 8°) and used to register functional MRI data to a standard brain space. FMRI data were collected using gradient-echo echo-planar imaging (EPI) with 2.0 mm isotropic resolution (FOV = 208 × 180 mm, matrix = 104 × 90, 72 slices, TR = 720 ms, TE = 33.1 ms, FA = 52°, multi-band factor = 8, 176 frames, ~ 2 m and 16 s/run).
As with our recent study of the HCP data (Li et al., 2020), imaging data were analyzed with Statistical Parametric Mapping (SPM8, Welcome Department of Imaging Neuroscience, University College London, U.K.). Standard image preprocessing was performed. Images of each individual subject were first realigned (motion corrected). A mean functional image volume was constructed for each subject per run from the realigned image volumes. These mean images were co-registered with the high-resolution structural MPRAGE image and then segmented for normalization with affine registration followed by nonlinear transformation. The normalization parameters determined for the structural volume were then applied to the corresponding functional image volumes for each subject. Finally, the images were smoothed with a Gaussian kernel of 4 mm at Full Width at Half Maximum.
2.4. Imaging data modeling
We modeled the BOLD signals to identify regional brain responses to negative vs. neutral stimuli. A statistical analytical block design was constructed for each individual subject, using a general linear model (GLM), by convolving the canonical hemodynamic response function (HRF) with a boxcar function in SPM. Realignment parameters in all six dimensions were entered in the model as covariates. The GLM estimated the component of variance that could be explained by each of the regressors.
In the first-level analysis, we constructed for individual subjects a statistical contrast of negative face versus neutral picture. These contrasts allowed us to evaluate brain regions that responded differently to viewing of negative face and neutral pictures. The contrast images (difference in β) of the first-level analysis were then used for the second-level group statistics. We conducted a one-sample t-test of the contrast (negative face vs. neutral shape) to identify regional responses to emotion processing and a two-sample t-test of the contrast (negative face minus neutral shape) to evaluate the difference between males and females with age and years of education as covariates. To examine how regional brain responses to emotion processing varied with individual emotion traits, we conducted a whole-brain multiple regression on the contrast (negative face minus neutral shape) each against the Anger-affect and Fear-affect score, with age and years of education as covariates. Following current reporting standards (Poldrack et al., 2008), all imaging results were evaluated with voxel p < 0.001, uncorrected, in combination with a cluster p < 0.05, corrected for family-wise error (FWE) of multiple comparisons, on the basis of Gaussian random field theory, as implemented in SPM.
In ROI analysis, we used MarsBar (http://marsbar.sourceforge.net/) to derive for each individual subject the activity (β contrast averaged across voxels) for the ROIs. Functional ROIs were defined based on clusters obtained from whole-brain analysis. All voxel activations were reported in Montreal Neurological Institute (MNI) coordinates.
In planned and post-hoc ROI analyses, we performed linear regressions to examine the relationship between ROI activities and performance measures as well as between ROI activities and Anger-/Fear-Affect scores. In the regressions for the entire sample, we included age, sex, and years of education as covariates. In the regressions for men and women separately, we included age and years of education as covariates. Where a significant finding was identified for men or women, we tested sex differences directly with a slope test (Zar, 1999) and showed two-tailed p values. As one has to set a threshold to identify the functional ROIs and the voxels of an ROI identified in say men may have a high β that just misses the cutoff in women, the sex differences in the correlations need to be tested directly.
2.5. Mediation analysis
As shown in the Results, we observed significant correlations, pair-wise between emotion traits, performance measures and RSC activities across men and women. Thus, we conducted mediation analyses to explore the inter-relationship between these measures, as with our previous studies (Le et al., 2019; Wang et al., 2020; Zhornitsky et al., 2019).
In a mediation analysis, the relation between the independent variable X and dependent variable Y, i.e. X → Y, is tested to see if it is significantly mediated by a variable M. The mediation test is performed by employing three regression equations (MacKinnon et al., 2007):
where a represents X → M, b represents M → Y (controlling for X), c’ represents X → Y (controlling for M), and c represents X → Y. The constants i1, i 2, i3 are the intercepts, and e1, e2, e3 are the residual errors. In the literature, a, b, c and c’ were referred as path coefficients or simply paths (MacKinnon et al., 2007; Wager et al., 2008), and we followed this notation. Variable M is said to be a mediator of the correlation X → Y if (c –c’), which is mathematically equivalent to the product of the paths a*b, is significantly different from zero (MacKinnon et al., 2007). If the product a* b and the paths a and b are significant, one concludes that X → Y is mediated by M. In addition, if path c’ is not significant, there is no direct connection from X to Y and that X → Y is completely mediated by M. Note that path b is the relation between Y and M, controlling for X, and should not be confused with the correlation coefficient between Y and M.
3. Results
3.1. Behavioral results and emotion traits
Fig. 1 shows the accuracy rate (AR) and reaction time (RT) across conditions. For AR, an ANOVA (sex × stimulus) showed a significant stimulus main effect (F = 22.83, p < 0.001) and sex × stimulus interaction (F = 5.40, p = 0.020), but not sex main effect (F = 3.52, p = 0.061). In post-hoc analyses both men’s and women’s AR was higher for the face than shape stimuli (p’s < 0.001) but the difference was larger in men than in women (Fig. 1(A)).
Fig. 1.
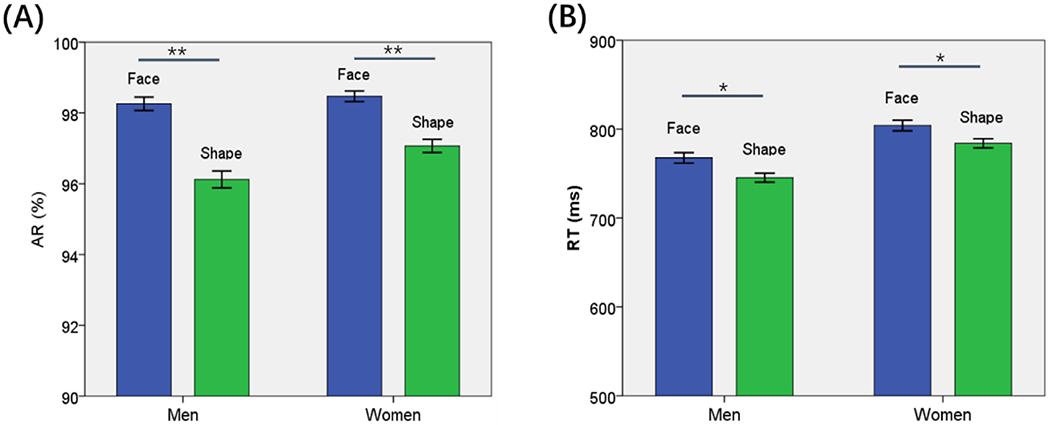
Behavioral results (mean ± SE) showed the (A) accuracy rate (AR) and (B) reaction time (RT) for face and shape stimuli each in men and women. AR was significantly higher for face than shape stimulus in both men and women but the difference was larger for men. Face trials showed slower RT than shape trials in both men and women. Women showed slower RT than men in both face and shape trials. **p < 0.001, *p < 0.05.
For RT, an ANOVA (sex × stimulus) showed a significant main effect of stimulus (F = 9.50, p = 0.002) and sex (F = 19.10, p < 0.001) but not sex × stimulus interaction (F = 0.81, p = 0.361). Women relative to men showed slower RT (p < 0.001). Both men (p = 0.004) and women (p = 0.011) showed slower RT during face than shape trials (Fig. 1(B)).
Together, these behavioral findings suggest that participants used more time but performed more accurately during face than during shape identification. However, across subjects, the difference in AR during face vs. shape identification (ARF-S) showed a negative correlation with RTF-S in both men (r = −0.194, p < 0.001) and women (r = −0.298, p < 0.001), suggesting that participants who were better in identifying face than shape stimuli also responded faster to face than to shape stimuli.
We next examined the relationship between personality traits and task performance with linear regressions of ARF-S and RTF-S against Anger-Affect and Fear-Affect score. We performed the analyses for men and women together (with age, sex, and years of education as covariates) as well as separately (with age and education as covariates). In men and women combined, the RTF-S was positively correlated with Anger-Affect score (r = 0.080, p = 0.013), and the ARF-S was negatively correlated with Fear-Affect score (r = −0.068, p = 0.034). For men only, the RTF-S was positively correlated with Anger-Affect score (r = 0.128, p = 0.006) and with Fear-Affect score (r = 0.118, p = 0.011). However, slopes tests failed to confirm sex differences in the latter two regressions (both p’s > 0.05). No other regressions showed significant results.
3.2. Brain activations to face vs. shape stimuli
In examining regional responses to negative face versus neutral shape pictures, we first conducted a one-sample t test on the entire cohort and on men and women separately. Supplementary Fig. S1 shows the results. To examine sex differences, we conducted a two-sample t test to compare men and women with age, years of education as covariates. Identifying negative emotional faces as compared with neutral shape pictures engaged higher activation of cortical and subcortical structures in men than women. At voxel p < 0.001, uncorrected, in combination with cluster-level p < 0.05, family-wise error corrected, these clusters included the fusiform gyrus, calcarine sulcus, middle frontal cortex, and both the anterior and posterior cingulate cortex. Conversely, women engaged higher activation in the middle temporal cortex and middle frontal cortex (Fig. 2). These clusters are summarized in Table 2. An additional analysis to compare men and women with age, years of education, as well as Anger-Affect and Fear-Affect scores as covariates yielded nearly identical findings (Supplementary Fig. S2).
Fig. 2.
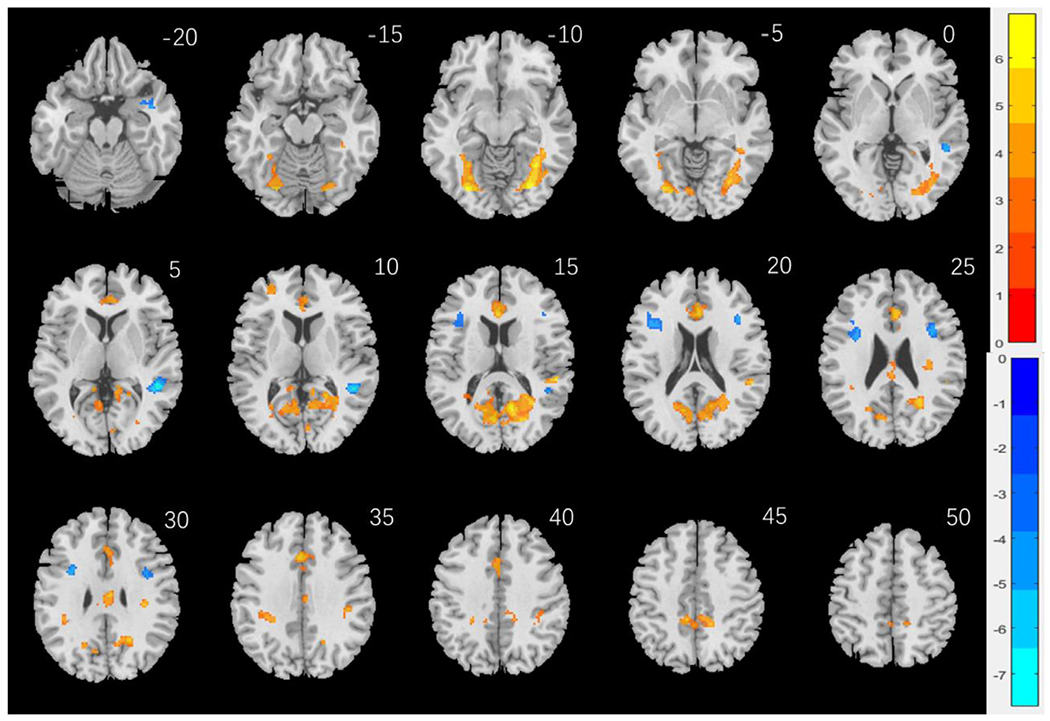
Sex differences in regional brain activations: Two-sample T test results of the contrast (face-shape) between men and women with age, years of education as covariates. Voxel p < 0.001, uncorrected. All clusters with cluster p < 0.05, corrected for family-wise error, are shown in Table 2. Color bars showed voxel T values; warm: men > women, cool: women > men. Clusters are overlaid on a T1 structural image in neurological orientation: right = right.
Table 2.
Regional activations in two-sample T-test of men vs. women.
| Region | Cluster size | Peak Voxel (Z) | Cluster FWE | MNI coordinate(mm) | ||
|---|---|---|---|---|---|---|
| P-value | X | Y | Z | |||
| Men > Women | ||||||
| Fusiform_L | 468 | 6.85 | 0.000 | −28 | −74 | −8 |
| Fusiform_R | 731 | 6.63 | 0.000 | 28 | −72 | −10 |
| Calcarine_R | 1591 | 6.15 | 0.000 | 24 | −70 | 14 |
| Cingulum_Ant_R | 588 | 6.00 | 0.000 | 4 | 28 | 24 |
| Temporal_Sup_R | 76 | 5.97 | 0.005 | 52 | −34 | 16 |
| Cingulum_Mid_R | 147 | 5.48 | 0.000 | 4 | −16 | 32 |
| Cingulum_Mid_L | 238 | 5.31 | 0.000 | −10 | −38 | 44 |
| Frontal_Mid_L | 50 | 4.70 | 0.014 | −30 | 50 | 12 |
| Precuneus_L | 37 | 4.02 | 0.038 | −10 | −44 | 14 |
| Women > Men | ||||||
| Temporal_Mid_R | 229 | 7.61 | 0.000 | 48 | −40 | 4 |
| Temporal_Inf_R | 44 | 6.88 | 0.044 | 40 | 6 | −24 |
| Middle Frontal C* | 251 | 5.10 | 0.000 | −32 | 6 | 28 |
| Middle Frontal C* | 157 | 5.08 | 0.000 | 40 | 22 | 22 |
Note: Brain regions were identified by reference to the Automated Anatomic Labeling or AAL Atlas (Tzourio-Mazoyer et al., 2002). We referred to (Duvernoy, 2009) for coordinates (*) that were not identified by the AAL. R: right; L: left; C: cortex.
We derived for individual subjects the β contrast of individual clusters – men > women or women > men – each as a region of interest (ROI) for a linear regression against Anger- and Fear-Affect score in men and in women, with age and years of education as covariates. The results showed that, at a p value corrected for the total number of tests – 0.05/(13 ROIs × 2 sexes × 2 scores) = 0.00096, none of the regressions were significant and only 4 of 52 regressions showed an uncorrected p < 0.05. These results suggested that individual variation in emotion traits was not reflected in the sex differences in these regional activities
Likewise, we performed linear regressions of the β contrasts of these individual ROIs against the ARF-S as well as RTF-S in men and women, separately, with age and years of education as covariates. Of the ROIs, the β contrast of the right middle frontal cortex (x=40, y=22, z=22, from the contrast women > men) showed a significant correlation with ARF-S in both men (r = −0.181, p = 0.000098) and women (r = −0.186, p = 0.000025), as well as with RTF-S in both men (r = 0.161, p = 0.000521) and women (r = 0.300, p = 5.254e–12) at the corrected threshold. We thus examined these two correlations with slope tests, which showed sex differences in the correlation with RTF-S (Z = −2.28, p = 0.0226) but not with ARF-S (Z = 0.08, p = 0.9362). Relative to men, women showed a stronger correlation of right middle frontal cortical activity with RTF-S during face vs. shape identification. These regressions are shown in Fig. 3.
Fig. 3.
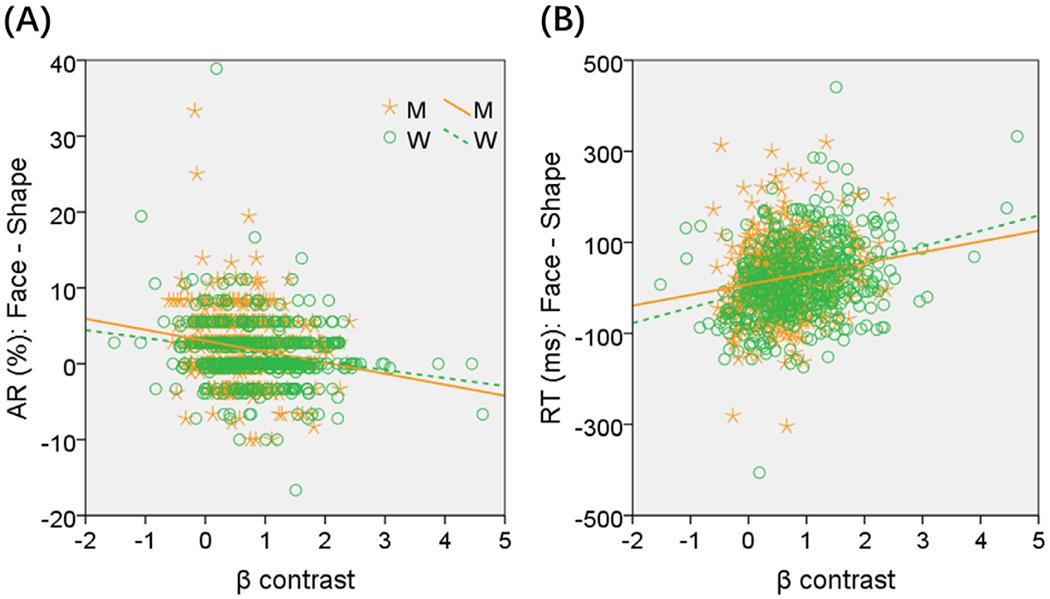
Correlations across men (M, orange) and women (W, green) between the β contrast of the right middle frontal cortex and (A) difference in accuracy, and (B) difference in mean RT (ms), during face vs. shape blocks. Slope tests (two-tailed, with years of age and education as covariates) showed sex differences in the correlations in (B): Z = −2.28, p = 0.0226.
3.3. Brain activations to face vs. shape stimuli in correlation with affect traits
We conducted a whole brain linear regression of contrast (face – shape) against the Anger-Affect and Fear-Affect scores across all subjects, with sex, age and years of education as covariates. No clusters met the threshold of voxel p < 0.001, uncorrected, in combination with cluster p < 0.05 FWE-corrected.
We next conducted a whole brain linear regression of contrast (face – shape) against the Anger-Affect and Fear-Affect scores for men and women separately, with age and years of education as covariates. For men, the left retrosplenial cortex (RSC; x = −8, y = −52, z = 16, volume = 472 mm3, Z = 4.46) showed activation in negative correlation with Anger-Affect score (Fig. 4(A)). Also for men, the right RSC (x = 8, y = −46, z = 18, volume = 728 mm3, Z = 4.40) and precuneus (x = 10, y = −56, z = 42, volume = 512 mm3, Z = 4.15), and left cerebellum (x = −14, y = −52, z = −24, volume = 656 mm3, Z = 4.47) showed activation in negative correlation with Fear-Affect score (Fig. 4(B)). No clusters showed responses in significant correlation with the Anger- or Fear-Affect score in women, at the same threshold.
Fig. 4.
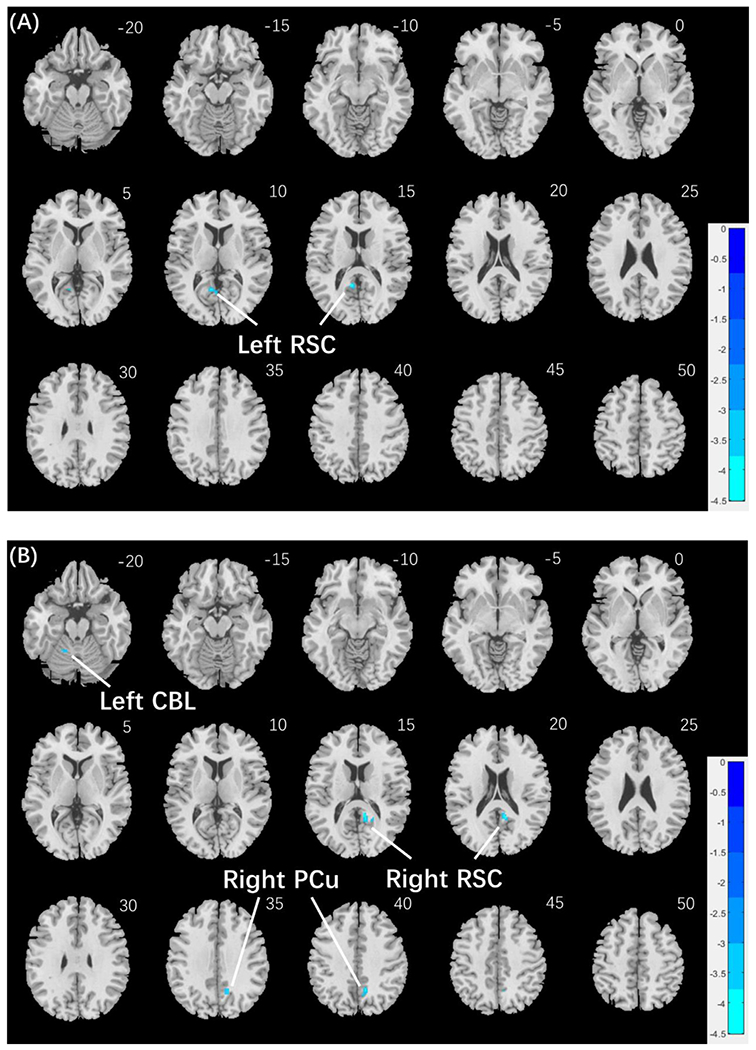
Regional responses to negative facial emotion vs. shape in correlation with emotion traits in men: whole-brain regression with age and years of education as covariates; p < 0.001, uncorrected, in combination with cluster p < 0.05 FWE-corrected. (A) The left retrosplenial cortex (RSC) showed response in negative correlation with Anger-Affect score in men. (B) The right RSC, right precuneus (PCu) and left cerebellum (CBL) showed responses in negative correlation with Fear-Affect score in men. Cold bars show voxel T values. No clusters showed significant correlations in women at the same threshold.
As men but not women showed regional responses in correlation with emotion traits, we computed the β contrast of each ROI – left RSC, right RSC, right precuneus, and left cerebellum – for all subjects, and compared men and women in the linear regressions with slope tests, in order to confirm the sex differences. The results showed left RSC responses in significant negative correlation with Anger-Affect score in men (r = −0.215, p < 0.001), as expected, but not in women (r = 0.047, p = 0.290). The sex difference was confirmed in a slope test (Z = −4.12, p < 0.001; Fig. 5A). Likewise, the right RSC (men: r = −0.238, p < 0.001; women: r = 0.053, p = 0.232), right precuneus (men: r = −0.202, p < 0.001; women: r = −0.022, p = 0.618), and left cerebellum (men: r = −0.266, p < 0.001; women: r = −0.016, p = 0.170) showed responses in significant negative correlation with the Fear-Affect score in men but not in women. The sex differences (right RSC: Z = −4.59, p < 0.001; right precuneus: Z = −2.83, p = 0.0047; left cerebellum: Z = −3.28, p = 0.001) were all confirmed in slope tests (Fig. 5(B)–(D)). Notably, compared to women, men also showed higher β contrast values for the left RSC (men: 0.16 ± 0.90; women: −0.11 ± 0.95; p < 0.001) and right RSC (men: 0.10 ± 0.69; women: −0.06 ± 0.70; p = 0.001) but not for the right PCu or left CBL (both p’s >0.662; two-sample t test with age and years of education as covariates).
Fig. 5.
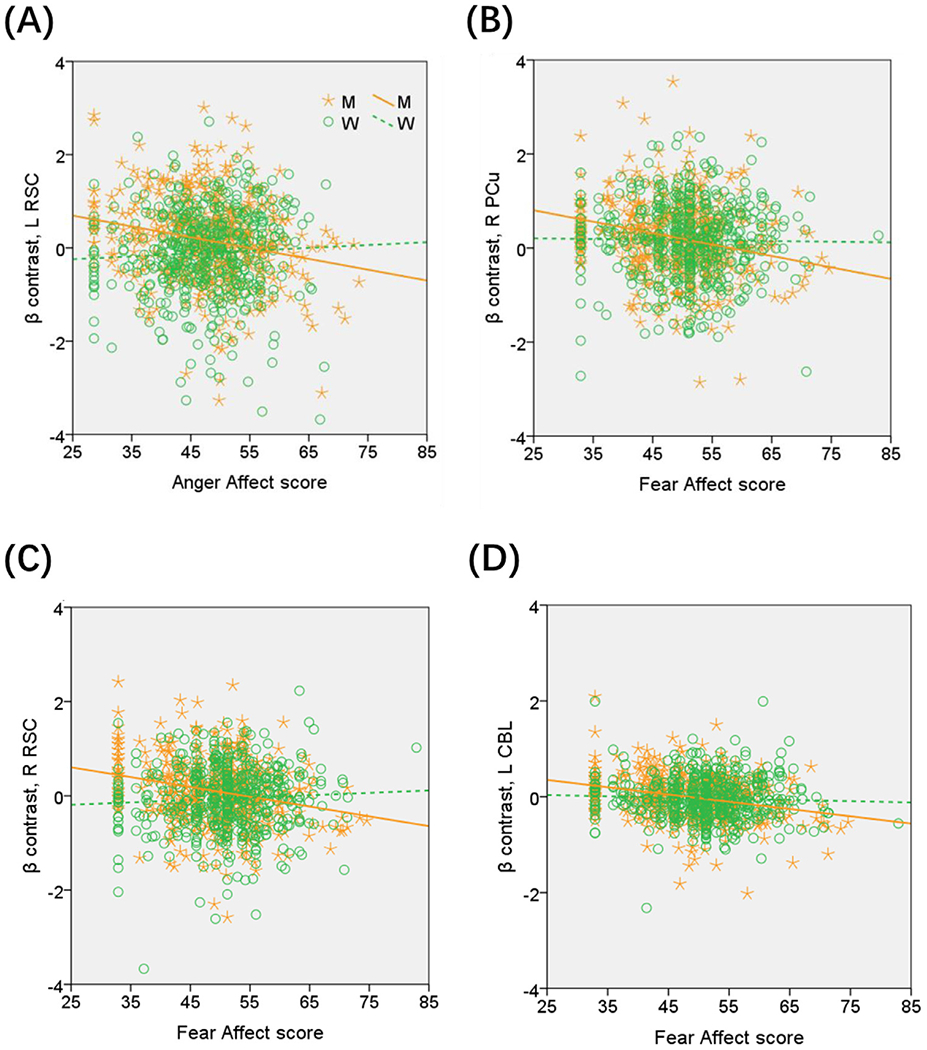
Region of interest analyses to confirm sex differences in the correlation of regional responses with emotion traits. Each data point represents one subject. Orange/green color: men (M)/women (W). The plots show sex differences in the regressions of the β contrast of (A) Left retrosplenial cortex (RSC) vs. Anger-Affect score (Z = −4.12, p < 0.001); (B) Right precuneus (PCu) vs. Fear-Affect score (Z = −2.83, p = 0.0047); (C) Right RSC vs. Fear-Affect score (Z = −4.59, p < 0.001); and (D) cerebellum (CBL) vs. Fear-Affect score (Z = −3.28, p = 0.001), all based on slope tests.
As described in Section 3.1, the RTF-S was positively correlated with Anger-Affect score and the ARF-S was negatively correlated with Fear-Affect score in men and women combined. Thus, although the left and right RSC were identified from the whole-brain regressions each against Anger- and Fear-Affect score in men, we explored whether the RSC responses were correlated with these performance measures across men and women. Across all subjects, the left RSC response was negatively correlated with RTF-S (r = −0.078, p = 0.015), and the right RSC response was positively correlated with ARF-S (r = 0.155, p < 0.001).
3.4. Mediation analyses
In men and women combined, we observed a significant correlation between Anger-Affect score and RTF-S (r = 0.080, p = 0.013), between the activity (face – shape) of the left RSC and RTF-S (r = −0.078, p = 0.015), and between the activity of the left RSC and Anger-Affect score (r = −0.075, p = 0.020). Thus, we conducted a mediation analysis to examine the inter-relationship between left RSC activity (β contrast), Anger-Affect score, and RTF-S. None of the six models showed significant mediation (all p’s > 0.125).
In men and women combined, we observed a significant correlation between Fear-Affect score and ARF-S (r = −0.068, p = 0.034), between the activity (face – shape) of the right RSC and ARF-S (r = 0.155, p < 0.001), and between the activity (face – shape) of the right RSC and Fear-Affect score (r = −0.095, p = 0.003). Thus, we conducted a mediation analysis to examine the inter-relationship between right RSC activity (β contrast), Fear-Affect score, and ARF-S.
Of the six possible models, we considered all except the two where ARF-S served as the independent variable, because the AR represents a behavioral outcome and, conceptually, is unlikely to drive brain response. Table 3 shows that only the model where right RSC β contrast mediates the relationship “Fear-Affect score → ARF-S ” demonstrated a significant and complete mediation effect. The two models not considered did not show significant mediation, either (both p’s > 0.069).
Table 3.
Mediation analysis: fear-affect, right RSC activity, and accuracy rate.
| Path a (X→M) | Path b (M→Y) | Path c (X→Y) | Path c’ (X→Y) | Mediation Path (c-c’) | |
|---|---|---|---|---|---|
| Model 1: X (Fear)→Y (R_RSC) mediated by M (Accuracy) | |||||
| β | 0.000 | 2.469 | −0.008 | −0.007 | −0.001 |
| p | 0.044 | 0.000 | 0.005 | 0.012 | 0.069 |
| Model 2: X (Fear)→Y (Accuracy) mediated by M (R_RSC) | |||||
| β | −0.008 | 0.009 | 0.000 | 0.000 | 0.000 |
| p | 0.005 | 0.000 | 0.044 | 0.110 | 0.023* |
| Model 3: X (R_RSC)→Y (Fear) mediated by M (Accuracy) | |||||
| β | 0.009 | −10.304 | −1.077 | −0.980 | − 0.096 |
| p | 0.000 | 0.108 | 0.007 | 0.015 | 0.131 |
| Model 4: X (R_RSC)→Y(Accuracy) mediated by M (Fear) | |||||
| β | −1.077 | 0.000 | 0.009 | 0.009 | 0.000 |
| p | 0.007 | 0.110 | 0.000 | 0.000 | 0.186 |
Significant mediation.
4. Discussion
We examined sex differences in regional brain responses during identification of negative emotional face versus neutral shape stimuli. Men relative to women showed higher activations in bilateral occipital, fusiform and cingulate gyri, retrosplenial cortex (RSC), and precuneus. Conversely, women as compared to men showed higher activations in bilateral middle frontal and right middle temporal cortex. The findings mirror those reported in many earlier imaging studies (Caseras et al., 2007; Domes et al., 2010; Garcia-Garcia et al., 2016; Kempton et al., 2009; Mak et al., 2009). In regional brain responses, men showed more significant modulations by anger and fear traits, relative to women. The left RSC and right RSC/precuneus each demonstrated activities during face vs. shape identification in negative correlation with Anger- and Fear-Affect scores in men only. Anger affect was positively correlated with prolonged RT in identifying face vs. shape target in men but not women. In contrast, women relative to men showed higher Fear-Affect score and higher activation in the right middle frontal cortex, which was more strongly correlated with prolonged RT during face vs. shape identification. Lastly, in men and women combined, the right RSC response mediated the correlation between fear affect and differences in accuracy rate in identifying face vs. shape stimuli. Together, these findings provide new evidence of sex-specific and sex-shared neural phenotypes of emotion traits in relation to negative emotion processing.
4.1. Retrosplenial cortex, precuneus and emotion processing
Along with the amygdala, hippocampus and prefrontal cortex, the RSC, posterior cingulate cortex (PCC) and precuneus (PCu) have been implicated in emotional (particularly fear) learning and memory in both preclinical (Todd et al., 2019; Vogt, 2019; Yousuf et al., 2020) and clinical (Holschneider et al., 2014; Kveraga et al., 2015; Van den Stock et al., 2014; Zhan et al., 2018) studies. For instance, fear induction with exposure to video clips elicited higher activity in bilateral anterior insula and right hemispheric parahippocampal gyrus (PHG), PCC, and PCu (Zhan et al., 2018). Evaluating negative emotional vs. non-emotional statements engaged higher activation of the PCC and PCu (Bruneau and Saxe, 2010). An earlier study showed higher responses of the PCu to fearful versus disgusted face pictures (Stark et al., 2007). Another fMRI study showed higher activation in bilateral extrastriate areas and right-hemispheric posterior PHG and lower activity in the right RSC during exposure to threating as compared to neutral visual scenes (Van den Stock et al., 2014). We replicated these findings in the current study (Supplementary Figure S1). Further, we showed that the right RSC and PCu respond to emotion vs. shape stimuli in negative correlation with fear trait in men, thus extending the literature by highlighting the neural correlates of individual variation in fear emotions. It is possible that we did not observe a correlate in the hippocampus/PHG because emotion target identification did not engage the memory processes as extensively as imagery or exposure to a movie. Individual variation in fear emotion may manifest differently when individuals are challenged with different emotion tasks.
The RSC, including the PCC, and PCu also respond to anger or behavioral contingencies that may trigger anger. For instance, in the Ultimatum game, the left-hemispheric PCC and PCu showed higher activity when participants retaliated for unfair offers (Klimecki et al., 2018). In an emotion recognition task, individuals born pre-term were less able to recognize anger emotions at low intensity, in link with diminished amygdala connectivity with the PCC/PCu, compared to healthy adults (Papini et al., 2016). Patients with multiple sclerosis, a condition with white matter pathology that disrupts inter-regional connectivity, demonstrated excess activations in the PCC/PCu during recognition specifically of angry and disgusted (vs. neutral) faces, in contrast to healthy controls, potentially in compensation for disrupted anatomical connectivity (Jehna et al., 2011). Other studies demonstrated altered PCC/PCu connectivity in association with the expression of anger and/or aggression in patients with borderline personality disorder (Quattrini et al., 2019; Ueltzhoffer et al., 2019). In the current findings, the left RSC showed higher activation to negative emotional vs. shape stimuli (Figure 2), and the extent of this activity was negatively correlated with anger affect in men (Fig. 3). Higher Anger-Affect score was also correlated with prolonged RT in identifying face vs. shape target in men (Section 3.1 Behavioral Results). Thus, men prone to anger may dampen the RT and response the left RSC to negative emotions, perhaps as a protective mechanism to avoid engagement in negative emotion processing?
Together, although the RSC, including the PCC, and PCu appear to respond to a wide range of emotions (Saarimaki et al., 2018), the afore-described studies provide evidence in support of left- and right-hemispheric RSC in processing anger and fear, respectively, in broad consistence with the current findings. Notably, although fear and anger represents distinct emotions, fear may promote anger and alter behavioral responses to emotional challenges (Zhan et al., 2015). In the study described above, activation of the right-hemispheric PCC and PCu during fear induction predicted the escalation of fear to anger (Zhan et al., 2018). It also seems intriguing that we observed a significant positive correlation between anger (and fear, though less strongly) affect and the RT (face – shape) as well as between left RSC response and RT (face – shape) in men. One is tempted to speculate that, in men, RSC response to negative emotion represents a trait neural marker of fear and anger trait, which impedes the identification of negative emotions, perhaps to prevent the escalation of emotional stimulation.
4.2. Sex differences in emotion processing and the neural markers of emotion traits
Both men and women showed higher accuracy rate in identifying face vs. shape target stimuli; however, men demonstrated more significant difference in the accuracy rate than women, as shown in the interaction effect. In brain imaging, men relative to women also demonstrated more significant differences in regional responses to identification of face vs. shape stimuli, in accord with earlier reports. However, the differences in regional responses did not seem to account for sex differences in the accuracy rate. In contrast, women as compared to men showed higher activations in bilateral middle frontal and middle temporal cortex. Higher activations of the right middle frontal cortex were correlated with prolonged RT in identifying face vs. shape target more significantly in women than in men. These findings are correlational in nature, but one may speculate that higher activation of right middle frontal cortex, a region central to executive control, may reflect more complex processing of emotional face stimuli, in broad accord with higher Fear-Affect score in women.
Women showed higher Fear-Affect score, compared to men, consistent with earlier studies (Reichenberger et al., 2019). This sex difference could be considered with the finding that women showed lower accuracy in identifying face (vs. shape) stimuli and with the correlation between middle frontal cortical activation and prolonged RT in face (vs. shape) blocks, but was otherwise not reflected in individual variation in fear trait. In contrast, although men and women did not differ in anger affect, individual variation in anger trait was reflected in left RSC response and prolonged RT to face (vs. shape) stimuli in men only. Together, these findings suggest that anger and fear each dominates in behavioral responses to emotional targets, and engages distinct neural substrates during these responses, in men and in women, respectively.
4.3. RSC response underlies difficulty in emotion target identification in fear-prone individuals
We showed that, in men and women combined, right RSC response to face (vs. shape) mediated the correlation between fear affect and diminished accuracy in identifying face (vs. shape) stimuli. The RSC may play a distinct role in impeding emotion target identification in fear-prone healthy individuals. The RSC has been implicated in the pathology of post-traumatic stress disorder (PTSD), with individuals of PTSD demonstrating higher stress-elicited RSC activity in link with symptom severity (Cwik et al., 2017; Sartory et al., 2013). Thus, it would appear that fear-prone healthy individuals are able to down-regulate RSC response to face stimuli, limiting the exposure to negative emotions, whereas individuals with PTSD are devoid of this protective mechanism. Indeed, people suffering PTSD showed higher physiological arousal to negative emotional stimuli (Wolf et al., 2009) and faster response in identifying negative facial and other stress-eliciting stimuli (Ashley and Swick, 2019). Thus, other than reflecting individual variation in emotion processing function in neurotypical populations, RSC response to negative emotions may potentially serve as a sex-shared neural marker of the severity of PTSD.
4.4. Limitations and conclusions of the study
Some limitations need to be considered for the study. First, participants showed an accuracy rate > 95% in emotion or shape identification, suggesting that the behavioral task might not be sufficiently challenging. It remains unclear whether the neural markers of emotion traits may be identified for women with a more difficult target identification task. Further, the HCP emotion task did not separate fearful and angry facial emotions in different blocks; thus, we were not able to investigate the neural responses specific to these emotions. Second, exposure to salient stimuli elicits physiological arousal (Wang et al., 2019; Zhang et al., 2012), which would have provided an objective index of behavioral engagement, in addition to accuracy rate and RT. Further, the physiological response may vary with individual dispositional trait (Le et al., 2019; Panayiotou et al., 2017; Yoshino et al., 2005), providing another venue to assess emotion processing. Third, the participants recruited for the HCP are known to have a variety of subclinical conditions, and it remains to be investigated how these clinical variables may have influenced the current findings.
In conclusion, the current study demonstrated sex differences in regional responses to negative emotions and how individual emotion traits may relate to RSC and PCu response to negative emotions in a target identification task. Men relative to women demonstrated more significant modulation in behavioral performance and regional activities by both anger and fear traits. Across men and women, the RSC response impedes negative emotion processing in fear-prone healthy individuals, a protective mechanism that may go awry in emotional disorders.
Supplementary Material
Funding support and acknowledgement
The current study is supported by NIH grants MH113134, DA023248, DA045189, AG067024 and AA021449 (C-SRL) and a scholarship from the China Scholarship Council to GL to visit Yale University.
Footnotes
Declaration of Competing Interest
The authors declare that they have no competing interests.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2020.117171.
References
- Albert PR, 2015. Why is depression more prevalent in women? J. Psychiatry Neurosci 40, 219–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley V. Swick D. 2019. Angry and fearful face conflict effects in post-traumatic stress disorder. Front. Psychol 10, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA. Valentino RJ, 2014. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol 35, 303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM. Burgess GC. Harms MP. Petersen SE. Schlaggar BL. Corbetta M. Glasser MF. Curtiss S. Dixit S. Feldt C. Nolan D, Bryant E. Hartley T, Footer O. Bjork JM, Poldrack R. Smith S. Johansen-Berg H. Snyder AZ. Van Essen DC, Consortium WU-MH, 2013. Function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage 80, 169–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody LR, Lovas GS, Hay DH, 1995. Gender differences in anger and fear as a function of situational context. Sex Roles 32, 47–78. [Google Scholar]
- Bruneau EG, Saxe R, 2010. Attitudes towards the outgroup are predicted by activity in the precuneus in Arabs and Israelis. Neuroimage 52, 1704–1711. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Ramsey NF. Tallent K. Bertolino A, Knable MB. Coppola R. Goldberg T, Gelderen P.v., Mattay VS, Frank JA, Moonen CT, Weinberger DR, 1998. Functional magnetic resonance imaging brain mapping in psychiatry: methodological issues illustrated in a study of working memory in schizophrenia. Neuropsychopharmacology 18, 186–196. [DOI] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JDE, 2002. Sex differences in the neural basis of emotional memories. Proc. Natl. Acad. Sci. USA 99, 10789–10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caseras X. Mataix-Cols D. An SK. Lawrence NS. Speckens A. Giampietro V. Brammer MJ, Phillips ML, 2007. Sex differences in neural responses to disgusting visual stimuli: implications for disgust-related psychiatric disorders. Biol. Psychiatry 62, 464–471. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Jacobs LF, 2009. Sex differences in directional cue use in a virtual landscape. Behav. Neurosci 123, 276–283. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Peristein WM, S.Braver T, Nystrom LE, C.Noll D, Jonides J, Smith EE, 1997. Temporal dynamics of brain activation during a working memory task. Nature 386, 604–608. [DOI] [PubMed] [Google Scholar]
- Colich NL, Williams ES, Ho TC, King LS, Humphreys KL, Price AN, Ordaz SJ, Gotlib IH, 2017. The association between early life stress and prefrontal cortex activation during implicit emotion regulation is moderated by sex in early adolescence. Dev. Psychopathol 29, 1851–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cwik JC, Sartory G, Nuyken M, Schurholt B, Seitz RJ, 2017. Posterior and prefrontal contributions to the development posttraumatic stress disorder symptom severity: an fMRI study of symptom provocation in acute stress disorder. Eur. Arch. Psychiatry Clin. Neurosci 267, 495–505. [DOI] [PubMed] [Google Scholar]
- Dan R, Canetti L, Keadan T, Segman R, Weinstock M, Bonne O, Reuveni I, Goelman G, 2019. Sex differences during emotion processing are dependent on the menstrual cycle phase. Psychoneuroendocrinology 100, 85–95. [DOI] [PubMed] [Google Scholar]
- Deng Y. Chang L. Yang M. Huo M. Zhou R. 2016. Gender differences i n emotional response: inconsistency between experience and expressivity. PLoS One 11, e0158666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Schulze L, Bottger M, Grossmann A, Hauenstein K, Wirtz PH, Heinrichs M, Herpertz SC, 2010. The neural correlates of sex differences in emotional reactivity and emotion regulation. Hum. Brain Mapp 31, 758–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM, 2009. The Human Brain, Second Edition Springer-Verlag, Wien/New York. [Google Scholar]
- Garcia-Garcia I, Kube J, Gaebler M, Horstmann A, Villringer A, Neumann J, 2016. Neural processing of negative emotional stimuli and the influence of age, sex and task-related characteristics. Neurosci. Biobehav. Rev 68, 773–793. [DOI] [PubMed] [Google Scholar]
- Gevins A, Morgan N, Bressler S, Cutillo B, White R, illes J, Greer D, Doyle J, Zeitlin G, 1987. Human neuroelectric patterns predict performance accuracy. Science 30, 580–585. [DOI] [PubMed] [Google Scholar]
- Habib M, Cassotti M, Moutier S, Houde O, Borst G, 2015. Fear and anger have opposite effects on risk seeking in the gain frame. Front. Psychol 6, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Liu Z, Wang J, Zhang D, 2018. Gender differences in processing fearful and angry body expressions. Front. Behav. Neurosci 12, 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel S, Metzger FG, Ehlis AC, Korell R, Alboji A, Haeussinger FB, Hagen K, Maetzler W, Eschweiler GW, Berg D, Fallgatter AJ, Consortium TS, 2013. Aging-related cortical reorganization of verbal fluency processing: a functional near-infrared spectroscopy study. Neurobiol. Aging 34, 439–450. [DOI] [PubMed] [Google Scholar]
- Hirnstein M, Hugdahl K, Hausmann M, 2019. Cognitive sex differences and hemispheric asymmetry: a critical review of 40 years of research. Laterality 24, 204–252. [DOI] [PubMed] [Google Scholar]
- Holschneider DP, Wang Z, Pang RD, 2014. Functional connectivity-based parcellation and connectome of cortical midline structures in the mouse: a perfusion autoradiography study. Front. Neuroinf 8, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehna M, Langkammer C, Wallner-Blazek M, Neuper C, Loitfelder M, Ropele S, Fuchs S, Khalil M, Pluta-Fuerst A, Fazekas F, Enzinger C, 2011. Cognitively preserved MS patients demonstrate functional differences in processing neutral and emotional faces. Brain Imaging Behav. 5, 241–251. [DOI] [PubMed] [Google Scholar]
- Kelly MM, Tyrka AR, Price LH, Carpenter LL, 2008. Sex differences in the use of coping strategies: predictors of anxiety and depressive symptoms. Depress Anxiety 25, 839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton MJ, Haldane M, Jogia J, Christodoulou T, Powell J, Collier D, Williams SC, Frangou S, 2009. The effects of gender and COMT Val158Met polymorphism on fearful facial affect recognition: a fMRI study. Int. J. Neuropsychopharmacol 12, 371–381. [DOI] [PubMed] [Google Scholar]
- Kim-Spoon J, Holmes C, Deater-Deckard K, 2015. Attention regulates anger and fear to predict changes in adolescent risk-taking behaviors. J. Child Psychol. Psychiatry 56, 756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinner VL, Het S, Wolf OT, 2014. Emotion regulation: exploring the impact of stress and sex. Front. Behav. Neurosci 8, 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimecki OM, Sander D, Vuilleumier P, 2018. Distinct brain areas involved in anger versus punishment during social interactions. Sci. Rep 8, 10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogler L, Gur RC, Derntl B, 2015. Sex differences in cognitive regulation of psychosocial achievement stress: brain and behavior. Hum Brain Mapp 36, 1028–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kret ME, De Gelder B, 2012. A review on sex differences in processing emotional signals. Neuropsychologia 50, 1211–1221. [DOI] [PubMed] [Google Scholar]
- Kring AM, Gordon AH, 1998. Sex differences in emotion: expression, experience, and physiology. J. Persnaliw Saial Psychol 74, 686–703. [DOI] [PubMed] [Google Scholar]
- Kveraga K, Boshyan J, Adams RB Jr., Mote J, Betz N, Ward N, Hadjikhani N, Bar M, Barrett LF, 2015. If it bleeds, it leads: separating threat from mere negativity. Soc. Cogn. Affect. Neurosci 10, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TM, Wang W, Zhornitsky S, Dhingra I, Zhang S, Li CR, 2019. Reward sensitivity and electrodermal responses to actions and outcomes in a go/no-go task. PLoS One 14, e0219147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner JS, Keltner D, 2001. Fear, anger, and risk. J. Person. Soc. Psychol 81, 146–159. [DOI] [PubMed] [Google Scholar]
- Li C.-s.R., Huang C-Y, Lin W.-y., Sun C-WV, 2007. Gender differences in punishment and reward sensitivity in a sample of Taiwanese college students. Person. Individ. Diff 43, 475–483. [Google Scholar]
- Li CS, Zhang S, Duann JR, Yan P, Sinha R, Mazure CM, 2009. Gender differences in cognitive control: an extended investigation of the stop signal task. Brain Imaging Behav. 3, 262–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Singh M, 2014. Sex differences in cognitive impairment and Alzheimer’s disease. Front. Neuroendocrinol 35, 385–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Zhang S, Le TM, Tang X, Li C-SR, 2020. Neural responses to reward: sex differences and individual variation in reward-driven impulsivity. Cereb Cortex Comm. tgaa025, 10.1093/texcom/tgaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TM, Zhornitsky S, Wang W, Ide J, Zhang S, Li CR, 2019. Posterior cingulate cortical response to active avoidance mediates the relationship between punishment sensitivity and problem drinking. J Neurosci. 39 (32), 6354–6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS, 2007. Mediation analysis. Annu. Rev. Psychol 58, 593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak AK, Hu ZG, Zhang JX, Xiao Z, Lee TM, 2009. Sex-related differences in neural activity during emotion regulation. Neuropsychologia 47, 2900–2908. [DOI] [PubMed] [Google Scholar]
- Mantyla T, 2013. Gender differences in multitasking reflect spatial ability. Psychol. Sci 24, 514–520. [DOI] [PubMed] [Google Scholar]
- McCarrey AC, An Y, Kitner-Triolo MH, Ferrucci L, Resnick SM, 2016. Sex differences in cognitive trajectories in clinically normal older adults. Psychol. Aging 31, 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGivern RF, Adams B, Handa RJ, Pineda JA, 2012. Men and women exhibit a differential bias for processing movement versus objects. PLoS One 7, e32238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz CJ, Wolf OT, 2017. Sex differences in stress effects on emotional learning. J. Neurosci. Res 95, 93–105. [DOI] [PubMed] [Google Scholar]
- Miller DI, Halpern DF, 2014. The new science of cognitive sex differences. Trends Cogn. Sci 18, 37–45. [DOI] [PubMed] [Google Scholar]
- Montagne B, Kessels RPC, Frigerio E, Hann E.H.F.d., Perrett DI, 2005. Sex differences in the perception of affective facial expressions: Do men really lack emotional sensitivity? Cogn. Process 136–141. [DOI] [PubMed] [Google Scholar]
- Munro CA, Winicki JM, Schretlen DJ, Gower EW, Turano KA, Munoz B, Keay L, Bandeen-Roche K, West SK, 2012. Sex differences in cognition in healthy elderly individuals. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn 19, 759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayiotou G, Karekla M, Georgiou D, Constantinou E, Paraskeva-Siamata M, 2017. Psychophysiological and self-reported reactivity associated with social anxiety and public speaking fear symptoms: Effects of fear versus distress. Psychiatry Res. 255, 278–286. [DOI] [PubMed] [Google Scholar]
- Papini C, White TP, Montagna A, Brittain PJ, Froudist-Walsh S, Kroll J, Karolis V, Simonelli A, Williams SC, Murray RM, Nosarti C, 2016. Altered resting-state functional connectivity in emotion-processing brain regions in adults who were born very preterm. Psychol. Med 46, 3025–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters M, Laeng B, Latham K, Jackson M, Zaiyouna R, Richardson C, 1995. A redrawn Vandenberg and Kuse mental rotations test: different versions and factors that affect performance. Brain Cogn. 28, 39–58. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Fletcher PC, Henson RN, Worsley KJ, Brett M, Nichols TE, 2008. Guidelines for reporting an fMRI study. Neuroimage 40, 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrini G, Pini L, Pievani M, Magni LR, Lanfredi M, Ferrari C, Boccardi M, Bignotti S, Magnaldi S, Cobelli M, Rillosi L, Beneduce R, Rossi G, Frisoni GB, Rossi R, 2019. Abnormalities in functional connectivity in borderline personality disorder: correlations with metacognition and emotion dysregulation. Psychiatry Res. Neuroimaging 283, 118–124. [DOI] [PubMed] [Google Scholar]
- Reed JL, Gallagher NM, Sullivan M, Callicott JH, Green AE, 2017. Sex differences in verbal working memory performance emerge at very high loads of common neuroimaging tasks. Brain Cogn. 113, 56–64. [DOI] [PubMed] [Google Scholar]
- Reichenberger J, Pfaller M, Forster D, Gerczuk J, Shiban Y, Muhlberger A, 2019. Men scare me more: gender differences in social fear conditioning in virtual reality. Front. Psychol 10, 1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repple J, Habel U, Wagels L, Pawliczek CM, Schneider F, Kohn N, 2018. Sex differences in the neural correlates of aggression. Brain Struct Funct. 223, 4115–4124. [DOI] [PubMed] [Google Scholar]
- Ritchie SJ, Cox SR, Shen X, Lombardo MV, Reus LM, Alloza C, Harris MA, Alderson HL, Hunter S, Neilson E, Liewald DCM, Auyeung B, Whalley HC, Lawrie SM, Gale CR, Bastin ME, McIntosh AM, Deary IJ, 2018. Sex differences in the adult human brain: evidence from 5216 UK biobank participants. Cereb. Cortex 28, 2959–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ, 2019. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology 44, 111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarimaki H, Ejtehadian LF, Glerean E, Jaaskelainen IP, Vuilleumier P, Sams M, Nummenmaa L, 2018. Distributed affective space represents multiple emotion categories across the human brain. Soc. Cogn. Affect. Neurosci 13, 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salsman JM, Butt Z, Pilkonis PA, Cyranowski JM, Zill N, Hendrie HC, Kupst MJ, Kelly MAR, Bode RK, Choi SW, Lai J-S, Griffith JW, Stoney CM, Brouwers P, Knox SS, Cella D, 2013. Emotion assessment using the NIH Toolbox. Am. Acad. Neurol 80, S76–S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartory G, Cwik J, Knuppertz H, Schurholt B, Lebens M, Seitz RJ, Schulze R, 2013. In search of the trauma memory: a meta-analysis of functional neuroimaging studies of symptom provocation in posttraumatic stress disorder (PTSD). PLoS One 8, e58150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylik R, Raman E, Szameitat AJ, 2018. Sex differences in emotion recognition and working memory tasks. Front. Psychol 9, 1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R, Zimmermann M, Kagerer S, Schienle A, Walter B, Weygandt M, Vaitl D, 2007. Hemodynamic brain correlates of disgust and fear ratings. Neuroimage 37, 663–673. [DOI] [PubMed] [Google Scholar]
- Sternberg S, 1966. High-speed scanning in human memory. Science 153, 652–654. [DOI] [PubMed] [Google Scholar]
- Todd TP, Fournier DI, Bucci DJ, 2019. Retrosplenial cortex and its role in cue-specific learning and memory. Neurosci. Biobehav. Rev 107, 713–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrubia R, Ávila C, Moltó J, Caseras X, 2001. The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Person. Individ. Diff 31, 837–862. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M, 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. [DOI] [PubMed] [Google Scholar]
- Ueltzhoffer K, Herpertz SC, Krauch M, Schmahl C, Bertsch K, 2019. Whole-brain functional connectivity during script-driven aggression in borderline personality disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 93, 46–54. [DOI] [PubMed] [Google Scholar]
- Van den Stock J, Vandenbulcke M, Sinke CB, de Gelder B, 2014. Affective scenes influence fear perception of individual body expressions. Hum. Brain Mapp 35, 492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg SG, Kuse AR, 1978. Mental rotations, a group test of three-dimensional spatial visualization. Perceptual. Motor Skills 47, 599–604. [DOI] [PubMed] [Google Scholar]
- Vogt BA, 2019. The cingulate cortex in neurologic diseases: history, structure, overview. Handb. Clin. Neurol 166, 3–21. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN, 2008. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 59, 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhornitsky S, Le TM, Dhingra I, Zhang S, Krystal JH, Li CR, 2019. Cue-elicited craving, thalamic activity, and physiological arousal in adult non-dependent drinkers. J. Psychiatr. Res 116, 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhornitsky S, Le TM, Zhang S, Li CR, 2020. Heart rate variability, cue-evoked ventromedial prefrontal cortical response, and problem alcohol use in adult drinkers. Biol Psychiatry Cogn Neurosci Neuroimaging 5 (6), 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Yucel M, Yap MB, Allen NB, 2011. Sex differences i n the neural correlates of emotion: evidence from neuroimaging. Biol. Psychol 87, 319–333. [DOI] [PubMed] [Google Scholar]
- Wolf EJ, Miller MW, McKinney AE, 2009. Emotional processing in PTSD: heightened negative emotionality to unpleasant photographic stimuli. J. Nerv. Ment. Dis 197, 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Zhao D, Wu Y, Tang P, Gu R, Luo YJ, 2018. Differentiating the influence of incidental anger and fear on risk decision-making. Physiol. Behav 184, 179–188. [DOI] [PubMed] [Google Scholar]
- Yoshino A, Kimura Y, Yoshida T, Takahashi Y, Nomura S, 2005. Relationships between temperament dimensions in personality and unconscious emotional responses. Biol. Psychiatry 57, 1–6. [DOI] [PubMed] [Google Scholar]
- Yousuf H, Ehlers VL, Sehgal M, Song C, Moyer JR Jr., 2020. Modulation of intrinsic excitability as a function of learning within the fear conditioning circuit. Neurobiol. Learn. Mem 167, 107132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH, 1999. Biostatistical Analysis, 4th ed Prentice-Hall, Inc., New Jersey. [Google Scholar]
- Zhan J, Ren J, Fan J, Luo J, 2015. Distinctive effects of fear and sadness induction on anger and aggressive behavior. Front. Psychol 6, 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan J, Ren J, Sun P, Fan J, Liu C, Luo J, 2018. The neural basis of fear promotes anger and sadness counteracts anger. Neural. Plast 2018, 3479059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Hu S, Chao HH, Luo X, Farr OM, Li CS, 2012. Cerebral correlates of skin conductance responses in a cognitive task. Neuroimage 62, 1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhornitsky S, Zhang S, Ide JS, Chao HH, Wang W, Le TM, Leeman RF, Bi J, Krystal JH, Li CR, 2019. Alcohol expectancy and cerebral responses to cue-elicited craving in adult nondependent drinkers. Biol Psychiatry Cogn Neurosci Neuroimaging 4 (5), 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


