Abstract
Background:
Early infant diagnosis of HIV (EID) improves child survival through earlier initiation of antiretroviral therapy (ART). In many settings, ART initiation is hindered by delays in testing performed in centralized labs. Point-of-care (PoC) platforms offer opportunities to improve the timeliness of ART initiation.
Methods:
We used a mathematical model to estimate the costs and performance of on-site PoC testing using three platforms (m-PIMA, GeneXpert IV, and GeneXpert Edge) compared to the standard of care (SoC). Primary outcomes included ART initiation within 60 days of sample collection, HIV-related mortality before ART initiation, and incremental cost-effectiveness ratios (ICERs).
Results:
PoC testing significantly increased ART initiation within 60 days (from 19% with SoC to 82–84% with PoC) and decreased HIV-related mortality (from 23% with SoC to 5% with PoC). ART initiation and mortality were similar across PoC platforms. When only used for EID and with high coverage of prevention of mother-to-child transmission (PMTCT) programs, ICERs for PoC testing compared to the SoC ranged from $430-$1097 per additional infant on ART within 60 days and from $1527-$3888 per death averted. PoC-based testing was more cost-effective in settings with lower PMTCT coverage, greater delays in the SoC, and when PoC instruments could be integrated with other disease programs.
Conclusions:
Our findings illustrate that PoC platforms can dramatically improve the timeliness of EID and linkage to HIV care. The cost-effectiveness of PoC platforms depends on the cost of PoC testing, existing access to diagnostic testing, and the ability to integrate PoC testing with non-EID programs.
Keywords: cost-effectiveness, pediatrics, early infant diagnosis, point-of-care testing, sub-Saharan Africa
Introduction
In 2019, 1.3 million pregnant women were living with HIV, most of whom lived in low- or middle-income countries [1]. Without access to drugs to prevent mother-to-child transmission (PMTCT), HIV-exposed infants may face cumulative transmission risks of 30–45% [2]. More than half of HIV-infected infants may die by the age of two years without treatment [3]. Timely diagnosis and early initiation of antiretroviral therapy (ART) are critical to reduce morbidity and mortality [4, 5]. To facilitate rapid treatment initiation, the World Health Organization (WHO) recommends early infant diagnosis (EID) for HIV-exposed infants through virological testing at six weeks and nine months of age [6, 7]. However, in 2019 only 60% of HIV-exposed infants received virological testing by eight weeks of age [1].
The challenge for EID in resource-constrained settings is the performance of virological testing in centralized labs typically located in urban centers. Centralized testing necessitates transportation of blood or dried blood spot samples from peripheral clinics to the lab, delivery of results back to healthcare workers, and subsequent notification of caregivers. While the WHO recommends this process be completed within four weeks of sample collection, in many settings results are delivered in eight weeks or longer [8–12].
The development of point-of-care (PoC) instruments for EID testing in peripheral health facilities has the potential to greatly reduce turnaround times and increase rapid ART initiation. Currently, two PoC or near-PoC assays have been prequalified by the WHO for EID testing: 1) m-PIMA (Abbott Laboratories, Chicago, Illinois); and 2) GeneXpert HIV-1 Qual (Cepheid Inc, Sunnyvale, California). Studies of PoC platforms have shown that they can provide same-day notification for more than 98% of tested infants [10, 11].
While PoC technologies may improve the timeliness and completeness of infant ART initiation, adopting such platforms will require substantial new investment, particularly in settings that have established centralized labs. Currently, little evidence exists to guide stakeholders from diverse settings regarding the cost-effectiveness of adopting a PoC platform instead of (or in addition to) centralized testing. We used mathematical models to estimate the epidemiological benefits, costs, and cost-effectiveness of adopting PoC testing with currently available platforms. We evaluated key factors that influence the cost-effectiveness of PoC testing and provide guidance about settings most likely to benefit from adoption of PoC platforms for EID.
Materials and Methods
Setting and Model Structure
A population living in a representative high burden country in sub-Saharan Africa where implementation of PoC testing is likely to be considered was simulated. The models considered a birth cohort of 25,000 HIV-exposed infants and simulated testing at six weeks and nine months of age (see Table 1 for parameter values) [6, 7]. HIV-exposed infants experienced a risk of HIV transmission until the nine-month test dependent on their age and the ART and PMTCT status of the mother and child. HIV-infected infants who were undiagnosed or did not initiate ART within 60 days of sample collection experienced age-dependent HIV-related mortality. HIV-infected infants not on treatment by nine months were followed until 18 months of age for ART initiation and HIV-related death.
Table 1:
Parameter Values
| Parameter | Base Case Value (Range)* | Ref. | Parameter | Base Case Value (Range)* | Ref. | |
|---|---|---|---|---|---|---|
| Transmission & Mortality Parameters | Assay Characteristic Parameters | |||||
| Probability of HIV Transmission | SoC Sensitivity and Specificity | 100% | Model Assumption | |||
| Among Infants First Tested at 6 Weeks (PMTCT) | 0.022 (0.005–0.04) | [23–25] | PoC Sensitivity/ Specificity | |||
| Among Infants First Tested at 6 Weeks (no PMTCT) | 0.2 (0.1–0.3) | [23, 25, 26] | m-PIMA | 98% (96–100%) / 99.9% (99.8–99.9%) | [13] | |
| New Transmission Between 6 Weeks and 9 Months | 0.017 (0.005–0.03) | [23, 27] | GeneXpert IV & Edge | 96% (93–99%) / 99.8% (99.7–99.9%) | [13] | |
| Among Infants First Tested at 9 Months (PMTCT) | 0.09 (0.04–0.14) | [23–25] | Economic Parameters | |||
| Among Infants First Tested at 9 Months (no PMTCT) | 0.3 (0.15–0.45) | [23, 25, 26] | 9-Month Capital Cost per Instrument (x1000 USD 2018)** | |||
| Probability of HIV-Related Mortality (Untreated) | SoC | $35 ($32-$38) | CHAI, see Supplement | |||
| Between 6 Weeks and 9 Months (Infected by 6 Weeks) | 0.23 (0.18–0.28) | [3] | m-PIMA | $4.8 ($4.4-$5.3) | CHAI, see Supplement | |
| Between 6 Weeks and 18 Months (Infected by 6 Weeks) | 0.39 (0.34–0.44) | [3] | GeneXpert IV | $5.2 ($4.7-$5.8) | CHAI, see Supplement | |
| Between 6 Weeks and 18 Months (Infected after 6 Weeks and Lost to Follow-Up) | 0.30 (0.25–0.35) | [3] | GeneXpert Edge | $1.8 ($1.6-$2.0) | CHAI, see Supplement | |
| Between 9 Months and 18 Months (Infected by 9 Months) | 0.16 (0.11–0.21) | [3] | Recurrent Costs per Test (USD 2018) | |||
| Testing and Treatment Parameters | SoC | $15 (14–16) | CHAI, see Supplement | |||
| Proportion Tested at 6 Weeks | 0.85 (0.83–0.87) | [28] | m-PIMA | $25 ($23-$27) | CHAI, see Supplement | |
| Proportion Returning at 9 Months (HIV-Negative at 6 Weeks) | 0.8 (0.7–0.9) | [23] | GeneXpert IV & Edge | $20 ($18-$22) | CHAI, see Supplement | |
| Proportion Returning after 1 Week for Tiebreaker (PoC-Only Algorithm) | 0.95 (0.3–0.97) | Model Assumption | Number of SoC Devices | |||
| PMTCT Coverage | SoC | 5 (4–6) | CHAI, see Supplement | |||
| High | 93% (84–100%) | [14] | PoC+SoC | 1 | Model Assumption | |
| Low | 48% (43–53%) | [14] | Proportion EID of All SoC Tests | |||
| Probability of Ever Initiating ART (by 18 Months of Age) | SoC | 0.50 (0.45–0.55) | CHAI, see Supplement | |||
| After Positive SoC PCR Test | 0.48 (0.43–0.53) | [10, 11] | PoC+SoC | 0.025 (0.023–0.027) | CHAI, see Supplement | |
| After Positive PoC Test | 0.95 (0.85–1) | [10, 11] | Number of PoC Devices | 150 (140–160) | CHAI, see Supplement | |
| Probability of Initiating ART within 60 Days | Proportion EID of all PoC Tests (Integrated Capital Costs) | |||||
| After Positive SoC PCR Test | 0.20 (0.13–0.27) | [10, 11] | m-PIMA | 25% | [16] | |
| After Positive PoC Test | 0.89 (0.85–0.94) | [10, 11] | GeneXpert IV & Edge | 15% | [16] | |
All values were sampled uniformly across the specified ranges
Nine-month capital costs were calculated by dividing lifetime capital costs by the estimated instrument lifetime and multiplying by 9 months (the duration of the testing period for a given infant). See Supplementary Methods (SDC 1) for further details.
On-site PoC testing was modeled and compared to the standard of care (SoC) (see SDC 1-Figure S1):
SoC testing:
Nucleic acid-based testing to detect HIV infection was performed by transporting samples to centralized labs. Following a delay of variable length, results were returned to the mother. After a positive result, infants initiated ART, and a second sample was collected for confirmatory testing at the centralized lab. To provide testing capacity for 25,000 infants, we assumed 4–6 polymerase chain reaction (PCR) instruments would be required and that EID testing accounted for 45–55% of all samples run on those instruments (see Table 1 and SDC 1-Table S2).
PoC testing:
EID testing was performed on site using PoC platforms to detect HIV infection, with the goal of same-day testing and results.[13] After a positive result, infants received same-day PoC confirmatory testing with ART initiation if positive. After a negative (discrepant) confirmatory result, infants returned one week later for a tiebreaker PoC test before initiating ART. We assumed that 140–160 PoC devices would be required to provide testing capacity for the simulated population (see Table 1 and SDC 1-Table S3).
An additional algorithm (PoC+SoC) was modeled to evaluate the impact of using the centralized lab as the tiebreaker test in the event of a discrepancy between the initial and confirmatory PoC test (see SDC 1-Figure S1C). As the results were similar to the PoC testing described above, they are only presented in the Supplementary Results.
As the proportion of women receiving PMTCT varies across settings, we simulated each algorithm in a setting of high (median 93%, the estimated coverage for East and Southern Africa in 2017) and low (median 48%, the estimated coverage for West and Central Africa in 2017) PMTCT coverage [14].
Diagnostic Platforms
For nucleic acid-based testing at the centralized lab in the SoC, we assumed use of a representative PCR platform (comparable to the Abbott m2000 [Abbott Laboratories, Chicago IL, USA) and Roche CAP/CTM [Roche Diagnostics, Basel, Switzerland] instruments). To illustrate an idealized comparison between PoC platforms and a diagnostic gold standard, a conservative simplifying assumption was made that the SoC PCR platform was 100% sensitive and specific but with delays between sample collection and ART initiation [10, 11].
We evaluated the epidemiologic and economic performance of three available PoC or near-PoC platforms: m-PIMA (Abbott Laboratories; Lake Forest, IL, USA) and GeneXpert HIV-1 Qual (Cepheid Inc. Sunnyvale CA, USA) performed on GeneXpert IV and GeneXpert Edge. Diagnostic accuracy and cost parameters for each platform are presented in Table 1. Further details on costs are included in the Supplementary Methods.
Epidemiological Outcomes
For each diagnostic platform, we estimated the proportion of HIV-infected infants initiating ART within 60 days of sample collection as a key indicator for infant outcomes. We also estimated the proportion of HIV-infected infants ever initiating ART by 18 months. Based on limited data available, we assumed no difference in the probability of ART initiation after a positive test result on any PoC platform (Table 1). We estimated excess 18-month mortality prior to ART initiation among HIV-infected infants (relative to HIV-uninfected infants) who went undiagnosed or started ART more than 60 days after specimen collection (see Supplemental Methods for further details).
Economic Outcomes
The economic evaluation took the perspective of the EID program, considering only the share of costs paid by the EID program during the nine-month period of testing for a given infant. Unit costs for each platform included one-time capital costs and recurrent costs per test. Capital costs included costs of the instruments, maintenance, freight, insurance, inspection, handling, clearance/shipping/distribution, and internet connectivity, annualized over useful lives of five to seven years per instrument (discounting was not applied). Recurrent costs included costs for reagents, consumables, human resources for sample collection and testing, transportation, and waste management (see SDC 1-Table S5). All costs were calculated in 2018 USD.
Capital costs of the SoC PCR platform were assumed to be shared between EID and HIV viral load testing programs weighted by the utilization of EID (the relative contribution of EID to all PCR tests performed). In the primary analysis, capital costs of the PoC platforms were assumed to be paid fully by EID programs, as may be the case for EID programs considering the initial purchase of PoC platforms. As a secondary analysis, use of the PoC platforms was integrated across programs. We assumed the proportion of testing allocated to EID with m-PIMA (capable of EID and HIV viral load testing) and GeneXpert IV/Edge (capable of EID, HIV viral load testing, and tuberculosis testing) would be 25% and 15%, respectively; capital costs were allocated to the EID program accordingly.
Cost-Effectiveness
For PoC testing, incremental effectiveness, costs, and cost-effectiveness ratios (ICERs) were calculated relative to outcomes under the SoC. Our primary cost-effectiveness outcome was cost per additional infant initiating ART within 60 days.
Measures of Uncertainty
We estimated uncertainty using a probabilistic approach. In our primary and secondary analyses, 10,000 sets of parameters were generated uniformly from across the distributions shown in Table 1 (see Supplemental Methods for further details) and used to estimate the incremental cost and effectiveness corresponding to each testing algorithm. Results are reported for the median outcome across all 10,000 parameter sets, with corresponding 95% uncertainty ranges (URs), defined as the 2.5th and 97.5th percentiles across those 10,000 simulations.
Sensitivity Analyses
To elucidate the key drivers of our primary findings, 100,000 new parameter sets were drawn across all model parameters (independent of specific PoC platforms) and used to simulate outcomes under each algorithm. Multivariable nonparametric partial rank correlation coefficients (PRCCs) were calculated to quantify the strength of correlation between individual parameter values and model outcomes, adjusting for all other parameter values. Based on these results, we performed two-way sensitivity analyses between critical parameters, where all other parameters were held at their median values while the two parameters in question were varied independently.
Additionally, we evaluated a combined scenario in which 80% of the infant population was seen in facilities equipped with PoC platforms and the remaining 20% was seen in facilities where SoC was available (see Supplemental Methods for further details).
All calculations and analyses were performed using R software version 3.5.0 (R Core Team, Vienna, Austria).
Results
Epidemiological Outcomes
Of 25,000 infants undergoing EID, we estimated that 21,400 would be tested starting at six weeks (95% UR: 20,900–21,800) and 3,600 would only be tested at nine months of age (95% UR: 3,200–4,100). A median of 1,534 and 3,508 infants were projected to be HIV-infected by nine months of age in settings of high and low PMTCT coverage, respectively (see Supplemental Table S7 for the number of infants infected at six weeks of age and diagnosed with each platform). With the SoC, we estimated that 42,147 and 41,777 PCR tests would be performed, corresponding to an average of 1.69 and 1.67 tests per infant in settings of high and low PMTCT coverage, respectively. With PoC testing, the number of tests performed ranged from 43,000–43,060 and from 43,677–43,773 in settings of high and low PMTCT coverage, respectively (see SDC 1-Table S8).
While the number of HIV infections decreased with higher PMTCT coverage, epidemiological outcomes in terms of proportions of infants initiating ART and HIV-related deaths were similar across PMTCT coverage settings (Figure 1A and 1B). PoC testing resulted in better epidemiologic outcomes than the SoC (Figure 1A and 1B). In settings of high PMTCT coverage, the proportion of HIV-infected infants initiating ART within 60 days increased from a median of 19% with the SoC to 82–84% with PoC testing. The proportion of HIV-infected infants initiating ART by 18 months increased from a median of 46% with the SoC to 87–89% with PoC testing. The proportion of HIV-infected infants dying by 18 months of age decreased from a median of 23% with the SoC to 5% with PoC testing.
Figure 1: Epidemiological Outcomes of Early Infant Diagnosis Testing Platforms.
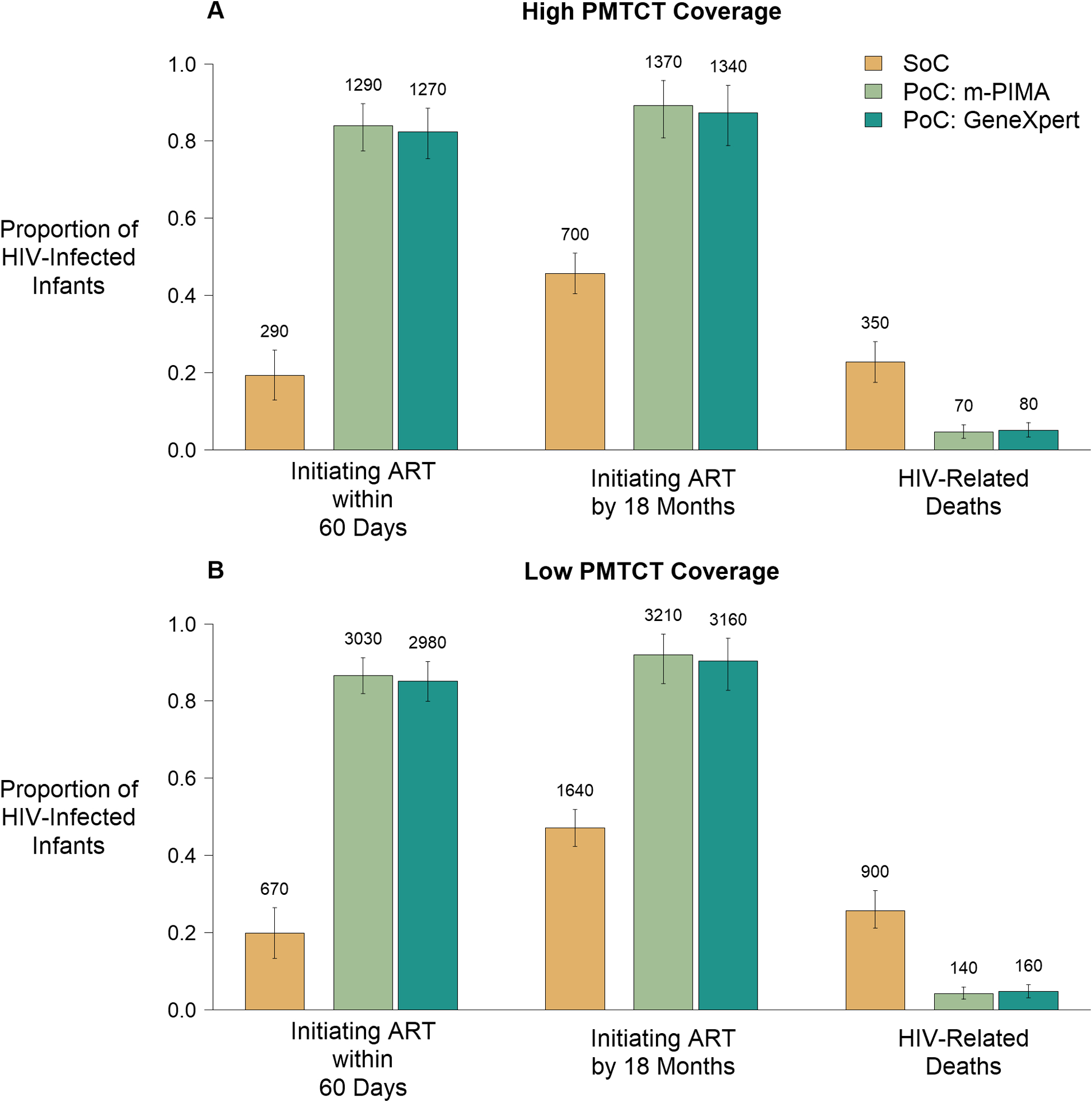
The epidemiologic outcomes of interest are plotted for each combination of early infant diagnosis (EID) platform and testing algorithm as a proportion of HIV-infected infants in each scenario. Cepheid platforms (GeneXpert IV and GeneXpert Edge) share assay characteristics resulting in identical epidemiological outcomes and are therefore combined. Data labels represent median numbers of HIV-infected infants experiencing each outcome. Error bars represent 95% uncertainty ranges. Panel A represents a setting of high coverage of prevention of mother-to-child-transmission (PMTCT) programs (median: 93% coverage), where a median of 1,534 infants were HIV-infected under the standard of care (SoC). Panel B represents a setting of low PMTCT coverage (median: 48% coverage) where a median of 3,493 infants were HIV-infected under the SoC.
Economic Outcomes
The number of EID tests, and thus total costs of testing, were similar by PMTCT coverage (Figure 2 and SDC 1-Table S8). In high PMTCT settings, the total costs per infant evaluated with the SoC were a median of $28.79. Total costs per infant evaluated for PoC testing were higher than the SoC when PoC platforms were only used for EID testing, ranging from a median of $45.34-$72.05, depending on the PoC platform (Figures 2A and 2C). While total costs per infant evaluated for PoC testing remained higher than the SoC when costs were integrated across programs, sharing of capital costs greatly reduced the total costs of testing from the EID program perspective, to a median of $36.09-$50.28 (Figure 2B and 2D). Total EID programmatic costs and total costs per test performed (median $17·08 for SoC and $26.35-$41.91 for PoC testing in high PMTCT settings with no shared capital costs) are further detailed in SDC 1 - Table S8.
Figure 2: Economic Outcomes of Early Infant Diagnosis Testing Platforms.
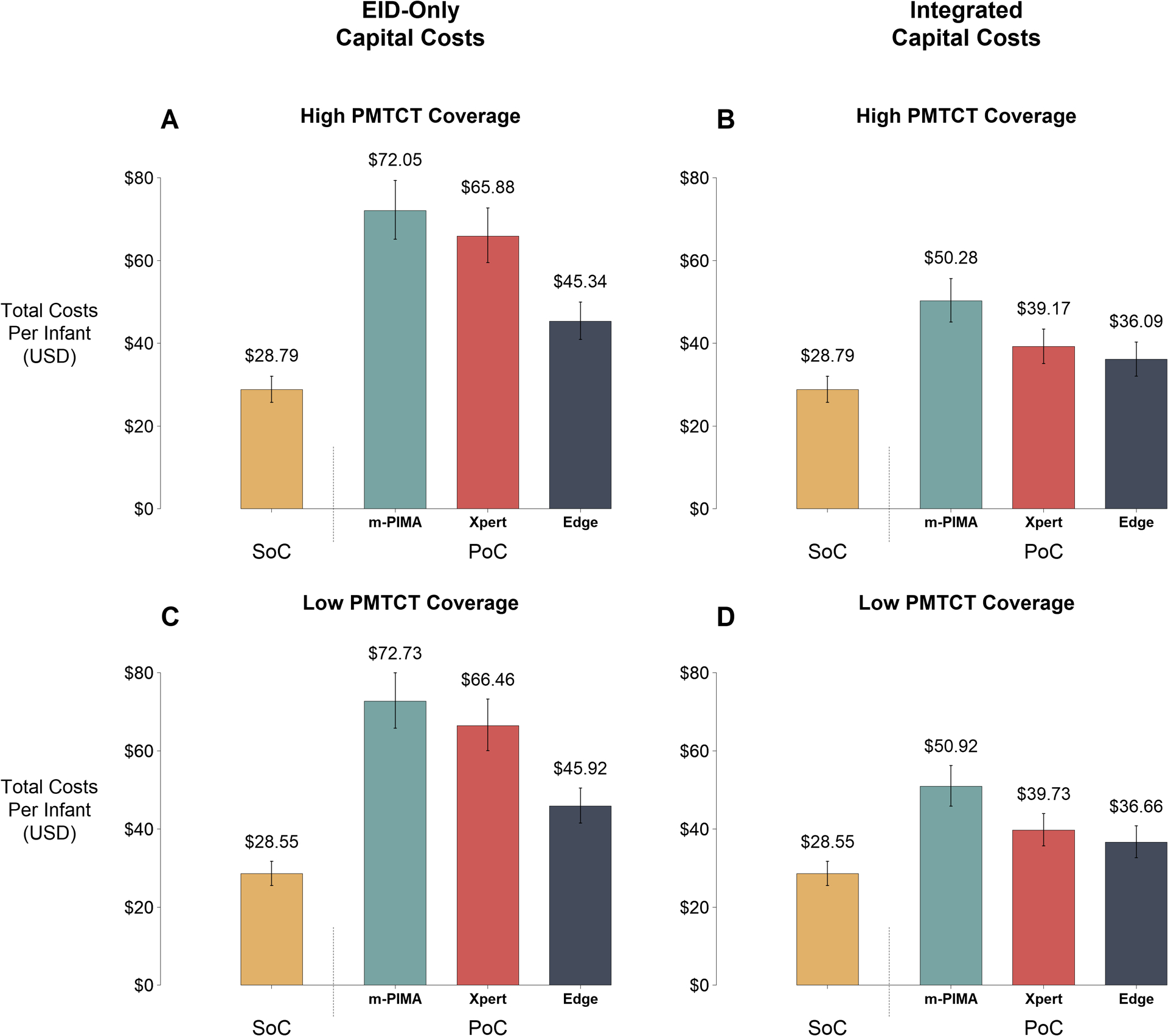
Total (capital and recurrent) programmatic costs per infant evaluated are plotted for the SoC and each PoC platform (m-PIMA, GeneXpert IV [Xpert], and GeneXpert Edge [Edge]). Data labels represent median costs per infant. Error bars represent 95% uncertainty ranges. Panels A and B represent a setting of high coverage of prevention of mother-to-child transmission (PMTCT) programs (median: 93% coverage), while Panels C and D represent a setting of low PMTCT coverage (median: 48% coverage). Left panels (A and C) illustrate scenarios where all capital costs are borne by the early infant diagnosis (EID) testing program, while right panels (B and D) illustrate scenarios where capital costs are integrated across EID, HIV viral load, and tuberculosis testing programs according to instrument utilization proportions. All costs are expressed in 2018 USD.
Cost-Effectiveness
Cost-effectiveness ratios were generally favorable to PoC testing compared to the SoC (Figure 3 and SDC1-Table S9; see also SDC1-Table S10 and Figure S2 for comparisons between platforms). In high PMTCT settings, median ICERs ranged from $430-$1,097 per additional HIV-infected infant initiating ART within 60 days, $655-$1,642 per additional infant initiating ART by 18 months, and $1,527-$3,888 per death averted compared to the SoC (Figure 3A). With integrated use of PoC platforms, cost-effectiveness ratios fell by approximately 50%, to $187-$543 per additional HIV-infected initiating ART within 60 days, $285-$815 per additional infant initiating ART by 18 months, and $664-$1929 per death averted compared to the SoC (Figure 3B and 3D). PoC testing was most cost-effective in low PMTCT settings, where testing costs were similar but more infants were infected, diagnosed, and treated (Figure 3C and 3D).
Figure 3: Incremental Cost-Effectiveness of Point-of-Care Platforms.
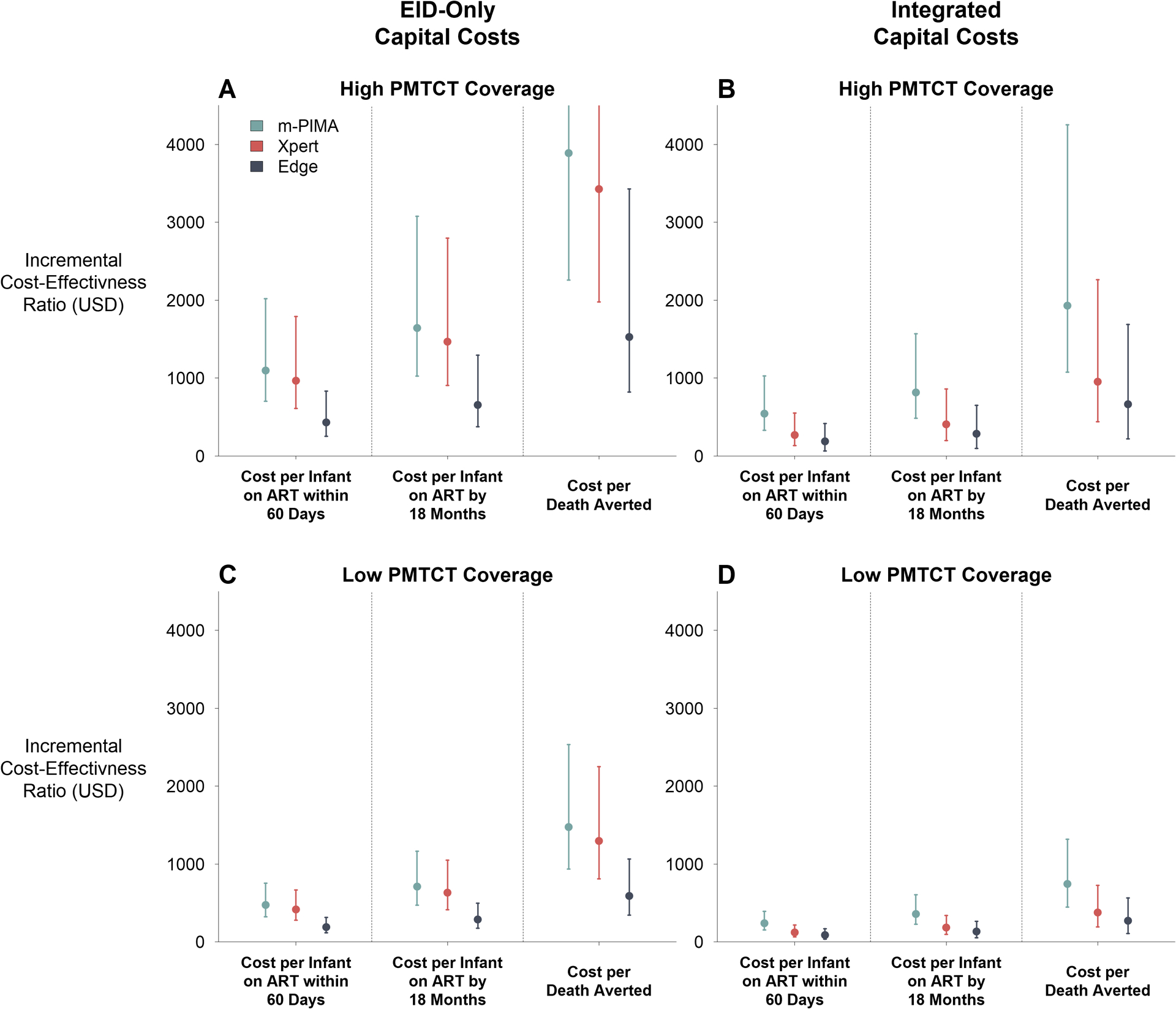
Incremental cost-effectiveness ratios (ICERs) are plotted for each PoC platform for each epidemiological outcome. All costs and outcomes are calculated as incremental values relative to the standard of care (SoC). Data labels represent median ICER values. Panels A and B represent a setting of high coverage of prevention of mother-to-child transmission (PMTCT) programs (median: 93% coverage), while Panels C and D represent a setting of low PMTCT coverage (median: 48% coverage). Left panels (A and C) illustrate scenarios where all capital costs are borne by the early infant diagnosis (EID) testing program, while right panels (B and D) illustrate scenarios where capital costs are integrated across EID, HIV viral load, and tuberculosis testing programs according to instrument utilization proportions. All costs are expressed in 2018 USD.
Considering these costs in the context of health expenditures in sub-Saharan Africa, the additional cost incurred by PoC testing as compared to the SoC amounted to 0.01–0.32% of per-capita health expenditures in select countries (SDC1 - Table S6).
Sensitivity Analyses
The cost-effectiveness of PoC testing was most influenced by parameters determining capital and recurrent costs, the probability of ART initiation within 60 days for the SoC, and the sensitivity of PoC assays (Figure 4A).
Figure 4: Sensitivity Analyses.
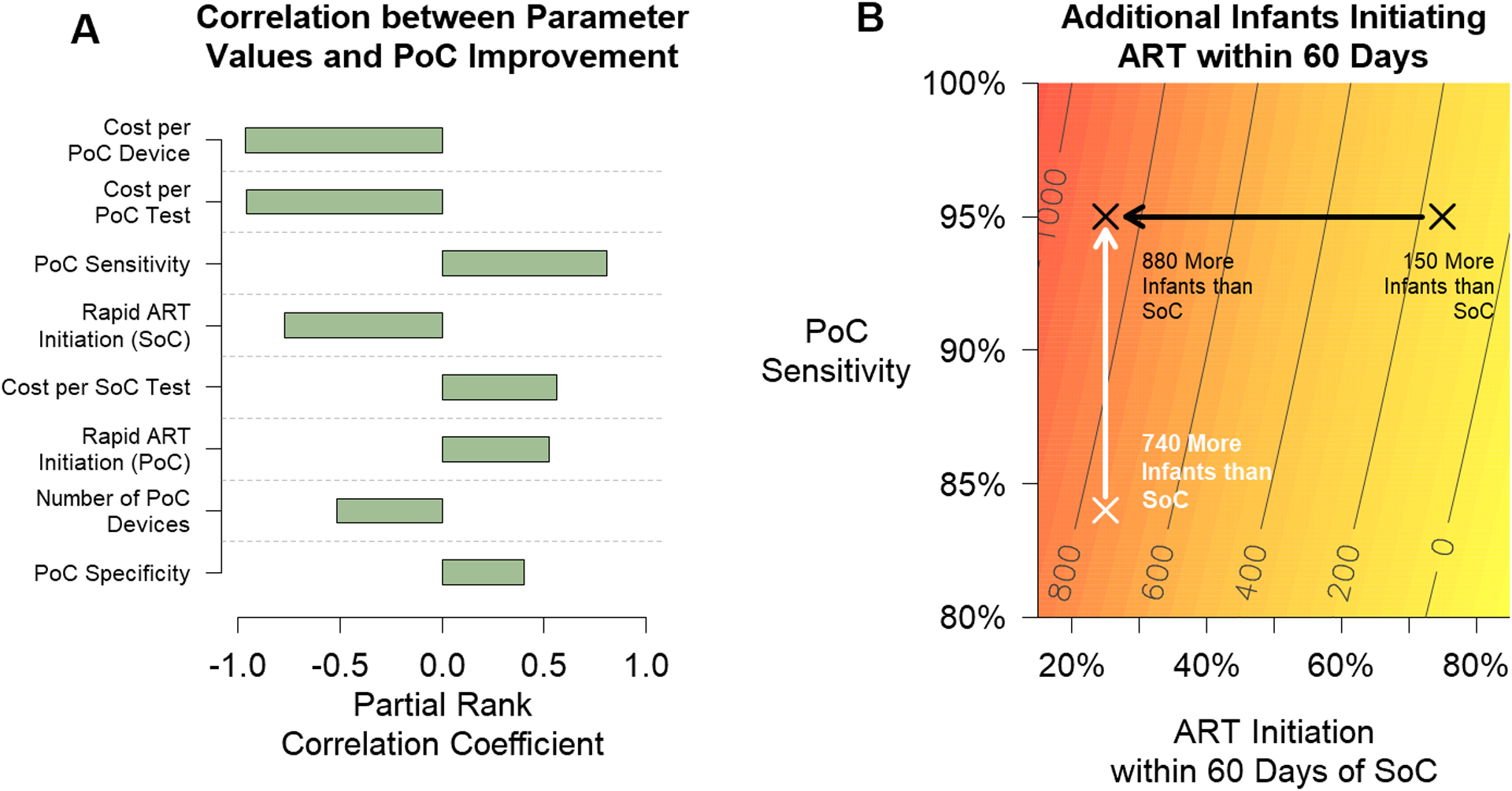
The cost-effectiveness of point-of-care (PoC) testing is most dependent on parameters related to PoC costs, ART initiation, and assay sensitivity. Panel A depicts multivariable partial rank correlation coefficients (PRCC) between values for input parameters and improvement in PoC cost-effectiveness – defined here as the difference in cost over the difference in the proportion of HIV-infected infants initiating ART within 60 days between the standard of care (SoC) and PoC testing. Parameters with coefficients closer to 1.0 or −1.0 are strongly correlated with the outcome of interest. Weakly correlated parameters (|PRCC|<0.2) have been excluded. Panel B illustrates the number of additional infants initiating ART within 60 days after adopting PoC testing compared with the SoC, as a function of PoC platform sensitivity and the probability that an infant initiates ART within 60 days of a positive result by the SoC. The white arrow shows the impact of increasing assay sensitivity from 84% to 95%, assuming that 25% of infants initiate ART within 60 days after testing with the SoC. The black arrow shows the impact of increasing the probability of ART initiation after testing with the SoC from 25% to 75%, assuming an assay sensitivity of 95%. For this analysis, we assumed 93% PMTCT coverage and that 89% of infants initiated ART within 60 days of a positive PoC test result.
The relative effectiveness of PoC testing varied depending on the programmatic performance of the SoC (Figure 4B and Figure S3). For example, settings with 75% and 25% of infants initiating ART within 60 days of testing with the SoC might expect 150 and 880 additional infants to initiate ART with PoC testing (Figure 4B, black arrow). The relative effectiveness of PoC testing also varied with the capital and recurrent costs. With reduced capital and recurrent costs, particularly with integrated capital costs across programs, PoC testing can be cost-saving (SDC 1-Figure S3).
The relative effectiveness of PoC testing also varied depending on the sensitivity of the PoC platform, with fewer infants initiating ART within 60 days with lower test sensitivity (Figure 4B, white arrow). While the PoC platforms evaluated have reported high sensitivity (>96%), new platforms for use in lower level health facilities may be developed with lower sensitivity, or current platforms may have lower sensitivity under programmatic conditions, and therefore expected improvements may be reduced. However, lower-sensitivity PoC platforms (e.g., as low as 80% sensitive) may still be cost-effective or cost-saving alternatives to the SoC in some settings if capital and recurrent costs can be reduced (SDC 1-Figure S4).
In the combined scenario where only 80% of the population had access to facilities with a PoC platform, epidemiological outcomes and costs were lower than the primary analysis, but incremental cost effectiveness was similar. For example, in settings of high PMTCT coverage with PoC platforms used for EID testing only, the proportion of HIV-infected infants initiating ART within 60 days ranged from a median of 70–71%, and median ICERs ranged from $1,528-$3,888 per death averted compared to the SoC (see SDC 1-Table S11).
Discussion
We provide a quantitative framework to guide policymakers and stakeholders considering the adoption of PoC platforms for EID in resource-limited settings. Our results indicate that such platforms are likely to increase ART initiation in early infancy and reduce mortality among HIV-infected infants, and that all three PoC platforms are similarly effective. In high PMTCT settings, ICERs for PoC testing ranged from $430-$1,097 per infant initiated on ART within 60 days and $1,527-$3,888 per death averted. Additional costs of PoC testing could more than double the overall cost of EID (e.g. from 0.04–0.21% to 0.06–0.53% of per-capita healthcare expenditure in select countries) and may be difficult to afford in countries where resources are highly constrained. Integrating use of PoC platforms across programs would decrease the share of health expenditures by 20–40% and would make EID more cost-effective and affordable.
The cost-effectiveness of PoC platforms was influenced by several factors. First, PoC platforms benefited a greater number of infants at a similar cost in settings with low PMTCT coverage, and thus were more cost-effective. Second, at current costs and with the modeled number of instruments, PoC platforms were more expensive to implement than the SoC when used exclusively for EID. However, current PoC platforms have the capacity to perform other tests, including HIV viral load and tuberculosis testing [15]. Multi-disease testing with the GeneXpert platform in Zimbabwe found that EID accounted for less than 9% of all PoC tests [16]. Integrating utilization of PoC instruments across programs, therefore, has the potential to reduce costs for EID programs and enhance cost-effectiveness, such that PoC testing could even be cost-saving. Third, sensitivity analyses illustrated that the cost-effectiveness of PoC platforms was highly dependent on capital and recurrent costs. With lower costs, PoC testing will become more cost-effective. Lastly, the cost-effectiveness of PoC platforms depended on the degree of improvement in ART initiation over the SoC, with the greatest benefits observed in settings with long delays for the SoC. When comparing the benefit of PoC platforms over the SoC, the timeframe of evaluation should be considered. In this analysis, we considered “rapid” ART initiation within 60 days of sample collection based on the availability of empirical data [10, 11]. However, PoC platforms provide the opportunity for same-day results and ART initiation, and thus offer the greatest improvements over the SoC in the first days and weeks following sample collection [10, 12]. Given the morbidity and mortality observed among HIV-infected infants prior to ART initiation in this and other studies [8, 17], and the limited ability of the SoC to return results within days of sample collection, the cost-effectiveness of PoC platforms for EID would be greater if a shorter definition of “rapid” testing were considered.
Our analysis also considered a combined implementation scenario as well as use of hypothetical PoC platforms with different characteristics than those currently available. The combined scenario may represent a realistic situation where PoC platforms are placed at selected health facilities that only cover a portion of the population, with the remaining population continuing with the SoC. As expected, health outcomes and costs for this combined scenario were intermediate between the SoC and PoC testing covering 100% of the population, whereas incremental cost-effectiveness was similar. Hypothetical PoC platforms were also considered as PoC technologies are changing rapidly. New platforms may become available with different costs and characteristics to consider when adopting PoC platforms for EID [18]. Our results illustrate the impact of a range of capital and recurrent costs on PoC cost-effectiveness, which can provide guidance for both developers and stakeholders. These results also indicate that PoC instruments with reduced sensitivity (as low as 80%) may still be cost-effective (and even cost-saving), provided they can increase early ART initiation compared to the SoC.
Our results add to a growing literature demonstrating the effectiveness and cost-effectiveness of PoC platforms for EID testing. Several studies have confirmed high accuracy and reliability of Abbott and Cepheid platforms in operational settings [19–21]. In addition, a cluster-randomized trial of the m-PIMA platform in Mozambique demonstrated that PoC testing improved ART initiation in rural and urban areas [10], a finding that was corroborated by pre-post observational cohorts in nine sub-Saharan African countries using m-PIMA and GeneXpert platforms [11, 12]. A recent model evaluating the cost-effectiveness of implementing the m-PIMA platform in Zimbabwe found PoC testing to be cost-effective (costs of less than 100% GDP per capita per year of life saved) [22]. Our results augment this literature by evaluating the cost-effectiveness of both WHO prequalified PoC assays across a range of programmatic (high/low PMTCT coverage; PoC and PoC+SoC algorithms) and economic (EID-only/integrated utilization) conditions using data sources from resource-limited settings and considering epidemiologic outcomes relevant to EID programs.
The results should be interpreted in the context of our analytic approach and assumptions. First, our analysis evaluated the incremental benefits of PoC platforms in linking HIV-infected infants to treatment; our approach did not consider treatment outcomes or costs, which may underestimate the long-term costs and benefits associated with PoC testing. Second, these results are dependent on parameter values selected. Important variations in these values may exist between settings, which could influence the cost-effectiveness of PoC platforms. To account for these variations, we used a robust statistical approach with estimates of uncertainty, multidimensional parameter sampling and bivariate and multivariate sensitivity analyses. Finally, we did not consider alternative intervention scenarios in which the performance of centralized labs was improved (for example, by investments in communication technology, transportation, or infrastructure), as such interventions are setting-dependent and difficult to cost from a health program perspective. However, increased investment in centralized labs could translate to improved health outcomes more broadly, and therefore cost-effectiveness of PoC testing should not be interpreted as evidence not to invest in lab infrastructure.
In summary, our results provide evidence to support PoC platforms as the primary means of EID testing across a range of settings, as programs offering PoC testing can expect significant clinical, epidemiological, and social benefits. Implementation of PoC platforms will likely require significant investment at current prices, but allowing integrated use of the platforms can help to decrease the costs for EID programs. These findings may serve to guide the development of international guidelines for PoC testing in the future.
Supplementary Material
Acknowledgements
We thank Seth McGovern and the Clinton Health Access Initiative for providing platform deployment and cost data to inform our analysis.
Conflicts of interest and sources of funding:
The authors have no conflicts of interest to declare. This work was supported by a contract from the World Health Organization and a grant from the National Institutes of Allergy and Infectious Disease (R01AI116324).
Footnotes
Supplemental Digital Content
SDC 1. WHO POC Supplement_2020_09_17.docx
This file provides the supplementary methods and results for the analysis.
References
- 1.UNAIDS. AIDSInfo. 2020. Available at: http://aidsinfo.unaids.org/ (September 11, 2020).
- 2.De Cock KM, Gilks CF, Lo YR, Guerma T. Can antiretroviral therapy eliminate HIV transmission? Lancet 2009; 373(9657):7–9. [DOI] [PubMed] [Google Scholar]
- 3.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 2004; 364(9441):1236–1243. [DOI] [PubMed] [Google Scholar]
- 4.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008; 359(21):2233–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotton MF, Violari A, Otwombe K, Panchia R, Dobbels E, Rabie H, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet 2013; 382(9904):1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection Recommendations for a public health approach. Second Edition 2016 Gevena, Switzerland: World Health Organization; 2016. [PubMed] [Google Scholar]
- 7.WHO. HIV diagnosis and ARV use in HIV-exposed infants: a programmatic update (Technical report). Geneva, Switzerland: World Health Organization; July 2018. [Google Scholar]
- 8.Sutcliffe CG, van Dijk JH, Hamangaba F, Mayani F, Moss WJ. Turnaround time for early infant HIV diagnosis in rural Zambia: a chart review. PLoS One 2014; 9(1):e87028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill MM, Hoffman HJ, Mokone M, Tukei VJ, Nchephe M, Phalatse M, et al. Assessing very early infant diagnosis turnaround times: Findings from a birth testing pilot in Lesotho. AIDS Res Treat 2017; 2017:2572594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jani IV, Meggi B, Loquiha O, Tobaiwa O, Mudenyanga C, Zitha A, et al. Effect of point-of-care early infant diagnosis on antiretroviral therapy initiation and retention of patients. AIDS 2018; 32(11):1453–1463. [DOI] [PubMed] [Google Scholar]
- 11.Mwenda R, Fong Y, Magombo T, Saka E, Midiani D, Mwase C, et al. Significant patient impact observed upon implementation of point-of-care early infant diagnosis technologies in an observational study in Malawi. Clin Infect Dis 2018; 67(5):701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianchi F, Cohn J, Sacks E, Bailey R, Lemaire JF, Machekano R, et al. Evaluation of a routine point-of-care intervention for early infant diagnosis of HIV: an observational study in eight African countries. Lancet HIV 2019. [DOI] [PubMed] [Google Scholar]
- 13.WHO. Information Note - HIV Diagnostics: Novel point-of-care tools for early infant diagnosis of HIV. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 14.UNAIDS. AIDSInfo. 2018. Available at: http://aidsinfo.unaids.org/.
- 15.WHO. Considerations for adoption and use of multidisease testing devices in integrated laboratory networks (Information note). Geneva, Switzerland: WHO: Global TB Programme and Department of HIV/AIDS; 2017. [Google Scholar]
- 16.Ndlovu Z, Fajardo E, Mbofana E, Maparo T, Garone D, Metcalf C, et al. Multidisease testing for HIV and TB using the GeneXpert platform: A feasibility study in rural Zimbabwe. PLoS One 2018; 13(3):e0193577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutcliffe CG, Thuma PE, van Dijk JH, Sinywimaanzi K, Mweetwa S, Hamahuwa M, et al. Use of mobile phones and text messaging to decrease the turnaround time for early infant HIV diagnosis and notification in rural Zambia: an observational study. BMC pediatrics 2017; 17(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunning L, Hsiao NY, Myer L. Point-of-care HIV early infant diagnosis: is test sensitivity everything? J Int AIDS Soc 2015; 18:20235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meggi B, Vojnov L, Mabunda N, Vubil A, Zitha A, Tobaiwa O, et al. Performance of point-of-care birth HIV testing in primary health care clinics: An observational cohort study. PLoS One 2018; 13(6):e0198344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabi I, Mahiga H, Mgaya J, Geisenberger O, Kastner S, Olomi W, et al. Accuracy and operational characteristics of Xpert human immunodeficiency virus point-of-care testing at birth and until week 6 in human immunodeficiency virus-exposed neonates in Tanzania. Clin Infect Dis 2019; 68(4):615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Opollo VS, Nikuze A, Ben-Farhat J, Anyango E, Humwa F, Oyaro B, et al. Field evaluation of near point of care Cepheid GeneXpert HIV-1 Qual for early infant diagnosis. PLoS One 2018; 13(12):e0209778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank SC, Cohn J, Dunning L, Sacks E, Walensky RP, Mukherjee S, et al. Clinical effect and cost-effectiveness of incorporation of point-of-care assays into early infant HIV diagnosis programmes in Zimbabwe: a modelling study. Lancet HIV 2019; 6(3):e182–e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinh TH, Mushavi A, Shiraishi RW, Tippett Barr B, Balachandra S, Shambira G, et al. Impact of timing of antiretroviral treatment and birth weight on mother-to-child human immunodeficiency virus transmission: Findings from an 18-month prospective cohort of a nationally representative sample of mother-infant pairs during the transition from Option A to Option B+ in Zimbabwe. Clin Infect Dis 2018; 66(4):576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilewo C, Karlsson K, Ngarina M, Massawe A, Lyamuya E, Swai A, et al. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr 2009; 52(3):406–416. [DOI] [PubMed] [Google Scholar]
- 25.Saounde Temgoua EM, Nkenfou CN, Zoung-Kanyi Bissek AC, Fokam J, Billong SC, Sosso SM, et al. HIV-1 early infant diagnosis is an effective indicator of the prevention of mother-to-child transmission program performance: Experience from Cameroon. Curr HIV Res 2015; 13(4):286–291. [DOI] [PubMed] [Google Scholar]
- 26.Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O’Sullivan MJ, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 1994; 331(18):1173–1180. [DOI] [PubMed] [Google Scholar]
- 27.Taha TE, Li Q, Hoover DR, Mipando L, Nkanaunena K, Thigpen MC, et al. Postexposure prophylaxis of breastfeeding HIV-exposed infants with antiretroviral drugs to age 14 weeks: updated efficacy results of the PEPI-Malawi trial. J Acquir Immune Defic Syndr 2011; 57(4):319–325. [DOI] [PubMed] [Google Scholar]
- 28.Maunga S, Moyo N, Mutembo S, Thuma PE, Moss WJ, Sutcliffe C. Early infant diagnosis of HIV infection and linkage to care in rural Zambia Zambia National Health Research Conference. Lusaka, Zambia; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


