Abstract
Significance.
It is difficult to determine the most efficacious refractive correction for individuals with Down syndrome using routine clinical techniques. New objective methods that optimize spectacle corrections for this population may reduce limitations on daily living by improving visual quality.
Purpose.
This manuscript describes the methods and baseline characteristics of study participants in a National Eye Institute sponsored clinical trial to evaluate objectively derived spectacle corrections in adults with Down syndrome. Inter-session repeatability of the primary outcome measure (distance visual acuity) is also reported.
Methods.
Adults with Down syndrome were enrolled into a 9 visit study to compare clinically derived spectacle corrections and two different objective spectacle corrections derived from wavefront aberration data. Spectacle corrections were randomized and dispensed for two months each. Distance visual acuity was measured with a Bailey-Lovie style chart. Inter-session repeatability of acuity was established by performing difference versus mean analysis from binocular acuity measures obtained through habitual corrections at visits 1 and 2.b
Results.
Thirty adults (mean ± standard deviation age = 29 ± 10 years) with a large range of refractive errors were enrolled. Presenting visual acuity at visit 1 was reduced (right eye: 0.47 ± 0.20 logMAR, left eye: 0.42 ± 0.17 logMAR). The mean difference between visits 1 and 2 was 0.02 ± 0.06 logMAR with a coefficient of repeatability (1.96 x within subject standard deviation) of 0.12 logMAR.
Conclusions.
This study seeks to investigate new strategies to determine optical corrections that may reduce commonly observed visual deficits in individuals with Down syndrome. The good inter-session repeatability of acuity found in this study (6 letters) indicates that, despite the presence of reduced acuity, adults with Down syndrome performed the outcome measure for this clinical trial reliably.
Reduced visual acuity is a common finding in individuals with Down syndrome, even in the absence of ocular pathology and when corrected with spectacles.1, 2 Individuals with Down syndrome commonly have high refractive error, particularly astigmatism,3–6 and elevated levels of higher-order optical aberrations.7 A previous study demonstrated improved visual acuity in participants with Down syndrome when tested with techniques that by-pass the eye’s optics,8 underscoring that current clinical techniques to determine optimum spectacle prescriptions are under-serving the Down syndrome community. Due to intellectual disability, individuals with Down syndrome may experience greater difficulty with the cognitively demanding aspects of a subjective refraction, leaving clinicians to base prescriptions on objective clinical measurements of lower-order refractive error (sphere & cylinder). This is problematic, given the fact that the lower and higher-order aberrations interact to impact retinal image quality and current objective clinical techniques do not account for these interactions.9–11 Thus, objective refraction techniques that consider the unique aberration structure of the individual eye offer a potential path to improved image quality.
The ability to measure whole eye wavefront error has resulted in significant efforts to better understand the impact of optical aberrations on retinal image quality. Various image quality metrics have been defined12 and tested as potential predictors of visual acuity in both normal and highly aberrated eyes.10, 13–16 Two such metrics utilized in the present work, visual Strehl ratio in the spatial domain (VSX) and pupil fraction tessellated (PFSt), have been reported to have a strong correlation with visual acuity,15 making them useful to predict the performance of a given refraction. Recent work has evaluated the use of VSX to identify best refractions by calculating the resultant VSX value for a given sphero-cylindrical refraction applied to the wavefront error of an eye and ranking the refractions by VSX value.17, 18 These VSX optimized refractions have been tested in typical patients using trial frames and were found to provide equivalent visual acuity to subjective refraction,17 but were also preferred over subjective refractions in the majority of subjects evaluated (72% of eyes). A study comparing VSX optimized refractions and subjective refraction in individuals with keratoconus had similar findings: equivalent visual acuity, but a preference for objective refraction for 73% of the eyes.18 The focus of the present work is to expand upon these previous findings and apply them to individuals with Down syndrome by evaluating prescriptions dispensed for extended periods of time.
This study is designed to compare performance of spectacle prescriptions identified by the optimization of two separate image quality metrics to clinically derived prescriptions in a clinical treatment trial of adults with Down syndrome. This manuscript provides the study design and baseline characteristics of individuals enrolled in the trial. In addition, this manuscript reports the inter-session repeatability of the primary outcome measure, distance visual acuity, for participants enrolled in the trial.
METHODS
Selection of Image Quality Metrics for Optimization
In designing the trial, studies were conducted to aid in the selection of specific image quality metrics to be utilized in the identification of the objective refractions. These studies leveraged previously established techniques that incorporated optical error into the appearance of a visual target10, 19, 20 to create distance acuity charts representing the image quality of eyes from patients with Down syndrome when mathematically corrected with refractions identified from optimization of different metrics.21 Typical observers viewed the charts and performed acuity measures for each refraction. This preliminary work demonstrated that refractive corrections optimized using image quality metrics were predicted to provide an improvement in visual acuity over the habitual corrections worn by individuals with Down syndrome.22 VSX was identified as one metric predicted to provide top performing refractions for the majority of eyes, and thus was adopted for the present study.22 However, given that VSX incorporates the neural contrast sensitivity function of a normal observer in its calculation, we sought to identify an additional metric that would not include a neural component, given that neural processing may differ in individuals with Down syndrome. As a result, PFSt was selected given that it frequently identified best refractions different from VSX, and given that these refractions were still predicted to improve visual acuity over habitual corrections.22
Study Aims
In launching this trial, a necessary goal was to establish the inter-session repeatability of the primary outcome measure, distance visual acuity (described in detail below), using measures obtained with habitual corrections prior to dispensing treatment to the participants. These data were collected at the initial study visit and the beginning of the dispensing visit, prior to exposing participants to new spectacle corrections.
The primary goal of this clinical trial is to evaluate the performance of spectacle prescriptions identified through both clinical refraction and the optimization of two retinal image quality metrics (VSX and PFSt) in a randomized, clinical treatment trial. Specifically, this study will:
Measure visual acuity outcomes of metric optimized spectacle prescriptions versus clinically derived prescriptions at an initial dispense visit.
Measure visual acuity outcomes of metric optimized spectacle prescriptions versus clinically derived prescriptions after two months of spectacle wear.
Measure spectacle compliance (average hours of wear) of metric optimized spectacle prescriptions versus clinically derived prescriptions over two months.
Regulatory Compliance
This study was approved by the University of Houston Committee for the Protection of Human Subjects and conducted at the University of Houston, College of Optometry. Parental/guardian permission was obtained for all participants, followed by participant assent. This study was registered on ClinicalTrials.gov (NCT03367793), in accordance with NIH policy. A data and safety monitoring board was not assigned to this trial, given the low risk of the intervention (spectacles), but a data and safety monitoring plan was submitted and participant safety monitored locally by the unmasked investigator. The baseline information reported here conforms to the CONSORT guidelines with both a completed checklist and enrollment report (Figure 1).
Figure 1.
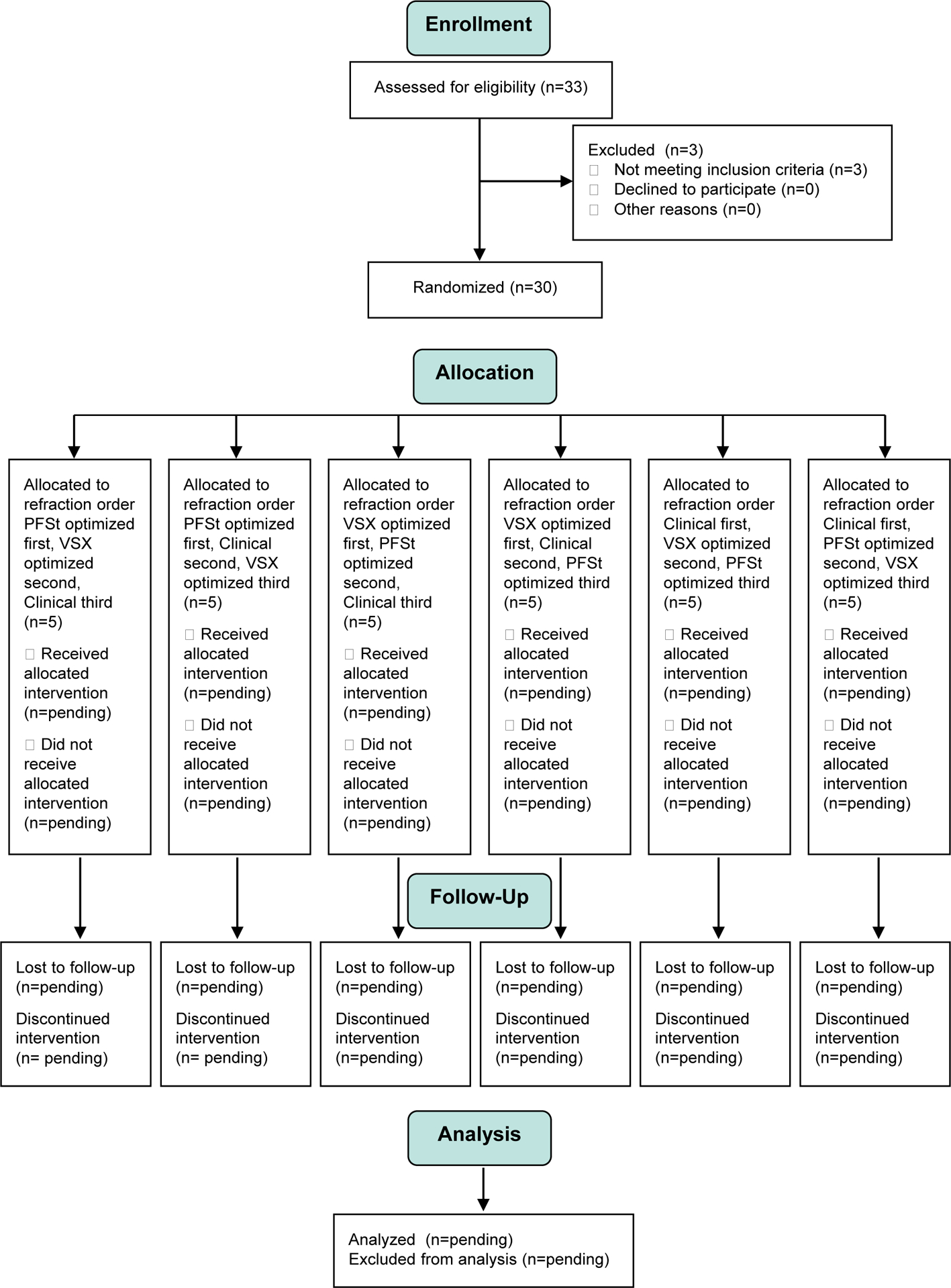
CONSORT diagram depicting participant enrollment and randomization. VSX = visual Strehl ratio in the spatial domain; PFSt = pupil fraction tessellated.
Eligibility Criteria
Eligibility criteria for the study included a diagnosis of Down syndrome (by parental/guardian report), minimum age of 18 years, and a willingness to undergo randomization. Exclusion criteria included a history of refractive or intraocular surgery, nystagmus (manifest, or observed upon occlusion of an eye), strabismic amblyopia (≥ 3 lines difference in distance visual acuity with the presence of strabismus), or anisometropic amblyopia (≥ 3 lines difference in distance visual acuity with > 1 diopter difference in spherical equivalent, or > 1.50 diopters difference in cylinder power). In addition, participants had to be able to be dilated, fixate several seconds for wavefront imaging, and have clear ocular media for image capture.
Recruitment
Participants were recruited from the investigator’s past study participants, the University of Houston’s University Eye Institute, local Down syndrome organizations, and word of mouth. Since many participants were pre-screened for eligibility criteria due to past participation in the investigator’s studies, or clinical examination at the University Eye Institute, the rate of eligibility was likely higher than recruitment from the general population. Recruitment began January 26, 2018 and concluded October 29, 2018.
Initial Study Visit
All initial study visits included a comprehensive eye examination conducted by a single investigator who is a licensed, pediatric optometrist with more than 35 years of experience examining children and individuals with special needs. The visit included determination of a clinical refraction (described below), visual acuity, binocular vision and ocular health assessments, and determination of participant eligibility. In addition to standard clinical measurements, pupil diameter was recorded with a dynamic, infrared photorefractor (PowerRef 3, Plusoptix, Nuremberg, Germany) during visual acuity measures (dim condition: room lights off with computerized display luminance of 380 cd/m2) and with all room lights and displays off (dark condition). Participants were then dilated with 1% tropicamide and 2.5% phenylephrine (separated by 4 to 6 minutes), after which topography, internal ocular health assessment, wavefront aberrometry, and an optional repeat of retinoscopy and autorefraction (at the clinician’s discretion) were performed.
Clinical Refraction
The investigator was not held to a strict protocol to determine the clinical refraction, but was asked to draw on her best clinical judgement and experience to optimize the refraction for each participant. Clinical measures used included lensometry of habitual spectacles, autorefraction (both dry and damp), retinoscopy (both dry and damp), and subjective refraction. During the examination, the investigator utilized findings of near visual acuity and accommodative lag to determine whether to prescribe a bifocal lens. Use of the phoropter, a trial frame, or loose lenses were all permitted in the determination of both the distance refraction and the added lens power. Accommodative lag testing was ordinarily performed through the clinical refraction, with a few instances of additional testing through the habitual correction. The decision to prescribe a bifocal lens was at the sole discretion of the clinical investigator conducting the initial study visit.
Metric Optimized Refractions
Following the initial study visit, 3 to 5 wavefront images per eye were re-sized to the individual participant’s average pupil diameter in dim illumination and the resultant images averaged using a custom program (Spectacle Sweep, University of Houston College of Optometry Core Programming Module, Houston, TX) written with MATLAB (MathWorks, Natick, MA). Spectacle Sweep was then used to apply refractions over a search range of 20,000 or more sphero-cylindrical combinations ranging from at least ±3 D in 0.25 D steps surrounding the participant’s habitual sphere correction and at least 0 to −4 DC in −0.25 DC steps (greater in cases of high habitual cylinder) for the entire range of cylindrical axes in 1 degree steps. For each refraction, the residual wavefront error was output, as well as the resultant value of each of the image quality metrics VSX and PFSt. Refractions were then sorted by metric value and each of the single refractions providing the best value for VSX and PFSt, respectively, were determined for each eye. For participants prescribed bifocals by the clinical investigator, the same added bifocal power was prescribed for each metric optimized refraction.
Production of Spectacles
Participants selected a single frame for use in the study and were permitted to have transitions, antireflective coating, and/or high-index lenses similarly added to all three prescriptions. For participants prescribed a bifocal, a flat-top bifocal fit 1–2mm above the lower lid was used. After frame selection, three identical frames (fit and color) were ordered and filled with each of the prescriptions (clinical, PFSt, and VSX). Once spectacles were produced, a site optician, the unmasked investigator, and an ancillary study member with optical training all independently performed lensometry to verify the spectacles were within the ANSI Z80.1 standards for manufacturing spectacles.23 Any lenses deemed to be out of tolerance were returned and spectacles refabricated to meet the standards.
Randomization and Masking
This study included three treatments with a crossover design. A sample size of 30 individuals with Down syndrome was enrolled and randomized into one of the 6 possible randomization orders for dispensing of the three different spectacle prescriptions for 2 months each. An equal number of participants received each treatment order. The unmasked study investigator opened a sealed envelope with the randomization order for each participant and marked each of the spectacles to indicate the order of randomization which was confirmed by an ancillary study member. All other study personnel, as well as study participants, were masked to the spectacle powers and methodology used to determine the prescription.
Initial Dispensing Visit
The goals of the initial dispensing visit were to:
Establish inter-session repeatability of visual acuity with the participant’s habitual refractive correction worn to the initial study visit (unaided if they did not have refractive correction)
Measure visual acuity to compare performance of the three experimental spectacle prescriptions upon first exposure
Determine whether each pair of spectacles meets the safety criteria to be dispensed for the two month treatment period
Safety criteria were defined to avoid dispensing a pair of spectacles that produced a visual experience worse than how the participant entered the study. To accomplish item 3, distance and near acuity, stereoacuity, and cover test were performed with each pair of spectacles and compared to the findings from the initial study visit. If any of the following was true for a pair of spectacles, it was deemed unsafe to dispense:
Binocular distance visual acuity more than 7 letters worse than presenting acuity at Visit 1
Binocular near visual acuity more than 1 line worse than presenting near acuity at Visit 1
Stereoacuity decreased by more than 2 levels from presenting stereoacuity at Visit 1 (Testing Levels: 800, 400, 200, 100, 60, and 40 arcsec)
Manifestation of strabismus that was previously not observed
If the failed spectacles were the first pair in the randomization order, the failed item was re-tested after all other spectacles were evaluated. Upon a second failure, that pair was deemed unsafe and the second pair in the randomization scheme dispensed instead. If the failed spectacles were the second or third pair in the randomization order, they were re-tested when the time arose for them to be dispensed, but if they failed again, they were not dispensed. The rationale to permit re-testing was related to the common occurrence of hyperopia in this population and the known clinical phenomenon that residual accommodative tonus will often produce blur upon initial exposure to a new or increased hyperopic correction.
Visit Schedule
The schedule for study visits and tests performed is shown in Table 1 with more detailed descriptions of tests provided in the text to follow. The timing of the visits was calculated from the date of the dispensing visit. The number of visits was determined by whether or not all three spectacle prescriptions met the safety criteria. For each pair of spectacles dispensed, participants returned for a 1 month and 2 month follow-up. All follow-up visits included measures of distance and near visual acuity, stereoacuity, and cover test. The 2-month follow-up visit also included a participant survey about perceptions of the spectacles, accommodative lag measures by monocular estimated method, and contrast sensitivity testing. The findings at the 2 month follow-up were considered the adapted findings and used in the analysis of the primary and secondary study outcomes.
Table 1.
Visit Schedule.
| Visit Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Visit Purpose | Baseline | Randomization | Specs 1 Month 1 |
Specs 1 Month 2, Dispense Specs 2 |
Specs 2 Month 1 |
Specs 2 Month 2, Dispense Specs 3 |
Specs 3 Month 1 |
Specs 3 Month 2 & Best Specs Identified & Dispensed |
Best Specs Month 6 |
| Planned Time from Randomization | N/A | 0 weeks | 4 (±1) weeks | 8 (±1) weeks | 12 (±1) weeks | 16 (±1) weeks | 20 (±1) weeks | 24 (±1) weeks | 50 (±2) weeks |
| Correction and Testing Order (tests listed below) |
Habitual | Habitual Autorefraction Specs 1 Specs 2 Specs 3 |
Specs 1 | Specs 1 | Specs 2 | Specs 2 | Specs 3 | Specs 3 Specs 1 Specs 2 Specs 3 |
Best Specs |
| Baseline | Outcome Specs 1 | Outcome Specs 2 | Outcome Specs 3 | Extended Follow-Up | |||||
| Clinical Exam with Dilation | X | ||||||||
| Vineland 2 Behavioral Assessment | X | ||||||||
| Grand Seiko Autorefraction | X | X | |||||||
| Distance Visual Acuity | X | X | X | X | X | X | X | X | X |
| Near Visual Acuity | X | X | X | X | X | X | X | X | X |
| Cover Test | X | X | X | X | X | X | X | X | X |
| Accommodative Lag by Monocular Estimate Method Retinoscopy | X | X | X | X | X | ||||
| StereoAcuity | X | X | X | X | X | X | X | X | X |
| Temperature Sensor Compliance Monitor | X | X | X | X | X | X | |||
| Participant Survey of Spectacle Perceptions | X | X | X | ||||||
| Contrast Sensitivity | X | X | X | ||||||
| Slit Lamp Examination | X | X | |||||||
| Zeiss Atlas Corneal Topography | X | X | X | ||||||
| Oculus Pentacam Corneal Topography | X | X | |||||||
| COAS HD Wavefront Aberrometry | X |
Visit schedule and tests performed for participants enrolled in the trial. Detailed descriptions of each test can be found in the body of the manuscript
Once the outcome measures were completed for the final randomized spectacles, participants were re-tested on distance and near visual acuity, stereoacuity, cover test, and accommodative lag for each pair of spectacles in their assigned randomization order. This was done to determine whether visual acuity had improved or degraded gradually over time, irrespective of the treatment. In addition, a masked clinical examiner compared performance of the spectacles by reviewing all clinical measures obtained throughout the study to determine which single prescription performed the best and should be dispensed for long-term follow-up. Both examiners and participants remained masked to the prescription worn for the extended follow-up and a final study visit was conducted 6 months later to determine whether additional gains in acuity were obtained with prolonged exposure to the ‘best’ performing refraction.
Due to the suspected association between keratoconus and Down syndrome,24, 25 and to assist in the determination of whether participants randomized into this study had progressive corneal changes that could impact refractive stability, corneal topography was performed at 3 time points throughout the study. A clinical investigator with expertise in the diagnosis and management of patients with keratoconus evaluated all participants with slit lamp examination at the final study visit and reviewed topography to determine whether changes had occurred. This investigator made a clinical judgement whether the participant was suspect for keratoconus (abnormal corneal topography with no slit lamp signs), or had keratoconus (presence of slit lamp signs). These data will potentially allow sub-analysis of study participants, as well as determination of whether corneal structure remained stable over time.
Study Measurements
Each study visit was attended by the unmasked investigator, a masked clinical examiner, and a masked non-clinical examiner. The unmasked examiner performed lensometry to verify spectacle lens powers and obtained ocular and health history updates from the participant and parent/guardian. Standardized study measurements included in the protocol are described below. The order of testing was not fixed with the exception that during the initial study visit that required dilation, all non-dilated procedures were performed prior to instillation of drops.
Vineland 2 Behavioral Assessment
The Vineland Adaptive Behavior Scales Second Edition (NCS Pearson, Inc. Ontario, Canada) was used to provide a measure of participant developmental ability. This survey was completed on paper by the parent/guardian and included categorical assessments of communication, daily living skills, socialization, and motor skills, which combined together to provide an assessment of overall adaptive behavior.
Grand Seiko Autorefraction
Unaided distance autorefraction was obtained (5 measures per eye) as participants viewed a 1.0 logMAR letter on a chart placed 4 meters away. All participants were measured without dilation, with optional additional measures post-dilation to assist in determining the clinical refraction.
Distance Visual Acuity:
Distance visual acuity served as the primary outcome measure and was performed using a logMAR style chart presented one line at a time on a high contrast LCD monitor (1200 × 1600 pixels; luminance: 380 cd/m2) at a viewing distance of 3.7 meters (controlled by use of a chin/forehead rest). This viewing distance does have a small accommodative demand (0.25D); however, any impact on acuity would be equally observed across all treatments tested. Charts composed of 5 letter lines of either the British standard 1968 recommended letters (D, E, F, H, N, P, R, U, V, Z) or a restricted set (H, O, T, V) with one repeated letter per line, were shown, depending upon the cognitive ability of the participant. A matching card was used if needed. The largest line size (0.8 logMAR) was presented first, continuing line by line until the participant made five total mistakes. Three investigators assisted in the measurement of acuity: a masked examiner who monitored participant position and solicited responses, a masked examiner who pointed to the monitor letter by letter and entered participant responses on a keyboard, and the unmasked examiner who wrote down responses as a back-up to the electronic data entry. Acuity was measured for each individual eye (order randomized at the initial study visit and maintained throughout the study), followed by a binocular acuity measure.
Near Visual Acuity
Near visual acuity was performed using the ATS4 Near Visual Acuity Test (Precision Vision, Woodstock, IL) which consists of the letters H, O, T, and V surrounded with crowding bars arranged in rows of four letters each.26 Acuity was tested binocularly by a masked examiner at 40cm with full room illumination in addition to a near lamp shone directly on the card. Testing began at the largest print size (1.30 logMAR) and threshold recorded as the smallest size at which 3 of 4 letters were correctly identified. Participants who had bifocal lenses were tested with and without their added correction.
Cover Test
Both unilateral and alternate cover test were performed at distance and near by a masked examiner using fixation targets appropriately sized for the participant’s acuity. The magnitude of the deviation was the highest prism power for which no visible movement occurred. Participants who had bifocal lenses were tested with and without their added correction for near cover test.
Monocular Estimate Method Retinoscopy
Monocular estimate method (MEM) retinoscopy was used to measure the accommodative lag at 40cm in the horizontal meridian for each eye separately. Participants were tested through their distance correction in primary gaze while viewing the Heine monocular estimate method cards (either the figures or grade level 1 card) as loose lenses were introduced to determine the power providing a neutral reflex.
Stereoacuity
All stereoacuity testing was performed at 40cm in full room illumination in addition to a near lamp shone directly on the book. The presence or absence of gross local stereo (59 arcmin) was assessed using the fly picture in the Stereo Fly test (Stereo Optical Company, Inc., Chicago, IL). Next, global stereopsis was assessed using the Randot Preschool Stereoacuity Test (Stereo Optical Company, Inc., Chicago, IL). Participants were first evaluated for test comprehension by matching a non-stereo shape printed on a card. The finest level at which 2 of 3 shapes were correctly identified was recorded, or NIL if the participant did not pass the coarsest level.
Compliance Monitor
Spectacle compliance (hours worn per day) was as a secondary outcome measure of this study as recorded with the ACR Systems Smart Button data logger (ACR Systems Inc., Surrey, BC Canada).27 Data loggers were placed in silicone mounts on the temple of participants’ spectacles and collected temperature every 20 minutes for 28 days. Data were downloaded at each visit and the logger reset and dispensed for another month. Temperature versus time plots were viewed independently by two examiners and marked for transitions of spectacles on and off using Temperature Log Viewer Version 2.0 (University of Houston College of Optometry Core Programming Module, Houston, TX). The total wear time in minutes, as identified by the two examiners, was averaged, and converted to average hours of wear per day.
Participant Survey
Participant perceptions of the spectacles were used as a secondary outcome measure in this study. At the 2 month follow-up visit for each pair of spectacles, participants completed a survey read to them by a masked examiner that consisted of three questions rated on a five item scale: Do you like wearing this pair of glasses? How well do you see with this pair of glasses when looking far away? How well do you see with this pair of glasses when looking up close? Participants also answered ‘yes’ or ‘no’ to the question: Do you see better with these glasses than without glasses?
Contrast Sensitivity
Contrast sensitivity was measured with the CamBlobs 2.1 worksheets (Precision Vision, Woodstock, IL).28 Participants were given a worksheet that included four columns and 25 rows (total of 100 cells) with a circle located at 1 of six positions within each cell (contrast range 0.95 to 2.15 logCS). Testing was performed in full room illumination with a near lamp directed at the worksheet. Working distance was not fixed, as per the standard administration of the test. Participants marked the location of each circle with a pen, or pointed with their finger. Testing began at the top row and the threshold was recorded as the lowest row at which 3 of 4 circles were correctly identified.
Slit Lamp Examination
Slit lamp examination was performed by a masked clinical examiner at both the initial and final study visit specifically to screen for optical opacities (corneal or lenticular) that may impact visual acuity, as well as by a clinician with specific training in the diagnosis and management of corneal disease at the final study visit to screen participants for subtle corneal signs associated with keratoconus.
Zeiss Atlas Corneal Topography (Carl Zeiss Meditec, Inc. Jena, Germany)
Measures were obtained on each eye with the goal of obtaining 3 high quality measurements per eye (full aperture, no distortion of the rings due to defocus or tear film, and with central fixation).
Oculus Pentacam Topography (Oculus, Inc. Arlington, WA)
Measures were obtained on each eye with the goal of obtaining 1 high quality measurement per eye as judged by the internal criterion set by the instrument for categorization of ‘OK’ image quality.
COAS-HD Wavefront Measurement (Johnson & Johnson Vision, Santa Ana, CA)
Measures were obtained beginning 30 minutes post dilation with the goal of obtaining 5 high quality measurements per eye. Shack-Hartmann spot images were evaluated for pupil diameter at least 5 mm (preferably 6 mm or greater), no areas of missing spots, or significant corneal reflex. Refractions included on the display by the aberrometer were also evaluated during image capture to identify images that may be of poor quality as evidenced by refractions differing sizably from fellow images. Wavefront measurements through the 10th radial order were exported for data analysis and metric optimized refraction determination. To provide a single value estimate of the magnitude of higher order aberrations for comparison with previously published cohorts, the higher order RMS of the average of the wavefront measures resized for a 4mm pupil diameter was calculated for each eye.
Statistical Analysis Plan
This manuscript reports the inter-session repeatability of distance visual acuity in our cohort of adults with Down syndrome. To assess the within-subject repeatability of visual acuity measures across visits 1 & 2, we estimated the within subject standard deviation which was then used to calculate repeatability (1.96 * within subject standard deviation).
For analysis of the clinical trial data, we will report descriptive statistics by period and treatment for primary outcome visual acuity (logMAR) and additional objective compliance and subjective prescription preference/quality. A mixed-effects linear modeling statistical approach will be used to compare differences in visual acuity among the three spectacle prescriptions with sequence and period as fixed effects and participant as a random effect to account for within-subject and between-subject variability, as well as to evaluate period and period by treatment effects. The overall effect of treatment via an overall F-test, which accounts for the covariance structure of the variance-covariance matrix, will be used in SAS (PROC GLIMMIX). Follow-up testing (via Tukey post-hoc) will be performed to further elucidate differences between experimental prescriptions. Should a period by treatment effect be detected, period 1 will be used for primary analysis. Assuming the objective measure of compliance, via temperature sensors, is continuous and normally distributed, we will adopt a similar statistical approach. Subjective prescription quality (or satisfaction) is based on a 5-point scale. We propose generalized estimating equations (GEE) to analyze the subjective quality outcome (ranging from 1–5) as a categorical response (e.g., as binary, we will define reported 4/5 as satisfactory; 0-otherwise).
Power Justification for Sample Size of 30
Primary Outcome Visual Acuity (logMAR)
For purposes of this power analysis, we assume a one-way, repeated measures design, ignoring the complexity of specifying sequence terms. Assuming an average correlation among the participants’ responses to the three treatments is 0.5, 30 participants is sufficient to obtain a moderate effect size, where the effect size is a function of Cohen’s estimated effect size for a one-way ANOVA and the average correlation among responses.29,30
Outcome Temperature Sensor
Using a similar power analysis approach, n=30 is justified to yield at least 80% power to detect larger effect sizes, assuming that the average correlation of compliance (measured as average hours of spectacle wear) across the three treatments is as small as 0.3.
Outcome Subjective Quality
To justify the sample size based on distribution-free methods in a 3×3 crossover with potential fixed effects is not common, and thus justification based on the binary ‘desirable to wear’ is presented here. Assuming no period effects and a binary outcome (i.e. reported value of 4 or 5 is deemed ‘desirable to wear’ and termed a successful outcome), power was computed from two separate McNemar’s tests, based on equality of discordant pairs, to compare proportion of successes among intervention prescriptions and the clinical prescription, assuming a conservative Type I error of 0.025. A sample size of n = 30 yields at least 82% power to detect a difference of 0.4.
RESULTS
Initial study visits were conducted on thirty-three participants. Three participants did not meet eligibility criteria due to either 1) macular disease, 2) corneal scarring in one eye, or 3) unwillingness to sit for study measurements using instruments with chin and forehead rests.
Thus, a total of 30 participants were randomized into the study. Baseline characteristics of these 30 participants are shown in Table 2 both by randomization group and total group. Participants had an average age of 29 ± 10 years (range = 18 to 52) and an even distribution between male and female. Participant race included 23 Caucasians, 4 African Americans, 1 Asian, 1 with more than one race, and 1 selecting other. Participant ethnicity included 23 non-Hispanics and 7 Hispanics. Patients were tested at the initial study visit using their habitual correction; 21 presented with spectacles for full-time wear and 9 presented and were tested unaided.
Table 2.
Baseline Characteristics.
|
Randomization Group |
PFSt VSX Clinical |
PFSt Clinical VSX |
VSX PFSt Clinical |
VSX Clinical PFSt |
Clinical PFSt VSX |
Clinical VSX PFSt |
Total |
|---|---|---|---|---|---|---|---|
| N | 5 | 5 | 5 | 5 | 5 | 5 | 30 |
| Age (years) | 28 ± 10 | 28 ± 12 | 24 ± 4 | 34 ± 9 | 30 ±13 | 32 ± 9 | 29 ± 10 |
| Gender (F = female, M = male) | F-4 M-1 |
F-1 M-4 |
F-3 M-2 |
F-3 M-2 |
F-1 M-4 |
F-3 M-2 |
F-15 M-15 |
| # Presenting with Spectacles | 4 | 4 | 2 | 3 | 4 | 4 | 21 |
| # with strabismus @ Distance (Cover Test) | 2 | 1 | 1 | 2 | 1 | 2 | 9 |
| # with strabismus @ Near (Cover Test) | 2 | 1 | 1 | 2 | 1 | 2 | 9 |
| # with Positive Finding on StereoFly | 3 | 3 | 3 | 2 | 4 | 4 | 19 |
| # with at least 800”on Preschool Randot | 1 | 0 | 2 | 2 | 1 | 2 | 8 |
| Autorefraction Spherical Equivalent OD (D) | −5.23 ± 4.75 | 0.98 ± 1.27 | −1.75 ± 4.37 | −3.73 ± 7.33 | +1.93 ± 3.07 | −1.68 ± 4.53 | −1.58 ± 4.88 |
| Autorefraction Spherical Equivalent OS (D) | −3.53 ± 3.73 | −0.05 ± 2.43 | −1.58 ± 4.55 | −2.68 ± 6.81 | +1.95 ± 2.52 | −2.03 ± 5.91 | −1.32 ± 4.59 |
| Autorefraction Cylinder OD (DC) | −1.35 ± 0.68 | −2.25 ± 1.08 | −1.50 ± 0.68 | −2.65 ± 2.91 | −3.65± 1.43 | −1.45 ±0.67 | −2.14 ± 1.58 |
| Autorefraction Cylinder OS (DC) | −2.05 ± 0.37 | −2.10 ± 0.42 | −1.95 ± 1.45 | −1.55 ± 1.81 | −3.60 ± 1.32 | −1.15 ± 0.68 | −2.07 ± 1.30 |
| Refractive Error Category # of Eyes (M = myopia, H = hyperopia, A = mixed astigmatism, E = emmetropia) |
M-8 H-1 A-0 E-1 |
M-1 H-4 A-5 E-0 |
M-4 H-3 A-3 E-0 |
M-4 H-3 A-3 E-0 |
M-1 H-6 A-3 E-0 |
M-5 H-4 A-1 E-0 |
M-23 H-21 A-15 E-1 |
| Accommodative Lag OD (D) | 1.94 ± 0.83 | 1.30 ± 0.57 | 0.80 ± 0.48 | 0.80 ± 0.54 | 1.75 ± 0.64 | 1.19 ± 0.31 | 1.28 ± 0.68 |
| Accommodative Lag OS (D) | 1.55 ± 0.78 | 1.05 ± 0.37 | 1.05 ± 0.54 | 1.10 ± 0.42 | 1.40 ± 0.95 | 1.00 ± 0.35 | 1.20 ± 0.60 |
| Distance Visual Acuity OD (logMAR) | 0.54 ± 0.15 | 0.44 ± 0.20 | 0.40 ± 0.18 | 0.48 ± 0.20 | 0.60 ± 0.29 | 0.34 ± 0.12 | 0.47 ± 0.20 |
| Distance Visual Acuity OS (logMAR) | 0.48 ± 0.11 | 0.43 ± 0.16 | 0.37 ± 0.20 | 0.40 ± 0.18 | 0.51 ± 0.24 | 0.34 ± 0.10 | 0.42 ± 0.17 |
| Interocular Difference in Distance Visual Acuity (logMAR) | 0.07 ± 0.07 | 0.10 ± 0.06 | 0.12 ± 0.08 | 0.11 ± 0.10 | 0.09 ± 0.08 | 0.10 ± 0.09 | 0.10 ± 0.07 |
| Distance Visual Acuity OU (logMAR) | 0.46 ± 0.16 | 0.38 ± 0.15 | 0.30 ± 0.14 | 0.40 ± 0.13 | 0.48 ± 0.29 | 0.29 ± 0.10 | 0.39 ± 0.17 |
| Near Visual Acuity OU (logMAR)* | 0.40 ± 0.16 | 0.40 ± 0.10 | 0.30 ± 0.07 | 0.36 ± 0.11 | 0.48 ± 0.24 | 0.26 ±0.13 | 0.37 ± 0.15 |
| Dim Pupil Diameter OD (mm) | 4.4 ± 1.0 | 4.2 ± 0.6 | 4.2 ± 0.8 | 4.8 ± 1.0 | 4.4 ± 0.5 | 4.3 ± 0.8 | 4.4 ± 0.8 |
| Dim Pupil Diameter OS (mm) | 4.2 ± 1.2 | 4.0 ± 0.8 | 4.3 ± 1.0 | 4.4 ± 0.9 | 4.4 ± 0.5 | 4.0 ± 0.9 | 4.2 ± 0.8 |
| Dark Pupil Diameter OD (mm) | 5.6 ± 0.9 | 5.1 ± 1.1 | 5.3 ± 0.7 | 5.7 ± 0.7 | 5.5 ± 0.4 | 5.2 ± 1.1 | 5.4 ± 0.8 |
| Dark Pupil Diameter OS (mm) | 5.5 ± 0.8 | 5.1 ± 0.7 | 5.6 ± 0.6 | 5.6 ± 0.8 | 5.4 ± 0.6 | 5.2 ± 1.1 | 5.4 ± 0.7 |
| Adaptive Behavior Standard Score | 65.2 ± 11.3 | 59.8 ± 16.6 | 55.6 ± 11.2 | 53.0 ± 22.4 | 66.2 ± 20.4 | 70.4 ± 21.1 | 61.7 ± 17.3 |
| Higher Order RMS for 4mm pupil OD (μm) | 0.27 ± 0.12 | 0.19 ± 0.10 | 0.17 ± 0.03 | 0.22 ± 0.05 | 0.24 ± 0.06 | 0.20 ± 0.08 | 0.21 ± 0.08 |
| Higher Order RMS for 4mm pupil OS (μm) | 0.23 ± 0.05 | 0.17 ± 0.10 | 0.17 ± 0.06 | 0.18 ± 0.06 | 0.20 ± 0.06 | 0.25 ± 0.08 | 0.20 ± 0.07 |
Baseline characteristics of randomized study participants. Randomization Group lists the order that spectacle refraction types were dispensed. OD = right eye, OS = left eye, OU = both eyes, D = diopter, DC = diopter cylinder, mm = millimeters, μm = micrometers; VSX = visual Strehl ratio in the spatial domain; PFSt = pupil fraction tessellated. *Near Visual Acuity is reported OU for 29 of 30 participants and OD for 1 participant
Consistent with the eligibility requirements, no participants had nystagmus, anisometropic amblyopia, or strabismic amblyopia; however, strabismus was present at both distance and near in 9 study participants (7 with eso deviations and 2 with exo deviations). Of these participants, the majority (n=7) had alternating strabismus, thus reducing the risk for development of strabismic amblyopia. Stereoacuity was overall poor in the study group, with 11 participants having no demonstrable stereoacuity on even the coarsest local stereotest (StereoFly). This included 7 participants with strabismus and 4 with phoric deviations.
The range of refractive error, as determined by non-dilated measures from the Grand Seiko autorefractor, was large (−15.25 to +6.00 D sphere) with all but two participants having at least one eye with −1.00 D cylinder power or more (range: −7.50 to −0.25 D cylinder) (Figure 2). Refractive error was classified for each eye based on the individual powers of the principal meridians and cylinder magnitude. Classification included 23 myopic eyes (both principal meridians of −0.50 D or more myopia), 21 hyperopic eyes (most plus meridian at least +1.00 D with the fellow meridian zero or greater), 15 eyes with mixed astigmatism (one principal meridian with plus power and the other with minus with at least 0.50 D cylinder), and 1 eye with emmetropia (both principal meridians falling between −0.50 to +1.00 D with less than 0.50 D cylinder). Higher order root-mean-square through the 10th radial order was also calculated based on analysis of a 4 mm pupil diameter. The average and standard deviation for all eyes combined was 0.20 ±0.07 microns (Figure 3). For context, these data are shown along with the 5 – 95% range of higher order root-mean-square (through at least the 6th radial order) reported for 1,690 eyes from age-matched individuals without Down syndrome in a published normative dataset.31
Figure 2A.
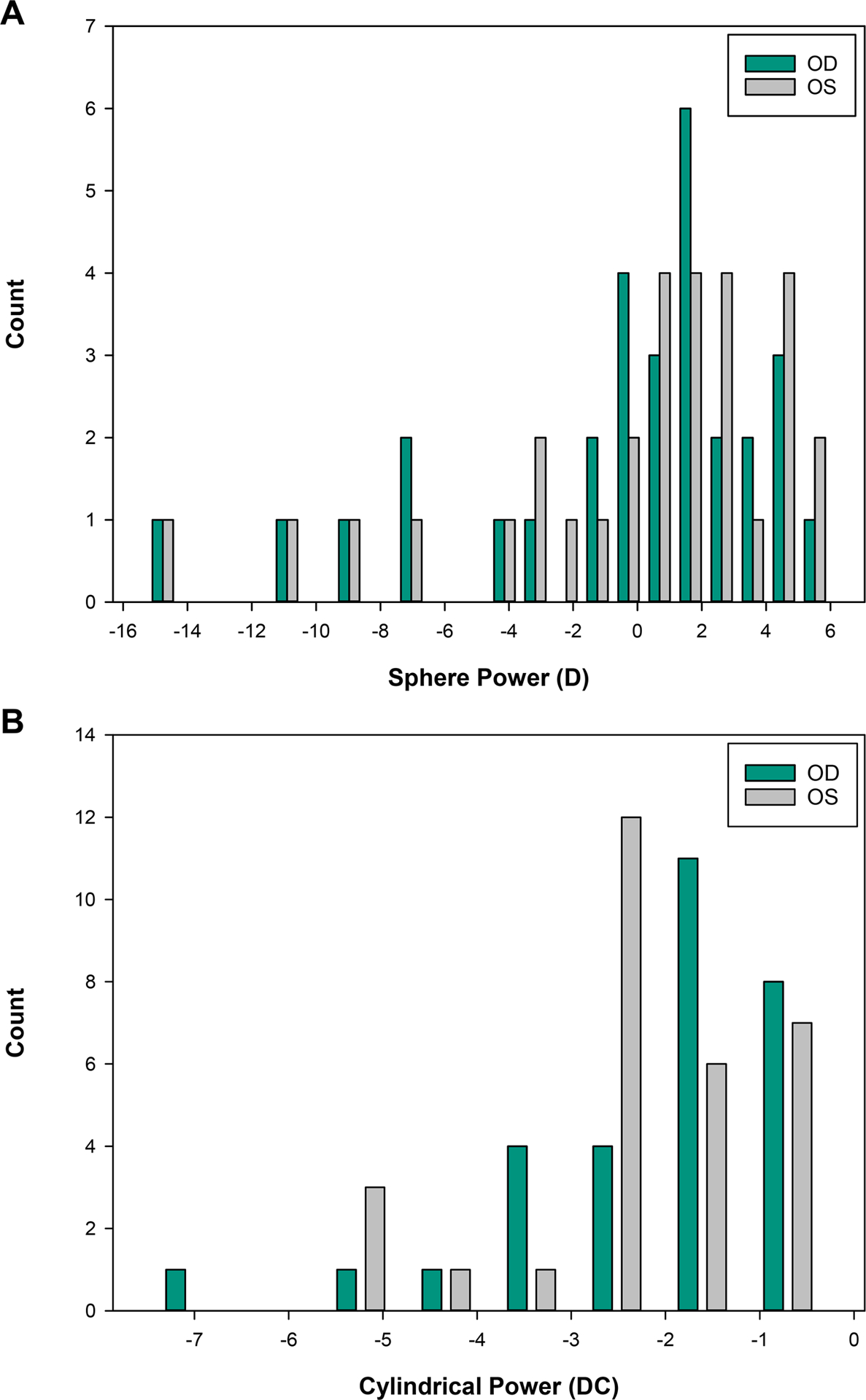
Distribution of participant spherical refractive power by non-dilated distance autorefraction obtained at the initial study visit. D = diopter Figure 2B. Distribution of participant cylindrical refractive power by non-dilated distance autorefraction obtained at the initial study visit. DC = diopter cylinder
Figure 3.
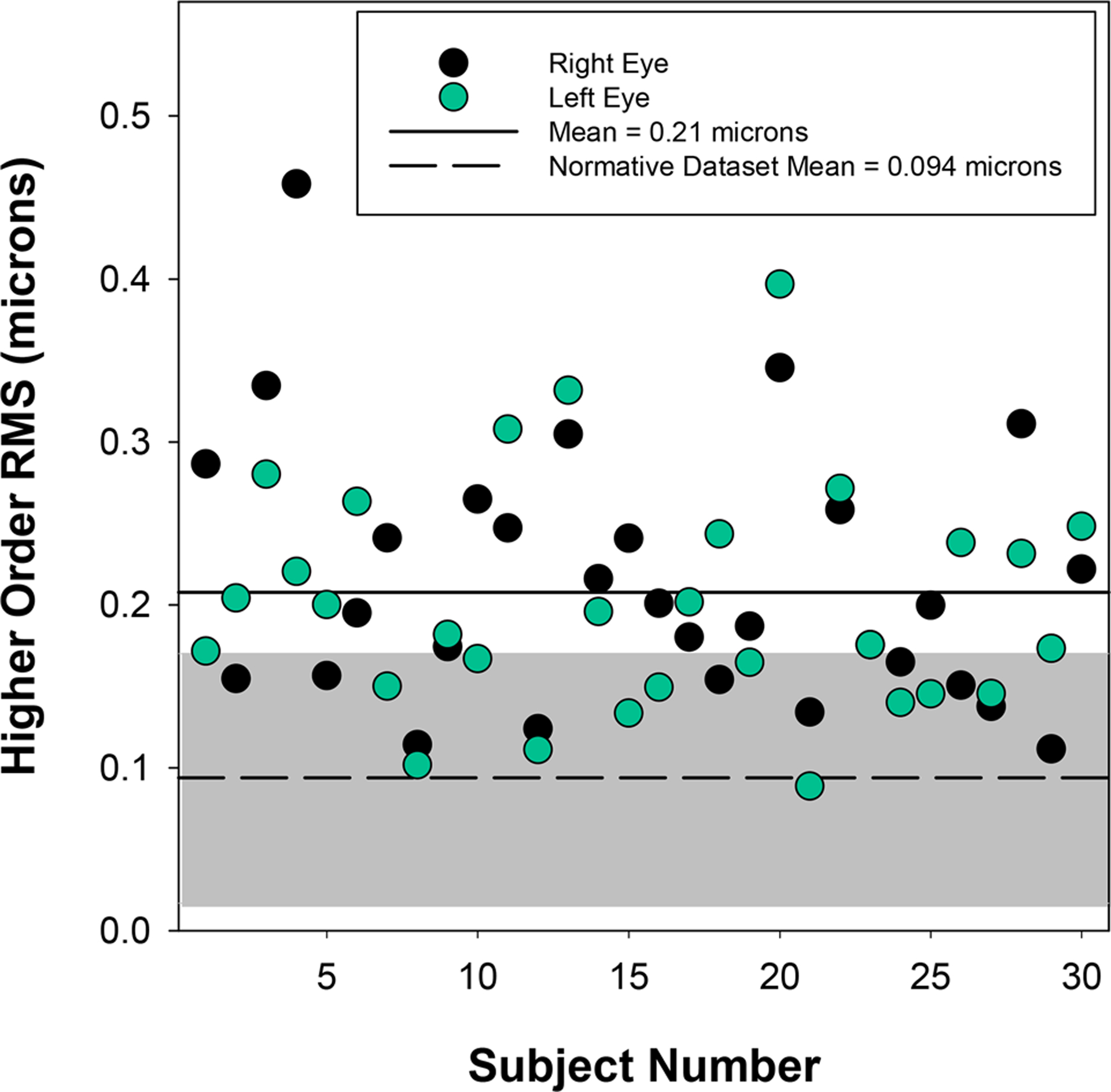
Higher order RMS through the 10th radial order calculated for a 4mm pupil diameter from dilated measures obtained with the COAS-HD wavefront aberrometer. The gray shaded region represents the 5 to 95% range of values reported for eyes (n = 1690) from an age-matched group of individuals without Down syndrome published in a normative database.31
Visual acuity with presenting correction using the logMAR style computerized testing system averaged 0.47 ± 0.20 logMAR for the right eye and 0.42 ± 0.17 logMAR for the left eye with an inter-ocular acuity difference of 0.10 ± 0.07 logMAR. Binocular acuity was 0.39 ± 0.19 logMAR at distance. Binocular acuity at near was 0.37 ± 0.15 logMAR using the ATS4 HOTV chart (Figure 4). Distance visual acuity testing was accomplished using the British Standard 1968 letter set for 29 participants and the HOTV letter set for 1 participant.
Figure 4.
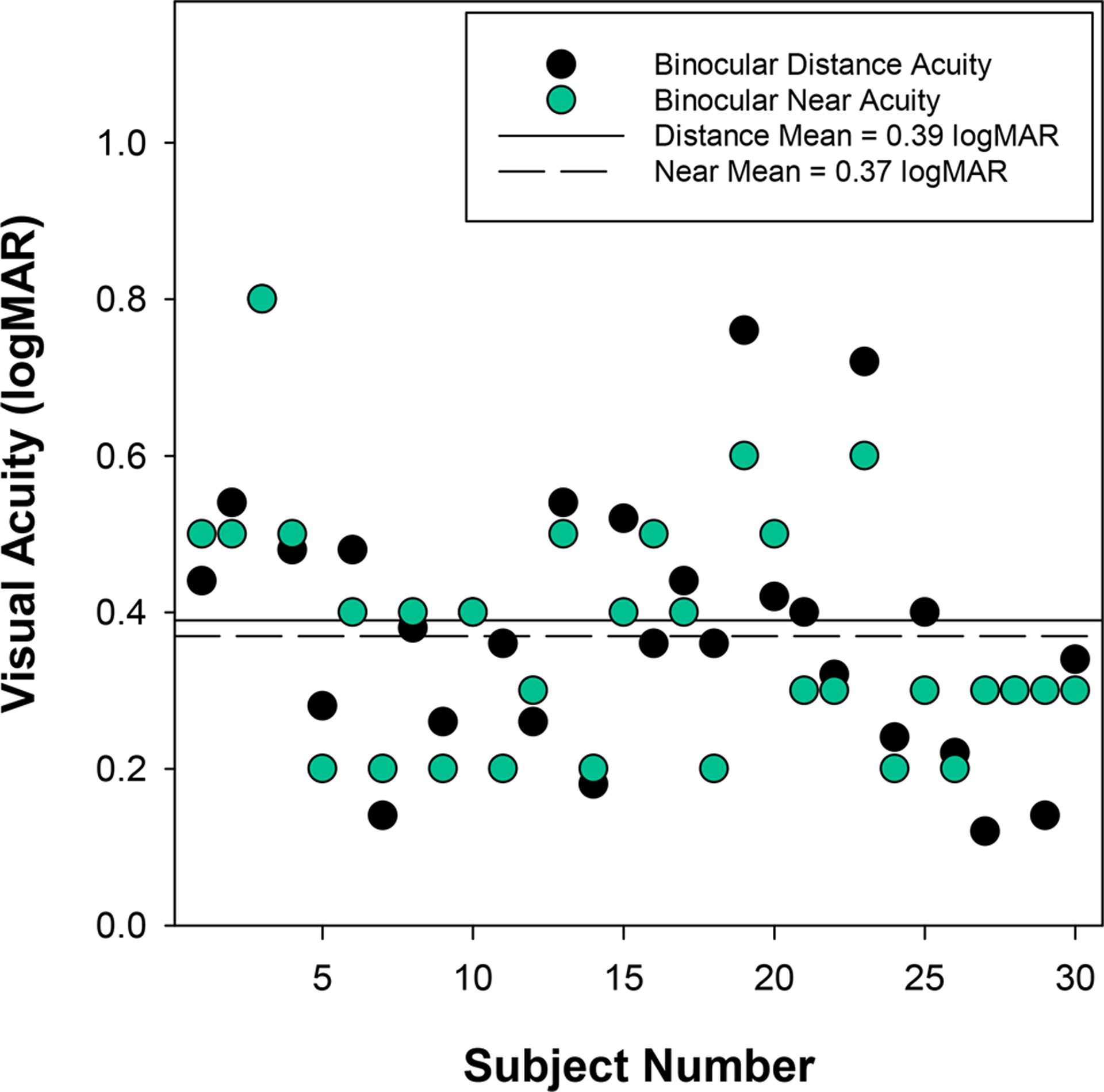
Binocular distance and near visual acuity measures obtained with presenting correction (or unaided if no presenting correction) at the initial enrollment visit. Note that the near visual acuity of participant 1 represents right eye acuity rather than binocular. Participants appearing to have only a near visual acuity measure had the same level of distance visual acuity (i.e. the symbols overlap).
Visual acuity with presenting correction was repeated at the dispensing visit which occurred an average of 35 days after the initial baseline visit (range = 19 to 71 days). The mean difference in distance binocular visual acuity between visits 1 and 2 was 0.02 ±0.06 logMAR (range = −0.10 to 0.14), giving a coefficient of repeatability (1.96 x within subject standard deviation) of 0.12 logMAR (6 letters) (Figure 5). Difference in acuity was not linearly related to magnitude of acuity and there was no evidence of a learning effect (i.e. better acuity at time 2). The one participant who was tested with the HOTV letter set had a difference in acuity between study visits of 0.04 logMAR. Eliminating this participant from the calculation of the coefficient of repeatability did not make a meaningful difference in the finding (0.12 logMAR with versus 0.13 logMAR without).
Figure 5.
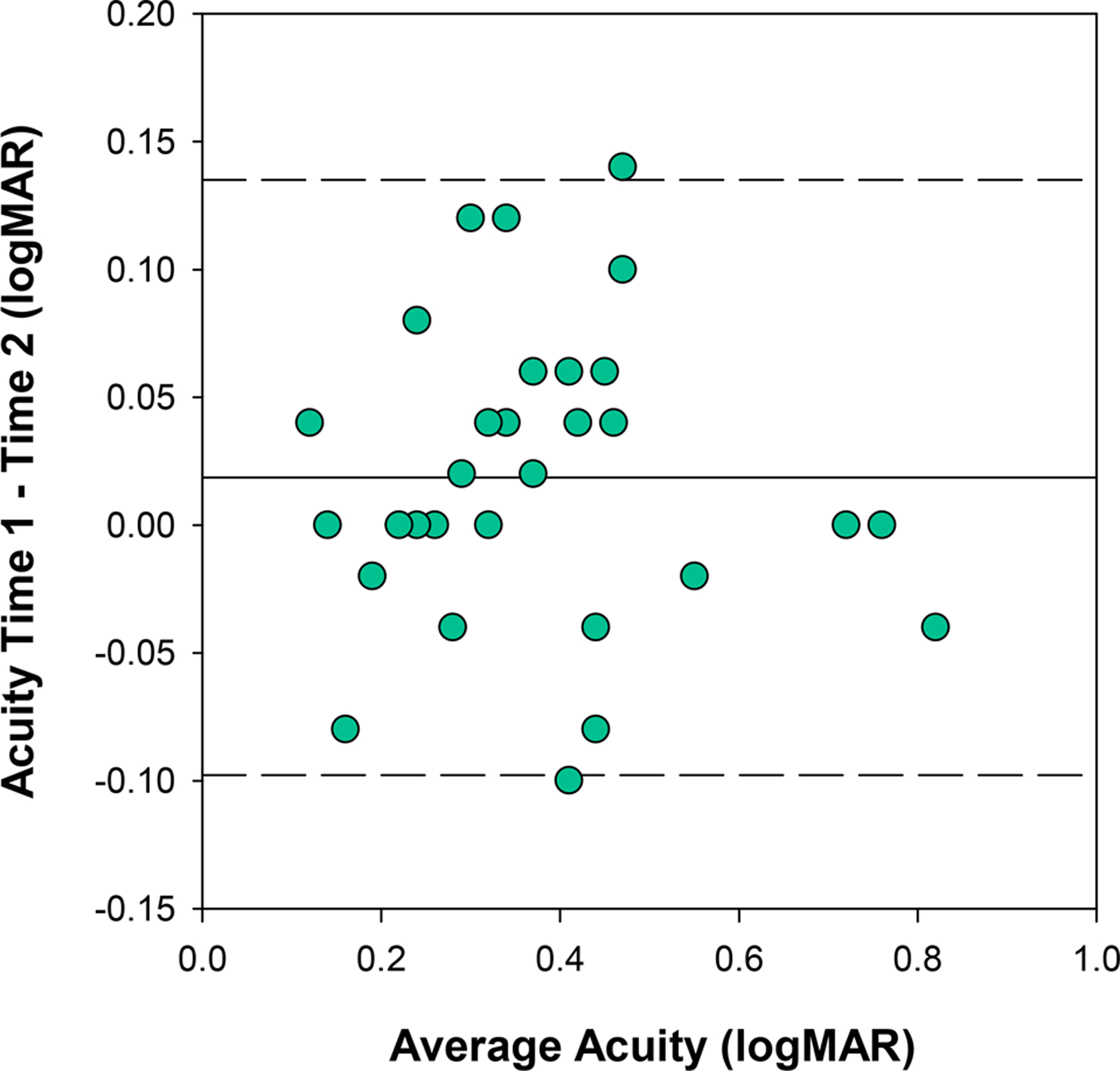
Comparison of distance visual acuity measures obtained with habitual corrections at visits 1 and 2 with 95% limits of agreement indicated by the dashed lines. Inter-session repeatability (1.96 x within subject standard deviation) of distance binocular visual acuity was 0.12 logMAR (6 letters).
Ninety spectacle prescriptions were produced for the thirty participants enrolled in this trial. Of those 90 prescriptions, only 1 failed one of the safety criteria on a re-test (reduction in distance visual acuity greater than 7 letters from presenting) and was not dispensed. All remaining 89 prescriptions were dispensed for two months each. Additional information about the prescription that failed the safety criteria will be disclosed in the primary outcome paper.
Participants in this study all had intellectual disability related to their diagnosis of Down syndrome, some of whom were non-verbal. However, all study participants were able to participate in the examination process, even for procedures requiring subjective responses, as these could be provided either verbally or through matching. Participant developmental ability was assessed through the parent/guardian responses on the Vineland 2 Adaptive Assessment (Table 2). Standard scores falling 2 or more standard deviations below the mean (<70) were classified as Low adaptive functioning (n = 18), scores falling between 1 and 2 standard deviations below the mean (70 – 85) were classified as Moderately Low adaptive functioning (n = 11), and scores falling within 1 standard deviation of the mean (85 – 115) were classified as Adequate (n = 1). The standard scores for this study cohort ranged from 22 to 99.
DISCUSSION
This study will be the first to evaluate objective spectacle prescribing methods based on wavefront measurements for individuals with Down syndrome. This work will evaluate prescriptions not only in the lab setting (acuity upon initial dispense), but also in a clinical dispensing trial following two months of adapted wear time. A standardized visual acuity testing procedure will serve as the outcome measure in addition to a newly developed objective assessment of spectacle compliance,27 offering valuable information regarding spectacle wear patterns in the study cohort. The objective spectacle wear monitor will address limitations observed in previous studies about the quality of survey data to assess spectacle compliance.
The primary outcome measure in this study is distance visual acuity measured with a computerized logMAR style chart. This method has previously been shown to have good within-session repeatability for 30 adults with Down syndrome (coefficient of repeatability of 0.13 logMAR (6.5 letters)).32 The inter-session repeatability of visual acuity reported in this study cohort is similar to the previously reported monocular intra-session repeatability (18 individuals participated in both studies). Thus the investigators believe that despite intellectual disability, all of the participants in this study were capable of reliably performing a rigorous acuity task to evaluate spectacle performance.
All of the participants in this study presented with reduced visual acuity and some level of refractive error in at least one eye. While it is possible that the nature of the study attracted participants who were known to have refractive or acuity concerns, the level of acuity reduction and the wide range of refractive errors observed in this cohort are consistent with other reports in the literature for this population.1, 4, 33 The participants in this study had higher order RMS wavefront errors that were elevated relative to values previously reported in typical individuals with healthy eyes,31, 34 albeit not as severe as that reported in individuals with keratoconus.35
The refractions evaluated in this study were determined either from standard clinical techniques, or analysis of dilated, wavefront measurements (1% tropicamide, 2.5% phenylephrine). While 1% tropicamide is known to leave some residual accommodative ability, a comparison of distance refraction with tropicamide versus cylopentolate found differences less than 0.25D at both 30 and 60 minutes post-dilation, and thus the use of tropicamide should be adequate for the present study population.36 Wavefront optimized refractions were always determined 30 minutes post-dilation, but not all clinical refractions included consideration of dilated measures (left to the clinical examiner’s discretion). However, in this population of adult participants with Down syndrome, accommodation is not anticipated to be a significant factor in the determination of refractive error, given the finding that the majority of individuals with Down syndrome have accommodative deficits, even in early childhood.2,37
Down syndrome is accompanied by a wide range of intellectual disability that includes minimal to severe developmental delays. While the cohort in this study did include individuals that were nonverbal, all participants were able to communicate with study investigators and follow instructions for completing study measurements, and thus the findings from this study may not be generalizable to the most severely impaired individuals with Down syndrome. Another limitation to the generalizability of the results is that individuals with nystagmus, a condition that has been observed in approximately 12 to 18% of individuals with Down syndrome,3, 33 were excluded from participation. This condition was excluded since the current instrumentation required for the metric optimized refractions requires participants to have good fixation. If the methodology shows promise in this subset of individuals with Down syndrome, further work will be needed to determine whether the methodology has any benefit for, or can be adapted to serve, individuals with nystagmus.
In summary, this study will provide a first look at the feasibility of utilizing objective spectacle prescribing methods based upon wavefront aberration measures to determine spectacle prescriptions for individuals with Down syndrome. The performance of those prescriptions will be compared to spectacle prescriptions determined by a clinical investigator with specific expertise examining populations with special needs, but that specific expertise is not commonly shared among all practitioners. Thus, equivalent outcomes between the objective prescriptions and the clinical prescriptions would indicate that an objective method is available that would allow all clinicians with access to the necessary equipment to provide refractions equivalent to those with decades of experience working with this population. In addition to benefitting persons with Down syndrome, this work may also translate to other populations unable to fully participate in the subjective refraction process (e.g. young children, intellectually disabled individuals), as well as those with elevated optical aberrations (e.g. corneal disease, poor surgical outcomes).
ACKNOWLEDGEMENTS
This work was funded by NIH EY0274580 awarded to HAA. The authors acknowledge the contributions of Hope Queener in the development of Temperature Log Viewer 2.0 and Spectacle Sweep, Chris Kuether in the design and production of the silicone mounts for the temperature sensor data loggers, and Lan Chi Nguyen for assistance in spectacle lens verification and treatment order labeling.
REFERENCES
- 1.Courage ML, Adams RJ, Reyno S, Kwa PG. Visual Acuity in Infants and Children with Down Syndrome. Dev Med Child Neurol 1994;36:586–93. [DOI] [PubMed] [Google Scholar]
- 2.Woodhouse JM, Pakeman VH, Saunders KJ, et al. Visual Acuity and Accommodation in Infants and Young Children with Down’s Syndrome. J Intellect Disabil Res 1996;40(Pt. 1):49–55. [DOI] [PubMed] [Google Scholar]
- 3.da Cunha RP, Moreira JB. Ocular Findings in Down’s Syndrome. Am J Ophthalmol 1996;122:236–44. [DOI] [PubMed] [Google Scholar]
- 4.Haugen OH, Hovding G, Lundstrom I. Refractive Development in Children with Down’s Syndrome: A Population Based, Longitudinal Study. Br J Ophthalmol 2001;85:714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Little JA, Woodhouse JM, Saunders KJ. Corneal Power and Astigmatism in Down Syndrome. Optom Vis Sci 2009;86:748–54. [DOI] [PubMed] [Google Scholar]
- 6.Knowlton R, Marsack JD, Leach NE, et al. Comparison of Whole Eye versus First-Surface Astigmatism in Down Syndrome. Optom Vis Sci 2015;92:804–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCullough SJ, Little JA, Saunders KJ. Higher Order Aberrations in Children with Down Syndrome. Invest Ophthalmol Vis Sci 2013;54:1527–35. [DOI] [PubMed] [Google Scholar]
- 8.Little JA, Woodhouse JM, Lauritzen JS, Saunders KJ. The Impact of Optical Factors on Resolution Acuity in Children with Down Syndrome. Invest Ophthalmol Vis Sci 2007;48:3995–4001. [DOI] [PubMed] [Google Scholar]
- 9.Applegate RA, Marsack JD, Ramos R, Sarver EJ. Interaction between Aberrations to Improve or Reduce Visual Performance. J Cataract Refract Surg 2003;29:1487–95. [DOI] [PubMed] [Google Scholar]
- 10.Cheng X, Bradley A, Thibos LN. Predicting Subjective Judgment of Best Focus with Objective Image Quality Metrics. J Vision 2004;4:310–21. [DOI] [PubMed] [Google Scholar]
- 11.de Gracia P, Dorronsoro C, Gambra E, et al. Combining Coma with Astigmatism Can Improve Retinal Image over Astigmatism Alone. Vision Res 2010;50:2008–14. [DOI] [PubMed] [Google Scholar]
- 12.Thibos LN, Hong X, Bradley A, Applegate RA. Accuracy and Precision of Objective Refraction from Wavefront Aberrations. J Vision 2004;4:329–51. [DOI] [PubMed] [Google Scholar]
- 13.Applegate RA, Marsack JD, Thibos LN. Metrics of Retinal Image Quality Predict Visual Performance in Eyes with 20/17 or Better Visual Acuity. Optom Vis Sci 2006;83:635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng X, Thibos LN, Bradley A. Estimating Visual Quality from Wavefront Aberration Measurements. J Refract Surg 2003;19:S579–84. [DOI] [PubMed] [Google Scholar]
- 15.Marsack JD, Thibos LN, Applegate RA. Metrics of Optical Quality Derived from Wave Aberrations Predict Visual Performance. J Vision 2004;4:322–8. [DOI] [PubMed] [Google Scholar]
- 16.Schoneveld P, Pesudovs K, Coster DJ. Predicting Visual Performance from Optical Quality Metrics in Keratoconus. Clin Exp Optom 2009;92:289–96. [DOI] [PubMed] [Google Scholar]
- 17.Hastings GD, Marsack JD, Nguyen LC, et al. Is an Objective Refraction Optimised Using the Visual Strehl Ratio Better Than a Subjective Refraction? Ophthalmic Physiol Opt 2017;37:317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shumard E, Hastings GD, Applegate RA, et al. Optimizing Spectacle Prescriptions for Patients with Keratoconus. Invest Ophthalmol Vis Sci 2017;58:4213. [Google Scholar]
- 19.Applegate RA, Ballentine C, Gross H, et al. Visual Acuity as a Function of Zernike Mode and Level of Root Mean Square Error. Optom Vis Sci 2003;80:97–105. [DOI] [PubMed] [Google Scholar]
- 20.Chan C, Smith G, Jacobs RJ. Simulating Refractive Errors: Source and Observer Methods. Am J Optom Physiol Opt 1985;62:207–16. [PubMed] [Google Scholar]
- 21.Ravikumar A, Marsack JD, Bedell HE, et al. Change in Visual Acuity Is Well Correlated with Change in Image-Quality Metrics for Both Normal and Keratoconic Wavefront Errors. J Vision 2013;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravikumar A, Benoit JS, Marsack JD, Anderson HA. Image Quality Metric Derived Refractions Predicted to Improve Visual Acuity Beyond Habitual Refraction for Patients with Down Syndrome. Transl Vis Sci Technol 2019;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Vision Council of America: Quick Reference Guide. American National Standards Institute (ANSI); ANSI Z80.1–2015; 2015. Available at: https://www.thevisioncouncil.org/sites/default/files/ANSI%20Z80%201-2015_Quick%20Reference%20v2.pdf. Accessed November 12, 2020. [Google Scholar]
- 24.Cullen JF, Butler HG. Mongolism (Down’s Syndrome) and Keratoconus. Br J Ophthalmol 1963;47:321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doyle SJ, Bullock J, Gray C, et al. Emmetropisation, Axial Length, and Corneal Topography in Teenagers with Down’s Syndrome. Br J Ophthalmol 1998;82:793–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Repka MX, Cotter SA, Beck RW, et al. A Randomized Trial of Atropine Regimens for Treatment of Moderate Amblyopia in Children. Ophthalmology 2004;111:2076–85. [DOI] [PubMed] [Google Scholar]
- 27.Lentsch MJ, Marsack JD, Anderson HA. Objective Measurement of Spectacle Wear with a Temperature Sensor Data Logger. Ophthalmic Physiol Opt 2018;38:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robson J, Raman R, Srinivasan R, Pardhan S. Contrast Sensitivity in Glaucoma Using Simple Disposable Printed (Camblobs) Charts. Invest Ophthalmol Vis Sci 2019;60:2488. [Google Scholar]
- 29.Green SA. Power Analysis in Repeated Measures Analysis of Variance with Heterogeneously Corrected Trials. Available at: https://files.eric.ed.gov/fulltext/ED320932.pdf. Accessed November 12, 2020. [Google Scholar]
- 30.Stevens J Applied Multivariate Statistics for the Social Sciences. Mahwah, NJ: Erlbaum; 1996. [Google Scholar]
- 31.Salmon TO, van de Pol C. Normal-Eye Zernike Coefficients and Root-Mean-Square Wavefront Errors. J Cataract Refract Surg 2006;32:2064–74. [DOI] [PubMed] [Google Scholar]
- 32.Ravikumar A, Benoit JS, Morrison KB, et al. Repeatability of Monocular Acuity Testing in Adults with and without Down Syndrome. Optom Vis Sci 2018;95:202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berk AT, Saatci AO, Ercal MD, et al. Ocular Findings in 55 Patients with Down’s Syndrome. Ophthalmic genetics 1996;17:15–9. [DOI] [PubMed] [Google Scholar]
- 34.Anderson HA, Ravikumar A, Benoit JS, Marsack JD. Impact of Pupil Diameter on Objective Refraction Determination and Predicted Visual Acuity. Transl Vis Sci Technol 2019;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kosaki R, Maeda N, Bessho K, et al. Magnitude and Orientation of Zernike Terms in Patients with Keratoconus. Invest Ophthalmol Vis Sci 2007;48:3062–8. [DOI] [PubMed] [Google Scholar]
- 36.Mutti DO, Zadnik K, Egashira S, et al. The Effect of Cycloplegia on Measurement of the Ocular Components. Invest Ophthalmol Vis Sci 1994;35:515–27. [PubMed] [Google Scholar]
- 37.Anderson HA, Manny RE, Glasser A, Stuebing KK. Static and Dynamic Measurements of Accommodation in Individuals with Down Syndrome. Invest Ophthalmol Vis Sci 2011;52:310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


