Abstract
Background
Internalizing mental disorders (IMDs) (depression, anxiety and post-traumatic stress disorder) have been associated with accelerated telomere length (TL) attrition; however, this association has not been investigated in the context of genetic variation that has been found to influence TL. We have previously reported an association between IMDs and accelerated TL attrition among Ugandan HIV+ children and adolescents. This study investigated the moderating effects of selected single nucleotide polymorphisms in the telomerase reverse transcriptase gene (TERT) (rs2736100, rs7726159, rs10069690 and rs2853669) and the telomerase RNA component gene (TERC) (rs12696304, rs16847897 and rs10936599) on the association between IMDs and TL, among Ugandan HIV+ children (aged 5–11 years) and adolescents (aged 12–17 years).
Results
We found no significant interaction between IMDs as a group and any of the selected SNPs on TL at baseline. We observed significant interactions of IMDs with TERT rs2736100 (p = 0.007) and TERC rs16847897 (p = 0.012), respectively, on TL at 12 months.
Conclusions
TERT rs2736100 and TERC rs16847897 moderate the association between IMDs and TL among Ugandan HIV+ children and adolescents at 12 months. Understanding the nature of this association may shed light on the pathophysiological mechanisms underlying advanced cellular aging in IMDs.
Keywords: Internalizing mental disorders, Telomere length attrition, TERT rs2736100, TERC rs16847897, HIV+ children and adolescents, Uganda
Background
Human immunodeficiency virus-positive (HIV+) children and adolescents suffer a considerable burden of internalizing mental disorders (IMDs) (namely, depression, anxiety and post-traumatic stress disorder) [1–3]. Studies undertaken both in the developed (Europe and the United States) and developing world (sub-Saharan Africa) among HIV+ children and adolescents have documented rates of major depressive disorder of between 12.7 and 40% [2–8], and rates of anxiety disorders of between 9 and 32.2% [1–3, 6]. For IMDs combined, rates of between 12 and 27% have been documented in Uganda and South Africa, respectively [1, 9]. Among people living with HIV/AIDS, IMDs have been associated with a number of other negative outcomes, including accelerated cellular aging [10], faster HIV disease progression [11, 12], poor adherence to medication [12, 13], risky sexual behavior [13, 14], poor linkage to care for newly diagnosed HIV+ persons [15], increased HIV transmission (through the promotion of HIV drug resistance) [16] and impaired academic and social functioning [13].
While considerable research has investigated the psychosocial risk factors for IMDs among HIV+ children and adolescents, there is a paucity of research on biological risk factors, including genetic factors. Recent genome-wide association (GWAS) studies have identified loci for depression [17], anxiety disorders [18] and post-traumatic stress disorder (PTSD) [19, 20], providing evidence for the role of genetic variation in the etiology of IMDs. Nevertheless, the etiology of IMDs, and the biochemical and molecular contributions in particular, are largely unknown.
IMDs are associated with increased mortality and age-related diseases, such as cancer, heart and cardiovascular disease [21–24]. IMDs are also highly comorbid with both psychiatric and somatic disorders, including those associated with advanced aging [25]. Depression has, for example, been reported to be associated with chronic diseases (e.g., type 2 diabetes mellitus and cardiovascular disease) [25, 26], as well as chronic inflammation [27]. In addition, higher mortality rates have been reported among patients with mental disorders (e.g., depression and other affective disorders) compared to the general population, with this mortality mainly due to the same age-related diseases, such as cancer, cardiac and cerebrovascular disease [22–24].
Several studies have investigated the association between telomeres, the protein-bound deoxyribonucleic acid (DNA) repeat structures at the ends of chromosomes, and IMDs [28]. Telomeres are important in protecting chromosomes from fusing together during mitosis, thus preventing loss of genetic data [29, 30]. They shorten progressively with each cell division, eventually leading to DNA damage responses, replicative senescence, or programmed cell death [31]. Since telomeres shorten with each cycle of cell division, telomere length (TL) provides a marker of biological aging [32]. Evidence for the role of TL among patients with IMDs and comorbid age-related diseases may offer insights into a novel potential mechanism for the excess morbidity and mortality associated with IMDs [33].
Shorter TL has been reported among patients with depression [34–41], anxiety disorders [34, 42, 43] and PTSD [44–47]. However, there has been comparatively little research into the relationship between TL and IMDs in the context of HIV infection, which has itself been associated with TL attrition [48, 49]. Shortening of telomeres in seropositive individuals may be due to HIV infection-associated increases in inflammatory and oxidative processes, both of which drive TL attrition [50, 51] or due to the effect of some nucleoside analogue reverse transcriptase inhibitors [50]. Furthermore, HIV-associated illness and stigma can act as a chronic psychological stressor to further drive TL attrition [52, 53].
We previously found evidence to suggest accelerated TL attrition that was driven by IMDs among Ugandan HIV+ children and adolescents [10]. There is, however, a dearth of data on the mechanisms by which IMDs lead to TL attrition. IMDs act as chronic stressors [54, 55], producing long-lasting biological adaptations that could potentially explain TL attrition due to IMDs [56]. Specifically, chronic stress can exert long-lasting effects on the hypothalamic–pituitary–adrenal (HPA) axis, such that previous experience of stress may prime a heightened response on subsequent stressor exposure [57]. Chronic stress also increases inflammatory signaling, which may in turn produce a pro-oxidative environment. Both inflammation and oxidative stress have been negatively associated with TL [51].
TL is partially heritable, with heritability estimates ranging between 44 and 80% [58, 59]. Therefore, genetic variation may contribute to telomere maintenance [60] and this genetic variation may confer risk for accelerated TL attrition. TL is maintained by telomerase, a catalytic enzyme with a protein component encoded by telomerase reverse transcriptase gene (TERT) and an RNA template component encoded by telomerase RNA component gene (TERC). Together, these act to add small DNA repeat segments to the end of chromosomes, thus counteracting TL attrition [30, 61]. A large genome-wide meta-analysis of 37,684 individuals found that TERT and TERC were amongst several loci found to influence mean TL [60], suggesting that genetic variation in these telomerase components has functional implications on TL.
The TERC rs12696304 G-allele and rs16847897 C-allele have been found to be associated with shorter TL by a candidate gene study on Swedish samples and a GWAS study on British samples [62, 63]. In addition, the TERT rs2736100 C-allele has been associated with shorter TL [60], while TERT rs7726159 AA genotype was associated with longer TL by a candidate gene study using White European samples [64].
We have previously determined an association between IMDs and TL in a sample of Ugandan HIV+ children and adolescents [10]. Specifically, TL was significantly longer in IMDs cases at baseline but did not differ from control TL at 12-month follow-up, suggesting that TL shortening over the one-year period was greater in participants with IMDs. Given that TERT and TERC are involved in TL maintenance [30], the present study investigated whether genetic variation in TERT (rs2736100, rs7726159, rs10069690 and rs2853669) and TERC (rs12696304, rs16847897 and rs10936599) moderated the association between IMDs and baseline and 12-month TL. Having observed that IMDs were driving accelerated TL attrition in the previous study [10], we modelled IMDs as the independent variable and TL as the dependent variable. We thus assessed for the interaction between IMDs and TERT and TERC on TL.
Methods
Study design
This case–control study was carried out in children (aged 5–11 years) and adolescents (12–17 years). A total of 368 cases with any internalizing mental disorder (IMD) and 368 age- and sex-matched controls were included. Both cases and controls were Ugandans. This study was nested within the previously described CHAKA study [1, 65], which enrolled 1339 HIV+ children and adolescents (855 children and 484 adolescents) in Uganda. This study complied with the Code of Ethics of the World Medical Association (Declaration of Helsinki). The CHAKA study obtained ethical and scientific clearance from the Uganda Virus Research Institute (UVRI) Science and Ethical Committee (#GC/127/15/06/459) and the Uganda National Council of Science and Technology (# HS 1601). The present study obtained approval from the Higher Degrees Research & Ethics Committee, School of Biomedical Sciences, College of Health Sciences, Makerere University (# SBS 421) and the Health Research Ethics Committee of Stellenbosch University (#S17/09/179). Study subjects who were diagnosed with significant psychiatric problems were referred to mental health units at Entebbe and Masaka government hospitals.
Study population
Study subjects were recruited from two HIV clinics in urban Kampala (Joint Clinic Research Centre (JCRC) and Nsambya Home Care) and three HIV clinics in rural Masaka (The AIDS Support Organization (TASO), Kitovu Mobile Clinic and Uganda Cares). All study subjects were on anti-retroviral therapy (ART).
Selection of cases and controls
Cases were all participants in CHAKA who met criteria for diagnosis of any IMD at baseline as per the diagnostic and statistical manual of mental disorders-edition 5 (DSM-5) referenced Child and Adolescent Symptom Inventory-5 (CASI-5) [66]. All cases at baseline were ascertained, and the cases were then stratified by site (one of two sites), sex, age category (one of three categories) and socio-economic status (SES) (one of three SES categories). This resulted in a total of 36 strata (2 × 2 × 3 × 3). In each stratum the number of cases was ascertained (e.g. for males in site 1 in the youngest age category and the lowest SES group there were 9 cases). An equal number of controls (HIV+ children and adolescents without any psychiatric disorder) were then randomly sampled from the stratum concerned (so for males in site 1 in the youngest age category and the lowest SES group we sampled 9 controls), thus the controls were frequency matched to the cases on site, sex, age and SES.
Inclusion and exclusion criteria
Inclusion criteria: (1) HIV-infected outpatients, registered with the HIV Clinics at JCRC or Nsambya Home Care at the Kampala study site and TASO, Kitovu mobile or Uganda Cares clinic at the Masaka site; (2) aged between 5 and 17 years at the time of enrolment; (3) conversant in English or Luganda, the language into which the assessment tools were translated; and (4) able to provide written informed consent/assent. Cases were HIV+ children and adolescents who had any depressive disorder (depression or dysthymia [persistent depressive disorder]), anxiety disorder or PTSD. Controls were age- and sex- matched HIV+ children and adolescents without any psychiatric disorder. Persistent IMDs were baseline cases that remained cases at 12 months while remitted ones were baseline cases that no longer qualified for a diagnosis at 12 months. Exclusion criteria: (1) seriously ill and unable to understand study procedures; and (2) any other psychiatric disorder other than the IMDs listed above.
Procedures
As part of the CHAKA study, children and assenting adolescents, as well as their parents/caregivers, were interviewed using a structured questionnaire. The questionnaire included, amongst others, socio-demographic characteristics and depression, PTSD and anxiety modules of the DSM-5 [66]. The CASI-5 was administered by trained psychiatric nurses or psychiatric clinical officers at two time points (baseline and 12 months). The CASI-5 lists the symptoms of a wide range of psychiatric disorders including MDD, generalized anxiety disorder, PTSD and attention-deficit/hyperactivity disorder among others. Individual CASI-5 items are rated on a four-point frequency of occurrence scale ranging from never (0) to very often (3). Though there are several CASI-5 scoring algorithms, in the present study we used symptom count cut-off scores, which reflect the prerequisite number of symptoms for a clinical diagnosis. At each study visit, 4 ml of blood from each study participant was collected via venipuncture into an EDTA vacutainer and subsequently stored at − 80 °C pending DNA extraction for genetics analyses.
Determination of genotypes for selected polymorphisms in telomerase reverse transcriptase gene and telomerase RNA component gene
DNA samples were genotyped for each selected SNP in each of TERT (rs2736100, rs7726159, rs10069690 and rs2853669) and TERC (rs12696304, rs16847897 and rs10936599), using a kompetitive allele-specific PCR (KASP) assay (LGC, Middelsex, United Kingdom). This genotyping chemistry allowed for bi-allelic discrimination of SNPs. The genotyped SNPs in each of TERT and TERC were analyzed for linkage disequillibrium (LD) by creating an LD map using the default Gabriel LD [67], implemented in Haploview software, version 4.2 [68]. For SNPs that were in LD, haplotypes were generated using the Haploview software.q
Power of the study
Using results from a study by Epel et al. [69], post hoc power calculations (described in [10]) indicated that our study was well-powered with 83.8% power to detect at least 5% reduction in mean TL between cases and controls. For the interaction analysis, assuming a type I error rate of 0.05, zero correlation of the outcome between cases and controls, 1.6 odds ratio of exposure in cases relative to controls, a 0.055 probability of exposure among controls and a 1:1 case: control ratio for 368 cases, our interaction analyses achieved a power of 87.5%.
Statistical methods
Statistical analyses were conducted using Stata 15 (StataCorp, TX, USA). Socio-demographic characteristics (including socio-economic status) were compared between cases and controls. Socio-economic status (SES) was generated from a scale of 9 household items owned (car, motorcycle, refrigerator, electricity, bicycle, radio, telephone, cupboard and flask). Each item was weighted in the respective order, a car carrying a maximum weight of 9 and a flask a minimum weight of 1. A total score of items was generated, whose median cut-off of 13 was used to classify low and high SES. A score less than 13 was classified as low SES, while that greater than 13 was classified as high SES. Our study group [70] has previously used household items as a measure of SES in rural settings of Uganda. A t-test was used to compare CD4 counts between cases and controls to account for any disparity in HIV disease progression.
Associations between the different socio-demographic factors and TL were tested using one-way analysis of variance (ANOVA) to determine potential confounders. Independent sample t-tests were used to assess the association between IMDs and TL both at baseline and 12 months. TERT and TERC genotypes were assessed for HWE using a likelihood ratio test. The genotypes were not validated as genotyping was done by a service provider using an automated SNP genotyping array. One-way ANOVA was used to assess for the association between each of the investigated SNPs or haplotype with baseline and 12 months TL, as well as the TL change. A likelihood ratio test was used to assess for interaction between each of the polymorphisms and IMDs on TL both at baseline and 12 months controlling for age and sex, with Bonferroni corrections for multiple testing. The likelihood ratio test was also used to assess for interaction between the generated TERT rs2736100-rs7726159 haplotypes and IMDs on TL both at baseline and 12 months controlling for age and sex as well. To perform a Bonferroni correction, the p value threshold of 0.05 was divided by 2 (number of separate tests) to yield a corrected threshold p value of 0.025. These interactions were performed on all the explanatory variables even without observing significant main effects in order to rule out the possibility of cross over interaction where significant interactions may be observed for non-significant main effects. For significant interactions, mean TL at 12 months was plotted against genotypes to elucidate the nature of the interaction terms. Where required, 95% confidence intervals were calculated.
Results
No significant differences were observed when socio-demographic variables were compared between case and control participants (Table 1).
Table 1.
Distribution of socio-demographic factors in cases and controls
| Variable (n) | Case n (%) | Control n (%) | p value |
|---|---|---|---|
| Sex | p = 0.111 | ||
| Male (342) | 160 (43.6) | 182 (49.5) | |
| Female (393) | 207 (56.4) | 186 (50.5) | |
| Site | p = 0.941 | ||
| Urban (415) | 208 (56.5) | 207 (56.3) | |
| Rural (321) | 160 (43.5) | 161 (43.7) | |
| Age | p = 0.374 | ||
| 5–11 years (389) | 202 (57.6) | 187 (54.2) | |
| 12– 17 years (307) | 149 (42.4) | 158 (45.8) | |
| Education level | p = 0.371 | ||
| No formal education (13) | 9 (2.5) | 4 (1.1) | |
| Primary (648) | 323 (88.0) | 325 (88.8) | |
| Secondary (72) | 35 (9.5) | 37 (10.1) | |
| Socio-economic status | p = 0.459 | ||
| Low (332) | 171 (46.5) | 161 (43.8) | |
| High (404) | 197 (53.5) | 207 (56.2) | |
| Mean CD4 count at baseline | 947.04 | 944.02 | p = 0.939 |
CD4: cluster of differentiation 4; primary = 0–7 years of formal education; secondary = 8–14 years of formal education; low socioeconomic status = 0–13; high socio-economic status = > 13 (see statistical methods section). All numbers that do not add up were due to missing data
The genotypes for all the selected SNPs were in Hardy–Weinberg equilibrium (HWE) (Table 2). None of TERC SNPs were in linkage disequilibrium (LD). However, TERT rs7726159 and rs2736100 were in LD, resulting in CT, CG and AG haplotypes (see Additional file 1: S1 and S2). The genotype frequencies for all the selected SNPs and the TERT rs2736100-rs7726159 haplotype freqencies are shown in Additional file 1: Table S1. The association between each investigated SNP and TL are shown in Table 2 below. None of the selected SNPs or the TERT rs2736100-rs7726159 haplotypes associated with baseline or 12 months TL or TL change; however, TERC 16847897 significantly associated with 12-month TL (Table 2).
Table 2.
p values for Hardy–Weinberg equilibrium and association of each selected single nucleotide polymorphism and the generated haplotypes with telomere length
| Single nucleotide polymorphism | p value of association with | HWE p value | ||
|---|---|---|---|---|
| Baseline TL | 12 months TL | TL change | ||
| TERC rs16847897 | 0.650 | 0.014 | 0.393 | 0.461 |
| TERC rs12696304 | 0.861 | 0.991 | 0.981 | 0.443 |
| TERC rs10936599 | 0.406 | 0.142 | 0.510 | 1 |
| TERT rs2736100 | 0.300 | 0.366 | 0.581 | 0.091 |
| TERT rs2853669 | 0.438 | 0.507 | 0.591 | 0.798 |
| TERT rs7726159 | 0.590 | 0.855 | 0.802 | 0.882 |
| TERT rs10069690 | 0.567 | 0.831 | 0.802 | 0.274 |
| TERT rs2736100-rs7726159 | 0.331 | 0.724 | 0.725 | N/A |
HWE: Hardy–Weinberg equilibrium; TERC: telomerase RNA component gene; TERT: telomerase reverse transcriptase gene; TERT rs2736100-rs7726159: TERT rs2736100 and rs7726159 haplotype; N/A: not applicable
Moderating effects of single nucleotide polymorphisms in telomerase reverse transcriptase gene and telomerase RNA component gene on the association between internalizing mental disorders and telomere length
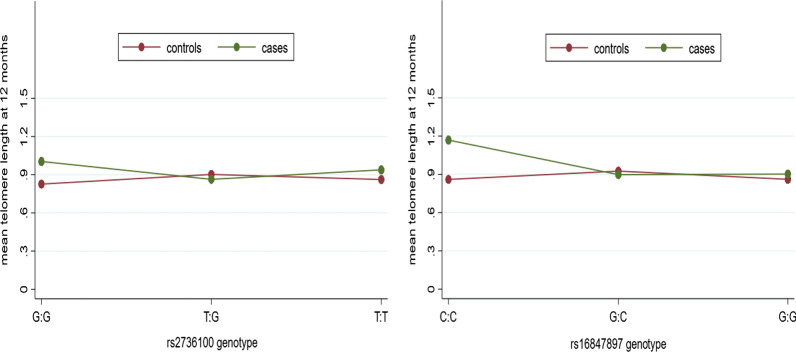
We found no moderating role for any of the selected SNPs and on the association between IMDs and TL at baseline (Table 3). We did find that TERT rs2736100 and TERC rs16847897 significantly moderated the association between IMDs and TL at 12 months (p = 0.007 and p = 0.012 respectively) (Table 3). For TERT rs2736100, mean TL was longer among cases as compared to controls, but only in those individuals with the GG genotype (n = 139; 82 cases, 57 controls) (Fig. 1). No significant difference in TL was observed between cases and controls who possessed either the TG (n = 330) or TT (n = 201) genotypes (Fig. 1). For TERC rs16847897, mean TL was longer in cases compared to controls, but only in individuals with the CC genotype (n = 44; 27 cases, 17 controls) (Fig. 1). No significant difference in TL was observed between cases and controls who possessed either the GC (n = 265) or GG (n = 373) genotypes (Fig. 1). None of the TERT rs2736100-rs7726159 haplotypes significantly moderated the association between IMDs and TL at either baseline (p = 0.379) or 12 months (p = 0.418).
Table 3.
Two-way analysis of variance for the interaction of internalizing mental disorders with rs2736100 and with s16847897 on telomere length at 12 months
| SNP | Obs | Variable | F | Bonferroni corrected p value (α = 0.025) |
|---|---|---|---|---|
| Baseline | ||||
| rs2736100 | 596 | IMDs | 15.52 | < 0.001 |
| rs2736100 | 0.98 | 0.375 | ||
| IMDs * rs2736100 | 1.15 | 0.318 | ||
| rs16847897 | 597 | IMDs | 2.53 | 0.112 |
| rs16847897 | 0.37 | 0.692 | ||
| IMDs * rs16847897 | 1.79 | 0.168 | ||
| 12 months | ||||
| rs2736100 | 511 | IMDs | 6.03 | 0.014 |
| rs2736100 | 0.44 | 0.645 | ||
| IMDs * rs2736100 | 4.95 | 0.007* | ||
| rs16847897 | 515 | IMDs | 7.25 | 0.007 |
| rs16847897 | 3.00 | 0.050 | ||
| IMDs * rs16847897 | 4.44 | 0.012* | ||
SNP: single nucleotide polymorphism; Obs: number of observations; IMDs * rs2736100: interaction of internalizing mental disorders with TERT rs2736100 on relative telomere length; IMDs * TERC rs16847897, interaction of internalizing mental disorders with rs16847897 on relative telomere length; *significant p value for the interaction term
Fig. 1.
Mean TL between cases and controls by genotype for TERT rs2736100 and TERC rs16847897 at 12 months. For TERT rs2736100 mean TL was significantly different between cases and controls, but only in individuals with the GG genotype while for TERC rs16847897, mean TL was significantly different between cases and controls but only in individuals with CC genotype
Discussion
Using the same samples of Ugandan HIV+ children and adolescents, we previously found that TL was longer at baseline in cases with IMDs, but that this difference did not remain 12 months later [10]. The present study built on those results and investigated whether selected polymorphisms within TERT and TERC interacted with IMDs to influence baseline and 12 months TL among the study participants. We found that TERT rs2736100 and TERC rs16847897 significantly moderated the association between IMDs and TL at 12 months. To our knowledge, this is the first sub-Saharan African study to investigate these interactions among HIV+ children and adolescents.
Telomeres are maintained by the telomerase enzyme, an enzyme whose catalytic protein component and RNA template is encoded by TERT and TERC, respectively [30, 61]. Given the role that TERT and TERC play in the structure of telomerase [71, 72], variations in genes encoding these components could influence TL. However, none of the investigated polymorphisms significantly influenced the association between IMDs and TL at baseline. We found a significant interaction between IMDs and TERT rs2736100 and TERC rs16847897 on TL at 12 months. This interaction points to a possible direct influence of IMDs on TL shortening. It is possible that chronic pathophysiological processes characteristic of IMDs, including inflammation, oxidative stress and HPA axis dysregulation [73], produced a cumulative burden over the one-year study period, with the resultant increase in allostatic load driving TL attrition. Plots of the interaction between IMDs and TL reveal that reductions in 12-month TL in cases are limited to participants carrying the TERT rs2736100 GT or TT genotype. Similarly, for TERC rs16847897, the GC or GG genotypes are associated with reduced TL at 12 months. These genotypes appear to accelerate TL attrition. However, it is hard to tease out the true effects of the genotypes on TL attrition in the present study, since the reduction in TL after 12 months was strongly significant in both cases and controls (p < 0.001 respectively), possibly due to the short 12 month period. Future studies with a longer study period are required to confirm our findings.
Although the functionality of these two SNPs is not well known, data from previous studies strongly suggest their involvement in disease-associated TL attrition (sporadic idiopathic pulmonary fibrosis), as well as pathophysiological mechanisms, such as inflammation, that are relevant to IMDs [74–76]. TERT rs2736100 is located within intron 2 of TERT, a position that has been described to be a putative regulatory region [77]. TERT rs2736100 has also been reported as a critical factor in TERT synthesis and activation [63]. TERT rs2736100 has previously been associated with diseases characterized by TL attrition, including lung cancer [78] and sporadic idiopathic pulmonary fibrosis [75, 76, 79]. TERT has also been reported to interact with natural factor kappa B (NF-κb) p65, where it activates NF-κb and increase metalloproteinases in cancer cells [74]. On the other hand, TERC 16847897 has also been reported as locus that could probably regulate TL. In line with findings of the present study, each copy of the TERC rs16847897 major G-allele (CG or GG) was associated with shorter mean TL in a Han Chinese population [80]. However, TERC rs16847897 CC genotype has also been associated with both shorter TL and lower TERT levels [81]. We have no direct explanation for this discrepancy. However, it is important to note that our findings result from a longitudinal interaction of the SNP with IMDs. The nature of this interaction needs to be elucidated in order to draw conclusions.
TERT rs2736100 and TERC rs18647897 loci appear to have regulatory functions and require further study. Although the nature of the interaction between IMDs and TERT and TERC on TL is not known, IMDs have been associated with increased oxidative stress [82, 83] and inflammatory markers, such as C-reactive protein and the pro-inflammatory cytokines interleukin-6 and tumor necrosis factor alpha [84, 85]. Oxidative stress leads to TL shortening through the inhibition of telomerase activity [73, 86, 87]. We hypothesize that IMD-related increases in oxidative stress and inflammatory responses produce a cumulative pathophysiological burden. The effect of this on TL may potentially be influenced by genetic variation in TERT and TERC.
Our study presents with limitations which deserve mention. First, the relationships between TL, IMDs and HIV are complex and, to a degree, reciprocal. HIV/AIDS may shorten TL via both infection-related pathophysiological processes (inflammation and oxidative stress), and its capacity to act as a chronic psychological stressor [52, 53]. Our study participants were also on ART, with some regimens, such as Effavirenz, reported to have psychiatric side effects [88]. Second, as we did not include an HIV- control group in our study, we can only speculate on the external validity of our findings. Nevertheless, since our results indicate that IMDs drive accelerated TL attrition [10], the SNP x IMD interactions observed in this study are potentially relevant to other populations. Further research would be required to investigate whether these findings are generalizable to Ugandan youths in the general population as well as other youth populations.. Third, we defined our cases as those individuals diagnosed with depressive disorder, any anxiety disorder or PTSD. The inclusion of PTSD (n = 60) in this sample is contentious, as the disorder has recently been excluded from the categorization of anxiety disorders in the DSM-5 [89]. This may have affected our findings. In order to elucidate the independent contribution of each particular disorder, future studies should analyze each IMD separately. Fourth, we did not control for population stratification at analysis since the study participants belonged to the Bagandan population group for which principal components analysis on GWAS data of a sample of over 4,000 individuals has shown that they are genetically similar, as principal components 1 and 2 have been reported to have explained only 0.3% and 0.1% of the genetic variation in a Bagandan general population cohort [90]. However, there is a possibility that some participants may not have been Baganda although they identified as Baganda and their inclusion could have caused population admixture that could have led to spurious results. Future studies should endeavor to control for population stratification.
Despite the limitations, our study has strengths that are worth mentioning. First, the study sample size was large enough (368 cases and 368 controls) to allow a sufficient power of greater than 80% for the association between IMDs and TL. Second, we measured TL at baseline and 12 months, a longitudinal aspect that allowed us to assess for causation. Third, TL undergoes a period of rapid attrition in the first five years of life, which is followed by relative stability until young adulthood [91, 92]. As our study participants ranged in age from 5 to 17 years and thus fell into this period of expected TL stability, the differences we identified are more likely to reflect valid influences of SNPs and IMDs, as opposed to chronological age effects.
Conclusions
We observed that TERT rs2736100 and TERC rs16847897 produce effects on 12-month TL in Ugandan HIV+ children and adolescents diagnosed with IMDs. The mechanisms through which TERT and TERC SNPs interact with IMDs to influence TL are not known. Understanding these mechanisms may help to unravel the biochemical processes that take place following onset of IMDs, which may aid the discovery of new drugs or drug targets for better management of these disorders. Furthermore, these mechanisms may also provide insight into the biochemical processes that underlie the comorbidity between IMDs, age-related conditions and metabolic diseases. Future functional studies are required to understand how TERT and TERC SNPs moderate the association between IMDs and accelerated TL attrition.
Supplementary Information
Additional file 1. Figures S1 and S2 show the linkage disequilibrium map of TERT and TERC single nucleotide polymorphisms respectively. Table S1 shows the genotype and aplotype frequencies for TERT and TERC single nucleotide polymorphisms.
Acknowledgements
Study subjects, Research assistants of the mental health project of MRC/UVRI & LSHTM Uganda Research Unit, Joint Clinical Research Council, Nsambya Home Care, TASO—Masaka, Kitovu Mobile Clinic, Uganda Cares—Masaka, Members of the Neuropsychiatric Genetics Laboratory at Stellenbosch University, Data and Statistics Section of the MRC/UVRI & LSHTM Uganda Research Unit, the National Research Foundation of South Africa.
Abbreviations
- µL
Microliter
- ANOVA
Analysis of variance
- CASI-5
Child and Adolescent Symptom Inventory-edition 5
- CD4
Cluster of differentiation 4
- DNA
Deoxyribonucleic acid
- DSM-5
Diagnostic and statistical manual of mental disorders-edition 5
- GWAS
Genome-wide association
- HIV/AIDS
Human immunodeficiency virus/Acquired immunodeficiency disease syndrome
- HIV+
Human immunodeficiency virus-positive
- HPA
Hypothalamic–pituitary–adrenal
- HWE
Hardy–Weinberg equilibrium
- IMD
Internalzing mental disorder
- IMDs
Internalizing mental disorders
- JCRC
Joint Clinical Research Council
- MDD
Major depressive disorder
- MRC/DfID
Medical Research Council/Department for International Development
- ng
Nano gram
- PTSD
Post-traumatic stress disorder
- qPCR
Quantitative polymerase chain reaction
- TL
Relative telomere length
- RNA
Ribonucleic acid
- s
Seconds
- S
Single copy gene
- SES
Socio-economic status
- SNP
Single nucleotide polymorphism
- SNPs
Single nucleotide polymorphisms
- TASO
The AIDS Support Organization
- TERC
Telomerase RNA component
- TERT
Telomerase reverse transcriptase
- TL
Telomere length
- UVRI
Uganda Virus Research Institute
Authors’ contributions
Concept: AK, SMJH, EK, SS; Data collection: AK, EK, SMJH, JSW, SS, Data analysis: WS, AK, RNN, SMJH, JSW, SS, MK, JL, EK; First draft: AK, SMJH, JSW, WS, EK, SS, MLJ; Final revision: AK, SMJH, JSW, EK, SS, WS, MLJ, RNN, PK, MK, JL; All authors read and approved the final manuscript.
Funding
The study was funded by Medical Research Council / Department for International Development—African Leadership Award to Prof. Eugene Kinyanda, Grant No.: MR/L004623/1, the Alliance for Global Health and Science of the Center for Emerging and Neglected Diseases, Grant No.: 50288/N7145, the South African Research Chairs Initiative in Post-traumatic Stress Disorder, funded by the Department of Science and Technology and the National Research Foundation of South Africa and the Africa Center of Excellence in Materials, Product Development and Nanotechnology (MAPRONANO ACE). AK received a doctoral bursary from the National Research Foundation of South Africa and is supported by both the South African Research Chairs Initiative in Post-traumatic Stress Disorder and the mental health project of MRC/UVRI and LSHTM Uganda Research unit. The funders played no role in the design of the study, collection, analysis and interpretation of the data or writing of the manuscript.
Availability of data and materials
All information gathered about study subjects and their samples is confidential, with access limited to the research team. However, upon request, data from the MRC/UVRI and LSHTM Uganda Research Unit is currently accessed under a data sharing policy via: http://www.mrcuganda.org/sites/default/files/publications/MRC_UVRI_Data_sharing_policyDecember2015.pdf.
Ethics approval and consent to participate
The study obtained ethics approval from the Health Research Committee of Stellenbosch University (# S17/09/179) and the higher Degrees Research & Ethics Committee, School of Biomedical Sciences, College of Health Sciences, Makerere University (# SBS 421). The parent study (CHAKA) obtained ethics approval from the Uganda Virus Research Institute (UVRI) Science and Ethical Committee (# GC/127/15/06/459) and the Uganda National Council of Science and Technology (# HS 1601). All parents/caregivers provided written informed consent for their children or adolescents to participate in the study and for a blood specimen to be withdrawn from them for the TL and other genetics analyses. Adolescents further provided written informed assent to participate in the study.
Consent for publication
No details, images or videos relating to any of the study subjects are included in this manuscript.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12920-020-00857-z.
References
- 1.Kinyanda E, Salisbury TT, Levin J, Nakasujja N, Mpango RS, Abbo C, Seedat S, Araya R, Musisi S, Gadow KD, Patel V. Rates, types and co-occurrence of emotional and behavioural disorders among perinatally HIV-infected youth in Uganda: the CHAKA study. Soc Psychiatry Psychiatr Epidemiol. 2019;54(4):415–425. doi: 10.1007/s00127-019-01675-0. [DOI] [PubMed] [Google Scholar]
- 2.Mellins CA, Elkington KS, Leu CS, Santamaria EK, Dolezal C, Wiznia A, Bamji M, Mckay MM, Abrams EJ. Prevalence and change in psychiatric disorders among perinatally HIV-infected and HIV-exposed youth. AIDS Care. 2012;24(8):953–962. doi: 10.1080/09540121.2012.668174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nachman S, Chernoff M, Williams P, Hodge J, Heston J, Gadow KD. Human immunodeficiency virus disease severity, psychiatric symptoms, and functional outcomes in perinatally infected youth. Arch Pediatr Adolesc Med. 2012;166(6):528–535. doi: 10.1001/archpediatrics.2011.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lwidiko A, Kibusi SM, Nyundo A, Mpondo BC. Association between HIV status and depressive symptoms among children and adolescents in the Southern Highlands Zone, Tanzania: a case-control study. PLoS ONE. 2018;13(2):e0193145. doi: 10.1371/journal.pone.0193145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim MH, Mazenga AC, Devandra A, Ahmed S, Kazembe PN, Yu X, Nguyen C, Sharp C. Prevalence of depression and validation of the Beck Depression Inventory-II and the Children's Depression Inventory-Short amongst HIV-positive adolescents in Malawi. J Int AIDS Soc. 2014;17(1):18965. doi: 10.7448/IAS.17.1.18965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamau JW, Kuria W, Mathai M, Atwoli L, Kangethe R. Psychiatric morbidity among HIV-infected children and adolescents in a resource-poor Kenyan urban community. AIDS Care. 2012;24(7):836–842. doi: 10.1080/09540121.2011.644234. [DOI] [PubMed] [Google Scholar]
- 7.Gadow KD, Angelidou K, Chernoff M, Williams PL, Heston J, Hodge J, Nachman S. Longitudinal study of emerging mental health concerns in youth perinatally infected with HIV and peer comparisons. J Dev Behav Pediatr. 2012;33(6):456. doi: 10.1097/DBP.0b013e31825b8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musisi S, Kinyanda E. Emotional and behavioural disorders in HIV seropositive adolescents in urban Uganda. East Afr Med J. 2009;86(1):16–24. doi: 10.4314/eamj.v86i1.46923. [DOI] [PubMed] [Google Scholar]
- 9.Woollett N, Cluver L, Bandeira M, Brahmbhatt H. Identifying risks for mental health problems in HIV positive adolescents accessing HIV treatment in Johannesburg. J Child Adolesc Ment Health. 2017;29(1):11–26. doi: 10.2989/17280583.2017.1283320. [DOI] [PubMed] [Google Scholar]
- 10.Kalungi A, Womersley JS, Kinyanda E, Joloba ML, Ssembajjwe W, Nsubuga RN, Levin J, Kaleebu P, Kidd M, Seedat S, Hemmings S. Internalizing mental disorders and accelerated cellular aging among HIV+ children and adolescents in Uganda. Front Genet. 2019;10:705. doi: 10.3389/fgene.2019.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chida Y, Vedhara K. Adverse psychosocial factors predict poorer prognosis in HIV disease: a meta-analytic review of prospective investigations. Brain Behav Immun. 2009;23(4):434–445. doi: 10.1016/j.bbi.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Ironson G, O’Cleirigh C, Fletcher MA, Laurenceau JP, Balbin E, Klimas N, Schneiderman N, Solomon G. Psychosocial factors predict CD4 and viral load change in men and women with human immunodeficiency virus in the era of highly active antiretroviral treatment. Psychosom Med. 2005;67(6):1013. doi: 10.1097/01.psy.0000188569.58998.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinyanda E, Levin J, Nakasujja N, Birabwa H, Nakku J, Mpango R, Grosskurth H, Seedat S, Araya R, Shahmanesh M, Patel V. Major depressive disorder: longitudinal analysis of impact on clinical and behavioral outcomes in Uganda. J Acquir Immune Defic Syndr. 2018;78(2):136–143. doi: 10.1097/QAI.0000000000001647. [DOI] [PubMed] [Google Scholar]
- 14.Springer SA, Dushaj A, Azar MM. The impact of DSM-IV mental disorders on adherence to combination antiretroviral therapy among adult persons living with HIV/AIDS: a systematic review. AIDS Behav. 2012;16(8):2119–2143. doi: 10.1007/s10461-012-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatia R, Hartman C, Kallen MA, Graham J, Giordano TP. Persons newly diagnosed with HIV infection are at high risk for depression and poor linkage to care: results from the Steps Study. AIDS Behav. 2011;15(6):1161–1170. doi: 10.1007/s10461-010-9778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Remien RH, Mellins CA. Long-term psychosocial challenges for people living with HIV: let's not forget the individual in our global response to the pandemic. AIDS. 2007;21(Suppl 5):S55–S63. doi: 10.1097/01.aids.0000298104.02356.b3. [DOI] [PubMed] [Google Scholar]
- 17.Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, Coleman JR, Hagenaars SP, Ward J, Wigmore EM, Alloza C. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22(3):343–352. doi: 10.1038/s41593-018-0326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier SM, Trontti K, Purves KL, Als TD, Grove J, Laine M, Pedersen MG, Bybjerg-Grauholm J, Bækved-Hansen M, Sokolowska E, Mortensen PB. Genetic variants associated with anxiety and stress-related disorders: a genome-wide association study and mouse-model study. JAMA Psychiatry. 2019;76(9):924–932. doi: 10.1001/jamapsychiatry.2019.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nievergelt CM, Maihofer AX, Klengel T, Atkinson EG, Chen CY, Choi KW, Coleman JR, Dalvie S, Duncan LE, Gelernter J, Levey DF. International meta-analysis of PTSD genome-wide association studies identifies sex-and ancestry-specific genetic risk loci. Nat Commun. 2019;10(1):1–6. doi: 10.1038/s41467-019-12576-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelernter J, Sun N, Polimanti R, Pietrzak R, Levey DF, Bryois J, Lu Q, Hu Y, Li B, Radhakrishnan K, Aslan M. Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in > 165,000 US veterans. Nat Neurosci. 2019;22(9):1394–1401. doi: 10.1038/s41593-019-0447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pratt LA, Druss BG, Manderscheid RW, Walker ER. Excess mortality due to depression and anxiety in the United States: results from a nationally representative survey. Gen Hosp Psychiatry. 2016;39:39–45. doi: 10.1016/j.genhosppsych.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Druss BG, Zhao L, Von Esenwein S, Morrato EH, Marcus SC. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599–604. doi: 10.1097/MLR.0b013e31820bf86e. [DOI] [PubMed] [Google Scholar]
- 23.Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42. [PMC free article] [PubMed] [Google Scholar]
- 24.Cuijpers P, Smit F. Excess mortality in depression: a meta-analysis of community studies. J Affect Disord. 2002;72(3):227–236. doi: 10.1016/S0165-0327(01)00413-X. [DOI] [PubMed] [Google Scholar]
- 25.Lotfaliany M, Agustini B, Kowal P, Berk M, Mohebbi M. Co-occurrence of depression with chronic diseases among the older population living in low-and middle-income countries: a compound health challenge. Ann Clin Psychiatry. 2019;31(2):95–105. [PubMed] [Google Scholar]
- 26.Mason DR, Beck PL, Muruve DA. Nucleotide-binding oligomerization domain-like receptors and inflammasomes in the pathogenesis of non-microbial inflammation and diseases. J Innate Immun. 2012;4(1):16–30. doi: 10.1159/000334247. [DOI] [PubMed] [Google Scholar]
- 27.Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem Res. 2007;32(10):1749–1756. doi: 10.1007/s11064-007-9385-y. [DOI] [PubMed] [Google Scholar]
- 28.Lindqvist D, Epel ES, Mellon SH, Penninx BW, Révész D, Verhoeven JE, Reus VI, Lin J, Mahan L, Hough CM, Rosser R. Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neurosci Biobehav Rev. 2015;55:333–364. doi: 10.1016/j.neubiorev.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA. 1992;89(21):10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12(10):1133–1138. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 31.Zlotorynski E. Telomere crisis activates autophagic death. Nat Rev Mol Cell Biol. 2019;20(3):133. doi: 10.1038/s41580-019-0105-7. [DOI] [PubMed] [Google Scholar]
- 32.Shay JW. Role of telomeres and telomerase in aging and cancer. Cancer Discov. 2016;6(6):584–593. doi: 10.1158/2159-8290.CD-16-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramunas J, Yakubov E, Brady JJ, Corbel SY, Holbrook C, Brandt M, Stein J, Santiago JG, Cooke JP, Blau HM. Transient delivery of modified mRNA encoding TERT rapidly extends telomeres in human cells. FASEB J. 2015;29(5):1930–1939. doi: 10.1096/fj.14-259531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verhoeven JE, van Oppen P, Penninx BW. Associations between depression, anxiety and telomere length in a large Dutch psychiatric cohort study. Tijdschr Psychiatr. 2017;59(6):350–359. [PubMed] [Google Scholar]
- 35.Lin PY, Huang YC, Hung CF. Shortened telomere length in patients with depression: a meta-analytic study. J Psychiatr Res. 2016;76:84–93. doi: 10.1016/j.jpsychires.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Schutte NS, Malouff JM. The association between depression and leukocyte telomere length: a meta-analysis. Depress Anxiety. 2015;32(4):229–238. doi: 10.1002/da.22351. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Rizo C, Fernandez-Egea E, Miller BJ, Oliveira C, Justicia A, Griffith JK, Heaphy CM, Bernardo M, Kirkpatrick B. Abnormal glucose tolerance, white blood cell count, and telomere length in newly diagnosed, antidepressant-naive patients with depression. Brain Behav Immun. 2013;28:49–53. doi: 10.1016/j.bbi.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verhoeven JE, Révész D, Epel ES, Lin J, Wolkowitz OM, Penninx BW. Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Mol Psychiatry. 2014;19(8):895–901. doi: 10.1038/mp.2013.151. [DOI] [PubMed] [Google Scholar]
- 39.Shalev I, Moffitt TE, Braithwaite AW, Danese A, Fleming NI, Goldman-Mellor S, Harrington H, Houts RM, Israel S, Poulton R, Robertson SP. Internalizing disorders and leukocyte telomere erosion: a prospective study of depression, generalized anxiety disorder and post-traumatic stress disorder. Mol Psychiatry. 2014;19(11):1163–1170. doi: 10.1038/mp.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Douillard-Guilloux G, Guilloux JP, Lewis DA, Sibille E. Anticipated brain molecular aging in major depression. Am J Geriatr Psychiatry. 2013;21(5):450–460. doi: 10.1016/j.jagp.2013.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kinser PA, Lyon DE. Major depressive disorder and measures of cellular aging: an integrative review. Nurs Res Pract. 2013;2013:469070. doi: 10.1155/2013/469070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verhoeven JE, Révész D, van Oppen P, Epel ES, Wolkowitz OM, Penninx BW. Anxiety disorders and accelerated cellular ageing. Br J Psychiatry. 2015;206(5):371–378. doi: 10.1192/bjp.bp.114.151027. [DOI] [PubMed] [Google Scholar]
- 43.Kananen L, Surakka I, Pirkola S, Suvisaari J, Lönnqvist J, Peltonen L, Ripatti S, Hovatta I. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLoS ONE. 2010;5(5):e10826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Avetyan D, Zakharyan R, Petrek M, Arakelyan A. Telomere shortening in blood leukocytes of patients with posttraumatic stress disorder. J Psychiatr Res. 2019;111:83–88. doi: 10.1016/j.jpsychires.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 45.Roberts AL, Koenen KC, Chen Q, Gilsanz P, Mason SM, Prescott J, Ratanatharathorn A, Rimm EB, Sumner JA, Winning A, De Vivo I. Posttraumatic stress disorder and accelerated aging: PTSD and leukocyte telomere length in a sample of civilian women. Depress Anxiety. 2017;34(5):391–400. doi: 10.1002/da.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, Metzler T, Lenoci M, Blackburn E, Neylan TC. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011;70(5):465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, Hu XZ, Benedek DM, Fullerton CS, Forsten RD, Naifeh JA, Li X, Li H, Benevides KN, Smerin S, Le T. The interaction between stressful life events and leukocyte telomere length is associated with PTSD. Mol Psychiatry. 2014;19(8):856–857. doi: 10.1038/mp.2013.141. [DOI] [PubMed] [Google Scholar]
- 48.Auld E, Lin J, Chang E, Byanyima P, Ayakaka I, Musisi E, Worodria W, Davis JL, Segal M, Blackburn E, Huang L. HIV infection is associated with shortened telomere length in Ugandans with suspected tuberculosis. PLoS ONE. 2016;11(9):e0163153. doi: 10.1371/journal.pone.0163153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oeseburg H, de Boer RA, van Gilst WH, van der Harst P. Telomere biology in healthy aging and disease. Pflüg Arch. 2010;459(2):259–268. doi: 10.1007/s00424-009-0728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lagathu C, Cossarizza A, Béréziat V, Nasi M, Capeau J, Pinti M. Basic science and pathogenesis of ageing with HIV: potential mechanisms and biomarkers. AIDS. 2017;31(2):S105–S119. doi: 10.1097/QAD.0000000000001441. [DOI] [PubMed] [Google Scholar]
- 51.Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075–1091. doi: 10.1176/appi.ajp.2015.15020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Varni SE, Miller CT, McCuin T, Solomon S. Disengagement and engagement coping with HIV/AIDS stigma and psychological well-being of people with HIV/AIDS. JSoc Clin Psychol. 2012;31(2):123–150. doi: 10.1521/jscp.2012.31.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Needham BL, Mezuk B, Bareis N, Lin J, Blackburn EH, Epel ES. Depression, anxiety and telomere length in young adults: evidence from the National Health and Nutrition Examination Survey. Mol Psychiatry. 2015;20(4):520. doi: 10.1038/mp.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dieleman GC, Huizink AC, Tulen JH, Utens EM, Creemers HE, van der Ende J, Verhulst FC. Alterations in HPA-axis and autonomic nervous system functioning in childhood anxiety disorders point to a chronic stress hypothesis. Psychoneuroendocrinology. 2015;51:135–150. doi: 10.1016/j.psyneuen.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 55.McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003;54(3):200–207. doi: 10.1016/S0006-3223(03)00177-X. [DOI] [PubMed] [Google Scholar]
- 56.Epel ES, Lithgow GJ. Stress biology and aging mechanisms: toward understanding the deep connection between adaptation to stress and longevity. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S10–S16. doi: 10.1093/gerona/glu055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aristizabal MJ, Anreiter I, Halldorsdottir T, Odgers CL, McDade TW, Goldenberg A, Mostafavi S, Kobor MS, Binder EB, Sokolowski MB, O’Donnell KJ. Biological embedding of experience: a primer on epigenetics. Proc Natl Acad Sci USA. 2020;117(38):23261–23269. doi: 10.1073/pnas.1820838116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Njajou OT, Cawthon RM, Damcott CM, Wu SH, Ott S, Garant MJ, Blackburn EH, Mitchell BD, Shuldiner AR, Hsueh WC. Telomere length is paternally inherited and is associated with parental lifespan. Proc Natl Acad Sci USA. 2007;104(29):12135–12139. doi: 10.1073/pnas.0702703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Broer L, Codd V, Nyholt DR, Deelen J, Mangino M, Willemsen G, Albrecht E, Amin N, Beekman M, De Geus EJ, Henders A. Meta-analysis of telomere length in 19 713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet. 2013;21(10):1163–1168. doi: 10.1038/ejhg.2012.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Codd V, Nelson CP, Albrecht E, Mangino M, Deelen J, Buxton JL, Hottenga JJ, Fischer K, Esko T, Surakka I, Broer L. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45(4):422–427. doi: 10.1038/ng.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang C, Meier UT. Architecture and assembly of mammalian H/ACA small nucleolar and telomerase ribonucleoproteins. EMBO J. 2004;23(8):1857–1867. doi: 10.1038/sj.emboj.7600181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Codd V, Mangino M, van der Harst P, Braund PS, Kaiser M, Beveridge AJ, Rafelt S, Moore J, Nelson C, Soranzo N, Zhai G. Common variants near TERC are associated with mean telomere length. Nat Genet. 2010;42(3):197–199. doi: 10.1038/ng.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Melin BS, Nordfjäll K, Andersson U, Roos G. hTERT cancer risk genotypes are associated with telomere length. Genet Epidemiol. 2012;36(4):368–372. doi: 10.1002/gepi.21630. [DOI] [PubMed] [Google Scholar]
- 64.Rode L, Nordestgaard BG, Bojesen SE. Long telomeres and cancer risk among 95 568 individuals from the general population. Int J Epidemiol. 2016;45(5):1634–1643. doi: 10.1093/ije/dyw179. [DOI] [PubMed] [Google Scholar]
- 65.Mpango RS, Kinyanda E, Rukundo GZ, Gadow KD, Patel V. Cross-cultural adaptation of the Child and Adolescent Symptom Inventory-5 (CASI-5) for use in central and south-western Uganda: the CHAKA project. Trop Dr. 2017;47(4):347–354. doi: 10.1177/0049475517724688. [DOI] [PubMed] [Google Scholar]
- 66.Gadow KD, Sprafkin J. Child and Adolescent Symptom Inventory-5 manual [Internet]. Stony Brook: Checkmate Plus; 2013 [cited 2020 Nov 31]. Available from: https://www.checkmateplus.com/product/casi5.htm. Accessed 39 June 2019.
- 67.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296(5576):2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 68.Barrett JC. Haploview: visualization and analysis of SNP genotype data. Cold Spring Harb Protoc. 2009;2009(10):pdb.ip71. doi: 10.1101/pdb.ip71. [DOI] [PubMed] [Google Scholar]
- 69.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kinyanda E, Waswa L, Baisley K, Maher D. Prevalence of severe mental distress and its correlates in a population-based study in rural south-west Uganda. BMC Psychiatry. 2011;11(1):97. doi: 10.1186/1471-244X-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen JL, Greider CW. Determinants in mammalian telomerase RNA that mediate enzyme processivity and cross-species incompatibility. EMBO J. 2003;22(2):304–314. doi: 10.1093/emboj/cdg024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee MS, Blackburn EH. Sequence-specific DNA primer effects on telomerase polymerization activity. Mol Cell Biol. 1993;13(10):6586–6599. doi: 10.1128/MCB.13.10.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu R, Woo J. Exploring the link between depression and accelerated cellular aging: telomeres hold the key. Res Rep Biochem. 2015;6:1–2. [Google Scholar]
- 74.Ding D, Xi P, Zhou J, Wang M, Cong YS. Human telomerase reverse transcriptase regulates MMP expression independently of telomerase activity via NF-κB-dependent transcription. FASEB J. 2013;27(11):4375–4383. doi: 10.1096/fj.13-230904. [DOI] [PubMed] [Google Scholar]
- 75.Mushiroda T, Wattanapokayakit S, Takahashi A, Nukiwa T, Kudoh S, Ogura T, Taniguchi H, Kubo M, Kamatani N, Nakamura Y, Pirfenidone Clinical Study Group A genome-wide association study identifies an association of a common variant in TERT with susceptibility to idiopathic pulmonary fibrosis. J Med Genet. 2008;45(10):654–656. doi: 10.1136/jmg.2008.057356. [DOI] [PubMed] [Google Scholar]
- 76.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, III, Lansdorp PM. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356(13):1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 77.Taylor J, Tyekucheva S, King DC, Hardison RC, Miller W, Chiaromonte F. ESPERR: learning strong and weak signals in genomic sequence alignments to identify functional elements. Genome Res. 2006;16(12):1596–1604. doi: 10.1101/gr.4537706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McKay JD, Hung RJ, Gaborieau V, Boffetta P, Chabrier A, Byrnes G, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P. Lung cancer susceptibility locus at 5p15. 33. Nat Genet. 2008;40(12):1404–1406. doi: 10.1038/ng.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, Rosenblatt RL, Shay JW, Garcia CK. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA. 2007;104(18):7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shen Q, Zhang Z, Yu L, Cao L, Zhou D, Kan M, Li B, Zhang D, He L, Liu Y. Common variants near TERC are associated with leukocyte telomere length in the Chinese Han population. Eur J Hum Genet. 2011;19(6):721–723. doi: 10.1038/ejhg.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Al Khaldi R, Mojiminiyi O, AlMulla F, Abdella N. Associations of TERC single nucleotide polymorphisms with human leukocyte telomere length and the risk of type 2 diabetes mellitus. PLoS ONE. 2015;10(12):e0145721. doi: 10.1371/journal.pone.0145721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11(6):851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 83.Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative–antioxidative systems. Hum Psychopharmacol. 2007;22(2):67–73. doi: 10.1002/hup.829. [DOI] [PubMed] [Google Scholar]
- 84.Musinguzi K, Obuku A, Nakasujja N, Birabwa H, Nakku J, Levin J, Kinyanda E. Association between major depressive disorder and pro-inflammatory cytokines and acute phase proteins among HIV-1 positive patients in Uganda. BMC Immunol. 2018;19(1):1. doi: 10.1186/s12865-017-0239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci. 2004;117(11):2417–2426. doi: 10.1242/jcs.01097. [DOI] [PubMed] [Google Scholar]
- 87.Von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–344. doi: 10.1016/S0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 88.Dalwadi DA, Ozuna L, Harvey BH, Viljoen M, Schetz JA. Adverse neuropsychiatric events and recreational use of efavirenz and other HIV-1 antiretroviral drugs. Pharmacol Rev. 2018;70(3):684–711. doi: 10.1124/pr.117.013706. [DOI] [PubMed] [Google Scholar]
- 89.American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-5. Arlingotn: American Psychiatric Association; 2013. [Google Scholar]
- 90.Gurdasani D, Carstensen T, Fatumo S, Chen G, Franklin CS, Prado-Martinez J, Bouman H, Abascal F, Haber M, Tachmazidou I, Mathieson I. Uganda genome resource enables insights into population history and genomic discovery in Africa. Cell. 2019;179(4):984–1002. doi: 10.1016/j.cell.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wojcicki JM, Shiboski S, Heyman MB, Elwan D, Lin J, Blackburn E, Epel E. Telomere length change plateaus at 4 years of age in Latino children: associations with baseline length and maternal change. Mol Genet Genomics. 2016;291(3):1379–1389. doi: 10.1007/s00438-016-1191-2. [DOI] [PubMed] [Google Scholar]
- 92.Frenck RW, Jr, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci USA. 1998;95(10):5607–5610. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Figures S1 and S2 show the linkage disequilibrium map of TERT and TERC single nucleotide polymorphisms respectively. Table S1 shows the genotype and aplotype frequencies for TERT and TERC single nucleotide polymorphisms.
Data Availability Statement
All information gathered about study subjects and their samples is confidential, with access limited to the research team. However, upon request, data from the MRC/UVRI and LSHTM Uganda Research Unit is currently accessed under a data sharing policy via: http://www.mrcuganda.org/sites/default/files/publications/MRC_UVRI_Data_sharing_policyDecember2015.pdf.