Abstract
Background
Current interest in using severe maternal morbidity (SMM) as a quality indicator for maternal healthcare will require the development of a standardized method for estimating hospital or regional SMM rates that includes adjustment and/or stratification for risk factors.
Objective
To perform a scoping review to identify methodological considerations and potential covariates for risk adjustment for delivery-associated SMM.
Search methods
Following the guidelines for Preferred Reporting Items for Systematic Reviews and Meta-analyses Extension for Scoping Reviews, systematic searches were conducted with the entire PubMed and EMBASE electronic databases to identify publications using the key term “severe maternal morbidity.”
Selection criteria
Included studies required population-based cohort data and testing or adjustment of risk factors for SMM occurring during the delivery admission. Descriptive studies and those using surveillance-based data collection methods were excluded.
Data collection and analysis
Information was extracted into a pre-defined database. Study design and eligibility, overall quality and results, SMM definitions, and patient-, hospital-, and community-level risk factors and their definitions were assessed.
Main results
Eligibility criteria were met by 81 studies. Methodological approaches were heterogeneous and study results could not be combined quantitatively because of wide variability in data sources, study designs, eligibility criteria, definitions of SMM, and risk-factor selection and definitions. Of the 180 potential risk factors identified, 41 were categorized as pre-existing conditions (e.g., chronic hypertension), 22 as obstetrical conditions (e.g., multiple gestation), 22 as intrapartum conditions (e.g., delivery route), 15 as non-clinical variables (e.g., insurance type), 58 as hospital-level variables (e.g., delivery volume), and 22 as community-level variables (e.g., neighborhood poverty).
Conclusions
The development of a risk adjustment strategy that will allow for SMM comparisons across hospitals or regions will require harmonization regarding: a) the standardization of the SMM definition; b) the data sources and population used; and c) the selection and definition of risk factors of interest.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40748-020-00123-1.
Keywords: Severe maternal morbidity, Maternal care, Obstetrics, Blood transfusion, Disparities, Quality indicators
Introduction
The tracking of severe maternal morbidity (SMM) has continued to evolve since it was first initiated by the World Health Organization (WHO) in 2004 as an alternative to maternal mortality surveillance for identifying failures and priorities in maternal health care [1]. By 2009, the WHO adopted a definition for a maternal near-miss (i.e., “a woman who nearly died but survived a complication that occurred during pregnancy, childbirth or within 42 days of termination of pregnancy”) and presented a list of identification criteria [2]. Using this approach, cases are first identified as having “potentially life-threatening conditions” associated with organ system dysfunction or failure. Surveillance relies on medical record review to document clinical, laboratory-based, or management-based SMM criteria. Case identification can occur retrospectively by identifying those who met criteria or prospectively by using a list of potentially life-threatening conditions.
A population-based approach to tracking SMM began in parallel to the WHO approach in 2005, when Wen et al. proposed a definition of SMM using population-based Canadian administrative data [3], which, although less specific compared to medical record data, was more feasible for routine monitoring. In 2008, Callaghan et al. at the United States (US) Centers for Disease Control and Prevention (CDC) published a definition using 15 conditions [4]. The CDC definition was expanded to 25 conditions in 2012 [5], and in 2015 when International Classification of Diseases, Clinical Modification, Version 9 (ICD-9) coding was upgraded to ICD-10, the SMM definition was reduced and consolidated to 21 and then to 18 conditions [6]. Roberts et al. in Australia contributed substantially to these efforts [7], and this work was further adapted in Canada by Joseph et al. [8]. Canadian and Australian SMM definitions were developed in ICD-10.
In the US, in addition to the CDC calculations of national, population-based trends for SMM using administrative data, facility-based SMM case audit (here referred to as “facility-based surveillance”) has been encouraged by both the CDC and the American College of Obstetricians and Gynecologists [9, 10]. In a recent review, Kuklina and Goodman promoted these complementary approaches, asserting that while case audits can go into depth to identify the causes of SMM and suggest avenues for prevention, population-based administrative data can be used not only to examine trends, but also to compare “hospitals, cities, or states” and to develop priorities for research and practice [11]. With funding from the Centers for Medicare and Medicaid services, the National Quality Forum, which provides standards for healthcare quality measurement in the US, has begun to explore the use of maternal morbidity and mortality measures to improve outcomes [12].
To make comparisons (e.g., by region or hospital) interpretable and amenable to policy directives and interventions, it will be necessary to develop a standardized method for adjusting for the most relevant risk factors. To address the research question of how an SMM measure might be adjusted for such comparisons, the objective of this scoping review was to describe what is currently known regarding the risk factors and methodological approaches for studying SMM using routinely collected population-based data.
Materials and methods
Data sources and search strategy
We performed a scoping literature review for predictors of delivery-admission SMM, using the definition provided by Anderson et al. [13]: “Scoping studies are concerned with contextualizing knowledge in terms of identifying the current state of understanding; identifying the sorts of things we know and do not know; and then setting this within policy and practice contexts.” This review conformed to guidelines for Preferred Reporting Items for Systematic Reviews and Meta-analyses Extension for Scoping Reviews (PRISMA-ScR) [14]. The PRISMA-ScR checklist is available in Additional file 1. Using the search term “severe maternal morbidity,” the search was conducted in PubMed and Embase, (both initiated in the late 1940’s), and included the entire databases through June 9, 2019. Since SMM is a composite measure that may include as few as one (e.g., intensive care unit admission) or more than 25 indicators, we used only this term (SMM) to narrow our search and retrieve articles using SMM definitions that were most relevant to population-based SMM tracking.
Inclusion and exclusion criteria
The inclusion criteria were that each study examined SMM as an outcome occurring during the delivery admission and that SMM was presented with risk factor adjustment or stratification. The exclusion criteria were the following: 1) case reports; 2) reviews, letters, or editorials; 3) errata; 4) methodological studies; 5) protocol only studies; 6) descriptive only studies; 7) quality improvement studies; 8) limited outcome studies (i.e., those that did not include a comprehensive SMM composite); 9) studies using SMM as a risk factor/predictor for further complications; 10) qualitative studies; 11) readmission SMM studies; 12) studies focused on SMM due to gynecological conditions (e.g., ectopic pregnancy or spontaneous abortion); 13) studies that relied predominantly on risk factors that were not routinely available in administrative data (e.g., clinical data, laboratory test results); 14) studies that relied on surveillance-based data collection methods (e.g., WHO-based methods); and 15) studies that were not in English. Citations identified through the searches were assessed by one reviewer and verified by another based on title and abstract using these pre-defined criteria. Duplicates were removed. Full text publications of potentially relevant citations were then examined by the same reviewers to assure that eligibility criteria were met.
Data extraction
Reviewer disagreements about study selection in the full text review and extraction phases were resolved by jointly re-examining studies and reaching mutual agreement. Information regarding each study was then extracted into a pre-defined database. Variables extracted included: study design and eligibility criteria; datasets used; SMM definition used; and risk factors used, including all patient-, hospital-, and community-level risk factors as reported.
Quality assessment
Risk of bias assessment is not a mandatory part of this review; however, for informative purposes, risk of bias was assessed using a version of the Newcastle-Ottawa Scale (NOS) [15] that was modified to evaluate observational cross-sectional studies relevant to the research question (Additional file 2). Risk of bias was assessed for a single outcome (SMM) within a study. Two reviewers assessed the articles for NOS criteria. Any discrepancy in scoring was resolved by joint re-examination to arrive at consensus. NOS scores were subdivided into those indicating high- (7–10), moderate- (5–6), and low-quality (1–4) of the publication with respect to the research question. Quality scores were used as part of the general assessment of the literature and did not affect the synthesis of results.
Synthesis of results
Risk factors were categorized into patient-, hospital-, and community-level groups. The patient-level risk factors were classified as pre-existing conditions (e.g., medical or behavioral risk factors), obstetrical conditions (e.g., multiple gestation), and non-clinical conditions (e.g., insurance type). The obstetrical conditions were further divided into those known to exist in the antepartum period (e.g., prior cesarean birth) versus those occurring in the intrapartum or postpartum periods of the delivery admission (e.g., dystocia, delivery mode).
The heterogeneity of the studies included in this review precluded any quantitative synthesis of the effect sizes (odds ratios or relative risks) of the identified risk factors. However, we present a comprehensive list of the risk factors for SMM that were reported in these publications and summarize: 1) the number of studies that used each risk factor; 2) the number of studies for which the effect size for a risk factor was reported to be statistically significant; and 3) the number of studies that did not report an effect size for the risk factor but did one of the following: a) included it in a risk adjustment model, b) stratified analyses by the risk factor, or c) excluded subsets of the population based on the risk factor. This information is presented with the understanding that it was not meaningful to compare or aggregate effect sizes across studies because of the wide heterogeneity of the study designs and covariates in the models.
The synthesis of results was organized by placing the 81 studies in the following categories defined by study approach: 1) Testing of multiple conditions for association with SMM without invoking a specific hypothesis; 2) Hypothesis testing of specific patient-level risk factors; 3) Hypothesis testing of hospital-level risk factors; and 4) Risk-adjusted SMM rates and trends. For purposes of this review, both maternal age and race/ethnicity were treated as pre-existing clinical risk factors.
Results
Study selection and characteristics
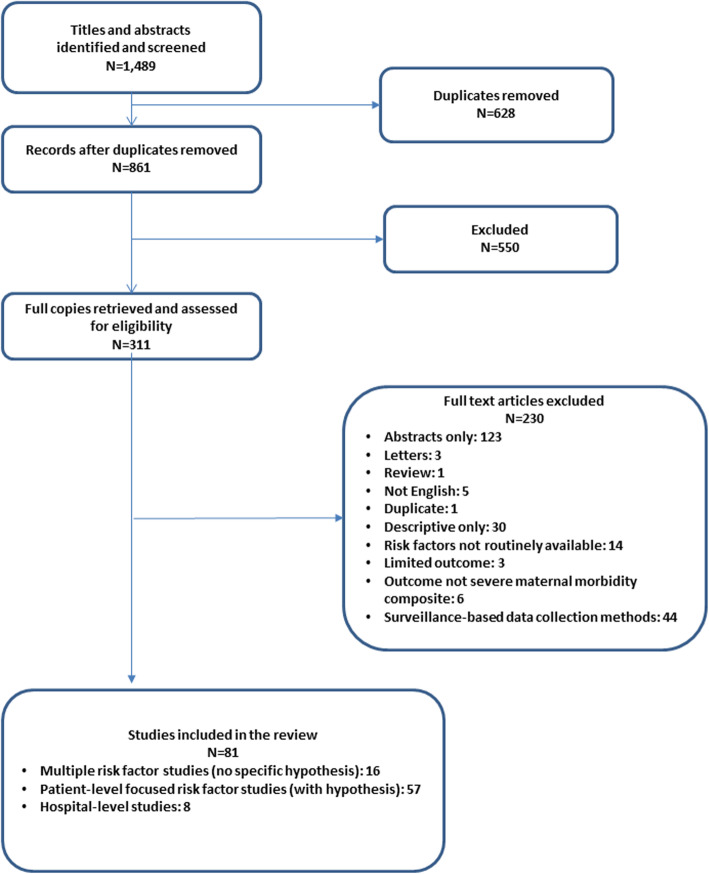
Results of the literature search are described in Fig. 1. A total of 1489 publications were identified by the original searches, and, of 861 unique titles, 81 (9.4%) met all inclusion and exclusion criteria after thorough review of the full text [3, 8, 16–94]. Table 1 summarizes selected study characteristics and Table 2 presents the risk factors used in the models. Most of the studies (n = 71, 87.7%) relied on hospital claims data, medical records, or discharge/birth certificate data from US hospitals. Although some studies sought to examine and identify general clinical risk factors as defined above (n = 16, 19.8%), most (n = 57, 70.4%) hypothesized an association of SMM with a specific risk factor, such as maternal age or race/ethnicity, or other pre-existing clinical condition; eight studies (9.9%) examined hospital-level risk factors, such as delivery volume. Over one-quarter of the studies (n = 22, 27.2%) limited the study population by patient characteristics (e.g., women at low risk) or hospital type (e.g., community hospitals).
Fig. 1.
Number of included publications by scoping review step
Table 1.
| Study Characteristic | Reference Number |
|---|---|
| Number and data source of included studies | |
| USa data (n = 50) | |
| National Inpatient Sample | 17,18,26,39,45,48,58,72 |
| National Readmission Database: 21 states | 32 |
| CMSb Medicaid (MAX) data | 22 |
| US claims data | 27,70 |
| US perinatal data networks | 28,43 |
| Hospital discharge data: 7 states | 33 |
| Hospital discharge data: Illinois | 84 |
| Hospital discharge data: Maryland | 81 |
| Hospital discharge ± birth certificate data: New York State | 19,44,53 |
| Hospital discharge + birth certificate data: 3 states | 29 |
| Hospital discharge + birth certificate data: Iowa | 38 |
| Hospital discharge + birth certificate data: New York City | 46,47,49,50,78 |
| Hospital discharge, birth, ± death certificate data: California | 36,40,52,55-57,68,69,76 |
| Hospital discharge, birth, + death certificate data: Georgia | 91 |
| Hospital discharge, birth, ± death certificate data: Washington | 42,62–64 |
| Hospital discharge + medical record data: 16 California hospitals | 51 |
| Hospital discharge + medical record data: Massachusetts | 21 |
| Hospital discharge + SARTc + vital statistics data: Massachusetts | 23 |
| Birth certificate data: Ohio | 71 |
| Birth certificate and SART data: 8 states | 67 |
| Medical record data single hospital: California | 93 |
| Medical record data single hospital: New York | 73 |
| Medical record data single hospital: Missouri | 83 |
| Medical record data single hospital: Tennessee | 41 |
| Non-US data (n = 31) | |
| Multiple countries including US | 60 |
| Multiple countries not including US | 90 |
| Japan | 16 |
| Korea | 75 |
| Sweden | 92 |
| Finland | 77 |
| France | 25,54,88 |
| Canada: Ontario | 34,35,80,89 |
| Canada: British Columbia | 61,85,86 |
| Canada: multiple provinces | 3,8,65,66,74,79,94 |
| Australia: New South Wales | 20,24,30,31,37,82,87 |
| Australia: Victoria | 59 |
| Study design and population | |
| Study design nested case-control (n = 6) | |
| Yes | 16,21,34,42,54,88 |
| Hospital-level risk factors with specific hypotheses (n = 8) | |
| Patient-level data adjusted for clustering among hospitals | 19,27,29,32,39 |
| Hierarchical models | 36,91 |
| Hospital-level data | 49 |
| Delivery discharges limited by: (n = 22) | |
| Community hospitals | 36 |
| Annual delivery volume less than 1000 | 45 |
| Low risk | 19,40,65,66 |
| Low risk breech presentation | 24 |
| Medicaid eligibility | 22 |
| Nulliparous | 85 |
| Prior cesarean delivery with parity = 1 | 94 |
| Prior cesarean delivery ≥3 | 73 |
| No prior cesarean delivery | 66 |
| Cesarean delivery 22–34 gestational weeks | 25 |
| Elective cesarean delivery | 16 |
| Preterm gestation | 20,68 |
| Term laboring patients with prolonged second stage | 74 |
| Preeclampsia | 27 |
| Hemorrhage | 80 |
| Race/ethnicity Hispanic or White | 46 |
| Race Black or White | 47,48 |
| SMMddefinition CDCe basis [7] (n = 37) | |
| 25 indicators | 17,18,23,29,33,36,38,42,46-53,58,60,68,69,71,72,75,76,91,93 |
| 18 indicators | 19,26,27,41,44,55-57,78,83,84 |
| SMM definition not using CDC basis (n = 44) | |
| Adapted from other US work (Bateman et al. [22]) | 32,39,45,61–63 |
| Adapted from Canadian work (Wen et al. [3]/Joseph et al. [8]) | 3,8,22,34,35,66,74,89,94 |
| Adapted from Australian work (Roberts et al. [8]) | 20,24,30,31,37,59,79,82,87 |
| Adapted from Swedish work (Wahlberg et al. [92]) | 90,92 |
| Adapted from Geller et al. (5 factor) [95] | 43,85 |
| Death | 80 |
| Intensive care unit admission | 21,25,28,40,53,65,67,71,73 |
| Other | 16,54,64,77,86,88 |
| Sensitivity analysis for use of transfusion in SMM definition (n = 15) | |
| Yes | 26,33,38,44,46,47,49,50,55-57,60,78,91,94 |
| Blood transfusion threshold was 4 units of packed red blood cells (n = 6) | |
| Yes | 43,51,54,85,88,93 |
| Blood transfusion not included in definition (n = 10) | |
| Yes | 22,32,37,39,45,77,80,86,90,92 |
| Key risk factors studied | |
| General risk factors (no hypotheses) (n = 16) | |
| Yes | 3,8,22,38,42-45,52,53,59,60,80,81,83,90 |
| Patient-level risk factors with specific hypotheses (n = 57) | |
| Race/ethnicity | 18,26,33,46-48,50,57,58,72 |
| BMIf or gestational weight gain | 35,55,62,71,73,78,86,88 |
| Maternal age | 63,76,85 |
| Preterm birth | 25,51,68 |
| Infertility/IVFg/ARTh | 23,34,54,67,70,93 |
| Preeclampsia | 64 |
| Hemorrhage | 37 |
| Obstructive sleep apnea | 28 |
| Congenital heart disease | 79 |
| Inflammatory bowel disease | 87 |
| Autoimmunity | 30 |
| Idiopathic arthritis | 31 |
| Rural vs. urban | 61 |
| Maternal birthplace or immigration country | 89,92 |
| Amphetamine/opioid use | 17 |
| Route of delivery | 20,21,24,41,56,66,74,77,84,94 |
| Induction of labor | 40,65,82 |
| Anesthesia for cesarean delivery | 16 |
| Off-hours delivery | 69,75 |
| Hospital-level risk factors with specific hypotheses (n = 8) | |
| Delivery volume | 27,29,39 |
| Level of care | 32,91 |
| Percent midwives delivering | 19 |
| Presence of laborist | 36 |
| Hospital quality indicators | 49 |
aUS United States; bCMS Centers for Medicare and Medicaid Services; cSociety for Assisted Reproductive Technology; dSMM severe maternal morbidity; eCDC Centers for Disease Control and Prevention; fBMI body mass index; gIVF in vitro fertilization; hART assisted reproductive technology
Table 2.
Risk factors tested for association with severe maternal morbidity
| Potential covariates for use in risk adjustment | Number of studies that used the variable in risk adjustment | Number of studies with a statistically significant result | Number of studies with a non-significant result | Number of studies where statistical significance was not reported |
|---|---|---|---|---|
| Pre-existing clinical | ||||
| Heart disease: including CHF,a,b CHD,a,c pulmonary hypertension,a ischemic heart disease,a valvular heart disease,a conduction disorders | 43 (53.1%) | 10 | 0 | 33 |
| Sickle cell diseasea | 11 (13.6%) | 1 | 0 | 10 |
| Collagen vascular disease: including SLEa,d and rare autoimmune, rheumatoid arthritis and other collagen vascular | 27 (33.3%) | 5 | 0 | 22 |
| HIVa | 21 (25.9%) | 3 | 0 | 18 |
| Chronic renal diseasea | 37 (45.7%) | 7 | 0 | 30 |
| Chronic hypertensiona | 68 (84.0%) | 20 | 0 | 48 |
| Chronic diabetesa | 67 (82.7%) | 15 | 1 | 51 |
| Chronic lung disease, including asthmaa | 31 (38.3%) | 5 | 1 | 25 |
| Thyroid disease, hypothyroidism | 8 (9.9%) | 0 | 0 | 8 |
| Maternal soft tissue condition: includes other uterine surgery, fibroids, cervical conditions | 5 (6.2%) | 0 | 0 | 5 |
| Pelvis abnormal | 1 (1.2%) | 0 | 0 | 1 |
| Gynecological conditions: e.g., endometriosis, PCOS,e peritoneal adhesions | 3 (3.7%) | 0 | 0 | 3 |
| Skin and subcutaneous tissues | 1 (1.2%) | 0 | 0 | 1 |
| Hospitalization in prior 5 years or during pregnancy | 4 (4.9%) | 1 | 0 | 3 |
| Drug abuse:a including amphetamine, opioid, other; one vs multiple substance, cocaine, combination with alcohol or smoking or mental health conditions | 27 (33.3%) | 4 | 1 | 22 |
| Alcohol abusea | 16 (19.8%) | 1 | 1 | 14 |
| Smoking | 34 (42.0%) | 6 | 7 | 21 |
| Mental health, including depression | 16 (19.8%) | 4 | 0 | 12 |
| Obesity class, BMI,f weight gain during pregnancy | 40 (49.4%) | 15 | 5 | 20 |
| Height | 2 (2.5%) | 0 | 0 | 2 |
| Weight gain | 3 (3.7%) | 2 | 1 | 0 |
| Liver disorders, including failure, hepatitis B or C | 11 (13.6%) | 2 | 0 | 9 |
| Digestive diseases, including IBDg | 9 (11.1%) | 1 | 2 | 6 |
| Seizures and other CNSh conditions, e.g., stroke, MSi | 12 (14.8%) | 2 | 0 | 10 |
| Blood diseases: including thrombocytopenia, coagulopathy, or anaemia | 16 (19.8%) | 3 | 0 | 13 |
| Fluid electrolyte disorders | 2 (2.5%) | 0 | 0 | 2 |
| Paralysis | 2 (2.5%) | 0 | 0 | 2 |
| Peripheral vascular disorders | 4 (4.9%) | 0 | 0 | 4 |
| Weight loss | 2 (2.5%) | 0 | 0 | 2 |
| Musculoskeletal conditions | 5 (6.2%) | 0 | 2 | 3 |
| Malignancy | 4 (4.9%) | 0 | 0 | 4 |
| History of organ transplant | 1 (1.2%) | 0 | 0 | 1 |
| High risk summary measure | 28 (34.6%) | 14 | 0 | 14 |
| VTE,j anticoagulant use (now or in past) | 4 (4.9%) | 1 | 0 | 3 |
| Hyperlipidemia | 1 (1.2%) | 0 | 0 | 1 |
| Disorders of the adrenal gland | 1 (1.2%) | 0 | 0 | 1 |
| Obstructive sleep apnea | 1 (1.2%) | 1 | 0 | 0 |
| Genital herpes | 4 (4.9%) | 0 | 0 | 4 |
| Cystic fibrosis | 2 (2.5%) | 0 | 1 | 1 |
| Maternal agea | 79 (97.5%) | 31 | 2 | 46 |
| Maternal race/ethnicity | 44 (54.3%) | 24 | 0 | 20 |
| N = 41 pre-existing clinical covariates | ||||
| Obstetrical Antepartum | ||||
| Parity (nulliparous vs grand multipara) and combinations with prior cesarean birth | 48 (59.3%) | 16 | 0 | 32 |
| Prior cesarean delivery,a number of prior cesareans | 48 (59.3%) | 13 | 2 | 33 |
| Prior preterm birth | 4 (4.9%) | 0 | 0 | 4 |
| Multiple gestationa | 69 (85.2%) | 15 | 2 | 52 |
| Preeclampsia:a including severe, mild, gestational, eclampsia | 47 (58.0%) | 13 | 1 | 33 |
| Placental conditions | 25 (30.9%) | 6 | 0 | 26 |
| Gestational diabetes | 29 (35.8%) | 4 | 5 | 20 |
| Assisted conception: invasive vs. non-invasive, IUI,k ovulation induction, IVF, ICSI,l diagnosed infertility, infertility treatment | 13 (16.0%) | 7 | 0 | 6 |
| Neonatal congenital anomalies or cancer | 14 (17.3%) | 0 | 0 | 14 |
| Fetal presentation | 14 (17.3%) | 2 | 2 | 10 |
| LGAm or SGAn fetus | 14 (17.3%) | 3 | 1 | 10 |
| Oligohydramnios/polyhydramnios | 6 (7.4%) | 1 | 0 | 5 |
| Male fetus | 5 (6.2%) | 0 | 0 | 5 |
| First trimester prenatal care, adequate prenatal care | 17 (21.0%) | 8 | 0 | 9 |
| Provider type (at PNC,o delivery) | 4 (4.9%) | 1 | 0 | 2 |
| Isoimmunization | 4 (4.9%) | 0 | 0 | 4 |
| Number of previous livebirths, number of previous miscarriages/previous miscarriage, ectopic, termination | 4 (4.9%) | 1 | 0 | 3 |
| Prior D&Cp | 1 (1.2%) | 0 | 0 | 1 |
| History of hemorrhage previous pregnancy | 2 (2.5%) | 2 | 0 | 0 |
| History of hypertensive disorder in a previous pregnancy | 1 (1.2%) | 1 | 0 | 0 |
| History of SGA | 1 (1.2%) | 0 | 0 | 1 |
| Group B streptococcus screen positive | 1 (1.2%) | 0 | 0 | 1 |
| N = 22 obstetrical antepartum covariates | ||||
| Intrapartum or postpartum | ||||
| Bishop score < 6 | 1 (1.2%) | 1 | 0 | 0 |
| Unengaged fetal head | 3 (3.7%) | 0 | 0 | 3 |
| Uterine rupture, prolapsed cord | 2 (2.5%) | 0 | 0 | 3 |
| Hemorrhage | 4 (4.9%) | 1 | 0 | 3 |
| Prior stillbirth or infant death | 4 (4.9%) | 0 | 0 | 4 |
| Stillbirth | 9 (11.1%) | 0 | 0 | 9 |
| Route of delivery: includes labor y/n, operative VD,q cesarean | 35 (43.2%) | 12 | 3 | 20 |
| Maternal indication for cesarean | 1 (1.2%) | 0 | 0 | 1 |
| Cesarean incision type | 1 (1.2%) | 0 | 1 | 0 |
| Induction | 12 (14.8%) | 5 | 0 | 7 |
| Cervical ripening | 1 (1.2%) | 1 | 0 | 0 |
| Epidural use | 3 (3.7%) | 0 | 0 | 3 |
| PROM,r PPROMs | 8 (9.9%) | 0 | 0 | 8 |
| Chorioamnionitis or maternal infection | 4 (4.9%) | 1 | 0 | 3 |
| Birth day: weekend, night | 6 (7.4%) | 3 | 2 | 1 |
| Gestational age at delivery, preterm birth, type of preterm birth | 33 (40.7%) | 6 | 0 | 27 |
| Preterm birth spontaneous vs indicated | 2 (2.5%) | 0 | 0 | 2 |
| Labor anomalies: prolonged second stage, oxytocin | 5 (6.2%) | 1 | 0 | 4 |
| General vs neuraxial anesthesia | 3 (3.7%) | 2 | 0 | 1 |
| Perineal trauma | 1 (1.2%) | 0 | 0 | 1 |
| Elective delivery | 6 (7.4%) | 0 | 0 | 6 |
| Fetal distress not in labor (separate from elective) | 1 (1.2%) | 0 | 0 | 1 |
| N = 22 intrapartum/postpartum covariates | ||||
| Other patient-level covariates | ||||
| Year of childbirth | 29 (35.8%) | 13 | 2 | 14 |
| Income | 2 (2.5%) | 1 | 0 | 1 |
| Rural | 4 (4.9%) | 2 | 0 | 2 |
| Insurance | 39 (48.1%) | 16 | 4 | 19 |
| Education | 21 (25.9%) | 7 | 0 | 14 |
| SESt | 5 (6.2%) | 1 | 0 | 4 |
| Foreign born | 16 (19.8%) | 7 | 1 | 8 |
| Language spoken | 1 (1.2%) | 0 | 0 | 1 |
| Refugee status | 1 (1.2%) | 0 | 0 | 1 |
| Duration of residence | 1 (1.2%) | 0 | 0 | 1 |
| Working | 2 (2.5%) | 0 | 2 | 0 |
| Married | 13 (16.0%) | 3 | 2 | 8 |
| Profession | 1 (1.2%) | 0 | 0 | 1 |
| Home birth | 1 (1.2%) | 0 | 0 | 1 |
| Transfer in from other hospital | 3 (3.7%) | 0 | 0 | 3 |
| N = 15 other patient-level covariates | ||||
| Hospital-level covariates | ||||
| Hospital level of maternal care | 2 (2.5%) | 1 | 1 | 0 |
| Hospital size or delivery volume | 17 (21.0%) | 6 | 3 | 8 |
| Hospitalist | 1 (1.2%) | 0 | 1 | 0 |
| Hospital ownership | 11 (13.6%) | 4 | 2 | 5 |
| Hospital teaching | 15 (18.5%) | 5 | 2 | 8 |
| Hospital urban/rural | 9 (11.1%) | 2 | 2 | 5 |
| Hospital percent high-risk | 1 (1.2%) | 1 | 0 | 0 |
| Hospital percent non-White | 1 (1.2%) | 1 | 0 | 0 |
| Hospital black-serving | 1 (1.2%) | 1 | 0 | 0 |
| Hospital percent Medicaid | 3 (3.7%) | 1 | 0 | 2 |
| Hospital coding intensity | 1 (1.2%) | 1 | 0 | 0 |
| Hospital percent midwife births | 1 (1.2%) | 0 | 1 | 0 |
| NICUu level | 4 (4.9%) | 2 | 1 | 1 |
| Hospital cesarean rate general endotracheal anesthesia | 1 (1.2%) | 0 | 0 | 1 |
| Hospital epidural rate | 1 (1.2%) | 0 | 0 | 1 |
| Hospital induction rate | 1 (1.2%) | 0 | 0 | 1 |
| Hospital percent NTSVv | 2 (2.5%) | 0 | 0 | 2 |
| Hospital percent early elective deliveries | 2 (2.5%) | 0 | 0 | 2 |
| Hospital Clinical Processes of Care quintiles | 1 (1.2%) | 0 | 0 | 1 |
| Hospital Patient Perspectives of Care quintiles | 1 (1.2%) | 0 | 0 | 1 |
| Hospital number triaged per day | 1 (1.2%) | 0 | 0 | 1 |
| Hospital number triaged per delivery | 1 (1.2%) | 0 | 0 | 1 |
| Hospital > 4 hospitals within 20 miles of residence | 1 (1.2%) | 0 | 0 | 1 |
| Hospital excellent doctor:nurse relationship | 1 (1.2%) | 0 | 0 | 1 |
| Hospital doctors/1000 deliveries | 1 (1.2%) | 0 | 0 | 1 |
| Hospital MFMw on staff | 1 (1.2%) | 0 | 0 | 1 |
| Hospital midwives available | 1 (1.2%) | 0 | 0 | 1 |
| Hospital anesthesia available 24/7 | 1 (1.2%) | 0 | 0 | 1 |
| Hospital anesthesia staff have no other responsibilities | 1 (1.2%) | 0 | 0 | 1 |
| Hospital equivalent staffing day and night | 1 (1.2%) | 0 | 0 | 1 |
| Hospital cesarean in main hospital operating room | 1 (1.2%) | 0 | 0 | 1 |
| Hospital radiology available 24/7 | 1 (1.2%) | 0 | 0 | 1 |
| Hospital blood bank available24/7 | 1 (1.2%) | 0 | 0 | 1 |
| Hospital massive transfusion protocol in place | 1 (1.2%) | 0 | 0 | 1 |
| Hospital pharmacist dedicated to L&Dx | 1 (1.2%) | 0 | 0 | 1 |
| Hospital Bakri Balloon available | 1 (1.2%) | 0 | 0 | 1 |
| Hospital epidural easy to get | 1 (1.2%) | 0 | 0 | 1 |
| Hospital adult critical care 24/7 | 1 (1.2%) | 0 | 0 | 1 |
| Hospital subspecialty intensive care units available | 1 (1.2%) | 0 | 0 | 1 |
| Hospital difficult to get consults | 1 (1.2%) | 0 | 0 | 1 |
| Hospital has NICU | 1 (1.2%) | 0 | 0 | 1 |
| Hospital central FHRy monitoring | 1 (1.2%) | 0 | 0 | 1 |
| Hospital emergency response team available to L&D | 1 (1.2%) | 0 | 0 | 1 |
| Hospital allow TOLACz | 1 (1.2%) | 0 | 0 | 1 |
| Hospital 100% of cesareans begun within 30 min | 1 (1.2%) | 0 | 0 | 1 |
| Hospital intermittent FHR monitoring < 50% of patients | 1 (1.2%) | 0 | 0 | 1 |
| Hospital doctors sign out to each other | 1 (1.2%) | 0 | 0 | 1 |
| Hospital formal rounds are conducted on L&D | 1 (1.2%) | 0 | 0 | 1 |
| Hospital drills and simulations required | 1 (1.2%) | 0 | 0 | 1 |
| Hospital FHR monitoring course required of doctors | 1 (1.2%) | 0 | 0 | 1 |
| Hospital tracking of haemorrhage occurs | 1 (1.2%) | 0 | 0 | 1 |
| Hospital tracking of infection occurs | 1 (1.2%) | 0 | 0 | 1 |
| Hospital tracking of 3rd & 4th degree lacerations occurs | 1 (1.2%) | 0 | 0 | 1 |
| Hospital has cesarean evaluation team | 1 (1.2%) | 0 | 0 | 1 |
| Hospital allows maternal transfers in | 1 (1.2%) | 0 | 0 | 1 |
| Hospital has a protocol for induction of labor | 1 (1.2%) | 0 | 0 | 1 |
| Hospital has a protocol for cesarean delivery | 1 (1.2%) | 0 | 0 | 1 |
| Hospital gives education regarding induction of labor | 1 (1.2%) | 0 | 0 | 1 |
| N = 58 hospital-level covariates | ||||
| Community-level covariates | ||||
| Region | 21 (25.9%) | 6 | 1 | 14 |
| Neighborhood poverty | 3 (3.7%) | 0 | 2 | 1 |
| Miles from zip code to hospital | 1 (1.2%) | 0 | 1 | 0 |
| Geographic designation of area urban/rural | 8 (9.9%) | 2 | 0 | 6 |
| County frequency of obstetricians/anesthesiologists | 1 (1.2%) | 0 | 1 | 0 |
| County frequency of births to teens | 1 (1.2%) | 0 | 1 | 0 |
| County frequency of unmarried women | 1 (1.2%) | 0 | 1 | 0 |
| County frequency of divorced females | 1 (1.2%) | 0 | 1 | 0 |
| County frequency of female family heads | 1 (1.2%) | 0 | 1 | 0 |
| County frequency of females with no insurance | 1 (1.2%) | 0 | 1 | 0 |
| County frequency of foreign-born persons | 1 (1.2%) | 0 | 1 | 0 |
| County frequency of persons with less than high school education | 1 (1.2%) | 0 | 1 | 0 |
| County frequency of non-White persons | 1 (1.2%) | 0 | 1 | 0 |
| County household income measure | 18 (22.2%) | 4 | 2 | 12 |
| County frequency of unemployed persons | 1 (1.2%) | 0 | 1 | 0 |
| County frequency of food stamp beneficiaries | 1 (1.2%) | 0 | 1 | 0 |
| County frequency of persons with no phone | 1 (1.2%) | 0 | 1 | 0 |
| County frequency of households with > 1 person/room | 1 (1.2%) | 0 | 1 | 0 |
| County number of days with good air | 1 (1.2%) | 0 | 1 | 0 |
| County number of deaths due to AIDSaa | 1 (1.2%) | 0 | 1 | 0 |
| County number of deaths due to MVAbb | 1 (1.2%) | 0 | 1 | 0 |
| County death suicide | 1 (1.2%) | 0 | 1 | 0 |
| N = 22 community-level covariates | ||||
| TOTAL: N = 180 covariates | ||||
aincluded in Bateman Comorbidity Index; bCHF congestive heart failure; cCHD congenital heart disease; dSLE systemic lupus erythematosus; ePCOS polycystic ovary syndrome; fBMI body mass index; gIBD inflammatory bowel disease; hCNS central nervous system; iMS multiple sclerosis; jVTE venous thromboembolism; kIUI intrauterine insemination; lICSI intracytoplasmic sperm injection; mLGA large for gestational age; nSGA small for gestational age; oPNC prenatal care; pD&C dilatation and curettage; qVD vaginal delivery; rPROM premature rupture of membranes; sPPROM preterm premature rupture of membranes; tSES socioeconomic status; uNICU neonatal intensive care unit; vNTSV nulliparous term singleton vertex; wMFM maternal fetal medicine specialist; xL&D labor and delivery area; yFHR fetal heart rate; zTOLAC trial of labor after cesarean; aaAIDS acquired immune deficiency syndrome; bbMVA motor vehicle accident
Risk of bias of included studies
Quality scoring is presented in Table 3. Of 10 potential points per study, the median score was 6 (range 3–10). There were 37 high-quality, 39 moderate-quality, and 5 low-quality studies.
Table 3.
Quality scoring of included studies (n = 81)
| REFERENCE NUMBER | LAST NAME OF FIRST AUTHOR | YEAR | SELECTION (MAX 3) *** | RISK FACTORS (MAX 4) **** |
OUTCOME (MAX 3) *** | TOTAL SCORE |
|---|---|---|---|---|---|---|
| [16] | ABE | 2018 | ** | ** | * | 5 |
| [17] | ADMON | 2018 | *** | ** | * | 6 |
| [18] | ADMON | 2018 | *** | ** | ** | 7 |
| [19] | ATTANASIO | 2017 | * | ** | * | 4 |
| [20] | BANNISTER-TYRRELL | 2015 | ** | ** | * | 5 |
| [21] | BARGER | 2013 | *** | ** | * | 6 |
| [22] | BATEMAN | 2013 | ** | *** | ** | 7 |
| [23] | BELANOFF | 2016 | *** | ** | * | 6 |
| [24] | BIN | 2016 | ** | ** | * | 5 |
| [25] | BLANC | 2019 | * | *** | * | 5 |
| [26] | BOOKER | 2018 | *** | *** | ** | 8 |
| [27] | BOOKER | 2018 | ** | ** | ** | 6 |
| [28] | BOURJEILY | 2017 | ** | *** | * | 6 |
| [29] | BOZZUTO | 2019 | *** | ** | * | 6 |
| [30] | CHEN | 2015 | ** | *** | * | 6 |
| [31] | CHEN | 2013 | ** | *** | * | 6 |
| [32] | CLAPP | 2018 | *** | *** | ** | 8 |
| [33] | CREANGA | 2014 | *** | ** | ** | 7 |
| [34] | DAYAN | 2019 | ** | *** | * | 6 |
| [35] | DAYAN | 2018 | * | *** | * | 5 |
| [36] | FELDMAN | 2015 | *** | *** | * | 7 |
| [37] | FORD | 2015 | ** | ** | ** | 6 |
| [38] | FREDERIKSEN | 2017 | *** | ** | ** | 7 |
| [39] | FRIEDMAN | 2016 | *** | *** | ** | 8 |
| [40] | GIBBS PICKENS | 2018 | *** | ** | * | 6 |
| [41] | GRASCH | 2017 | * | *** | * | 5 |
| [42] | GRAY | 2012 | *** | *** | * | 7 |
| [43] | GROBMAN | 2014 | *** | **** | *** | 10 |
| [44] | GUGLIELMINOTTI | 2019 | *** | ** | ** | 7 |
| [45] | HEHIR | 2013 | ** | *** | ** | 7 |
| [46] | HOWELL | 2017 | ** | ** | ** | 6 |
| [47] | HOWELL | 2016 | ** | *** | ** | 7 |
| [48] | HOWELL | 2016 | *** | ** | * | 6 |
| [49] | HOWELL | 2014 | ** | *** | ** | 7 |
| [50] | HOWLAND | 2019 | ** | *** | ** | 7 |
| [8] | JOSEPH | 2010 | ** | ** | * | 5 |
| [51] | KILPATRICK | 2016 | *** | ** | *** | 8 |
| [52] | KORST | 2014 | *** | *** | * | 7 |
| [53] | LAZARIU | 2017 | *** | ** | * | 6 |
| [54] | LE RAY | 2019 | ** | *** | *** | 8 |
| [55] | LEONARD | 2019 | *** | *** | ** | 8 |
| [56] | LEONARD | 2019 | *** | *** | ** | 8 |
| [57] | LEONARD | 2019 | *** | *** | ** | 8 |
| [58] | LIESE | 2019 | *** | ** | ** | 7 |
| [59] | LINDQUIST | 2015 | ** | *** | * | 6 |
| [60] | LIPKIND | 2019 | ** | ** | ** | 6 |
| [61] | LISONKOVA | 2016 | ** | ** | * | 5 |
| [62] | LISONKOVA | 2017 | *** | *** | * | 7 |
| [63] | LISONKOVA | 2017 | *** | *** | * | 7 |
| [64] | LISONKOVA | 2014 | *** | *** | * | 7 |
| [65] | LIU | 2013 | ** | * | * | 4 |
| [66] | LIU | 2007 | ** | ** | * | 5 |
| [67] | LUKE | 2019 | *** | ** | * | 6 |
| [68] | LYNDON | 2019 | *** | ** | * | 6 |
| [69] | LYNDON | 2015 | *** | ** | * | 6 |
| [70] | MARTIN | 2016 | ** | *** | * | 6 |
| [71] | MASTERS | 2018 | *** | *** | * | 7 |
| [72] | METCALFE | 2018 | *** | *** | * | 7 |
| [73] | MOURAD | 2014 | ** | ** | * | 5 |
| [74] | MURACA | 2019 | * | * | * | 3 |
| [75] | NAM | 2019 | ** | ** | * | 5 |
| [76] | OSMUNDSON | 2016 | *** | ** | * | 6 |
| [77] | PALLASMAA | 2014 | ** | ** | * | 5 |
| [78] | PLATNER | 2019 | ** | *** | ** | 7 |
| [79] | RAMAGE | 2019 | *** | * | * | 5 |
| [80] | RAY | 2018 | ** | *** | * | 6 |
| [81] | REID | 2018 | ** | *** | ** | 7 |
| [82] | ROBERTS | 2009 | ** | ** | * | 5 |
| [83] | ROSENBLOOM | 2017 | ** | **** | * | 7 |
| [84] | ROY | 2019 | *** | ** | * | 6 |
| [85] | SCHUMMERS | 2018 | ** | **** | * | 7 |
| [86] | SCHUMMERS | 2015 | ** | **** | ** | 8 |
| [87] | SHAND | 2016 | ** | *** | * | 6 |
| [88] | SIDDIQUI | 2019 | ** | **** | ** | 8 |
| [89] | URQUIA | 2017 | ** | * | * | 4 |
| [90] | URQUIA | 2015 | * | * | ** | 4 |
| [91] | VANDERLAAN | 2019 | *** | *** | ** | 8 |
| [92] | WAHLBERG | 2013 | ** | *** | ** | 7 |
| [93] | WANG | 2016 | **** | *** | 7 | |
| [3] | WEN | 2005 | ** | *** | * | 6 |
| [95] | YOUNG | 2018 | ** | * * | * | 5 |
Synthesis of results by study approach
Testing of multiple conditions for association with SMM
The 16 publications in this category are described in Table 2. Four of these publications attempted to describe the accuracy of the models using various statistical techniques [22, 43, 44, 83]. All studies used maternal age and 11 used race/ethnicity. In several studies, maternal age [3, 27, 33, 47, 69, 81, 82] and parity [3, 42, 53, 82] appeared to have a U- or J-shaped relationship with SMM, requiring categorization into three or more groups or appropriate selection of the functional form (e.g., polynomial or logistic) for the association of these covariates with SMM. Two studies used no pre-existing risk factors [3, 90] and one used no obstetrical risk factors [60]. Of the 15 studies that did use obstetrical risk factors, four included intrapartum risk factors [3, 38, 44, 53].
Hypothesis testing of specific risk factors for SMM
There were 57 studies in this category, and the key risk factors used in modelling are listed in Table 2. As with the studies that tested multiple conditions, maternal age and race/ethnicity were common covariates, as was body mass index (BMI). Where BMI was treated as an independent risk factor, it appeared to have a U-shape. Patients who were underweight and those who were obese had increased risk [42, 53, 55, 62, 63].
There were 10 publications that focused specifically on race [18, 26, 33, 46–48, 50, 57, 58, 72]. SMM rates of Black women have been found to be higher than those of White women, even among those with no comorbidities. In a study by Admon et al., among women with no physical or behavioral health conditions, the SMM rate of non-Hispanic Black women was nearly twice that of non-Hispanic White women [18]. Among women with two or more chronic health conditions, non-Hispanic Black women again had an SMM rate that was nearly twice the rate of non-Hispanic White women. Viewed another way, over time, Metcalfe et al. examined trends of SMM rates by race/ethnicity and found that adjustment for race did not change the SMM trends for 5-year periods between 1993 and 2012, over and above adjustment for comorbidity [72]. Similarly, Leonard et al., in a California study [26], and Booker et al., in a study of older women [57], examined SMM rates over time and found that all racial groups experienced rising SMM; SMM was strongly affected by the presence of comorbidities; and the SMM increases for Black and White women were proportionate. Furthermore, Howland et al. demonstrated that Black-White disparities persisted in the highest income and educational groups [50]. Taken together, these studies suggest that there is a baseline difference in SMM between Black and White women that has not been explained.
Several studies [23, 34, 54, 67, 70, 93] tested whether infertile women were at increased risk of SMM. All found an increased risk for SMM among those receiving infertility treatments, cautioning that this increased risk may be attributable to multiple gestation; however, one publication found SMM risk to be elevated among singleton gestations [70].
There have been separate approaches to including drug, alcohol, and/or tobacco use as covariates in SMM models using administrative data. Some studies incorporated these conditions within a risk factor category labelled mental health while others treated these as separate risk factors. However, the sensitivity of administrative data for this information has been reported to be low [96, 97].
Fifteen studies examined specific intrapartum risk factors for their contribution to SMM: induction of labor [40, 65, 82], off-hours delivery [69, 75], route of delivery [20, 24, 41, 56, 66, 74, 77, 84, 94], and anesthesia type for cesarean delivery [16]. Others included intrapartum risk factors as covariates in the context of other hypotheses or in trying to explain the variation in SMM [21, 25, 37, 41, 46–48, 61, 63, 67, 76, 79]. Multiple investigators specifically used risk-adjustment models that only included antepartum risk factors for SMM to avoid adjustment for differences in patient management.
Hypothesis testing of hospital-level risk factors for SMM
Eight studies focused on hospital-level risk factors [19, 27, 29, 32, 36, 39, 49, 91]. There were few consistent findings. Three studies focused on annual hospital delivery volume and had mixed results [27, 29, 39]. Three other investigations tested various hypotheses regarding an association between the following specific hospital characteristics and SMM and found no association: the use of laborists in community hospitals [36], hospital quality indicators [49], and the percent of practitioners doing deliveries at the hospital that were midwives [19].
Given patients with the same high-risk conditions, it has been assumed that delivery at higher level hospitals will lead to less SMM. However, evidence for this supposition is limited. Two studies attempted to find an association between hospital resources and SMM. In both cases, hospital resource levels were studied as proxies for levels of maternal care, which are proposed designations for hospitals based on their resources and staffing [98]. Vanderlaan et al. used American Hospital Association data indicating the risk level of patients cared for by the hospital [91], and Clapp et al. assigned risk levels to patients based on Bateman’s Obstetrical Comorbidity Index and then rated hospitals as high versus low acuity based on their percentages of high-risk patients [32]. In spite of extensive sensitivity analyses, Vanderlaan et al. found no relationship between these proxy resource levels and SMM [91]. Clapp et al. found that high-risk patients had a higher absolute risk of SMM at low-acuity hospitals when compared with high-risk patients at high-acuity centers; however, 95% confidence intervals overlapped and no p-value for the comparison was reported [32].
SMM rates and trends
Twenty-seven of the included studies presented SMM rates. Several examined trends of SMM rates over the years [17, 26, 38, 45, 72], reporting rising rates of both SMM and associated comorbidities. Some investigators disaggregated SMM rates and reported rates of the various indicators [18, 89]. SMM rates were highly dependent on the SMM definitions, study populations, and adjustment models. For example, some investigators built on the CDC definitions [52, 61–64]; others used broad definitions that included maternal intensive care unit admission [21, 25, 28, 40, 53, 65, 67, 71, 73]. A number of studies extended SMM case finding to 42 days postpartum or readmission with SMM.
In the last 5 years, and particularly with the use of administrative data wherein the number of units of packed red blood cells cannot be reliably ascertained, investigators have recognized that blood transfusion accounts for a large proportion of the SMM cases, and, consequently, whether or not it is included in the SMM definition substantially affects the SMM rate and its interpretability [7]. Fifteen studies did sensitivity analyses to display trends or determine if the effect sizes of risk factors were confirmed when transfusion was eliminated from the SMM definition. Trends from year to year were less likely to show statistical differences, and most studies (with some exceptions [50, 55, 78, 94] showed minimal to no changes in the magnitude of risk factors when excluding transfusion. Another 10 did not include transfusion in their SMM definition, nine studies using a maternal ICU admission did not separate transfusion out, and six used medical chart review to assure that at least 4 units of packed red blood cells were used to qualify as meeting the SMM definition (Table 1).
From the seven studies using administrative data with unrestricted delivery populations and including transfusion in the SMM definition [3, 8, 38, 44, 53, 60, 81], SMM rates varied from 0.44% [3] to 2.55% [53]. Using the US National Inpatient Sample [99], the CDC reported the most recent SMM rates from 2014 as 1.44% with transfusions and 0.35% when using the definition excluding transfusions [7]. The overall rate of SMM increased 200% from 1993 to 2014 when transfusion was included and 20% in the same time period when transfusion was excluded.
Maternal death is not an exclusion criterion for the CDC definition of SMM [7]. Some studies specifically included maternal death whether or not SMM was reported. One posited that the coding of death without SMM must be erroneous, and, therefore, excluded such cases [70]. Friedman et al. studied both SMM and death, finding that: 1) 78.7% of deaths in the dataset had been identified as having SMM (these deaths were referred to as “failure to rescue”); and 2) 1.0% of patients with SMM died [39]. This study did not extend the SMM definition to include post-discharge follow-up. In a study by Ray et al., 68.0% of deaths in a population-based delivery cohort had been identified as having SMM [80].
Discussion
Principal findings
This review identified 81 studies of SMM that relied on risk adjustment of routinely collected population-based data. Although the key search term was deliberately chosen to be “severe maternal morbidity” in an attempt to identify studies that incorporated similar outcomes, only 37 (45.7%) used an SMM definition with a CDC basis; the SMM definitions used in the remaining studies varied to a much larger extent. The inclusion of blood transfusions (yes/no) in the SMM definition added a layer of complexity to the comparability of these analyses, given that, in various studies, more than half of the SMM cases had this single indicator of SMM. Such heterogeneity was also evident in the principal datasets used (e.g., claims data, electronic medical record or medical record data, administrative data in both ICD-9 and ICD-10), which may have included linkages to other datasets (e.g., infertility, birth certificate, hospital surveys, census data). Study populations also differed with respect to the definition of a delivery admission and, depending on the purpose of the study, the inclusion and exclusion criteria.
The covariates used for risk adjustment also varied extensively (n = 180, Table 2), not only with respect to the choice of covariates, but also with respect to their definitions (e.g., BMI as a continuous, ordinal, or binary variable). Interpretation of the results also depends on the study design (e.g., subset of deliveries included) and model specification (e.g., other covariates included). Some studies attempted to limit the types of covariates to patient-level conditions that would be apparent prior to the childbirth admission, while others attempted to develop more explanatory models for SMM and included intrapartum variables such as dystocia and delivery mode. Several studies used hospital characteristics (e.g., delivery volume, ownership, or teaching status) or community-level variables (e.g., median household income, percent foreign-born by zip code or county) to make comparisons more interpretable or models more explanatory. Consequently, effect sizes (odds ratios and relative risks) could not be synthesized in a meaningful way.
Interpretation
The call for facility-based surveillance of SMM through case review [10, 11] remains critical for identifying SMM causes, so that prevention strategies and interventions can be developed, implemented, and tested prospectively. In addition, there remains a role for population-based administrative data to describe and monitor the SMM burden [12]. The use of administrative data enables the development of standard SMM rates that can be used to describe trends and disparities and, potentially, to make comparisons across regions and hospitals. Such comparisons can highlight regions or hospitals with disproportionate burdens and can potentially provide insight regarding the quality of pregnancy care for those with SMM and/or the resources needed to address SMM. This use of administrative data at the population level can also inform decisions regarding the potential for public health interventions, such as improving the availability of preconception care [100], and can be used to track their success. Demonstrated success could mean more resources can be deployed to scale-up effective interventions and attenuate the SMM burden.
The results of this review point to several areas that are in need of development for the continued evolution of SMM tracking using population-based data. First is the standardization of the SMM definition. In the US, this definition has been gravitating toward that used by the CDC. However, differences remain across recent US studies, particularly with respect to the inclusion of blood transfusion. The role of transfusions in the administrative definition of SMM needs further evaluation and standardization because the rise in transfusions is due largely to quality improvement efforts to decrease mortality from postpartum hemorrhage [101]. It is apparent that blood products are increasingly being used as part of a secondary prevention effort and that such usage in practice (which is life-saving) conflicts with the interpretation of the SMM measure as a poor outcome.
The second area in need of development is the standardization of the content and size of datasets used for hospital or regional comparisons. Hospital discharge datasets appear to be the best choice because they are relatively similar and nearly universally available. The marginal benefit for the addition of linked patient-level datasets, such as the birth certificate data, may be too resource-intensive for some states. The linkage of a basic subset of hospital variables such as ownership, delivery volume, and teaching status, could be gleaned from a variety of sources and maintained in a central location for consistent use. The importance of community-level variables (e.g., by census tract, zip code, county) has not been well-explored in the literature and needs further evaluation, especially as it relates to the potential for public health intervention and ability to impact SMM rates. Community-level summary measures (e.g., median income, rural status) were frequently used as proxies for patient- or hospital-level comparisons and were relatively infrequently reported as contributing to risk adjustment models.
Third is the selection and definition of risk factors of interest. This will depend on the purpose of the risk adjustment. For the purpose of comparing hospital SMM rates, we suggest that models should adjust for case-mix using the risk factors known upon admission but without including those variables describing intrapartum management (e.g., route of delivery) because these variables are under the control of a given hospital and there is no need to keep them balanced across hospitals. Hospital-level factors, such as resources or staffing characteristics, should also be excluded if hospitals are being ranked. The “within” hospital correlation in SMM can be addressed using clustered standard errors. A more serviceable comparison can be achieved by comparing only hospitals of the same type (e.g., teaching hospitals or community hospitals). By confining hospital comparisons to a group with a similar type, the average hospital for that type yields a better representation of the group compared with an average hospital in a group composed of diverse hospital types. On the other hand, if the purpose is to predict the SMM risk, it is reasonable for these models to include intrapartum-, hospital-, and community-level risk factors to increase explanatory power.
Furthermore, the inclusion of patient-level non-clinical variables (e.g., insurance type, educational level) in SMM risk adjustment models deserves reflection. Such variables may be potential proxies for unmeasured clinical risk factors (e.g., malnutrition), measures of access to higher quality of care, or sources of variation due to discrimination. The risk adjustment purpose and the hypothesized source for the variation in SMM risk due to such variables should determine their use in modelling. For example, for hospital comparisons, use of these covariates would not be appropriate given that they would credit hospitals for poor care given to disadvantaged patients.
Limitations of the review
As discussed in depth above, a limitation of this review is the study heterogeneity, which prevents meaningful synthesis of effect sizes. More narrow inclusion criteria may have allowed for increased detail regarding the relative importance of specific risk factors, such as race/ethnicity and prematurity.
Conclusions
This review identified multiple potential risk factors associated with SMM. The heterogeneity of the studies precluded any quantitative synthesis of the effect sizes (odds ratios or relative risks) of the identified risk factors. The development of a risk adjustment strategy that will allow for SMM comparisons across hospitals or regions will require harmonization regarding the standardization of the SMM definition, the datasets and population used, and the selection and definition of risk factors of interest. The ability to perform such comparisons would contribute to the capacity of public health systems to monitor SMM trends and disparities and to develop strategies to decrease the SMM burden. Administrative data comparisons also allow for evaluating the potential for interventions, tracking their success, and estimating the resources needed to scale-up effective interventions.
Supplementary Information
Additional file 1. Newcastle-Ottawa Quality Assessment Scale (adapted for cross sectional studies with healthcare data for research question: What are the risk factors for severe maternal morbidity associated with the delivery admission?
Additional file 2. Preferred Reporting Items for Systematic reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) Checklist.
Acknowledgments
None.
Abbreviations
- AIDS
Acquired immune deficiency syndrome
- ART
Assisted reproductive technology
- BMI
Body mass index
- CDC
Centers for Disease Control and Prevention
- CHF
Congestive heart failure
- CHD
Congenital heart disease
- CNS
Central nervous system
- CMS
Centers for Medicare and Medicaid Services
- D&C
Dilatation and curettage
- FHR
Fetal heart rate
- IBD
Inflammatory bowel disease
- ICD-9,10
International Classification of Diseases, Clinical Modification, versions 9,10
- ICSI
Intracytoplasmic sperm injection
- IUI
Intrauterine insemination
- IVF
In vitro fertilization
- L&D
Labor and delivery area
- LGA
Large for gestational age
- MFM
Maternal fetal medicine specialist
- MS
Multiple sclerosis
- MVA
Motor vehicle accident
- NICU
Neonatal intensive care unit
- NOS
Newcastle-Ottawa Scale
- NTSV
Nulliparous term singleton vertex
- PCOS
Polycystic ovary syndrome
- PNC
Prenatal care
- PRISMA-ScR
Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping reviews
- PROM
Premature rupture of membranes
- PPROM
Preterm premature rupture of membranes
- SART
Society for Assisted Reproductive Technology
- SES
Socioeconomic status
- SGA
Small for gestational age
- SLE
Systemic lupus erythematosus
- SMM
Severe maternal morbidity
- TOLAC
Trial of labor after cesarean
- VD
Vaginal delivery
- VTE
Venous thromboembolism
- US
United States
- WHO
World Health Organization
Authors’ contributions
Conceptualization: LMK,KDG,LAN,SS,DJR,JLT,NG,MF; Data curation: LMK,MF; Formal analysis: LMK, MF; Funding acquisition: LMK,KDG,LAN,DJR,JLT,MF; Investigation: LMK,KDG,LAN,SS,DJR,JLT,NG,MF; Methodology: LMK,KDG,NG,MF; Project administration: LAN, KDG,SS,DJR,JLT; Resources: LMK,MF; Supervision: KDG,LAN,DJR,MF; Validation: LMK, MF; Visualization: LMK,KDG,LAN,DJR,JLT,MF; Writing-original draft preparation: LMK, MF; Writing-review and editing: LMK,KDG,LAN,SS,DJR,JLT,NG,MF. The authors read and approved the final manuscript.
Authors’ information
Dr. Kimberly Gregory is Helping Hand of Los Angeles Mariam Jacobs Chair in Maternal Fetal Medicine in the Department of Obstetrics and Gynecology at Cedars-Sinai Medical Center.
Funding
Funding source: California Department of Public Health, Maternal, Child and Adolescent Health Division, contract number 18–10003. (LMK, KDG, LAN, and MF received the award.) role of the sponsor: DJR and JLT were involved in the study design, data collection and analysis, decision to publish, and preparation of the manuscript. The original funding source for this award is the Title V Maternal and Child Health Services Block Grant from the Maternal and Child Health Bureau/Health Resources & Services Administration.
Availability of data and materials
No data or materials were used for the publication of this article beyond the review of those articles referenced.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors report no conflicts of interest and the following disclosures. LMK and MF are shareholders in Maternal Metrics, Inc. LMK, MF, KDG, and LAN are recipients of federal and state grants for work regarding obstetrical outcomes. DJR and JLT are employees of the California Department of Public Health (CDPH). The remaining authors have nothing to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization . Beyond the numbers: reviewing maternal deaths and complications to make pregnancy safer. Geneva: World Health Organization; 2004. [Google Scholar]
- 2.Say L, Souza JP, Pattinson RC, for the WHO working group on Maternal Mortality and Morbidity classifications Maternal near miss – towards a standard tool for monitoring quality of maternal health care. Best Pract Res Clin Obstet Gynaecol. 2009;23:287–296. doi: 10.1016/j.bpobgyn.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Wen SW, Huang L, Liston R, Heaman M, Baskett T, Rusen ID, et al. Severe maternal morbidity in Canada, 1991-2001. CMAJ. 2005;173(7):759–764. doi: 10.1503/cmaj.045156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callaghan WM, MacKay AP, Berg CY. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991–2003. Am J Obstet Gynecol. 2008;199:133.e1–133.e8. doi: 10.1016/j.ajog.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029–1036. doi: 10.1097/AOG.0b013e31826d60c5. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. How does CDC identify severe maternal morbidity. Available from: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/smm/severe-morbidity-ICD.htm. Accessed 1 Sept 2020.
- 7.Roberts CL, Cameron CA, Bell JC, Algert CS, Morris JM. Measuring maternal morbidity in routinely collected health data: development and validation of a maternal morbidity outcome indicator. Med Care. 2008;46(8):786–794. doi: 10.1097/MLR.0b013e318178eae4. [DOI] [PubMed] [Google Scholar]
- 8.Joseph KS, Liu S, Rouleau J, Kirby RS, Dramer MS, Sauve R, et al. Severe maternal morbidity in Canada, 2003 to 2007: surveillance using routine hospitalization data and ICD-10ca codes. J Obstet Gynaecol Can. 2010;32(9):837–846. doi: 10.1016/S1701-2163(16)34655-2. [DOI] [PubMed] [Google Scholar]
- 9.Callaghan WM, Grobman WA, Kilpatrick SJ, Main EK, D’Alton M. Facility-based identification of women with severe maternal morbidity: it is time to start. Obstet Gynecol. 2014;123:978–981. doi: 10.1097/AOG.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilpatrick SJ, Berg C, Bernstein P, Bingham D, Delgado A, Callaghan WM, et al. Standardized severe maternal morbidity review: rationale and process. Obstet Gynecol. 2014;124:361–366. doi: 10.1097/AOG.0000000000000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuklina EV, Goodman DA. Severe maternal or near miss morbidity: implications for public health surveillance and clinical audit. Clin Obstet Gynecol. 2018;61(2):307–318. doi: 10.1097/GRF.0000000000000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Quality Forum . National Quality Forum launches new project to improve maternal morbidity and mortality outcomes. 2019. [Google Scholar]
- 13.Anderson S, Allen P, Peckham S, Goodwin N. Asking the right questions: scoping studies in the commissioning of research on the organization and delivery of health services. Health Res Policy Sys. 2008;6:7. doi: 10.1186/1478-4505-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) website. Available from: http://www.prisma-statement.org/Extensions/ScopingReviews. Accessed 1 Sept 2020.
- 15.Wells GA, Shea B, O'Connell D, Robertson J, Peterson J, Welch V et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 1 Sept 2020.
- 16.Abe H, Sumitani M, Uchida K, Ikeda T, Matsui H, Fushimi K, et al. Association between mode of anaesthesia and severe maternal morbidity during admission for scheduled caesarean delivery: a nationwide population-based study in Japan, 2010-2013. Br J Anaesth. 2018;120(4):779–789. doi: 10.1016/j.bja.2017.11.101. [DOI] [PubMed] [Google Scholar]
- 17.Admon LK, Bart G, Kozhimannil KB, Richardson CR, Dalton VK, Winkelman TNA. Amphetamine- and opioid-affected births: incidence, outcomes, and costs, United States, 2004-2015. Am J Public Health. 2018;109(1):148–154. doi: 10.2105/AJPH.2018.304771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Admon LK, Winkelman TNA, Zivin K, Terplan M, Mhyre JM, Dalton VK. Racial and ethnic disparities in the incidence of severe maternal morbidity in the United States, 2012-2015. Obstet Gynecol. 2018;132(5):1158–1166. doi: 10.1097/AOG.0000000000002937. [DOI] [PubMed] [Google Scholar]
- 19.Attanasio L, Kozhimannil KB. Relationship between hospital-level percentage of midwife-attended births and obstetric procedure utilization. J Midwifery Womens Health. 2018;63(1):14–22. doi: 10.1111/jmwh.12702. [DOI] [PubMed] [Google Scholar]
- 20.Bannister-Tyrrell M, Patterson JA, Ford JB, Morris JM, Nicholl MC, Roberts CL. Variation in hospital caesarean section rates for preterm births. Aust N Z J Obstet Gynaecol. 2015;55(4):350–356. doi: 10.1111/ajo.12351. [DOI] [PubMed] [Google Scholar]
- 21.Barger MK, Nannini A, DeJoy S, Wisner K, Markenson G. Maternal and newborn outcomes following uterine rupture among women without versus those with a prior cesarean. J Matern Fetal Neonatal Med. 2013;26(2):183–187. doi: 10.3109/14767058.2012.725790. [DOI] [PubMed] [Google Scholar]
- 22.Bateman BT, Mhyre JM, Hernandez-Diaz S, Huybrechts KF, Fischer MA, Creanga AA, et al. Development of a comorbidity index for use in obstetric patients. Obstet Gynecol. 2013;122(5):957–965. doi: 10.1097/AOG.0b013e3182a603bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belanoff C, Declercq ER, Diop H, Gopal D, Kotelchuck M, Luke B, et al. Severe maternal morbidity and the use of assisted reproductive technology in Massachusetts. Obstet Gynecol. 2016;127(3):527–534. doi: 10.1097/AOG.0000000000001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bin YS, Roberts CL, Ford JB, Nicholl MC. Outcomes of breech birth by mode of delivery: a population linkage study. Aust N Z J Obstet Gynaecol. 2016;56(5):453–459. doi: 10.1111/ajo.12488. [DOI] [PubMed] [Google Scholar]
- 25.Blanc JN, Resseguier JN, Goffinet F, Lorthe E, Kayem G, Delorme P, et al. Association between gestational age and severe maternal morbidity and mortality of preterm cesarean delivery: a population-based cohort study. Am J Obstet Gynecol. 2019;220(4):399.e1–399.e9. doi: 10.1016/j.ajog.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Booker WA, Gyamfi-Bannerman C, Sheen JJ, Wright JD, Siddiq Z, D’Alton ME, et al. Maternal outcomes by race for women aged 40 years or older. Obstet Gynecol. 2018;132(2):404–413. doi: 10.1097/AOG.0000000000002751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Booker WA, Ananth CV, Wright JD, Siddiq A, D’Alton ME, Cleary KL, et al. Trends in comorbidity, acuity, and maternal risk associated with preeclampsia across obstetric volume settings. J Matern Fetal Neonatal Med. 2018;32(16):2680–2687. doi: 10.1080/14767058.2018.1446077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bourjeily G, Danilack VA, Bublitz MH, Lipkind H, Muri J, Caldwell D, et al. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort. Sleep Med. 2017;38:50–57. doi: 10.1016/j.sleep.2017.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bozzuto L, Passarella M, Lorch S, Srinivas S. Effects of delivery volume and high-risk condition volume on maternal morbidity among high-risk obstetric patients. Obstet Gynecol. 2019;133(2):261–268. doi: 10.1097/AOG.0000000000003080. [DOI] [PubMed] [Google Scholar]
- 30.Chen JS, Roberts CL, Simpson JM, March LM. Pregnancy outcomes in women with rare autoimmune diseases. Arthritis Rheumatol. 2015;67(12):3314–3323. doi: 10.1002/art.39311. [DOI] [PubMed] [Google Scholar]
- 31.Chen JS, Ford JB, Roberts CL, Simpson JM, March LM. Pregnancy outcomes in women with juvenile idiopathic arthritis: a population-based study. Rheumatology. 2013;52(6):1119–1125. doi: 10.1093/rheumatology/kes428. [DOI] [PubMed] [Google Scholar]
- 32.Clapp MA, James KE, Kaimal AJ. The effect of hospital acuity on severe maternal morbidity in high-risk patients. Am J Obstet Gynecol. 2018;219(1):111.e1–111.e7. doi: 10.1016/j.ajog.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Creanga AA, Bateman BT, Kuklina EV, Callaghan WM. Racial and ethnic disparities in severe maternal morbidity: a multistate analysis, 2008–2010. Am J Obstet Gynecol. 2014;210(5):435.e1–435.e8. doi: 10.1016/j.ajog.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 34.Dayan N, Joseph KS, Fell DB, Laskin CA, Basso O, Park AL, et al. Infertility treatment and risk of severe maternal morbidity: a propensity score-matched cohort study. CMAJ. 2019;191(5):e118–e127. doi: 10.1503/cmaj.181124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dayan N, Fell DB, Guo Y, Wang H, Velez MP, Spitzer K, et al. Severe maternal morbidity in women with high BMI in IVF and unassisted singleton pregnancies. Hum Reprod. 2018;33(8):1548–1556. doi: 10.1093/humrep/dey224. [DOI] [PubMed] [Google Scholar]
- 36.Feldman DS, Bollman DL, Fridman M, Korst LM, El Haj IS, Fink A, et al. Do laborists improve delivery outcomes for laboring women in California community hospitals? Am J Obstet Gynecol. 2015;213(4):587.e581–587.e587. doi: 10.1016/j.ajog.2015.05.051. [DOI] [PubMed] [Google Scholar]
- 37.Ford JB, Patterson JA, Seeho SK, Roberts CL. Trends and outcomes of postpartum haemorrhage, 2003-2011. BMC Pregnancy Childbirth. 2015;15:334. doi: 10.1186/s12884-015-0788-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frederiksen BN, Lillehoj CJ, Kane DJ, Goodman D, Rankin K. Evaluating Iowa severe maternal morbidity trends and maternal risk factors: 2009-2014. Matern Child Health J. 2017;21(9):1834–1844. doi: 10.1007/s10995-017-2301-4. [DOI] [PubMed] [Google Scholar]
- 39.Friedman AM, Ananth CV, Huang Y, D'Alton ME, Wright JD. Hospital delivery volume, severe obstetrical morbidity, and failure to rescue. Am J Obstet Gynecol. 2016;215(6):795.e1–795.e714. doi: 10.1016/j.ajog.2016.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibbs Pickens CM, Kramer MR, Howards PP, Badell ML, Caughey AB, Hogue CJ. Term elective induction of labor and pregnancy outcomes among obese women and their offspring. Obstet Gynecol. 2018;131(1):12–22. doi: 10.1097/AOG.0000000000002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grasch JL, Thompson JL, Newton JM, Zhai AW, Osmundson SS. Trial of labor compared with cesarean delivery in superobese women. Obstet Gynecol. 2017;130(5):994–1000. doi: 10.1097/AOG.0000000000002257. [DOI] [PubMed] [Google Scholar]
- 42.Gray KE, Wallace ER, Nelson KR, Reed SD, Schiff MA. Population-based study of risk factors for severe maternal morbidity. Paediatr Perinat Epidemiol. 2012;26(6):506–514. doi: 10.1111/ppe.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grobman WA, Bailit JL, Rice MM, Wapner RJ, Reddy UM, Varner MW, et al. Frequency of and factors associated with severe maternal morbidity. Obstet Gynecol. 2014;123(4):804–810. doi: 10.1097/AOG.0000000000000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guglielminotti J, Landau R, Wong CA, Li G. Patient-, hospital-, and neighborhood-level factors associated with severe maternal morbidity during childbirth: a cross-sectional study in New York state 2013-2014. Matern Child Health J. 2019;23(1):82–91. doi: 10.1007/s10995-018-2596-9. [DOI] [PubMed] [Google Scholar]
- 45.Hehir MP, Ananth CV, Wright JD, Siddiq Z, D'Alton ME, Friedman AM. Severe maternal morbidity and comorbid risk in hospitals performing <1000 deliveries per year. Am J Obstet Gynecol. 2017;216(2):179.e1–179.e12. doi: 10.1016/j.ajog.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 46.Howell EA, Egorova NN, Janevic T, Balbierz A, Zeitlin J, Hebert PL. Severe maternal morbidity among Hispanic women in New York City: investigation of health disparities. Obstet Gynecol. 2017;129(2):285–294. doi: 10.1097/AOG.0000000000001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Howell EA, Egorova NN, Balbierz A, Zeitlin J, Hebert PL. Site of delivery contribution to black-white severe maternal morbidity disparity. Am J Obstet Gynecol. 2016;215(2):143–152. doi: 10.1016/j.ajog.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howell EA, Egorova N, Balbierz A, Zeitlin J, Hebert PL. Black-white differences in severe maternal morbidity and site of care. Am J Obstet Gynecol. 2016;214(1):122.e1–122.e7. doi: 10.1016/j.ajog.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Howell EA, Zeitlin J, Hebert PL, Balbierz A, Egorova N. Association between hospital-level obstetric quality indicators and maternal and neonatal morbidity. JAMA. 2014;312(15):1531–1541. doi: 10.1001/jama.2014.13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howland RE, Angley M, Won SH, Wilcox W, Searing H, Liu SY, et al. Determinants of severe maternal morbidity and its racial/ethnic disparities in New York City, 2008-2012. Matern Child Health J. 2019;23(3):346–355. doi: 10.1007/s10995-018-2682-z. [DOI] [PubMed] [Google Scholar]
- 51.Kilpatrick SJ, Abreo A, Gould J, Greene N, Main EK. Confirmed severe maternal morbidity is associated with high rate of preterm delivery. Am J Obstet Gynecol. 2016;215(2):233.e1–233.e7. doi: 10.1016/j.ajog.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 52.Korst LM, Fridman M, Lu MC, Mitchell C, Lawton E, Griffin F, et al. Monitoring childbirth morbidity using hospital discharge data: further development and application of a composite measure. Am J Obstet Gynecol. 2014;211(3):268.e1–268.e16. doi: 10.1016/j.ajog.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 53.Lazariu V, Nguyen T, McNutt LA, Jeffrey J, Kacica M. Severe maternal morbidity: a population-based study of an expanded measure and associated factors. PLoS One. 2017;12(8):e0182343. doi: 10.1371/journal.pone.0182343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Ray C, Pelage L, Seco A, Bouvier-Colle MH, Chantry AA, Deneux-Tharaux C, et al. Risk of severe maternal morbidity associated with in vitro fertilisation: a population-based study. BJOG. 2019;126(8):1033–1041. doi: 10.1111/1471-0528.15668. [DOI] [PubMed] [Google Scholar]
- 55.Leonard SA, Carmichael SL, Main EK, Lyell DJ, Abrams B. Risk of severe maternal morbidity in relation to prepregnancy body mass index: roles of maternal co-morbidities and caesarean birth. Paediatr Perinat Epidemiol. 2020;34(4):460–468. doi: 10.1111/ppe.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leonard SA, Main EK, Carmichael SL. The contribution of maternal characteristics and cesarean delivery to an increasing trend of severe maternal morbidity. BMC Pregnancy Childbirth. 2019;19(1):16. doi: 10.1186/s12884-018-2169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leonard SA, Main EK, Scott KA, Profit J, Carmichael SL. Racial and ethnic disparities in severe maternal morbidity prevalence and trends. Ann Epidemiol. 2019;33:30–36. doi: 10.1016/j.annepidem.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liese KL, Mogos M, Abboud S, Decocker K, Koch AR, Geller SE. Racial and ethnic disparities in severe maternal morbidity in the United States. J Racial Ethn Health Disparities. 2019;6(4):790–798. doi: 10.1007/s40615-019-00577-w. [DOI] [PubMed] [Google Scholar]
- 59.Lindquist AC, Kurinczuk JJ, Wallace EM, Oats J, Knight M. Risk factors for maternal morbidity in Victoria, Australia: a population-based study. BMJ Open. 2015;5(8):e007903. doi: 10.1136/bmjopen-2015-007903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lipkind HS, Zuckerwise LC, Bragan Turner E, Collins JJ, Campbell KH, Reddy UM, et al. Severe maternal morbidity during delivery hospitalization in a large international administrative database, 2008-2013: a retrospective cohort. BJOG. 2019;126(10):1223–1230. doi: 10.1111/1471-0528.15818. [DOI] [PubMed] [Google Scholar]
- 61.Lisonkova S, Haslam MD, Dahlgren L, Chen I, Synnes AR, Lim KI. Maternal morbidity and perinatal outcomes among women in rural versus urban areas. CMAJ. 2016;188(17–18):e456–e465. doi: 10.1503/cmaj.151382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lisonkova S, Muraca GM, Potts J, Liauw J, Chan WS, Skoll A, et al. Association between prepregnancy body mass index and severe maternal morbidity. JAMA. 2017;318(18):1777–1786. doi: 10.1001/jama.2017.16191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lisonkova S, Potts J, Muraca GM, Razaz N, Sabr Y, Chan WS, et al. Maternal age and severe maternal morbidity: a population-based retrospective cohort study. PLoS Med. 2017;14(5):e1002307. doi: 10.1371/journal.pmed.1002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lisonkova S, Sabr Y, Mayer C, Young C, Skoll A, Joseph KS. Maternal morbidity associated with early-onset and late-onset preeclampsia. Obstet Gynecol. 2014;124(4):771–781. doi: 10.1097/AOG.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 65.Liu S, Liston RM, Joseph KS, Heaman M, Sauve R, Kramer MS. Gestational age-specific severe maternal morbidity associated with labor induction. Am J Obstet Gynecol. 2013;209(3):209.e1–209.e8. doi: 10.1016/j.ajog.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 66.Liu S, Liston RM, Joseph KS, Heaman M, Sauve R, Kramer MS. Maternal mortality and severe morbidity associated with low-risk planned cesarean delivery versus planned vaginal delivery at term. CMAJ. 2007;176(4):455–460. doi: 10.1503/cmaj.060870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luke B, Baker VL, Doody KJ. Risk of severe maternal morbidity by maternal fertility status: a US study in 8 states. Am J Obstet Gynecol. 2019;220(2):195.e1–195.e12. doi: 10.1016/j.ajog.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lyndon A, Baer RJ, Gay CL, El Ayadi AM, Lee HC, Jelliffe-Pawlowski L. A population-based study to identify the prevalence and correlates of the dual burden of severe maternal morbidity and preterm birth in California. J Matern Fetal Neonatal Med. 2019. 10.1080/14767058.2019.1628941. [DOI] [PubMed]
- 69.Lyndon A, Lee HC, Gay C, Gilbert WM, Gould JB, Lee KA. Effect of time of birth on maternal morbidity during childbirth hospitalization in California. Am J Obstet Gynecol. 2015;213(5):705.e1–705.11. doi: 10.1016/j.ajog.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin AS, Monsour M, Kissin DM, Jamieson DJ, Callaghan WM, Boulet SL. Trends in severe maternal morbidity after assisted reproductive technology in the United States, 2008-2012. Obstet Gynecol. 2016;127(1):59–66. doi: 10.1097/AOG.0000000000001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Masters HR, Housley E, van Hook JW, DeFranco E. Maternal obesity is an independent risk factor for intensive care unit admission during delivery hospitalization. Am J Perinatol. 2018;35(14):1423–1428. doi: 10.1055/s-0038-1660460. [DOI] [PubMed] [Google Scholar]
- 72.Metcalfe A, Wick J, Ronksley P. Racial disparities in comorbidity and severe maternal morbidity/mortality in the United States: an analysis of temporal trends. Acta Obstet Gynecol Scand. 2018;97(1):89–96. doi: 10.1111/aogs.13245. [DOI] [PubMed] [Google Scholar]
- 73.Mourad M, Silverstein M, Bender S, Melka S, Klauser CK, Gupta S, et al. The effect of maternal obesity on outcomes in patients undergoing tertiary or higher cesarean delivery. J Matern Fetal Neonatal Med. 2015;28(9):989–993. doi: 10.3109/14767058.2014.941284. [DOI] [PubMed] [Google Scholar]
- 74.Muraca GM, Sabr Y, Lisonkova S, Skoll A, Brant R, Cundiff GW, et al. Morbidity and mortality associated with forceps and vacuum delivery at outlet, low, and midpelvic station. J Obstet Gynaecol Can. 2019;41(3):327–337. doi: 10.1016/j.jogc.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 75.Nam JY, Lee SG, Nam CM, Park S, Jang SI, Park EC. The effect of off-hour delivery on severe maternal morbidity: a population-based cohort study. Eur J Pub Health. 2019;29(6):1031–1036. doi: 10.1093/eurpub/ckz013. [DOI] [PubMed] [Google Scholar]
- 76.Osmundson SS, Gould JB, Butwick AJ, Yeaton-Massey A, El-Sayed YY. Labor outcome at extremely advanced maternal age. Am J Obstet Gynecol. 2016;214(3):362.e1–362.e7. doi: 10.1016/j.ajog.2015.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pallasmaa N, Ekblad U, Gissler M, Alanen A. The impact of maternal obesity, age, pre-eclampsia and insulin dependent diabetes on severe maternal morbidity by mode of delivery-a register-based cohort study. Arch Gynecol Obstet. 2015;291(2):311–318. doi: 10.1007/s00404-014-3352-z. [DOI] [PubMed] [Google Scholar]
- 78.Platner MH, Ackerman C, Howland RE, Xu X, Pettker CM, Illuzzi JL, et al. Gestational weight gain and severe maternal morbidity at delivery hospitalization. Obstet Gynecol. 2019;133(3):515–524. doi: 10.1097/AOG.0000000000003114. [DOI] [PubMed] [Google Scholar]
- 79.Ramage K, Grabowska K, Silversides C, Quan H, Metcalfe A. Association of adult congenital heart disease with pregnancy, maternal, and neonatal outcomes. JAMA Netw Open. 2019;2(5):e193667. doi: 10.1001/jamanetworkopen.2019.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ray JG, Park AL, Dzakpasu S, Dayan N, Deb-Rinker P, Luo W, et al. Prevalence of severe maternal morbidity and factors associated with maternal mortality in Ontario, Canada. JAMA Netw Open. 2018;1(7):e184571. doi: 10.1001/jamanetworkopen.2018.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reid LD, Creanga AA. Severe maternal morbidity and related hospital quality measures in Maryland. J Perinatol. 2018;38(8):997–1008. doi: 10.1038/s41372-018-0096-9. [DOI] [PubMed] [Google Scholar]
- 82.Roberts CL, Ford JB, Algert CS, Bell JC, Simpson JM, Morris JM. Trends in adverse maternal outcomes during childbirth: a population-based study of severe maternal morbidity. BMC Pregnancy Childbirth. 2009;9:7. doi: 10.1186/1471-2393-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosenbloom JI, Tuuli MG, Stout MJ, Young OM. Woolfolk, Macones GA, et al. a prediction model for severe maternal morbidity in laboring patients at term. Am J Perinatol. 2019;36(1):8–14. doi: 10.1055/s-0038-1626716. [DOI] [PubMed] [Google Scholar]
- 84.Roy A, Peaceman A, Son M, Feinglass J. Maternal obstetric complication rates remain high in Illinois: a retrospective study, 2010–2015. Jt Comm J Qual Patient Saf. 2019;45(1):24–30. doi: 10.1016/j.jcjq.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 85.Schummers L, Hutcheon JA, Hacker MR, VanderWeele TJ, Williams PL, McElrath TF, et al. Absolute risks of obstetric outcomes risks by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29(3):379–387. doi: 10.1097/EDE.0000000000000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schummers L, Hutcheon JA, Bodnar LM, Lieberman E, Himes KP. Risk of adverse pregnancy outcomes by prepregnancy body mass index: a population-based study to inform prepregnancy weight loss counseling. Obstet Gynecol. 2015;125(1):133–143. doi: 10.1097/AOG.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shand AW, Chen JS, Selby W, Solomon M, Roberts CL. Inflammatory bowel disease in pregnancy: a population-based study of prevalence and pregnancy outcomes. BJOG. 2016;123(11):1862–1870. doi: 10.1111/1471-0528.13946. [DOI] [PubMed] [Google Scholar]
- 88.Siddiqui A, Azria E, Howell EA, Deneux-Tharaux C, Langer B, Dupont C, et al. Associations between maternal obesity and severe maternal morbidity: findings from the French EPIMOMS population-based study. Paediatr Perinat Epidemiol. 2019;33(1):7–16. doi: 10.1111/ppe.12522. [DOI] [PubMed] [Google Scholar]
- 89.Urquia ML, Wanigaratne S, Ray JG, Joseph KS. Severe maternal morbidity associated with maternal birthplace: a population-based register study. J Obstet Gynaecol Can. 2017;39(11):978–987. doi: 10.1016/j.jogc.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 90.Urquia ML, Glazier RH, Mortensen L, Nybo-Andersen AM, Small R, Davey MA, et al. Severe maternal morbidity associated with maternal birthplace in three high-immigration settings. Eur J Pub Health. 2015;25(4):620–625. doi: 10.1093/eurpub/cku230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vanderlaan J, Rochat R, Williams B, Dunlop A, Shapiro SE. Associations between hospital maternal service level and delivery outcomes. Womens Health Issues. 2019;29(3):252–258. doi: 10.1016/j.whi.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 92.Wahlberg A, Roost M, Haglund B, Hogberg U, Essen B. Increased risk of severe maternal morbidity (near-miss) among immigrant women in Sweden: a population register-based study. BJOG. 2013;120(13):1605–1611. doi: 10.1111/1471-0528.12326. [DOI] [PubMed] [Google Scholar]
- 93.Wang ET, Ozimek JA, Greene N, Ramos L, Vyas N, Kilpatrick SJ, et al. Impact of fertility treatment on severe maternal morbidity. Fertil Steril. 2016;106(2):423–426. doi: 10.1016/j.fertnstert.2016.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Young CB, Liu S, Muraca GM, Sabr Y, Pressey T, Liston RM, et al. Mode of delivery after a previous cesarean birth, and associated maternal and neonatal morbidity. CMAJ. 2018;190(18):e556–e564. doi: 10.1503/cmaj.170371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Geller SE, Rosenberg D, Cox S, Brown M, Simonson L, Kilpatrick S. A scoring system identified near-miss maternal morbidity during pregnancy. J Clin Epidemiol. 2004;57:716–720. doi: 10.1016/j.jclinepi.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 96.Lain SJ, Hadfield RM, Raynes-Greenow CH, Ford JB, Mealing NM, Algert CS, et al. Quality of data in perinatal population health databases: a systematic review. Med Care. 2012;50(4):e7–e20. doi: 10.1097/MLR.0b013e31821d2b1d. [DOI] [PubMed] [Google Scholar]
- 97.Yasmeen S, Romano PS, Schembri ME, Keyzer JM, Gilbert WM. Accuracy of obstetric diagnoses and procedures in hospital discharge data. Am J Obstet Gynecol. 2006;194:992–1001. doi: 10.1016/j.ajog.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 98.American College of Obstetricians and Gynecologists (ACOG) Levels of maternal care. Obstetric Care Consensus No. 2. Obstet Gynecol. 2015;125(2):502–515. doi: 10.1097/01.AOG.0000460770.99574.9f. [DOI] [PubMed] [Google Scholar]
- 99.Agency for Healthcare Research and Quality. National Inpatient Sample Overview. Available from: https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed 1 Sept 2020.
- 100.Cauldwell M, Ghonim S, Uebing A, Swan L, Steer PJ, Gatzoulis M, et al. Preconception counseling, predicting risk and outcomes in women with mWHO 3 and 4 heart disease. Int J Cardiol. 2017;234:76–80. doi: 10.1016/j.ijcard.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 101.California Maternal Quality Care Collaborative. OB Hemorrhage Toolkit v2.0. Available from: https://www.cmqcc.org/resources-tool-kits/toolkits/ob-hemorrhage-toolkit. Accessed 1 Sept 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Newcastle-Ottawa Quality Assessment Scale (adapted for cross sectional studies with healthcare data for research question: What are the risk factors for severe maternal morbidity associated with the delivery admission?
Additional file 2. Preferred Reporting Items for Systematic reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) Checklist.
Data Availability Statement
No data or materials were used for the publication of this article beyond the review of those articles referenced.