Abstract
Background
Dysregulated lncRNA PCAT6 was discovered in many cancers excluding pituitary adenomas (PA). Therefore, we explored the role of PCAT6 in PA in this research.
Methods
Abnormally expressed miRNAs were analyzed by bioinformatics and RT-qPCR. The target and regulator of miR-139-3p were determined by bioinformatics, dual-luciferase reporter assay, or RIP. The correlation among PCAT6, miR-139-3p, and BRD4 was further analyzed. The viability, apoptosis, cell cycle distribution of PA cells, as well as their ability to invade, migrate, and proliferate, were tested after transfection through CCK-8, flow cytometry, transwell, wound healing, and colony formation assays. After construction of transplanted-tumor model in nude mice, cell apoptosis in the tumor was detected by TUNEL. The expressions of PCAT6, BRD4, miR-139-3p, and apoptosis-related factors in PA tissues, cells, or tumor tissues were detected by RT-qPCR, Western blot, or IHC.
Results
PCAT6 and BRD4 were high-expressed but miR-139-3p was low-expressed in PA. Both the 3′-untranslated regions of PCAT6 and BRD4 mRNAs were demonstrated to contain a potential binding site for miR-139-3p. PCAT6 was positively correlated to BRD4, and miR-139-3p was negatively correlated to PCAT6 and BRD4. MiR-139-3p mimic, shPCAT6 and siBRD4 inhibited the viability, migration, invasion, and proliferation of PA cells while inducing apoptosis. MiR-139-3p mimic and shPCAT6 inhibited the cell cycle progression of PA cells, decreased the weight and volume of the xenotransplanted tumor, and reduced the levels of Bcl-2 and BRD4 while enhancing the levels of Bax, miR-139-3p, and Cleaved caspase-3. MiR-139-3p inhibitor caused the opposite effect of miR-139-3p mimic and further reversed the effect of shPCAT6 on on PA cells.
Conclusion
PCAT6 regulated the progression of PA via modulating the miR-139-3p/BRD4 axis, which might provide a novel biomarker for the prevention, diagnosis, and treatment of PA.
Keywords: Pituitary adenomas, lncRNA PCAT6, miR-139-3p, BRD4, Xenotransplanted-tumor
Background
Pituitary adenoma (PA), a common tumor in neurosurgery, originates from the parenchymal cells of adenohypophysis and accounts for about 10–15% of intracranial tumors [1]. Approximately 1/3 of PA patients show malignant biological behavior including aggressive growth, wrapping, and invasion of the surrounding tissue structure, which can cause neurological and endocrine symptoms; this kind of PA is clinically called invasive PA (IPA) [1–3]. The pituitary gland is located in the sellar region that is surrounded by important nerves and blood vessels, which explains the high incidence of skull base bone erosion, tumor necrosis, and cystic stroke in IPA patients who are seriously affected by the disease in terms of quality of life [4, 5]. At present, surgical resection is still the main clinical treatment of PA, but due to the incomplete removal of tumor cells, numerous patients are faced with the risk of neoplasm recurrence and distant metastasis; what is worse, a large proportion of patients with PA were in an advanced stage at diagnosis [1, 3, 6]. Therefore, finding a novel approach for the early prevention and treatment of PA is necessary.
Nowadays, lncRNAs have been recognized as the emerging role in the development of most cancers and in modulating the proliferation, metastasis, and apoptosis of cancer cells including PA cells [3, 7, 8]. For instance, lncRNAs MEG3 and HOTAIR were found associated with the development of non-invasion PA (NIPA) into IPA, as evidence by the decrease in MEG3 level and increase in HOTAIR level during the development transformation of normal pituitary into NIPA and eventually into IPA tissues [9]; lncRNA H19 was up-regulated in IPA, and thereby could be used as a biomarker for PA diagnosis [10]; the knockdown of lncRNA AFAP1-AS1 could suppress cell growth and induce apoptosis in PA [11]; moreover, lncRNA SNHG1 had a high level in IPA and could activate the Wnt/beta-catenin pathway in IPA [12]. In addition, previous research has also proved that lncRNA PCAT6 was abnormally expressed in different types of cancers such as cervical cancer, liver cancer, and ovarian cancer, and had the ability to modulate the processes of these cancers [13–15]. To our knowledge, however, the level and role of PCAT6 in the development of PA have not been reported. Besides, although evidence has exhibited that lncRNAs could target some miRNAs to adjust miRNA-mediated targets and further play a role in physiological and pathological processes [3, 16], there is still a lack of research on the target miRNA of PCAT6.
Therefore, in the current study, we searched for the target miRNA of PCAT6 in PA and further explored its functions in the progression of PA.
Methods
Ethics statement
The use of clinical samples and the animal experiments in this study were approved by the Ethics Committee of Beijing Tiantan Hospital (BT20190530S). The patients who donated the samples signed informed consent.
Clinical tissues
Cancer tissues and adjacent normal tissues were obtained from 20 patients with IPA; cancer tissues were also obtained from 20 patients with NIPA who had undergone an operation at Beijing Tiantan Hospital between March 2018 and March 2019.
Cell culture
Rat PA cell lines RC-4B/C (CRL-1903) and GH3 (CCL-82.1) were bought from ATCC (MD, USA). RC-4B/C cells were grown in DMEM complete medium which consisted of 500 ml of DMEM basic medium (11995065, Gibco, MA, USA) with 10% (v/v) FBS (16140071, Gibco). GH3 cells were grown in F-12K complete medium which consisted of 500 ml of F-12K basic medium (30-2004, ATCC) with 10% (v/v) FBS. All cells were cultured in a humid atmosphere at 37 °C with 5% CO2.
RNA immunoprecipitation (RIP) assay
After RC-4B/C and GH3 cells were collected and washed with cold PBS, the cells were lysed with RIP lysis buffer (RG129S) which was purchased from Beyotime (Shanghai, China). Then the cell lysates were mixed with anti-Argonaute2 magnetic beads (anti-AGO2; MA514861, Thermo, MA, USA) or mouse IgG (31203, Thermo) which was used as the negative control. After the beads were collected, total RNAs were extracted and the expressions of PCAT6 and miR-139-3p were detected by RT-qPCR. The cell lysate without any treatment acted as the input.
Lentiviral infection and lipofectamine transfection
For lentiviral infection, shPCAT6 lentiviral and shRNA negative control (shNC) lentiviral vectors were packaged by GenePharma (Shanghai, China), and the sequence of shPCAT6 was 5′-GGTGTCTCCATCCTCATTC-3′. Before infection, 1.0 × 106 RC-4B/C and GH3 cells were separately poured into a 6-well plate (2 ml of complete medium in each well). After the cell confluence attained 80%, the previous medium was replaced by 2 ml of basic medium. Then 2 μl of shPCAT6 or shNC lentiviral vector was added into the medium and mixed with the cells thoroughly. After culture for 48 h, the transfected cells were collected for later use.
For lipofectamine transfection, small interfering RNA for BRD4 (siBRD4; 5′-GACTAGAAACTTCCCAAATGTCT-3′) and siNC (5′-GCGACCAA CGCCTTGATTG-3′) were also synthesized by GenePharma. MiR-139-3p mimic (miR10004735-1-5; 5′-TGAGGTTGTCCCGGCGCAGAGGT-3′), inhibitor (miR20004735-1-5; 5′-ACTCCAACAGGGCCGCGTCTCCA-3′), mimic control (MC; miR01102-1-1), and inhibitor control (IC; miR2N0000002-1-5) were purchased from RIBOBIO (Guangzhou, China). Before infection, 1.0 × 106 RC-4B/C and GH3 cells were separately poured into 6-well plates (2 ml of complete medium in each well). After the cell confluence attained 80%, the previous medium was replaced by 1.8 ml of basic medium. Then 2 μg of siBRD4, siNC, mimic, and inhibitor were separately added into 100 μl of Opti-MEM basic medium (31985070, Gbico). Meantime, 2 μl of lipofectamine 3000 (BMB1385, BOMEI BIOTECHNOLOGY, Hefei, China) was mixed with 100 μl of Opti-MEM basic medium. After a thorough mixing, the two media were stood for 10 min, and then separately added into each well of the 6-well plate and evenly mixed with the cells. After 48 h of culture, the transfected cells were collected for later use.
Luciferase reporter assay
The PCAT6 wide-type (PCAT6-WT) sequence that contained miR-139-3p binding site (5′-CCAGCCCGCTCCGTGAGTGCCCACGTCTCCA-3′), the PCAT6 mutant sequence (PCAT6-MUT; 5′-GAGAGACTCGATACCTCATAGCA-3′), the SDCBP-WT sequence that contained miR-139-3p binding site (5′-GGAAATGTAGCTGAACGTCTCCA-3′), the SDCBP-MUT sequence (5′-GGAAATGTAGCTGAAGAAGGATC-3′), the BRD4-WT sequence that contained miR-139-3p binding site (5′-CCGAAATGGTGTGATCGTCTCCT-3′), and the BRD4-MUT sequence (5′-CCGAAATGGTGTGATAAGGCAGT-3′) were first separately cloned into the pGL3-basic vector (60908-3961y, TIANDZ, Beijing, China). Then, 3.0 × 104 cells were placed into a 48-well plate (300 μl of complete medium in each well). After the cell confluence attained 80%, the cells were co-transfected with one of the above vectors and miR-139-3p mimic. After transfection, the cells were treated with a Luciferase Reporter Assays Substrate Kit (ab228530, Abcam, CA, USA) for the detection of luciferase activity under a SpectraMax reader (Molecular Devices, Shanghai, China).
CCK-8 assay
CCK-8 reagent (K1018) was bought from APExBIO (Houston, USA) and diluted with PBS (SH30256.01, HyClone, MA, USA) to a concentration of 0.5 mg/ml. RC-4B/C and GH3 cells were separately suspended in complete medium after transfection to adjust the cell concentration to 1.0 × 104/ml. Then 100 μl of cell suspension was added into a 96-well plate and cultured for 48 h. Afterwards, the previous medium in the 96-well plate was replaced by 100 μl of CCK-8 for another 4 h incubation. Lastly, the absorbance of each well of the 96-well plate was read using a microplate reader (Multiskan FC, Thermo) at 450 nm.
Wound healing assay
After transfection, cells were suspended in complete medium to adjust the cell concentration to 4.0 × 105/ml. Then 2 ml of cell suspension was added into a 6-well plate. After the wells were filled with cells, wounds of the same width were created in each well. Then the previous medium was replaced by 2 ml of basic medium. The images of the wounds were documented at 0 and 48 h under a BX53 optical microscope (Olympus, Japan). The data were analyzed using Image J 1.8.0 software.
Transwell assay
After transfection, cells were suspended in basic medium to adjust the cell concentration to 2.0 × 105/ml. Then 200 μl of cell suspension was added into transwell chambers (354234) which were bought from Corning (NY, USA). The cell-containing chambers were then embedded into a 24-well plate, and subsequently added with 700 μl of complete medium. After 48 h of culture, the cells inside the chambers were wiped off, while the cells outside the chambers were stained with crystal violet (R019731, Rhawn, Shangai, China) for 20 min. Finally, the stained cells were observed and the images were documented under a BX53 optical microscope (Olympus, Japan). The data were analyzed using Image J 1.8.0 software.
Colony formation assay
After transfection, cells were suspended in basic medium to adjust the cell concentration to 500/ml. Then 2 ml of cell suspension was added into a 6-well plate. The cells were then cultured for 14 days and the basic medium was renewed every three days. Afterwards, the cell colonies were fixed with methanol (R007536, Rhawn) for 15 min, stained with crystal violet for 15 min, and washed with PBS. Finally, the cell colonies were observed and the images were documented with a camera (EOS M50, Canon, Japan).
Flow cytometry
For cell apoptosis analysis, cells were suspended in complete medium after transfection to adjust the cell concentration to 4.0 × 105/ml. Then 2 ml of cell suspension was added into a 6-well plate and cultured for 48 h. After being stained with Annexin V-FITC (BCT-XAP2102-TT01, Adipogen, San Diego, USA) for 15 min, the cells were further stained with Propidium Iodide (PI; P8080, Solarbio, Beijing, China) for 20 min. Lastly, the apoptosis of the cells was analyzed under a FACSCaliburTM flow cytometer (BD Biosciences, San Jose, CA, USA).
For cell cycle distribution analysis, cells were suspended in complete medium after transfection to adjust the cell to 4.0 × 105/ml. Then 2 ml of cell suspension was added into a 6-well plate and cultured for 48 h. After being stained with 75% ethanol (E99941, Rhawn) overnight, the cells were further stained with PI for 20 min. Lastly, the cell cycle distribution of the cells was analyzed by a FACSCaliburTM flow cytometer (BD Biosciences, San Jose, CA, USA).
Western blot assay
In short, 2 × 106 transfected cells were incubated with NP-40 (N8031, Solarbio) for 15 min at 4 ℃, followed by centrifugation at 4 ℃ (14,000 × g, 20 min). After the supernatant (total protein of cells) was collected, a BCA kit (P0009) bought from Beyotime was used to measure the protein concentration. Then, the protein (25 µg) was mixed with 5 × loading buffer (C05-03001, Bioss, Wuhan, China), and the mixture was subsequently added to the 10 wells containing 12% gels which were synthesized using an SDS-PAGE Kit (C-0047, Bioss). Also, 4 µl of marker (PR1910, Solarbio) was specially added to the first lane of each gel. The protein was then separated inside the gels at 100 V for 150 min and subsequently transferred to the surface of 0.22 μm NC membranes (W015-2, Jiancheng, Nanjing, China). After being blocked with 5% defatted milk, the membranes were incubated with the following relative primary antibodies at 4 ℃ for 16 h: BRD4 (1:1500, 152 kDa, ab128874, Abcam), Bcl-2 (1:3000, 26 kDa, ab59348, Abcam), Bax (1:3000, 21 kDa, ab32503, Abcam), Cleaved caspase-3 (1:3000, 17 kDa, ab2302, Abcam), and GAPDH (1:3000, 36 kDa, ab8245, Abcam). The next day, the membranes were further incubated with rabbit secondary antibody (1:10000, ab205718, Abcam) or mouse secondary antibody (1:10000, ab205719, Abcam) for 2 h at room temperature. Lastly, 200 μl of detection solution (P0019, Beyotime) was added to the surface of all membranes, and then the image signal was detected using the Image Lab 3.0 Software (Bio-Ras, CA, USA).
Establishment of subcutaneously xenografted tumor mice model
Twelve nude mice (species: BALB/c; sex: male; age: 6 weeks; weight: 20 ± 2 g) were bought from SLAC (Shanghai, China). All the mice were fed in SPF grade feeding rooms under a 12 h light/dark cycle with free access to food and water. After 4 days of feeding, the mice were randomly divided into four groups: the shNC group (n = 3), the shPCAT6 group (n = 3), the MC group (n = 3), and the mimic group (n = 3).
In short, 2 × 106 GH3 cells which were stably transfected with shNC, shPCAT6, MC, or miR-139-3p mimic were resuspended in 100 μl of PBS. Then the cell-containing PBS was mixed with 100 μl of Matrigel (354248, Corning). Subsequently, the cells in the mixed solution were subcutaneously injected into the right forelimb (above axillary fossa) of the nude mice in the four groups. Then the mice were normally fed and the tumor of the mice was observed every day. The tumor volume was measured and calculated by the formula: tumor volume (mm3) = major diameter (L) × (minor diameter)2 (W2)/2. Thirty-five days later, the mice were anesthetized with 2% sodium pentobarbital (B005, Jiancheng) at the dose of 50 mg/kg and then sacrificed by cervical dislocation. Finally, the tumor tissues were photographed, weighed, and stored for later use.
RNA extraction and RT-qPCR
Total RNAs were extracted from clinical PA tissues and PA cell lines using TRIzol (15596, Invitrogen) for analysis of the expressions of BRD4 mRNA and lncRNA PCAT6. Cytoplasmic and nuclear RNAs were extracted from PA cell lines using cytoplasmic and nuclear RNAs isolation regent (37400, NORGEN BIOTEK, Thorold, Canada) for analysis of the expression location of PCAT6. Besides, miRNAs were extracted from clinical PA tissues, PA cell lines, and xenografted tumors of the nude mice using miRNA isolation regent (DP501, TianGEN, Beijing, China) for the analysis of miRNA expressions. After extraction, a total of 500 ng of RNAs were collected and reverse-transcribed into cDNA using cDNA synthesis regent (AE301-02, TransGen, Shanghai, China). Then 1 μl of cDNA, 2 μl of primers of the targeted gene (Table 1), 5 μl of qPCR supermix (AQ601-01, TransGen), and 2 μl of RNase-free H2O (RF001, Real-Times, Beijing, China) were mixed. Finally, the amplification reaction of cDNA in the mixed solution was detected and documented using the Q6 system (Applied Biosystems, CA, USA), and the expression levels of the RNAs were also quantified using the Q6 system by the 2−△△CT method. The expressions of GAPDH and U6 were set as the internal controls for gene mRNA and miRNA levels, respectively.
Table 1.
RT-qPCR primers
| Target gene | Forward primers, 5′–3′ | Reverse primers, 5′–3′ |
|---|---|---|
|
Human miR-137 Human miR-139-3p Rat miR-139-3p |
ACGCGTAGTCGAGGAGAGTA GCCCTGTTGGAGTGTCGTAT GCCCTGTTGGAGGTCGTATC |
CGGCAATTGCACTGGATACG GTATCCAGTGCGTGTCGTGG GTATCCAGTGCGTGTCGTGG |
|
Human miR-31 Human miR-507 |
GACGATCTCCTCAGCACCTG GAAGTGCCATGCATGTGTCT |
CGGCAATTGCACTGGATACG CGGCAATTGCACTGGATACG |
|
Human miR-542-3p Human miR-571 |
AAGTCGTATCCAGTGCAATTGC GGCTCTTGAGTTGGCCATCT |
GTCGTATCCAGTGCGTGTCG CGGCAATTGCACTGGATACG |
|
Human miR-578 Human miR-580 Human miR-617 Human miR-658 Human PCAT6 Human BRD4 Rat BRD4 Human U6 Rat U6 Human GAPDH Rat GAPDH |
TCCCGGACAACAAGAAGCTC GGTTCCGGTCAGAAATTGTCG GTGGCCATTTCCTACCACCT GGAAGTAGGTCCGTTGGTCG CAGGAACCCCCTCCTTACTC ACCTCCAACCCTAACAAGCC CCTCCCAAATGTCTACAACGC CTCGCTTCGGCAGCACA GACCTCTATGCCAACACAGT GGAGCGAGATCCCTCCAAAAT AGGTCGGTGTG AACGGATTTG |
CGGCAATTGCACTGGATACG TGTCGTGGAGTCGGCAATTG CGGCAATTGCACTGGATACG CGGCAATTGCACTGGATACG CTAGGGATGTGTCCGAAGGA TTTCCATAGTGTCTTGAGCACC TGAGCAGATATTGCAGTTGGTT AACGCTTCACGAATTTGCGT AGTACTTGCGCTCAGGAGGA GGCTGTTGTCATACTTCTCATGG TGTAGACCATGTAGTTGAGGTCA |
TUNEL staining
Cell apoptosis in mice tumor tissues was examined by TUNEL staining using a reaction kit (c1091, Beyotime) which contained marker buffer, streptavidin-HRP solution, and DAB buffer. In brief, after being fixed on a glass slide, the mice tumor tissue slices were incubated with Proteinase K (ST532, Beyotime) for 20 min, followed by 20 min incubation with wash buffer (P0106, Beyotime). Then the tissue slices were blocked with block solution (P0100B, Beyotime) for 20 min. After 60 min of incubation with the marker buffer in the dark, the tissue slices were covered with stop buffer for 10 min. Then the streptavidin-HRP solution and the DAB buffer were separately used to incubate the tissue slices for 30 min. After washing with PBS, the image of the tissue slices was recorded with a BX53 optical microscope (Olympus, Japan).
Immunohistochemical
After being fixed on a glass slide, the mice tumor tissue slices were soaked in citrate antigen repair solution (AR0024, Boster, Wuhan, China) and heated in a microwave oven for 8 min. Then the tissue slides were cooled naturally at room temperature and incubated with BRD4 (1:400, ab128874, Abcam) at 4 °C overnight. The next day, the tissue slides were incubated with the secondary antibody (ab205718, Abcam) for 2 h and stained with the DAB buffer for 35 min. After 2 min of washing under tap water, hematoxylin (H810910, Macklin) was added to stain the nuclei of the cells for 4 min. Finally, after the tissue slides were sealed, the images of the tissue slices were recorded using a BX53 optical microscope (Olympus, Japan).
Statistical analysis
Data in this study were analyzed using student’s t-test and one-way ANOVA. LSD and Dunnet’s were applied as post-hoc tests. Pearson analysis was used to analyze the correlations between PCAT6 and miR-139-3p, PCAT6 and BRD4, and miR-139-3p and BRD4. The analysis software was SPSS 20.0. Statistical data were presented as mean ± SD. P < 0.05 represented that the data were statistically significant.
Results
Up-regulated PCAT6 in IPA tissues was negatively correlated to miR-139-3p
To further investigate the relation between the expressions of PCAT6 and miR-139-3p, we subsequently detected PCAT6 expression in IPA tissues (Fig. 1a). It was observed that the expression of PCAT6 was higher in IPA tissues than that in the Normal group (P < 0.05); meanwhile, as expected, miR-139-3p expression in IPA tissues was lower than that in normal pituitary tissues (P < 0.05, Fig. 1b). Then we analyzed the correlation between PCAT6 and miR-139-3p expressions in normal pituitary and IPA tissues (Fig. 1c, d), and found that the expression of PCAT6 in both normal and IPA tissues was negatively correlated with the expression of miR-139-3p.
Fig. 1.
Up-regulated PCAT6 in IPA tissues was negatively correlated to miR-139-3p. a The expressions of PCAT6 in normal pituitary tissues and IPA tissues were detected by RT-qPCR. GAPDH was used as an internal control. b The expressions of miR-139-3p in normal pituitary tissues and IPA tissues were detected by RT-qPCR. U6 was used as an internal control. c, d The correlation between the expressions of PCAT6 and miR-139-3p in normal pituitary tissues and IPA tissues was analyzed using Pearson correlation analysis. (***P < 0.001, vs. MC). IPA invasive pituitary adenomas
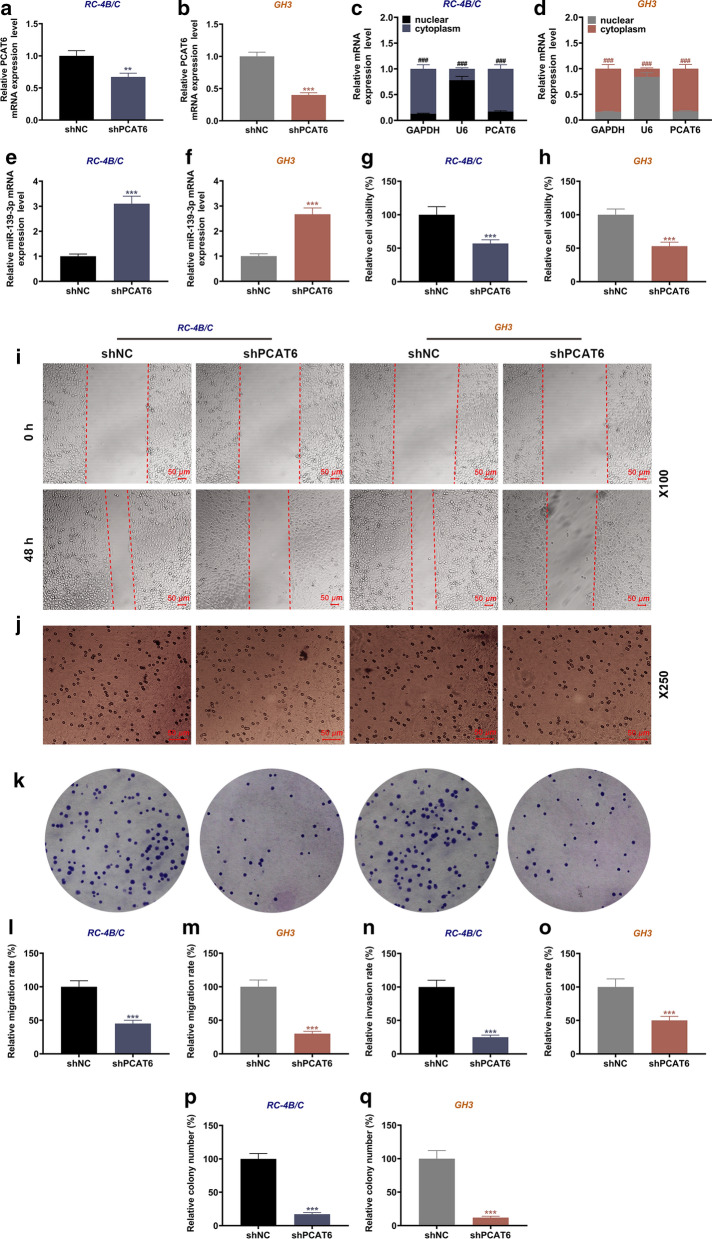
PCAT6 was mainly located in the cytoplasm, and it regulated cell viability, migration, invasion, proliferation, and miR-139-3p expression in PA cells
According to the previous results, PCAT6 was commonly high-expressed in tumor tissues of PA patients, but the specific functions and potential mechanism of PCAT6 in PA progression is still unclear. Therefore, we then investigated the main location of PCAT6 in PA cells, and found that PCAT6 was mainly expressed in the cytoplasm (Fig. 2c, d). Then we transfected shPCAT6 into the PA cells (Fig. 2a, b), and it turned out that the expression of PCAT6 was significantly decreased after transfection as compared with the shNC group (P < 0.01). We also discovered that shPCAT6 up-regulated the expression of miR-139-3p in both RC-4B/C and GH3 (Fig. 2e, f). Mechanically, we detected the effect of PCAT6 on the biological functions of PA cells. As exhibited in Fig. 3g, h, shPCAT6 remarkably inhibited the viability of RC-4B/C and GH3 cells when compared with the shNC group (P < 0.001). Meantime, the ability of the two cells to migrate, invade, and proliferate were also suppressed by shPCAT6 as compared to the shNC group (P < 0.001, Fig. 2i–q).
Fig. 2.
PCAT6 was mainly located in the cytoplasm, and it regulated cell viability, migration, invasion, proliferation, and miR-139-3p expression in PA cells. a, b The transfection efficiencies of shPCAT6 in RC-4B/C and GH3 cells were determined by RT-qPCR. GAPDH was used as an internal control. c, d The expression location of PCAT6 in RC-4B/C and GH3 cells were determined by RT-qPCR. GAPDH and U6 were used as internal controls. e, f The expressions of miR-139-3p in RC-4B/C and GH3 cells after transfection were detected by RT-qPCR. U6 was used as an internal control. g, h The viability of RC-4B/C and GH3 cells after transfection was detected by CCK-8 assay. i, l–m The migration of RC-4B/C and GH3 cells after transfection was detected by wound healing assay (Magnification × 100). j, n, o The invasion of RC-4B/C and GH3 cells after transfection was detected by transwell assay (Magnification × 250). k, p, q The proliferation of RC-4B/C and GH3 cells after transfection was detected by colony formation assay. (***P < 0.001, vs. shNC; ###P < 0.001, vs. nuclear). shNC negative control of shRNA
Fig. 3.
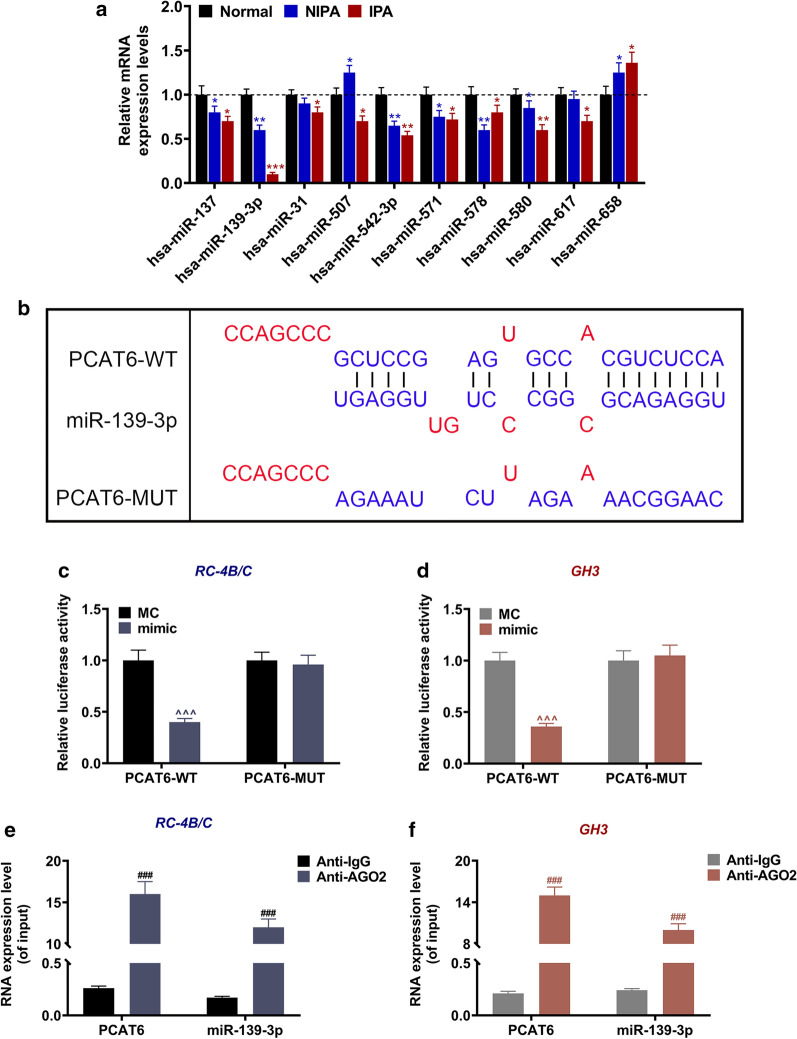
Down-regulated miR-139-3p was targeted by PCAT6 in PA. a The expression levels of miR-137, miR-139-3p, miR-542-3p, miR-571, miR-578, miR-580, miR-31, miR-617, miR-658, and miR-507 in normal pituitary, NIPA, and IPA tissues were analyzed by RT-qPCR. U6 was used as an internal control. b The putative binding site between miR-486-3p and circFLNA was predicted by LncBase Predicted v.2. c, d The results of luciferase assay validated that lncRNA PCAT6 targeted miR-139-3p in PA cells RC-4B/C and GH3. e, f The relationship between PCAT6 and miR-139-3p was further assessed by RIP assay. (*P < 0.05, **P < 0.01, vs. Normal; ^^^P < 0.001, vs. MC; ###P < 0.01, vs. Anti-IgG). PA pituitary adenomas, IPA invasive pituitary adenomas, NIPA non-invasive pituitary adenomas, RIP RNA immunoprecipitation
Down-regulated miR-139-3p was targeted by PCAT6 in PA
After identifying the dysregulated miRNAs in PA in the GEO2R database using the GSE46294 dataset, we further performed RT-qPCR to verify the expressions of the top ten miRNAs in normal, NIPA, and IPA tissues. The results (Fig. 3a) exhibited that when compared with the Normal group, six miRNAs (miR-137, miR-139-3p, miR-542-3p, miR-571, miR-578, and miR-580) were lowly expressed in both NIPA and IPA tissues (P < 0.05); two miRNAs (miR-31 and miR-617) had low expression in IPA tissues (P < 0.05); miR-658 had higher expression in both NIPA and IPA tissues (P < 0.05); and miR-507 expression was higher in NIPA tissues and lower in IPA tissues (P < 0.05). Furthermore, the bioinformatics analysis predicted that miR-139-3p was targeted by PCAT6, given that miR-139-3p had binding sites for PCAT6 (Fig. 3b). Therefore, we further conducted luciferase reporter assay with PA cells (RC-4B/C and GH3) to verify this prediction (Fig. 3c, d). The results exhibited that the luciferase activity in both cells transfected with PCAT6-WT and miR-139-3p mimic were reduced compared to the MC group (P < 0.001); however, the luciferase activity in both cells transfected with PCAT6-MUT and miR-139-3p mimic had no change as compared to the MC group. The RIP data (Fig. 3e, f) further proved this target relationship, as evidenced by the fact that the levels of PCAT6 and miR-139-3p in cells incubated with Anti-AGO2 were enhanced as compared to the Anti-IgG group (P < 0.001).
ShPCAT6 and miR-139-3p mimic inhibited tumor growth and BRD4 expression in tumor tissues, and promoted apoptosis and miR-139-3p expression in tumor tissues
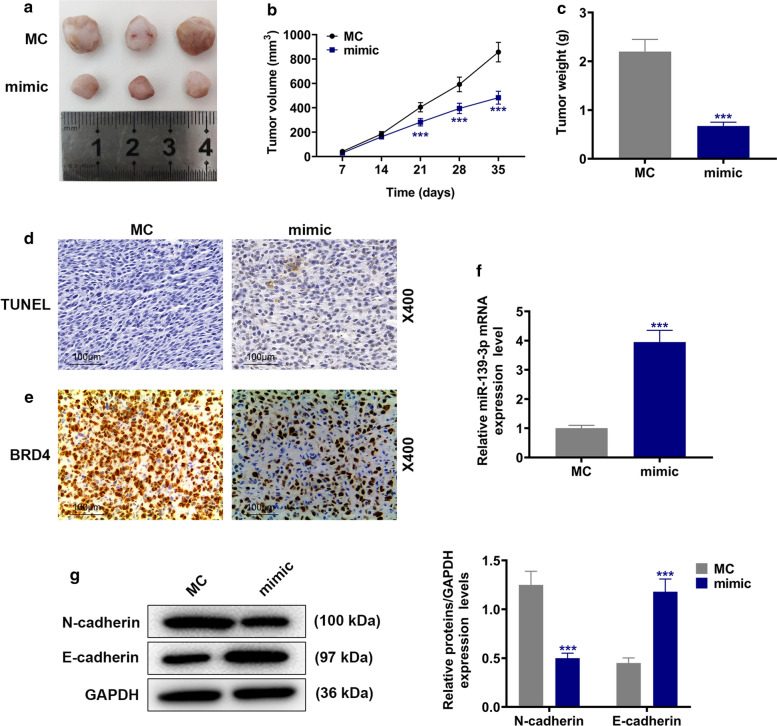
In order to verify the present findings, we further conducted animal experiments in mice subcutaneous-xenotransplanted tumor model (Figs. 4a and 5a). It was found that shPCAT6 inhibited the increase in tumor volume (Fig. 4b) and tumor weight (Fig. 4c) when compared with the shNC group (P < 0.001); consistently, miR-139-3p mimic also produced an inhibitory effect on the increase in tumor volume (Fig. 5b) and tumor weight (Fig. 5c) when compared with the MC group (P < 0.001). In addition, shPCAT6 and miR-139-3p mimic also induced cell apoptosis in tumor tissues (Figs. 4d and 5d). As exhibited in Figs. 4e and 5e, the expression of BRD4 in tumor tissues was down-regulated by both shPCAT6 and miR-139-3p mimic. Moreover, miR-139-3p expression in tumor tissues was increased by shPCAT6 and miR-139-3p mimic when compared with the shNC group (P < 0.001, Fig. 4f) and the MC group (P < 0.001, Fig. 5f) respectively. To investigate the changes of EMT-related expressions, we also detected the protein levels of E-cadherin and N-cadherin, and the results suggested that both the silence of PCAT6 and miR-139-3p overexpression could promote the progression of EMT (P < 0.001, Figs. 4g and 5g). All the phenomena confirmed the previous findings in vitro that PCAT6 had the capacity to modulate the progression of PA by targeting miR-139-3p/BRD4.
Fig. 4.
ShPCAT6 inhibited tumor growth and BRD4 expression, and promoted apoptosis and miR-139-3p expression in tumor tissues. a–c The picture of the solid tumor (a) was exhibited; tumor volume (b) and tumor weight (c) were calculated. d Cell apoptosis in tumor tissues was detected by TUNEL staining (Magnification × 400). e The expression of BRD4 in tumor tissues was detected by immunohistochemical (Magnification × 400). f The expression of miR-139-3p was detected by RT-qPCR. g The protein levels of EMT-related proteins were detected by Western blot. U6 was used as an internal control. (***P < 0.001, vs. shNC). shNC shRNA negative control
Fig. 5.
MiR-139-3p mimic inhibited tumor growth and BRD4 expression, and promoted apoptosis and miR-139-3p expression in tumor tissues. a–c The picture of the solid tumor (a) was exhibited; tumor volume (b) and tumor weight (c) were calculated. d Cell apoptosis in tumor tissues was detected by TUNEL staining (Magnification × 400). e The expression of BRD4 in tumor tissues was detected by immunohistochemical (Magnification × 400). f The expression of miR-139-3p was detected by RT-qPCR. U6 was used as an internal control. g The protein levels of EMT-related proteins were detected by Western blot. (***P < 0.001, vs. MC). shNC mimic control
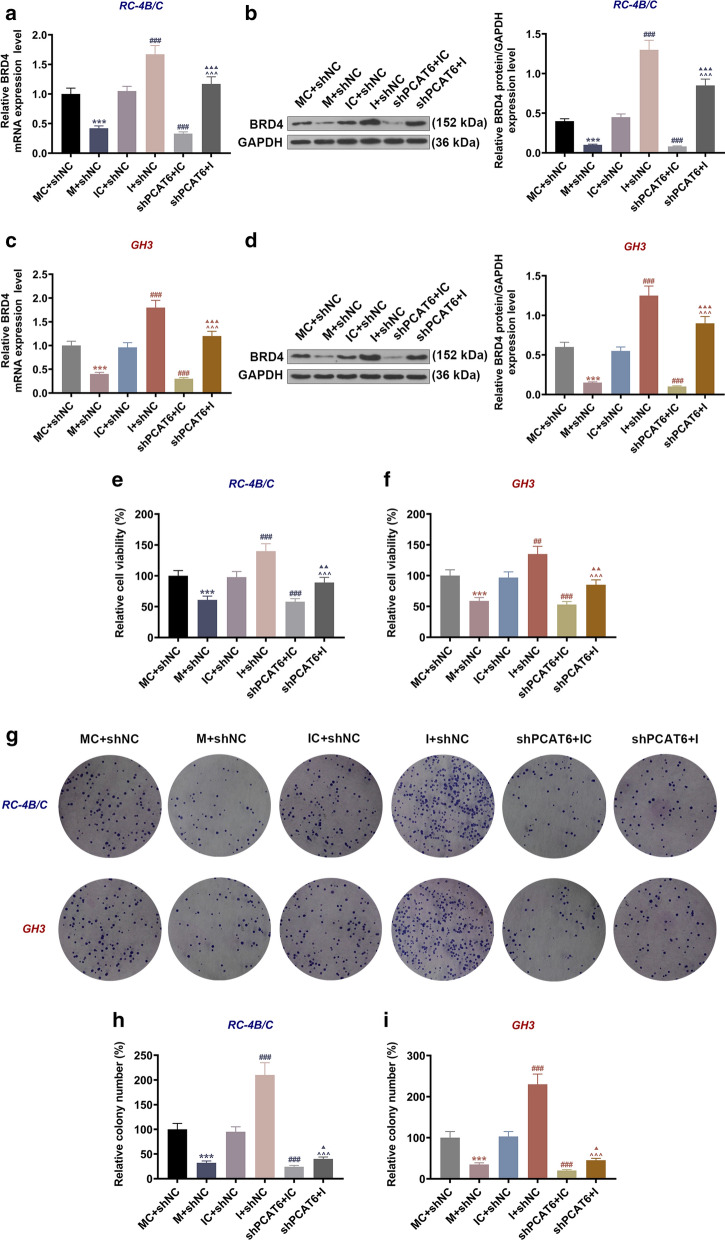
MiR-139-3p inhibitor reversed the inhibitory effect of shPCAT6 on BRD4 expression and the viability and proliferation of PA cells
The previous analysis has demonstrated the promoting effects of PCAT6 on the proliferation, migration and invasion of PA cells, but how the interaction of PCAT6 and miR-139-3p affected PA progression is still unclear. As Fig. 6a–d showed, the gene and protein levels of BRD4 in RC-4B/C and GH3 cells were reduced by miR-139-3p mimic as compared to the IC + siNC group (P < 0.001); when compared with the IC + shNC group, BRD4 expression was enhanced in the I + shNC group (P < 0.001) but decreased in the shPCAT6 + IC group (P < 0.001); moreover, BRD4 level in the shPCAT6 + I group was lower than that in the I + shNC group (P < 0.001) and higher than that in the shPCAT6 + IC group (P < 0.001). Similar changes were observed in cell viability and proliferation (Fig. 6e–i): the viability and proliferation of RC-4B/C and GH3 cells were inhibited by miR-139-3p mimic as compared to the IC + siNC group (P < 0.001); when compared with the IC + shNC group, cell viability and proliferation were increased in the I + shNC group (P < 0.001) but decreased in the shPCAT6 + IC group (P < 0.001); meanwhile, the viability and proliferating ability of cells in the shPCAT6 + I group were lower than those in the I + shNC group (P < 0.001) and higher than those in the shPCAT6 + IC (P < 0.05). The phenomena unveiled that miR-139-3p mimic and shPCAT6 suppressed BRD4 expression and the viability and proliferation of RC-4B/C and GH3 cells, whereas miR-139-3p inhibitor had the opposite effect and further reversed the inhibitory effect of shPCAT6 on PA cells.
Fig. 6.
MiR-139-3p inhibitor reversed the inhibitory effect of shPCAT6 on BRD4 expression, and the viability and proliferation of PA cells. a, c The expressions of BRD4 in RC-4B/C and GH3 cells after ransfection were detected by RT-qPCR. GAPDH was used as an internal control. b, d The expressions of BRD4 in RC-4B/C and GH3 cells after transfection were detected by Western blot. GAPDH was used as an internal control. e, f The viability of RC-4B/C and GH3 cells after transfection was detected by CCK-8 assay. g–i The proliferation of RC-4B/C and GH3 cells after transfection was detected by colony formation assay. (***P < 0.001, vs. MC + shNC; ##P < 0.01, ###P < 0.001, vs. IC + shNC; ^^^P < 0.001, vs. I + shNC; ▲P < 0.05, ▲▲P < 0.01, ▲▲▲P < 0.001, vs. shPCAT6 + IC). M: miR-139-3p mimic, MC mimic control, I miR-139-3p inhibitor, IC inhibitor control, shNC shRNA negative control
MiR-139-3p inhibitor reversed the regulatory effect of shPCAT6 on apoptosis, cell cycle distribution, and the expressions of apoptosis-related factors in PA cells
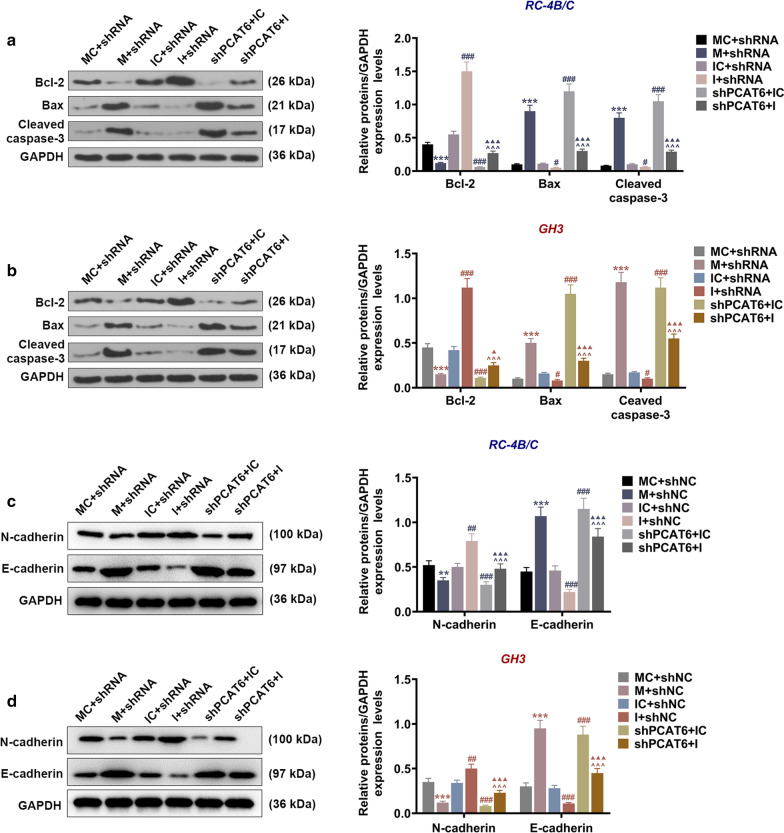
We then checked the changes in the apoptosis and cell cycle distribution of PA cells after transfection of miR-139-3p inhibitor, mimic, and shPCAT6. According to Fig. 7a, c, d, the apoptosis rates of PA cells were increased by miR-139-3p mimic as compared to the IC + siNC group (P < 0.001); when compared with the IC + shNC group, the apoptosis rates were reduced in the I + shNC group (P < 0.01) but enhanced in the shPCAT6 + IC group (P < 0.001); in addition, the apoptosis rates in shPCAT6 + I group were higher than those in the I + shNC group (P < 0.001) and lower than those in the shPCAT6 + IC group (P < 0.001). These results suggested that miR-139-3p mimic and shPCAT6 enhanced the apoptosis of PA cells, while miR-139-3p inhibitor had the opposite effect and further reversed the promoting effect of shPCAT6 on the apoptosis of PA cells. We subsequently determined the levels of apoptosis-related factors and EMT-associated factors (Fig. 8) in RC-4B/C and GH3 cells, and the results further verified the above discoveries: miR-139-3p mimic and shPCAT6 down-regulated Bcl-2 and N-cadherin expressions yet up-regulated Bax, Cleaved caspase-3 and E-cadherin expressions, whereas miR-139-3p inhibitor resulted in an opposite effect of miR-139-3p mimic and further reversed the effect of shPCAT6 on these factors. Through the detection of cell cycle distribution (Fig. 7b, e, f), we observed that miR-139-3p mimic and shPCAT6 induced G0/G1 phase arrest as compared to the MC + shNC group (P < 0.05) and the IC + shNC group (P < 0.01); miR-139-3p inhibitor promoted cell transition from G1 to S phase as compared to the IC + shNC group (P < 0.01); in addition, miR-139-3p inhibitor further reversed the effect of shPCAT6 on cell cycle distribution as compared with the shPCAT6 + IC group (P < 0.01).
Fig. 7.
MiR-139-3p inhibitor reversed the regulatory effect of shPCAT6 on the apoptosis and cell cycle distribution of PA cells. a, c, d The apoptosis of RC-4B/C and GH3 cells after transfection was detected by flow cytometry. b, e, f The cell cycle distribution of RC-4B/C and GH3 cells after transfection was detected by flow cytometry. (*P < 0.05, **P < 0.01, ***P < 0.001, vs. MC + shNC; ##P < 0.01, ###P < 0.001, vs. IC + shNC; ^^P < 0.01, ^^^P < 0.001, vs. I + shNC; ▲P < 0.05, ▲▲P < 0.01, ▲▲▲P < 0.001, vs. shPCAT6 + IC). M miR-139-3p mimic, MC mimic control, I miR-139-3p inhibitor, IC inhibitor control, shNC shRNA negative control
Fig. 8.
MiR-139-3p inhibitor reversed the regulatory effect of shPCAT6 on the expressions of apoptosis-related factors in PA cells. a, b The expressions of Bcl-2, Bax, and Cleaved caspase-3 in RC-4B/C and GH3 cells after transfection were detected by Western blot assay. c, d The protein levels of E-cadhein and N-cadherin were detected by Western blot. (***P < 0.001, vs. MC + shNC; #P < 0.05, ###P < 0.001, vs. IC + shNC; ^^^P < 0.001, vs. I + shNC; ▲P < 0.05, ▲▲▲P < 0.001, vs. shPCAT6 + IC). M miR-139-3p mimic, MC mimic control, I miR-139-3p inhibitor, IC inhibitor control, shNC shRNA negative control
BRD4 was high-expressed in IPA tissues and was targeted by miR-139-3p in PA cells
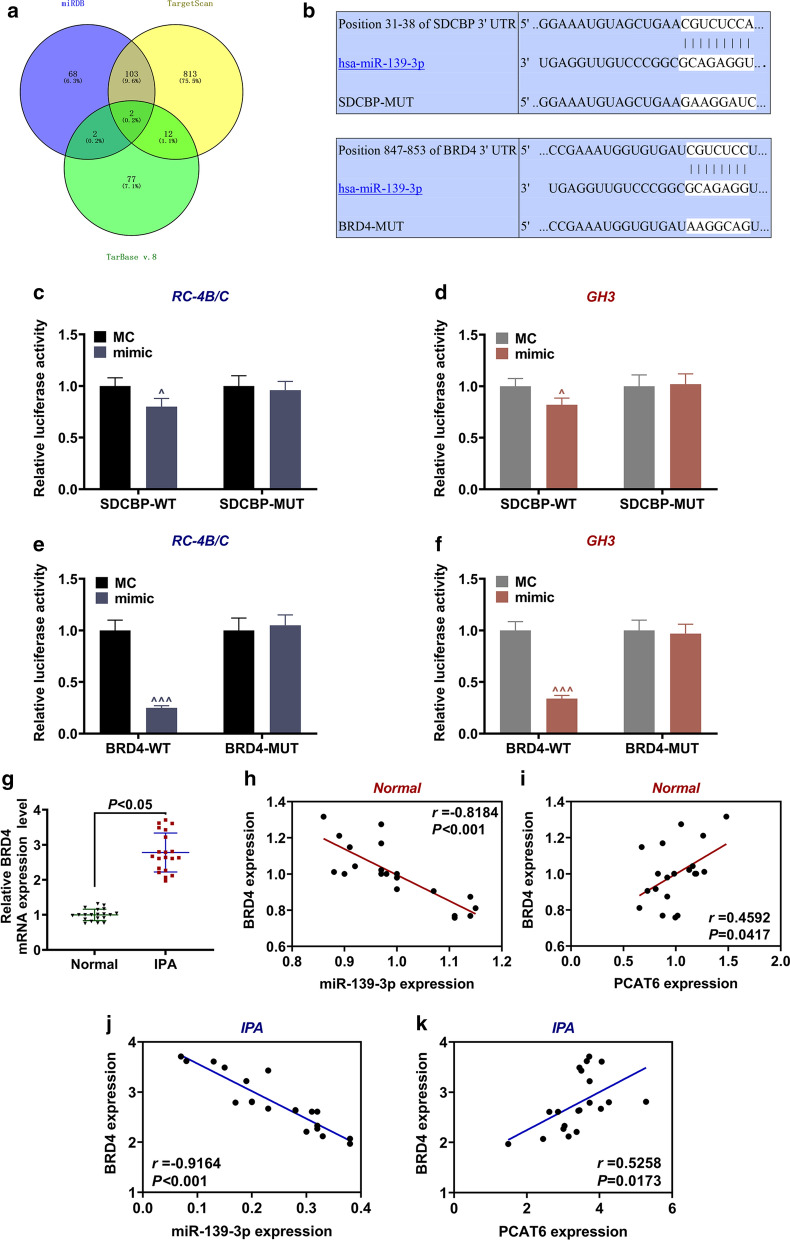
The mRNAs that were possibly targeted by miR-139-3p were then predicted by miRDB, TargetScan, and TarBase v.8 (Fig. 9a). Besides, TargetScan 7.2 also predicted that SDCBP and BRD4 could be targeted by miR-139-3p, given that the 3′UTRs of SDCBP and BRD4 had the binding sites for miR-139-3p (Fig. 9b). Then luciferase reporter assay was performed to verify this prediction (Fig. 9e, f). The results exhibited that the luciferase activity in cells co-transfected with miR-139-3p mimic and SDCBP-WT or BRD4-WT was reduced as compared to the MC group (P < 0.05); however, no difference in luciferase activity was detected in cells co-transfected miR-139-3p mimic and SDCBP-MUT or BRD4-MUT as compared to the MC group. Considering that BRD4 had a relatively strong binding ability to miR-139-3p, we then probed into the correlation between BRD4 and miR-139-3p. It was found that the expression of BRD4 in IPA tissues was higher than that in normal pituitary tissues (P < 0.05, Fig. 4g). Moreover, the expression of BRD4 in normal pituitary tissues and IPA tissues showed a negative correlation with miR-139-3p expression (Fig. 9h, j) and a positive correlation with PCAT6 expression (Fig. 9i, k).
Fig. 9.
BRD4 was high-expressed in IPA and was targeted by miR-139-3p in PA cells. a The mRNAs that were possibly targeted by miR-139-3p were then predicted by miRDB, TargetScan, and TarBase v.8. b The putative binding site between miR-139-3p and SDCBP or BRD4 was predicted by Targetscan7.2. c, d The results of luciferase assay validated that miR-139-3p targeted SDCBP in PA cells RC-4B/C and GH3. e, f The results of luciferase assay validated that miR-139-3p targeted BRD4 in PA cells RC-4B/C and GH3. g The expressions of BRD4 in normal pituitary and IPA tissues were detected by RT-qPCR. GAPDH was used as an internal control. h, j The correlation between BRD4 expression and miR-139-3p expression in normal pituitary and IPA tissues was analyzed by Pearson correlation analysis. i, k The correlation between BRD4 expression and PCAT6 expression in normal pituitary and IPA tissues was analyzed by Pearson correlation analysis. (^P < 0.05, ^^^P < 0.001, vs. MC). IPA invasive pituitary adenomas, MC mimic control
SiBRD4 reversed the promoting effect of miR-139-3p inhibitor on BRD4 expression and the viability, migration, invasion, and proliferation of PA cells
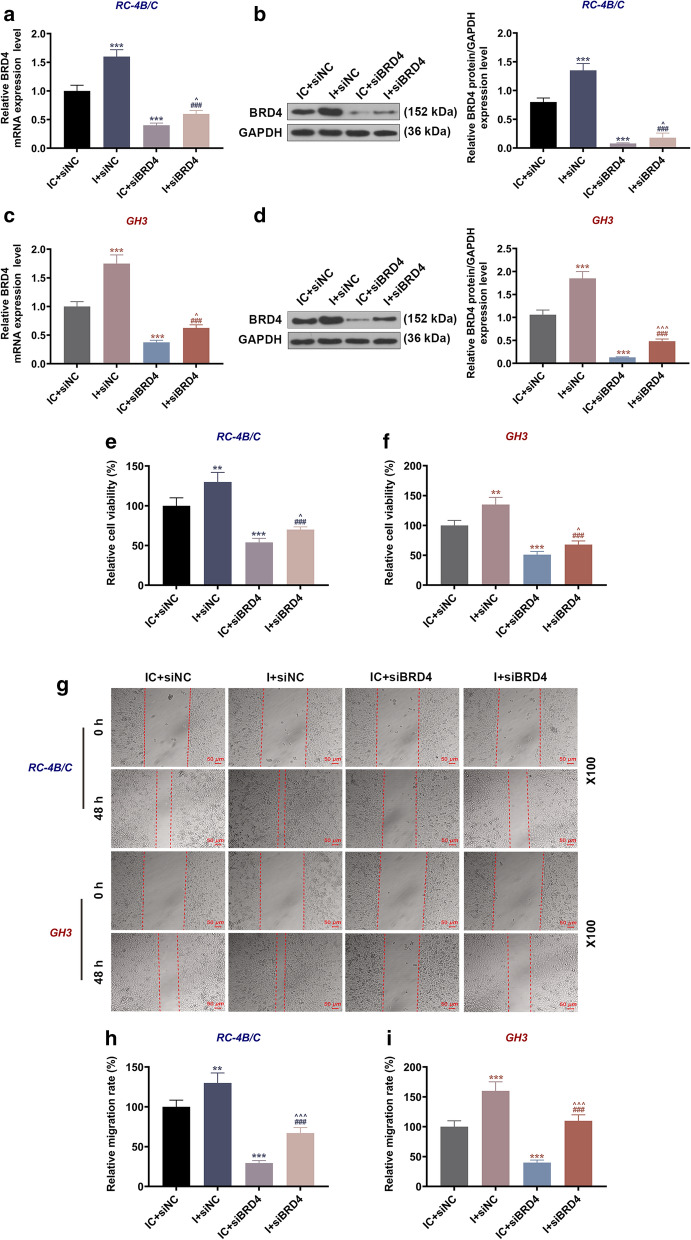
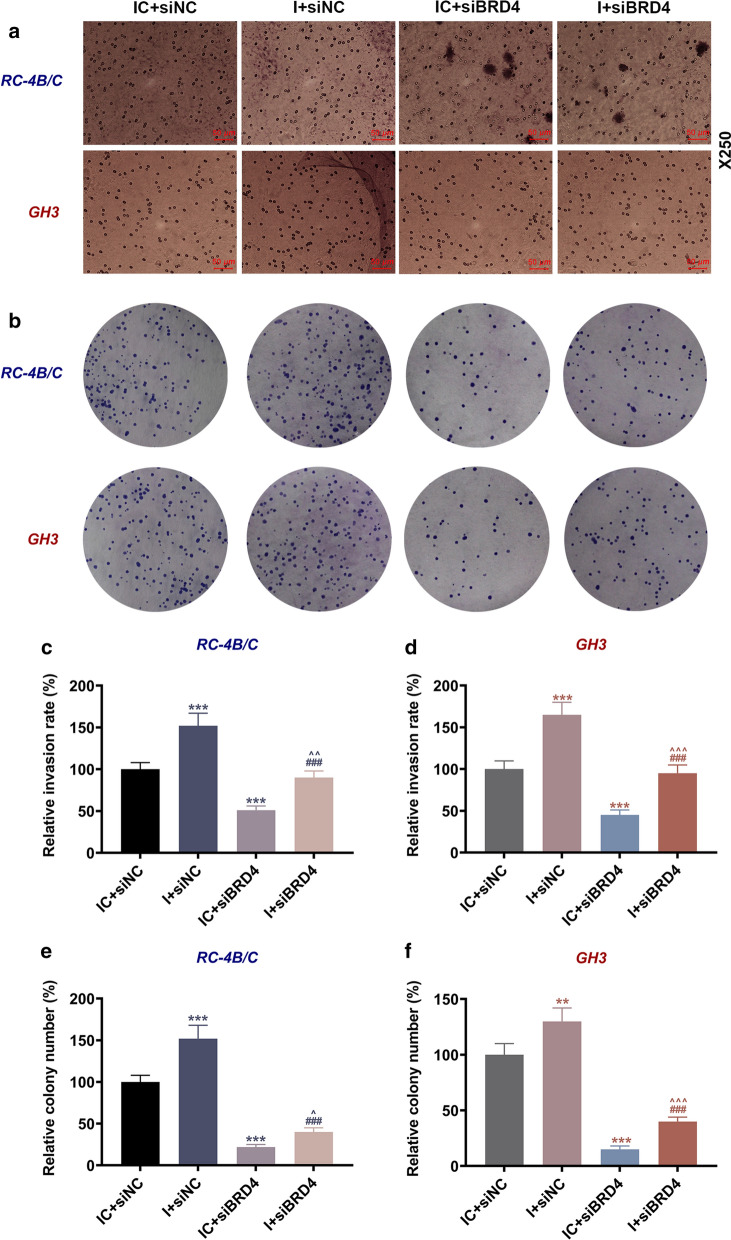
We have demonstrated the interaction of BRD4 and miR-139-3p, but it has not been clarified whether BRD4 was involved in the regulatory effects of miR-139-3p on PA progression. Hence, we next detected the effect of miR-139-3p on the expression of BRD4 in PA cells. As illustrated in Fig. 10a–d, the transcription and translation levels of BRD4 in both kinds of PA cells were up-regulated by miR-139-3p inhibitor but down-regulated by siBRD4 when compared with the IC + siNC group (P < 0.001); by contrast, those in the I + siBRD4 group were decreased as compared with the I + siNC group (P < 0.001) and increased when compared to the IC + siBRD4 group (P < 0.05). The results suggested that siBRD4 could reverse the promoting effect of miR-139-3p mimic on BRD4 expression. As for cell viability and migration (Fig. 10e–i), the viability and relative migration rate in the two cells were increased by miR-139-3p inhibitor but decreased by siBRD4 when compared with the IC + siNC group (P < 0.01); meantime, the viability and relative migration rate in the I + siBRD4 group were lower than those in the I + siNC group (P < 0.001) and higher than those in the IC + siBRD4 group (P < 0.05). Besides, the relative invasion and proliferation rates (Fig. 11) of RC-4B/C and GH3 cells were increased by miR-139-3p inhibitor but decreased by siBRD4 when compared with the IC + siNC group (P < 0.01), and the relative invasion and proliferation rates in the I + siBRD4 group were lower than those in the I + siNC group (P < 0.001) and higher than those in the IC + siBRD4 group (P < 0.05).
Fig. 10.
SiBRD4 reversed the promoting effect of miR-139-3p inhibitor on BRD4 expression, and the viability and migration of PA cells. a, c The expressions of BRD4 in RC-4B/C and GH3 cells after transfection were detected by RT-qPCR. GAPDH was used as an internal control. b, d The expressions of BRD4 in RC-4B/C and GH3 cells after transfection were detected by Western blot. GAPDH was used as an internal control. e, f The viability of RC-4B/C and GH3 cells after transfection was detected by CCK-8 assay. g–i The migration of RC-4B/C and GH3 cells after transfection was detected by wound healing assay (Magnification × 100). (**P < 0.01, ***P < 0.001, vs. IC + siNC; ###P < 0.001, vs. I + siNC; ^P < 0.05, ^^^P < 0.001, vs. I + siBRD4). I miR-139-3p inhibitor, IC inhibitor control, siNC small interfering RNA negative control
Fig. 11.
SiBRD4 reversed the promoting effect of miR-139-3p inhibitor on the invasion and proliferation of PA cells. a, c, d The invasion of RC-4B/C and GH3 cells after transfection was detected by transwell assay (Magnification × 250). b, e, f The proliferation of RC-4B/C and GH3 cells after transfection was detected by colony formation assay. (***P < 0.001, vs. IC + siNC; ###P < 0.001, vs. I + siNC; ^P < 0.05, ^^P < 0.01, ^^^P < 0.001, vs. I + siBRD4). I miR-139-3p inhibitor, IC inhibitor control, siNC small interfering RNA negative control
Discussion
Much evidence revealed that abnormal levels of miRNAs are closely related to the development of various diseases and cancers, including PA [16, 17]. Therefore, we used the GSE46294 dataset to identify the dysregulated miRNAs in PA in the GEO2R database, and further verified the levels of the top ten miRNAs in normal, NIPA, and IPA tissues. Among these dysregulated miRNAs, miR-139-3p which was lowly expressed in both IPA and PA tissues was previously proved to play a key role in the progression of multiple diseases [18–20]. Since the role of miR-139-3p in PA has not been reported, we chose miR-139-3p for further analysis in this study. There were several regulators of miR-139-3p in the modulation of cancers; for instance, circ_0031288 and lncRNA TP73-AS1 could target miR-139-3p in cervical cancer and retinoblastoma respectively [18, 21]. In this study, we for the first time discovered that miR-139-3p was also a target of lncRNA PCAT6, and PCAT6 was up-regulated in IPA and was negatively correlated with miR-139-3p. Like miR-139-3p, PCAT6 also exerted a crucial function in several diseases such as lung, liver, and ovarian cancers [13, 14, 22], yet its role in PA has not been studied.
Most of the obstacles in the treatment of PA and IPA are ascribed to the rapid proliferation and metastasis of PA cells as well as their low apoptosis rate [3, 6]. Studies proved that PCAT6 and miR-139-3p could regulate these biological activities of cancer cells. For example, PCAT6 had the capacity to promote the proliferation and invasion of cholangiocarcinoma cells [23], and miR-139-3p could induce apoptosis and suppress metastasis in cervical cancer [24]. Several previous studies suggested that PCAT6 functions as an oncogene in different cancer types. In 2018, PCAT6 was found high-expressed in patients with non-small cell lung cancer (NSCLC), and the knockdown of PCAT6 showed a significantly inhibitory effect on the proliferation, migration and invasion of NSCLC cells [13]. Similarly, a recent study provided a range of evidence that highlighted the potential roles of PCAT6: it could not only serve as a valuable prognostic indicator for patients with osteosarcoma (OS), but also aggravate the malignant phenotype of OS cells via the regulation of the miR-143-3p/ZEB1 axis [25]. In this study, we discovered that shPCAT6 and miR-139-3p mimic suppressed the viability, migration, invasion, and proliferation of PA cells while inducing apoptosis; miR-139-3p inhibitor had the opposite effect of miR-139-3p mimic and could reverse the effect of shPCAT6 on PA cells. In view of the close relations between cell proliferation and the regulation of cell cycle distribution, and between apoptosis and the regulation of key factors in apoptosis (Bcl-2, Bax, Cleaved caspase-3) [26–28], we further detected cell cycle distribution and the expressions of the apoptosis-related proteins, and discovered that miR-139-3p mimic and shPCAT6 could induce cell cycle arrest in G0/G1 phase, suppress Bcl-2 expression, and enhance Bax and Cleaved caspase-3 expressions in PA cells. Besides, the previous studies also found that high expression of lncRNA PCAT6 showed a close association with tumor occurrence and development in ovarian cancer as well as the chemoresistance of cervical cancer [14, 15]. Our study focused on the regulatory effects of PCAT6 expression on cell biological behaviors in PA. As mentioned above, miR-139-3p inhibitor reversed the effect of shPCAT6 on PA cells. All these phenomena revealed that PCAT6 targeted miR-139-3p to modulate the progression of proliferation, migration, invasion, and apoptosis in PA cells.
The effect of miR-139-3p in cancer could be mediated by targeting certain mRNAs [19, 24, 29]. For instance, the carcinogenic effect of miR-139-3p in colorectal cancer was mediated by targeting RAB1A [19]; miR-139-3p targeted NOB1 in cervical cancer to regulate cell apoptosis and metastasis [24]; in addition, miR-139-3p targeted MMP11 in bladder cancer [29]. Here, we further discovered that miR-139-3p could target SDCBP and BRD4. Previous study confirmed that BRD4, whose effect was found up-regulated in PA, could regulate the growth of PA tumor in mice [30, 31], and therefore, we chose BRD4 for further analysis in the current study. BRD4 has been widely reported to be involved in the development of various cancer types, including multiple myeloma [32], small cell lung cancer [33], and ovrican cancer [34]. The expression of BRD4 could notably affect multiple biological characteristics via different approaches. For example, BRD4 was involved in the effects of circ_0007841/miR-338-3p axis on multiple myeloma progression [32]. Furthermore, BRD4 also played an important role in the sensitivity of multiple myeloma cells to bortezomib [35]. Similarly, the up-regulation of BRD4 in PA tissues was also observed in this study, and furthermore, the expression of BRD4 was found negatively correlated with miR-139-3p expression and positively correlated with PCAT6 expression. Based on these findings, we speculated that the effect of PCAT6 in PA might be mediated by regulating the miR-139-3p/BRD4 axis. To confirm this supposition, multiple functional experiments were further conducted, and the results demonstrated that siBRD4 weakened the capacity of PA cells to migrate, invade, and proliferate, and reversed the effect of miR-139-3p inhibitor on PA cells, which thus attested to our conjecture.
We also established a subcutaneous xenotransplanted-tumor model to further prove the present findings. Our results revealed that shPCAT6 and miR-139-3p mimic inhibited the growth of PA tumor in nude mice, and induced cell apoptosis in PA tumors while suppressing the expression of BRD4. All the phenomena we discovered in vivo further verified that up-regulation of PCAT6 could promote the progression of PA in vitro and in vivo by targeting the miR-139-3p/BRD4 axis.
Our findings in this research proved that PCAT6 could promote the progression of PA both in vitro and in vivo through targeting the miR-139-3p/BRD4 axis. Our research also provided a novel biomarker for the prevention, diagnosis, and treatment of PA.
Acknowledgements
Not applicable.
Abbreviations
- PA
Pituitary adenomas
- IPA
Invasive PA
- NIPA
Non-invasion PA
- RIP
RNA immunoprecipitation
- shNC
ShRNA negative control
- PCAT6-WT
PCAT6 wide-type
Authors’ contributions
PZ and JC designed the research study. BL, DN and HW performed the research. CL, SG and YZ analyzed the data. PZ and JC wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Beijing Natural Science Foundation under [grant number 7162034].
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The application of clinical samples and the animal tests were ratified by the Ethics Committee of Beijing Tiantan Hospital (BT20190530S). The patients whose samples were used in this study signed informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1186/s12935-024-03398-y
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peng Zhao and Jianhua Cheng contributed equally to this work
Change history
6/14/2024
This article has been retracted. Please see the Retraction Notice for more detail: 10.1186/s12935-024-03398-y
References
- 1.Jin Z, Wu X, Wang Y. Clinical study of endoscopic treatment of a sellar pituitary adenomas with sellar diaphragm defect. BMC Neurol. 2020;20(1):129. doi: 10.1186/s12883-020-01690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guerrero-Perez F, Marengo AP, Vidal N, Villabona C. Pituitary adenomas with changing phenotype: a systematic review. Exp Clin Endocrinol Diabetes. 2020 doi: 10.1055/a-1120-8277. [DOI] [PubMed] [Google Scholar]
- 3.Xue YH, Ge YQ. Construction of lncRNA regulatory networks reveal the key lncRNAs associated with Pituitary adenomas progression. Math Biosci Eng. 2020;17(3):2138–2149. doi: 10.3934/mbe.2020113. [DOI] [PubMed] [Google Scholar]
- 4.Rajaratnam S, Jeyaseelan L, Rajshekhar V. Delayed hyponatremia following surgery for pituitary adenomas: an under-recognized complication. Neurol India. 2020;68:340. doi: 10.4103/0028-3886.280637. [DOI] [PubMed] [Google Scholar]
- 5.Sommerfelt H, Sagberg LM, Solheim O. Impact of transsphenoidal surgery for pituitary adenomas on overall health-related quality of life: a longitudinal cohort study. Br J Neurosurg. 2019;33(6):635–640. doi: 10.1080/02688697.2019.1667480. [DOI] [PubMed] [Google Scholar]
- 6.Marino AC, Taylor DG, Desai B, Jane JA., Jr Surgery for pediatric pituitary adenomas. Neurosurg Clin N Am. 2019;30(4):465–471. doi: 10.1016/j.nec.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Zhu H, Guo J, Shen Y, Dong W, Gao H, Miao Y, Li C, Zhang Y. Functions and mechanisms of tumor necrosis factor-alpha and noncoding RNAs in bone-invasive pituitary adenomas. Clin Cancer Res. 2018;24(22):5757–5766. doi: 10.1158/1078-0432.CCR-18-0472. [DOI] [PubMed] [Google Scholar]
- 8.Mohebi M, Ghafouri-Fard S, Modarressi MH, Dashti S, Zekri A, Kholghi-Oskooei V, Taheri M. Expression analysis of vimentin and the related lncRNA network in breast cancer. Exp Mol Pathol. 2020;115:104439. doi: 10.1016/j.yexmp.2020.104439. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Li C, Liu C, Yu S, Zhang Y. Expression of the long non-coding RNAs MEG3, HOTAIR, and MALAT-1 in non-functioning pituitary adenomas and their relationship to tumor behavior. Pituitary. 2015;18(1):42–47. doi: 10.1007/s11102-014-0554-0. [DOI] [PubMed] [Google Scholar]
- 10.Lu T, Yu C, Ni H, Liang W, Yan H, Jin W. Expression of the long non-coding RNA H19 and MALAT-1 in growth hormone-secreting pituitary adenomas and its relationship to tumor behavior. Int J Dev Neurosci. 2018;67:46–50. doi: 10.1016/j.ijdevneu.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Tang H, Hou B, Ye Z, Ling C, Guo Y. Knockdown of long non-coding RNA AFAP1-AS1 inhibits growth and promotes apoptosis in pituitary adenomas. Int J Clin Exp Pathol. 2018;11(3):1238–1246. [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Wang G, Gao Y, Zhao C, Li X, Zhang F, Jiang C, Wu B. Lnc-SNHG1 activates the TGFBR2/SMAD3 and RAB11A/Wnt/beta-catenin pathway by sponging MiR-302/372/373/520 in invasive pituitary tumors. Cell Physiol Biochem. 2018;48(3):1291–1303. doi: 10.1159/000492089. [DOI] [PubMed] [Google Scholar]
- 13.Cui LH, Xu HR, Yang W, Yu LJ. lncRNA PCAT6 promotes non-small cell lung cancer cell proliferation, migration and invasion through regulating miR-330-5p. OncoTargets Ther. 2018;11:7715–7724. doi: 10.2147/OTT.S178597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong FR, Lv YH, Yao HM, Zhang HY, Zhou Y, Liu SE. LncRNA PCAT6 promotes occurrence and development of ovarian cancer by inhibiting PTEN. Eur Rev Med Pharmacol Sci. 2019;23(19):8230–8238. doi: 10.26355/eurrev_201910_19132. [DOI] [PubMed] [Google Scholar]
- 15.Ma Z, Gu G, Pan W, Chen X. LncRNA PCAT6 accelerates the progression and chemoresistance of cervical cancer through up-regulating ZEB1 by sponging miR-543. OncoTargets Ther. 2020;13:1159–1170. doi: 10.2147/OTT.S232354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu C, Huang L, Xu F, Li P, Li P, Hu F. LncRNA PCAT6 promotes tumor progression in osteosarcoma via activation of TGF-beta pathway by sponging miR-185-5p. Biochem Biophys Res Commun. 2020;521(2):463–470. doi: 10.1016/j.bbrc.2019.10.136. [DOI] [PubMed] [Google Scholar]
- 17.Sur D, Coza O, Havasi A, Cainap C, Burz C, Vlad C, Balacescu O, Alexandru I, Lisencu C. Exosomal miRNAs in colorectal cancer: the carriers of useful news. J BUON. 2020;25(1):23–34. [PubMed] [Google Scholar]
- 18.Xu YJ, Yu H, Liu GX. Hsa_circ_0031288/hsa-miR-139–3p/Bcl-6 regulatory feedback circuit influences the invasion and migration of cervical cancer HeLa cells. J Cell Biochem. 2020;121:4251. doi: 10.1002/jcb.29650. [DOI] [PubMed] [Google Scholar]
- 19.Pei FL, Cao MZ, Li YF. Circ_0000218 plays a carcinogenic role in colorectal cancer progression by regulating miR-139-3p/RAB1A axis. J Biochem. 2020;167(1):55–65. doi: 10.1093/jb/mvz078. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Y, Zhou C, He Q. High miR-139-3p expression predicts a better prognosis for hepatocellular carcinoma: a pooled analysis. J Int Med Res. 2019;47(1):383–390. doi: 10.1177/0300060518802727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia Z, Yang X, Wu S, Feng Z, Qu L, Chen X, Liu L, Mayr Y. LncRNA TP73-AS1 down-regulates miR-139-3p to promote retinoblastoma cell proliferation. Biosci Rep. 2019;39(5):BSR20190475. doi: 10.1042/BSR20190475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo Y, Lin J, Zhang Y, Dai G, Li A, Liu X. LncRNA PCAT6 predicts poor prognosis in hepatocellular carcinoma and promotes proliferation through the regulation of cell cycle arrest and apoptosis. Cell Biochem Func. 2020;38:895. doi: 10.1002/cbf.3510. [DOI] [PubMed] [Google Scholar]
- 23.Xin Y, He X, Zhao W, Zhan M, Li Y, Xiao J, He K, Lu L. LncRNA PCAT6 increased cholangiocarcinoma cell proliferation and invasion via modulating miR-330-5p. Am J Transl Res. 2019;11(9):6185–6195. [PMC free article] [PubMed] [Google Scholar]
- 24.Huang P, Xi J, Liu S. MiR-139-3p induces cell apoptosis and inhibits metastasis of cervical cancer by targeting NOB1. Biomed Pharmacother. 2016;83:850–856. doi: 10.1016/j.biopha.2016.07.050. [DOI] [PubMed] [Google Scholar]
- 25.Wu K, Feng Q, Li L, Xiong Y, Liu S, Liu J, Wu Q. Long-noncoding RNA PCAT6 aggravates osteosarcoma tumourigenesis via the MiR-143-3p/ZEB1 axis. OncoTargets Ther. 2020;13:8705–8714. doi: 10.2147/OTT.S258415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang H, Liu JX, Shang HX, Lin S, Zhao JY, Lin JM. Qingjie Fuzheng granules inhibit colorectal cancer cell growth by the PI3K/AKT and ERK pathways. World J Gastrointest Oncol. 2019;11(5):377–392. doi: 10.4251/wjgo.v11.i5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu X, Chen Z, Li X. Diosgenin inhibits the proliferation, migration and invasion of the optic nerve sheath meningioma cells via induction of mitochondrial-mediated apoptosis, autophagy and G0/G1 cell cycle arrest. J BUON. 2020;25(1):508–513. [PubMed] [Google Scholar]
- 28.Fakhrabadi HG, Rabbani-Chadegani A, Ghadam P, Amiri S. Protective effect of bleomycin on 5-azacitidine induced cytotoxicity and apoptosis in mice hematopoietic stem cells via Bcl-2/Bax and HMGB1 signaling pathway. Toxicol Appl Pharmacol. 2020;396:114996. doi: 10.1016/j.taap.2020.114996. [DOI] [PubMed] [Google Scholar]
- 29.Yonemori M, Seki N, Yoshino H, Matsushita R, Miyamoto K, Nakagawa M, Enokida H. Dual tumor-suppressors miR-139-5p and miR-139-3p targeting matrix metalloprotease 11 in bladder cancer. Cancer Sci. 2016;107(9):1233–1242. doi: 10.1111/cas.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fielitz K, Althoff K, De Preter K, Nonnekens J, Ohli J, Elges S, Hartmann W, Kloppel G, Knosel T, Schulte M, et al. Characterization of pancreatic glucagon-producing tumors and pituitary gland tumors in transgenic mice overexpressing MYCN in hGFAP-positive cells. Oncotarget. 2016;7(46):74415–74426. doi: 10.18632/oncotarget.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi C, Ye Z, Han J, Ye X, Lu W, Ji C, Li Z, Ma Z, Zhang Q, Zhang Y, et al. BRD4 as a therapeutic target for nonfunctioning and growth hormone pituitary adenoma. Neuro Oncol. 2020;22:1114. doi: 10.1093/neuonc/noaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Lin Q, Song C, Ma R, Li X. Circ_0007841 promotes the progression of multiple myeloma through targeting miR-338-3p/BRD4 signaling cascade. Cancer Cell Int. 2020;20:383. doi: 10.1186/s12935-020-01475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szczepanski AP, Zhao Z, Sosnowski T, Goo YA, Bartom ET, Wang L. ASXL3 bridges BRD4 to BAP1 complex and governs enhancer activity in small cell lung cancer. Genome Med. 2020;12(1):63. doi: 10.1186/s13073-020-00760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng XY, Yuan J, Wang C, Zeng D, Yong JH, Jiang XY, Lan H, Xiao SS. circCELSR1 facilitates ovarian cancer proliferation and metastasis by sponging miR-598 to activate BRD4 signals. Mol Med. 2020;26(1):70. doi: 10.1186/s10020-020-00194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li G, Zheng YH, Xu L, Feng J, Tang HL, Luo C, Song YP, Chen XQ. BRD4 inhibitor nitroxoline enhances the sensitivity of multiple myeloma cells to bortezomib in vitro and in vivo by promoting mitochondrial pathway-mediated cell apoptosis. Ther Adv Hematol. 2020;11:2040620720932686. doi: 10.1177/2040620720932686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.