Abstract
Background
The nucleotide analogue prodrug remdesivir was among the first antiviral therapies to be tested in randomized controlled trials (RCTs) for COVID-19. We performed a meta-analysis to understand efficacy and safety.
Methods
We searched PubMed, EMBASE, Cochrane library, and ClinicalTrials.gov databases (from January 1, 2020 to November 5, 2020). We included RCTs comparing the efficacy and safety of remdesivir to control/placebo in COVID-19. Two independent investigators abstracted data, assessed the quality of evidence, and rated the certainty of evidence.
Results
A total of 4 RCTs with 7334 patients with COVID-19 were included. At a follow-up of 28–29 days from randomization, very low certainty evidence showed that use of remdesivir compared with control group (placebo and/or standard of care) was not associated with a significant decrease in time to clinical improvement (standardized mean difference −0.80 day; [CI, −2.12, 0.53]). However, moderate certainty of evidence showed that remdesivir was associated with higher rates of recovered patients (risk difference [RD] 0.07 [0.05, 0.08]) and discharged patients (RD 0.07 [0.03, 0.11]) and lower rates of developing serious adverse events (RD -0.05 [−0.10, −0.01]) compared with control. Moderate and very low certainty of evidence showed there was no significant difference in deaths at 28–29 days follow-up (RD -0.01 [−0.03, 0.01]) and developing any adverse events (RD 0.01 [−0.17, 0.19]) between both groups, respectively.
Conclusion
Patients given remdesivir are more likely to demonstrate recovery and were associated with higher rates of hospital discharge, but not with significant reduction in mean time to clinical improvement or mortality.
Keywords: Remdesivir, COVID-19, SARS-Cov-2
1. Introduction
The devastating impact of the coronavirus disease 2019 (COVID-19) pandemic including social, economic, and health effects, caught the world unprepared [1]. As of November 2020, there have been over 1,300,000 deaths worldwide from COVID-19. While the scientific community mobilizes to identify vaccine and therapeutic candidates, public health interventions such as contact tracing, isolation, quarantine, face mask use, and social distancing have been widely adopted to preventing transmission.
Remdesivir is an adenosine nucleotide analogue prodrug that abrogates viral replication by inducing chain termination after its incorporation into the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA-dependent RNA polymerase (RdRp) enzyme [2]. At the start of the pandemic, remdesivir was one of very few nucleoside/nucleotide analogue therapeutics with human safety data that had been shown to overcome the proofreading exoribonuclease activity of the coronavirus RdRp to effectively inhibit a broad range of coronaviruses [3,4]. With encouraging SARS-CoV-2-specific in vitro efficacy data, remdesivir became one of the earliest direct-acting antiviral therapeutics to enter randomized clinical trials (RCTs) for COVID-19 [2,5]. Its efficacy in preventing respiratory disease with early treatment was demonstrated shortly thereafter in a primate model of COVID-19 [6].
There are currently two RCTs that have assessed the efficacy of remdesivir vs placebo and/or standard of care in individuals with severe COVID-19, one RCT in individuals with moderate COVID-19, and one RCT that included both moderate and severe COVID-19 [5,[7], [8], [9]]. Patients were considered to have severe disease if their peripheral capillary oxygen saturation was equal or less than 94% on room air with evidence of pneumonia and considered to have moderate disease if their saturation was more than 94% with evidence of pneumonia.
Due to the need for greater certainty in the evidence base for use of remdesivir compared to standard treatment based on disease severity and to strengthen evidence of risks and benefits, we conducted a meta-analysis to evaluate the effect of remdesivir in the treatment of moderate and severe COVID-19.
2. Methods
This meta-analysis was conducted following the Cochrane Collaboration guidelines and reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) [10,11]. The protocol was registered at PROSPERO: CRD42020181509.
2.1. Data sources and searches
The literature search was performed without language restriction using the following electronic databases of PubMed, EMBASE, Cochrane library, and ClinicalTrials.gov from January 1, 2020 to November 5, 2020. The search strategy included broad search terms: “Remdesivir”, “COVID-19”, “SARS-CoV-2”, and “Coronavirus” (Appendix Table 1).
2.2. Study selection
The prespecified inclusion criteria were (1) RCTs comparing remdesivir treatment of any duration to placebo/control (2) RCTs reporting any of the following; time to clinical recovery, number of recovered patients, mortality, or adverse events. There were no limitations on the language, sample size or follow-up duration of RCTs. We excluded observational studies from this meta-analysis.
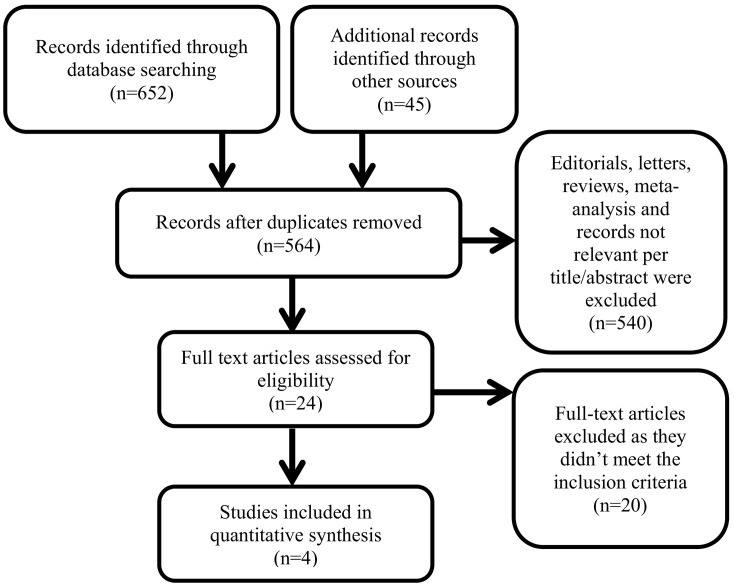
After removing the duplicates and following the selection criteria, we screened the remaining articles at the title and abstract level and then at the full text level (Fig. 1 ). The process of study search and selection was performed independently by 2 investigators (A.A. and A.B.) Any conflicts were resolved by a third investigator (A.J.).
Fig. 1.
Details of the search results.
2.3. Data extraction, and quality assessment
Two investigators (A.A. and A.B.) abstracted the data using prespecified data collection forms and resolved any discrepancies by consensus after discussing it with a third investigator (A.J.). The following information was abstracted from eligible RCTs: first author, characteristics of the trials and participants, time to recovery, number of events and sample sizes and follow-up duration.
Two unblinded investigators (A.A. and A.B.) independently assessed the potential risks of bias of the RCTs using the Cochrane Risk of Bias Tool at the study level as well as at outcome level (Appendix Fig. 1) [12,13].
The main outcomes of interest were mean time to clinical improvement, and the rate of recoveries, discharges from hospital, deaths, serious adverse events, and any adverse events through 28–29 days of follow up. Patients were considered to be recovered in our study if they were discharged alive from the hospital or were admitted without oxygen requirements (for infection control purposes); this definition is compatible with the definition of recovery in most included trials.
2.4. Data synthesis and analysis
Two reviewers (A.A and A.B,) assessed the certainty of the evidence using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach (GRADEpro GDT) (https://gdt.gradepro.org/app/), which was classified as high, moderate, low, or very low (Appendix Table 2 and 3) [14].
We calculated the mean and standard deviation for the time to clinical improvement from the median and interquartile ranges that were provided in the selected trials as described by Wan et al. [15]. Clinical improvement was defined as improvement of 2 or more points on the severity ordinal scale. In Spinner et al. [9], the 5-day and 10-day remdesivir arms were pooled together in one arm. Estimates in this meta-analysis were pooled using inverse variance random-effects model due to heterogeneity in the included population and treatment duration [16]. The Paule–Mandel method was used for estimation of τ [2]. We applied Hartung–Knapp/Sidik-Jonkman small-sample adjustments considering the number of studies [17]. We reported effect sizes as standardized mean difference (SMD) for the time to clinical improvement outcome and risk difference (RD) for other outcomes with 95% confidence interval (CI). The RD was reported as it gives better representation of absolute risk and doesn't overestimate the size of the effects [18]. Odds ratio was not reported in our analysis as we are evaluating data from clinical trials and using odds ratio in this kind of studies could be misleading [19]. We used I 2 statistics to measure the extent of unexplained statistical heterogeneity: I 2 greater than 50% was considered a high degree of between-study statistical heterogeneity [20]. We did not examine the publication bias as we were underpowered to detect publication bias due to small number of studies. The 95% CIs that did not cross 0 were considered statistically significant. We used R studio for all analyses in this study.
2.5. Sensitivity analysis
We performed a sensitivity analysis limited to patients with moderate COVID-19 for time to clinical improvement and rates of recoveries and deaths (data were pooled from Spinner et al. [9], and patients with moderate severity in Beigel et al. [7] and Pan et al. [21]). We also performed another sensitivity analysis by excluding one trial at a time and repeating the analysis (leave-one-out analysis) for all outcomes. Given the small number of studies, meta-regression analysis was not done.
2.6. Role of the funding source
The study received no funding.
3. Results
We reviewed 564 articles for eligibility, 4 RCTs encompassing 7334 patients were selected (Fig. 1). There was some variation among RCTs with regards to designs and characteristics of participants (Table 1 ). A summary of baseline characteristics and comorbidities of the included patients are available in appendix (Appendix Table 4). Wang et al. [5] (double-blinded RCT) included patients with severe COVID-19 and showed a trend towards faster time to clinical improvement that did not reach statistical significance; however because this trial was stopped early (due to the decline in new cases in China), it may have been underpowered to detect an effect. The Adaptive COVID-19 Treatment Trial Part 1 (ACTT-1) by Beigel et al. [7] (double-blinded RCT) included 1062 participants with severe COVID-19 and demonstrated that remdesivir shortened the time to recovery compared to placebo in individuals with severe COVID-19. Spinner et al. [9] (open label RCT) included patients with moderate COVID-19 with oxygen saturation of more than 94% on room air. It compared 5-day remdesivir treatment to 10-day remdesivir treatment to control (standard care) and showed a significant difference in clinical status in patients using remdesivir but of uncertain clinical importance; however, it did not adjust or control for severity of disease and the use of other COVID-19 therapeutics. Pan et al. [8] (open label RCT) is the biggest trial and included COVID-19 patients with different severities, it compared 10-day treatment of remdesivir to control (standard care) and showed no mortality benefit of using remdesivir.
Table 1.
Characteristics of included trials.
| Wang [5] | Beigel [7] | Spinner [8] | Pan (Solidarity) [9] | |
|---|---|---|---|---|
| Centers | 10 sites in Wuhan, China | 60 sites and 13 subsites in the United States, Mexico, Europe, and Asia | 105 sites in the United States, Europe, and Asia | 405 sites in 30 countries (all over the world) |
| Number of patients | 236 | 1063 | 584 | 5451 |
| Enrollment initiation | February 2020 | February 2020 | March 2020 | March 2020 |
| Enrollment completion | March 2020 | April 2020 | April 2020 | October 2020 |
| Date of publication | April 2020 | May 2020 | August 2020 | November 2020 (preprint) |
| Population | Patients hospitalized with SARS-CoV-2 infection with an interval from symptom onset to enrolment of 12 days or less, and evidence of pneumonia | Patients hospitalized with SARS-CoV-2 infection with evidence of lower respiratory tract involvement | Patients hospitalized with severe SARS-CoV-2 infection and moderate COVID-19 pneumonia (pulmonary infiltrates and room-air oxygen saturation > 94%) | Patients hospitalized with SARS-CoV-2 infection |
| Trial type | Double-blind; multicenter; RCT | Double-blind; multicenter; RCT | Open-label; multicenter; RCT | Open-label; multicenter; RCT |
| Inclusion criteria |
|
|
|
|
| Exclusion criteria |
|
|
|
|
| Randomization sequence | SAS software, version 9.4 (Stratified) | Interactive web response system | Interactive web response system | Cloud-based GCP-compliant clinical data management system) |
| Treatments |
|
|
|
|
| Primary outcome | Time to clinical improvement (two-point reduction in patients' admission status on a six-point ordinal scale, or live discharge from the hospital, whichever came first) within 28 days after randomization | The time to recovery, defined by either discharge from the hospital or hospitalization for infection control purposes only | Clinical status assessed on the 7-point ordinal scale on study day 11 | All-cause mortality |
| Results of the primary outcome | Remdesivir was not superior to placebo in shortening time to clinical improvement | Remdesivir was superior to placebo in shortening the time to recovery | Remdesivir (5-day course) was superior to standard care in terms of care associated with higher odds of better clinical status | Remdesivir was not superior to standard care in reducing all-cause mortality |
| Follow up | 28 days | 29 days | 28 days | 28 days |
| Funding | National Key Research and Development Program of China | National Institute of Allergy and Infectious Diseases and others | Gilead Sciences | World health organization and participating countries |
ALT = Alanine aminotransferase; AST = Aspartate aminotransferase; COVID-19 = Coronavirus Disease 2019; PCR = Polymerase chain reaction; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; ULN=Upper limit of normal.
Overall, the proportion of enrolled women varied from 35 to 44%. The prevalence of hypertension ranged from 38% to 50%, and diabetes 21% to 44%. There were 24.3% patients with diabetes in the remdesivir group while there were 26.1% patients with diabetes in the control group. Systemic corticosteroids were administered to 40.4% of the patients in the remdesivir arm and to 42.3% of the patients in the control arm. (Appendix Table 4). Severity ordinal scale in this meta-analysis was designed to be compatible with the scales used in all included trials and has 6 categories: (1) Patients not requiring admission to the hospital (2) Admitted to hospital but not requiring supplemental oxygen (3) Admitted to hospital and requiring supplemental oxygen (4) Admitted to hospital and requiring high-flow nasal cannula or non-invasive mechanical ventilation (5) Admitted to hospital and requiring extracorporeal membrane oxygenation or invasive mechanical ventilation and (6) Death. The severity score of the included patients ranged from 2 to 5 at baseline; patients with severity score of two were 27.8% (remdesivir) vs. 25.4% (control), patients with severity score of three and four were 62.1% (remdesivir) vs. 63.5% (control), while those with severity score of five were 10.1% (remdesivir) vs. 11.1% (control). The number of events were abstracted from the included trials at a range of 28–29 days.
The GRADE certainty of evidence [14] ranged from very low to moderate as we found some issues applying indirectness (combining 5-day and 10-day treatments of remdesivir in Spinner et al. [9]), inconsistency, and imprecision (wide confidence interval) for the studied outcomes in our analysis (Appendix Tables 2 and 3).
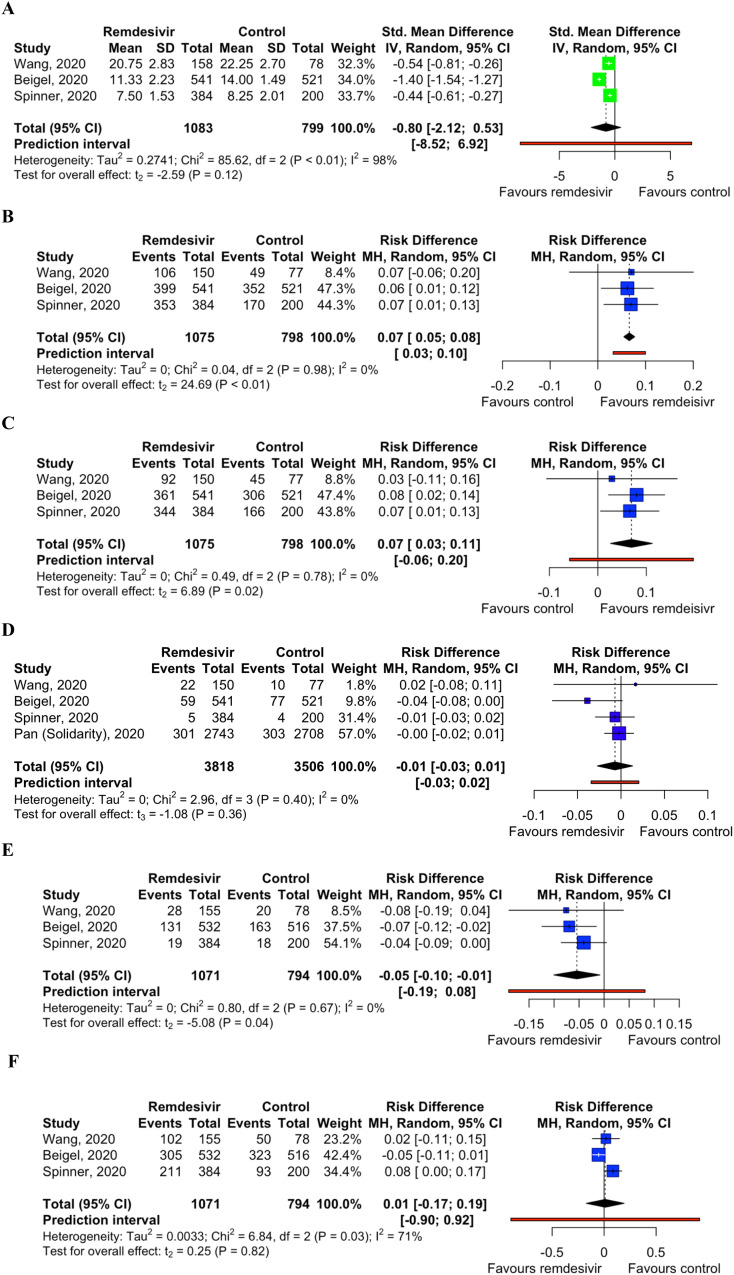
Very low certainty of evidence showed that the use of remdesivir was not associated with a statistically significant standardized mean difference (SMD) of time to clinical improvement (SMD -0.80 day; 95% CI -2.12 to 0.53; p = 0.12; I 2 = 98%). However, moderate certainty of evidence showed that remdesivir was associated with higher rates of recovered patient (RD 0.07; 95% CI 0.05 to 0.08; p < 0.01; I 2 = 0%); and discharged patients (RD 0.07; 95% CI 0.03 to 0.11; p = 0.02; I 2 = 0%) and lower rates of developing serious adverse events (RD -0.05; 95% CI -0.10 to −0.01; p = 0.04; I 2 = 0%) compared with control. Moderate certainty of evidence showed that there was no statistically significant difference in deaths (RD -0.01; 95% CI -0.03 to 0.01; p = 0.36; I 2 = 0%); and very low certainty of evidence showed that there was no statistically significant difference in developing any adverse events (RD 0.01; 95% CI -0.17 to 0.19; p = 0.82; I 2 = 71%) between both groups (Fig. 2 A to F).
Fig. 2.
A. Forest plot of mean time to clinical improvement. B. Forest plot of recoveries. C. Forest plot of discharges from hospital. D. Forest plot of deaths. E. Forest plot of any serious adverse events. F. Forest plot of any adverse events.
4. Sensitivity analysis (moderate COVID-19)
Sensitivity analysis limited to patients with moderate COVID-19 showed the following: very low certainty of evidence showed that use of remdesivir was not associated with statistically significant SMD of time to clinical improvement (SMD -0.36 day; 95% CI -1.90 to 1.18; p = 0.21; I 2 = 36%), moderate certainty of evidence showed no statistically significant decrease in rates of death (RD 0.00; 95% CI -0.01 to 0.01; p = 0.20; I 2 = 0%), but very low certainty of evidence showed statistically significant increase in recovery rates (RD 0.07; 95% CI 0.02 to 0.11; p = 0.03; I 2 = 0%) (Appendix Figs. 2-A to 2-C).
5. Sensitivity analysis (Leave-one-out analysis)
Leave-one-out analysis was done by excluding one trial a time and repeating the analysis. For all studied outcomes, excluding any of the included trials makes the results statistically non-significant except for the serious adverse events where excluding Spinner et al. [9] keeps the results significant due to lower number of adverse events reported in this trial (Appendix Table 5).
6. Discussion
Our meta-analysis showed that COVID-19 patients receiving remdesivir had significantly higher rates of recovery and hospital discharge with lower rates of developing serious adverse events compared to patients receiving standard of care/placebo. However, there were no significant differences in the remaining outcomes (standardized mean difference in time to clinical improvement, and rates of deaths or developing any adverse events). We did sensitivity analysis limited to patients with moderate COVID-19 from Spinner et al. [9] and a group of patients with moderate COVID-19 who were included in Beigel et al. [7] and Pan et al. [21] which showed that this category of patients are more likely to demonstrate recovery if they were given remdesivir without significant effect on mean time to clinical improvement. However, this analysis was with lower power and certainty of evidence than the main analysis due to lower number of included patients. Mortality was the only outcome that was reported in all included studies, and none of the studies showed significant decrease of mortality but were not adequately powered for mortality outcome.
In our analysis, we pooled the 5-day and 10-day treatment of remdesivir together, so our study cannot recommend certain duration of treatment. However, a head to head comparison between 5-day and 10-day remdesivir treatment was done in Goldman et al. [22] (excluded from our quantitative analysis as it didn't include a control arm) which showed no significant difference between both regimens, and in Spinner et al. [9] which showed significant difference favouring the 5-day regimen. Network meta-analysis comparing 5-day to 10-day remdesivir treatment to control/placebo group was not performed in our study as there are only two studies [9,22] comparing 5-day to 10-day remdesivir treatment in different population (Spinner et al. [9] included patients with moderate CVOID-10 infection and Goldman et al. [22] included patients with severe COVID-19 infection), and both were open label trials, and had high discontinuation rate of remdesivir in the 10-day treatment group of more than 50%. The Infectious Disease Society of America (IDSA) currently recommends a 5-day duration in patients on supplemental oxygen, and 10-day treatment for patients on mechanical ventilation or Extracorporeal Membrane Oxygenation (ECMO) [23].
Wang et al. [5] was the first double blinded RCT and randomized patients with an interval from symptoms onset to enrollment of 12 days or less; it had 158 patients in the remdesivir arm while 78 patients received placebo. The study was terminated early due to disease control in China and safety concerns as more adverse events were reported in the remdesivir compared to placebo arm (12% vs 5%). It indicated a possible trend towards clinical benefit in remdesivir group and hence necessitating larger sample size for confirmation. Corticosteroid was used in 65% of patients who received remdesivir and in 68% of patients in placebo arm which may have confounded the results.
Beigel et al. [7] (ACTT-1) randomized 1062 patients hospitalized with COVID-19 and evidence of lower respiratory tract infection to remdesivir or placebo. The study demonstrated that remdesivir was superior to placebo in shortening the time to recovery in hospitalized COVID-19 patients and odds of improvement (secondary endpoint) was also significantly favouring remdesivir over placebo. There was a trend towards survival benefit at day 29 but didn't reach to statistically significant point. The beneficial effect of remdesivir was mainly noticed in participants who were within the severe disease stratum but not requiring mechanical ventilation or ECMO at enrollment which may suggest starting remdesivir early in the disease course or it could be due to lower number of patients who were enrolled in this category that didn't reach to enough power to demonstrate significant effect. The primary outcome was changed after the trial was started from a comparison of the eight-category ordinal scale scores to the time to recovery. In our analysis, we didn't include the time to recovery (defined as either discharge from the hospital or hospitalization for infection-control purposes only) which was the primary outcome in this trial. Instead, we included time to clinical improvement (improvement of at least of 2 points on the severity ordinal scale) to be compatible and more homogenous with other included trials.
Spinner et al. trial [9] was open label trial, and was the only trial to include adolescents (at age of ≥12). This trial was designed for patients with moderate COVID-19 (no oxygen requirements) though around 15% of patients required oxygen at enrollment. It randomized 596 patients with COVID-19 and moderate pneumonia in 1:1:1 ratio to receive 10-day course of remdesivir, a 5-day course of remdesivir, or standard care infection. It showed that patients randomized to 5-day but not 10-day treatment duration had a statistically significant difference in clinical status. However, the difference was of uncertain clinical importance. A subgroup analysis excluding the patients who required oxygen at baseline still showed a significant difference favouring remdesivir over standard care. The 10-day treatment group in this study could be affected by the assigned duration of remdesivir treatment as rates of discharge peaked on the day after the end of dosing in both groups. The discontinuation rate was very high in the 10-day treatment arm (120 out of 197 and the median duration of treatment was 6 days). The primary end point was changed after starting the study from discharge rate to ordinal scale.
Pan et al. [21] (Solidarity trial) is the biggest trial (open label RCT) and was conducted by WHO in 30 countries. It didn't show a decrease of in-hospital mortality, although there was a trend of decreasing mortality in those not on a ventilator. The main limitation of this study is that there was no follow up after discharge and didn't report other outcomes like time to clinical improvement and adverse events.
In general, remdesivir has a fair safety profile. Our analysis showed lower serious adverse events among patients in the remdesivir arm and no difference in any adverse events rates. Wang et al. [5] and Spinner et al. [9] trials showed higher rates while Beigel et al. [7] showed lower rates of any adverse events among patients in the remdesivir group. All the trials that reported adverse events showed lower rates of serious adverse events among patients in the remdesivir arm. Hepatotoxicity is usually the main concern and was identified in Phase 1 trials (unknown mechanism) and were found to be related to the dose and duration of remdesivir treatment. It will be difficult to evaluate hepatotoxicity in COVID-19 as the disease itself can cause elevation in liver enzymes and no evidence of higher rates of hepatotoxicity was found among the included trials in our analysis. Nephrotoxicity was noticed in nonclinical studies but the included trials in our analysis didn't show evidence of renal toxicity in remdesivir group. The safety profile of remdesivir in pediatric and pregnant patients is still unclear, and further trials evaluating these population will help in understanding its safety [24].
The United States Food and Drug Administration (FDA) initially approved the use of remdesivir for the treatment of COVID-19 in light of the results of Beigel et al. [7], Spinner et al. [9], and Goldman et al. [22] trials. After publishing the results of Pan et al. [8] trial, the FDA updated their approval of using remdesivir for COVID-19 though it was the biggest trial and didn't show mortality benefit. This update was based on the fact that Beigel et al. [7] was better designed to evaluate a time to recovery endpoint compared to Pan et al. [8] trial that didn't evaluate this outcome [25].
Our study results are compatible with some results of observational studies: (1) Olender et al. [26] compared the remdesivir arms (5-day and 10-day treatment) used in the Goldman et al. study with an ongoing retrospective standard-care cohort study (GS-US-540-5807 study). This comparison showed significant higher recovery rates (74.4% vs 59.9%) and lower death rates (7.6% vs 12.5%) among remdesivir arm compared to standard care treatment in patients with severe COVID-19. (2) Lin et al. [27] (accessed as a preprint) included stratified risk states (low, moderate, and severe) of COVID-19 patients, and it showed statistically significant higher odds of discharge and lower risks of death among the remdesivir group in all risk states compared to standard care.
Our current study has several important strengths. Our meta-analysis is the first to evaluate multiple efficacy and safety outcomes including the time to clinical improvement which is the primary outcome in most of the included trials by pooling the results of all available RCTs. We used the Paule–Mandel method in our analysis, and we applied Hartung–Knapp/Sidik-Jonkman small-sample adjustments due to the small number of trials included in the current analysis which is consistent with general recommendations [28].
However, our findings should be considered in the context of several limitations. First, the heterogeneity of some of the outcomes we included were high which could be due to methodological heterogeneity of the included trials, the heterogeneity of the population, and the duration of remdesivir use. Second, one of the included trials was an open label trial and didn't use placebo, and one was terminated prematurely. Third, the mean time to clinical improvement was not provided in the included trials and was calculated from the median and ranges as recommended which assumes that the included population has normal distribution [15]. Fourth, this is a study level meta-analysis and a patient level meta-analysis in future would provide better evidence by better exploring the possible confounders of the results.
Further trials are evaluating remdesivir in inhaled form (NCT04539262 and NCT04480333), in combination with tocilizumab (NCT04409262), in combination with baricitinib (ACTT-2), in combination with intranasal interferon (ACTT-3) and in different populations such as pediatric patients (NCT04431453) and adult outpatients (NCT04501952). All these trials will help in evaluating the efficacy and safety of remdesivir in COVID-19 patients. The overall management of COVID-19 has improved as experience with the condition has been gained. The differences in the timing of the studies and implementation of newer management methodologies can influence the differences seen within the analyzed studies, the data obtained, and the ability to translate these findings to future clinical uses.
7. Conclusion
In conclusion, remdesivir use in patients with moderate or severe COVID-19 was associated with significant increase in rates of recovery and hospital discharge and lower rates of serious adverse events. However, there was no significant difference in mean time to clinical improvement and mortality. These results suggest the need of more RCTs to evaluate the role of remdesivir in COVID-19 patients.
Funding
The authors did not receive any specific funding for this work. Dr. Michos is partially supported by the (unrestricted) Blumenthal Scholars Preventive Cardiology Fund at Johns Hopkins.
Disclosures
None of the authors report any disclosures.
Author agreement statement
Authors declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We understand that the Corresponding Author is the sole contact for the Editorial process. He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cct.2021.106272.
Appendix A. Supplementary data
Supplementary material
References
- 1.Haleem A., Javaid M., Vaishya R. Effects of COVID-19 pandemic in daily life. Curr. Med. Res. Pract. 2020;10(2):78–79. doi: 10.1016/j.cmrp.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon C.J., Tchesnokov E.P., Woolner E., et al. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020;295(20):6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulangu S., Dodd L.E., Davey R.T.J., et al. A randomized, controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheahan T.P., Sims A.C., Graham R.L., et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9(396) doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Zhang D., Du G., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet (LondonEngland) 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williamson B.N., Feldmann F., Schwarz B., et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. bioRxiv Prepr. Serv. Biol. April 2020 doi: 10.1101/2020.04.15.043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19 — Final report. N. Engl. J. Med. May 2020 doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan H, Peto R, Henao-Restrepo A-M, et al. Repurposed antiviral drugs for Covid-19 - Interim WHO Solidarity Trial Results. N. Engl. J. Med. December 2020:NEJMoa2023184. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed]
- 9.Spinner C.D., Gottlieb R.L., Criner G.J., et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: A randomized clinical trial. JAMA. August 2020 doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 11.van Tulder M., Furlan A., Bombardier C., Bouter L. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine (Phila Pa 1976) 2003;28(12):1290–1299. doi: 10.1097/01.BRS.0000065484.95996.AF. [DOI] [PubMed] [Google Scholar]
- 12.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;l4898:366. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 13.Higgins J.P.T., Altman D.G., Gotzsche P.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;d5928:343. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyatt G., Oxman A.D., Akl E.A., et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 15.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barili F., Parolari A., Kappetein P.A., Freemantle N. Statistical primer: heterogeneity, random- or fixed-effects model analyses? Interact. Cardiovasc. Thorac. Surg. 2018;27(3):317–321. doi: 10.1093/icvts/ivy163. [DOI] [PubMed] [Google Scholar]
- 17.Veroniki A.A., Jackson D., Viechtbauer W., et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res. Synth. Methods. 2016;7(1):55–79. doi: 10.1002/jrsm.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noordzij M., van Diepen M., Caskey F.C., Jager K.J. Relative risk versus absolute risk: one cannot be interpreted without the other. Nephrol. Dial. Transplant. 2017;32(suppl_2):ii13–ii18. doi: 10.1093/ndt/gfw465. [DOI] [PubMed] [Google Scholar]
- 19.Deeks J. When can odds ratios mislead? Odds ratios should be used only in case-control studies and logistic regression analyses. BMJ. 1998;317(7166):1155–1157. doi: 10.1136/bmj.317.7166.1155a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner R.M., Davey J., Clarke M.J., Thompson S.G., Higgins J.P. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int. J. Epidemiol. 2012;41(3):818–827. doi: 10.1093/ije/dys041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan H., Peto R., Karim Q.A., et al. Repurposed antiviral drugs for COVID-19 –interim WHO SOLIDARITY trial results. N. Engl. J. Med. January 2020 doi: 10.1101/2020.10.15.20209817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldman J.D., Lye D.C.B., Hui D.S., et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N. Engl. J. Med. May 2020 doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Clin. Infect. Dis. doi: 10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed]
- 24.US Food and Drug Administration Remdesivir (Veklury) Approval for the Treatment of COVID-19. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/214787Orig1s000Sumr.pdf Combined Cross-Discipline Team Leader, Division Director, and ODE Director Summary Review. Accessed on December 26th, 2020.
- 25.US Food and Drug Administration Remdesivir (Veklury) Approval for the Treatment of COVID-19—The Evidence for Safety and Efficacy. 2020. https://www.fda.gov/drugs/news-events-human-drugs/remdesivir-veklury-approval-treatment-covid-19-evidence-safety-and-e (accessed 26.25.20)
- 26.Olender S.A., Perez K.K., Go A.S., et al. Remdesivir for severe COVID-19 versus a cohort receiving standard of care. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. July 2020 doi: 10.1093/cid/ciaa1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin T.-Y., Chang W.-J., Hsu C.-Y., et al. Impacts of remdesivir on dynamics and efficacy stratified by the severity of COVID-19: a simulated two-arm controlled study. medRxiv. January 2020 doi: 10.1101/2020.05.17.20104711. [DOI] [Google Scholar]
- 28.Knapp G., Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat. Med. 2003;22(17):2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material