We conducted a multicenter retrospective cohort study of metabolic dysfunction‐associated hepatocellular carcinoma. In this cohort, cirrhosis was not associated with poorer survival, while history of liver imaging was.

Abstract
Metabolic‐associated fatty liver disease (MAFLD) is a major cause of liver‐related complications, including hepatocellular carcinoma (HCC). While MAFLD‐related HCC is known to occur in the absence of cirrhosis, our understanding of MAFLD‐related HCC in this setting is limited. Here, we characterize MAFLD‐related HCC and the impact of cirrhosis and screening on survival. This was a multicenter, retrospective, cohort study of MAFLD‐related HCC. MAFLD was defined based on the presence of race‐adjusted overweight, diabetes, or both hypertension and dyslipidemia in the absence of excess alcohol use or other underlying cause of liver disease. The primary outcome of interest was overall survival, and the primary dependent variables were cirrhosis status and prior HCC screening. We used Kaplan‐Meier methods to estimate overall survival and Cox proportional hazards models and random forest machine learning to determine factors associated with prognosis. This study included 1,382 patients from 11 centers in the United States and East/Southeast Asia. Cirrhosis was present in 62% of patients, but under half of these patients had undergone imaging within 12 months of HCC diagnosis. Patients with cirrhosis were more likely to have early stage disease but less often received curative therapy. After adjustment, cirrhosis was not associated with prognosis, but the presence of cancer‐related symptoms at diagnosis was associated with poorer prognosis. Conclusion: Cirrhosis was not associated with overall survival in this cohort of MAFLD‐related HCC, while diagnosis in the presence of symptoms was associated with poorer prognosis. The HCC surveillance rate in patients with MAFLD‐related HCC was disappointingly low in a multicenter cohort.
Abbreviations
- ALBI
albumin‐bilirubin
- BCLC
Barcelona Clinic Liver Cancer
- CI
confidence interval
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- MAFLD
metabolic‐associated fatty liver disease
- MELD
Model for End‐Stage Liver Disease
- NFS
nonalcoholic fatty liver disease fibrosis score
Hepatocellular carcinoma (HCC) is the third most common cause of cancer death worldwide.( 1 , 2 ) The most rapidly growing cause of HCC in developed countries is nonalcoholic fatty liver disease or metabolic‐associated fatty liver disease (MAFLD),( 3 , 4 ) which is projected to become a leading cause of cirrhosis and HCC.( 5 , 6 , 7 , 8 , 9 , 10 ) However, MAFLD‐related HCC has been relatively poorly characterized. MAFLD differs from other common causes of HCC, such as chronic viral hepatitis and heavy alcohol use, in that there are no simple, highly effective therapies directed against MAFLD. There is also no simple consistent way to diagnose MAFLD in patients with cirrhosis and HCC because MAFLD may have “burned out,” with hepatic steatosis no longer evident in advanced stage. Patients with MAFLD frequently have cardiovascular and renal comorbidities, which may preclude cancer‐directed therapy or increase the risk of complications following therapy.( 11 , 12 , 13 ) How these factors affect the natural history of MAFLD‐related HCC compared to other etiologies of HCC is not clear. In addition, disease awareness of MAFLD among affected persons is extremely poor, estimated to be about 5% based on data from a nationally representative sample of Americans from 2011 to 2016.( 14 ) Disease recognition is low even among patients with MAFLD‐related cirrhosis, and this lack of awareness is associated with presentation with advanced‐stage HCC.( 15 )
Similar to chronic hepatitis B and unlike most other causes of liver disease, MAFLD‐related HCC can arise in the absence of cirrhosis.( 16 , 17 ) In the case of chronic hepatitis B‐related HCC, cirrhosis is associated with poorer prognosis.( 18 , 19 ) The effect of cirrhosis on MAFLD‐related HCC has been investigated only in small cohorts and primarily in Caucasian populations.( 17 , 20 , 21 , 22 ) Unlike chronic hepatitis B, current practice guidelines do not recommend routine HCC surveillance for noncirrhotic MAFLD because the incidence of HCC in this population is too low to justify surveillance.( 23 , 24 , 25 , 26 , 27 , 28 , 29 ) While diagnosis by screening/surveillance rather than when symptoms develop has been associated with improved prognosis in other causes of liver disease and cirrhosis overall,( 18 , 30 ) it is not known whether this is true in patients with MAFLD‐related cirrhosis. A clearer understanding of the effects of screening history or diagnosis under surveillance versus by symptoms in MAFLD‐related cirrhosis may help inform screening guidelines.
The goal of this study was to characterize a large diverse cohort of MAFLD‐related HCC, focusing on the effects of cirrhosis and surveillance on prognosis, and to create a survival model.
Patients and Methods
Study Design and Patient Population
We performed an international, multicenter, retrospective, cohort study of MAFLD‐related HCC at 11 medical centers. Details of the centers are shown in Supporting Table S1. The study period was from 2005 to 2017. Inclusion criteria were MAFLD and a first diagnosis of HCC, as verified by chart review. As recommended by a recent international consensus forum,( 3 , 4 ) MAFLD was diagnosed based on any of the following: diabetes, race‐adjusted overweight (body mass index ≥23 for Asians and ≥25 for non‐Asians), or both dyslipidemia and hypertension. We also excluded participants with other etiologies of liver disease, such as viral hepatitis, excessive alcohol use, and autoimmune diseases as we have already reported findings for patients with liver diseases of other etiologies. HCC was diagnosed based on 2010 American Association for the Study of Liver Diseases criteria.( 31 ) Presence of steatosis was not a requirement as it is often not present by the time patients develop cirrhosis and HCC. Patients with prior HCC or liver transplant were excluded.
This study was approved by the Institutional Review Board at Stanford University (Stanford, CA) and at each of the other participating centers.
Definition of Cirrhosis and Screening
Laboratory values, imaging findings, and HCC treatments were obtained from individual medical records. Patients were designated as having cirrhosis based on histologic or noninvasive elastographic methods demonstrating fibrosis stage 4, clinical portal hypertension (i.e., otherwise unexplained splenomegaly or platelet count <120,000/μL, ascites, or varices on imaging/endoscopy), prior hepatic decompensation (hepatic encephalopathy, ascites, variceal gastrointestinal bleeding), or decreased synthetic function (total bilirubin >2.0 mg/dL or international normalized ratio >1.2 without alternative explanation).
Screening was estimated using two methods. First, we assessed whether patients had had liver imaging within the year before HCC diagnosis; it could not be reliably determined whether these imaging studies were obtained for screening purposes or other indications. Second, we assessed the scenario in which patients were diagnosed: without symptoms (implying that diagnosis was made based on screening) versus with symptoms of HCC, such as new‐onset or worsening hepatic decompensation, abdominal pain, or weight loss at the time of HCC diagnosis.
Tumor Staging and Survival Outcomes
Tumor characteristics were determined by multiphase contrast‐enhanced computed tomography or magnetic resonance imaging. Patients were followed from the date of diagnosis with HCC and either date of death or last follow‐up. For U.S. sites, the medical record was supplemented by a National Death Index registry search up to March 19, 2019. The National Death Index registry is a centralized database of death record information on file in state vital statistics offices; it has over 90% completion for most states and 99% for the state of California where the Stanford cohort is located.( 32 )
Statistical Analysis
Descriptive statistics were reported as percentage for categorical variables and mean ± SD or median (interquartile range) for continuous variables. Normally distributed continuous variables were compared by the Student t test, and Wilcoxon rank‐sum statistics were applied when continuous variables were not normally distributed. The chi square test was used to compare categorical variables.
For time‐to‐event analyses, the primary outcome was overall survival. Follow‐up was calculated for each patient as the time from dates of HCC diagnosis to the date of death or to the date when patients were last known to be alive. Mortality rates by various disease states were calculated and expressed per 100 person‐years. The Kaplan‐Meier method was used to estimate overall survival of patients; those lost to follow‐up were censored. Differences in overall survival among subgroups were compared using the log‐rank test. A Cox proportional hazards model was developed to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) relating cirrhosis, imaging history, and other risk factors with overall survival. For the multivariable model, we used the albumin‐bilirubin (ALBI) score as a measurement for liver function and Milan criteria for tumor staging instead of the Child‐Pugh Model for End‐Stage Liver Disease (MELD) score or Barcelona Clinic Liver Cancer (BCLC) stage because we had the most complete data for the ALBI score and Milan criteria. We conducted lead‐time analyses, as reported, using an estimated sojourn of 6 months.( 30 , 33 )
Statistical significance was defined as two‐tailed P < 0.05. All statistical analyses were performed using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria; www.r‐project.org) with the tidyverse package (www.tidyverse.org).
In addition, we used machine learning to determine factors associated with prognosis. The dependent variable was overall survival based on a rank‐based method to account for censored data.( 34 ) Values that were missing in over half of patients were removed; following this step, missing values were imputed using the “mice” package with five imputations and a maximum of 50 iterations.( 35 ) The machine learning algorithm used was a random forest model with tune length 5 with the “party” package version 1.3.3.( 36 ) We used the Stanford University Medical Center, Ogaki Municipal Hospital, and National University Health System cohorts as validation cohorts because together they provided a balance of U.S. and Asian patients and constituted approximately 27% of the overall cohort size. All other centers’ data were used as the training cohort. The model was trained using 10‐fold cross‐validation on the training cohort and then applied to the validation cohort. We chose the top three nonredundant contributors to survival and added them into a risk score based on their relative importance.
Results
Overall Characteristics and Treatment
In total, 1,382 patients with MAFLD‐related HCC met study inclusion criteria and were included in this study. Overall characteristics for the cohort are shown in Table 1. The cohort was 68% men, 46% Asian, 34% Caucasian, and 7% Hispanic, with a mean age of 67 years. Cirrhosis was present in 62% of patients. There was no difference in age or sex between patients with or without cirrhosis, but patients with cirrhosis were more frequently Caucasian or Hispanic and less frequently Asian, more frequently had diabetes, and were less frequently obese. Having undergone abdominal imaging within the year before HCC diagnosis was more common among patients with cirrhosis (30% vs. 16%). Compared to patients without cirrhosis, patients with cirrhosis had smaller maximal tumor size (median 3.3 vs. 5.7 cm; P < 0.001); they also more frequently had early stage (BCLC 0 or A) disease (43% vs. 29%) and less frequently had intermediate‐stage (BCLC B) disease (27% vs. 46%; P < 0.001 for both) (Table 2). Despite this, patients with cirrhosis less often received potentially curative therapy (i.e., resection, ablation, or liver transplant) and more often received liver‐directed therapy with radiation or transarterial therapy (Table 2).
TABLE 1.
Demographic, Clinical, and Laboratory Characteristics Based on Cirrhosis Status
| Trait | Overall | No Cirrhosis | Cirrhosis | P Value |
|---|---|---|---|---|
| N = 1,382 | n = 470 | n = 770 | ||
| Age (n = 1,030) | 67.0 ± 10.9 | 67.0 ± 11.7 | 66.9 ± 10.5 | 0.97 |
| % male | 68.4% | 71.3% | 65.70% | 0.045 |
| Ethnicity | ||||
| Asian | 46.2% | 61.1% | 44.3% | <0.001 |
| Living in Asia | 42.1% | 56.4% | 40.0% | <0.001 |
| Living in United States | 4.1% | 4.7% | 4.3% | 0.78 |
| Caucasian | 33.9% | 28.5% | 35.1% | 0.018 |
| Hispanic | 7.0% | 4.2% | 10.1% | <0.001 |
| African American | 0.8% | 0.9% | 0.7% | 0.74 |
| Other | 3.0% | 2.8% | 3.4% | 0.62 |
| Screening (n = 313) | 25.0% | 15.8% | 30.2% | 0.012 |
| Mean Child‐Pugh‐Turcotte score | N/A | N/A | 7.0 (6.0, 8.0) | N/A |
| Comorbidities | ||||
| Diabetes mellitus (n = 948) | 60.7% | 49.0% | 68.3% | <0.001 |
| Hypertension (n = 899) | 60.4% | 58.4% | 61.3% | 0.35 |
| Dyslipidemia (n = 403) | 44.9% | 55.0% | 41.0% | 0.009 |
| Coronary artery disease (n = 830) | 24.2% | 27.1% | 22.2% | 0.096 |
| Chronic obstructive pulmonary disease (n = 620) | 2.7% | 4.0% | 2.2% | 0.16 |
| Chronic kidney disease (n = 488) | 10.6% | 12.5% | 9.5% | 0.26 |
| Myocardial infarction (n = 210) | 10.7% | 11.2% | 10.5% | 0.83 |
| Congestive heart failure (n = 211) | 9.4% | 10.0% | 9.2% | 0.82 |
| Cerebrovascular disease (n = 208) | 9.1% | 5.7% | 11.2% | 0.17 |
| Obesity (n = 720) | 54.0% | 56.7% | 51.3% | 0.17 |
| Laboratory values | ||||
| White blood cell count (K/µL) (n = 773) | 6.1 (4.6, 8.1) | 6.9 (5.5, 8.6) | 5.5 (4.1, 7.3) | <0.001 |
| Hemoglobin (g/dL) (n = 752) | 12.8 (11.1, 14.0) | 13.2 (11.6, 14.4) | 12.4 (10.7, 13.8) | <0.001 |
| Platelet count (K/µL) (n = 778) | 160.5 (109.0, 227.8) | 207.5 (154.0, 268.0) | 121.0 (82.5, 187.0) | <0.001 |
| Creatinine (mg/dL) (n = 750) | 0.9 (0.8, 1.2) | 0.9 (0.8, 1.2) | 1.0 (0.8, 1.2) | 0.59 |
| Sodium (mEq/L) (n = 371) | 139.0 (136.0, 141.0) | 139.0 (136.0, 140.5) | 139.0 (136.0, 141.0) | 0.84 |
| International normalized ratio (n = 731) | 1.1 (1.0, 1.2) | 1.0 (1.0, 1.1) | 1.1 (1.0, 1.3) | <0.001 |
| Aspartate aminotransferase (U/L) (n = 747) | 50.0 (35.0, 82.0) | 44.0 (31.0, 81.0) | 54.0 (39.0, 82.0) | <0.001 |
| Alanine aminotransferase (U/L) (n = 832) | 39.0 (26.0, 60.0) | 39.0 (26.0, 63.0) | 39.0 (27.0, 58.8) | 0.71 |
| Total bilirubin (mg/dL) (n = 853) | 0.9 (0.6, 1.5) | 0.7 (0.5, 1.0) | 1.1 (0.7, 2.0) | <0.001 |
| Alkaline phosphatase (U/L) (n = 650) | 123.5 (85.0, 207.2) | 110.0 (74.0, 190.0) | 130.5 (90.0, 211.0) | 0.002 |
| ALBI index (n = 678) | 2.0 (1.0, 2.0) | 2.0 (1.0, 2.0) | 2.0 (2.0, 2.0) | <0.001 |
| MELD‐sodium score (n = 681) | 9.2 (7.5, 12.3) | 7.6 (6.5, 10.0) | 10.2 (8.2, 13.7) | <0.001 |
Data are depicted as mean ± SD, median (interquartile range), or percentages.
TABLE 2.
Tumor Characteristics Based on Cirrhosis Status
| Trait | Overall | No Cirrhosis | Cirrhosis | P Value |
|---|---|---|---|---|
| N = 1,382 | n = 470 | n = 770 | ||
| Alpha‐fetoprotein (ng/dL) (n = 762) | 16.0 (4.4, 404.1) | 24.5 (4.2, 478.7) | 13.4 (4.5, 374.0) | 0.64 |
| Maximum tumor size (cm) (n = 917) | 4.0 (2.3, 7.6) | 5.7 (3.5, 9.8) | 3.3 (2.1, 6.0) | <0.001 |
| Tumor number (n = 922) | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | 0.01 |
| Unifocal cancer (n = 922) | 68.6% | 74.4% | 66.3% | 0.005 |
| Within Milan criteria (n = 859) | 52.5% | 55.4% | 53.1% | 0.46 |
| BCLC stage (n = 643) | ||||
| 0 | 6.2% | 5.4% | 6.0% | |
| A | 33.1% | 23.5% | 37.1% | <0.001 |
| B | 32.5% | 46.2% | 26.7% | |
| C | 21.5% | 24.2% | 19.8% | |
| D | 6.8% | 0.7% | 10.4% | |
| Treatment | ||||
| Any cancer treatment | 71.2% | 76.0% | 70.5% | 0.042 |
| Resection | 24.6% | 39.4% | 14.7% | <0.001 |
| Liver transplant | 4.1% | 0.0% | 6.9% | <0.001 |
| Ablation | 8.8% | 3.4% | 12.6% | <0.001 |
| Transarterial/radiation therapy | 43.2% | 35.4% | 50.3% | <0.001 |
| Systemic | 6.4% | 7.0% | 7.3% | 0.91 |
| Supportive care only | 28.8% | 24.% | 29.5% | 0.042 |
Data are depicted as mean ± SD, median (interquartile range), or percentages. Some patients underwent multiple treatments.
We estimated the effect of screening in two ways. First, we assessed the effect of abdominal imaging (ultrasound or contrast‐enhanced computed tomography or magnetic resonance imaging) within the year before HCC diagnosis; data were available in 23% of patients (n = 313; Supporting Table S2). Second, we investigated the scenario in which HCC was diagnosed, this being asymptomatic (implying that HCC was diagnosed by screening) versus symptomatic, including new/worsening hepatic decompensation or abdominal pain at time of diagnosis (Supporting Table S3). Data on diagnosis scenario were available in 51% of patients (n = 706). With both the comparisons of imaging versus no imaging and symptoms versus no symptoms, there were no differences in age, sex, or presence of diabetes or obesity. Patients with recent imaging or with no symptoms had smaller and/or fewer tumors, more frequently had early stage disease based on BCLC stage, and were more likely to receive curative therapy.
Survival
We next turned our attention to factors associated with overall survival. Unadjusted mortality in multiple subgroups are shown in Table 3. Cirrhosis did not affect overall survival (HR, 1.14; 95% CI, 0.94‐1.37) (Table 4; Fig. 1A). Other factors associated with poorer survival included greater age, higher MELD score, Child‐Pugh score, and BCLC stage; being within Milan criteria, receiving curative therapy, Asian race, and more recent period of diagnosis (2008 and later) were associated with better prognosis (Table 4). History of abdominal imaging within a year of HCC diagnosis was associated with improved prognosis (HR, 0.55; 95% CI, 0.37‐0.82), whereas symptoms at HCC diagnosis were associated with poorer outcomes (HR, 2.16; 95% CI, 1.68‐2.78) (Table 4; Fig. 1B,C). After lead‐time adjustment with an estimated sojourn of 6 months, the association between symptoms and mortality remained significant (HR, 1.66; 95% CI, 1.28‐2.13; P < 0.001) while that between history of imaging and mortality did not (HR, 0.70; 95% CI, 0.47‐1.03; P = 0.07).
TABLE 3.
Mortality in Selected Subgroups
| Group | Total Number | Deaths | Person‐Years of Follow‐Up | Mortality per 100 Person‐Years |
|---|---|---|---|---|
| Overall | ||||
| All patients | 1,382 | 588 | 1,932.5 | 30.4 |
| No cirrhosis | 470 | 184 | 639.5 | 28.8 |
| Cirrhosis (all) | 770 | 347 | 1,071.3 | 32.4 |
| Cirrhosis (Child‐Pugh A) | 234 | 100 | 418 | 23.9 |
| Cirrhosis (Child‐Pugh B) | 247 | 111 | 225.3 | 49.3 |
| Cirrhosis (Child‐Pugh C) | 49 | 33 | 18.6 | 177.4 |
| BCLC stage | ||||
| 0/A | 329 | 97 | 586.6 | 16.5 |
| B | 266 | 121 | 319.7 | 37.8 |
| C/D | 225 | 147 | 148.9 | 98.7 |
| ALBI index | ||||
| 1 | 341 | 107 | 574.6 | 18.6 |
| 2 | 574 | 270 | 662.3 | 40.8 |
| 3 | 118 | 72 | 82.9 | 86.9 |
| Treatment type | ||||
| Resection | 340 | 99 | 650.4 | 15.2 |
| Liver transplant | 56 | 10 | 194.3 | 5.1 |
| Ablation | 121 | 42 | 249.2 | 16.9 |
| Transarterial/radiation therapy | 596 | 270 | 909.4 | 29.7 |
| Systemic | 89 | 53 | 106.4 | 49.8 |
| Supportive care only | 398 | 204 | 322.5 | 63.3 |
TABLE 4.
Factors Associated With Mortality
| Trait | Unadjusted HR (95% CI) | P Value | Adjusted HR (95% CI) n = 220 | P Value |
|---|---|---|---|---|
| Age | 1.02 (1.01‐1.03) | <0.001 | 1.02 (1.01‐1.03) | <0.001 |
| Male sex | 1.02 (0.85‐1.23) | 0.84 | ||
| Race | ||||
| Non‐Asian | 1 (referent) | 1 (referent) | ||
| Asian | 0.70 (0.59‐0.83) | <0.001 | 0.87 (0.68‐1.11) | 0.25 |
| Hypertension | 1.49 (1.24‐1.81) | <0.001 | 1.05 (0.81‐1.36) | 0.7 |
| Dyslipidemia | 1.19 (0.86‐1.64) | 0.29 | ||
| Diabetes | 1.22 (1.01‐1.46) | 0.035 | 0.89 (0.7‐1.12) | 0.31 |
| Myocardial infarction | 1.27 (0.68‐2.34) | 0.45 | ||
| Imaging <12 months before diagnosis | 0.55 (0.37‐0.82) | 0.003 | ||
| Symptoms at diagnosis | 2.16 (1.68‐2.78) | <0.001 | ||
| Period of diagnosis | ||||
| 2007 and earlier | 1 (referent) | 1 (referent) | ||
| 2008 and later | 0.74 (0.62‐0.89) | 0.001 | 0.75 (0.6‐0.95) | 0.018 |
| Cirrhosis | 1.14 (0.94‐1.37) | 0.18 | 1.08 (0.85‐1.39) | 0.52 |
| Child‐Pugh score (per point) | 1.42 (1.34‐1.50) | <0.001 | ||
| MELD (per point) | 1.04 (1.03‐1.05) | <0.001 | ||
| ALBI index | ||||
| 1 | 1 (referent) | 1 (referent) | ||
| 2 | 2.05 (1.62‐2.60) | <0.001 | 1.79 (1.36‐2.35) | <0.001 |
| 3 | 4.28 (3.12‐5.89) | <0.001 | 3.96 (2.71‐5.79) | <0.001 |
| BCLC stage | ||||
| 0/A | 1 (referent) | |||
| B | 2.15 (1.64‐2.80) | <0.001 | ||
| C/D | 5.05 (3.88‐6.56) | <0.001 | ||
| Within Milan criteria | 0.34 (0.28‐0.41) | <0.001 | 0.34 (0.27‐0.43) | <0.001 |
| Curative therapy | 0.31 (0.25‐0.38) | <0.001 |
FIG. 1.
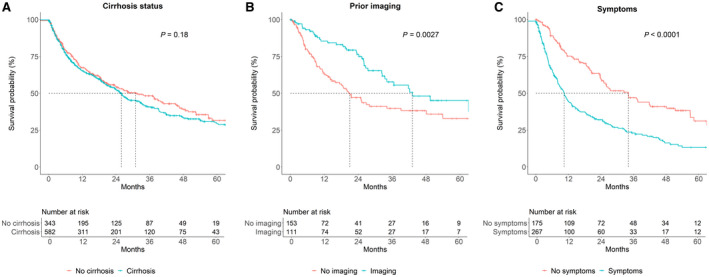
Overall survival. Stratified by (A) cirrhosis status, (B) history of imaging before HCC diagnosis, and (C) presence of symptoms at HCC diagnosis.
We stratified by cirrhosis status and either receipt of imaging in the year before HCC diagnosis or symptoms. Among patients both with and without cirrhosis, there was an insignificant trend toward improved survival based on screening history (Supporting Fig. S1A,B), whereas presence of symptoms was associated with poorer survival in both groups (Supporting Fig. S2A,B). Patients with symptoms and without cirrhosis had poorer survival than those with symptoms and with cirrhosis (Supporting Figs. S1D, S2D); there was no difference in survival based on cirrhosis status based on having undergone screening/surveillance or among asymptomatic patients.
We constructed a multivariate Cox proportional hazards model for overall survival that included cirrhosis. The model was adjusted for factors associated with overall survival on univariate analysis at P < 0.10. To avoid collinearity with ALBI score and Milan criteria, Child‐Pugh and MELD scores, BCLC stage, and treatment type were not included in the multivariable model. On this analysis, cirrhosis was not associated with survival.
When presence of symptoms at HCC diagnosis was added to the model, symptoms were associated with poorer overall survival (HR, 1.82; 95% CI, 1.30‐2.53; P < 0.001; Supporting Table S4). There was a trend toward an association between prior imaging and improved prognosis (HR, 0.61; 95% CI, 0.38‐1.00; P = 0.051; Supporting Table S5). However, many patients had missing data on both symptoms and prior imaging.
We conducted a sensitivity analysis where we used the nonalcoholic fatty liver disease fibrosis score (NFS) to determine fibrosis based on cutoff >0.675 indicating advanced fibrosis/cirrhosis (fibrosis stage 3‐4), <−1.455 indicating no significant fibrosis (stage 0‐2), and a score from −1.455 to 0.675 as indeterminate. NFS >0.675 was not associated with increased mortality relative to NFS <−1.455 (HR, 0.84; 95% CI, 0.61‐1.15) or relative to NFS ≤0.675 (HR, 1.21; 95% CI, 0.97‐1.52).
Machine Learning
Finally, we constructed a random forest machine learning model to identify prognostic factors in an attempt to create a risk score for survival in MAFLD‐related HCC. The factors most strongly associated with rank‐based survival were treatment type, Child‐Pugh score, and maximal tumor size (Supporting Fig. S3). Based on these results, we created a score that allocated up to 3 points based on therapy type, 1 point for maximal tumor size <4.3 cm (median tumor size in the derivation cohort), and 1 point for Child‐Pugh score <6 (median score in the derivation cohort) (Fig. 2A). This score predicted three distinct risk groups in both the training and validation cohorts (Fig. 2B,C). Notably, in a Cox proportional hazards model incorporating treatment type, tumor size, and Child‐Pugh score, variance inflation factors were low (<2 for all), indicating no meaningful multicollinearity.
FIG. 2.
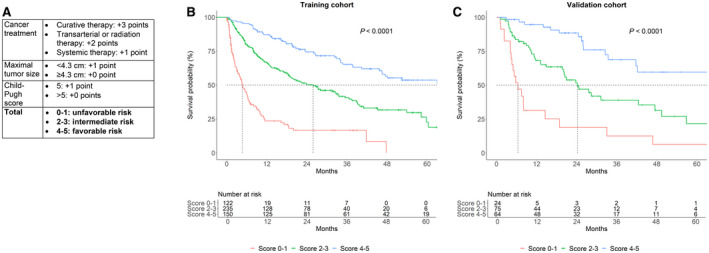
Risk score for cirrhosis survival. (A) Score definition. Treatment types were hierarchical, with curative therapy superseding transarterial/radiation therapy, which superseded systemic therapy. (B,C) Outcomes based on score (0‐1, 2‐3, or 4‐5) in the (B) derivation and (C) validation cohorts. The validation cohort included all patients from Stanford University Medical Center, National University Hospital of Singapore, and Ogaki Municipal Hospital; the derivation cohort included patients from all other centers.
Discussion
We have comprehensively characterized a large and diverse international cohort of patients with MAFLD HCC, focusing on the effect of cirrhosis on survival outcomes. Unlike chronic hepatitis B, which is also strongly associated with noncirrhotic HCC, there was no significant difference in survival of patients with MAFLD HCC with cirrhosis versus those without cirrhosis. The reason for this may be due to the lower likelihood of HCC surveillance in patients with MAFLD without known cirrhosis because HCC screening guidelines do not include this population. Indeed, in a sensitivity analysis stratifying by the presence of imaging in the year before HCC diagnosis or the presence of symptoms (two factors used as surrogates for HCC screening), patients without HCC screening and without cirrhosis actually had poorer survival than those without screening and with cirrhosis.
It is not clear why survival in MAFLD‐related HCC is unaffected by cirrhosis status, which is different than what has been reported in chronic hepatitis B‐related HCC,( 19 , 37 ) although consistent with the limited earlier literature in MAFLD‐related HCC.( 17 , 20 , 21 , 22 ) One potential explanation is that the superior liver function in patients without cirrhosis was offset by the later presentation due to a decrease in screening; however, cirrhosis status was not associated with mortality after adjustment for meeting Milan criteria (Table 4). While the competing risk of cardiovascular disease may be greater in patients with MAFLD, we did not see an association between history of diabetes or myocardial infarction and overall survival, which would argue against this hypothesis (Table 4). Another possibility is that risk of recurrent HCC is lower in MAFLD‐related cirrhosis than in other causes of cirrhosis; the rate of HCC recurrence in MAFLD following curative therapy has not been well characterized.( 23 ) Finally, misclassification of cirrhosis status may be more frequent in MAFLD‐related HCC than in other causes of HCC; for instance, noninvasive scores and transient elastography may have lower sensitivity in excluding advanced fibrosis than in other etiologies of cirrhosis.( 38 ) In a sensitivity analysis, advanced fibrosis as determined by NFS was not associated with mortality. Further research will be required to better assess reasons for the differences observed between viral and MAFLD‐related HCC based on cirrhosis status.
Given this study design, we are unable to directly address the question of whether patients with MAFLD without cirrhosis should undergo HCC surveillance. While the risk of HCC in noncirrhotic MAFLD remains incompletely characterized due to difficulties in diagnosing MAFLD at the population level, it is virtually certain to be below the threshold of 0.2%/year that is usually recommended to justify surveillance in the absence of cirrhosis.( 23 , 26 ) There may be subsets of patients with noncirrhotic MAFLD in which the HCC incidence is high enough to warrant surveillance, for example, presence of the patatin‐like phospholipase domain containing 3 isoleucine to methionine substitution at position 148 (PNPLA3 I148M) variant,( 39 , 40 , 41 ) but at this point the evidence for this or any other purported predisposing factor is insufficient. In addition, HCC surveillance can lead to unnecessary follow‐up cross‐sectional imaging, invasive biopsies, and patient anxiety.( 42 ) Further prospective studies on the role of screening in MAFLD will be required. If a high‐risk population of patients with noncirrhotic MAFLD could be identified, our data suggest that screening will result in superior outcomes because (with the absence of routine surveillance) our patients with MAFLD HCC without cirrhosis tended to have larger tumors and despite better liver function were no more likely to receive cancer‐directed therapy than those with cirrhosis, presumably due to their more advanced stage.
Notably, HCC screening rates were low in this cohort; even in patients with cirrhosis, only 30% underwent imaging in the year before HCC diagnosis. In comparison, previous studies in cirrhosis of mixed etiology showed roughly 50% adherence to surveillance,( 43 ) although this varies greatly across practice settings and depending on definitions of surveillance adherence.( 33 ) Our study is based on data from academic medical centers, and it may be that with no effective treatment for MAFLD, patients with MAFLD may be less likely to be followed in a liver disease practice. Limited patient awareness of MAFLD may also lead to poor linkage to care at both the primary care and referral care levels.( 14 )
Limitations of this study include its retrospective nature; in particular, it is not possible to reliably determine indications for imaging procedures and why surveillance was or was not performed. Also, biopsy was not performed in the majority of patients, so we could not distinguish between simple steatosis and nonalcoholic steatohepatitis, which may be especially relevant in patients without cirrhosis. We also lack sufficient data for additional factors, such as statin use, whose effects are often dose and duration dependent, or markers to assess for the severity of metabolic syndrome over a long period of time.( 44 , 45 ) Strengths of this study include that it was large and included patients from several different countries and diverse ethnicities. In addition, the outcome of overall survival is objective, and definitions of the major outcomes and predictors were standardized across the centers.
In conclusion, we found that history of liver imaging was associated with improved prognosis in patients with MAFLD‐related HCC but that cirrhosis had no significant effect on survival. Further studies will be required to more clearly identify which patients with noncirrhotic MAFLD, if any, may benefit from HCC surveillance. Additional efforts are also needed to improve adherence to HCC surveillance in patients with MAFLD cirrhosis.
Supporting information
Supplementary Material
VLC was supported by the University of Michigan Training in Basic and Translational Digestive Sciences (grant 5T32DK094775), National Natural Science Foundation of China (grant 81770607 to Q.Z.), Agency for Science, Technology, and Research Biomedical Research Council (grant H17/01/a0/003 to Y.Y.D.), National Center Institute (grant P50CA210964 to L.R.R.), and Kaohsiung Medical University Research Center Grant Center for Cancer Research (grant KMU‐TC108A04 to M‐L.Y.).
Potential conflict of interest: Dr. Dai consults, advises, and is on the speakers’ bureau for AbbVie; he advises and is on the speakers’ bureau for Gilead and is on the speakers’ bureau for MSD. Dr. Hsu advises, is on the speakers’ bureau for, and received grants from Gilead; he is on the speakers’ bureau for AbbVie and BMS. Dr. Huang received grants from Exxon Mobile. Dr. Leong received grants from Gilead. Dr. Nguyen consults for and received grants from Pfizer, Glycotest, Enanta, Gilead, and Vir. Dr. Roberts consults for AstraZeneca Pharmaceuticals; he advises and received grants from Bayer, Exact Sciences, and Gilead; he advises GRAIL Inc., QED Therapeutics Inc., and TAVEC and received grants from BTG International, Glycotest, RedHill Biopharma, Target PharmaSolutions, and Wako Diagnostics. Dr. Toyoda is on the speakers’ bureau for AbbVie, MSD, and Bayer. Dr. Yang consults for Exact Sciences. Dr. Dan advises and received grants from Novartis; he advises Boeringher Ingelheim and received grants from Novo Nordisk. Dr. Yu consults for, advises, is on the speakers’ bureau for, and received grants from AbbVie, Abbott, BMS, Gilead, and Merck; he consults for, advises, and received grants from Ascletis and Roche. The other authors have nothing to report.
References
- 1. American Cancer Society . Global Cancer Facts & Figures, 2nd edn. https://www.cancer.org/research/cancer‐facts‐statistics/global.html. Published 2011. [Google Scholar]
- 2. Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J Hepatol 2020;72:250‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eslam M, Sanyal AJ, George J. MAFLD: a consensus‐driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020;158:1999‐2014.e1. [DOI] [PubMed] [Google Scholar]
- 4. Eslam M, Newsome PN, Anstee QM, Targher G, Gomez MR, Zelber‐Sagi S, et al. A new definition for metabolic dysfunction‐associated fatty liver disease: an international expert consensus statement. J Hepatol 2020;73:202‐209. [DOI] [PubMed] [Google Scholar]
- 5. Valery PC, Laversanne M, Clark PJ, Petrick JL, McGlynn KA, Bray F. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology 2018;67:600‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014;59:2188‐2195. [DOI] [PubMed] [Google Scholar]
- 7. Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015;62:1723‐1730. [DOI] [PubMed] [Google Scholar]
- 8. Estes C, Anstee QM, Arias‐Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016‐2030. J Hepatol 2018;69:896‐904. [DOI] [PubMed] [Google Scholar]
- 9. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loomba R, Wong R, Fraysse J, Shreay S, Li S, Harrison S, et al. Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: a real world analysis of Medicare data. Aliment Pharmacol Ther 2020;51:1149‐1159. [DOI] [PubMed] [Google Scholar]
- 11. Le MH, Yeo YH, Henry L, Nguyen MH. Nonalcoholic fatty liver disease and renal function impairment: a cross‐sectional population‐based study on its relationship from 1999 to 2016. Hepatol Commun 2019;3:1334‐1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park H, Dawwas GK, Liu X, Nguyen MH. Nonalcoholic fatty liver disease increases risk of incident advanced chronic kidney disease: a propensity‐matched cohort study. J Intern Med 2019;286:711‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non‐alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta‐analysis. J Hepatol 2016;65:589‐600. [DOI] [PubMed] [Google Scholar]
- 14. Le MH, Yeo YH, Cheung R, Wong VW, Nguyen MH. Ethnic influence on nonalcoholic fatty liver disease prevalence and lack of disease awareness in the United States, 2011‐2016. J Intern Med 2020;287:711‐722. [DOI] [PubMed] [Google Scholar]
- 15. Bertot LC, Jeffrey GP, Wallace M, MacQuillan G, Garas G, Ching HL, et al. Nonalcoholic fatty liver disease‐related cirrhosis is commonly unrecognized and associated with hepatocellular carcinoma. Hepatol Commun 2017;1:53‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ertle J, Dechene A, Sowa JP, Penndorf V, Herzer K, Kaiser G, et al. Non‐alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer 2011;128:2436‐2443. [DOI] [PubMed] [Google Scholar]
- 17. Mohamad B, Shah V, Onyshchenko M, Elshamy M, Aucejo F, Lopez R, et al. Characterization of hepatocellular carcinoma (HCC) in non‐alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol Int 2016;10:632‐639. [DOI] [PubMed] [Google Scholar]
- 18. Chen VL, Le AK, Kim NG, Kim LH, Nguyen NH, Nguyen PP, et al. Effects of cirrhosis on short‐term and long‐term survival of patients with hepatitis B‐related hepatocellular carcinoma. Clin Gastroenterol Hepatol 2016;14:887‐895.e1. [DOI] [PubMed] [Google Scholar]
- 19. Chen VL, Yeh ML, Le AK, Jun M, Saeed WK, Yang JD, et al. Anti‐viral therapy is associated with improved survival but is underutilised in patients with hepatitis B virus‐related hepatocellular carcinoma: real‐world east and west experience. Aliment Pharmacol Ther 2018;48:44‐54. [DOI] [PubMed] [Google Scholar]
- 20. Leung C, Yeoh SW, Patrick D, Ket S, Marion K, Gow P, et al. Characteristics of hepatocellular carcinoma in cirrhotic and non‐cirrhotic non‐alcoholic fatty liver disease. World J Gastroenterol 2015;21:1189‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bengtsson B, Stal P, Wahlin S, Bjorkstrom NK, Hagstrom H. Characteristics and outcome of hepatocellular carcinoma in patients with NAFLD without cirrhosis. Liver Int 2019;39:1098‐1108. [DOI] [PubMed] [Google Scholar]
- 22. Kodama K, Kawaguchi T, Hyogo H, Nakajima T, Ono M, Seike M, et al. Clinical features of hepatocellular carcinoma in nonalcoholic fatty liver disease patients without advanced fibrosis. J Gastroenterol Hepatol 2019;34:1626‐1632. [DOI] [PubMed] [Google Scholar]
- 23. Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, et al. Risk of hepatocellular cancer in patients with non‐alcoholic fatty liver disease. Gastroenterology 2018;155:1828‐1837.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kanwal F, Singal AG. Surveillance for hepatocellular carcinoma: current best practice and future direction. Gastroenterology 2019;157:54‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Younes R, Bugianesi E. Should we undertake surveillance for HCC in patients with NAFLD? J Hepatol 2018;68:326‐334. [DOI] [PubMed] [Google Scholar]
- 26. Stine JG, Wentworth BJ, Zimmet A, Rinella ME, Loomba R, Caldwell SH, et al. Systematic review with meta‐analysis: risk of hepatocellular carcinoma in non‐alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther 2018;48:696‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loomba R, Lim JK, Patton H, El‐Serag HB. AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology 2020;158:1822‐1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kanwal F, Kramer JR, Li L, Dai J, Natarajan Y, Yu X, et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology 2020;71:808‐819. [DOI] [PubMed] [Google Scholar]
- 29. Natarajan Y, Kramer JR, Yu X, Li L, Thrift AP, El‐Serag HB, et al. Risk of cirrhosis and hepatocellular cancer in patients with non‐alcoholic fatty liver disease and normal liver enzymes. Hepatology 2020. 10.1002/hep.31157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen VL, Singal AG, Tapper EB, Parikh ND. Hepatocellular carcinoma surveillance, early detection, and survival in a privately‐insured US cohort. Liver Int 2020;40:947‐955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bruix J, Sherman M; American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trepo E, Romeo S, Zucman‐Rossi J, Nahon P. PNPLA3 gene in liver diseases. J Hepatol 2016;65:399‐412. [DOI] [PubMed] [Google Scholar]
- 33. Choi DT, Kum HC, Park S, Ohsfeldt RL, Shen Y, Parikh ND, et al. Hepatocellular carcinoma screening is associated with increased survival of patients with cirrhosis. Clin Gastroenterol Hepatol 2019;17:976‐987.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang Z, Zhang H, Boss J, Goutman SA, Mukherjee B, Dinov ID, et al.; Pooled Resource Open‐Access ALS Clinical Trials Consortium . Complete hazard ranking to analyze right‐censored data: an ALS survival study. PLoS Comput Biol 2017;13:e1005887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sv B, Groothuis‐Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw 2011;45:1‐67. [Google Scholar]
- 36. Hothorn T, Hornik K, Strobl C, Zeileis A. Party: a laboratory for recursive partytioning https://cran.r‐project.org/web/packages/party/index.html. Published 2015. [Google Scholar]
- 37. Chayanupatkul M, Omino R, Mittal S, Kramer JR, Richardson P, Thrift AP, et al. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J Hepatol 2017;66:355‐362. [DOI] [PubMed] [Google Scholar]
- 38. Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta‐analysis. Hepatology 2017;66:1486‐1501. [DOI] [PubMed] [Google Scholar]
- 39. Yang J, Trepo E, Nahon P, Cao Q, Moreno C, Letouze E, et al. PNPLA3 and TM6SF2 variants as risk factors of hepatocellular carcinoma across various etiologies and severity of underlying liver diseases. Int J Cancer 2019;144:533‐544. [DOI] [PubMed] [Google Scholar]
- 40. Burza MA, Pirazzi C, Maglio C, Sjoholm K, Mancina RM, Svensson PA, et al. PNPLA3 I148M (rs738409) genetic variant is associated with hepatocellular carcinoma in obese individuals. Dig Liver Dis 2012;44:1037‐1041. [DOI] [PubMed] [Google Scholar]
- 41. Gellert‐Kristensen H, Richardson TG, Davey Smith G, Nordestgaard BG, Tybjaerg‐Hansen A, Stender S. Combined effect of PNPLA3, TM6SF2, and HSD17B13 variants on risk of cirrhosis and hepatocellular carcinoma in the general population. Hepatology 2020. 10.1002/hep.31238. [DOI] [PubMed] [Google Scholar]
- 42. Atiq O, Tiro J, Yopp AC, Muffler A, Marrero JA, Parikh ND, et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology 2017;65:1196‐1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao C, Jin M, Le RH, Le MH, Chen VL, Jin M, et al. Poor adherence to hepatocellular carcinoma surveillance: a systematic review and meta‐analysis of a complex issue. Liver Int 2018;38:503‐514. [DOI] [PubMed] [Google Scholar]
- 44. Thrift AP, Natarajan Y, Liu Y, El‐Serag HB. Statin use after diagnosis of hepatocellular carcinoma is associated with decreased mortality. Clin Gastroenterol Hepatol 2019;17:2117‐2125.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simon TG, Duberg AS, Aleman S, Hagstrom H, Nguyen LH, Khalili H, et al. Lipophilic statins and risk for hepatocellular carcinoma and death in patients with chronic viral hepatitis: results from a nationwide Swedish population. Ann Intern Med 2019;171:318‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


