Abstract
Hepatocellular carcinoma (HCC) is the third‐leading cause of cancer‐related death worldwide, with a growing incidence and poor prognosis. While some recent studies suggest an inverse association between aspirin use and reduced HCC incidence, other data are conflicting. To date, the precise magnitude of risk reduction—and whether there are dose‐dependent and duration‐dependent associations—remains unclear. To provide an updated and comprehensive assessment of the association between aspirin use and incident HCC risk, we conducted a systematic review and meta‐analysis of all observational studies published through September 2020. Using random‐effects meta‐analysis, we calculated the pooled relative risks (RRs) and 95% confidence intervals (CIs) for the association between aspirin use and incident HCC risk. Where data were available, we evaluated HCC risk according to the defined daily dose of aspirin use. Among 2,389,019 participants, and 20,479 cases of incident HCC, aspirin use was associated with significantly lower HCC risk (adjusted RR, 0.61; 95% CI, 0.51‐0.73; P ≤ 0.001; I2 = 90.4%). In subgroup analyses, the magnitude of benefit associated with aspirin was significantly stronger in studies that adjusted for concurrent statin and/or metformin use (RR, 0.45; 95% CI, 0.28‐0.64) versus those that did not (P heterogeneity = 0.02), studies that accounted for cirrhosis (RR, 0.49; 95% CI, 0.45‐0.52) versus those that did not (P heterogeneity = 0.02), and studies that confirmed HCC through imaging/biopsy (RR, 0.30; 95% CI, 0.15‐0.58) compared with billing codes (P heterogeneity < 0.001). In four studies, each defined daily dose was associated with significantly lower HCC risk (RR, 0.98; 95% CI, 0.97‐0.98), corresponding to an 8.4% risk reduction per year of aspirin use. Conclusion: In this comprehensive systematic review and meta‐analysis, aspirin use was associated with a significant reduction in HCC risk. These benefits appeared to increase with increasing dose and duration of aspirin use.
Abbreviations
- BMI
body mass index
- CI
confidence intervals
- DDD
defined daily dose
- DM
diabetes mellitus
- HBV
hepatitis B virus
- HCC
Hepatocellular carcinoma
- HCV
hepatitis C virus
- NAFLD
nonalcoholic fatty liver disease
- NHIRD
National Health Insurance Research Database
- RCT
randomized control trial
- RR
relative risk
Despite the decline in overall cancer‐related mortality over the past decade, hepatocellular carcinoma (HCC) incidence and mortality continue to increase in the United States and worldwide.( 1 ) Among those who develop HCC, the prognosis remains grim, with limited treatment options and 5‐year survival of 12%,( 2 , 3 ) underscoring the need for effective primary prevention strategies.
While the benefits of aspirin for the prevention of colorectal cancer are well‐established,( 4 ) a growing body of recent evidence suggests that aspirin may also help to prevent other cancers,( 5 , 6 , 7 , 8 ) including HCC, through diverse potential mechanisms including the inhibition of cyclooxygenase enzymes, or through pleiotropic effects on inflammatory lipids, platelets, and the gut microbiome.( 9 , 10 , 11 ) However, to date, observational studies focused on aspirin use and HCC risk have yielded conflicting results, with some demonstrating an inverse association between aspirin use and HCC risk, and others finding null associations.( 5 , 6 , 7 , 25 ) Furthermore, inconsistencies in study design, and variability in definitions of aspirin exposure, outcomes or comorbidities, have rendered it difficult to compare effect sizes across studies. As a result, prior meta‐analyses on this topic have included only a fraction of published studies,( 8 , 26 , 27 , 28 ) which can result in biased estimates of effect with limited generalizability.
Thus, to provide the most comprehensive and updated estimates for the potential association between aspirin use and incident HCC risk, we performed a systematic review and meta‐analysis of all observational studies, to evaluate aspirin use—including dose and duration of use—in relation to the risk of developing HCC.
Methods
Search Strategy
A medical librarian (L.P.) undertook a systematic literature search of PubMed, Embase, and Web of Science (inception through September 1, 2020), to identify all existing studies that evaluated aspirin use in relation to HCC incidence. The full PubMed search strategy is available in Supporting Table S1. The title and abstract of all identified studies were screened by two independent reviewers (Z.M. and A.A.), and studies that did not address the research question were excluded. All remaining articles were subsequently reviewed in full against the selection criteria. References were also examined to identify additional studies of relevance. Any discrepancies were resolved by consensus between the two reviewers, or with the input of a third reviewer (T.S.).
Eligibility Criteria
The systematic review and meta‐analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) guidelines.( 29 ) We included all human clinical studies written in English that evaluated aspirin exposure and incident HCC risk, and that reported effect estimates (i.e., relative risks [RRs], odds ratios [ORs], or hazard ratios [HRs]) for the association between aspirin use and HCC incidence, or provided sufficient data for their calculation. This also included studies focused primarily on alternative medication exposures, but nevertheless included an effect estimate for aspirin use or data for its calculation. After the initial records were screened by title and abstract, we reviewed 62 full‐text articles and excluded 43 papers for the following reasons: (1) incorrect study design (n = 24; preclinical study, review without original research, or letter to editor or commentary); (2) abstract only (n = 12); (3) wrong patient population (n = 3; patients with established liver cancer), or (4) overlapping study populations (n = 4). For the studies with overlapping populations, we chose to include the study with the largest sample size, as outlined in detail in the Supporting Appendix. After these exclusions, 19 studies were included in the meta‐analysis. Supporting Fig. S1 summarizes the study selection process.
Data Abstraction
Two independent reviewers (Z.M. and A.A.) conducted critical appraisal and extracted data according to a prespecified data collection form. Specifically, the following data were extracted from each study: first author, publication year, study period, study design, geographic region, number of aspirin‐exposed subjects, number of unexposed subjects, method of defining aspirin exposure, dose and/or duration of aspirin use (if reported), method of ascertaining HCC, etiology of underlying liver disease (if known), proportion with cirrhosis (if reported), number of incident HCC cases among aspirin user and nonuser groups, concomitant use of nonsteroidal anti‐inflammatory drugs, metformin or statin medications (if reported), other covariates included in multivariable models or propensity‐score matching, HRs/RRs/ORs and 95% confidence intervals (CIs) before and after adjustment for confounders, and the database used to obtain patient information. Among studies reporting dose and/or duration of aspirin use, we extracted data regarding the defined daily dose (DDD) of aspirin; or if the DDD was not reported, then we calculated the DDD based on the dosage and number of filled 30‐day prescriptions or the duration of daily aspirin use.
Statistical Analysis
We used the established random effects model described by DerSimonian and Laird to calculate pooled RR and 95% CIs. ( 30 ) Wherever possible, we used adjusted risk estimates from the included studies to account for confounding variables. Between‐study heterogeneity was calculated using Cochran’s Q test and I2 statistic. All P values were two‐tailed, and P < 0.05 was considered statistically significant for all analyses except for tests of heterogeneity and publication bias. We considered heterogeneity to be statistically significant with P < 0.10 and an I2 statistic >50%. A P value of <0.10 was used as a conservative threshold to test for heterogeneity, given that some sample sizes were relatively small and certain subgroups contained a small number of studies, as recommended by prior publications,( 31 , 32 ) including the Cochrane handbook.( 33 ) To identify potential sources of heterogeneity, we performed subgroup analyses according to strata defined by study design, geographic region, definition of aspirin exposure, minimum required duration of aspirin exposure, HCC ascertainment, cohort versus case‐control studies, studies including patients with viral hepatitis, studies including patients with cirrhosis, and studies that simultaneously accounted for statins and metformin. In additional sensitivity analyses, we evaluated whether study region or study design accounted for the observed heterogeneity of results. Publication bias was assessed using a funnel plot and Egger’s test. Qualitative inspection of funnel plots was completed by evaluating the logarithmic odds ratio versus the standard errors.( 31 ) Finally, using a range of assumed control risks for incident HCC, we calculated the number needed to treat (NNT), to prevent one case of incident HCC from an established algorithm.( 34 ) The hazard difference was assumed to represent a valid approximation of the risk difference, given the rarity of incident HCC diagnoses (i.e., event rates <5% per year).( 35 ) All analyses were performed using STATA software 15.1 (StataCorp 2017, College Station, TX).
Results
Search Results
Among the 12,536 unique studies identified through our search strategy, 19 observational studies met the criteria for inclusion. These included two prospective cohort studies, 12 retrospective cohort studies and five case‐control studies, with a total of 2,389,019 total participants and 20,479 incident cases of HCC. Eight studies were removed after initial search, as they were exact duplicates (identified through multiple search engines), and four studies were excluded from the primary meta‐analysis due to overlapping populations.( 5 , 36 , 37 , 38 ) Supporting Fig. S1 shows the flow diagram adapted from PRISMA( 29 ) for study selection.
Study Characteristics
Table 1 outlines the characteristics of included studies. Among the 2,389,019 subjects, 29% (701,297) were exposed to aspirin. Most studies accounted for sex (15 of 19) and age (14 of 19), while a smaller proportion of studies adjusted for other medications (10 of 19), body mass index (BMI) (4 of 19), alcohol use (6 of 19), smoking status (5 of 19), and other comorbidities (7 of 19), and 11 of 19 studies accounted for cirrhosis (with 4 of 19 adjusting for cirrhosis in multivariable models). Most studies from the United States and Europe included subjects with hepatitis C virus (HCV) infection, nonalcoholic fatty liver disease or unknown causes of underlying liver disease, while most studies conducted in Asia included subjects with hepatitis B virus (HBV) infection. The median Newcastle‐Ottawa scale (NOS) score was 7, and 16 of 19 studies were considered high‐quality (NOS > 7) (Supporting Table S2).
TABLE 1.
Baseline Characteristics of Observational Studies Included in the Meta‐analysis
| Study (Author, Year) | Region | Study Design | Mean Age | HCC Cases (n) # | Aspirin Group (n) | Total Number | Aspirin Assessment | HCC Assessment ‡ | Covariates || | Accounted for Cirrhosis | Exposure Group Balance |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lee, T, 2020 | Taiwan | Retrospective cohort | 63.2 | 436 | 2,478 | 7,434 | Prospective/prescription | ICD‐9 code 155 | 1, 2, 5, 11‐13, 17, 23, 28 | Y | PS |
| Shen, 2020 | USA | Case control | NR | 673 | 676 | 1,839 | Retrospective/self‐reported | US State Cancer Registry ¶ | 1, 2, 10, 25, 26, 27 | Y | NR |
| Shin, 2020 | Korea | Retrospective cohort | 64.6 | 133 | 224 | 949 | Prospective/prescription | Imaging or biopsy | 1, 2,11, 15, 17 | Y | PS |
| Simon, 2020 | Sweden | Retrospective cohort | 45 | 1,612 | 14,205 | 50,275 | Prospective/prescription | ICD code | 2, 3, 5, 6, 8, 12‐14, 29 | Y | IPTW |
| Du, 2019 | China | Retrospective cohort | 45 | 41 | 59 | 264 | Prospective/prescription | AFP and imaging or biopsy | NR | Y | NR |
| Lee, T, 2019 | Korea | Retrospective cohort | 58.8 | 697 | 2,123 | 10, 615 | Prospective/prescription | ICD‐9 M code 155 | 1, 2, 3, 5, 12‐14 | Y | PS |
| Tsoi, 2019 | Hong Kong | Retrospective cohort | 67.5 | 9,370 | 204,170 | 612,509 | Prospective/prescription | ICD‐9 M code 155 | 1, 2, 12 | N | IPTW |
| Hwang, 2018 | Korea | Retrospective cohort | 50 | 2,336 | 64,782 | 460,755 | Prospective/prescription | ICD‐10 code C22.0 § | 1, 2, 4, 7‐ 9, 12, 13, 17‐19 | Y | PS |
| Lin, 2018 | Taiwan | Retrospective cohort | NR | 110 | 3,576 | 18,243 | Prospective/prescription | ICD‐9 M code 155 | 1, 2, 12, 13, 21 | N | NR |
| Tseng, 2018 | Taiwan | Retrospective cohort | NR | 1,750 | 23,112 | 43,800 | Prospective/prescription | ICD‐9 M code 155 | 1, 2, 3, 6, 13, 22 | Y | NR |
| Lee, M, 2017 | Korea | Retrospective cohort | 55.2 | 63 | 343 | 14,392 | Prospective/prescription | AFP and imaging or biopsy | 1‐3, 5, 11‐12, 15, 16, 17 | Y | PS |
| Lee, T, 2017 | Taiwan | Retrospective cohort | 52.69 | NR | 5,602 | 18,080 | Prospective/prescription | ICD‐9 M code 155 | 1‐3, 12‐13, 17 | N | NR |
| Kim, 2017 | Korea | Case control | NR | 229 | 390 | 1,374 | Prospective/prescription | ICD‐10 code C22.0 § | 1, 2, 3, 12 | Y | NR |
| Yang, 2016 | UK | Case control | 67 | 1,195 | 1,670 | 5,835 | Prospective/prescription* | Read code | 3, 4, 6, 8, 9, 12, 13 | Y | NR |
| Petrick, 2015 | USA | Prospective cohort | NR | 346 | 258,179 | 803,248 | Prospective/self‐reported* | ICD‐0‐3 histology codes 8170‐8175 | 1‐4, 8‐10, 29 | N | NR |
| Sahasrabuddhe, 2012 | USA | Prospective cohort | 62.8 | 250 | 89,585 | 300,504 | Prospective/self‐reported* | ICD‐9 and ICD‐10 code | 1‐4, 9, 8‐10 | N | NR |
| Chiu, 2011 | Taiwan | Case control | 66.1 | 1,166 | 162 | 2,332 | Retrospective/prescription* | ICD‐9 M code 155 | NR | N | NR |
| Friis, 2003 | Denmark | Retrospective cohort | 70 | 21 | 29,470 | 29,470 † | Prospective/prescription | ICD‐7 code | NR | N | NR |
| Coogan, 2000 | USA | Case control | NR | 51 | 491 | 7,101 | Retrospective/self‐reported* | AFP and imaging or biopsy | 1, 2, 8, 9, 10, 30‐33 | N | NR |
Included both aspirin and nonsteroidal anti‐inflammatory drugs.
Aspirin users were compared with the population in North Jutland County, Denmark (total number was not reported).
ICD code did not specify morphology (M) or histology unless otherwise specified.
Authors with C22 codes did not specify topography versus morphology (M) codes.
Variables adjusted for with either multivariable regression analysis, PS, or IPTW: 1, age; 2, sex; 3, diabetes mellitus; 4, BMI; 5, cirrhosis; 6, hepatitis B/C; 7, physical activity; 8, alcohol; 9, smoking; 10, race; 11, MELD; 12, other medications (i.e., statins and/or metformin); 13, comorbidities; 14, nucleoside analogues; 15, CTP score; 16, hep level; 17, lab tests such as cholesterol, plasma glucose, AST/ALT, albumin, PLT; 18, socioeconomic status; 19, Charlson comorbidity index score; 20, diet; 21, urbanization; 22, occupation; 23, HCV diagnosis year; 24, number of years of aspirin use; 25, education; 26, household income; 27, marital status; 28, index date to start of follow‐up; 29, cohort; 30, religion; 31, education; 32, family history of digestive cancer; and 33, interview year.
NJ, NYC, and CT State Cancer Registry.
Due to no specification of HCC case number from Lee T (2017), the HCC case numbers are slightly less than the true total cohort number for each subgroup in Table 2.
Abbreviations: AFP, alpha‐fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTP, Child‐Turcotte‐Pugh; hep level, hepatitis B or C viral load; IPTW, inverse probability of treatment weighting; M code, morphology code; MELD, Model for End‐Stage Liver Disease; NR, not reported; NYC, New York City; PLT, platelet.
Aspirin Use and HCC Risk
Overall, random‐effects meta‐analysis demonstrated that aspirin users had a 39% lower risk of developing incident HCC, compared with nonusers, after accounting for confounding variables (pooled adjusted RR, 0.61; 95% CI, 0.51‐0.73; P ≤ 0.001), with considerable heterogeneity (Cochran’s Q test P ≤ 0.001; I2 = 90.4%) (Fig. 1). We identified no evidence of publication bias through visual inspection of the funnel plot (Supporting Fig. S2) or through quantitative testing, using Egger’s test (P = 0.78).
FIG. 1.
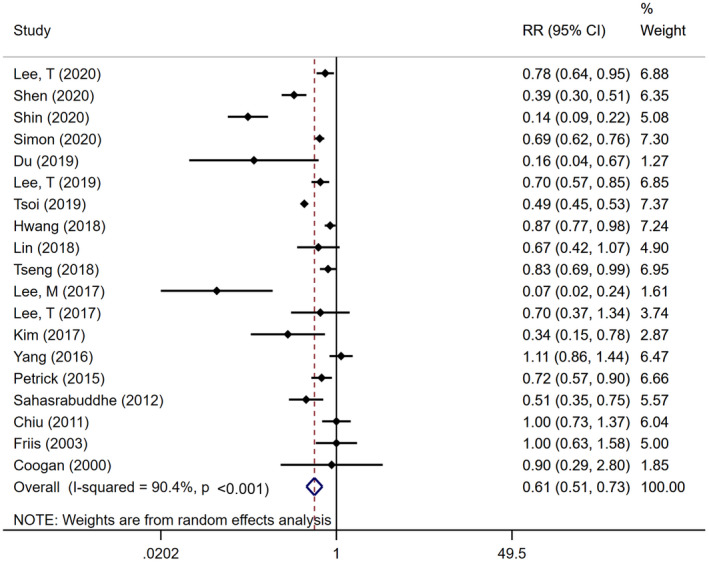
Forest plot of aspirin use and risk of HCC development among 19 observational studies, including 2,389,019 participants and overall relative risks with respective weightings. The pooled results of included studies illustrate a significant relative reduction in risk of HCC in participants who used aspirin (RR, 0.61; 95% CI, 0.51‐0.73).
Subgroup and Sensitivity Analyses
We performed subgroup analyses to evaluate potential sources of heterogeneity (Table 2). Among studies that accounted for concurrent statin and/or metformin use (n = 10), aspirin was associated with a significant, 55% lower risk of incident HCC (RR, 0.45; 95% CI, 0.28‐0.64), with moderate heterogeneity (I2 = 50.7%). In contrast, among studies that did not account for those other hepatoprotective medications (n = 9), the RR for aspirin use was 0.71 (95% CI, 0.60‐0.86), with substantial heterogeneity (I2 = 89.3%). Among studies that accounted for underlying cirrhosis (n = 11), aspirin use was associated with a significant, 51% lower HCC risk (RR, 0.49; 95% CI, 0.45‐0.52), with minimal heterogeneity (I2 = 21.2%); however, among the eight studies that did not account for underlying cirrhosis, the observed RR was 0.68 (95% CI, 0.51‐0.82), with substantial heterogeneity (I2 = 90.8%). Furthermore, among studies that simultaneously accounted for both cirrhosis and use of statins and/or metformin (n = 7),( 6 , 12 , 14 , 15 , 20 , 22 , 36 ) aspirin use was associated with a significant, 45% reduced risk of HCC (RR, 0.55; 95% CI, 0.49‐0.60), with minimal heterogeneity (I2 = 8.7%), suggesting that, together, those two factors might explain the significant heterogeneity in our primary analysis.
TABLE 2.
Subgroup Analysis to Identify Sources of Heterogeneity
| Subgroup Analysis | No. of Studies | No. of HCC Cases | Total No. of Subjects | RR (95% CI) | Tests of Heterogeneity | Heterogeneity Between Groups (P) | |
|---|---|---|---|---|---|---|---|
| I2 | P | ||||||
| Region | |||||||
| European/U.S. | 7 | 4,148 | 1,198,272 | 0.70 (0.54‐0.90) | 82.8% | <0.001 | P = 0.43 |
| Asian | 12 | 16,331 | 1,190,747 | 0.55 (0.42‐0.71) | 92.6% | <0.001 | |
| HCC ascertainment | |||||||
| Not confirmed (diagnostic code) | 13 | 19,172 | 1,561,226 | 0.74 (0.62‐0.87) | 89.2% | <0.001 | P < 0.001 |
| Confirmed (imaging/pathology) | 6 | 1,307 | 827,793 | 0.30 (0.15‐0.58) | 51.0% | <0.001 | |
| Minimum required duration of aspirin use | |||||||
| >4 weeks | 15 | 18,488 | 1,837,235 | 0.60 (0.49‐0.74) | 92.0% | <0.001 | P = 0.65 |
| No minimum duration | 4 | 1,991 | 1,107,458 | 0.66 (0.47‐0.93) | 71.2% | 0.015 | |
| Aspirin ascertainment | |||||||
| Prospective | 16 | 18,569 | 2,377,747 | 0.61 (0.50‐0.74) | 91.0% | <0.001 | P = 0.77 |
| Retrospective | 3 | 1,890 | 11,272 | 0.67 (0.31‐1.46) | 89.9% | <0.001 | |
| Cohort vs. case control | |||||||
| Cohort study | 14 | 17,165 | 2,370,538 | 0.59 (0.49‐0.72) | 52.4% | <0.001 | P = 0.67 |
| Case control | 5 | 3,314 | 18,481 | 0.68 (0.39‐1.18) | 89.3% | <0.001 | |
| Definition of aspirin exposure | |||||||
| Prescription data | 15 | 19,159 | 1,276,327 | 0.63 (0.51‐0.77) | 91.8% | <0.001 | P = 0.54 |
| Self‐reported data | 4 | 1,320 | 1,112,692 | 0.55 (0.38‐0.80) | 75.4% | 0.007 | |
| Accounted for viral hepatitis | |||||||
| Yes | 10 | 9,032 | 596,583 | 0.69 (0.58‐0.83) | 84.8% | <0.001 | P = 0.54 |
| No/unspecified | 9 | 11,447 | 1,792,436 | 0.54 (0.39‐0.75) | 86.9% | <0.001 | |
| Accounted for cirrhosis † | |||||||
| Cirrhosis | 11 | 9,165 | 597,532 | 0.49 (0.45‐0.52) | 21.2% | <0.001 | P = 0.02 |
| No cirrhosis/unspecified | 8 | 11,314 | 1,791,487 | 0.68 (0.51‐0.82) | 90.8% | 0.002 | |
| Accounted for hepatoprotective medications * | |||||||
| Yes | 10 | 16,048 | 1,199,512 | 0.45 (0.28‐0.64) | 50.7% | <0.001 | P = 0.02 |
| No | 9 | 4,431 | 1,189,507 | 0.71 (0.60‐0.86) | 89.3% | <0.001 | |
| Exposure group balance | |||||||
| PS or IPTW | 7 | 14,647 | 1,156,929 | 0.56 (0.43‐0.72) | 94.9% | <0.001 | P = 0.29 |
| None | 12 | 5,832 | 1,232,090 | 0.67 (0.51‐0.88) | 78.6% | <0.001 | |
Statins and/or metformin.
Studies could account for cirrhosis by including cirrhosis as a covariate in the multivariable model, or by including cirrhosis as a matching variable to balance exposure groups, or by including a cirrhosis‐only population for analysis.
Abbreviation: IPTW, inverse probability weighting.
Several additional factors contributed to the observed heterogeneity. First, among the six studies in which HCC was confirmed by imaging and/or biopsy,( 6 , 7 , 13 , 25 , 37 , 38 ) aspirin use was associated with a 70% reduced HCC risk (95% CI, 0.15‐0.58), with moderate heterogeneity (I2 = 51.0%). In contrast, among the 13 studies that defined HCC using diagnostic codes,( 12 , 36 ) aspirin users had a 26% lower risk of HCC (95% CI, 0.62‐0.87), with substantial heterogeneity (I2 = 89.2%). Second, among cohort studies (n = 14), aspirin use was significantly associated with reduced HCC risk (RR, 0.59; 95% CI, 0.49‐0.72), with moderate heterogeneity (I2 = 52.4%), whereas substantial heterogeneity was found among the five case control studies (RR, 0.68; 95% CI, 0.39‐1.18; I2 = 89.3%). Finally, when we restricted the analyses exclusively to populations with established cirrhosis (n = 6 studies), aspirin use was associated with 31% lower HCC risk, with no heterogeneity (RR, 0.69; 95% CI, 0.62‐0.75; I2 = 0%). In contrast, no significant differences were found in subgroups defined by geographic region, assessment of viral hepatitis, required duration of aspirin usage (i.e., >4 weeks vs. no minimum), method of aspirin ascertainment (i.e., prospective vs. retrospective), or the definition of aspirin exposure (i.e., self‐report vs. prescription) (all P heterogeneity > 0.10; Table 2).
To assess whether any individual study might exert a disproportionate influence on the overall summary RR, we compared our findings after excluding each individual study in turn; no single study was found to substantially affect the main summary estimate (Supporting Fig. S3).
Exploratory Dose‐Response and Duration‐Response Analysis
Four studies( 5 , 7 , 12 , 15 ) reported risk of incident HCC according to dose and/or duration of aspirin use, from which we calculated continuous aspirin DDD exposures, as outlined in the Supporting Appendix. Meta‐analysis of these eligible studies revealed a significant, dose‐dependent and duration‐dependent inverse association between increasing aspirin DDD and reduced HCC risk. Specifically, each additional aspirin DDD contributed to a significant 0.02% reduction in HCC risk (adjusted RR, 0.98; 95% CI, 0.97‐0.98), which corresponds to an 8.4% risk reduction per year of daily aspirin use. The results showed substantial heterogeneity (Cochran’s Q2 test = 8.57; P = 0.036; I2 = 65.0%). There was no evidence of publication bias either quantitatively (P = 0.78) or qualitatively (Supporting Fig. S2). Given the small number of eligible studies, further subgroup analyses were not undertaken.
Number Needed to Treat
Due to substantial between‐study heterogeneity, it was not possible to calculate a single pooled estimate of the NNT with aspirin to prevent one case of incident HCC. Nevertheless, if we assume an HCC incidence rate of 0.6 per 100 person‐years among adults with noncirrhotic chronic HBV infection,( 39 , 40 ) and assume a 39% lower observed HCC risk with aspirin use, then an estimated 427 adults with noncirrhotic chronic HBV infection would need to be treated with aspirin for 1 year to prevent one case of incident HCC. Alternatively, if we restricted the population to higher‐risk patients with cirrhosis (in whom the annual HCC incidence rate is between 2% and 4%), then even if we assumed a conservative annual HCC incidence rate of 2% per year, only 128 adults with cirrhosis would need to be treated with aspirin for 1 year, to prevent one case of HCC.
Discussion
In this systematic review and meta‐analysis of more than 19,000 incident HCC cases recorded in over 2.3 million participants across seven different countries, aspirin use was associated with a significant, 39% lower risk of developing incident HCC. The HCC risk reduction associated with aspirin was similar in both Asian and Western populations, and in patients with and without viral hepatitis. Subgroup analyses further demonstrated that the apparent benefits of aspirin were most homogenous in well‐phenotyped populations that simultaneously accounted for cirrhosis and for concurrent use of other hepatoprotective medications. Additionally, our secondary dose‐response meta‐analysis of more than 1.3 million adults and over 4,294 incident HCC cases demonstrated that the inverse association between aspirin use and HCC incidence was highly dose‐dependent and duration‐dependent, such that 1 year of daily aspirin use contributed to a significant, 8.4% reduction in HCC risk. Importantly, the likelihood of significant selection or publication bias was very small. Overall, these findings provide further rationale for the design of randomized control trials (RCTs) to evaluate the safety and efficacy of aspirin therapy in at‐risk patients with chronic liver disease.
Although two recent meta‐analyses have addressed the association between aspirin use and HCC risk,( 26 , 27 ) those previous meta‐analyses each included fewer than 50% of published observational studies.( 12 , 13 , 17 , 18 , 23 , 24 , 36 , 37 , 38 ) Furthermore, neither of those previous meta‐analyses addressed key HCC risk factors, including viral hepatitis, cirrhosis, concurrent use of statins or metformin, or the methods by which aspirin use was ascertained or HCC cases were confirmed. This is important, because failing to account for those factors could yield biased and imprecise risk estimates, with limited generalizability. In contrast, by incorporating 11 additional studies that were not part of the most recent published meta‐analysis, ( 26 ) and by conducting detailed subgroup and stratified analyses, the current study is able to provide a more comprehensive assessment of aspirin use in relation to HCC risk.
The association between aspirin use and HCC risk was most homogenous in studies that simultaneously accounted for underlying cirrhosis and concurrent use of other hepatoprotective medications, as well as in studies that confirmed HCC diagnoses with appropriate imaging or pathology. It is well‐established that HCC risk is dependent on the severity of underlying liver disease; thus, accounting for underlying cirrhosis could address potential selection bias. Reliance on administrative diagnostic codes to define HCC may also introduce misclassification, which in turn could substantially attenuate risk estimates. Finally, it has been hypothesized that the combined use of aspirin together with either statins or metformin could act synergistically to prevent hepatocarcinogenesis.( 20 , 23 ) Indeed, in vitro studies of HCC cell lines have demonstrated that co‐administration of simvastatin and a cyclooxygenase‐2 inhibitor, NS398, act synergistically to activate apoptosis and inhibit proliferation.( 41 )
The apparent benefits of aspirin were similar in both Asian and Western populations, and in patients with and without established viral hepatitis. Although this could suggest that the chemopreventive effects of aspirin might be broadly generalizable, caution must be stressed. In Asia, HBV infection represents the dominant HCC risk factor, whereas in the United States and Europe, the dominant risk factors include chronic HCV infection, alcohol use, and nonalcoholic fatty liver disease (NAFLD). Prior studies have found that aspirin may exert unique, HBV‐specific hepatoprotective and chemopreventive benefits, by reducing hepatic infiltration of CD8+ T cells and other pro‐inflammatory cells.( 42 ) Furthermore, among published studies in Western populations, all but one( 12 ) have lacked comprehensive data regarding underlying HBV or HCV infection, cirrhosis, or the relative contribution of prescription aspirin versus over‐the‐counter (OTC) aspirin. As the United States and parts of Europe have widely available OTC aspirin, it is possible that studies relying on prescription claims or medical record data in those regions may introduce exposure misclassification, by missing a proportion of aspirin users. Finally, it has been suggested that aspirin use and prescribing patterns may differ in Asia, compared with Western countries( 43 ); thus, without careful accounting for both selection and indication bias, it is possible that aspirin users included in previous studies were at inherently lower risk of developing HCC, compared with nonusers.( 14 , 43 )
The association between aspirin use and HCC risk was modestly attenuated but remained statistically significant in analyses restricted only to patients with cirrhosis. It has been previously suggested that aspirin might exert its hepatoprotective effects early in the course of chronic liver disease, by preventing or delaying the development of liver fibrosis.( 44 ) However, physicians are also less likely to prescribe aspirin to patients with cirrhosis, out of concern for bleeding; this raises the important possibility that selection bias or confounding by indication could have affected our results. Although the apparent benefits of aspirin were similar in studies that used propensity‐score approaches to balance treatment groups,( 6 , 12 , 14 , 15 , 16 , 36 , 37 ) it is nevertheless impossible to exclude these potential biases. This in turn highlights the known limitations of observational studies and underscores the need for well‐designed RCTs to test the safety and efficacy of aspirin therapy across the full spectrum of chronic liver disease.
Due to its potent antiplatelet effects, the bleeding risks associated with aspirin represent an important consideration in patients with chronic liver disease. Previous RCTs have demonstrated that aspirin therapy for the primary prevention of cardiovascular disease increases the risk of major gastrointestinal and extracranial bleeding (0.10% vs. 0.07% per year in nonusers; P < 0.0001)( 45 ); however, those trials excluded patients with cirrhosis. At present, data regarding the potential aspirin‐related bleeding risks in patients with liver disease are limited. In this meta‐analysis, only four retrospective cohort studies, three of which conducted in patients with chronic viral hepatitis and one conducted in patients with alcoholic cirrhosis,( 37 ) specifically evaluated bleeding events associated with aspirin use.( 6 , 12 , 36 , 37 ) All four studies found a null association between daily aspirin use and excess bleeding risk, including a single exploratory comparison of patients with and without cirrhosis. However, given the small sample sizes and exploratory nature of those analyses, further research is needed to fully characterize the potential risks of aspirin in patients with liver disease of varying degrees of severity.
To our knowledge, this represents the most comprehensive meta‐analysis to date that is focused on aspirin use in relation to incident HCC risk. It is strengthened by the inclusion of over 2.3 million participants from seven countries, which increases generalizability and permits calculation of more precise risk estimates. Furthermore, by demonstrating a significant dose‐response and duration‐response relationship, we provide additional support for a causal association between aspirin use and reduced HCC incidence.
We acknowledge several limitations. First, our findings are based exclusively on observational studies; thus, we cannot exclude the possibility of residual confounding and bias related to immortal time, selection, or the underlying indications for aspirin use. However, among the few previous studies that were conducted in unselected, population‐based cohorts with careful accounting for those factors, the estimates were similar. Second, we acknowledge that there was substantial heterogeneity among the 19 included studies; however, this was no longer present among studies that carefully accounted for both underlying cirrhosis and concurrent use of other hepatoprotective medications. Nevertheless, there remain other important potential sources of heterogeneity that should be assessed in future studies, including individual major HCC risk factors (e.g., HBV, HCV, alcohol use, NAFLD or nonalcoholic steatohepatitis [NASH]), modifiers of HCC risk (e.g., fibrosis severity, adequacy of viral suppression in HBV, HCV eradication), or environmental and lifestyle factors (e.g., BMI, diabetes, smoking, diet). Third, the 19 included studies varied widely in the covariates used for multivariable models, and most (9 of 19) did not adjust for concomitant use of statins or metformin, two medications that have been independently associated with HCC risk reduction. Moreover, very few studies ascertained data regarding adherence to HCC screening recommendations. Fourth, we did not contact investigators to request unpublished data. Finally, although we conducted subgroup analyses in patients with cirrhosis, we lacked sufficient data to examine aspirin use across individual fibrosis stages, and only one study specifically evaluated aspirin use and HCC risk among patients with established NAFLD or NASH. Thus, future prospective studies are needed in well‐phenotyped populations with NAFLD/NASH, to assess whether aspirin might offer unique benefits in that growing patient population.
In conclusion, this systematic review and meta‐analysis demonstrates that aspirin use is associated with a significant dose‐dependent and duration‐dependent reduction in the risk of developing incident HCC. The apparent benefits of aspirin were similar in Asian and Western populations, and in patients with and without established viral hepatitis. Although HCC risk reduction was modestly attenuated in patients with cirrhosis, significant benefit nevertheless persisted. Well‐designed, prospective studies are needed to evaluate the safety of aspirin therapy across the complete spectrum of chronic liver disease, and to characterize the optimal dose, duration of use, and timing of aspirin initiation or discontinuation, to achieve maximal benefit while incurring minimal risks. Our findings support the planning of RCTs to confirm these observations.
Supporting information
Supplementary Material
Supported by the National Institutes of Health (NIH DK122104) and the American Association for the Study of Liver Diseases Clinical and Translational Research Fellowship in Liver Disease.
Potential conflict of interest: Dr. Chung received grants from Gilead, AbbVie, Merck, BMS, Boehringer, Roche, and Janssen. Dr. Corey consults for and received grants from BMS. She advises Novo Nordisk. She received grants from Boehringer‐Ingelheim.
References
- 1. Mokdad AH, Dwyer‐Lindgren L, Fitzmaurice C, Stubbs RW, Bertozzi‐Villa A, Morozoff C, et al. Trends and patterns of disparities in cancer mortality among US counties, 1980‐2014. JAMA 2017;317:388‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lepage C, Capocaccia R, Hackl M, Lemmens V, Molina E, Pierannunzio D, et al. Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999‐2007: results of EUROCARE‐5. Eur J Cancer 2015;51:2169‐2178. [DOI] [PubMed] [Google Scholar]
- 3. Tan D, Yopp A, Beg MS, Gopal P, Singal AG. Meta‐analysis: underutilisation and disparities of treatment among patients with hepatocellular carcinoma in the United States. Aliment Pharmacol Ther 2013;38:703‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bibbins‐Domingo K, Force USPST . Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2016;164:836‐845. [DOI] [PubMed] [Google Scholar]
- 5. Simon TG, Ma Y, Ludvigsson JF, Chong DQ, Giovannucci EL, Fuchs CS, et al. Association between aspirin use and risk of hepatocellular carcinoma. JAMA Oncol 2018;4:1683‐1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee M, Chung GE, Lee JH, Oh S, Nam JY, Chang Y, et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology 2017;66:1556‐1569. [DOI] [PubMed] [Google Scholar]
- 7. Petrick JL, Sahasrabuddhe VV, Chan AT, Alavanja MC, Beane‐Freeman LE, Buring JE, et al. NSAID use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the Liver Cancer Pooling Project. Cancer Prev Res 2015;8:1156‐1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qiao Y, Yang T, Gan Y, Li W, Wang C, Gong Y, et al. Associations between aspirin use and the risk of cancers: a meta‐analysis of observational studies. BMC Cancer 2018;18:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kern MA, Schubert D, Sahi D, Sahi D, Schöneweiß MM, Moll I, et al. Proapoptotic and antiproliferative potential of selective cyclooxygenase‐2 inhibitors in human liver tumor cells. Hepatology 2002;36(4 Pt 1):885‐894. [DOI] [PubMed] [Google Scholar]
- 10. Beloribi‐Djefaflia S, Vasseur S, Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016;5:e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chan TA, Morin PJ, Vogelstein B, Kinzler KW. Mechanisms underlying nonsteroidal antiinflammatory drug‐mediated apoptosis. Proc Natl Acad Sci USA 1998;95:681‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simon TG, Duberg AS, Aleman S, Chung RT, Chan AT, Ludvigsson JF. Association of aspirin with hepatocellular carcinoma and liver‐related mortality. N Engl J Med 2020;382:1018‐1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Du ZQ, Zhao JZ, Dong J, Bi JB, Ren YF, Zhang J, et al. Effect of low‐dose aspirin administration on long‐term survival of cirrhotic patients after splenectomy: a retrospective single‐center study. World J Gastroenterol 2019;25:3798‐3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee TY, Hsu YC, Tseng HC, Yu SH, Lin JT, Wu MS, et al. Association of daily aspirin therapy with risk of hepatocellular carcinoma in patients with chronic hepatitis B. JAMA Intern Med 2019;179:633‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hwang IC, Chang J, Kim K, Park SM. Aspirin use and risk of hepatocellular carcinoma in a National Cohort Study of Korean Adults. Sci Rep 2018;8:4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsoi KKF, Ho JMW, Chan FCH, Sung JJY. Long‐term use of low‐dose aspirin for cancer prevention: a 10‐year population cohort study in Hong Kong. Int J Cancer 2019;145:267‐273. [DOI] [PubMed] [Google Scholar]
- 17. Lin YS, Yeh CC, Huang SF, Chou YS, Kuo LT, Sung FC, et al. Aspirin associated with risk reduction of secondary primary cancer for patients with head and neck cancer: a population‐based analysis. PLoS One 2018;13:e0199014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tseng CH. Metformin and risk of hepatocellular carcinoma in patients with type 2 diabetes. Liver Int 2018;38:2018‐2027. [DOI] [PubMed] [Google Scholar]
- 19. Lee TY, Wu JC, Yu SH, Lin JT, Wu MS, Wu CY. The occurrence of hepatocellular carcinoma in different risk stratifications of clinically noncirrhotic nonalcoholic fatty liver disease. Int J Cancer 2017;141:1307‐1314. [DOI] [PubMed] [Google Scholar]
- 20. Kim G, Jang SY, Han E, Lee YH, Park SY, Nam CM, et al. Effect of statin on hepatocellular carcinoma in patients with type 2 diabetes: a nationwide nested case‐control study. Int J Cancer 2017;140:798‐806. [DOI] [PubMed] [Google Scholar]
- 21. Sahasrabuddhe VV, Gunja MZ, Graubard BI, Trabert B, Schwartz LM, Park Y, et al. Nonsteroidal anti‐inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst 2012;104:1808‐1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang B, Petrick JL, Chen J, Hagberg KW, Sahasrabuddhe VV, Graubard BI, et al. Associations of NSAID and paracetamol use with risk of primary liver cancer in the Clinical Practice Research Datalink. Cancer Epidemiol 2016;43:105‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chiu HF, Ho SC, Chen CC, Yang CY. Statin use and the risk of liver cancer: a population‐based case–control study. Am J Gastroenterol 2011;106:894‐898. [DOI] [PubMed] [Google Scholar]
- 24. Friis S, Sørensen HT, McLaughlin JK, Johnsen SP, Blot WJ, Olsen JH. A population‐based cohort study of the risk of colorectal and other cancers among users of low‐dose aspirin. Br J Cancer 2003;88:684‐688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coogan PF, Rosenberg L, Palmer JR, Strom BL, Zauber AG, Stolley PDL, et al. Nonsteroidal anti‐inflammatory drugs and risk of digestive cancers at sites other than the large bowel. Cancer Epidemiol Biomarkers Prev 2000;9:119‐123. [PubMed] [Google Scholar]
- 26. Wang S, Yu Y, Ryan PM, Dang M, Clark C, Kontogiannis V, et al. Association of aspirin therapy with risk of hepatocellular carcinoma: a systematic review and dose‐response analysis of cohort studies with 2.5 million participants. Pharmacol Res 2020;151:104585. [DOI] [PubMed] [Google Scholar]
- 27. Tao Y, Li Y, Liu X, Deng Q, Yu Y, Yang Z. Nonsteroidal anti‐inflammatory drugs, especially aspirin, are linked to lower risk and better survival of hepatocellular carcinoma: a meta‐analysis. Cancer Manag Res 2018;10:2695‐2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bosetti C, Santucci C, Gallus S, Martinetti M, La Vecchia C. Aspirin and the risk of colorectal and other digestive tract cancers: an updated meta‐analysis through 2019. Ann Oncol 2020;31:558‐568. [DOI] [PubMed] [Google Scholar]
- 29. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015: elaboration and explanation. BMJ 2015;349:g7647. [DOI] [PubMed] [Google Scholar]
- 30. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7:177‐188. [DOI] [PubMed] [Google Scholar]
- 31. Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet 1991;337:867‐872. [DOI] [PubMed] [Google Scholar]
- 32. Suchmacher M, Geller M. Practical Biostatistics With Microsoft Excel: A User‐Friendly Approach for Evidence‐Based Medicine. London: Elsevier; 2012. [Google Scholar]
- 33. Higgins J, Thomas J, eds.Cochrane Handbook for Systemic Reviews of interventions. https://handbook‐5‐1.cochrane.org/chapter_9/9_5_2_identifying_and_measuring_heterogeneity.htm. Accessed September 15, 2020.
- 34. Higgins J, Thomas J, eds.Cochrane Handbook for Systemic Reviews of Interventions. https://training.cochrane.org/handbook/current/chapter‐15. Accessed April 2020.
- 35. Mayne TJ, Whalen E, Vu A. Annualized was found better than absolute risk reduction in the calculation of number needed to treat in chronic conditions. J Clin Epidemiol 2006;59:217‐223. [DOI] [PubMed] [Google Scholar]
- 36. Lee TY, Hsu YC, Tseng HC, Lin JT, Wu MS, Wu CY. Association of daily aspirin therapy with hepatocellular carcinoma risk in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol 2020;18:2784‐2792.e7. [DOI] [PubMed] [Google Scholar]
- 37. Shin S, Lee SH, Lee M, Kim JH, Lee W, Lee HW, et al. Aspirin and the risk of hepatocellular carcinoma development in patients with alcoholic cirrhosis. Medicine (Baltimore) 2020;99:e19008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shen Y, Risch H, Lu L, Ma X, Irwin ML, Lim JK, et al. Risk factors for hepatocellular carcinoma (HCC) in the northeast of the United States: results of a case‐control study. Cancer Causes Control 2020;31:321‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol 2008;48:335‐352. [DOI] [PubMed] [Google Scholar]
- 40. Sherman M, Bruix J, Porayko M, Tran T, Committee APG. Screening for hepatocellular carcinoma: the rationale for the American Association for the Study of Liver Diseases recommendations. Hepatology 2012;56:793‐796. [DOI] [PubMed] [Google Scholar]
- 41. Lee SJ, Hwang JW, Yim H, Yim HJ, Woo SU, Suh SJ, et al. Synergistic effect of simvastatin plus NS398 on inhibition of proliferation and survival in hepatocellular carcinoma cell line. J Gastroenterol Hepatol 2014;29:1299‐1307. [DOI] [PubMed] [Google Scholar]
- 42. Sitia G, Iannacone M, Guidotti LG. Anti‐platelet therapy in the prevention of hepatitis B virus‐associated hepatocellular carcinoma. J Hepatol 2013;59:1135‐1138. [DOI] [PubMed] [Google Scholar]
- 43. Fox RK, Taddei TH, Kaplan DE. Aspirin use and risk of hepatocellular carcinoma in hepatitis B. JAMA Intern Med 2019;179:640‐641. [DOI] [PubMed] [Google Scholar]
- 44. Simon TG, Henson J, Osganian S, Masia R, Chan AT, Chung RT, et al. Daily aspirin use associated with reduced risk for fibrosis progression in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2019;17:2776‐2784.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Antithrombotic Trialists C, Baigent C, Blackwell L, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta‐analysis of individual participant data from randomised trials. Lancet 2009;373:1849‐1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


