Abstract
Background
Many countries consistently fail to achieve the target influenza vaccine coverage rate (VCR) of 75% for populations at risk of complications, recommended by the World Health Organization and European Council. We aimed to identify factors for achieving a high VCR in the scope of four benchmark countries with high influenza VCRs: Australia, Canada, UK and USA.
Methods
Publicly available evidence was first reviewed at a global level and then for each of the four countries. Semi-structured interviews were then conducted with stakeholders meeting predefined criteria. Descriptive cluster analyses were performed to identify key factors and pillars for establishing and maintaining high VCRs.
Results
No single factor led to a high VCR, and each benchmark country used a different combination of tailored approaches to achieve a high vaccine coverage. In each country, specific triggers were important to stimulate changes that led to improved vaccine coverage. A total of 42 key factors for a successful influenza vaccination programme were identified and clustered into five pillars: (1) Health Authority accountability and strengths of the influenza programme, (2) facilitated access to vaccination, (3) healthcare professional accountability and engagement, (4) awareness of the burden and severity of disease and (5) belief in influenza vaccination benefit. Each benchmark country has implemented multiple factors from each pillar.
Conclusion
A wide range of factors were identified from an evaluation of four high-performing benchmark countries, classified into five pillars, thus providing a basis for countries with lower VCRs to tailor their own particular solutions to improve their influenza VCR.
Keywords: benchmark, influenza vaccine, vaccine coverage rate
Introduction
Influenza infection constitutes a significant burden on health systems and societies globally.1 The burden of disease is much higher than can be estimated based on diagnosed influenza cases, since many cases remain undiagnosed and cases that present as complications after viral shedding has resolved will be missed. Much of this burden could be prevented with higher vaccination coverage rates (VCRs). Vulnerable populations, such as those with chronic non-communicable diseases, including diabetes or chronic obstructive pulmonary disease, or older adults (aged 65 years and over), are particularly at risk of severe outcomes, making them a target for high coverage rates.2
The WHO Global Influenza Strategy 2019–2030 has reiterated its aim for the highest possible level of influenza prevention, control and preparedness to safeguard the health of all people, with a key aspect being to improve the global VCR.3 The WHO and the European Council both recommend a target VCR of 75% for populations at risk of complications following influenza infection (i.e. older adults, anyone aged 6 months and over with a chronic medical condition and pregnant women), and those liable to transmit influenza to such populations (i.e. healthcare providers (HCPs) and social care workers).4,5 However, even when influenza vaccination recommendations and funding are available, many countries globally consistently fail to achieve the target VCR. In a 2019 assessment of 30 countries, the European Commission highlighted that none met the 75% VCR target.6 Furthermore, hospitalisations increase during the influenza season and an analysis of the European situation has shown achieving the 75% target VCR can avoid approximately 40–50% of extra influenza-related hospitalisations and deaths as well as preventing many cardiovascular complications.7
Previous research has evaluated key barriers and facilitators to influenza vaccination coverage from different perspectives for the WHO target groups (older adults, patients with chronic conditions, pregnant women and HCPs) across a wide range of low-, medium- and high-performing countries. Systematic meta-analyses have reviewed key factors identified in the existing literature,8 with nuanced conclusions on the possible impact on VCR.9 The evolution of VCR over time is influenced by a complex combination of factors and previous work has highlighted the impact of programmatic factors,10–12 policy amenable factors13 and socio-psychological determinants. The latter are often evaluated through HCPs and/or patient surveys evidencing drivers and barriers to vaccination.14
The aim of our analysis was to identify factors that have been instrumental in achieving a high influenza VCR in the scope of four benchmark countries in order to provide an organised list of factors that could be used by countries with lower VCRs to help improve and strengthen their own VCRs.
Materials and methods
Selection of benchmark countries
In order to facilitate the research, only English-speaking countries were included in the analysis. Additional prerequisites were good access to public health records and documented progression from a low to a high influenza VCR (close to 75%) in older adults. At the time of the analysis, only two countries (Mexico and South Korea) had achieved the 75% VCR threshold but were not included as they are not English speaking. Four benchmark countries were chosen that fulfilled these prerequisites: Australia, Canada, UK and USA.
Research methodology
We used a two-step process to identify the factors that underpin high influenza VCRs in the four benchmark countries. First, a review was conducted of the available evidence in published literature and publicly available reports from health institutions, including health authority (HA) reports, academic literature, market research reports and expert conference presentations, to objectively document the historical perspective for each country. This was done initially at a global level to establish a catalogue and clustering of factors.11,12,15 The evaluation was subsequently fine-tuned and confirmed based on a country-level evaluation but a systematic review approach was not used.
Secondly, following the review of publicly available evidence in the four benchmark countries, semi-structured telephone interviews of key informants, stakeholders and experts of approximately 1 hour in duration were conducted by Corporate Value Associates (Paris, France) to further detail the understanding of the growth of VCR in each country. Key informants were important nodes in the network of individuals related to influenza VCR-related activities, whereas stakeholders played a role in influenza VCR-related activities and experts were knowledgeable about influenza VCR-related activities in their own country but not necessarily part of the network of key informants; these included public authorities, HCPs at General Practice (GP) surgeries and pharmacies, patient/healthcare associations and influenza experts. A list of potential interviewees was compiled based on the level of knowledge and expertise of the influenza programme in a given country whilst ensuring that the sample was representative of the different stakeholders. Each stakeholder was contacted separately (snowball sampling was not used) and included if they agreed to participate. The interviews were semi-structured to allow flexibility due to variability between countries and stakeholders’ expertise but were not modified during the course of the study and were conducted between August 2018 and December 2018.
Table 1 summarises the topics covered by the interviews, the types of information collected and the sources. In each country (Table 2), the main source of information for the review of publicly available evidence was HA reports. Specific information sources are referenced in Figure 1 (Australia), Figure 2 (Canada), Figure 3 (UK) and Figure 4 (USA). For the semistructured interviews, the main source of information in Australia and the UK was HCPs; in Canada and the USA, this was more evenly distributed amongst the different sources (Table 2).
Table 1.
Interview topics, questions and types of information collected, and sources.
| Topic | Questions and type of information | Example sources |
|---|---|---|
| Immunisation programme |
|
|
|
|
|
|
|
|
| Vaccination impact |
|
|
Table 2.
Sources of information for review of publicly available information and semi-structured interviews by country.
| Type of research Source of information |
Country | |||
|---|---|---|---|---|
| Australia | Canada | UK | USA | |
| Review of publicly available evidence | 59 documents | 88 documents | 37 documents | 65 documents |
| Health authority reports | 54% | 51% | 43% | 71% |
| Published literature | 22% | 14% | 22% | 5% |
| Unpublished data and analysis | 8% | 24% | 11% | 12% |
| Market research reports | 5% | 5% | 11% | 6% |
| Other | 11% | 6% | 13% | 6% |
| Semi-structured interviews | 14 interviews | 8 interviews | 9 interviews | 7 interviews |
| Health authorities | 21% | 25% | 33% | – |
| Healthcare providers | 43% | 12% | 44% | 13% |
| Patient associations or groups | – | 25% | 11% | 29% |
| Key opinion leaders | 21% | 25% | 11% | 29% |
| Medical societies | 14% | – | – | 29% |
| Other | 1% | 13% | 1% | – |
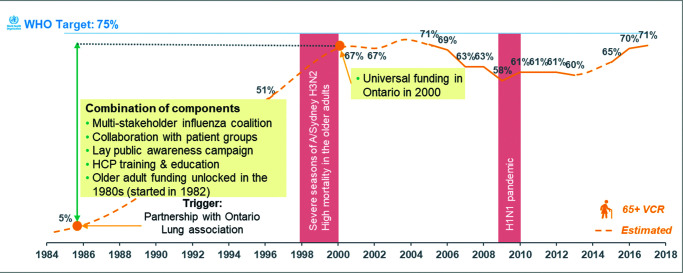
Figure 1. Evolution of influenza VCR in Australia (population ≥65 years, 1990–2017).
Sources: Hampson (1999),32 Health Stats NSW (2018),33 Hendry et al. (2017).34
HA, Health Authority; HCP, healthcare provider; KOLs, key opinion leaders; VCR, vaccination coverage rate.
Figure 2. Evolution of influenza VCR in Canada (population ≥65 years, 1976–2017).
Sources: Kwong et al. (2007),35 Buchan et al. (2016),36 Government of Canada (2016),37 Government of Canada (2019),38 Public Health Agency of Canada (2019).39
HCP, healthcare provider; VCR, vaccination coverage rate.
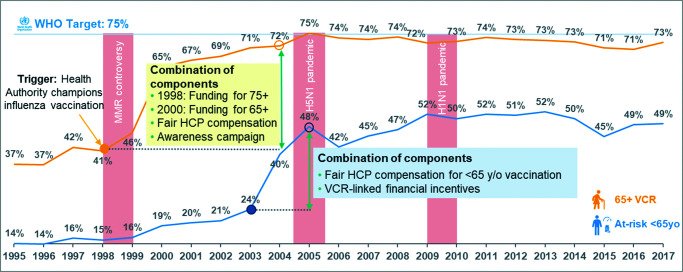
Figure 3. Evolution of influenza VCR in the UK (population ≥65 years and at risk <65 years, 1995–2018).
Sources: Joseph et al. (2017),40 Public Health England (2017),41 Public Health England (2018).42
HCP, healthcare provider; VCR, vaccination coverage rate.
Figure 4. Evolution of influenza VCR in the USA (population ≥65 years and general population, 1988–2018).
Sources: Centers for Disease Control and Protection (1993),43 Centers for Disease Control and Protection (2008),44 Centers for Disease Control and Prevention (2018),45 Lu et al. (2013).46
HCP, healthcare provider; VCR, vaccination coverage rate.
Data analyses and subsequent reclustering of factors
Secondary clustering and the definition of key pillars essential for establishing and maintaining a high VCR were performed after the initial two steps. Interviews were conducted and analysed sequentially, that is, interviews were not analysed and modified in an ongoing manner during the study. No quantified metric gap assessment was performed. All analyses were descriptive and no statistical comparisons were performed.
Outcomes of the interviews conducted were analysed qualitatively in the context of previous evaluations of factors for improving VCR.12,15 The identification of components for achieving a successful influenza VCR and the definition of pillars was discussed and agreed by a panel of influenza experts and researchers from each country that is represented by the authorship and acknowledgements of this article.
Results
Key factors and pillars for achieving a high influenza VCR
A total of 42 key factors (policies, programmatic factors and policy-amenable factors) were identified for a successful influenza vaccination programme. These were clustered into five main pillars (Figure 5 and Table 3) (in no order of priority), as follows:
Figure 5.

The five inter-connected influenza VCR pillars linked by three fundamental elements.
Table 3.
Key factors for the 5 pillars for a high influenza VCR.
|
Pillar 1 Health authority accountability and strengths of the influenza immunisation programme |
Pillar 2 Facilitated access to vaccination |
Pillar 3 HCP accountability and engagement |
Pillar 4 Awareness of influenza burden and severity of disease |
Pillar 5 Belief in influenza vaccination benefits |
|---|---|---|---|---|
| HA leaders willing to champion influenza vaccination | Access to multiple vaccination settings | Regular HCP education and training by multiple stakeholders | Structured and robust influenza surveillance network | Overall trust in influenza vaccine safety and effectiveness |
| VCR targets set at national and regional levels for recommended populations | Multiple HCPs allowed to vaccinate target population | Fair and specific HCP compensation per vaccination | Reliable collection and dissemination of data on influenza burden | Trust in the influenza vaccine as the most effective prevention |
| Nationwide regular monitoring of patient VCR at vaccination site/HCP level by HA | Convenient and seamless vaccination journey for all target populations | Attractive VCR-linked financial incentive for HCP | Proven evidence of the economic direct and indirect impacts of influenza | Public trust in HA and HCP communication |
| Data collection and reporting on HCP vaccination status | No financial barriers to getting immunised (i.e. no out-of-pocket expenses or cash layout) | Individual vaccination status visibility across providers (e.g. GP, pharmacist) | Published data on influenza-related disruption of the healthcare system and company productivity | Positive media coverage of vaccines |
| HCP VCR as part of performance criteria in hospitals and primary care | Awareness of vaccine recommendations by target populations | Competition through publication of VCR at vaccination area/HCP level | Coordinated multistakeholder communication campaigns | Effectively dealing with active anti-vax groups |
| Multistakeholder coalition supporting influenza immunisation | Reminder call-to-action communications to target groups by multiple stakeholders | Mandatory/strongly recommended HCP vaccination | Patient associations actively support influenza vaccination | Monitoring and responsiveness vaccine disinformation |
| Investment in pandemic preparedness | Vaccine dedicated refrigerators at vaccination setting (e.g. GP practice) | Simple influenza vaccine procurement process for GPs | Target populations motivated to get vaccinated | |
| Systematic assessment of cost-efficiency of VCR initiatives | HCP pop-up notification to vaccinate eligible individuals | HCP associations actively endorsing influenza vaccination | ||
| Regional HAs willingness to develop new initiatives to drive VCR | Availability of influenza vaccines (including cold chain management) in close proximity to the patients | Clear guidance about vaccine-specific usage per target population | ||
| Sustainable procurement system to ensure appropriate vaccine supply | ||||
| Funding of flu vaccinations for all recommended groups |
GP, general practitioner; HA, Health Authority; HCP, healthcare provider; VCR, vaccination coverage rate.
HA accountability and strengths (defined as efficiency, relevance and robustness of the influenza programme)
Facilitated access to vaccination
Healthcare professional accountability and engagement
Awareness of the burden and severity of disease
Belief in influenza vaccination benefits
Three fundamental elements (Figure 5) were generally applicable for each country and so were considered to be of particular importance and a priority for the implementation of a successful vaccination programme, namely
a multifaceted strategy, consisting of the implementation of numerous policies and interventions in a short period of time;
multistakeholder accountability, in which all stakeholders were mobilised and coordinated to have a maximum impact on vaccine uptake and
data collection and communication, whereby all countries had robust VCR and/or influenza burden data-generation systems to manage and monitor the impact of the national vaccination programme and were able to communicate this effectively.
Some of the 42 key factors were specific to decentralised health systems (Australia, Canada and the US), for example, influenza coalitions and data on the burden of disease driving conviction, whereas some were specific to centralised health systems (UK), for example, HAs leading stakeholder alignment, VCR targets and monitoring. However, overall, the high VCRs were all achieved through long-term, resolute HA commitment.
VCR evolution for each country: key findings
The evolution of VCR for older adults and at-risk populations in each country was characterised by step-changes following the implementation of a combination of factors triggered by a key event that raised the sense of urgency to take action. Subsequently, programmes were further strengthened and continuously improved with the addition of other factors to maintain high VCRs.
In Australia (Figure 1), media scare stories regarding influenza in 1990/1991 created a surge in vaccine demand and a concomitant shortage of supply. This resulted in vaccination of some healthy individuals at the expense of higher risk groups and led to closer collaboration between HAs and healthcare providers and a better understanding of manufacturing and logistical restraints. An Influenza Specialist Group (multistakeholder coalition) was created for educational purposes and it rolled out awareness campaigns in mass media and events in public settings for the general public whilst targeting the medical community through specialised press and events facilitated by key overseas speakers. The impact of such activities was monitored through media metrics as well as by annual patient attitudinal surveys. These activities resulted in a VCR rise in older adults from approximately 30% to 60–70%, which influenced authorities in two southern states to introduce free influenza vaccination for older adults. Later in the 1990s, state funding was followed by national funding and promotional efforts further improved VCR, which has since remained high. Further funding and policy reforms (e.g. changes to eligibility for free influenza vaccination) for all at-risk groups increased influenza VCR at a national level and were important during the H1N1 pandemic crisis around 2010, which led to funding for non-elderly at-risk individuals, a recommendation for the paediatric population, and the introduction of pharmacy vaccination. Other events, for example, a severe influenza season in 2017, have further driven public influenza awareness and vaccine uptake.
In Canada (Figure 2), the swine flu scare of 1976 stimulated the development of a pandemic plan that included ensuring vaccine-manufacturing capability. This, together with increasing appreciation of the burden of influenza, led to the development of a national surveillance system for influenza, government funding, and a national procurement process for seasonal vaccine for at-risk populations. A patient association, backed by industry, initiated the creation of a coalition to raise awareness of influenza vaccination from the mid-1980s, initially on a small scale in Ontario (the Ontario Lung Association) and later on a larger scale nationally (Canadian Public Health Association). Parallel campaigns in the USA also assisted in education and awareness. A Canadian national consensus conference was organised in 1993, in response to a VCR stalled at 50%, which led to national targets for vaccine coverage and multiple other recommendations for improvement. Together, these explain VCR growth from approximately 5% to 67% over 15 years. A Universal Influenza Immunisation Programme was adopted by Ontario in 2000, and later by other provinces following severe influenza seasons and high mortality in older adults in the late 1990s. VCR then declined as promotional programmes reduced in intensity, the 2009 H1N1 pandemic resulted in confusion with two vaccines being recommended simultaneously (although in the longer term, the H1N1 pandemic led to the introduction of pharmacy vaccination), and media coverage of limited vaccine efficacy against A(H3N2) strains increased. After the 2014/2015 season, reinvestment in education and vaccine promotion as well as increasing access to vaccination in pharmacies led to improved VCRs nationally.
In the UK (Figure 3), the Department of Health and Social Care established an efficient adult influenza programme in the 1990s. Data on the burden of influenza in older adults were used to advocate for the reimbursement of influenza vaccination by the NHS, the creation of fair compensation specific to vaccination to remunerate GPs and the launch of an awareness campaign, and VCR rose from 41% to 65% in 2 years. Since the 2000s, VCR data have been collected regularly to ensure appropriate financial compensation of GPs according to the number of eligible individuals vaccinated to monitor the performance of GP surgeries and potentially take supportive action where necessary and to inform evidence-based recommendations for continuous improvement and maintenance of a high VCR. Additional VCR-linked financial incentives were introduced to reach out proactively to at-risk groups (with the introduction of influenza VCR to the Quality and Outcomes Framework), namely patients with coronary heart disease, stroke/transient ischemia, chronic obstructive pulmonary disease and diabetes. This call and recall system introduced for at-risk patients contributed to a step-change in VCR in at-risk groups aged <65 years from 24% to 48% between 2003 and 2005. Despite having one of the world’s best-performing influenza immunisation programmes, the UK has continued to add factors, such as allowing pharmacists to administer the influenza vaccine to individuals aged 18 years and above and licensing new enhanced vaccines. In addition, public awareness campaigns during the H1N1 pandemic led to high public awareness and resulted in expanded HA vaccination recommendations to include children and the morbidly obese as well as the introduction of pharmacy vaccination.
In the USA (Figure 4), a Medicare pilot study in the early 1990s in ten states showed that VCR increased following the implementation of a range of factors (vaccine reimbursement, GP fair compensation, patient reminders, HCP education, and public awareness campaigns) and that this would be cost-effective assuming at least 40% VCR and 21–42% vaccine effectiveness.16 This prompted Medicare to roll out this approach to all US states, which led to an increased VCR17 and overall cost-savings.18 Medicare concluded that the cost per year of life gained due to influenza vaccination was substantially below the cost of other preventative interventions and included influenza vaccination under the Part B insurance from 1993 for adults aged 65 years and older. This was followed by funding from Medicare to promote vaccine awareness and uptake, and an expansion of providers authorised to administer vaccines, including compensation and financial incentives for practitioners, patient reminders, HCP education, and awareness campaigns, which led to improved VCR amongst older adults throughout the 1990s from <40% to 60–65%. In the early 2000s, problems with vaccine supply19 triggered a reaction from the US Government. Pandemic preparedness underpinned the strategy to secure a stable supply base and demand for seasonal influenza vaccines through higher levels of VCR. Later, a multistakeholder influenza coalition was created20 with support from the Centers for Disease Control and Prevention (CDC) and the American Medical Association, and is currently managed by the Immunization Action Coalition, the Office of Infectious Disease Policy of the Department of Health and Human Services, and the CDC. Whilst vaccination policy in the USA remains primarily driven by the CDC and their immunisation advisory committee,21 the multistakeholder coalition plays a key role in the coordination of communication messages and the assessment of root causes of implementation challenges amongst stakeholder groups. The expansion of policy from older adults to those aged 50 years or above, then to children from 6 months to 4 years of age, to all children and, finally, to everyone aged 6 months and above in response to the 2009 H1N1 pandemic22 along with, to a lesser extent, the broadened access through pharmacy-based immunisation23 were key to increasing VCR in the USA. Layered with this have been improvements in laboratory diagnosis of influenza and more diverse treatment options, including the use of antiviral treatments based on rapid test results, and improved surveillance of influenza has also been instrumental in the expansion of the CDC policy and increased VCR. However, overcoming vaccine hesitancy or refusal, influenced by reports of low vaccine efficacy, continues to be a challenge to further increasing VCR.
Discussion
Suboptimal vaccination coverage is a complex issue that can be influenced by socio-demographic, programmatic and socio-psychological factors (which consistently explain most of the variance in past influenza vaccination behaviour13) that have previously been organised by access, affordability, awareness, acceptance and activation.15 Although socio-psychological factors have been widely explored to understand the patient or HCP perspectives regarding motivations or barriers to vaccination,24 fewer studies or meta-analyses have specifically documented the actions taken by multiple relevant stakeholders to improve vaccine uptake.25
Our analysis focused on four benchmark countries in which we aimed to identify the programmatic factors and policy actions that underpinned successful increases in VCR in older adults to achieve close to the 75% target. This was achieved through an evaluation of country-specific documentation and stakeholder interviews rather than by using a systematic review approach. Each country started from a low VCR and achieved high vaccine coverage in older adults. It is crucial to note that each country achieved a successful VCR by using different approaches tailored to their country and/or health system specificities. We do not propose, therefore, a ‘silver bullet’ to be applied universally, neither did we aim to define such an endpoint, but rather we provide a set of five structural pillars from which we would recommend that multiple components could be selected to improve VCR in a given country depending on that country’s particularities. A country’s status as low, medium or high income may directly affect its VCR and its ability to implement certain of the cited components; it is therefore important to view the results of this research as a pool of components and not as an exhaustive checklist or defined combination of key success factors.
The 5 pillars and 42 components that we describe provide an influenza-specific schema that is detailed, demonstrable and practical. This list of possible components is actionable and solution-focused in terms of mechanisms for increasing VCR, and is complementary to and enriches the findings of the WHO evaluation of the introduction of influenza vaccination.26 Whilst annual influenza vaccination programmes have specificities that may not be evident in other vaccination programmes, our analysis may also provide some common factors and important insights for increasing the VCR of other vaccines. As such, we invite any country seeking to improve its VCR to perform a critical appraisal of its influenza vaccination programme against the list of components in each pillar. In this way, missing factors dependent on a country’s specificities can be identified and discussed before implementation.
In particular, the novelty of this research is that we have shown that each benchmark country has (1) implemented a combination of factors concomitantly, (2) mobilised multiple stakeholders to create their own solution for success and (3) generated and monitored VCR and/or burden of disease data to measure the success of those key changes. We have identified these as three fundamental elements for success and would recommend that any country aiming to improve its influenza VCR considers their implementation. Previously, a European study catalogued 17 policy elements (policies and policy actions) in different influenza programmes across Europe and showed some elements linked to higher VCRs in older adults (e.g. reminder systems, strong official recommendations and ease of access) but others did not have the expected effect (e.g. a summit meeting of experts before or during an annual vaccination campaign).11,12 A European Commission report has described vaccine uptake and barriers in EU member states, and country-specific research in the UK,27 US,28 Australia29 and Canada30 has identified socio-psychological barriers to vaccine uptake. Our benchmark country experience has identified 42 programmatic, policy and policy-amenable solution-oriented factors for success. However, we did not identify any single factor that accounted for successfully achieving a high VCR. Various triggers were identified, including political will, vaccine shortage or a severe epidemic reported in the media, which can be further reinforced by fact-based cost-effectiveness studies and alignment of interests between stakeholders. Additionally, in each country, the 2009 H1N1 pandemic acted as a trigger to some extent, leading to improvements such as expanded vaccination recommendations and pharmacy vaccination. The development of advocacy coalitions may be an effective driver of awareness as well as of the implementation of recommendations and guidelines through mobilisation and coordination of stakeholders from different sectors. Subsequently, following the implementation of the factors for success, one-off step-changes can result in improved VCR, but continuous improvement by steadily refining existing factors or adding more factors is also vital to build a resilience into the system that ensures sustained high VCR. Examples of factors used for fine tuning and maintaining VCR are strong communication of vaccine safety and the impact of vaccine effectiveness and related questions in the media and from the public domain. For instance, in the USA, the discussion around vaccine effectiveness has included other issues, such as quality of life, the incidence of severe illness and hospitalisations prevented by vaccination, and has been used to support the concept of vaccine-preventable disability.
Some factors for the five pillars can be applied universally, including national and regional VCR targets for recommended populations together with nationwide and local VCR monitoring, which can be on a weekly basis (Pillar 1), ensuring seamless access to vaccination with reminders send to all target populations (Pillar 2), ensuring regular HCP education and training as well as potential financial sanctions linked to low VCR (Pillar 3), and implementing coordinated media campaigns targeting both HCPs and the public with impact measured through behavioural science assessments (Pillars 4 and 5). However, whilst five pillars have been identified and some factors are common to each country, an important finding is that each benchmark country used different factors and no country has reached an ideal situation on each of the pillars.
Specific factors that apply particularly to centralised systems such as the UK represent an ‘enforcement’ approach, including HAs leading stakeholder alignment and implementing VCR targets and close performance monitoring with support available for underperforming GP practices. Those that apply particularly to decentralised systems, such as Australia, Canada and the USA, represent a ‘cooperative’ approach and include the creation of coalitions to coordinate and align stakeholders and the use of VCR and burden of disease data and HCP quality-improvement measures to drive vaccine conviction and uptake.
A commitment from stakeholders is required that goes beyond simply increasing funding; cultural shifts are instrumental, with both vaccine providers and recipients needing to recognise the benefits and importance of vaccination and a high VCR. Vaccination hesitancy remains a challenge to HCPs and public officials; however, the implementation of factors that improve service delivery to make it easy and convenient, communications informed by social and behavioural insights, and mobilisation of many partners may effectively take public attitudes beyond simple acceptance to the ultimate goal of active demand for vaccination.31 In addition, monitoring and assessment to enable a cycle of continual improvement of vaccination programmes also appears important to maintain resilient, high VCRs. In this regard, the use of VCR data and target setting and the implementation of information systems are critical and particularly evidenced by the UK experience.
The study has a number of limitations. First, it was limited to four English-speaking, high-income countries. This preselection was made based the ability to identify factors that have been integral to achieving high VCRs and, importantly, access to public health records was facilitated by the inclusion of only English-speaking countries. As such, the findings may only be applicable to high-income countries with similar resources to those included in the analysis and remain to be tested on middle- and low-income countries. In the future, this framework could be enriched by further analyses of other high-performing countries. Second, it is likely that there may be differences in VCR and the solution for success in different areas within individual countries, for example, interstate differences in Australia and Canada or between counties in the UK. The analysis stops at the country level and regional differences could be explored further. Third, there is the possibility of unconscious bias related to the selection of stakeholders. Lastly, the analysis is at a macro level in high-income countries, with recommendations to improve influenza VCR generally, and it is possible that differences could exist between at-risk populations, particularly between high- and low-income countries. In this context, future research is planned to evaluate VCR in low-performing countries.
In conclusion, the aim of the study to provide an organised list of components was met by describing a wide range of factors for improving VCR from an evaluation of four high-performing benchmark countries. As there is no single solution for success that is applicable to all countries, the classification of these factors into five distinct pillars for success provides a basis for countries with lower VCRs to tailor their own solutions by selecting components that could be implemented to improve VCR in a particular country. It is important to stress that no single combination of components will be applicable for all countries; rather, a country could evaluate its own performance and systems and identify any components that could be added to approaches that may already be in place. Developing the right combination of factors or elements, from each of the five pillars, in country-specific policies could lead to a rapid change in VCR if implemented appropriately.
Acknowledgements
The authors would like to thank all interviewees for their time and valuable insights into vaccination practices. This manuscript was prepared with the assistance of a professional medical writer, Dr Andrew Lane (Lane Medical Writing, Lyon, France), in accordance with the European Medical Writers Association guidelines and Good Publication Practice and funded by Sanofi Pasteur.
Footnotes
Contributions: AB: Participated in data interpretation and reviewed and approved the manuscript. FBP: Participated in the study design, highlighted key data and report for sourcing, interpreted the findings, structured the key messages and comparative overview, outlined, reviewed and approved the manuscript. AH: Participated in data acquisition and analysis and reviewed and approved the manuscript. GK: Participated in the study design, highlighted key data and report for sourcing, interpreted the findings, structured the key messages and comparative overview, outlined, reviewed, and approved the manuscript. JEM: Participated in country-specific interviews related to the influenza vaccination program in Canada. Highlighted reports from the Public Health Agency of Canada on a recent survey of current contributors and barriers to vaccine uptake in Canada. Reviewed and approved manuscript and added key references. AMcG: Participated in data acquisition and analysis and reviewed and approved the manuscript. TRdF: Structured and performed the comparative analysis across the four high-performing countries, sourced and reviewed the available evidence, carried out the interviews and codeveloped the 5-pillar framework and the list of factors. Interpreted the findings, structured the key messages, reviewed, and approved the manuscript. MR: Participated in country-specific interviews related to the influenza vaccination programs in the United States. Highlighted the work of the National Adult and Influenza Summit (NAIIS), CDC, HHS – National Vaccine Advisory Committee, coalitions and the engagement of pharmacists as members of immunisation neighbourhoods. Reviewed and approved the manuscript. HS: Participated in data analysis and interpretation and reviewed and approved the manuscript. LJT: Participated in data acquisition and analysis and reviewed and approved the manuscript. AT: Participated in the study design, highlighted key data and report for sourcing, interpreted the findings, structured the key messages and comparative overview, outlined, reviewed, and approved the manuscript. OV: Structured and performed the comparative analysis across the four high-performing countries, sourced and reviewed the available evidence, carried out the interviews and codeveloped the 5-pillar framework and the list of factors. Interpreted the findings, structured the key messages, reviewed and approved the manuscript. All authors are accountable for the accuracy and integrity of the manuscript.
Disclosure and potential conflicts of interest: No author received any direct payment from Sanofi Pasteur with regard to their contributions to this manuscript but could receive expenses for conference attendance for the presentation of data from this study. AT and FBP are employees of Sanofi Pasteur. AT currently works with UNICEF but the views expressed in this article are of AT and not UNICEF. AB has participated in advisory boards (Sanofi Pasteur), educational events (Sanofi Pasteur and Seqirus), and telephone interviews (Parexel). AB is an employee of Public Health England (PHE) but the views expressed in this article are of AB and not PHE. AH is a member and former Chairperson of the Immunisation Coalition (formerly the Influenza Specialist Group), an independent Australian not-for-profit organisation receiving financial support from influenza vaccine manufacturers, including Sanofi Pasteur. GK is President of the British Global and Travel Health Association and National Immunisation Lead of the Royal College of General Practitioners and has participated in advisory boards or lectured at meetings organised by Sanofi Pasteur, MSD, Seqirus, Pfizer, AstraZeneca, European Scientific Working Group on Influenza (ESWI) and UK’s National Health Service. He chairs the RAISE Pan-European Influenza Group and is Member of the ESWI Board of Directors. Additionally, GK is a former Editor in Chief of Drugs in Context and is currently Associate Editor for Primary Care. JMcE’s institution has received on her behalf honoraria and consulting fees from Sanofi for scientific presentations/participation in advisory boards and data monitoring boards (DMBs) and related travel costs, and holds an NIH R01AG048023 grant (independent of industry) comparing high-dose versus standard-dose fluzone in older adults. Her institution has also received honoraria for her presentations and participation in advisory boards and travel costs from GSK, Merck, Pfizer, ResTORbio, and Medicago for her role as a clinical trial lead from VBI and Jansen, and consulting fees for participation in DMBs for GSK and Merck. AMcG has received grants and personal fees from Sanofi Pasteur, GlaxoSmithKline, and Seqirus, outside the submitted work. TRdeF and OV are employees of Corporate Value Associates, Paris. MR’s wife is an employee of Merck. HS has received funding for investigator-driven research and fees to present at conferences/workshops and develop resources (bio-CSL/Sequiris, GSK and Sanofi Pasteur). LJT has no conflicts of interest to disclose. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2020/10/dic.2020-9-5-COI.pdf
Funding declaration: The work was funded by Sanofi Pasteur, Lyon, France.
Correct attribution: Copyright © 2021 Kassianos G, Banerjee A, Baron-Papillon F, Hampson AW, McElhaney JE, McGeer A, Rigoine de Fougerolles T, Rothholz M, Seale H, Tan LJ, Thomson A, Vitoux O. https://doi.org/10.7573/dic.2020-9-5. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.World Health Organization. Vaccines against influenza WHO position paper – November 2012. [Accessed September 28, 2020];Wkly Epidemiol Rec. 2012 87(47):461–476. https://www.who.int/wer/2012/wer8747.pdf?ua=1. [Google Scholar]
- 2.World Health Organization. Influenza (seasonal) 2014. [Accessed September 10, 2020]. http://www.who.int/mediacentre/factsheets/fs211/en/
- 3.World Health Organization. Global influenza strategy 2019–2030. [Accessed September 28, 2020]. https://apps.who.int/iris/handle/10665/311184.
- 4.European Union Council. Council recommendation of 22 December 2009 on seasonal influenza vaccination (2009/1019/EU) [Accessed September 28, 2020]. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:348:0071:0072:EN:PDF.
- 5.European Centre for Disease Prevention and Control. Risk groups for severe influenza. [Accessed September 28, 2020]. https://ecdc.europa.eu/en/seasonal-influenza/prevention-and-control/vaccines/risk-groups.
- 6.European Commission. State of health in the EU: companion report. 2019. [Accessed September 28, 2020]. http://www.ehealthnews.eu/download/publications/6014-state-of-health-in-the-eu-companion-report-2019.
- 7.Gurfinkel EP, de la Fuente RL, Mendiz O, Mautner B. Influenza vaccine pilot study in acute coronary syndromes and planned percutaneous coronary interventions: the FLU Vaccination Acute Coronary Syndromes (FLUVACS) Study. Circulation. 2002;105(18):2143–2147. doi: 10.1161/01.cir.0000016182.85461.f4. [DOI] [PubMed] [Google Scholar]
- 8.Thomas RE, Russell ML, Lorenzetti DL. Systematic review of interventions to increase influenza vaccination rates of those 60 years and older. Vaccine. 2010;28(7):1684–1701. doi: 10.1016/j.vaccine.2009.11.067. [DOI] [PubMed] [Google Scholar]
- 9.Brondi L, Higgins M, Noori T, et al. Drivers and barriers of seasonal influenza vaccination in the European Union and European Economic area (EU/EEA): a systematic review. Eur J Public Health. 2013;23(Suppl 1):ckt126.339. doi: 10.1093/eurpub/ckt126.339. [DOI] [Google Scholar]
- 10.European Commission. The organization and delivery of vaccination services in the European Union. [Accessed September 28, 2020]. https://ec.europa.eu/health/sites/health/files/vaccination/docs/2018_vaccine_services_en.pdf.
- 11.Blank P, Schwenkglenks M, Szucs TD. The impact of European vaccination policies on seasonal influenza vaccination coverage rates in the elderly. Hum Vaccin Immunother. 2012;8(3):328–335. doi: 10.4161/hv.18629. [DOI] [PubMed] [Google Scholar]
- 12.Blank PR, van Essen GA, Ortiz de Lejarazu R, et al. Impact of European vaccination policies on seasonal influenza vaccination coverage rates: an update seven years later. Hum Vaccin Immunother. 2018;14(11):2706–2714. doi: 10.1080/21645515.2018.1489948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheelock A, Miraldo M, Thomson A, Vincent C, Sevdalis N. Evaluating the importance of policy amenable factors in explaining influenza vaccination: a cross-sectional multinational study. BMJ Open. 2017;7(7):e014668. doi: 10.1136/bmjopen-2016-014668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmid P, Rauber D, Betsch C, Lidolt G, Denker ML. Barriers of influenza vaccination intention and behavior - a systematic review of influenza vaccine hesitancy, 2005–2016. PloS One. 2017;12(1):e0170550. doi: 10.1371/journal.pone.0170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomson A, Robinson K, Vallee-Tourangeau G. The 5As: a practical taxonomy for the determinants of vaccine uptake. Vaccine. 2016;34(8):1018–1024. doi: 10.1016/j.vaccine.2015.11.065. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Perspectives in disease prevention and health promotion final results: Medicare influenza vaccine demonstration – selected states, 1988–1992. [Accessed September 28, 2020];Morb Mortal Wkly Rep. 1993 42(31):601–604. https://www.cdc.gov/mmwr/preview/mmwrhtml/00021340.htm. [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Increasing influenza vaccination rates for Medicare beneficiaries – Montana and Wyoming. [Accessed September 28, 2020.];Morb Mortal Wkly Rep. 1994 44(40):741–744. https://www.cdc.gov/mmwr/preview/mmwrhtml/00039252.htm. [PubMed] [Google Scholar]
- 18.Nichol KL, Margolis KL, Wuorenma J, Von Sternberg T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N Engl J Med. 1994;331(12):778–784. doi: 10.1056/NEJM199409223311206. [DOI] [PubMed] [Google Scholar]
- 19.Lister S, Williams E. Influenza vaccine shortages and implications. 2004. [Accessed September 28, 2020]. https://maloney.house.gov/sites/maloney.house.gov/files/documents/olddocs/govreform/012704CRS_flu.pdf.
- 20.National Adult and Influenza Immunization Summit. [Accessed September 28, 2020]. https://www.izsummitpartners.org/
- 21.Centers for Disease Control and Prevention. Seasonal Influenza (flu): Summary: ‘Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) – United States, 2020–2021. [Accessed September 28, 2020]. https://www.cdc.gov/flu/professionals/acip/summary/summary-recommendations.htm.
- 22.Centers for Disease Control and Prevention. CDC’s Advisory Committee on Immunization Practices (ACIP) Recommends Universal Annual Influenza Vaccination. [Accessed September 28, 2020]. https://www.cdc.gov/media/pressrel/2010/r100224.htm.
- 23.Drozd EM, Miller L, Johnsrud M. Impact of pharmacist immunization authority on seasonal influenza immunization rates across states. Clin Ther. 2017;39(8):1563–1580.e1517. doi: 10.1016/j.clinthera.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Nagata JM, Hernandez-Ramos I, Kurup AS, Albrecht D, Vivas-Torrealba C, Franco-Paredes C. Social determinants of health and seasonal influenza vaccination in adults >/=65 years: a systematic review of qualitative and quantitative data. BMC Public Health. 2013;13:388. doi: 10.1186/1471-2458-13-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeung MP, Lam FL, Coker R. Factors associated with the uptake of seasonal influenza vaccination in adults: a systematic review. J Public Health. 2016;38(4):746–753. doi: 10.1093/pubmed/fdv194. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Influenza vaccine post-introduction evaluation (IPIE) [Accessed September 28, 2020]. https://www.who.int/immunization/research/development/ipie_influenza_post_introduction_evaluation/en/
- 27.Dexter LJ, Teare MD, Dexter M, Siriwardena AN, Read RC. Strategies to increase influenza vaccination rates: outcomes of a nationwide cross-sectional survey of UK general practice. BMJ Open. 2012;2(3) doi: 10.1136/bmjopen-2011-000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okoli GN, Abou-Setta AM, Neilson CJ, Chit A, Thommes E, Mahmud SM. Determinants of seasonal influenza vaccine uptake among the elderly in the United States: a systematic review and meta-analysis. Gerontol Geriatr Med. 2019;5 doi: 10.1177/2333721419870345. 2333721419870345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyda A, Karki S, Hayen A, et al. Influenza and pneumococcal vaccination in Australian adults: a systematic review of coverage and factors associated with uptake. BMC Infect Dis. 2016;16(1):515. doi: 10.1186/s12879-016-1820-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy M, Sherrard L, Dube E, Gilbert NL. Determinants of non-vaccination against seasonal influenza. [Accessed September 28, 2020.];Health Rep. 2018 29(10):12–22. https://www150.statcan.gc.ca/n1/pub/82-003-x/2018010/article/00003-eng.pdf. [PubMed] [Google Scholar]
- 31.World Health Organization. Assessment report of the global vaccine action plan: strategic advisory group of experts on Immunization. 2018. [Accessed September 28, 2020]. WHO/IVB/18.11. https://apps.who.int/iris/handle/10665/276967.
- 32.Hampson AW. Vaccination of the older adult: the Australian experience. Vaccine. 1999;17(Suppl 1):S63–S66. doi: 10.1016/S0264-410X(99)00109-7. [DOI] [PubMed] [Google Scholar]
- 33.HealthStats NSW. Influenza and pneumococcal disease immunisation. [Accessed September 28, 2020]. http://www.healthstats.nsw.gov.au/Indicator/com_flupneumoimmu_age/com_flupneumoimmu_comparison?&topic=Influenza&topic1=topic_res&code=com_flu%20res_inf.
- 34.Hendry A, Hull B, Dey A, et al. NSW annual immunisation coverage report. 2017. [Accessed September 28, 2020]. https://www.health.nsw.gov.au/immunisation/Documents/2017-annual-coverage-report.pdf.
- 35.Kwong JC, Rosella LC, Johansen H. Trends in influenza vaccination in Canada, 1996/1997 to 2005. [Accessed September 28, 2020.];Health Rep. 2007 18(4):9–19. https://www150.statcan.gc.ca/n1/pub/82-003-x/2006010/article/vaccination/10355-eng.pdf. [PubMed] [Google Scholar]
- 36.Buchan SA, Kwong JC. Trends in influenza vaccine coverage and vaccine hesitancy in Canada, 2006/07 to 2013/14: results from cross-sectional survey data. CMAJ Open. 2016;4(3):E455–E462. doi: 10.9778/cmajo.20160050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Government of Canada. Vaccine uptake in Canadian adults: results from the 2014 adult National Immunization Coverage Survey. [Accessed September 28, 2020]. https://www.canada.ca/en/public-health/services/publications/healthy-living/vaccine-uptake-canadian-adults-results-2014-adult-national-immunization-coverage-survey.html.
- 38.Government of Canada. Immunization coverage and registries. [Accessed September 28, 2020]. https://www.canada.ca/en/public-health/services/immunization-coverage-registries.html#a1.
- 39.Public Health Agency of Canada. Seasonal influenza vaccine coverage in Canada, 2017–2018. [Accessed September 28, 2020]. http://publications.gc.ca/collections/collection_2019/aspc-phac/HP40-198-2018-eng.pdf.
- 40.Joseph C, Goddard N, Gelb D. Influenza vaccine uptake and distribution in England and Wales using data from the General Practice Research Database, 1989/90–2003/04. J Public Health. 2005;27(4):371–377. doi: 10.1093/pubmed/fdi054. [DOI] [PubMed] [Google Scholar]
- 41.Public Health England. Seasonal influenza vaccine update in GP patients: winter season 2016 to 2017. [Accessed September 28, 2020]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/613452/Seasonal_influenza_vaccine_uptake_in_GP_patients_winter_season_2016_to_2017.pdf.
- 42.Public Health England. Seasonal influenza vaccine update in GP patients: winter season 2017 to 2018. [Accessed September 28, 2020]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/710416/Seasonal_influenza_vaccine_uptake_in_GP_patients_winter_season_2017_to_2018..pdf.
- 43.Centers for Disease Control and Prevention. Perspectives in disease prevention and health promotion final results: medicare influenza vaccine demonstration – selected states, 1988–1992. [Accessed September 28, 2020.];Morb Mortal Wkly Rep. 1993 42(31):601–604. https://www.cdc.gov/mmwr/preview/mmwrhtml/00021340.htm. [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention. Self-reported influenza vaccination coverage trends 1989–2008 among adults by age group, risk group, race/ethnicity, health-care worker status, and pregnancy status, United States, National Health Interview Survey (NHIS) [Accessed September 28, 2020]. https://www.cdc.gov/flu/pdf/professionals/nhis89_08fluvaxtrendtab.pdf.
- 45.Centers for Disease Control and Prevention. Influenza vaccine coverage (FluVaxView) [Accessed September 28, 2020]. https://www.cdc.gov/flu/fluvaxview/index.htm.
- 46.Lu PJ, Singleton JA, Euler GL, Williams WW, Bridges CB. Seasonal influenza vaccination coverage among adult populations in the United States, 2005–2011. Am J Epidemiol. 2013;178(9):1478–1487. doi: 10.1093/aje/kwt158. [DOI] [PMC free article] [PubMed] [Google Scholar]