Abstract
Introduction
Smoke-free homes (SFHs), the voluntary adoption of home smoking restrictions, are associated with reduced secondhand smoke exposure. However, SFHs are uncommon in permanent supportive housing (PSH) for formerly homeless adults, who have fivefold higher smoking rates than the general population. We pilot-tested a brief intervention to increase voluntary adoption of SFHs among PSH residents in the San Francisco Bay Area.
Aims and Methods
We pilot-tested a brief intervention to increase voluntary adoption of SFHs among PSH residents in the San Francisco Bay Area. Rest of the methods, PSH residents (n = 100) and staff (n = 62) from 15 PSH sites participated in the intervention between October 2017 and February 2018. Research staff provided counseling to PSH residents on how to adopt an SFH and trained PSH staff on how to counsel residents on smoking cessation. The primary outcome was self-reported voluntary adoption of an SFH for ≥90 days, and the secondary outcome was carbon monoxide-verified PPA at 6-month follow-up. PSH staff completed the Smoking Knowledge, Attitudes, and Practices survey at baseline and 3-month follow-up.
Results
At 6 months, 31.3% of PSH residents had adopted an SFH (vs. 13.0% at baseline) and 16.9% reported carbon monoxide-verified PPA. A positive attitude toward an SFH policy was associated with increased odds of SFH adoption (adjusted odds ratio = 8.68, 95% confidence interval: 2.42, 31.17). Voluntary SFH adoption was associated with increased PPA (adjusted odds ratio = 26.27, 95% confidence interval: 3.43, 201.30). PSH staff reported improved attitudes toward and self-efficacy in delivering cessation care, and decreased barriers to discussing smoking cessation among PSH residents between baseline and 3-month follow-up.
Conclusions
In this single-arm study, a brief intervention increased SFH adoption and PPA among PSH residents.
Implications
To date, few interventions have addressed SFHs and their association with tobacco use among PSH residents. A “ground-up” approach that relies on buy-in from residents and that promotes voluntary SFHs is an innovative way to increase smoke-free living environments in PSH. This approach could pave a pathway for smoke-free policy implementation in these sites. PSH can play a role in reducing the burden of tobacco use by empowering its residents to adopt voluntary SFHs, which could increase smoking cessation among residents.
Introduction
In 2017, over 376,000 people in the US resided in permanent supportive housing (PSH),1 which is subsidized housing with closely linked voluntary supportive services for formerly homeless individuals.2 Housing First, the most common approach, prioritizes housing stability over preconditions of abstinence or requirements to engage in services prior to entry into housing or to maintain housing.2 PSH residents who have an income pay 30% of their monthly income on rent, with government subsidies covering the difference. Over 70% of PSH residents live with mental health, physical conditions, and/or substance use disorders.3,4 Cigarette smoking is higher among populations living in PSH compared with the general population; over 50% of PSH residents report current smoking.5 Smoking-related cancer, cardiovascular, and pulmonary diseases contribute to over 60% of the all-cause mortality among these populations.6
Smoking also incurs a financial burden. PSH residents who smoke spend, on average, 12% of their monthly income on cigarettes.5 Smoking expenditure can pose an opportunity cost, limiting scarce funds for essentials such as food or housing.7 The considerable health- and financial-impact point to the urgent need for interventions to reduce the burden of tobacco use among this population.
Effective tobacco control interventions include smoke-free policies and access to cessation assistance (eg, behavioral counseling, pharmacotherapy). Most PSH sites do not have mandated smoke-free policies restricting indoor smoking, nor do they provide cessation interventions.8 In contrast, mandated policies restricting indoor smoking are present in other types of multiunit housing such as the US Department of Housing and Urban Development’s public housing authority housing.9,10 There are several reasons for the absence of smoke-free policies in PSH. First, there is concern that a mandated smoke-free policy might contradict Housing First’s harm reduction framework if PSH providers use eviction as a punitive consequence to a smoke-free policy violation.8 Second, PSH residents’ high rates of mental health conditions, substance use, and cognitive impairments may present challenges to policy adherence. Third, lack of appropriate repercussions to violations is another barrier as eviction is unethical for homeless-experienced individuals.8
However, the promotion of a voluntary smoke-free home (SFH) could increase access to smoke-free living in PSH.11 An SFH is the adoption of a voluntary no-smoking rule in one’s home.12 SFHs are associated with reduced exposure to secondhand smoke among nonsmokers13 and reduced consumption, increased quit attempts, and reduced smoking relapse among smokers.12,14 SFHs have been shown to reduce cigarette smoking behaviors among low-income vulnerable groups with high proportion of mental health and/or substance use disorders.15,16 Voluntary SFHs may be helpful in settings such as PSH where there are challenges to implementing smoke-free policies, and could be the first step to implementing a building-wide smoke-free policy.
In this single-arm study, we developed and pilot-tested a multifaceted SFH intervention to increase the voluntary adoption of SFHs among PSH residents. Guided by social cognitive theory (SCT) and our formative work,8,17 the brief intervention consisted of counseling for PSH residents on how to adopt SFHs and a training for PSH staff on how to deliver brief smoking cessation counseling. We hypothesized that the SFH intervention would increase SFH adoption, which had the potential to increase point prevalence smoking abstinence (PPA) among PSH residents.
Methods
Participants and Setting
We partnered with six San Francisco Bay Area agencies that provided PSH to over 4000 formerly homeless individuals. In our formative work, we focused on 23 sites where clientele had high smoking rates and interventions to reduce tobacco use could be beneficial.8,17 Of these 23 sites, we selected 15 sites (three from Agency 1; three from Agency 2; four from Agency 3; two from Agency 4; two from Agency 5; one from Agency 6) that were willing and had the capacity to pilot-test the SFH intervention in their sites.8 Two sites were required to adopt smoke-free policies because of local ordinances; one enforced the policy.
We recruited PSH resident and staff participants between October 2017 and February 2018 using purposive sampling based on interest and availability to participate. Eligible PSH residents were aged 18 years or older, current smokers (smoked 100 cigarettes or more in their lifetime and within the past 7 days), able to provide informed consent, and planned to live in the PSH property for 12 or more months. Eligible PSH staff were employed (case manager, counselor, director, nurse, property manager, social worker) at the sites, aged 18 years or older, and able to provide informed consent. All study procedures took place in a private room at the PSH site. The institution’s committee on human research approved all study procedures (IRB #16-1992).
Interventions
SFH Intervention for PSH Residents
In our formative work, we used the SCT to identify environmental, personal, and behavioral factors that influenced the adoption of SFHs.8,17 For example, social norms around smoking, smoke-free policies, and access to cessation resources were environmental-level factors. Personal factors included stress, mental health or substance use disorders, or lack of knowledge about tobacco use or cessation. Behavioral factors include incentive motivations, self-efficacy, or practices to change behavior. Using this formative work,8,17 we mapped out intervention content to the following constructs in the SCT: behavioral capability (imparting knowledge/skills), reinforcements (internal/external reinforcements, incentivization), expectations (goal settings), and self-efficacy (materials to increase self-efficacy).
Research staff administered a one-time, 1-hour, face-to-face counseling session to PSH residents that consisted of (1) a step-by-step guide on how to adopt a voluntary SFH adapted from previous health communication materials,11 (2) information and infographics on the harmful consequences of secondhand smoke, thirdhand smoke, and e-cigarettes,18–20 (3) presentation and discussion of the nine Food and Drug Administration’s proposed graphic cigarette pack warning labels,21 (4) interactive worksheet on calculating expenditures related to smoking, and (5) pledges to designate one’s home as smoke-free.11 Additionally, we provided financial incentives for the amount of $25.00 at 3- and 6-month follow-up to PSH residents who had (1) adopted an SFH at follow-up when they reported no SFH at baseline or (2) maintained an SFH at follow-up and had biochemically verified smoking reduction or cessation when they reported an SFH at baseline.
PSH Staff Intervention
We invited PSH staff who engaged directly with residents to participate in a group training on how to deliver brief cessation counseling. Although participation was voluntary, agency leadership at each site encouraged all PSH staff to attend. To facilitate attendance, we conducted trainings during prescheduled staff meetings. Research staff delivered a one-time, 1.5-hour training to PSH staff on how to provide brief cessation counseling.22 The training addressed the following topics: tobacco use in the homeless population, nicotine addiction, brief smoking cessation counseling (ask, advise, and refer; 2As and R),23 and moderate intensity smoking cessation counseling (ask, advise, assess, assist, and arrange; 5As),24 tobacco cessation medications, local cessation resources, and counseling on how to implement an SFH. The training also included a practicum on identifying ways to integrate cessation services into other support services such as (1) procedures for screening and tracking smoking, (2) policies that could trigger referrals to cessation services, (3) approaches to discussing the financial impact of smoking, and (4) strategies to discussing cessation in the context of tobacco-related health and financial consequences.
Data Collection
Research staff administered a questionnaire to PSH residents at baseline, and 3- and 6-month follow-up, and to PSH staff at baseline and at 3-month follow-up. All participants received a $15.00 gift card for completing the baseline questionnaire and $10.00 for each follow-up questionnaire. These amounts were based on our prior work with this population.25–27 In total, PSH resident participants could receive up to $85.00, inclusive of the $25.00 incentive for voluntarily adopting an SFH at 3- and 6-month follow-up. Participants were informed about the potential to receive these incentives during informed consent.
Measures
PSH Resident Sociodemographics and Other Covariates
We asked PSH residents to self-report their age, gender (female, male, transgender), race/ethnicity, education (< high school, high school/GED, some college, college/professional training), and household income (disability, interests, salary, SSI, pensions, public assistance). We asked them to report a history of mental health (anxiety, depression, PTSD, schizophrenia) and/or chronic health conditions (asthma, bronchitis, cancer, COPD, coronary artery disease, diabetes, hypertension, stroke). PSH residents reported lifetime and past 30-day use of alcohol, cannabis, cocaine and/or crack cocaine, prescription and/or illicit opiates, and amphetamines.
Cigarette Smoking Behaviors
PSH residents reported whether they smoked daily or some days, the number of days smoked in the past 7 days, and the number of cigarettes smoked on each smoking day. We used this information to estimate average daily cigarette consumption. PSH residents reported the time to first cigarette after waking (≤5, 6–30, 31–60, >60 min). We reported nicotine dependence using the heaviness of smoking index (HSI),28 categorized as low (0–2), moderate (3–4), or high (5–6) dependence.
Tobacco-Related Expenditures
We asked PSH residents to report their household income from all sources and the amount of money spent on cigarettes in the past week to calculate tobacco-related monthly expenditures.
Smoking Cessation Intention and Attempts
PSH residents reported their intention to quit smoking (“Never expect to quit,” “May quit in the next 6-months,” “Will quit in the next 6-months,” and “Will quit in the next month”), whether they had made a quit attempt in the past year, and the length of the last quit attempt. At each follow-up, participants reported whether they had attempted to quit since the last visit and the length of that attempt.
Attitudes Toward Smoke-Free Policies
Using questions from prior studies, we created a five-item scale measuring attitudes toward smoke-free policies.29 We worded statements as: “I believe this property should have an outside area where smoking is not allowed,” “I support a smoking ban where smoking is not allowed in any indoor areas of the property, including my apartment,” “Banning smoking in the property would improve the health of tenants,” “Banning smoking in the property would reduce smoking among tenants,” and “I believe this property should have an outside area where smoking is allowed.” We averaged responses (score 0–4) with a higher score indicating a favorable attitude (α = .54).
Noncigarette Tobacco Use
We asked participants about lifetime and past 30-day use of noncigarette tobacco products including blunts, cigarillos, e-cigarettes, roll-your-own tobacco, and smokeless tobacco. We asked whether they had used any combustible noncigarette tobacco products in their homes in the past 30 days.
Measures Related to SFH Adoption
We asked PSH residents whether they had adopted an SFH in the past 90 days (“In the past 3-months, have you previously tried to establish a smoke-free rule in your home/apartment?”) and the length of adoption (“For how many days were you smoke-free?”).
Primary and Secondary Outcomes
Our primary outcome was self-reported, voluntary adoption of an SFH for ≥90 days at 6-month follow-up. We created an ordinal variable that included three categories: no SFH, SFH for <90 days, and SFH for ≥90 days. We chose 90 days for the primary outcome because it indicated a complete ban on smoking in one’s home during the 90-day interval in between the study follow-up visits. Those who reported a ban for <90 days may have relapsed back to smoking in their homes at the time of the 3- or 6-month assessment. Our secondary outcome was PPA at 6-month follow-up. We defined PPA as a self-report of not smoking at all and having no cigarette consumption. We verified self-reported PPA with expired breath carbon monoxide (CO) using a handheld monitor, and designated abstinence as CO ≤ 5 parts per million [ppm])30 (Bedfont EC50 Smokerlyser; Bedfont Scientific Ltd.). Other outcomes were SFH adoption for ≥90 days at 3 months and PPA at 3-month follow-up.
PSH Staff Assessment
We asked PSH staff to report their age, sex (male, female, transgender), race/ethnicity, and education (< high school, high school/GED, some college, college/professional training). We asked PSH staff whether they smoked 100 cigarettes in their lifetime, and those who had, reported whether they smoked daily, some days or not at all. We administered the Smoking Knowledge, Attitudes, and Practices (S-KAP) survey31 at baseline and 3-month follow-up. The S-KAP scales (score 1–5) assessed knowledge of tobacco-related harm (knowledge; α = .89), beliefs about offering smoking cessation advice (beliefs; α = .69), barriers to providing cessation services (barriers; α = .57), beliefs of one’s own efficacy in promoting smoking cessation (efficacy; α = .61), and practices related to providing cessation advice and assistance in the past month (practices; α = .87).
Statistical Analysis
We reported sample and tobacco use characteristics for PSH residents and staff using median and interquartile range (IQR) for continuous variables and chi-square statistic for categorical variables. We reported the primary outcome of SFH adoption for ≥90 days and the secondary outcome of PPA at 6-month follow-up. We examined cigarette consumption and tobacco-attributable income by SFH adoption. We examined factors associated with self-reported adoption of an SFH (any SFH vs. none) and PPA, using mixed-effects logistic regression models, clustering by participant and PSH site, and accounting for correlation in responses within PSH residents. For SFH adoption model, we adjusted for attitudes toward smoke-free policies. For PPA model, we adjusted for any SFH adoption. We adjusted for age, sex, race/ethnicity, mental and chronic health conditions, HSI, and alcohol and cannabis use in all models. For PSH staff, we compared mean scores of the S-KAP scale between baseline and 3-month follow-up using a paired sample t-test. We performed analyses with STATA 14.2 (College Station, TX).
Results
Sample Characteristics and Tobacco Use Behaviors Among PSH Residents
We screened 108 PSH residents for eligibility; 100 met eligibility criteria, completed the intervention, and were included in the analysis (85.0% and 83.0% retention rates at 3 and 6 months, respectively, Supplementary Figure S1). Among PSH residents, median age was 58.5 (IQR 51.5–65.0) years, and 70.0% were racial/ethnic minorities (Table 1). The majority of participants reported a mental health disorder (73.0%) or chronic health condition (76.0%). PSH residents reported high rates of lifetime substance use (99.0%), with 78.0% reporting one or more forms of current use (49.0%, alcohol; 17.0%, amphetamines, 50.0% cannabis; 19%, cocaine and/or crack; 11.0%, opiates).
Table 1.
Demographic and Characteristics of Permanent Supportive Housing Residents (N = 100)
| Characteristic | n (%) |
|---|---|
| Age, y—median (IQR) | 58.5 (51.5–65.0) |
| Sex | |
| Female | 34 (34.0) |
| Male | 65 (65.0) |
| Transgender | 1 (1.0) |
| Race/ethnicity | |
| Hispanic | 13 (13.0) |
| Non-Hispanic Black | 49 (49.0) |
| Non-Hispanic Pacific Islander | 3 (3.0) |
| Non-Hispanic White | 30 (30.0) |
| Other/two races or more | 5 (5.0) |
| Educationa | |
| Less than high school | 23 (23.2) |
| High school or equivalent (GED) | 32 (32.3) |
| Some college | 28 (28.2) |
| College, professional training | 16 (16.2) |
| Yearly incomeb | |
| ≤$30,000 | 91 (92.9) |
| >$30,000 | 7 (7.1) |
| Years in facility—median (IQR) | 4.0 (1.6–8.0) |
| Mental health condition | |
| Anxiety | 44 (44.0) |
| Depression | 64 (64.0) |
| PTSD | 36 (36.0) |
| Schizophrenia | 22 (22.0) |
| Chronic health condition | |
| Asthma | 31 (31.0) |
| Bronchitis | 30 (30.0) |
| Cancer | 15 (15.0) |
| COPD | 30 (30.0) |
| Coronary artery disease | 16 (16.0) |
| Diabetes | 21 (21.0) |
| Hypertension | 46 (46.0) |
| Stroke | 16 (16.0) |
| Cannibis use (lifetime) | 92 (92.0) |
| Cannibis use in past 30 d | 50 (53.8) |
| Alcohol use (lifetime) | 99 (99.0) |
| Alcohol use in past 30 d | 48 (48.5) |
| Opiates use (lifetime)a | 32 (32.3) |
| Opiates use in past 30 d | 11 (34.4) |
| Amphetamines use (lifetime)a | 44 (44.0) |
| Amphetamines use in past 30 d | 17 (38.6) |
| Cocaine/crack use (lifetime)a | 77 (77.8) |
| Cocaine/crack use in past 30 d | 19 (24.7) |
COPD = chronic obstructive pulmonary disease; IQR = interquartile range; PTSD = post-traumatic stress disorder.
aOne participant did not respond.
bTwo participants did not respond.
The majority (78.0%) of PSH residents reported daily smoking and consuming a median of nine cigarettes per day (IQR 4.0–12.5, Table 2). Most PSH residents reported the current use of alternative tobacco products, with combustible tobacco being the highest (42.0%, roll-your-own; 26.0%, cigarillos; 17.0%, blunts) and smokeless tobacco being the lowest (1.0%). Only 8% reported current e-cigarette use.
Table 2.
Cigarette Smoking and Other Noncigarette Tobacco Use Patterns Among Permanent Supportive Housing Residents (N = 100)
| n (%) | |
|---|---|
| Cigarette use | |
| Daily smoking in past 7 da | 78 (78.0) |
| Less than daily smoking in past 7 d | 22 (22.0) |
| Daily cigarette consumption—median (IQR) | 9.0 (4.0–12.5) |
| Heaviness of smoking index (HSI) | |
| Low dependence (0–2) | 63 (63.0) |
| Moderate dependence (3–4) | 35 (35.0) |
| High dependence (5–6) | 2 (2.0) |
| Percent monthly income spent on cigarettes—median (IQR) | 10.0 (4.4–17.4) |
| Any SFH | 6.1 (3.2–8.6) |
| No SFH | 10.9 (4.7–20.0) |
| Smoking cessation intention | |
| Never expect to quit | 17 (17.0) |
| May quit in next 6 mo | 56 (56.0) |
| Will quit in next 6 mo | 17 (17.0) |
| Will quit in the next month | 9 (9.0) |
| Proportion attempting to quit in past year | 55 (55.0) |
| Longest quit attempt in past year (d)—median (IQR) | 9 (4.0–18.0) |
| Attitudes toward smoke-free policies—median (IQR) | 2.2 (1.8–2.6) |
| Noncigarette tobacco use | |
| Blunts use | |
| Lifetime | 49 (49.0) |
| Past 30 d | 17 (34.7) |
| Inside apartment | 14 (14.0) |
| Cigarillos use | |
| Lifetime | 69 (69.0) |
| Past 30 d | 26 (37.7) |
| Inside apartment | 15 (57.7) |
| E-cigarette use | |
| Lifetime | 46 (46.0) |
| Past 30 d | 8 (17.4) |
| Inside apartment | 7 (87.5) |
| Roll-your-own tobacco use | |
| Lifetime | 88 (88.0) |
| Past 30 d | 42 (47.7) |
| Inside apartment | 32 (76.2) |
| Smokeless tobacco usea | |
| Lifetime | 22 (22.2) |
| Past 30 d | 1 (4.5) |
SFH = smoke-free home; Lifetime = any use ever in life; IQR = interquartile range.
aOne participant did not respond.
SFH Adoption and PPA Among PSH Residents
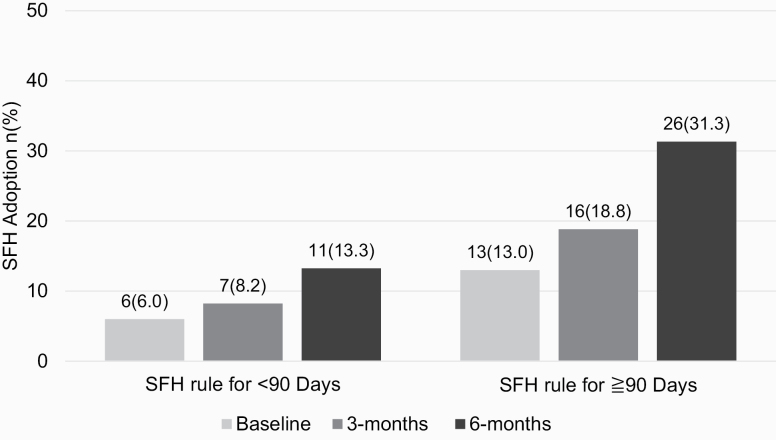
At baseline, 13.0% (n = 13) reported SFH adoption for ≥90 days, and for these participants, the follow-up focused on sustaining SFH adoption. The proportion who reported having voluntarily adopted an SFH for ≥90 days at 6 months was 31.1% (n = 26, Figure 1). At 3 months, 18.8% (n = 16) reported an SFH for ≥90 days. The proportion of residents who received financial incentives for any SFH adoption was 20.5% (n = 17) at 3 months and 39.8% (n = 33) at 6 months.
Figure 1.
Proportion of permanent supportive housing residents who reported voluntary adoption of a smoke-free home (SFH) for <90 d or ≥90 d.
Of the 13 participants who were smoke free at baseline, 8 were smoke free at 3- and 6-month follow-up. Of the 87 participants who did not have an SFH at baseline, 6 had an SFH at both 3- and 6-month follow-up, whereas 12 had an SFH at only 6-month follow-up (ie, 26 total who were smoke free at 6-month follow-up).
Overall, PSH residents’ daily cigarette consumption was lower at follow-up compared with baseline (median [IQR], 9.0 [4.0–12.5] at baseline, 5.0 [2.0–10.0] at 3 months, and 5.0 [2.0–10.0] at 6 months). However, those with an SFH reported half the daily cigarette consumption compared with those without an SFH (any SFH, baseline 5.0 [4.0–10.0], 3 months 3.0 [1.0–10.0], and 6 months 2.0 [0.0–5.0] vs. no SFH, baseline 10.0 [5.0–14.0], 3 months 6.0 [2.0–10.0], 6 months 7.0 [3.0–10.0]). Compared with baseline, where median (IQR) smoking-related expenditure was 10.0% (4.4–17.4), median expenditures reduced to 7.8% (2.6–17.8) and 5.3% (2.4–15.4) at 3 and 6 months, respectively. PSH residents with an SFH reported lower median tobacco expenditures compared with those without an SFH (any SFH, baseline 6.1% [3.2–13.0], 3 months 4.4% [0.0–8.6], and 6 months 4.0% [1.8–8.0] vs. no SFH, baseline 10.9% [4.7–20.0], 3 months 8.9 [4.0–18.2], and 6 months 7.0 [3.1–18.9]).
Compared with baseline, CO-verified PPA at 6-month follow-up was 16.9% and at 3-month follow-up was 7.1% (see Supplementary Figure S2).
Factors Associated With Any SFH Adoption and PPA Among PSH Residents
PSH residents had higher odds of adopting an SFH at 6 months (adjusted odds ratio [aOR] 10.82, 95% CI: 2.60, 45.02) compared with baseline (Table 3). PSH residents who had a positive attitude toward a smoke-free policy had higher odds of SFH adoption (aOR 8.68, 95% CI: 2.42, 31.17). In the model on PPA, residents who adopted any SFH were more likely to report PPA (aOR 26.27, 95% CI: 3.43, 201.30).
Table 3.
Factors Associated With Smoke-Free Home (SFH) Adoption and Point Prevalence Abstinence (PPA) Among Permanent Supportive Housing Residents (N = 100 for Both Models)
| SFH | PPA | |||
|---|---|---|---|---|
| aORa (95% CI) | p | aORa (95% CI) | p | |
| Visit (baseline referent) | ||||
| 3 mo | 2.97 (0.89, 9.91) | .07 | 16.46 (1.48, 183.36) | <.05 |
| 6 mo | 10.82 (2.60, 45.02) | .001 | 19.17 (1.41, 261.04) | <.05 |
| Age | 1.06 (0.99, 1.13) | .13 | 0.98 (0.90, 1.06) | .57 |
| Gender (female referent) | ||||
| Male/transgender | 1.84 (0.41, 8.36) | .13 | 1.26 (0.20, 8.00) | .80 |
| Race/ethnicity (NH White referent) | ||||
| Hispanic | 0.15 (0.01, 2.28) | .17 | 0.29 (0.01, 9.18) | .48 |
| Non-Hispanic Black | 0.49 (0.09, 2.63) | .40 | 1.21 (0.16, 8.92) | .85 |
| Other/two races or more | 0.26 (0.02, 2.88) | .27 | 1.66 (0.11, 25.75) | .72 |
| Attitudes toward smoke-free policies | 8.68 (2.42, 31.17) | .001 | ||
| Smoke-free home | 26.27 (3.43, 201.30) | <.01 | ||
| HSI (low dependence [0–2] referent) | ||||
| Moderate dependence (3–4) | 0.29 (0.07, 1.22) | .09 | ||
| High dependence (5–6) | 0.12 (0.00, 26.46) | .45 | ||
| Mental health condition | 1.78 (0.46, 6.92) | .47 | 3.47 (0.53, 22.59) | .19 |
| Chronic health condition | 0.88 (0.18, 4.23) | .87 | 1.05 (0.14, 8.05) | .97 |
| E-cigarette useb | 0.25 (0.01, 5.59) | .46 | 7.63 (0.43, 135.26) | .17 |
| Cigarillo useb | 0.34 (0.06, 1.91) | .22 | 0.16 (0.01, 3.02) | .22 |
| Blunt useb | 0.27 (0.04, 1.95) | .19 | ||
| Cannabis useb | 0.53 (0.15, 1.89) | .52 | 3.47 (0.64, 18.65) | .15 |
| Alcohol useb | 0.95 (0.30, 3.03) | .93 | 0.08 (0.11, 0.59) | <.05 |
Transgender resident participant (n = 1) was combined with male category, and NHPI resident participants (n = 3) were combined with “other/two races or more” for analyses. Bold type is p < .05 or less. aOR = adjusted odds ratio; HSI = heaviness of smoking index; Mental health = ever diagnosed with a mental health condition; NH = non-Hispanic; current = noncigarette tobacco or substance use in the past 30 d; CI = confidence interval.
aAdjusted for age, gender, race/ethnicity, and agency.
bUse in the past 30 d.
S-KAP Scores Among PSH Staff
In total, 98 PSH staff were screened for eligibility; of those, 62 were eligible and completed the intervention (85.4% retention rate at 3 months). Among PSH staff, the median age was 40.0 (IQR 23.0–70.0) years, 58.0% were female, and 51.7% belonged to racial/ethnic minority group (Supplementary Table S1). Over one half (51.6%) of PSH staff reported lifetime cigarette use, and 37.5% of lifetime users reported current smoking. Between baseline and 3 months, attitudes (mean 2.25 ± 0.58 vs. 2.45 ± 0.56; p < .01) toward and self-efficacy (2.02 ± 0.57 vs. 2.33 ± 0.60; p < .01) in delivering cessation care increased, whereas barriers (2.18 ± 0.43 vs. 1.97 ± 0.49; p = .01) to discussing smoking cessation decreased (Supplementary Figure S3). There were no significant changes in knowledge or practices.
Discussion
In this single-arm pilot study of currently smoking PSH residents, 31.3% reported voluntary SFH adoption and 16.9% reported PPA at 6 months after a brief SFH intervention. The proportion who had adopted an SFH adoption was double that from baseline, and the PPA estimate was similar to what has been achieved in cessation trials with behavioral counseling and pharmacotherapy among homeless adults (9%–18% PPA).32,33 Our findings highlight the feasibility of implementing an SFH intervention to increase voluntary adoption of SFHs, which in turn, may have increased PPA among this vulnerable population.
Introducing mandated smoke-free policies in PSH is fraught with concerns that policies may contradict PSH’s Housing First approach. PSH staff have suggested that policies could lead to unintended consequences such as increasing evictions for people who violate a smoke-free policy or increasing unsheltered homeless if people chose to leave their housing.2 This would contradict PSH’s primary goal to keep individuals stably housed. However, our study suggests that an individually directed intervention, guided by a “ground-up” approach that relied on resident buy-in and that provided residents with the tools necessary to voluntarily become smoke free, may be a feasible first step to increasing smoke-free living in PSH.
Previous studies among homeless adults and PSH residents have shown a positive association between a mandated smoke-free policy and reduced tobacco use.5,29 Although the focus of our study was not on a mandated smoke-free policy, persons who adopted a voluntary SFH had an increased likelihood of achieving PPA. There are several mechanisms by which SFHs increase PPA. SFHs promote an environment with minimal triggers to smoking, thereby increasing cessation attempts and reducing relapse to smoking.14,34 Smokers who adopt home smoking restrictions also report a smoke-free social network.14 An SFH rule may make it harder for smokers to smoke cigarettes on demand; therefore, an SFH may reduce smoking intensity prior to a quit attempt.14 SFHs may also pose challenges to smoking previously favorite cigarettes (eg, after a meal), potentially reducing consumption and/or relapse back to smoking after a quit attempt.14,16
Consistent with previous findings,35 PSH residents who adopted SFHs also reported decreased consumption, which resulted in lower median tobacco-related expenditures. At 6 months, median tobacco-related expenditure dropped by almost half among all participants, particularly among those with an SFH. An SFH can also increase efficacy of cessation medications and increase quit attempts among smokers.36 Future iterations of this intervention could benefit from increasing access to cessation medications to facilitate smoking cessation among smokers who adopt an SFH.
Low-income adults are less likely to adopt SFHs compared with adults in higher socioeconomic positions.37 However, quit rates are comparable between low- and higher-income individuals who have adopted an SFH,16 suggesting that SFHs can mitigate the income disparity in smoking cessation.37 Our study, along with others,11,15,37 highlights the need for interventions that increase adoption of SFHs in low-income households to increase smoke-free living and cessation rates among this population.
Our study targeted only cigarette smokers, given that over 50% of individuals living in PSH are current cigarette smokers.17 In a previous study of a PSH site that had adopted a mandated smoke-free policy, there was overall support for the policy.5 However, although current smokers reported lower levels of support pre-policy compared with nonsmokers, the proportional increase in support for the policy after implementation was greatest among current smokers.5 In our study, we found a 20% increase in support for a smoke-free policy that restricted smoking indoors between baseline and 6-month follow-up. PSH residents enrolled in the study had to anticipate being stably housed for at least 12 months; this period of housing stability could have increased their support for a smoke-free policy. Moreover, a favorable attitude toward a smoke-free policy was associated with increased SFH adoption in our study.
Unlike other studies, our study did not include nonsmokers.11,38,39 Many nonsmokers in PSH sites may temporarily live with smokers, or have friends or visitors who smoke in their homes. Thus, promoting smoke-free living environments will also require the support of nonsmokers designating their homes as smoke free. If a critical mass of both smoking and nonsmoking residents in a building adopted SFHs, then this social norm effect could potentially lead to a voluntary, building-wide smoke-free policy. Although we focused on individual adoption of SFHs among smokers in PSH, subsequent research will explore whether a social norm or network effect of voluntary adoption of SFHs among smokers and nonsmokers could pave a pathway for a building-wide smoke-free policy.11
This study had several strengths. We developed a strong relationship with our PSH partners, highlighted by the fact that almost all PSH services staff participated in the trainings. PSH sites facilitated study procedures by providing a private space on-site for data collection and assisting in recruitment efforts. Intervention materials were freely available and developed previously by public health organizations that were leaders in tobacco-related health communications (eg, Centers for Disease Control and Prevention).
However, our study also has limitations. The small sample sizes limited our ability to examine other factors (eg, mental/physical health, substance use) and their association with SFH adoption and PPA. The odds ratio estimates for SFH adoption and PPA were statistically significant, but they had wide CI intervals, highlighting imprecision in these estimates. We sampled purposively and did not include a control group. Our study sample may not be representative of the general population residing in PSH who are smokers, as evidenced by the fact that our sample included more light than heavy smokers. SFH adoption was self-reported; participants may have been primed to report an SFH by virtue of viewing intervention materials on secondhand and thirdhand smoke. We were unable to assess the extent to which incentives influenced self-reported adoption of an SFH. The incentivization scheme did not biochemically validate SFH adoption and was tailored to residents who had self-reported an SFH at baseline. In future iterations of this research, we will explore biochemical verification (eg, passive air nicotine and/or air quality feedback).40,41
Our single-arm study showed that a brief intervention increased voluntary adoption of SFHs among PSH residents, which may have led to an increase in PPA. To date, few interventions have addressed SFHs and cessation among PSH residents, despite evidence of the negative tobacco-related health and financial impact. A “ground-up” approach that relies on resident buy-in by promoting voluntary SFHs provides an innovative way to increase smoke-free living environments in PSH and could pave a pathway for smoke-free policy implementation in these sites. PSH can play a role in reducing the burden from tobacco use by empowering residents to adopt voluntary SFHs, which could, in turn, increase cessation behaviors among this population.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Funding
This research and M.V. were supported by the Tobacco-Related Disease Research Program (grant 251P-0015), and A.D. was supported in part by National Cancer Institute (grant CA-113710).
Declaration of Interests
None declared.
References
- 1. Henry M, Mahathey A, Morrill T, et al. . The 2018 Annual Homeless Assessment Report (AHAR) to Congress. Part 1: Point-in-Time Estimates of Homelessness. In: U.S. Department of Housing and Urban Development, ed. Washington, DC; 2018. Available from https://files.hudexchange.info/resources/documents/2018-AHAR-Part-1.pdf. Accessed June 13, 2019. [Google Scholar]
- 2. Tsemberis S. Housing First: ending homelessness, promoting recovery and reducing costs. In: Ellen IG, O’Flaherty B, eds. How to House the Homeless. New York, NY: Russell Sage Foundation; 2010;1:37–56. [Google Scholar]
- 3. Stringfellow EJ, Kim TW, Gordon AJ, et al. . Substance use among persons with homeless experience in primary care. Subst Abus. 2016;37(4):534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bauer LK, Baggett TP, Stern TA, O’Connell JJ, Shtasel D. Caring for homeless persons with serious mental illness in general hospitals. Psychosomatics. 2013;54(1):14–21. [DOI] [PubMed] [Google Scholar]
- 5. Petersen AB, Stewart HC, Walters J, Vijayaraghavan M. Smoking policy change within permanent supportive housing. J Community Health. 2018;43(2):312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Henwood BF, Cabassa LJ, Craig CM, Padgett DK. Permanent supportive housing: addressing homelessness and health disparities? Am J Public Health. 2013;103 (Suppl 2):S188–S192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baggett TP, Rigotti NA, Campbell EG. Cost of smoking among homeless adults. N Engl J Med. 2016;374(7):697–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alizaga NM, Nguyen T, Petersen AB, Elser H, Vijayaraghavan M. Developing tobacco control interventions in permanent supportive housing for formerly homeless adults. Health Promot Pract. 2019:1–11. doi: 10.1177/1524839919839358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pizacani BA, Maher JE, Rohde K, Drach L, Stark MJ. Implementation of a smoke-free policy in subsidized multiunit housing: effects on smoking cessation and secondhand smoke exposure. Nicotine Tob Res. 2012;14(9):1027–1034. [DOI] [PubMed] [Google Scholar]
- 10. U.S. Department of Housing and Urban Development. Smoke-Free Policies in Public Housing Available from: https://www.hud.gov/sites/documents/12-25PIHN.PDF. Published 2012. Accessed June 13, 2019.
- 11. Kegler MC, Bundy L, Haardörfer R, et al. . A minimal intervention to promote smoke-free homes among 2-1-1 callers: a randomized controlled trial. Am J Public Health. 2015;105(3):530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. IARC Working Group on the Evaluation of the Effectiveness of Smoke-free Policies. Evaluating the Effectiveness of Smoke-Free Policies. IARC Handbooks of Cancer Prevention, Tobacco Control. Vol. 13 Lyon, France: WHO Press; 2009. [Google Scholar]
- 13. Rosen LJ, Myers V, Winickoff JP, Kott J. Effectiveness of interventions to reduce tobacco smoke pollution in homes: a systematic review and meta-analysis. Int J Environ Res Public Health. 2015;12(12):16043–16059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mills AL, Messer K, Gilpin EA, Pierce JP. The effect of smoke-free homes on adult smoking behavior: a review. Nicotine Tob Res. 2009;11(10):1131–1141. [DOI] [PubMed] [Google Scholar]
- 15. Semple S, Turner S, O’Donnell R, et al. . Using air-quality feedback to encourage disadvantaged parents to create a smoke-free home: results from a randomised controlled trial. Environ Int. 2018;120:104–110. [DOI] [PubMed] [Google Scholar]
- 16. Vijayaraghavan M, Messer K, White MM, Pierce JP. The effectiveness of cigarette price and smoke-free homes on low-income smokers in the United States. Am J Public Health. 2013;103(12):2276–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petersen AB, Elser H, Nguyen T, Alizaga NM, Vijayaraghavan M. Smoke-free or not: attitudes toward indoor smoke-free policies among permanent supportive housing residents. Am J Health Promot. 2020;34(1):32–41. [DOI] [PubMed] [Google Scholar]
- 18. Breatheeasymaine.org. Thirdhand Smoke Available from: https://breatheeasymaineorg/wp-content/uploads/2018/07/BE_RackCardThirdhandpdf. Published 2018. Accessed November 8, 2017.
- 19. Initiative T. Secondpaw Smoke Kills Available from: https://truthinitiativeorg/topics/policy-and-intervention/secondhand-smoke. Published 2018. Accessed November 8, 2017.
- 20. Centers for Disease Control and Prevention (CDC). Secondhand Smoke Can Infiltrate Into Other Units Through Hallways and Stairwells Available from: https://www.cdc.gov/tobacco/infographics/secondhand-smoke/index.htm. Accessed November 8, 2017.
- 21. Food and Drug Administration HHS. Required warnings for cigarette packages and advertisements. Final rule. Fed Regist. 2011;76(120): 36628–36777. [PubMed] [Google Scholar]
- 22. Vijayaraghavan M, Guydish J, Pierce JP. Building tobacco cessation capacity in homeless shelters: a pilot study. J Community Health. 2016;41(5):998–1005. [DOI] [PubMed] [Google Scholar]
- 23. Schroeder SA. What to do with a patient who smokes. JAMA. 2005;294(4):482–487. [DOI] [PubMed] [Google Scholar]
- 24. Anderson JE, Jorenby DE, Scott WJ, Fiore MC. Treating tobacco use and dependence: an evidence-based clinical practice guideline for tobacco cessation. Chest. 2002;121(3):932–941. [DOI] [PubMed] [Google Scholar]
- 25. Vijayaraghavan M, Tieu L, Ponath C, Guzman D, Kushel M. Tobacco cessation behaviors among older homeless adults: results from the HOPE HOME study. Nicotine Tob Res. 2016;18(8):1733–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vijayaraghavan M, Olsen P, Weeks J, McKelvey K, Ponath C, Kushel M. Older African American homeless-experienced smokers’ attitudes toward tobacco control policies – results from the HOPE HOME study. Am J Health Promotion. 2018;32(2):381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vijayaraghavan M, Penko J, Vittinghoff E, Bangsberg DR, Miaskowski C, Kushel MB. Smoking behaviors in a community-based cohort of HIV-infected indigent adults. AIDS Behav. 2014;18(3):535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kozlowski LT, Porter CQ, Orleans CT, Pope MA, Heatherton T. Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug Alcohol Depend. 1994;34(3): 211–216. [DOI] [PubMed] [Google Scholar]
- 29. Vijayaraghavan M, Pierce JP. Interest in smoking cessation related to a smoke-free policy among homeless adults. J Community Health. 2015;40(4):686–691. [DOI] [PubMed] [Google Scholar]
- 30. SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. [DOI] [PubMed] [Google Scholar]
- 31. Delucchi KL, Tajima B, Guydish J. Development of the Smoking Knowledge, Attitudes, and Practices (S-KAP) Instrument. J Drug Issues. 2009;39(2):347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shelley D, Cantrell J, Wong S, Warn D. Smoking cessation among sheltered homeless: a pilot. Am J Health Behav. 2010;34(5):544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldade K, Whembolua GL, Thomas J, et al. . Designing a smoking cessation intervention for the unique needs of homeless persons: a community-based randomized clinical trial. Clin Trials. 2011;8(6):744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hyland A, Higbee C, Travers MJ, et al. . Smoke-free homes and smoking cessation and relapse in a longitudinal population of adults. Nicotine Tob Res. 2009;11(6):614–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pierce JP, White MM, Messer K. Changing age-specific patterns of cigarette consumption in the United States, 1992–2002: association with smoke-free homes and state-level tobacco control activity. Nicotine Tob Res. 2009;11(2):171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gilpin EA, Messer K, Pierce JP. Population effectiveness of pharmaceutical aids for smoking cessation: what is associated with increased success? Nicotine Tob Res. 2006;8(5):661–669. [DOI] [PubMed] [Google Scholar]
- 37. Vijayaraghavan M, Benmarhnia T, Pierce JP, et al. . Income disparities in smoking cessation and the diffusion of smoke-free homes among U.S. smokers: results from two longitudinal surveys. PLoS One. 2018;13(7):e0201467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Williams RS, Stollings JH, Bundy Ł, et al. . A minimal intervention to promote smoke-free homes among 2-1-1 callers: North Carolina randomized effectiveness trial. PLoS One. 2016;11(11):e0165086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mullen PD, Savas LS, Bundy ŁT, et al. . Minimal intervention delivered by 2-1-1 information and referral specialists promotes smoke-free homes among 2-1-1 callers: a Texas generalisation trial. Tob Control. 2016;25(Suppl 1):i10–i18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilson I, Semple S, Mills LM, et al. . REFRESH–reducing families’ exposure to secondhand smoke in the home: a feasibility study. Tob Control. 2013;22(5):e8. [DOI] [PubMed] [Google Scholar]
- 41. Klepeis NE, Hughes SC, Edwards RD, et al. . Promoting smoke-free homes: a novel behavioral intervention using real-time audio-visual feedback on airborne particle levels. PLoS One. 2013;8(8):e73251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.