Abstract
Asthma and obstructive sleep apnea (OSA) are highly prevalent chronic conditions, and both are associated with systemic hypertension. Additionally, asthma and OSA reciprocally interact, mutually exacerbating each other. In this study, we tested the effect of allergen-induced lower airway inflammation and concurrent chronic intermittent hypoxia (CIH) on systemic blood pressure (BP), pulmonary function, and proinflammatory cytokines, in a rat model. Brown Norway rats were exposed to 43 days of normoxia (NORM) or CIH, concurrent with weekly house dust mite (HDM) challenges. BP was measured 1 day after the last HDM challenge. On day 44, pulmonary function was tested, and blood for Th-2 and Th-1 cytokine levels was collected. HDM significantly increased mean (P = 0.002), systolic (P = 0.003), and diastolic (P = 0.004) BP compared with saline-challenged controls. Higher mean BP significantly correlated to increased total respiratory system resistance (R2 = 0.266, P = 0.002), driven by an association with parenchymal tissue dampening (R2 = 0.166, P = 0.016). HDM relative to saline-challenged controls increased the expression of serum IL-6 (P = 0.008), but no relationships of systemic BP with IL-6 or any other cytokines were found. CIH did not alter the allergen-induced responses on BP, although it tended to increase the expression of serum IL-6 (P = 0.06) and monocyte chemoattractant protein-1 (MCP-1, P = 0.09), regardless of HDM challenge. Chronic allergen-induced airway inflammation results in systemic hypertension that is correlated to the degree of distal airway obstruction induced by the allergen. These effects do not appear to be explained by the associated systemic inflammation.
Keywords: asthma, hypertension, inflammation, intermittent hypoxia, lung, obstructive, sleep apnea, systemic
INTRODUCTION
Asthma, obstructive sleep apnea (OSA), and systemic hypertension (HTN) are some of the most prevalent chronic diseases that substantially contribute to annual health care costs and overall patient morbidity and mortality in the United States (10). Asthma affects ∼8% of the middle-aged population, while clinically significant OSA affects 5–14% of middle-aged adults (23, Center for Disease Control and Prevention: National Center for Health Statistics. US Department of Health and Human Services. Fast Stats A to Z. 2018; Available at https://www.cdc.gov/nchs/fastats/asthma.htm. Twenty nine percent of U.S. adults have diagnosable HTN (13).
Several lines of evidence suggest that asthma may predispose to systemic hypertension. Our group has recently shown a prevalence of HTN in asthmatic subjects higher than the estimated population prevalence (12). Among 812 asthmatic subjects, 46 yr old on average, 191 (24%) had HTN, whereas the population prevalence for this age group at the time had been estimated at 14%. Worse lower airway obstruction, as measured by forced expiratory volume in the first second of forced vital capacity as percentage of predicted value (FEV1%predicted), was associated with an increased prevalence of HTN. Moreover, in those with comorbid OSA, the prevalence of HTN was even higher, of 55%. Several putative explanations have been put forth. One concerns “spillover” systemic inflammation from the airways (27). An inverse relationship has been shown between FEV1%predicted and systemic inflammatory markers (27), and, in turn, systemic inflammation in the setting of chronic lung disease is a risk factor for cardiovascular disease (28). In addition, OSA, which is more prevalent in asthmatics, via its hallmark feature [chronic intermittent hypoxia (CIH)] could aggravate asthma and also cause HTN (27).
Accumulating evidence suggests that systemic inflammation may be the pathogenic link between lower airway obstruction and cardiovascular disease. Traditionally, allergic, IL-5, IL-4, and IL-13 centric Th-2 type of inflammation has been principally implicated in asthma pathobiology (15). More recently, a heterogeneity in the airway inflammation of asthma is increasingly recognized, with non-Th-2 pathways being also involved, leading in >50% of patients with persistent asthma to a more severe phenotype and fatal events (15). The lower airway inflammation of asthma leads to the release of Th-1 mediators, such as IL-6, IL-1β, and TNF-α, by the bone marrow in the systemic circulation (34), at levels that correlate with the degree of lower airway inflammation (14). Additionally, in OSA, a persistent low-grade Th-1 type of inflammation, related particularly to its intermittent hypoxia feature, is well recognized, leading to the associated cardiovascular consequences of the disease. Although a direct role of Th-2 inflammation in the systemic pathology associated with asthma is largely not studied, mild but chronic Th-1 systemic inflammation increases the risk of hypertension and cardiovascular mortality (2). IL-6, IL-1β, and TNF-α increase arterial wall stiffness (2). In addition, they impair the ability of the endothelium to secrete nitric oxide, a potent vasodilator, leading to endothelial dysfunction and chronic impairment of vasodilation (2, 28). Impaired endothelium-dependent vasodilation of the arterial circulation precedes the development of hypertension in the offspring and relatives of hypertensive patients (35). Moreover, proinflammatory cytokines, such as IL-6 and TNF-α, promote secretion of C-reactive protein by hepatocytes, which is also associated with worse hypertension, independent of other risk factors (2).
However, the current literature is limited by primarily cross-sectional, clinical, or population-based studies, and by lack of experimental investigations directly testing causal relationships of asthma in cardiovascular pathology. In this study, we aimed to test the effects of allergic lower airway inflammation on systemic hypertension and left ventricular (LV) mass in rats. In addition, we tested the ability of CIH to modulate these effects, and the role of systemic Th-1 (related to both allergic inflammation and CIH) and Th-2 (related to allergic inflammation) cytokines in these relationships. We hypothesized that rats with allergic lower airway inflammation will have higher systemic blood pressure and LV mass compared with rats without lower airway inflammation and that this effect will be amplified by CIH. Last, that systemic levels of Th-2 (IL-4, IL-5, IL-13, and eotaxin) and Th-1 [IL-1β, IL-6, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), and interferon-γ] proinflammatory cytokines will relate to both, measures of lower airway obstruction and systemic blood pressures.
METHODS
Animals.
Brown Norway (strain 091) male rats (n = 10–12/group), 51–75 days old, were purchased from Charles River Laboratories (Wilmington, MA). Animals were housed two to four per cage, with standard rat chow and drinking water provided ad libitum. All procedures received approval by the Animal Care and Use Committees of the University of Wisconsin and William S. Middleton Memorial Veterans’ Affairs Hospital.
Experimental protocol.
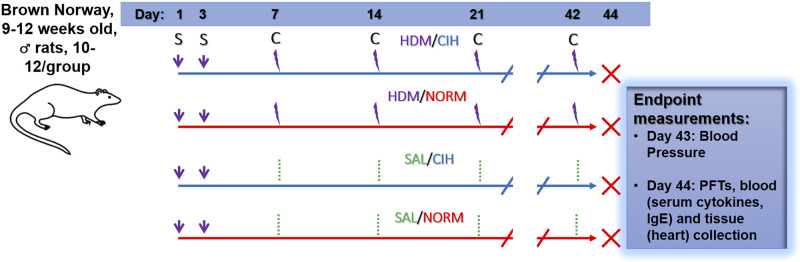
Study design and end-point measures are summarized in Fig. 1. On days 1 and 3 of the experiment, rats were sensitized with house dust mite (HDM) and then placed under normoxia (NORM) or chronic intermittent hypoxia (CIH) exposure for 43 days. On day 7, 14, 21, 28, 35, and 42 of the CIH exposure, animals were challenged with either HDM or sterile saline solution (SAL). On day 43, blood pressure was measured. On day 44, animals were subjected to pulmonary function testing and euthanized for blood and heart collection.
Fig. 1.
Experimental design. Brown Norway 9- to 12-wk-old male rats were exposed to 43 days of normoxia (NORM) or chronic intermittent hypoxia (CIH), concurrent with house dust mite (HDM) sensitization (S) on days 1 and 3. On day 7, and weekly thereafter, animals were challenged (C) with intratracheal HDM or saline (SAL) instillations. On day 43, blood pressure was measured, and the animals were returned to their exposure chambers. On the morning of day 44, the animals were removed from the chambers for end-point [pulmonary function testing (PFT) , blood for serum cytokines and IgE levels, and heart extraction] collection.
House dust mite sensitization and challenges.
HDM extract (B82; Stallergenes Greer) was reconstituted with sterile saline. Animals were sensitized by intratracheal instillation of 5 µg total HDM protein in 250 µL saline on days 1 and 3. HDM challenges were performed by intratracheal instillation of 10 µg HDM total protein in 250 µL saline. Control animals received intratracheal instillations of 250 µL SAL alone. Challenges commenced on day 7 of the experiment and thereafter, were administered weekly.
Chronic intermittent hypoxia exposure.
CIH exposure was performed essentially as we previously published (5), for 43 days (Fig. 1). Previous studies in rats have shown that CIH exposures as short as 2 days (21) and 5 wk (7) are sufficient to induce systemic hypertension and increase LV mass, respectively (7, 8, 21). On day 1, after the HDM sensitization, rats in their home cages were placed into a Plexiglass chamber and exposed to intermittent hypoxia for 10 h/day (from 0600 to 1600), daily. Oxygen concentration in the chamber was monitored using a heated zirconium sensor (Fujikura America, Pittsburgh, PA) connected to solenoid valves that controlled the flow of oxygen and nitrogen. The valves were operated by a microprocessor-controlled timer. Rats were exposed to 30 s of 10–11% inspired oxygen every 2 min, yielding 30 hypoxic episodes/h. Control rats were housed under normoxic conditions, adjacent to the hypoxia chamber. Chamber and room temperatures were maintained at 24 ± 1°F, and relative humidity was maintained between 20 and 70%. Ad libitum access was provided to standard rat chow and water. On the morning of day 44, the animals were transported from the CIH exposure room to the testing facility, for end-point experiments (Fig. 1).
Noninvasive blood pressure.
Noninvasive blood pressure was measured on the morning (0900–1200) of day 43 (Fig. 1), by the volume pressure recording method (11) with the CODA noninvasive blood pressure system (Kent Scientific Corporation), using the manufacturer’s default parameters for rats. In each session, three sets of 10 cycles were performed with a 10-s break between sets (a “cycle” is defined as one inflation and deflation of the occluding cuff). The first 15 cycles were used to acclimate the animal to restraint and tail cuffs; data from these cycles were discarded. Data from cycles 16–30 were checked for presence of artifacts and designated “valid” or “invalid.” Systolic, diastolic, and mean blood pressure from valid measurements were averaged, and the average values were recorded as the data points for that session. Before the end point blood pressure data collection day, on days 41 and 42, the animals were acclimated to the restraining devices and tail cuffs for 15–20 min/day. During these acclimation sessions, the rats were placed in the restraint devices, instrumented with the tail cuffs, and kept on the warming platform in a quiet room while under constant supervision by the experimenter.
Pulmonary function testing.
Pulmonary function testing was conducted essentially as previously described (4), on day 44. Briefly, animals were anesthetized by intraperitoneal injection of urethane (2.0 g/kg body wt), fitted with a metal endotracheal cannula (Harvard Apparatus), and placed on a FlexiVent FX4 system (Scireq), allowing partitioning of the overall respiratory system resistance (Rrs) in its components, Newtonian resistance (central airway, RN), and tissue impedance (distal airway resistance, G). Animals were mechanically ventilated (10 mL/kg body wt tidal volume, 90 breaths/min, 3 cmH2O positive end-expiratory pressure) and paralyzed with succinylcholine chloride (4 mg/kg body wt) to eliminate breathing effort artifact during testing.
Heart dissection and Fulton index.
After pulmonary function tests, animals were euthanized by exsanguination, under anesthesia. The heart was removed en bloc. The right ventricle along with atrium were dissected at the septum. The left ventricle, left atrium, and septum were weighed en bloc, separately from the right ventricle and right atrium en bloc. Because the mass of the atria are within the sensitivity error of our scale, we recorded these weights as the weights of the right ventricle (RV) and left ventricle plus septum (LVS). Fulton index was calculated as RV/LVS.
Serum cytokine levels.
Serum cytokine levels of Th-2 (IL-4, IL-5, IL-13, and eotaxin) and Th-1 (IL-1β, IL-6, MCP-1, MIP-1α, and interferon-γ) were measured using a custom-built multiplex Rat Cytokine Milliplex Panel (RECYTMAG-65K; Millipore Sigma, Burlington, MA) according to the manufacturer’s instructions. Cytokine concentration (pg/mL) was extrapolated from an internal standard curve using median fluorescence intensity. Assay sensitivities were 4.9 (for IL-4, IL-5, IL-13, and eotaxin), 2.4 (for IL-1β and MIP-1α), 73.2 (for IL-6), 29.3 (for MCP-1), and 16.6 (for interferon-γ) pg/mL.
Serum HDM-specific IgE titers.
Serum HDM-specific IgE titers were determined using a sandwich ELISA method. HDM extract proteins were biotinylated using the EZ-Link Micro Sulfo-NHS-Biotinylation Kit (21925; ThermoFisher Scientific), using 2.77 × 10−7 mol biotin for every 200 μg total protein in the HDM extract and following the manufacturer’s instructions. ELISA plates were coated with 2.5 μg/mL Mouse anti-Rat IgE (MA5–16810; ThermoFisher Scientific) in 1× PBS, 0.05% Tween 20, by incubating at 4°C overnight on a plate shaker. The wells were blocked with 1% (wt/vol) bovine serum albumin in 1× PBS, 0.05% Tween 20 (“Blocking Buffer”) and washed thoroughly with 1× PBS, 0.05% Tween 20. Rat sera, diluted 1:5 in Blocking Buffer, were loaded in the plate wells and incubated at 4°C overnight on a plate shaker. Serial 1:5 dilutions of a control serum (from an HDM-challenged Brown Norway rat with confirmed allergen-induced airway inflammation) were used as a standard curve. After the overnight incubation and several washes with 1× PBS, 0.05% Tween 20, biotinylated HDM extract (3 μg total protein/1 mL; diluted in Blocking Buffer) was added to the wells and incubated at room temperature for 1 h. This was followed by incubation with 1:1,500 Extravidin-HRP (E2886; Millipore Sigma, Burlington, MA) in Blocking Buffer and development of the chromogenic reaction with TMB reagent (T0440; Millipore Sigma). Absorbance at 450 nm was used to extrapolate the anti-HDM-specific IgE (α-HDM IgE) concentration.
Statistical analysis.
Results were graphed using Prism 7.0 software (GraphPad). Analyses were conducted using two-way ANOVA, with Holm-Sidak post hoc tests for comparisons between treatment groups. Associations of blood pressures with either airway physiological measures or systemic cytokine levels were tested using linear regression. Results with P < 0.05 were considered to be significant and those with P = 0.05–0.10 were considered trends. Data are summarized as means ± SE. Summary statistics are presented along with general treatment (HDM or CIH) effects from two-way ANOVA (followed, if significant or a trend, by Holm-Sidak post hoc test results for comparisons of the 4 groups) or with R2 and P value from the regression analyses.
RESULTS
Chronic airway inflammation increased systemic blood pressures.
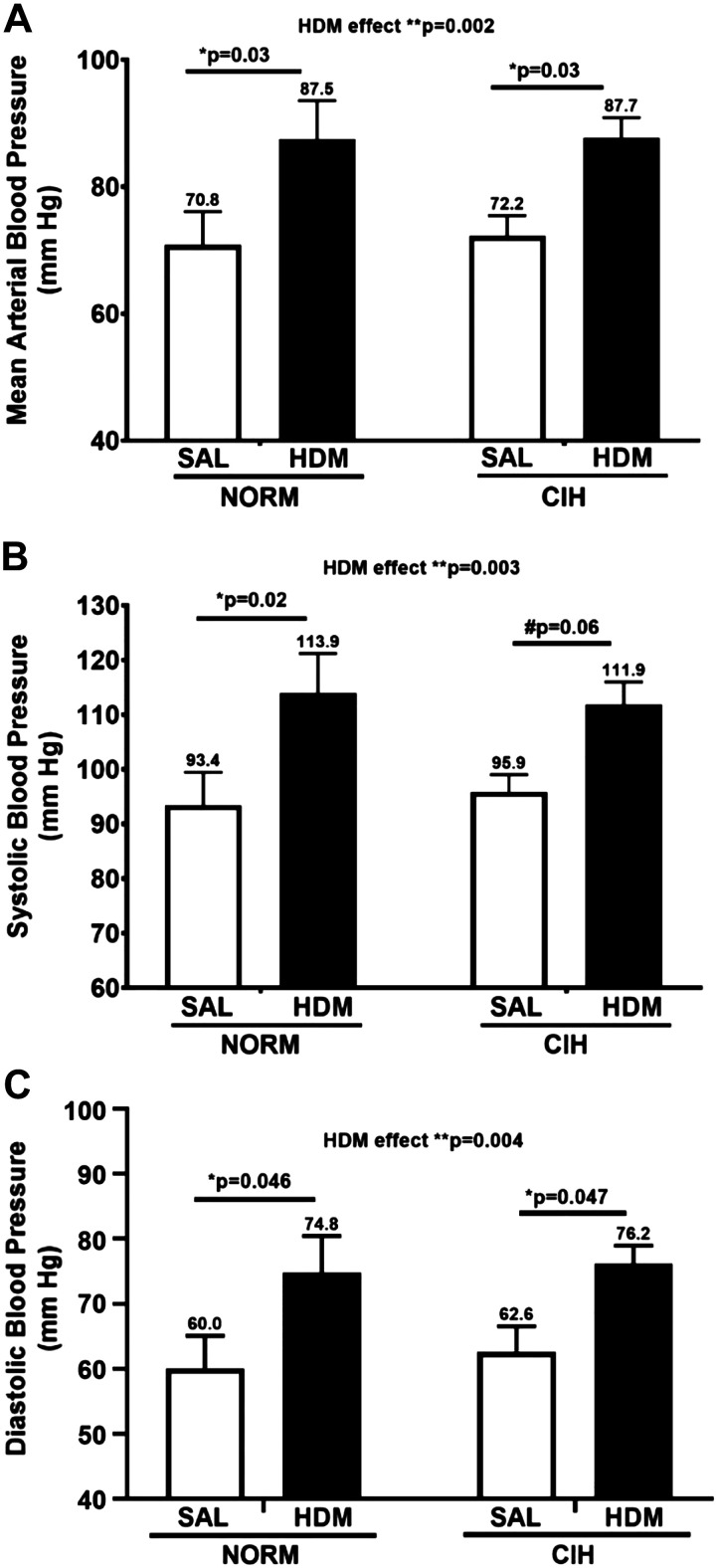
Relative to SAL-challenged animals, rats with allergic lower airway inflammation demonstrated higher mean blood pressure (MAP, mean + SEM: 87.6 ± 3.4 vs. 71.5 ± 3.1, general HDM effect P = 0.002, 2-way ANOVA; Fig. 2A), and both systolic (SBP, 112.9 ± 4.1 vs. 94.6 ± 3.6, general HDM effect P = 0.003, 2-way ANOVA; Fig. 2B) and diastolic (DBP, 75.5 ± 3.1 vs. 61.2 ± 3.2 mmHg, general HDM effect P = 0.004, 2-way ANOVA; Fig. 3C) blood pressures, regardless of CIH exposure. These HDM vs. SAL-induced differences in blood pressures were rather large, 16.1 ± 4.7 for MAP, 18.3 ± 5.7 for SBP, and 14.2 ± 4.6 mmHg for DBP. These effects were not influenced by CIH, since the HDM/NORM and HDM/CIH rats had similar MAP, SBP, and DBP(Fig. 2, A–C). Table 1 presents the four-group statistics for blood pressures and the differences between HDM- and SAL-challenged groups, under normoxic and CIH conditions, respectively. As shown in Table 1, again, these group differences were consistently greater than 13 mmHg. Notably, the difference in SBP between HDM/NORM- and SAL/NORM-challenged rats reached as much as 20.5 mmHg, on average (Table 1).
Fig. 2.
Chronic house dust mite (HDM)-induced airway inflammation increased arterial blood pressure compared with saline (SAL)-treated control. A: mean arterial blood pressure; B: systolic blood pressure; C: diastolic arterial blood pressure. NORM, normoxia; CIH, chronic intermittent hypoxia. N = 9–12 animals/group. P values: Holm-Sidak post hoc tests after 2-way ANOVA. *0.01 ≤ P < 0.05; **P < 0.01; #trend (0.05 ≤ P ≤ 0.10). Data are presented as means ± SE.
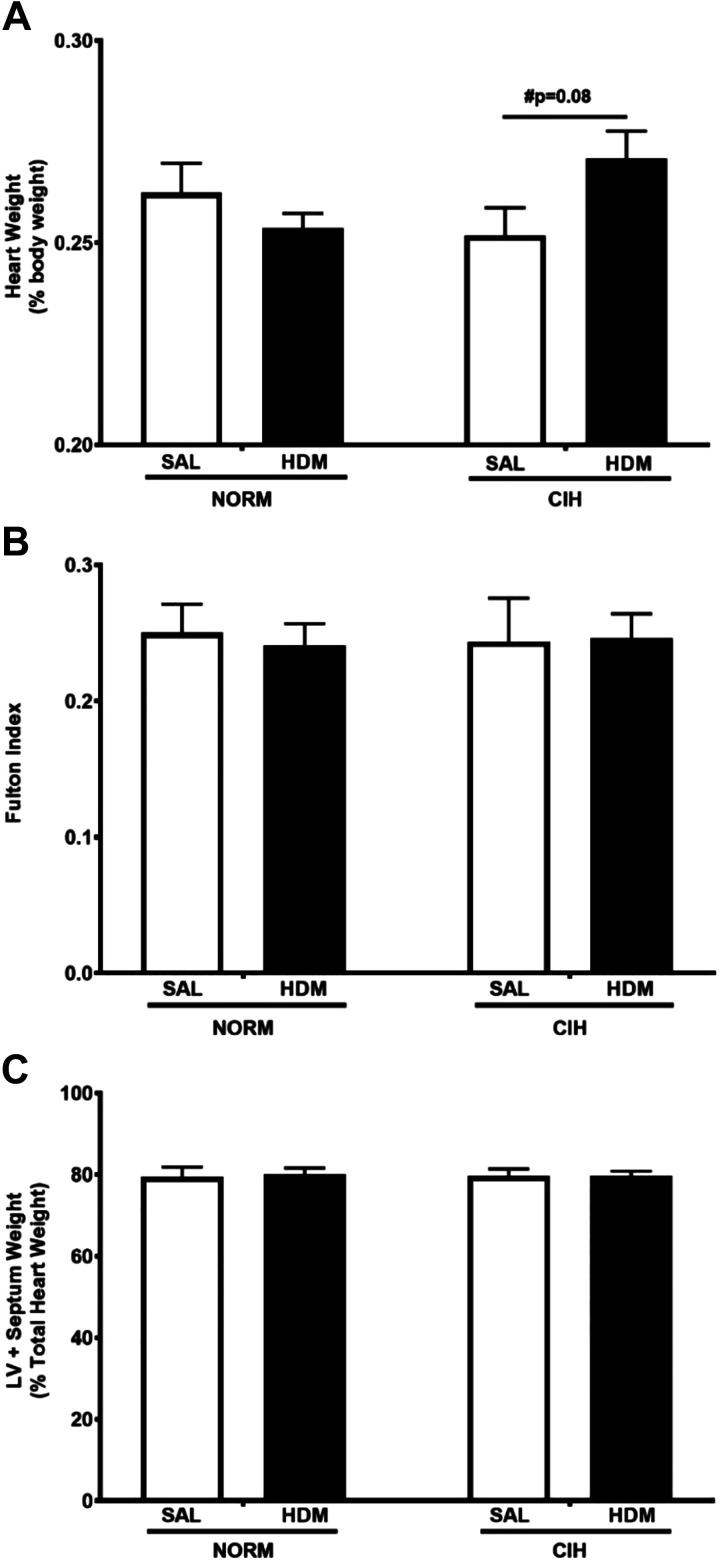
Fig. 3.
Chronic house dust mite-induced airway inflammation tended to increase heart weight in chronic intermittent hypoxia (CIH)-exposed animals. No statistically significant differences among any treatment groups were noted in Fulton index or in left ventricle (LV) plus septum weight. A: heart weight, expressed as %total body weight. N = 10 animals/group. P values: Holm-Sidak post hoc tests after 2-way ANOVA. #Trend (0.05 ≤ P ≤ 0.1). B: Fulton index (ratio of weight of right ventricle/weight of left ventricle plus septum). C: left ventricle plus septum weight (%total heart wt). SAL, saline; HDM, house dust mite; NORM, normoxia. Data are presented as means ± SE.
Table 1.
Group statistics in systemic blood pressures and group differences between HDM- and SAL-challenged rats under normoxic and CIH conditions
| MAP |
SBP |
DBP |
||||
|---|---|---|---|---|---|---|
| Group | Differences between groups | Group | Differences between groups | Group | Differences between groups | |
| SAL/NORM | 70.8 ± 5.2 (43–109.5) |
93.4 ± 6.1 (59–132.4) |
60.0 ± 5.0 (35–98.7) |
|||
| HDM/NORM | 87.5 ± 6.1 (58.6–125.5) |
16.6 ± 6.6* | 113.9 ± 7.3 (73.4–155.2) |
20.5 ± 7.8* | 74.8 ± 5.6 (51.9–111.1) |
14.8 ± 6.3* |
| SAL/CIH | 72.2 ± 3.2 (60.8–84.6) |
95.8 ± 3.1 (82.8–108.9) |
62.6 ± 4.0 (44.4–75.8) |
|||
| HDM/CIH | 87.7 ± 3.2 (69.2–110.6) |
15.5 ± 6.7* | 111.9 ± 4.1 (92.1–143.6) |
15.6 ± 8.2# | 76.2 ± 2.8 (58.2–94.7) |
13.6 ± 6.6* |
Data are means ± SE with ranges in parentheses. HDM, house dust mite; CIH, chronic intermittent hypoxia; MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; SAL, saline; NORM, normoxia.
0.01 ≤ P < 0.05;
P = 0.06.
A trend for increased heart weight in HDM- and CIH-treated rats.
Among the CIH-exposed animals, HDM-treated rats tended to have higher heart weight (expressed as a percentage of body weight) than the SAL-treated controls (P = 0.08, Fig. 3A). No differences in heart weight due to HDM treatment alone were observed in NORM-exposed rats (P = 0.34). Neither HDM nor CIH exposure had an effect on body weight of the animals (2-way ANOVA P = 0.35 for HDM and P = 0.40 for CIH). No differences in the Fulton index (RV weight/LVS weight, Fig. 3B) or in LVS mass (Fig. 3C) were detected. These findings suggest that both ventricles may be proportionally affected by the combination of HDM and CIH.
Chronic HDM and CIH affected serum Th-1 cytokine levels.
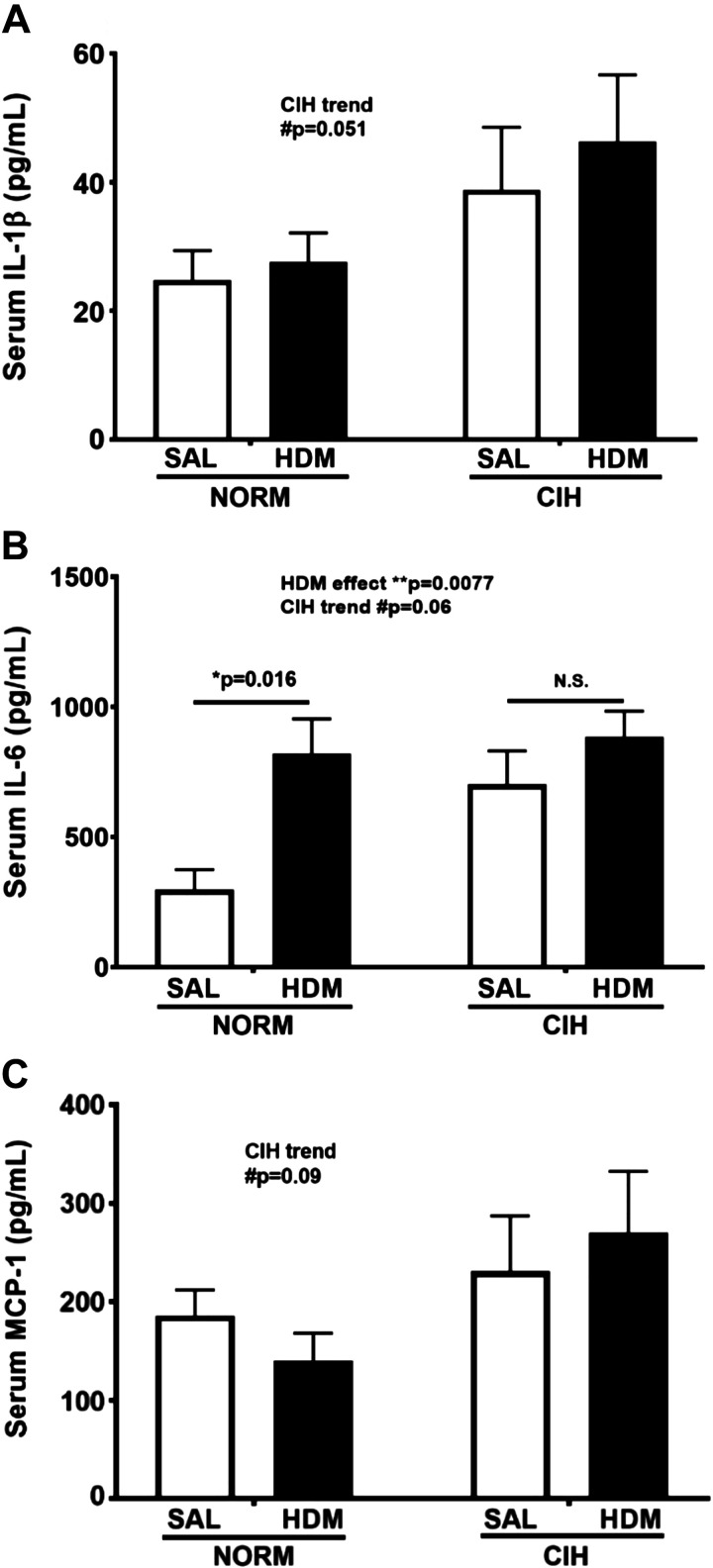
There was a trend toward elevated IL-1β in CIH- vs. NORM-exposed animals, regardless of allergen treatment (general CIH effect P = 0.051; 2-way ANOVA; Fig. 4A). Likewise, serum IL-6 levels tended to be elevated in CIH-exposed, compared with NORM-exposed, animals, regardless of allergen treatment (general CIH effect P = 0.06; 2-way ANOVA; Fig. 4B). Serum IL-6 was significantly elevated in HDM-treated animals compared with SAL-treated controls (general HDM effect P = 0.0077, 2-way ANOVA; Fig. 4B). This difference was especially apparent in HDM/NORM-exposed animals (P = 0.016; Fig. 4B), whereas in HDM/CIH-exposed animals it did not reach significance, likely because of the elevated IL-6 levels in the SAL/CIH animals. A trend for an increase in MCP-1 levels with CIH exposure was also noted, regardless of the allergen treatment (general CIH effect P = 0.09; 2-way ANOVA; Fig. 4C). No effects of either CIH or HDM were seen on MIP-1α (P = 0.19 for CIH, P = 0.99 for HDM; data not shown).
Fig. 4.
Serum cytokine levels in house dust mite (HDM)- and/or chronic intermittent hypoxia (CIH)-exposed animals. A: CIH exposure resulted in a trend for increased serum IL-1β concentration. B: HDM exposure significantly increased serum IL-6 concentration. C: CIH tended to increase serum monocyte chemoattractant protein-1 (MCP-1) levels. SAL, saline; NORM, normoxia. N = 5–6 animals/group. P values: 2-way ANOVA for overall effects of HDM and CIH treatments, Holm-Sidak post hoc tests for comparisons between groups. *0.01 ≤ P < 0.05; **P < 0.01; #trend (0.05 ≤ P ≤ 0.1). Data are presented as means ± SE.
None of the Th-2 cytokines, IL-4 (general HDM effect P = 0.22, 2-way ANOVA), IL-5 (P = 0.12), IL-13 (P = 0.61), or eotaxin (P = 0.26), levels were significantly different in HDM vs. SAL-challenged animals (data not shown). The levels of serum interferon-γ were detectable in four rat samples only, which precluded its inclusion in any further analyses.
Higher systemic blood pressures were associated with worse lower airway obstruction, but not with serum cytokines.
As expected, HDM compared with SAL challenge induced a significant increase in Rrs (general HDM effect P = 0.03, 2-way ANOVA), with trends noted for each of its components, central (RN, P = 0.09) and distal (G, P = 0.08) airways resistance (data not shown). CIH did not significantly alter these physiological measures.
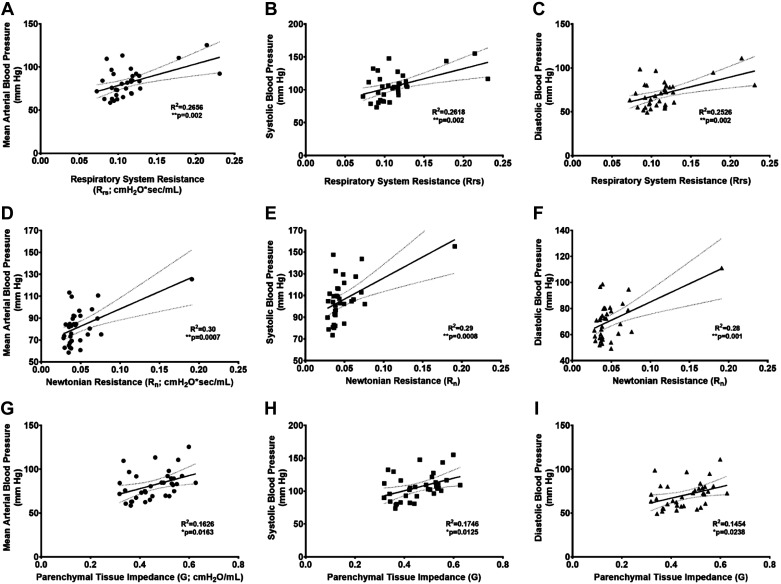
The significantly elevated Rrs was positively associated with systemic MAP (R2 = 0.266, P = 0.002; Fig. 5A), systolic (R2 = 0.262, P = 0.002; Fig. 5B, and diastolic (R2 = 0.253, P = 0.002; Fig. 5C) blood pressures. Three data points, all from HDM-challenged rats, seem to drive these relationships. In reanalyses with exclusion of these three data points, the statistical significance of these relationships was lost [MAP (R2 = 0.037, P = 0.288), systolic (R2 = 0.062, P = 0.172), and diastolic (R2 = 0.022, P = 0.413) blood pressures]. Significant positive associations were also seen for central airway resistance (RN) with MAP (R2 = 0.296, P = 0.0007; Fig. 5D), systolic (R2 = 0.295, P = 0.0008; Fig. 5E) and diastolic (R2 = 0.281, P = 0.001; Fig. 5F). These associations seemed to be driven by one rat data point, also from the HDM/NORM-challenged group, which demonstrated the highest blood pressures (MAP = 125.5, SBP = 155.2, and DBP = 111.1 mmHg) and RN (0.191 cmH2O·s−1·mL−1) values among all animals. Rerunning the analysis without this data point weakened these relationships for MAP (R2 = 0.099, P = 0.068) and systolic (R2 = 0.15, P = 0.023) and diastolic (R2 = 0.065, P = 0.146) blood pressures. However, none of these animals demonstrated any detectable clinical sickness, or any other reason to exclude from final analyses, and we reason these animals were particularly sensitive to the HDM challenges. Finally, higher tissue impedance (G) significantly related with higher blood pressure measures, MAP (R2 = 0.166, P = 0.016; Fig. 5G), systolic (R2 = 0.175, P = 0.013; Fig. 5H), and diastolic (R2 = 0.145, P = 0.024; Fig. 5I), and these data were much more normally distributed than Rrs and RN.
Fig. 5.
Worse distal airway obstruction induced by allergic airway inflammation was associated with higher systemic blood pressure. Increased total airway resistance (RRS) predicted higher mean (A), systolic (B), and diastolic (C) arterial blood pressure. A rise in central airway (Newtonian) resistance associated with elevated mean (D), systolic (E), and diastolic (F) arterial blood pressure. Parenchymal tissue impedance (G) was positively associated with mean (G), systolic (H), and diastolic (I) arterial blood pressure. Animals from all treatment groups are plotted. R2 and P values from linear regression analysis. *0.01 ≤ P < 0.05; **P < 0.01.
No significant associations of blood pressure measures with serum IL-1β, IL-6, MCP-1, or IL-4, IL-5, IL-13, and eotaxin levels were found (data not shown).
HDM-specific IgE was significantly increased in HDM-challenged animals.
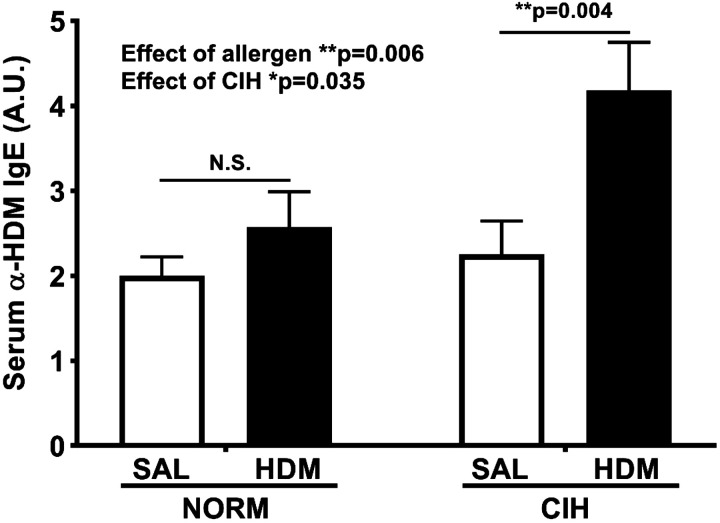
As expected, serum levels of HDM-specific IgE were significantly elevated in HDM-challenged rats compared with SAL-treated controls (general HDM effect P = 0.006, 2-way ANOVA; Fig. 6). A weak general effect of CIH was also noted (P = 0.035, 2-way ANOVA; Fig. 6). The HDM/CIH animals demonstrated the highest IgE levels, nearly a twofold increase relative to HDM/SAL and SAL/HDM groups (P = 0.004, Holm-Sidak post-hoc test; Fig. 6); however, no significant interaction of HDM with CIH was detected (P = 0.118 for the interaction term).
Fig. 6.
House dust mite (HDM)-specific IgE was significantly increased in HDM-challenged animals. SAL, saline; NORM, normoxia. N = 7–9 animals/group. P values from 2-way ANOVA for overall effects of HDM and chronic intermittent hypoxia (CIH) treatments, Holm-Sidak post hoc tests for comparisons between groups. *0.01 ≤ P < 0.05; **P < 0.01. Data are presented as means ± SE.
DISCUSSION
Because previous literature suggests that asthma may contribute to development of systemic HTN, in the present study, we tested the effects of allergic lower airway inflammation (representative of allergic asthma) on blood pressure in rats, and the role of systemic inflammation in mediating these relationships. We found that lower airway inflammation led to: 1) increased systemic blood pressures (Fig. 2); 2) increased expression of serum IL-6 and IL-1β (Fig. 4), which are known to result in cardiovascular remodeling and systemic hypertension (6, 26); 3) significant elevation in distal airway resistance, which correlated with higher systemic blood pressures (Fig. 5, G–I); and 4) no correlation of either Th-2 (IL-4, IL-5, IL-13 and eotaxin) or Th-1 (IL-1β, IL-6, MCP-1 and MIP-1α) proinflammatory cytokine levels with systemic blood pressures. These findings suggest an important link between distal airway processes and systemic cardiovascular risk, which does not appear to be explained by the associated systemic inflammation. The elevated systemic blood pressure may be directly induced by cross talk between lung inflammation and centers regulating the cardiovascular function.
We observed that rats with allergic lower airway inflammation demonstrated higher systemic blood pressures, in relationship to the degree of airways obstruction. These experimental findings align well with results from clinical studies showing that individuals with asthma are more likely to have hypertension (9, 12), which is one of the strongest modifiable risk factors for cardiovascular disease (CVD). In addition, long-standing asthma increases the risk for incident CVD events, such as myocardial infarction, angina, stroke, coronary revascularization, heart failure, and CVD death (29, 31). Moreover, the pathology starts early in life, with evidence of subclinical arterial injury at the carotid artery level in adolescents with asthma or other atopic disease, relative to their counterparts without such diseases (30). It is known that reduced FEV1% is a risk factor for cardiovascular mortality independent of traditional risk factors (27). Likewise, in patients with asthma, the relationship between reduced FEV1% and diagnosis of hypertension was nearly linear (12). Conversely, use of inhaled corticosteroids at lowest doses, compared with no inhaled corticosteroid use, was associated with a reduced risk for hypertension [0.44 (0.22–0.90)]. Adequate control of airway obstruction in asthmatic subjects may represent an important avenue for blood pressure development and, thus CVD risk reduction, in this patient population.
Contrary to our hypothesis, CIH did not modulate the effects of allergy on LV and systemic blood pressures. However, coexposure with HDM tended to increase the overall heart weight compared with animals with allergic airway inflammation alone. Corroborated with lack of differences in LVS mass and Fulton index, the data suggest both ventricles may become proportionally hypertrophic in response to CIH/HDM coexposure. In previous studies, CIH exposure, of similar duration to ours (4–6 wk), increased heart-to-body weight ratio and LV-to-total heart weight ratio in rats. The increase in LV dimension was associated with increased cavity volume and dysfunction, on echography and catheterization (7). In a follow-up study, CIH vs. normoxia led to greater surface area and length of unloaded resting isolated myocytes, consistent with the presence of cardiac hypertrophy previously observed at the whole heart level (23). However, the CIH/allergic inflammation coexposure may additionally affect the right heart. With 4 wk of CIH/ovalbumin coexposure in Brown Norway rats, we have shown airway and parenchymal remodeling, leading to airway dysfunction (5). Although we have not measured blood gases, pulmonary vascular hemodynamics, or RV function, it is possible that they are affected. Future studies assessing for changes in morphology and function of both heart cavities, in this model, are necessary. Second, lack of a CIH effect on the systemic blood pressure raises questions for either an insufficient stimulus or host susceptibility. We believe the CIH intensity we used (30 episodes/h, down to of 10%, 10 h/day, daily for 43 days) was sufficient to elicit a systemic vascular response, should one exist in this rat strain. This is because in other rat strains such as the Sprague-Dawley and Wistar, CIH of less intensity and for shorter periods than ours, i.e., 14 days (20, 21, 24) and 10 days (3), respectively, consistently produced a significant pressor response, including during the rat’s light cycle (when they were not under CIH exposure). This initial pressor response remained unaltered with longer, up to 56 days, exposure periods (24). By not observing similar effects in our rats, the data herein suggest strain differences in vascular behavior. In this study, we had to use the Brown Norway rat strain because of its utility as a consistent model of allergic inflammation. It is known that Brown Norway rats differ from other rat strains in that they exhibit attenuated ventilatory responses, across the respiratory system. Notably, compared with Sprague-Dawley, Brown Norway rats demonstrate reduced carotid body sensing under baseline conditions and severely reduced responses to hypoxic challenge (22). Additionally, these rats exhibit reduced hypercapnic ventilatory responses because of deficiencies in medullary raphe neuromodulators, which when supplemented normalize the CO2 chemoreflex (25). Last, compared with Fisher and Lewis rats, Brown Norway rats have reduced levels of spinal phrenic and hypoglossal plasticity in response to hypoxia, mainly because of deficiency in specific serotonergic receptors (1). Therefore, we believe that the lack of CIH-induced hypertension in these animals is likely another result of strain-specific genetic differences dictating the physiology. Although an effect of CIH on systemic blood pressure was not noted in this study, in combination with allergic-induced airway obstruction, our data suggest that it may nevertheless exert deleterious effects on the pulmonary vascular bed and, thus, on the right heart.
We found that allergic airway inflammation induced a systemic inflammatory response manifested by elevation of IL-6. However, although we observed an association between blood pressure measures with distal airway resistance, in contrast to our hypothesis, no relationships of blood pressure with levels of serum cytokines were demonstrated. These observations suggest that other mechanisms than systemic inflammation underlie the physiological relationships observed. Indeed, inflammatory processes originating in the lung can lead to inflammation in the central nervous system (CNS). For example, 48 h after acute bleomycin-induced acute lung injury in rats, Jacono et al. (16) detected Th-1 cytokine expression in brain stem regions responsible for cardiopulmonary control, at levels correlated with changes in ventilatory pattern observed following the onset of lung injury. Whereas increased systemic levels of proinflammatory cytokines had already been detected as early as 6 h following lung injury, their levels did not correlate with alterations in ventilatory pattern. Several systemic inflammation-independent immune-to-brain communication pathways were put forward, such as: 1) neural transport of inflammatory mediators from the lung through afferent fibers of the vagi and 2) cytokine production within the CNS by the resident brain macrophage-microglia (16). Indeed, activation of brain stem microglia following acute bleomycin-induced lung injury has been recently demonstrated in adult rats. Notably, microglia assumed its known “active” morphotype, characterized by hyperramified appendages, throughout the nucleus tractus solitarii and several surrounding regions, where it colocalized with an augmented expression of IL-1β and its downstream transcriptional target, cyclooxygenase-2 (18, 19). Conversely, daily intraperitoneal injection of minocycline, which is permeable to the blood-brain barrier and inhibits microglia/macrophages function, prevented the hyper-ramification and abolished the neurophysiological changes evoked by lung injury (19).
Additionally, Th-1 type inflammation in the lung appears to sensitize the carotid body as well, which may lead to an enhanced baroreceptor response and elevated blood pressures. Following bleomycin instillation in rats (5 days), before lung fibrosis and arterial hypoxemia ensued, Jacono et al. (17) found heightened hypoxic and hyperoxic ventilatory responses in anesthetized spontaneously breathing bleomycin-treated relative to control animals. The enhanced hypoxic response persisted following bilateral vagotomy but was eliminated following bilateral carotid sinus nerve sectioning. It remains to be tested whether Th-2 predominant inflammatory processes in the lung have similar effects on the brain stem and carotid body.
Our study was several limitations. The tail cuff method of acquiring blood pressure, although noninvasive and free of anesthesia requirements, provides only a snapshot in time of the blood pressure, which we acquired only on day 43, before the final end-point testing on day 44 (Fig. 1). Additionally, it requires animal restraint, which introduces the stress of restraint as a potential confounder, and requires acclimation of animals to restraint for few days before end-point collection. Telemetry signals from implanted pressure sensors would allow for continuous monitoring of blood pressure for the entire time period of the experiment. However, for these initial investigation of the effects of HDM-induced airway inflammation on blood pressure, we elected to use the simpler, less invasive, and more readily available in our center technique of tail cuff pressure recordings (11). In future investigations, it is possible to implant telemetry sensors in each animal (33), allowing for separate monitoring of systemic and pulmonary blood pressures. Another limitation relates to the allergic exposure, which may not have been intense enough to produce sufficient lung pathology. This is suggested by findings in normoxic rats, where comparable levels of serum IgE (Fig. 6) and Th-2 cytokines (data not shown) were noted in HDM- and SAL-challenged animals. Nevertheless, significant group differences between HDM- and SAL-challenged normoxic animals were noted in systemic IL-6 levels (Fig. 4B) and blood pressures (Fig. 2), suggesting that even this low-grade allergic airway exposure had significant systemic consequences. In future experiments, a more intense than weekly HDM challenge may allow observing more significant lung pathology and, thereby, systemic consequences. Last, only blood and not bronchoalveolar lavage fluid (BALF) collection was feasible in this study for measurement of cytokine levels; therefore, the role of the airway compartment in the blood pressure responses could not be assessed. Compartment-specific differences in cytokine expression do exist. In the aforementioned study by Jacono et al. (16), IL-1β and TNF-α were increased in BALF but not in the serum, whereas IL-6 was not elevated in the BALF 48 h after bleomycin-induced lung injury. BALF analysis would provide a more accurate reflection of airway inflammation and should be part of future studies.
In summary, asthma and OSA represent important risk factors and avenues to intervene for hypertension and cardiovascular mortality risk reduction, in addition to the traditional cardiovascular risk factors. Cohort studies suggest that individuals with asthma and those with OSA have higher risk of hypertension. Our findings support this notion, since rats with lower airway inflammation representative of allergic asthma had higher systemic blood pressures, with group differences consistently over 13 mmHg for all of the BP measures. In addition, rats with concurrent allergic airway inflammation and CIH exposure had heavier hearts compared with those with only allergic airway inflammation, likely representative of biventricular hypertrophy. There was also an association between worsening distal airway resistance and higher systemic blood pressure. Relationships between levels of systemic inflammatory cytokine tested with systemic blood pressures were not found, suggesting that neuroimmune interactions between lung inflammation and centers regulating the cardiovascular tone need to be considered. These findings support important links between distal airway processes and systemic cardiovascular risk, warranting further experimental mechanistic investigations. Although an effect of CIH was not noted in this protocol, in combination with allergy, CIH may exert deleterious effects on the right side of the heart; further studies of the right heart-lung interactions during concurrent allergic and CIH exposures are necessary.
GRANTS
This work was supported by Merit Review Award No. 1I01BX002880-01A2 (to M. Teodorescu) from the United States Department of Veterans Affairs, Biomedical Laboratory Research and Development Service, with additional resources and use of facilities at the William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin.
DISCLOSURES
M. Teodorescu has received funding from Boehringer Ingelheim Pharmaceuticals Inc., for an Investigator Initiated Project on the role of obstructive sleep apnea on responses to antifibrotic medications in patients with idiopathic pulmonary fibrosis, which is unrelated to this article. The content of this article is solely the responsibility of the authors and does not represent the views of the Department of Veterans Affairs or the United States Government.
AUTHOR CONTRIBUTIONS
O.B. and M.T. conceived and designed research; A.C. and O.B. performed experiments; O.B. analyzed data; A.C., O.B. and M.T. interpreted results of experiments; O.B. and M.T. prepared figures; A.C., O.B. and M.T. drafted manuscript; A.C., O.B. and M.T. edited and revised manuscript; A.C., O.B. and M.T. approved final version of manuscript.
REFERENCES
- 1.Baker-Herman TL, Bavis RW, Dahlberg JM, Mitchell AZ, Wilkerson JE, Golder FJ, Macfarlane PM, Watters JJ, Behan M, Mitchell GS. Differential expression of respiratory long-term facilitation among inbred rat strains. Respir Physiol Neurobiol 170: 260–267, 2010. doi: 10.1016/j.resp.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bautista LE. Inflammation, endothelial dysfunction, and the risk of high blood pressure: epidemiologic and biological evidence. J Hum Hypertens 17: 223–230, 2003. doi: 10.1038/sj.jhh.1001537. [DOI] [PubMed] [Google Scholar]
- 3.Bazilio DS, Bonagamba LGH, Moraes DJA, Machado BH. Cardiovascular and respiratory profiles during the sleep-wake cycle of rats previously submitted to chronic intermittent hypoxia. Exp Physiol 104: 1408–1419, 2019. doi: 10.1113/EP087784. [DOI] [PubMed] [Google Scholar]
- 4.Braun RK, Broytman O, Braun FM, Brinkman JA, Clithero A, Modi D, Pegelow DF, Eldridge M, Teodorescu M. Chronic intermittent hypoxia worsens bleomycin-induced lung fibrosis in rats. Respir Physiol Neurobiol 256: 97–108, 2018. doi: 10.1016/j.resp.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broytman O, Braun RK, Morgan BJ, Pegelow DF, Hsu PN, Mei LS, Koya AK, Eldridge M, Teodorescu M. Effects of chronic intermittent hypoxia on allergen-induced airway inflammation in rats. Am J Respir Cell Mol Biol 52: 162–170, 2015. doi: 10.1165/rcmb.2014-0213OC. [DOI] [PubMed] [Google Scholar]
- 6.Capra V, Bäck M, Barbieri SS, Camera M, Tremoli E, Rovati GE. Eicosanoids and their drugs in cardiovascular diseases: focus on atherosclerosis and stroke. Med Res Rev 33: 364–438, 2013. doi: 10.1002/med.21251. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Einbinder E, Zhang Q, Hasday J, Balke CW, Scharf SM. Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am J Respir Crit Care Med 172: 915–920, 2005. doi: 10.1164/rccm.200504-560OC. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Zhang J, Gan TX, Chen-Izu Y, Hasday JD, Karmazyn M, Balke CW, Scharf SM. Left ventricular dysfunction and associated cellular injury in rats exposed to chronic intermittent hypoxia. J Appl Physiol (1985) 104: 218–223, 2008. doi: 10.1152/japplphysiol.00301.2007. [DOI] [PubMed] [Google Scholar]
- 9.Dogra S, Ardern CI, Baker J. The relationship between age of asthma onset and cardiovascular disease in Canadians. J Asthma 44: 849–854, 2007. doi: 10.1080/02770900701752391. [DOI] [PubMed] [Google Scholar]
- 10.Farley TA, Dalal MA, Mostashari F, Frieden TR. Deaths preventable in the U.S. by improvements in use of clinical preventive services. Am J Prev Med 38: 600–609, 2010. doi: 10.1016/j.amepre.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Feng M, Whitesall S, Zhang Y, Beibel M, D’Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens 21: 1288–1291, 2008. doi: 10.1038/ajh.2008.301. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson S, Teodorescu MC, Gangnon RE, Peterson AG, Consens FB, Chervin RD, Teodorescu M. Factors associated with systemic hypertension in asthma. Lung 192: 675–683, 2014. doi: 10.1007/s00408-014-9600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon-Moran D. Hypertension Prevalence and Control Among Adults: United States, 2015–2016. NCHS Data Brief 289: 1–8, 2017. [PubMed] [Google Scholar]
- 14.Halász A, Cserháti E, Kósa L, Cseh K. Relationship between the tumor necrosis factor system and the serum interleukin-4, interleukin-5, interleukin-8, eosinophil cationic protein, and immunoglobulin E levels in the bronchial hyperreactivity of adults and their children. Allergy Asthma Proc 24: 111–118, 2003. [PubMed] [Google Scholar]
- 15.Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med 377: 965–976, 2017. doi: 10.1056/NEJMra1608969. [DOI] [PubMed] [Google Scholar]
- 16.Jacono FJ, Mayer CA, Hsieh YH, Wilson CG, Dick TE. Lung and brainstem cytokine levels are associated with breathing pattern changes in a rodent model of acute lung injury. Respir Physiol Neurobiol 178: 429–438, 2011. doi: 10.1016/j.resp.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacono FJ, Peng YJ, Nethery D, Faress JA, Lee Z, Kern JA, Prabhakar NR. Acute lung injury augments hypoxic ventilatory response in the absence of systemic hypoxemia. J Appl Physiol (1985) 101: 1795–1802, 2006. doi: 10.1152/japplphysiol.00100.2006. [DOI] [PubMed] [Google Scholar]
- 18.Litvin DG, Denstaedt SJ, Borkowski LF, Nichols NL, Dick TE, Smith CB, Jacono FJ. Peripheral-to-central immune communication at the area postrema glial-barrier following bleomycin-induced sterile lung injury in adult rats. Brain Behav Immun 87: 610–633, 2020. doi: 10.1016/j.bbi.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Litvin DG, Dick TE, Smith CB, Jacono FJ. Lung-injury depresses glutamatergic synaptic transmission in the nucleus tractus solitarii via discrete age-dependent mechanisms in neonatal rats. Brain Behav Immun 70: 398–422, 2018. doi: 10.1016/j.bbi.2018.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcus NJ, Li YL, Bird CE, Schultz HD, Morgan BJ. Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: role of the angiotensin II type 1 receptor. Respir Physiol Neurobiol 171: 36–45, 2010. doi: 10.1016/j.resp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcus NJ, Olson EB Jr, Bird CE, Philippi NR, Morgan BJ. Time-dependent adaptation in the hemodynamic response to hypoxia. Respir Physiol Neurobiol 165: 90–96, 2009. doi: 10.1016/j.resp.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng YJ, Makarenko VV, Nanduri J, Vasavda C, Raghuraman G, Yuan G, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. Inherent variations in CO-H2S-mediated carotid body O2 sensing mediate hypertension and pulmonary edema. Proc Natl Acad Sci USA 111: 1174–1179, 2014. doi: 10.1073/pnas.1322172111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 177: 1006–1014, 2013. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philippi NR, Bird CE, Marcus NJ, Olson EB, Chesler NC, Morgan BJ. Time course of intermittent hypoxia-induced impairments in resistance artery structure and function. Respir Physiol Neurobiol 170: 157–163, 2010. doi: 10.1016/j.resp.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puissant MM, Echert AE, Yang C, Mouradian GC Jr, Novotny T, Liu P, Liang M, Hodges MR. RNASeq-derived transcriptome comparisons reveal neuromodulatory deficiency in the CO2 insensitive brown Norway rat. J Physiol 593: 415–430, 2015. doi: 10.1113/jphysiol.2014.285171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Thorax 64: 631–636, 2009. doi: 10.1136/thx.2008.105577. [DOI] [PubMed] [Google Scholar]
- 27.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest 127: 1952–1959, 2005. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 28.Sinisalo J, Paronen J, Mattila KJ, Syrjälä M, Alfthan G, Palosuo T, Nieminen MS, Vaarala O. Relation of inflammation to vascular function in patients with coronary heart disease. Atherosclerosis 149: 403–411, 2000. doi: 10.1016/S0021-9150(99)00333-0. [DOI] [PubMed] [Google Scholar]
- 29.Tattersall MC, Barnet JH, Korcarz CE, Hagen EW, Peppard PE, Stein JH. Late-onset asthma predicts cardiovascular disease events: the Wisconsin sleep cohort. J Am Heart Assoc 5: 5, 2016. doi: 10.1161/JAHA.116.003448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tattersall MC, Evans MD, Korcarz CE, Mitchell C, Anderson E, DaSilva DF, Salazar LP, Gern JE, Jackson DJ, Lemanske RF Jr, Stein JH. Asthma is associated with carotid arterial injury in children: The Childhood Origins of Asthma (COAST) Cohort. PLoS One 13: e0204708, 2018. doi: 10.1371/journal.pone.0204708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tattersall MC, Guo M, Korcarz CE, Gepner AD, Kaufman JD, Liu KJ, Barr RG, Donohue KM, McClelland RL, Delaney JA, Stein JH. Asthma predicts cardiovascular disease events: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol 35: 1520–1525, 2015. doi: 10.1161/ATVBAHA.115.305452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Thatcher SE, Cassis LA. Blood pressure monitoring using radio telemetry method in mice. Methods Mol Biol 1614: 75–85, 2017. doi: 10.1007/978-1-4939-7030-8_7. [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama A, Kohno N, Fujino S, Hamada H, Inoue Y, Fujioka S, Ishida S, Hiwada K. Circulating interleukin-6 levels in patients with bronchial asthma. Am J Respir Crit Care Med 151: 1354–1358, 1995. doi: 10.1164/ajrccm.151.5.7735584. [DOI] [PubMed] [Google Scholar]
- 35.Zizek B, Poredos P, Videcnik V. Endothelial dysfunction in hypertensive patients and in normotensive offspring of subjects with essential hypertension. Heart 85: 215–217, 2001. doi: 10.1136/heart.85.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]