Abstract
Reproductive hormones have significant nonreproductive physiological effects, including altering fluid regulation. Our purpose was to explore the impact of sex and menstrual cycle (MC) phase on volume-regulatory responses to 24-h fluid restriction (24-h FR). Participants (men: n = 12, 20 ± 2 yr; women: n = 10, 20 ± 1 yr) were assigned two randomized and counterbalanced fluid prescriptions [Euhy: euhydrated, urine specific gravity (USG) < 1.020; Dehy: 24-h FR, USG > 1.020]. Men completed both (MEuhy, MDehy), while women completed both in the late-follicular (days 10–13; FDehy, FEuhy) and midluteal (days 18–22; LDehy, LEuhy) phases. We measured body mass, plasma and urine osmolality (Posm, Uosm), urine specific gravity (USG), urine color (Ucol), and serum copeptin; 24-h FR yielded mild dehydration without influence of sex or MC (P > 0.05). Copeptin increased in men following Dehy (pre: 8.2 ± 5.2, post: 15.8 ± 12.6, P = 0.04) but not in women (FDehy pre: 4.3 ± 1.6, post: 10.5 ± 6.9, P = 0.06; LDehy pre: 5.6 ± 3.5, post: 10.4 ± 6.2, P = 0.16). In FDehy, Posm increased following FR (pre: 288 ± 2, post: 292 ± 1, P = 0.03) but not in men (pre: 292 ± 3, post: 293 ± 2, P = 0.46). No MC differences were observed between body mass loss, Posm, Uosm, USG, and copeptin (P > 0.05). These results suggest that volume-regulatory responses to 24-h FR were present in men but not in women, without apparent effects of the menstrual cycle.
Keywords: body mass loss, sex differences, copeptin, estrogen
INTRODUCTION
Mild dehydration (1–2% body mass loss) has been previously observed to impact cognitive function and mood (1, 10, 33), exercise performance (13), and chronically may impact overall health (16). Fluid restriction (FR) can be used to investigate mild dehydration while minimizing potential confounders such as ambient heat and/or exercise. Although the level of hydration can change with activity, diet, and environment, it is unclear whether female sex or reproductive hormones have specific influences on volume-regulatory responses to mild dehydration. Executive function has been observed to be decreased by mild dehydration in women (27). Shirreffs et al. (20) observed decreased alertness and similar levels of body mass loss (BML) in men and women when investigating hydration status in progressive FR, but reproductive hormone status was not reported. Armstrong et al. (2) recently developed hydration guidelines for women within the placebo phase of oral contraceptive use, the first diagnostic and clinical guidelines specific to women to date; however, those guidelines do not consider endogenous or exogenous hormonal variation. Hormonal status may alter the accuracy of any such guidelines, particularly if hormonal variation affects mechanisms involved in body fluid regulation.
Female sex hormones have been observed to interact with body fluid-regulatory hormones throughout the menstrual cycle (22), possibly resulting in differences in body mass change, thirst perception, or hormonal responses to hydration challenges (11). Arginine vasopressin (AVP) is a fluid-regulatory hormone that responds primarily to osmotic stress (17, 18). Increases in estrogen, which occur at distinct time points across the menstrual cycle, are related to increases in AVP concentration (9). Additionally, a decrease in the osmotic threshold for thirst and AVP secretion has been observed in the midluteal phase when both estrogen and progesterone are high, with no evidence suggesting the threshold shift led to increased fluid retention (7, 28, 32). Similarly, dehydrating stimuli have been shown to increase copeptin, a surrogate marker of AVP, in investigations including women but only in the early follicular phase of the menstrual cycle (31).
In research regarding volume regulation in young women, menstrual cycle comparisons have often been made between the early follicular phase and the midluteal phase, where estradiol and progesterone are both low or both elevated, respectively (15), leaving possible differences due to unopposed estrogen uninvestigated. For example, Stachenfeld et al. (23) found no differences in hydration status or BML with acute, exercise-induced dehydration between early follicular and midluteal phases of the menstrual cycle; however, mild dehydration via FR was not evaluated in this all-female data set. In this context, it would be helpful to determine whether variation exists in the late follicular phase when estrogen is high, independent of progesterone, and when both estrogen and progesterone are both high in the midluteal phase (15).
At present, responses to milder dehydration have not been examined, nor has the impact of interactions between estrogen and progesterone fully been assessed. Accordingly, the present study investigated the impact of sex and menstrual cycle phase on physiological volume-regulatory responses to a 24-h fluid restriction (24-h FR). We hypothesized 1) that integrative physiological volume-regulatory responses to 24-h FR are different in women compared with men, and 2) that the high “unopposed” estrogen status of the late follicular phase would lead to increased fluid retention and decreased BML. To address these hypotheses, we measured BML and indexwes of dehydration and fluid volume regulation following 24-h FR in men and in women in the late follicular and midluteal phases of the menstrual cycle.
MATERIALS AND METHODS
Subjects.
Twelve men and 10 women participated in this investigation. Participants were recruited from a healthy, college-aged population via University emails, flyers, and word of mouth. All subjects were screened for contraindications to 24-h FR including any chronic diseases (e.g., chronic kidney disease, diabetes, etc.) before enrollment in the study. Self-reported menstrual history questionnaires were collected from female participants to determine menstrual status. Women were excluded if they were currently using or had utilized hormonal birth control within 6 mo or were not considered to be eumenorrheic (25–35 days per cycle) based on their self-reported menstrual history questionnaires.
Ethical approval.
Following a recruiting informational session, all participants provided written informed consent before participation. All study protocols and procedures were conducted in accordance with the Declaration of Helsinki and were evaluated and approved by the Institutional Review Board at the University of Connecticut under Protocol Nos. H18-220 and H19-049.
Protocol and procedures.
Individuals arrived at the Human Performance Laboratory at the University of Connecticut and were provided with instructions for their assigned fluid prescription and provided prefluid prescription blood samples and body mass measurements. All blood samples were collected by a trained phlebotomist. This study utilized two different fluid prescriptions. Participants were not informed of their fluid prescription before arriving at the laboratory to reduce a possible effect of fluid loading before dehydration. Trial order was randomized and counterbalanced, and trials were performed at least 72 h apart to prevent possible influence of one hydration prescription on another.
For the euhydrated (Euhy) prescription, participants were asked to consume fluid and food in order to arrive to the laboratory 24 h later in a Euhy state [urine specific gravity (USG) <1.020) where researchers provided guidance to consume additional ∼500 mL of fluid the evening before trials. Before the Euhy trials, all participants were instructed to avoid caffeine and alcohol for 12 and 24 h, respectively. In the dehydrated (Dehy) prescription, participants were instructed not to consume fluid or any foods with high water content for 24 h before their next visit. Participants recorded all food and fluid consumed over this 24-h fluid prescription period to verify adherence to fluid prescription.
Participants were asked to refrain from physical activity during the 24-h fluid prescription time period to prevent any confounding influence of exercise. Individuals were provided a clean container to collect their urine for 24 h and were instructed to record all food and fluids consumed over the 24-h period for assessment of compliance. At the conclusion of the 24-h period, participants provided a first morning urine sample and thirst rating before their arrival at the laboratory. Thirst ratings were reported using a nine-point Likert scale that participants had been familiarized with during baseline testing (8). Following 24-h fluid prescription upon arrival at the laboratory, we collected blood samples, and nude body mass was measured to assess percent body mass change (%BML) from prefluid prescription body mass measurements (Ohaus Defender 3000; Ohaus, Parsippany, NJ).
Men performed two trials, one Euhy fluid prescription (MEuhy) and one Dehy fluid prescription (MDehy). Women were tested over two or three menstrual cycles based on block randomization and trial order where women completed late follicular and midluteal trials at least 7 days apart. Women performed four total trials, one Euhy and one Dehy in the late follicular (FDehy and FEuhy, days 10–13) and midluteal (LDehy and LEuhy, days 18–22) phases of the menstrual cycle.
Menstrual cycle verification.
Visits to the laboratory were scheduled on the basis of self-reported menstrual cycle start dates and then verified post hoc via enzyme-linked immunoassay (17β-estradiol and progesterone; ALPCo Diagnostics, Salem, NH). Late follicular phase testing was completed between days 10 and 14, and midluteal phase testing was completed between days 18 and 22, where the first day of menstruation was counted as day 1. All trials were at least 72 h apart, and women were tested only one time within the late follicular and midluteal phase windows. Sex hormone concentration was assessed from plasma samples collected following 24-h fluid prescription.
Urine and biomarker measurements.
As urinary and hydration biomarkers, plasma osmolality (Posm), copeptin concentration, urine specific gravity (USG), and urine color (Ucol) were assessed before and after 24-h fluid prescription. USG and Ucol are often utilized to clinically assess dehydration. Posm and Uosm were analyzed using freezing point depression osmometry following the manufacturer’s recommendations (OsmoPRO; Advanced Instruments, Norwood, MA). USG was assessed utilizing a light refractometer (Atago A 300CL, Japan), and Ucol was assessed utilizing a validated urine color chart (3, 4). We utilized copeptin as a surrogate marker for AVP, which is consistent with previous literature, to assess dehydration stress (31). Copeptin (CT-pro-AVP) has been identified and recently utilized as a surrogate for AVP because it is released in equimolar ratios (14). Copeptin has been shown to have parallel responses to AVP within a 28-h fluid restriction as well as in response to other dehydration stimuli that result in osmotic stress (5, 31). Serum copeptin concentration was analyzed from samples collected during the pretesting and posttesting periods with an automated immunofluorescent Copeptin proAVP KRYPTOR assay via the fully automated random access immunoassay KRYPTOR Compact PLUS analyzer (BRAHMS, Thermo Fisher, Hennigsdort, Germany). Assay calibration was performed before analysis, and quality controls were included on each plate. Copeptin fold change was calculated by dividing postfluid prescription values by prefluid prescription to assess the relative change and effect of the fluid prescription. Fold change calculations help to account for inherent variability in premeasures, given the known endogenous variability between men and women.
Statistical analysis.
Statistical analysis was conducted using SPSS software (v.26; IBM, Armonk, NY). We assessed whether data were normally distributed by utilizing a Shapiro–Wilk test, and any variables that were not normally distributed were analyzed using nonparametric tests. Specifically, sex differences in copeptin concentration and fold change were assessed using the Mann–Whitney U test. Data from women were initially pooled and compared with men’s to determine overall sex differences, and then men’s data were compared with data from women separated by menstrual phase. Differences within sexes, between hydration conditions, pre- and postfluid prescription, and menstrual cycle phase in copeptin concentration and fold change were assessed using a Friedman test with nonparametric repeated measures. Any significant differences within the Friedman analysis were further analyzed using Wilcoxon signed rank sum test. Sex differences were computed for BML and urine and blood markers of hydration status using one-way analysis of variance (ANOVA), since these data were confirmed to be normally distributed. Differences between menstrual cycle phases, hydration status, and pre- and postfluid prescription for all other normally distributed variables were completed utilizing a 2 × 2 repeated-measures ANOVA with Bonferroni pairwise comparisons. Pearson product correlations were calculated between hydration biomarkers and female sex hormone concentration. Bonferroni post hoc tests were utilized for males and for females during the follicular and luteal phases of the menstrual cycle. Data are presented as means ± SD, and significance was set a priori at P ≤ 0.05.
RESULTS
Twelve men and 10 women participated in this investigation. Subject characteristics are found in Table 1. Progesterone was significantly higher in the luteal phase for both trials (P < 0.05), whereas estrogen was not different between phases (P > 0.05). Female sex hormone concentrations can be found in Table 2.
Table 1.
Subject characteristics
| Age, yr | Weight, kg | Height, cm | |
|---|---|---|---|
| Men, n = 12 | 20 ± 2 | 70.1 ± 10.5*** | 173.4 ± 6.0*** |
| Women, n = 10 | 20 ± 1 | 57.0 ± 7.3 | 163.8 ± 5.6 |
Data presented are means ± SD.
P < 0.05 between sexes.
Table 2.
Female sex hormone concentrations
| Follicular Dehydrated | Follicular Euhydrated | Luteal Dehydrated | Luteal Euhydrated | |
|---|---|---|---|---|
| Estrogen, pg/mL | 112.33 ± 14.70 | 122.40 ± 16.56 | 130.82 ± 24 | 121.48 ± 24.48 |
| Progesterone, ng/mL | 1.156 ± 0.31 | 0.915 ± 0.18 | 5.190 ± 1.56# | 4.498 ± 1.38# |
Data presented are means ± SD.
P < 0.05 between both follicular phase trials.
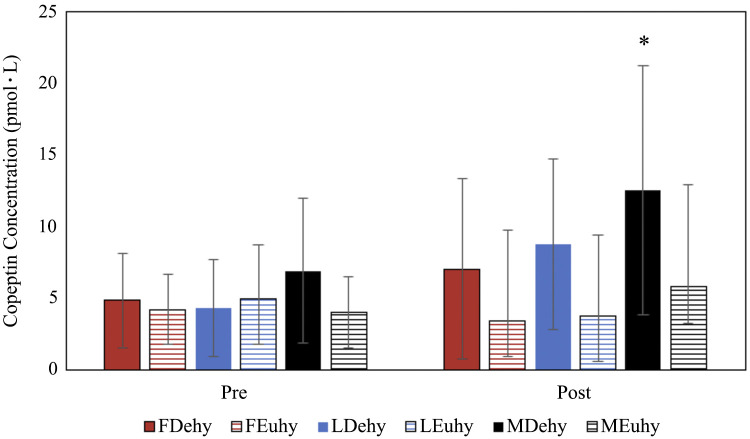
Absolute BML, %BML, USG, Ucol, and Uosm clinically indicate mild dehydration (Table 3). These data indicate that 24-h FR was an effective stimulus to elicit mild dehydration with no influence of sex or menstrual cycle phase on the extent of the dehydration. First morning thirst rating was also greater following Dehy compared with Euhy in all groups. Men exhibited significantly greater copeptin following Dehy (Fig. 1), and men had significantly higher copeptin concentration pre-Dehy than women when data were pooled [data presented median (interquartile range); men: 6.90(5.06–8.70), women: 4.48(3.50–6.27), P = 0.038]. There were no differences in copeptin pre or postfluid prescription based on menstrual cycle phase (Fig. 1).
Table 3.
Hydration biomarkers after fluid prescription by sex and menstrual phase
| Follicular |
Luteal |
Men |
||||
|---|---|---|---|---|---|---|
| Dehy | Euhy | Dehy | Euhy | Dehy | Euhy | |
| Body mass change, % | −1.15 ± 0.85** | 0.31 ± 1.63 | −0.74 ± 0.41** | 0.25 ± 1.28 | −1.18 ± 0.91** | 0.39 ± 1.12 |
| Body mass loss, kg | −0.69 ± 0.54** | 0.12 ± 0.92 | −0.42 ± 0.22** | 0.13 ± 0.74 | −0.84 ± 0.69** | 0.25 ± 0.81 |
| USG post | 1.023 ± 0.002** | 1.014 ± 0.002 | 1.022 ± 0.003** | 1.009 ± 0.002 | 1.028 ± 0.001** | 1.014 ± 0.002 |
| Post first morning thirst rating | 8 ± 1** | 4 ± 1 | 8 ± 1** | 3 ± 0 | 7 ± 1** | 4 ± 1 |
| Ucol | 4 ± 2 | 3 ± 2 | 4 ± 2** | 2 ± 1 | 6 ± 1** | 4 ± 1 |
| 24-h Urine volume, L | 0.675 ± 0.220** | 1.635 ± 0.330 | 1.000 ± 0.240** | 1.840 ± 0.250 | 0.763 ± 0.094** | 1.432 ± 0.237 |
| 24-h Uosm, mosmol/kg H2O | 653 ± 185** | 396 ± 179 | 661 ± 215** | 338 ± 144 | 882 ± 227** | 633 ± 241 |
Data presented are means ± SD. Dehy, dehydration; Euhy, euhydration; USG, urine specific gravity; Ucol, urine color; Uosm, urine osmolality.
P < 0.05 between Dehy and Euhy measures.
Fig. 1.
Copeptin concentration pre- and post-fluid prescription calculated utilizing Mann–Whitney U tests (sex differences) and Friedman tests (pre to post and menstrual cycle comparisons). Data presented as median with interquartile range (IQR). *P < 0.05 from pre to post. FDehy, follicular phase dehydrated trial; FEuhy, follicular phase euhydrated trial; LDehy, luteal phase dehydrated trial; LEuhy, luteal phase euhydrated trial; MDehy, men dehydrated trial, MEuhy, men euhydrated trial.
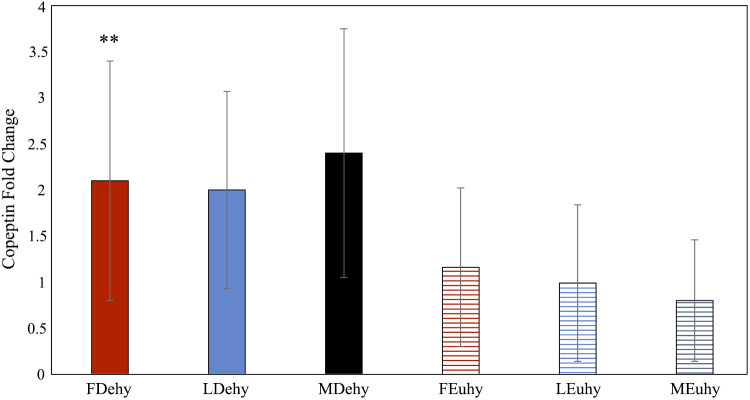
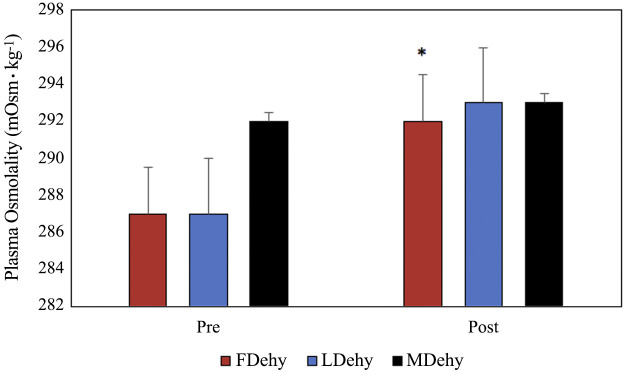
Fold chance in copeptin was significantly greater in FDehy women compared with FEuhy women, with no observed effect of sex or menstrual cycle phase (Fig. 2). Posm significantly increased after 24-h FR in FDehy women (F = 7.635, P = 0.03) and trended toward significance in LDehy women (F = 5.535, P < 0.06), but not in men (F = 0.600, P = 0.46) (Fig. 3). Posm decreased for men and women in the luteal phase following euhydrated fluid prescription (MEuhy pre: 293 ± 2, post: 289 ± 2, F = 13.582, P < 0.01; LEuhy pre: 290 ± 2, post: 289 ± 2, F = 22.773, P = 0.03), but not for women in the follicular phase (FEuhy pre: 292 ± 1, post: 289 ± 3, F = 5.022, P = 0.07). Copeptin concentration post-Dehy was significantly correlated with %BML across all groups (r = −0.562, P = 0.003). There was no relationship present between estrogen and pre-FP copeptin (r = 0.093, P = 0.597). No other correlations between variables including female sex hormones were statistically significant.
Fig. 2.
Pre- to post-copeptin fold change calculated utilizing Mann–Whitney U tests (sex differences) and Friedman tests (pre to post and menstrual cycle comparisons). Data presented as median with interquartile range (IQR). **P < 0.05 between Dehy and Euhy condition. FDehy, follicular phase dehydrated trial; FEuhy, follicular phase euhydrated trial; LDehy, luteal phase dehydrated trial; LEuhy, luteal phase euhydrated trial; MDehy, men dehydrated trial, MEuhy, men euhydrated trial.
Fig. 3.
Plasma osmolality (Posm) pre- and post-dehydration calculated via one-way and repeated-measures ANOVA. Data presented are means ± SE. *P < 0.05 between pre- and post-fluid prescription measures. FDehy, follicular phase dehydrated trial; FEuhy, follicular phase euhydrated trial; LDehy, luteal phase dehydrated trial; LEuhy, luteal phase euhydrated trial; MDehy, men dehydrated trial, MEuhy, men euhydrated trial.
DISCUSSION
In this study, we measured two major components of maintaining fluid balance, Posm and copeptin response. These two components are related, with increased Posm likely prompting a copeptin response that would in turn cause fluid retention, decreasing Posm and preventing greater BML. Our major findings were that 24-h FR yielded similar fluid volume loss across all groups and elicited a greater copeptin response in men compared with women (regardless of hormone status). The higher copeptin levels in men likely contributed to the maintenance of Posm in this group. In contrast, women had smaller copeptin responses and significant increases in Posm with 24-h FR, and there were no observed differences between menstrual cycle phases. FR induced between 0.74 and 1.18% BML and significant increases in biological markers of dehydration, including USG, Ucol, and Uosm without any apparent influence of sex or menstrual cycle phase. It is possible that the %BML observed in this study was not a strong enough stimulus to elicit a fluid-regulatory response in women based on the FR effects of female sex hormones (25, 30).
Despite the lack of statistical differences in copeptin concentration after FR between men and women, we noted that men exhibited higher copeptin concentration at baseline. This was consistent with previous observations of sex differences at baseline in both copeptin and AVP (19, 24, 31). Copeptin concentration significantly increased in men after FR but was not different for women after FR in either phase of the menstrual cycle. Across all groups, copeptin concentration post-FR was significantly correlated with BML from the dehydration, consistent with the idea that it is an appropriate marker of responses to dehydration stress. Although the observed increases in copeptin in men would theoretically alter urine volume and Uosm, those factors are subject to wide interindividual variability; thus, no differences were observed in our data set. Fold change of copeptin, which was analyzed to account for premeasure differences given the wide variability of baseline copeptin concentrations, did not appear to be affected by sex or menstrual phase. However, fold change was observed to be statistically significant in women following FDehy compared with FEuhy but not in LDehy despite no differences in concentration in either menstrual phase. Copeptin responses to dehydration in the follicular phase may have been augmented due to a larger dehydration stimulus in this phase that was masked by large interindividual differences in %BML across our group of women.
Whereas our male subjects did present with higher Posm before dehydration, their maintenance of Posm following 24-h FR was likely related to their copeptin response. Copeptin was not increased by FR for women in either phase, but FDehy women did present with higher Posm fafter 24-h FR, which may have contributed to the larger fold change in copeptin that was observed. LDehy Posm increases following 24-h FR showed a trend that did not reach statistical significance. We do not believe this was due to a direct hormonal effect but rather a possible effect of different levels of dehydration reached within the two phases. However, it is possible that the combination of estrogen and progesterone limited the level of dehydration reached in the LDehy trials, since estradiol and progesterone tend to promote fluid retention (22, 25, 26, 30).
Although we did not observe a statistical effect of sex on copeptin or Posm levels, it is striking that copeptin levels significantly increased in men (pre- vs. post-FR), whereas they did not change in women in response to this mild dehydration stressor. Mechanistic differences between the sexes may account for the practical differences in copeptin and Posm responses that we observed. This is consistent with the previously observed sex difference in threshold shifts and sensitivity of AVP responses to changes in Posm (29). Stachenfeld et al. (29) previously investigated variation in threshold for onset of AVP between sexes in the early follicular and the midluteal phases in women, using hypertonic saline infusion to stimulate AVP release (29). They found that men had greater sensitivity to changes in Posm for AVP secretion than women did in both the early follicular and the midluteal phases of the menstrual cycle (29). These previously observed differences in sensitivity are consistent with the sex differences observed in our present data, where copeptin in men was more responsive to the mild dehydration stimulus. However, multiple differences in study design between the study of Stachenfeld et al. and the present study preclude further direct comparison of our results. Although our Dehy trial did elicit significantly increased Posm in women only, no relationship was observed in the present study between Posm and post-FR copeptin concentration.
Experimental design considerations.
The current study design allowed for investigating effects of estrogen with and without progesterone, since we studied women in the late follicular phase, when estrogen is high, and in the midluteal phase, when both estrogen and progesterone are high. This allowed for the assessment of any possible additive effects that progesterone might have on body fluid balance, as previously proposed (26, 30). Our results show no sex or menstrual cycle phase differences in body fluid regulation following 24-h FR, either in BML from 24-h FR, urinary indexes, Posm, or copeptin concentration, which suggests that progesterone does not have independent or additive effects on body fluid regulation in the setting of mild passive dehydration. As an important note, progesterone concentration in the midluteal phase in our study was slightly lower than expected values for this phase of the menstrual cycle (21). While we did observe a four- to fivefold increase in progesterone, the midluteal phase is ordinarily characterized by progesterone concentrations of more than 6 ng/mL (21). It is possible that the lack of menstrual cycle differences was observed due to the lower than expected progesterone concentrations or that progesterone effects as previously theorized (26, 30) may be dose dependent. Progesterone has also been observed to downregulate estrogen receptors in some tissues (12), which may impact the fluid-regulatory effects across the menstrual cycle. Additionally, greater levels of dehydration may lead to possible sex and menstrual cycle differences, but yielding greater levels of dehydration via fluid restriction would require a longer FR period. It is also possible that mechanistic variation exists between the sexes, with differences in contributing factors affecting outcomes associated with dehydration stimuli, but those assessments were beyond the scope of this investigation.
Despite the previously reported positive relationship between estrogen and AVP/copeptin (6, 9), no significant relationship was reported between sex hormone concentration (estrogen or progesterone) and baseline concentration or change in copeptin. This may be related to possible inherent variation in copeptin concentration in women and the use of two menstrual cycle phases with high estrogen concentration. The lack of variation in baseline copeptin concentration between menstrual phases is consistent with previously reported results from Blum et al. (6).
Perspectives and Significance
Our present results suggest that 24-h FR yields similar levels of dehydration between men and women in both the late follicular and midluteal phases of the menstrual cycle, suggesting no significant impact of female sex hormones at this relatively mild level of dehydration. Men do appear to have volume-regulatory responses to 24-h FR, reflected by both increased copeptin and maintained Posm. These responses were not observed in women in either menstrual phase, a sex difference that may reflect a relatively less sensitive vasopressin response in healthy young women compared with men (29). Thus, we report here that even a mild dehydration stimulus unveils sex differences in volume-regulatory responses. We believe our results emphasize the need for careful consideration of sex differences in research, clinical, and athletic decision-making regarding fluid volume regulation.
DISCLAIMERS
The views, opinions, and/or findings contained in this article are those of the authors and should not be construed as an official United States Department of the Army position, or decision, unless so designated by other official documentation. Approved for public release, distribution unlimited. Citations of commercial organizations and trade names in this report do not constitute an official Department of the Army endorsement or approval of the products or services of these organizations.
DISCLOSURES
G.E.W.G., M.C.M., C.R.B., M.L.P., and D.J.C. were employed by the Korey Stringer Institute at the University of Connecticut at the time the research took place. S.A.K. has provided scientific consultation to Danone Research and Quest Diagnostics and has active research grants with Danone Research and Standard Process. D.J.C. has served as expert witness and received consulting honoraria from Clif Bar, Sports Innovation Laboratories, and the National Football League, funding from Gatorade, and royalties from Jones and Bartlett, Springer, LWW, Wolters-Kluwer Publishers, Up-to-Date, and Routledge/Taylor & Francis Group. A.T.C. and N.C. have no conflicts, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
G.E.G. and N.C. conceived and designed research; G.E.G., M.C.M., C.R.B., and M.L.P. performed experiments; G.E.G. and A.T.C. analyzed data; G.E.G., A.T.C., S.A.K. and N.C. interpreted results of experiments; G.E.G. prepared figures; G.E.G. drafted manuscript; G.E.G., A.T.C., M.C.M., C.R.B., M.L.P., S.A.K., N.C., and D.J.C. edited and revised manuscript; G.E.G., A.T.C., M.C.M., C.R.B., M.L.P., S.A.K., N.C., and D.J.C. approved final version of manuscript.
REFERENCES
- 1.Armstrong LE, Ganio MS, Casa DJ, Lee EC, McDermott BP, Klau JF, Jimenez L, Le Bellego L, Chevillotte E, Lieberman HR. Mild dehydration affects mood in healthy young women. J Nutr 142: 382–388, 2012. doi: 10.3945/jn.111.142000. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong LE, Johnson EC, Munoz CX, Swokla B, Le Bellego L, Jimenez L, Casa DJ, Maresh CM. Hydration biomarkers and dietary fluid consumption of women. J Acad Nutr Diet 112: 1056–1061, 2012. doi: 10.1016/j.jand.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong LE, Maresh CM, Castellani JW, Bergeron MF, Kenefick RW, LaGasse KE, Riebe D. Urinary indices of hydration status. Int J Sport Nutr 4: 265–279, 1994. doi: 10.1123/ijsn.4.3.265. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong LE, Herrera Soto JA, Hacker FT Jr, Casa DJ, Kavouras SA, Maresh CM. Urinary indices during dehydration, exercise, and rehydration. Int J Sport Nutr 8: 345–355, 1998. doi: 10.1123/ijsn.8.4.345. [DOI] [PubMed] [Google Scholar]
- 5.Balanescu S, Kopp P, Gaskill MB, Morgenthaler NG, Schindler C, Rutishauser J. Correlation of plasma copeptin and vasopressin concentrations in hypo-, iso-, and hyperosmolar states. J Clin Endocrinol Metab 96: 1046–1052, 2011. doi: 10.1210/jc.2010-2499. [DOI] [PubMed] [Google Scholar]
- 6.Blum CA, Mirza U, Christ-Crain M, Mueller B, Schindler C, Puder JJ. Copeptin levels remain unchanged during the menstrual cycle. PLoS One 9: e98240, 2014. doi: 10.1371/journal.pone.0098240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calzone WL, Silva C, Keefe DL, Stachenfeld NS. Progesterone does not alter osmotic regulation of AVP. Am J Physiol Regul Integr Comp Physiol 281: R2011–R2020, 2001. doi: 10.1152/ajpregu.2001.281.6.R2011. [DOI] [PubMed] [Google Scholar]
- 8.Engell DB, Maller O, Sawka MN, Francesconi RN, Drolet L, Young AJ. Thirst and fluid intake following graded hypohydration levels in humans. Physiol Behav 40: 229–236, 1987. doi: 10.1016/0031-9384(87)90212-5. [DOI] [PubMed] [Google Scholar]
- 9.Forsling ML, Akerlund M, Strömberg P. Variations in plasma concentrations of vasopressin during the menstrual cycle. J Endocrinol 89: 263–266, 1981. doi: 10.1677/joe.0.0890263. [DOI] [PubMed] [Google Scholar]
- 10.Ganio MS, Armstrong LE, Casa DJ, McDermott BP, Lee EC, Yamamoto LM, Marzano S, Lopez RM, Jimenez L, Le Bellego L, Chevillotte E, Lieberman HR. Mild dehydration impairs cognitive performance and mood of men. Br J Nutr 106: 1535–1543, 2011. doi: 10.1017/S0007114511002005. [DOI] [PubMed] [Google Scholar]
- 11.Giersch GEW, Charkoudian N, Stearns RL, Casa DJ. Fluid balance and hydration considerations for women: review and future directions. Sports Med 50: 253–261, 2020. doi: 10.1007/s40279-019-01206-6. [DOI] [PubMed] [Google Scholar]
- 12.Lessey BA, Palomino WA, Apparao KB, Young SL, Lininger RA. Estrogen receptor-alpha (ER-alpha) and defects in uterine receptivity in women. Reprod Biol Endocrinol 4, Suppl 1: S9, 2006. doi: 10.1186/1477-7827-4-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maughan RJ. Impact of mild dehydration on wellness and on exercise performance. Eur J Clin Nutr 57, Suppl 2: S19–S23, 2003. doi: 10.1038/sj.ejcn.1601897. [DOI] [PubMed] [Google Scholar]
- 14.Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 52: 112–119, 2006. doi: 10.1373/clinchem.2005.060038. [DOI] [PubMed] [Google Scholar]
- 15.Owen JA., Jr Physiology of the menstrual cycle. Am J Clin Nutr 28: 333–338, 1975. doi: 10.1093/ajcn/28.4.333. [DOI] [PubMed] [Google Scholar]
- 16.Perrier ET. Shifting focus: from hydration for performance to hydration for health. Ann Nutr Metab 70, Suppl 1: 4–12, 2017. doi: 10.1159/000462996. [DOI] [PubMed] [Google Scholar]
- 17.Robertson GL, Athar S. The interaction of blood osmolality and blood volume in regulating plasma vasopressin in man. J Clin Endocrinol Metab 42: 613–620, 1976. doi: 10.1210/jcem-42-4-613. [DOI] [PubMed] [Google Scholar]
- 18.Robertson GL, Shelton RL, Athar S. The osmoregulation of vasopressin. Kidney Int 10: 25–37, 1976. doi: 10.1038/ki.1976.76. [DOI] [PubMed] [Google Scholar]
- 19.Share L, Crofton JT, Ouchi Y. Vasopressin: sexual dimorphism in secretion, cardiovascular actions and hypertension. Am J Med Sci 295: 314–319, 1988. doi: 10.1097/00000441-198804000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Shirreffs SM, Merson SJ, Fraser SM, Archer DT. The effects of fluid restriction on hydration status and subjective feelings in man. Br J Nutr 91: 951–958, 2004. doi: 10.1079/BJN20041149. [DOI] [PubMed] [Google Scholar]
- 21.Speroff L, Vande Wiele RL. Regulation of the human menstrual cycle. Am J Obstet Gynecol 109: 234–247, 1971. doi: 10.1016/0002-9378(71)90872-6. [DOI] [PubMed] [Google Scholar]
- 22.Stachenfeld NS. Sex hormone effects on body fluid regulation. Exerc Sport Sci Rev 36: 152–159, 2008. doi: 10.1097/JES.0b013e31817be928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stachenfeld NS, DiPietro L, Kokoszka CA, Silva C, Keefe DL, Nadel ER. Physiological variability of fluid-regulation hormones in young women. J Appl Physiol (1985) 86: 1092–1096, 1999. doi: 10.1152/jappl.1999.86.3.1092. [DOI] [PubMed] [Google Scholar]
- 24.Stachenfeld NS, Gleim GW, Zabetakis PM, Nicholas JA. Fluid balance and renal response following dehydrating exercise in well-trained men and women. Eur J Appl Physiol Occup Physiol 72: 468–477, 1996. doi: 10.1007/BF00242277. [DOI] [PubMed] [Google Scholar]
- 25.Stachenfeld NS, Keefe DL. Estrogen effects on osmotic regulation of AVP and fluid balance. Am J Physiol Endocrinol Metab 283: E711–E721, 2002. doi: 10.1152/ajpendo.00192.2002. [DOI] [PubMed] [Google Scholar]
- 26.Stachenfeld NS, Keefe DL, Palter SF. Estrogen and progesterone effects on transcapillary fluid dynamics. Am J Physiol Regul Integr Comp Physiol 281: R1319–R1329, 2001. doi: 10.1152/ajpregu.2001.281.4.R1319. [DOI] [PubMed] [Google Scholar]
- 27.Stachenfeld NS, Leone CA, Mitchell ES, Freese E, Harkness L. Water intake reverses dehydration associated impaired executive function in healthy young women. Physiol Behav 185: 103–111, 2018. doi: 10.1016/j.physbeh.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 28.Stachenfeld NS, Silva C, Keefe DL, Kokoszka CA, Nadel ER. Effects of oral contraceptives on body fluid regulation. J Appl Physiol (1985) 87: 1016–1025, 1999. doi: 10.1152/jappl.1999.87.3.1016. [DOI] [PubMed] [Google Scholar]
- 29.Stachenfeld NS, Splenser AE, Calzone WL, Taylor MP, Keefe DL. Selected contribution: sex differences in osmotic regulation of AVP and renal sodium handling. J Appl Physiol (1985) 91: 1893–1901, 2001. doi: 10.1152/jappl.2001.91.4.1893. [DOI] [PubMed] [Google Scholar]
- 30.Stachenfeld NS, Taylor HS. Progesterone increases plasma volume independent of estradiol. J Appl Physiol (1985) 98: 1991–1997, 2005. doi: 10.1152/japplphysiol.00031.2005. [DOI] [PubMed] [Google Scholar]
- 31.Szinnai G, Morgenthaler NG, Berneis K, Struck J, Müller B, Keller U, Christ-Crain M. Changes in plasma copeptin, the c-terminal portion of arginine vasopressin during water deprivation and excess in healthy subjects. J Clin Endocrinol Metab 92: 3973–3978, 2007. doi: 10.1210/jc.2007-0232. [DOI] [PubMed] [Google Scholar]
- 32.Vokes TJ, Weiss NM, Schreiber J, Gaskill MB, Robertson GL. Osmoregulation of thirst and vasopressin during normal menstrual cycle. Am J Physiol Regul Integr Comp Physiol 254: R641–R647, 1988. doi: 10.1152/ajpregu.1988.254.4.R641. [DOI] [PubMed] [Google Scholar]
- 33.Wittbrodt MT, Millard-Stafford M. Dehydration impairs cognitive performance: a meta-analysis. Med Sci Sports Exerc 50: 2360–2368, 2018. doi: 10.1249/MSS.0000000000001682. [DOI] [PubMed] [Google Scholar]