Abstract
The role of hypoxia-inducible factor (HIF)-1 in pancreatic β-cell response to intermittent hypoxia (IH) was examined. Studies were performed on adult wild-type (WT), HIF-1α heterozygous (HET), β-cell-specific HIF-1−/− mice and mouse insulinoma (MIN6) cells exposed to IH patterned after blood O2 profiles during obstructive sleep apnea. WT mice treated with IH showed insulin resistance, and pancreatic β-cell dysfunction manifested as augmented basal insulin secretion, and impaired glucose-stimulated insulin secretion and these effects were absent in HIF-1α HET mice. IH increased HIF-1α expression and elevated reactive oxygen species (ROS) levels in β-cells of WT mice. The elevated ROS levels were due to transcriptional upregulation of NADPH oxidase (NOX)-4 mRNA, protein and enzymatic activity, and these responses were absent in HIF-1α HET mice as well as in β-HIF-1−/− mice. IH-evoked β-cell responses were absent in adult WT mice treated with digoxin, an inhibitor of HIF-1α. MIN6 cells treated with in vitro IH showed enhanced basal insulin release and elevated HIF-1α protein expression, and these effects were abolished with genetic silencing of HIF-1α. IH increased NOX4 mRNA, protein, and enzyme activity in MIN6 cells and disruption of NOX4 function by siRNA or scavenging H2O2 with polyethylene glycol catalase blocked IH-evoked enhanced basal insulin secretion. These results demonstrate that HIF-1-mediated transcriptional activation of NOX4 and the ensuing increase in H2O2 contribute to IH-induced pancreatic β-cell dysfunction.
Keywords: glucose-stimulated insulin secretion, hypoxia-inducible factor, NADPH oxidases, obstructive sleep apnea, reactive oxygen species
INTRODUCTION
Obstructive sleep apnea (OSA) is a widespread respiratory disorder characterized by brief (tens of seconds), repetitive interruptions of breathing occurring either due to complete (apnea) or partial (hypopnea) obstruction of the upper airway during sleep. Population-based studies showed strong association between severity of OSA and incidence of type 2 diabetes (T2D) (19, 20). Repetitive interruption of breathing results in chronic intermittent hypoxia (IH), which is a major contributing factor for T2D in OSA (23, 33). Mice treated with chronic IH, patterned after blood O2 profiles occurring with OSA, exhibit several features of T2D including insulin resistance, as indicated by an elevated homeostatic model assessment (HOMA) index, an established marker of insulin resistance (3, 35, 37) as well as β-cell dysfunction manifested as elevated basal insulin secretion and impaired glucose-stimulated insulin secretion (GSIS) (37). However, the molecular mechanisms by which IH evokes β-cell dysfunction are not known.
The hypoxia‐inducible factor (HIF) family of transcriptional activators plays critical role in cellular responses to hypoxia. HIF-1 was the first identified member of the HIF family followed by HIF-2 (29). HIF‐1 and HIF-2 are heterodimeric proteins composed of an O2‐regulated α subunit and a constitutively expressed β subunit (28). Pancreatic β-cells express low levels of HIF-1α, whereas HIF-2α expression was undetectable (5). GSIS is impaired in mice with β-cell-specific deletion of Arnt/Hif1b, a dimerization component of HIF-1α or in mice with selective deletion of HIF-1α in β-cells (2, 7, 9). Overexpression of HIF-1α in β-cells also exhibits impaired GSIS (6). In contrast, overexpression of HIF-2α in β-cells had no obvious effect on glucose homeostasis (4). These studies suggest that either increased or decreased HIF-1α levels result in disrupted glucose homeostasis and pathophysiology of β-cells.
Recent studies have shown that HIF-1 mediates autonomic pathologies caused by chronic IH (27). IH increases HIF-1α expression in the central and peripheral nervous system associated with regulation of cardio-respiratory functions. HIF1α+/− heterozygous (HET) mice, which are partially deficient in HIF1α expression, develop normally and are indistinguishable from wild-type (WT) littermates under basal conditions (11, 39). However, HIF-1α HET mice exhibit absence of IH-evoked sympathetic nerve activation and hypertension (26). Given that disrupted HIF-1α function in β-cells affects glucose homeostasis (6, 7), we tested the hypothesis that HIF-1 activation by IH mediates insulin resistance and β-cell dysfunction. This possibility was tested in HIF-1α HET as well as in β-cell-specific HIF-1α−/− mice and with targeted gain and loss of HIF-1α function in mouse insulinoma (MIN6) cells, which exhibit many properties of pancreatic β-cells (10).
METHODS
Experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Chicago (approved protocol #71811). Experiments were performed on adult male wild-type (C57BL6), HIF-1α HET (breeding pair obtained from Dr. Semenza) and β-cell-specific HIF1α−/− mice weighing 20–30 g. Information on HIF-1α HET mice including their origin and characterization is provided in our earlier publications (11, 26).
Generation of β-Cell-Specific HIF1α−/− Mice
β-HIF1α−/− mice were generated using the Cre-lox approach. Briefly, HIF-1α-floxed mice (The Jackson Research Laboratory, Stock No: 007561) were crossbred with Cre recombinase expressing mice, under control of the mouse insulin promoter (Ins1creErt2) (The Jackson Research Laboratory, Stock No: 026802). To induce recombination, HIF-1αflox Ins1creErt2 mice were treated with tamoxifen (100 mg·kg−1·day−1 ip) for 10 consecutive days before exposure to IH. Stock solutions of tamoxifen (T5648; Sigma-Aldrich, St. Louis, MO) were prepared in corn oil (20 mg/mL).
Exposure to Intermittent Hypoxia
The protocols for exposing mice to IH were essentially the same as described previously (26). Briefly, unsedated wild-type (WT) and HIF-1α HET or β-cell HIF1α−/− mice were placed in a specialized chamber and exposed to alternating cycles of hypoxia (15 s of ∼5% O2 followed by 5 min of room air, 9 episodes/h; 8 h/day) for 30 days. Parallel experiments were performed on mice exposed to alternating cycles of room air (controls) instead of IH in the same chambers. The duration of the gas flow was regulated by timer-controlled solenoid valves. Ambient O2 and CO2 levels in the chamber were continuously monitored, and the CO2 levels were maintained at ∼0.1%.
Measurement of Fasting Plasma Insulin and Glucose
Mice were fasted overnight and anesthetized with inhalation of isoflurane (Abbott Laboratories, Abbott Park, IL). Blood samples were collected by cardiac puncture. Plasma was separated, aliquoted, and stored at −80°C until further analysis. Insulin levels were determined by ultrasensitive mouse insulin ELISA kit (detection limit 0.05 ng/mL; Mercodia, Uppsala, Sweden), and glucose levels were analyzed using glucose oxidase assay kit (Pointe Scientific Inc., Canton, MI).
Exposure of Pancreatic Islets and MIN6 Cells to In Vitro IH
Pancreatic islets were isolated from anesthetized mice by distending the pancreas with intraductal injection of collagenase solution (1.0 mg/mL) after occluding the distal end close to the duodenum. Pancreatic islets were separated by Ficoll step gradient density as described previously (12, 37). Isolated islets and MIN6 cells (original clone provided by Dr. Louis Phillipson, University of Chicago) were cultured in 10% CO2 and 90% room air (20% O2) incubator at 37°C in DMEM growth medium supplemented with 15% fetal bovine serum, 1% penicillin–streptomycin, and 2 µM β-mercaptoethanol. Experiments were performed on islets serum starved overnight in RPMI-1640 medium containing 2.8mM glucose and 0.1% bovine serum albumin. Cells were exposed to in vitro IH (alternating cycles of 1.5% O2 for 30 s followed by 20% O2 for 5 min at 37°C) as described previously (40). Ambient O2 levels in the IH chamber were monitored by an O2 analyzer (Alpha Omega Instruments, Huston, TX). Insulin secretion was determined in the medium by mouse insulin ELISA kit (Mercodia, Uppsala, Sweden) in triplicate and normalized to islet number or mg protein. Protein was determined by Bradford assay (Bio-Rad, Hercules, CA). In the experiments involving treatment with drugs, cells were preincubated 30 min before and during IH exposure with either drug or vehicle.
Glucose- and KCl-Stimulated Insulin Secretion
Twenty islets of comparable size were distributed per well in nonadherent 48-well plates and incubated in Krebs Ringer’s bicarbonate (KRB) buffer containing 2 mM glucose or 2 mM KCl for 1 hour. The buffer was then replaced with KRB buffer containing 20 mM glucose or 30 mM KCl and incubated for 1 hour. Insulin abundance in the supernatant was determined by an ultrasensitive mouse insulin ELISA kit (Mercodia, Uppsala, Sweden) in triplicate.
Measurement of Insulin Content in the Islets
About 50 comparable-sized islets were selected and homogenized in acid ethanol (0.18 M HCl in 96% vol/vol ethanol) at 4°C. The homogenates were diluted 200 times to prevent interactions of ethanol with ELISA. Insulin levels were determined by mouse insulin ELISA kit (Mercodia, Uppsala, Sweden).
Immunocytochemistry
Anesthetized mice were perfused transcardially with ice-cold heparinized phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. Pancreatic tissue was harvested and mounted in OCT (Optimal Cutting Temperature) compound (Tissue Tek, VWR Scientific, Radnor, PA). Sections of 8 μm were cut and stored at −80°C until further analysis. MIN6 cells plated on polylysine-coated coverslips were exposed to either room air (normoxia) or IH, followed by fixation with 1% paraformaldehyde. Fixed pancreatic sections or MIN6 cells were washed three times in PBS and blocked with 20% normal goat serum and 0.2% Triton X-100 in PBS for 30 min and then incubated with anti HIF-1α antibody (1/500 dilution, #NB100–479, Novus Biologicals, Centennial, CO) or NOX4 (1/500 dilution; NB110–58851, Novus Biologicals, Centennial, CO) or insulin (1/2000, Sigma, St. Louis, MO). Antibody binding was detected using fluorescein isothiocyanate-conjugated secondary antibody (1/250, Molecular Probes, Eugene, OR). For morphometric analysis, HIF-1α positive cells were counted in each islet (3–4 islets per section/4 sections separated by an interval of 50 µm/animal) using IMAGE J (NIH, Bethesda, MD). Data are represented as the percent number of cells stained for HIF-1α divided by total number of cells stained with DAPI per islet.
Genetic Silencing
Recombinant lentivirus was generated by transfecting HEK293T cells with the third-generation packaging plasmid (Addgene, Watertown, MA), recombinant pLKO-1 expression vector encoding short hairpin HIF-1 RNA (shRNA) (provided by Dr. Semenza), or non-targeting control shRNA and Trans IT-Virus GEN transfection reagent (Mirus Bio LLC, Madison, WI). NOX4 siRNA lentivector was purchased from Applied Biological Material Inc. (Richmond, Canada). Lentiviral supernatants were harvested and passed through a 0.45-μm filter, and aliquots were frozen at −80°C. Lentiviral transfected MIN6 cells were cultured for 48 h before exposure to IH.
Immunoblot Analysis
Cell extracts from islets (∼250 islets from one mouse) or MIN6 cells (20 μg) prepared in radioimmunoprecipitation assay (RIPA) buffer (phosphate buffer, pH 7.4 containing 150mM NaCl, 1% triton X-100, 1% sodium deoxycholate, 0.1% SDS, 5 mM EDTA, and protease inhibitor cocktail) were fractionated by polyacrylamide-SDS gel electrophoresis. Nuclear extracts of MIN6 cells were prepared using nuclear extraction kit (Active Motif, Carlsbad, CA). Briefly, cells (1 × 106) were homogenized in 200 µL of hypotonic buffer provided in the kit and centrifuged at 850 g for 10 min at 4°C. The pellet was resuspended in 200 µL of hypotonic buffer, and small sample is checked under the microscope to verify that cells have been efficiently lysed and that nuclei have been released. The suspension is centrifuged at 14,000 g for 30 s. The nuclear pellet was resuspended in 40 µL of provided complete lysis buffer and fractionated by polyacrylamide-SDS gel electrophoresis. Immunoblots were probed with monoclonal HIF-1α (1/1000 dilution; NB100–123, Novus Biologicals, Centennial, CO), polyclonal NOX4 (1/1000; NB110–58851, Novus Biologicals, Centennial, CO), monoclonal TBP (#668306, Bio legend, San Diego, CA), or monoclonal tubulin (1/5000; #T6199, Sigma, St. Louis, MO) antibodies, followed by corresponding HRP-conjugated secondary antibody detected by the Clarity Western ECL substrate kit (Bio-Rad, Hercules, CA). Immunoblots were scanned and quantified using an Odyssey Fc imaging system (LI-COR, Lincoln, NE). Protein expression in islets from β-cell HIF-1α−/− mice was determined using WES apparatus (Protein Simple, San Jose, CA).
Real-Time RT-PCR
NOX4 mRNA was analyzed in pancreatic islets or MIN6 cells by quantitative real-time RT-PCR with mouse-specific primers, with 18S RNA as a housekeeping gene and SsoFast Evagreen Supermix (Bio-Rad, Hercules, CA) as a fluorogenic binding dye as described previously (22). Primer sequences for real-time PCR amplification are as follows: NOX4 (NM_015760.5); forward: TGTTGGGCCTAGGATTGTGTT; reverse: AGGGACCTTCTGTGATCCTCG. 18S (NR_003278); forward: CGCCGCTAGAGGTGAAATTC; reverse: CGAACCTCCGACTTTCGTTCT.
Measurement of Malondialdehyde Levels
Malondialdehyde (MDA) levels were determined as previously described (13). Briefly, ∼250 islets were homogenized in 10 volumes of 20 mM phosphate buffer (pH 7.4) at 4°C and centrifuged at 500 g for 10 min at 4°C. MDA levels were measured in the cell lysates in triplicate using thiobarbituric acid reactive substances (TBARS) assay kit (Cayman Chemical Company; Ann Arbor, MI) and reported as nanomoles of MDA formed per milligram of protein.
Measurement of NADPH Oxidase Activity
NADPH oxidase activity in the membrane-enriched protein fractions was determined as rate of superoxide dismutase-inhibitable cytochrome c reduction as described previously (13). Briefly, 50 µg of membrane protein lysate (∼500 islets pooled from 2 mice) was added to 150 µM cytochrome c and 100 µM NADPH in 25 mM HEPES buffer (pH 7.0). The assay was performed in the presence and absence of superoxide dismutase (200 units/mL) at 37°C for 30 min in triplicate. Cytochrome c reduction was measured by monitoring the absorbance at 550 nm. NADPH oxidase activity was calculated based on extinction coefficient 21 mmol·L−1·cm−1 and expressed as nanomoles per minute per milligram protein.
Measurement of H2O2
H2O2 was measured using a commercially available Amplex Red assay kit (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. Briefly, either the cell lysate prepared in phosphate buffer or a known amount of H2O2 was incubated in the dark with Amplex Red (100 μM) and horseradish peroxidase (1 U/mL) for 30 min at room temperature, and the absorbance of the solution was measured at 560 nm. The concentration of H2O2 was determined from the standard curve and expressed as nmol per milligram of protein.
Statistical Analysis
Data are expressed as mean ± SE from mice (6–8 animals per group) and three to five independent cell culture experiments. Statistical analysis was performed by analysis of variance (ANOVA). The Wilcoxon–Mann–Whitney test was used for analysis of normalized data. P values < 0.05 were considered significant.
RESULTS
Absence of IH-Induced Insulin Resistance in HIF-1α HET Mice
Homeostasis model assessment (HOMA) is a widely used and validated assay for measuring insulin resistance in OSA patients (16) and experimental animals (3, 37). Mice treated with 30 days of IH (IH30d) showed insulin resistance as evidenced by elevated HOMA index (37). To assess whether HIF-1α contributes to insulin resistance by IH, fasting plasma insulin and blood glucose levels were determined in unsedated age and gender matched littermate wild-type (WT) and HIF-1α HET mice treated with IH30d as described previously (26). Insulin resistance was determined by HOMA using the following formula: fasting insulin (μU/mL) × fasting glucose (mg/dL)/22.5. Consistent with a previous report (37), WT mice treated with IH30d exhibited elevated fasting insulin, unaltered fasting glucose levels, and elevated HOMA index (Fig. 1, A–C). Control HIF-1α HET mice reared in room air had lower fasting plasma insulin and modest elevation of fasting plasma glucose levels compared with WT controls (Fig. 1, A and B). IH30d had no significant effect on either fasting plasma insulin or glucose levels in HIF-1α HET mice, and as a consequence, HOMA index was unaltered (Fig. 1, A–C). These results demonstrate that HIF-1α HET mice exhibit absence of IH-evoked insulin resistance.
Fig. 1.
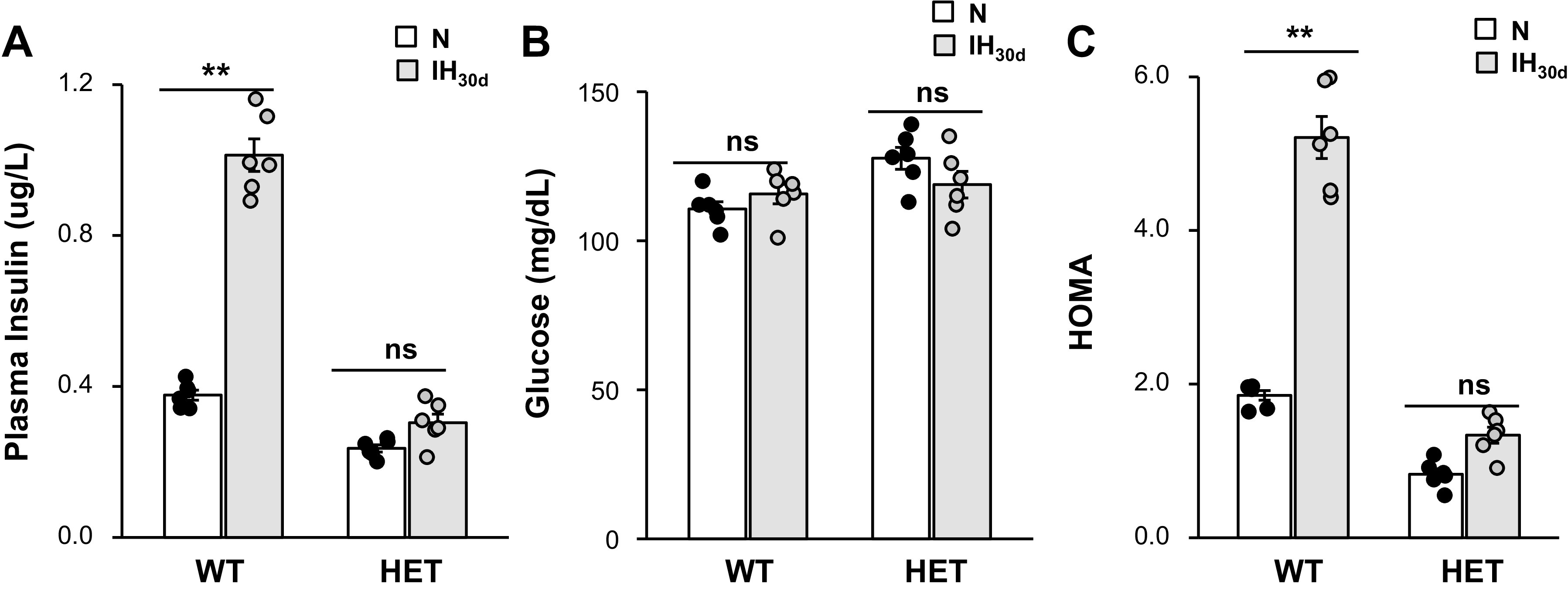
Effect of intermittent hypoxia (IH) on fasting plasma insulin, glucose, and homeostatic model assessment (HOMA) in wild-type (WT) and hypoxia-inducible factor (HIF-1α) heterozygous (HET) mice exposed to 30 days of IH (IH30d) or room air (normoxia, N). Fasting plasma insulin (A) and blood glucose (B) were measured as described in Methods. C: HOMA, an index of insulin resistance was calculated using the following formula: fasting plasma insulin (µU/mL) × fasting glucose (mg/dL)/22.5. Shown are the data from n = 8 animals as means ± SE; **P value < 0.01; ns, not significant (P value > 0.05) as determined by one-way ANOVA test.
HIF-1α Contributes to IH-Evoked Enhanced Basal Insulin Release from β-Cells
Our previous study (37) reported elevated basal insulin secretion from islets of IH-treated WT mice, which may account for the elevated fasting plasma insulin levels (Fig. 1A). The results showed that fasting plasma insulin levels were not elevated in IH-treated HIF-1α HET mice. Therefore, we hypothesized that HIF-1α contributes to enhanced basal insulin release by IH. To assess this possibility, we first determined the effects of IH on HIF-1α expression in pancreatic β-cells by immunocytochemistry and quantified by morphometric analysis. IH30d-treated WT mice showed increased HIF-1α-like immunoreactivity in pancreatic islets, as compared with room air-treated controls, and this effect was absent in HIF-1α HET mice (Fig. 2A). Morphometric analysis showed that ∼50% of β-cells from IH-treated WT mice were positive for HIF-1α-like immunoreactivity, whereas only 10% of IH-treated HIF-1α HET islets showed HIF-1α-like immunoreactivity (Fig. 2B). To further assess the effects of IH, HIF-1α protein expression was analyzed by immunoblot assay. Control WT islets showed modest HIF-1α protein which was undetectable in control HET mice reared in room air (Fig. 2C). HIF-1α expression increased significantly in IH-treated WT islets compared with that in HIF-1α HET islets (8-fold vs. 2-fold, respectively; P < 0.01; Fig. 2C).
Fig. 2.
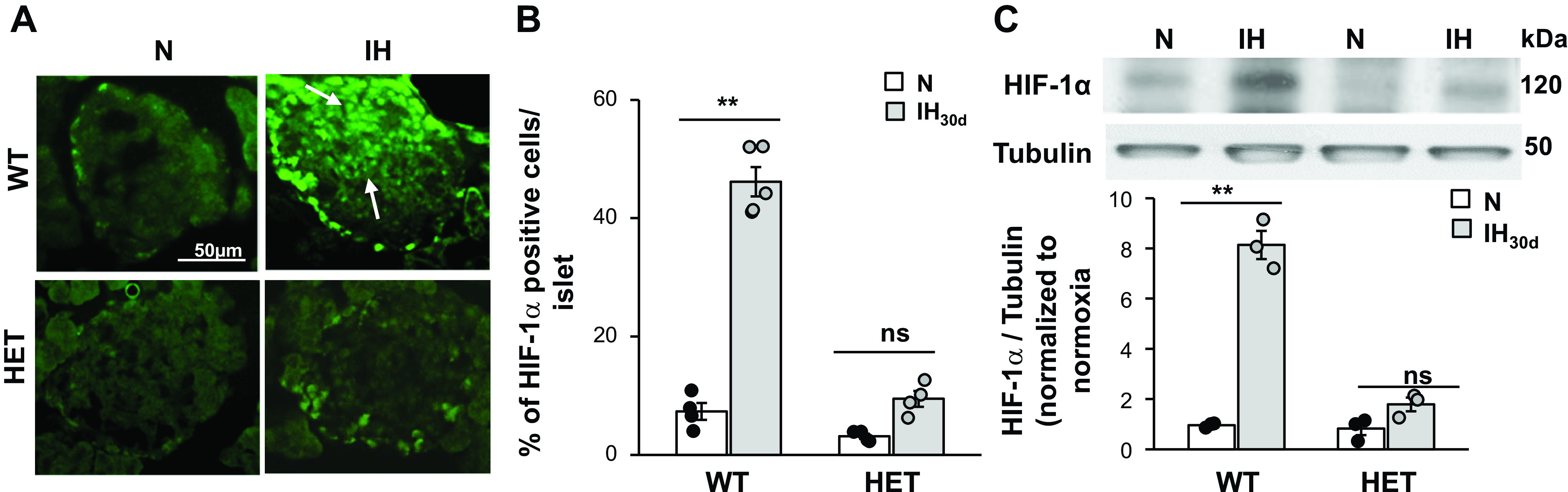
Intermittent hypoxia (IH) increases hypoxia-inducible factor (HIF-1α) protein abundance in β-cells of wild-type (WT) but not in HIF-1α heterozygous (HET) mice. A: representative example of a pancreatic islet stained for HIF-1α protein in WT and HIF-1α HET mice treated with IH30d or room air (normoxia, N). B: morphometric analysis of HIF-1α in β-cells. Data represent percent of HIF-1α expressing cells divided by number of cells stained by DAPI in each islet. Number of cells was determined from 16 to 20 islets from each mouse; n = 4 mice in each group. C: top, representative immunoblot of HIF-1α protein in islets from room air control (N) and IH30d-treated WT and HIF-1α HET mice. Tubulin protein was monitored as a loading control. Bottom, densitometric analysis of the immunoblots (means ± SE; n = 4 mice). **P value < 0.01; ns, not significant (P value > 0.05).
To determine whether HIF-1α contributes to elevated basal insulin secretion by IH, insulin release from pancreatic islets of WT and HIF-1α HET mice was determined as described in methods. Basal insulin levels were 4.5-fold higher in IH-treated WT islets, and this effect was absent in IH-treated HIF-1α HET mice (Fig. 3A). Immunocytochemical and ELISA analyses showed comparable insulin expression in pancreatic islets of WT and HIF-1α HET mice suggesting that absence of IH-induced basal insulin release was not secondary to decreased basal insulin content in HIF-1α HET mice (Fig. 3, B and C). Taken together, these results suggest that HIF-1α contributes to enhanced basal insulin release by IH.
Fig. 3.
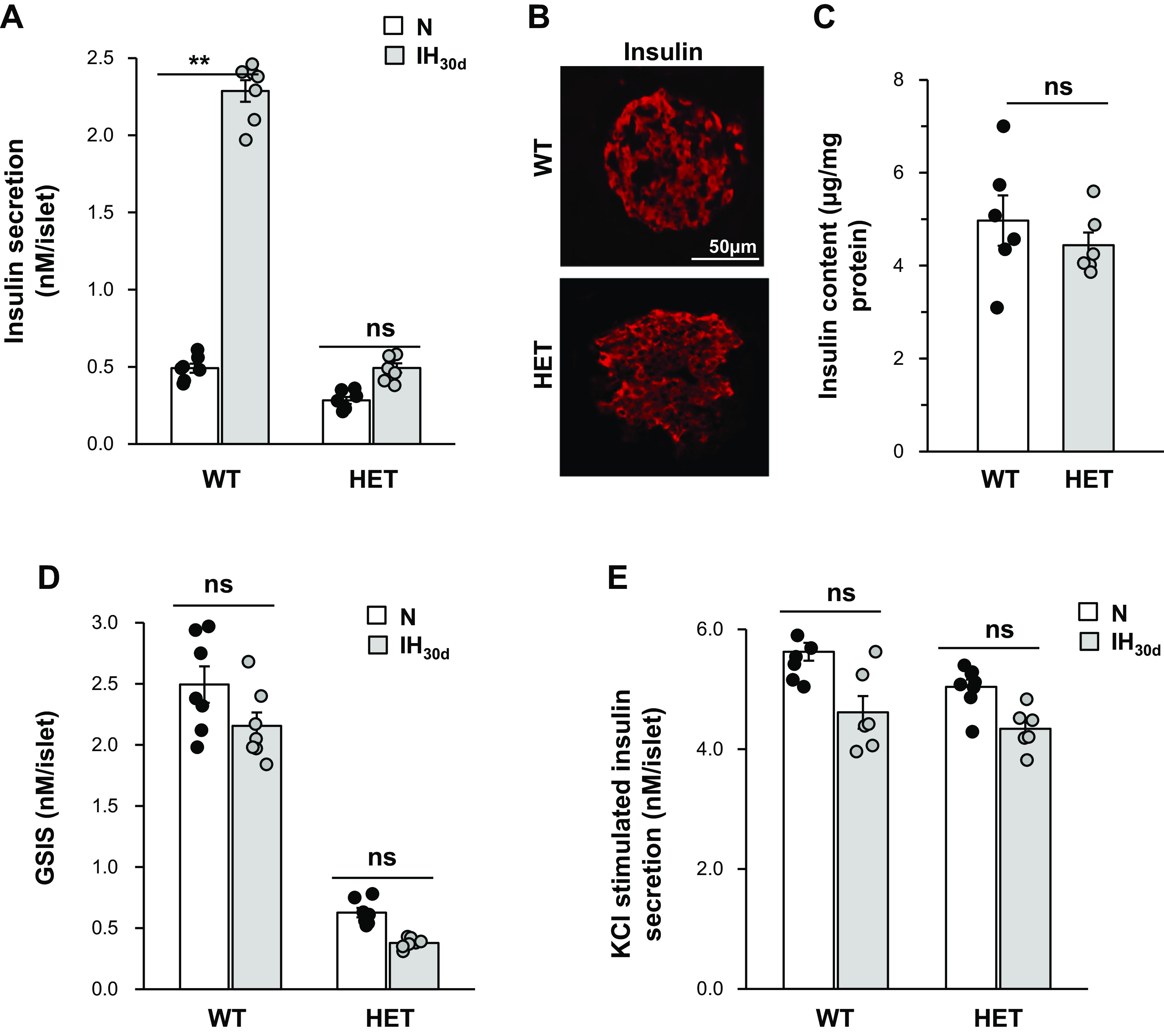
Pancreatic β-cell responses to intermittent hypoxia (IH). Basal insulin secretion and glucose-stimulated insulin secretion (GSIS) were determined in islets from wild-type (WT) and hypoxia-inducible factor 1 (HIF-1α) heterozygous (HET) mice treated with IH30d or room air (normoxia, N). A: basal insulin secretion was determined by ELISA (Mercodia, Uppsala, Sweden) and normalized to islet number. B: representative example of a pancreatic islet stained for insulin protein in WT and HIF-1α HET mice. C: quantitative measurement of insulin content in WT and HIF-1α HET islets by ELISA. D and E: insulin secretion was determined by ELISA in response to 20 mM glucose and 30 mM KCl, and the data were normalized to islet number. Shown are the data from n = 6 animals as means ± SE. **P value < 0.05; ns, not significant (P value > 0.05).
In addition to basal insulin release, IH impairs glucose-stimulated insulin secretion (GSIS) (37). To assess whether HIF-1 contributes to impaired GSIS by IH, insulin secretion was determined in response to 20 mM glucose from islets of IH-treated WT and HIF-1α HET mice; 20 mM glucose elicited approximately fourfold increase in insulin secretion in control WT islets and this response was impaired in HIF-1α HET islets (Fig. 3D). However, 20 mM glucose had no further stimulatory effect on insulin secretion in either WT or HIF-1α HET mice treated with IH (Fig. 3D). On the other hand, 30 mM KCl, a nonselective secretagogue, elicited comparable insulin release from islets of WT and HIF-1α HET mice treated with either room air or IH (Fig. 3E).
Absence of Reactive Oxygen Species Generation by IH in β-Cells of HIF-1α HET Mice
We then examined the mechanism(s) underlying HIF-1α-dependent-enhanced basal insulin release by IH. Increased reactive oxygen species (ROS) production has been implicated in evoking augmented basal insulin secretion by IH (37). We determined whether HIF-1α contributes to increased ROS generation by IH. To this end, malondialdehyde (MDA) levels, which represent oxidized lipids (34), were measured as an index of ROS generation in pancreatic islets from control and IH-treated mice (24). Basal MDA levels were ∼50% lower in HIF-1α HET compared with those in WT islets (Fig. 4A). IH increased MDA levels in WT but not in HIF-1α- HET islets (Fig. 4A).
Fig. 4.
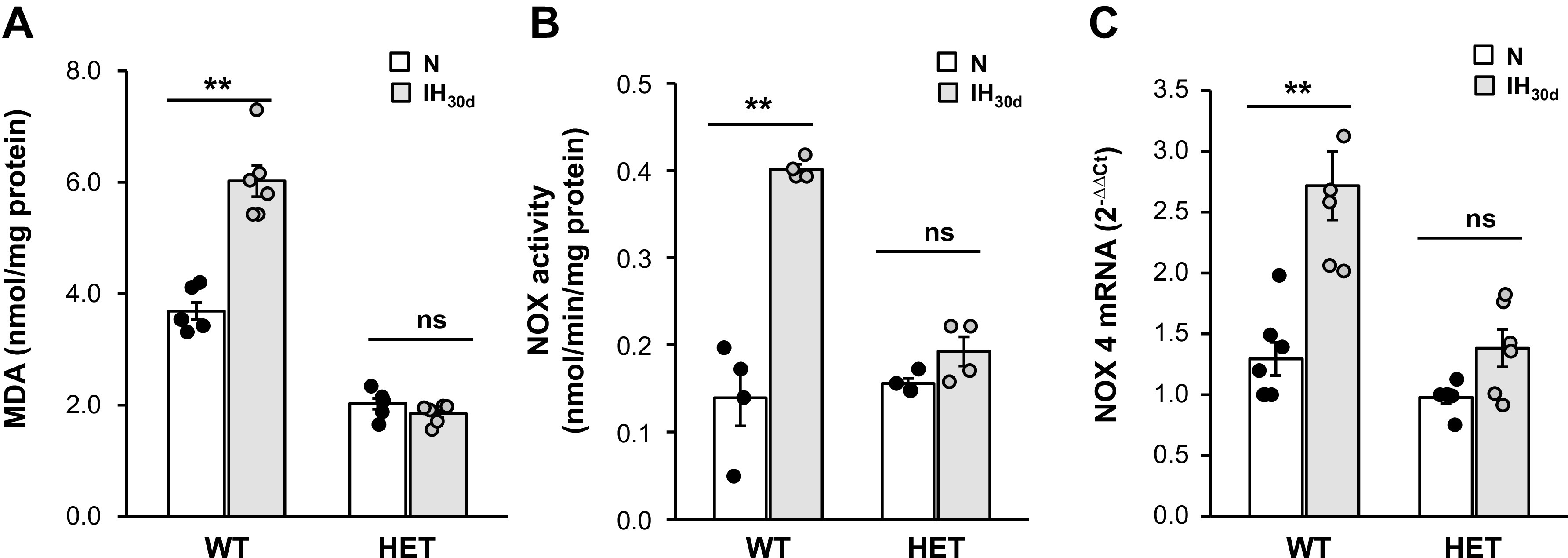
Intermittent hypoxia (IH) increases reactive oxygen species (ROS) levels, NADPH oxidase (NOX) enzyme activity and NOX 4 mRNA in β-cells of wild-type (WT) but not in hypoxia-inducible factor (HIF-1α) heterozygous (HET) mice. A: malondialdehyde (MDA) levels were determined in pancreatic islets as an index of ROS levels. B: NOX enzyme activity. C: NOX4 mRNA levels in islets from WT and HIF-1α HET mice exposed to room air (normoxia, N) or IH30d. Data from n = 6 animals as means ± SE. **P value <0.05; ns, not significant (P value > 0.05).
We next investigated how HIF-1α contributes to increased ROS generation by IH. Activation of prooxidant enzymes is an important source of ROS production. NADPH oxidase (NOX)-4, which is a major prooxidant enzyme in β-cells (17), is a HIF-1α target gene (8). We therefore determined whether IH activates NOX4 gene in a HIF-1-dependent manner. NOX4 mRNA and enzymatic activity were determined in β-cells of WT and HIF-1α HET mice. IH increased NOX4 enzyme activity and mRNA abundance by ∼2.7- and 2.0-fold, respectively, in WT islets, and these responses were absent HIF-1α HET mice (Fig. 4, B and C).
To further establish the role of HIF-1α, islets harvested from WT mice were treated with digoxin (100 nM), a potent inhibitor of HIF-1α (43), and then exposed to IH60. Digoxin treatment blocked IH-induced increase in insulin secretion (Fig. 5A). More importantly, IH-evoked increases in HIF-1α, NOX4 protein, mRNA, and enzyme activities were all absent in digoxin-treated islets (Fig. 5, B, C and D).
Fig. 5.
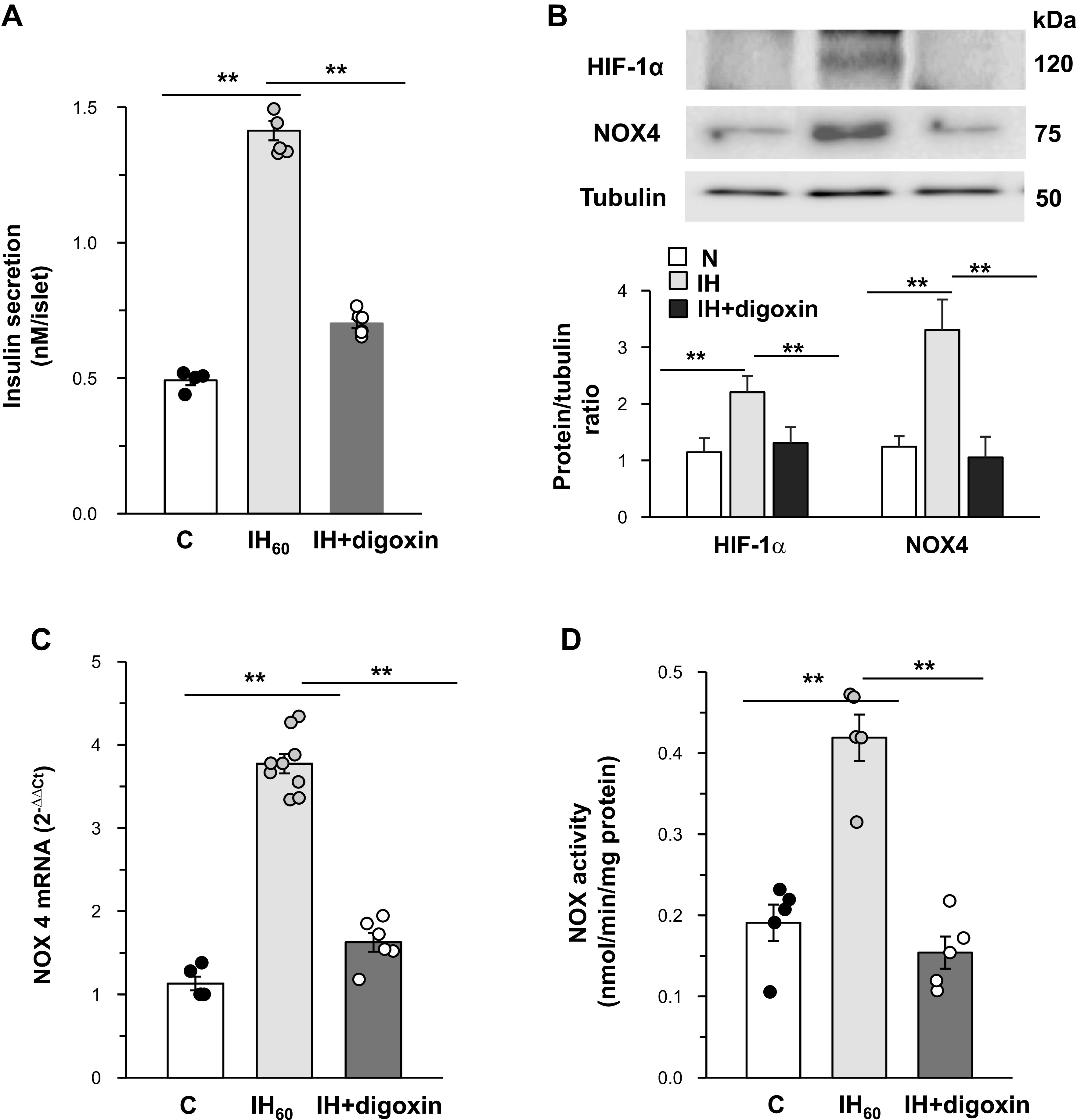
Hypoxia-inducible factor (HIF)-dependent upregulation of NADPH oxidase (NOX4) mediates increased basal insulin release by intermittent hypoxia (IH). Islets harvested from wild-type (WT) mice (n = 8) were treated with either vehicle (control, C) or digoxin (100 nM) and exposed to room air or 60 cycles of IH (IH60) (each cycle consisting alternating cycles of 30 s hypoxia and 5 min of room air). A: basal insulin secretion normalized per islet. B: Top: representative immunoblot showing HIF-1α, NOX4, and tubulin protein levels in islets. Bottom: quantitative densitometric analysis. Data represent means ± SE from n = 4 mice in each group. C and D: NOX4 mRNA expression and NOX enzyme activity in pancreatic islets. **P value < 0.05.
Thus far, studies were performed on mice with global partial deficiency of HIF-1α or mice with pharmacological blockade of HIF-1α protein. To assess the effect of targeted disruption of HIF-1α in pancreatic islets, β-cell-specific HIF-1α−/− mice were generated using Cre-lox approach and exposed to IH30d. Islets from β-HIF-1α−/− mice showed complete absence of HIF-1α protein as compared with the islets from control mice (Fig. 6A), demonstrating efficient knockdown of HIF-1α in β-cells. WT mice treated with IH30d showed increased HIF-1α as well as NOX4 protein and NOX4 mRNA expression in pancreatic islets, and these responses were absent in β-cell-specific HIF-1α−/− mice exposed to IH30d (Fig. 6, A and B). β-HIF-1α−/−mice reared in room air showed lower fasting plasma insulin levels, as compared with HIF-1αflox Ins1creErt2 mice, and IH had no effect on plasma insulin levels in β- HIF-1α−/− mice (Fig. 6C).
Fig. 6.
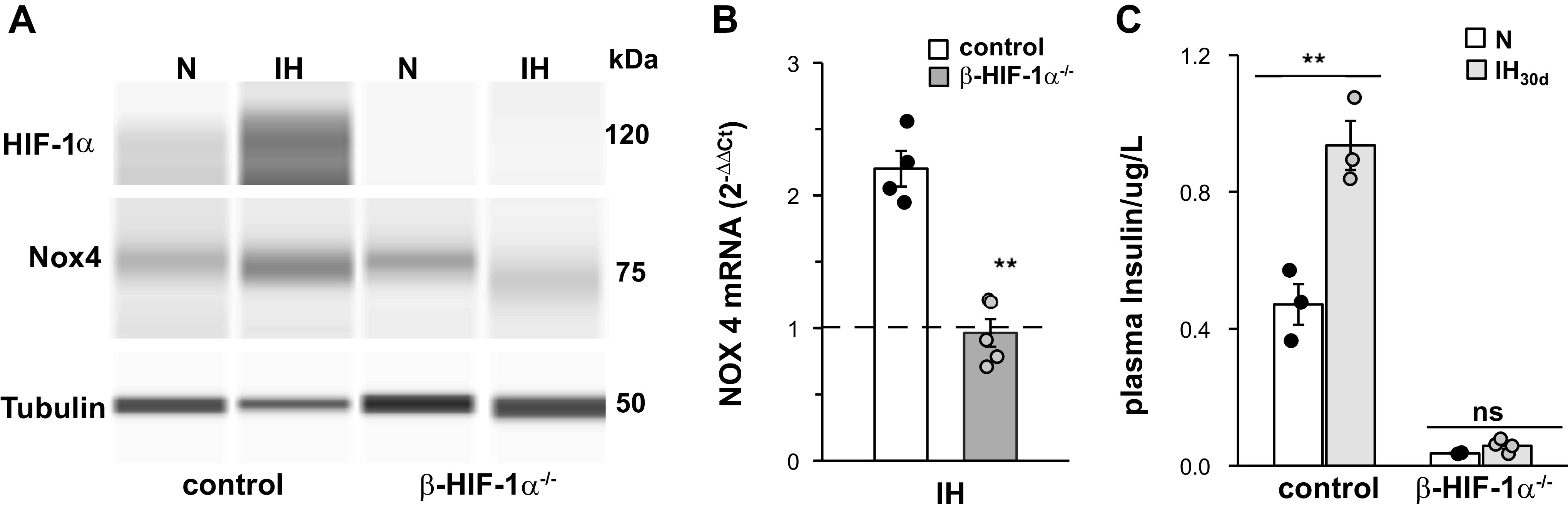
Absence of intermittent hypoxia (IH)-induced increase in hypoxia-inducible factor (HIF-1α), NADPH oxidase (NOX4) protein, NOX4 mRNA, and fasting plasma insulin levels in β-cell-specific HIF-1−/− mice exposed to IH30d. A: HIF-1α and NOX4 protein; B: NOX4 mRNA; and C: fasting plasma insulin levels in HIF-1α floxIns1CreERT mice with (β-HIF-1α−/−) and without (control) tamoxifen treatment. Data with means ± SE are shown (n = 4). **P value < 0.01; ns, not significant (P value > 0.05).
Effect of Targeted Loss and Gain of HIF-1α in MIN6 Cells
To further analyze the effects of IH on HIF-1α-dependent increase in insulin secretion; studies were performed on mouse insulinoma 6 (MIN6) cells with either targeted disruption (loss of function) or increased HIF-1α expression (gain of function). MIN6 cells, which exhibit many properties of native pancreatic β-cells (10), were exposed to increasing cycles of in vitro IH with each cycle consisting of 30 s of hypoxia followed by 5 min of normoxia as described previously (37). Cells treated with 60 and 120 cycles of IH (IH60 and IH120) showed robust insulin secretion (Fig. 7A). Based on these results, IH60, which evoked submaximal insulin secretion, was chosen for all further experiments.
Fig. 7.
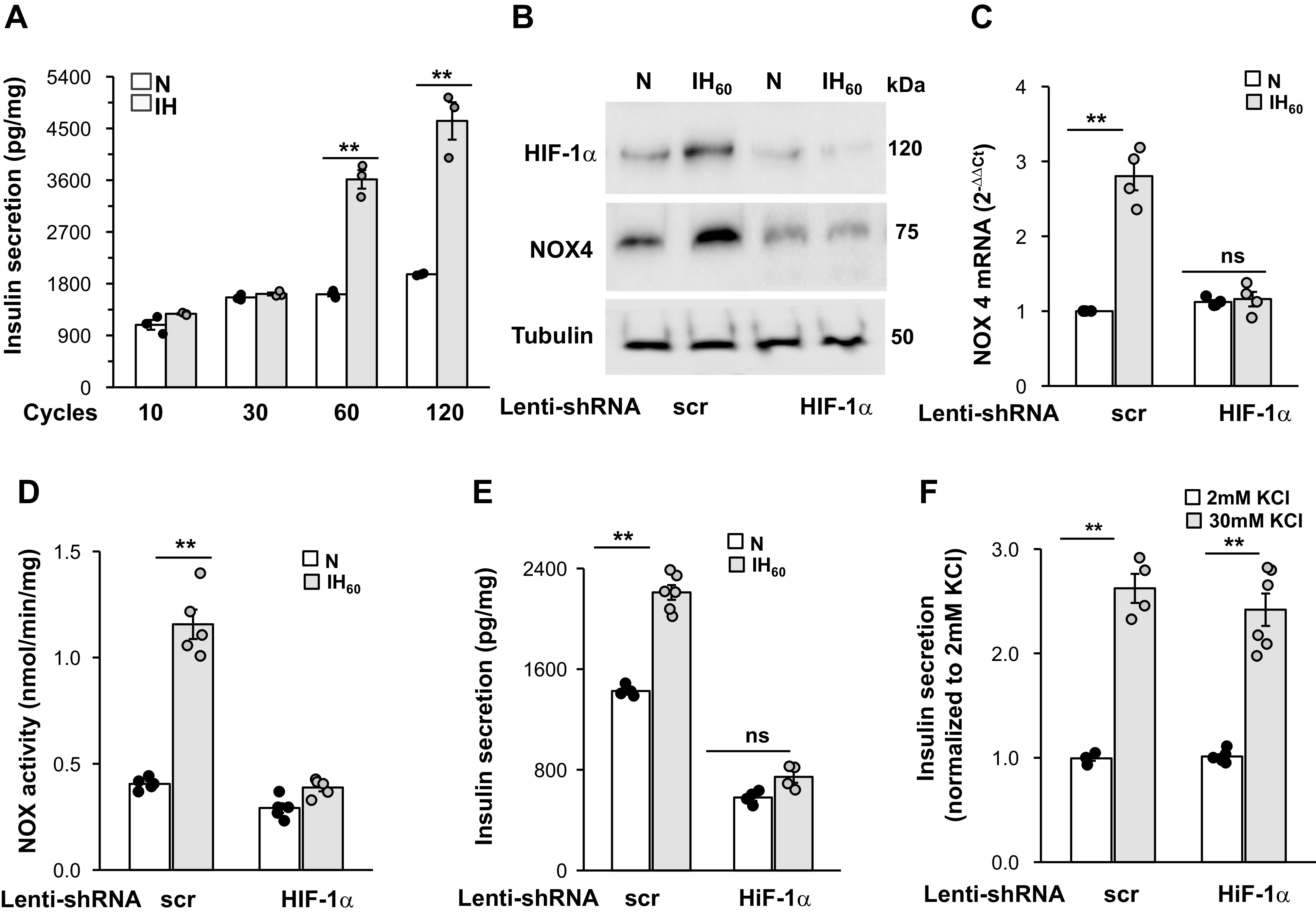
Silencing hypoxia-inducible factor (HIF-1α) prevents intermittent hypoxia (IH)-induced increase in NADPH oxidase (NOX4) protein, mRNA, enzyme activity, and baseline insulin release in mouse insulinoma (MIN6) cells. A: MIN6 cells were exposed to increasing cycles of in vitro IH. Insulin release was measured in the media after terminating IH. Data were normalized to milligram (mg) of protein. B: representative immunoblot of HIF-1α and NOX4 along with tubulin protein. C: NOX4 mRNA; D: NOX enzyme activity in MIN6 cells treated with lentiviral short hairpin HIF-1 RNA (shRNA) targeted to HIF-1α or scrambled (scr) RNA and exposed to 60 cycles of IH (IH60) or normoxia (N); E: insulin release in response to IH (normalized to mg protein); and F: insulin secretion responses to 30 mM KCl (normalized to 2mM KCl) in the media from MIN6 cells treated with lentiviral siRNA targeted to HIF-1α or scrambled (scr) RNA and exposed to IH60 or normoxia (N). Data presented are means ± SE from five independent experiments. **P < 0.01; ns, Not significant (P value > 0.05).
Loss of HIF-1α function.
MIN6 cells treated with lentivirus containing a short hairpin RNA (shRNA) targeted to either HIF-1α or a scrambled (control) RNA sequence were exposed to either IH60 or room air (control). IH-evoked HIF-1α expression, elevated NOX4 mRNA abundance, protein and enzyme activity, as well as enhanced basal insulin release, were all absent in HIF-1α shRNA treated cells. (Fig. 7, B–E). On the other hand, insulin release evoked by 30 mM KCl was comparable between scrambled (scr) and shRNA-treated cells (Fig. 7F).
Gain of HIF-1α function.
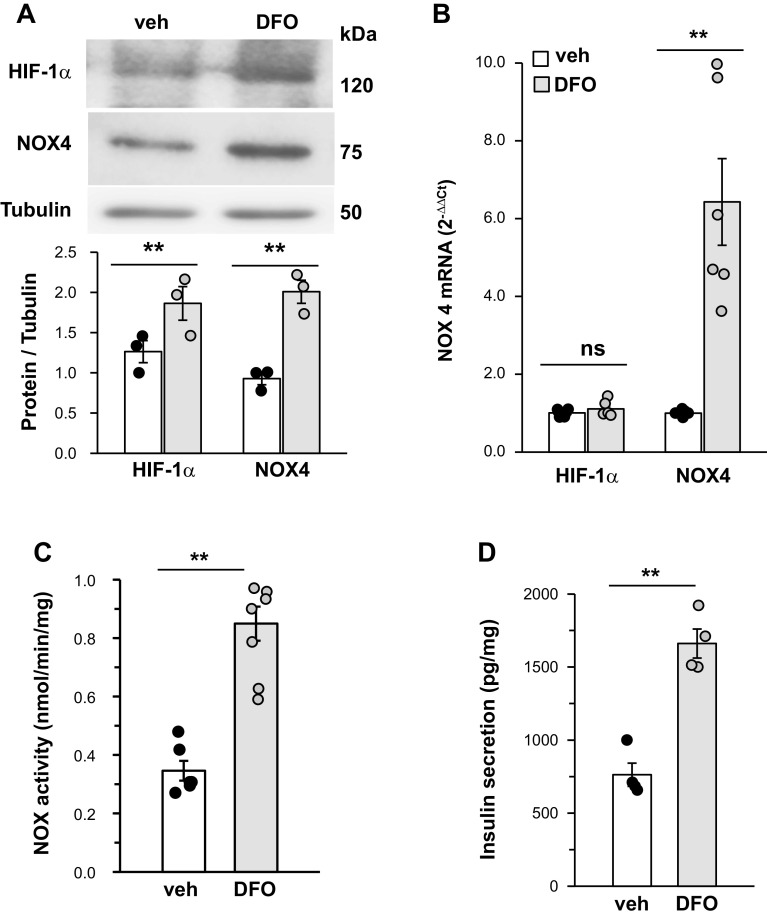
To assess gain of HIF-1α function, MIN6 cells cultured in room air were treated with an iron chelator, desferrioxamine (DFO, 500 µM) (36), for 24 h. DFO-treated cells showed elevated HIF-1α protein expression; increased NOX4 mRNA, protein, and NOX enzyme activity; and elevated basal insulin release, a phenotype similar to that seen in IH60-exposed MIN6 cells treated with a vehicle (Fig. 8, A–D).
Fig. 8.
Desferrioxamine (DFO) (500 µM for 24 h) treatment of mouse insulinoma (MIN6) cells mimics the effect of intermittent hypoxia (IH). A: top. representative immunoblot showing hypoxia-inducible factor (HIF-1α) and NADPH oxidase (NOX4) protein expression in MIN6 cells treated with either vehicle or DFO. Bottom, quantitative densitometric analysis of immunoblot assay. B: NOX4 mRNA; C: NOX enzyme activity; and D: insulin release from MIN6 cells treated with DFO. Data shown are means ± SE from six individual experiments. **P < 0.05; ns, not significant (P value > 0.05).
NOX4-Derived H2O2 Contributes to IH-Evoked Insulin Secretion
H2O2 is the main reactive oxygen species generated by NOX4 (18, 32). As ROS were implicated in enhanced basal insulin release (37), we examined whether NOX4-derived H2O2 contributes to this response. H2O2 abundance was determined in IH60-treated cells with silencing RNA (siRNA) targeted to NOX4. Immunocytochemical analysis showed increased NOX4-like immunoreactivity (Fig. 9A, top), and elevated NOX4 protein in nuclear fractions, as well as increased NOX enzyme activity in IH60 treated MIN6 cells (Fig. 9, A and B), and these responses were absent in cells treated with siRNA targeted to NOX4 (Fig. 9A, top and bottom;Fig. 9B).
Fig. 9.
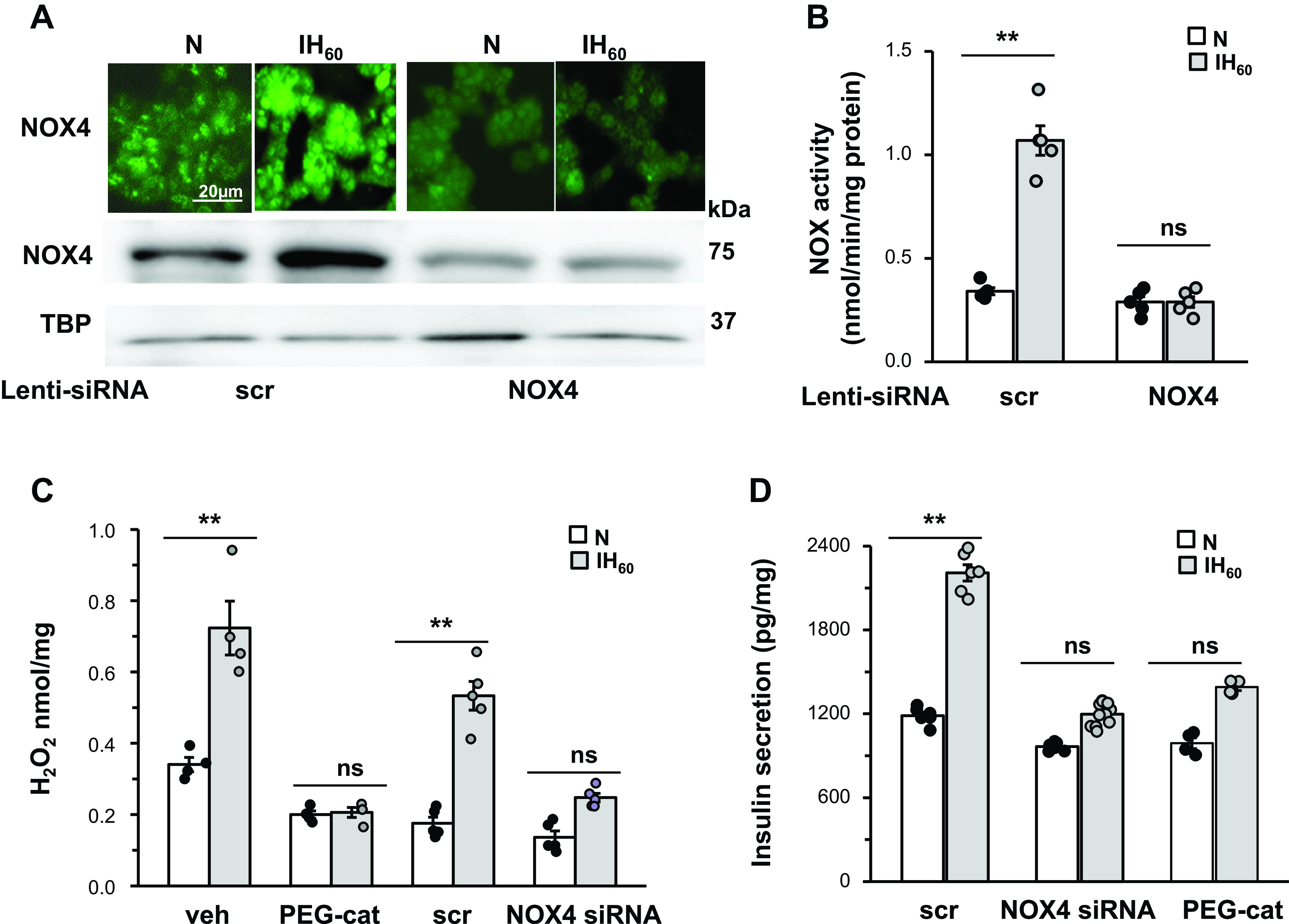
NADPH oxidase (NOX-4)-derived H2O2 contributes to intermittent hypoxia (IH)-evoked increase in basal insulin release. A: NOX4 protein expression determined by immunocytochemistry (top) and immunoblot of the nuclear cell extracts (bottom), tata binding protein (TBP) was used as loading control. B: NOX enzyme activity in MIN6 cells treated with lentiviral siRNA targeted to NOX4 or scrambled (scr) RNA and exposed to IH60 or room air (normoxia, N). C: H2O2 levels as measured by amplex red hydrogen peroxide assay kit (Invitrogen, Carlsbad, CA). D: insulin release from MIN6 cells treated with PEG-catalase (cell permeable H2O2 scavenger, 350U/mL) or lentiviral siRNA targeted to NOX4 or scrambled (scr) RNA and exposed to IH60 or normoxia (N). Data presented are means ± SE (n = 5 individual experiments). **P < 0.05; ns, not significant (P value > 0.05). PEG, polyethylene glycol.
H2O2 abundance increased in scrambled RNA-treated IH60-exposed cells and this response was markedly attenuated in either NOX4 siRNA-treated cells or in the presence of propylene glycol-conjugated catalase [polyethylene glycol (PEG) catalase], a cell membrane permeable H2O2 scavenger (Fig. 9C). The enhanced basal insulin secretion evoked by IH60 was blocked by treating cells either with NOX4 siRNA or with PEG-catalase (Fig. 9D).
DISCUSSION
The present study establishes a role for HIF-1 in pancreatic β-cell responses to IH patterned after blood O2 saturation profiles during OSA. Our results demonstrate that IH increases HIF-1α protein in mouse pancreatic β-cells. Similar induction of HIF-1α by IH was also reported in other cells such as carotid body glomus cells (14), adrenal medullary chromaffin cells, and neurons in the nTS and rostral ventrolateral medulla (25). On the other hand, IH was ineffective in inducing HIF-1α in the cerebellum (41), suggesting that IH increases HIF-1α in a cell selective manner. The effects of IH on HIF-1α expression in the central and peripheral nervous system are indirect and require neuronal activation (25). The finding that IH increases HIF-1α expression in MIN6 cells indicates a direct effect of IH on β-cells. We previously (21, 42) reported that increased generation of reactive oxygen species (ROS) by xanthine oxidase and subsequent increase in protein synthesis by mammalian target of rapamycin (mTOR), as well as decreased degradation by prolyl hydroxylase, contribute to elevated HIF-1α by IH in rat pheochromocytoma (PC)-12 cells. Further studies are needed to determine whether similar signaling mechanism(s) also contribute to IH-induced increase in HIF-1α in β-cells.
HIF-1α activation by IH was associated with elevated basal insulin release from β-cells, a finding consistent with an earlier report (37). In striking contrast, β-cells of HIF-1α HET mice treated with IH showed neither an increase in HIF-1α protein nor an enhanced insulin release. These findings suggest that persistent activation of HIF-1α by IH contribute to elevated basal insulin release. The absence of enhanced insulin release by IH in HIF-1α HET mice might conceivably be due to developmental adaptations resulting from partial deficiency of HIF-1α since birth. However, such a possibility appears unlikely because β-cells of adult WT mice treated with digoxin, a pharmacological inhibitor of HIF-1α also showed absence of IH-induced enhanced insulin release. HET mice used in the present study represent global partial knock down of HIF-1α and not selective loss of HIF-1α in β-cells. However, inducible β-cell selective disruption of HIF-1α also showed loss of HIF-1α activation and enhanced basal insulin release by IH. These findings taken together demonstrate that increased HIF-1α contributes to enhanced basal insulin secretion by IH from β-cells.
HIF-1-dependent enhanced basal insulin release by IH has adverse physiological consequences. First, the enhanced basal insulin release might in part contribute to IH-induced impaired GSIS by imposing excessive functional demand and exhaustion of the β-cell secretory capacity. Second, the elevated fasting plasma insulin levels resulting from enhanced basal insulin release lead to IH-induced insulin resistance which is a hallmark feature of T2D.
T2D in patients with OSA is linked to other comorbidities such as hypertension (31). Given that IH is ineffective in inducing hypertension in HIF-1α HET mice (26), the loss of β-cell responses to IH in HIF-α HET mice may be secondary to the absence of IH evoked hypertension. The findings are as follows: isolated islets from IH-treated HIF-1α HET mice, which are devoid of influence from systemic hypertension, still showed absence of enhanced basal insulin release; and targeted silencing of HIF-1α in MIN6 cells also showed loss of enhanced insulin release in response to IH demonstrate that absence of enhanced basal insulin release from IH-treated HIF-1α-deficient β-cells is not secondary to hypertension.
Our results provide insights into cellular mechanisms underlying HIF-1-dependent altered β-cell function by IH. An earlier study suggested ROS as an important cellular mechanism underlying IH-induced enhanced insulin release from β-cells (37). The following observations suggest that HIF-1α contributes to increased ROS generation by IH in β-cells: IH-induced increase in ROS generation was absent in HIF-1α HET mice; treatment of mice with digoxin, which blocked IH-increased HIF-1α expression also blocked ROS generation by IH as measured by NOX enzyme activity in β-cells; and IH-induced ROS generation was absent in MIN-6 cells with genetic silencing of HIF-1α. Collectively, these findings suggest that HIF-1α by facilitating ROS production in β-cells mediates enhanced basal insulin release by IH.
How might HIF-1α facilitate ROS production by IH in β-cells? Cellular ROS levels depend on balanced activities of prooxidant and antioxidant enzymes. NOX4 is a major prooxidant enzyme in pancreatic β-cells, and antioxidant capacity is relatively low in β-cells as compared with that in other cells (15). IH increased prooxidant capacity in WT β-cells as indicated by increased NOX4 mRNA and protein and enzymatic activity, and these effects were absent in HIF-1α HET pancreatic islets. The absence of NOX4 upregulation by IH in HIF-1α HET mice in unlikely due to developmental adaptations resulting from partial deficiency of HIF-1α since birth, because digoxin, which blocks IH-induced HIF-1α expression in adult mice, also showed absence of IH-induced increase in NOX4 mRNA, protein and enzyme activity. Similar results were also observed in mice with targeted disruption of HIF-1α in β-cells. Moreover, MIN6 cells with loss of HIF-1α function showed absence of IH-induced increase in NOX4 mRNA, protein, and enzyme activity, whereas gain of HIF-1α function mimicked the effects of IH in room air-treated control MIN6 cells. Given that HIF-1 is a potent activator of NOX4 transcription (8), our results suggest that increased ROS production by IH involves HIF-1-dependent transcriptional activation of NOX4. Our earlier study indicated that the effects of IH on β-cell function involve increased ROS production in the mitochondria (37). A study by Khan et al. (13) showed that NOX-dependent increase in ROS inhibits mitochondrial electron transport chain at complex I, further increasing ROS in the mitochondria. Whether similar cross talk between NOX and the mitochondrial complex leading to ROS-induced ROS also occurs in IH treated β-cells requires further study.
NOX4 generates hydrogen peroxide (H2O2) (18, 32). Our study identified H2O2 as a major reactive oxygen species mediating the effects of IH in β-cells. NOX4-dependent ROS generation has been implicated in β-cell function under basal conditions (38). Persistent ROS generation by NOX4 might lead to hyper secretion of insulin resulting in β-cell dysfunction (1, 17). Consistent with such a possibility genetic silencing of NOX4 or treating cells with PEG-catalase, a membrane permeable scavenger of H2O2 prevented the elevated H2O2 levels and blocked enhanced insulin release by IH. Although the present study identified NOX4 as one of the genes regulated by HIF-1 in the context of IH, HIF-1 also regulates several other genes including glucose transporters and enzymes associated with glucose metabolism (30), which might contribute in part to impaired glucose homeostasis by IH.
GRANTS
This work was supported by the National Institutes of Health Grant P01-HL-44454. Xuefeng Shi is a Visiting Scholar from Qing Hai Provincial People’s Hospital, China, and is funded by CSC (China Scholarship Council).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.N. conceived and designed research; N.W., X.S., S.A.K., and B.W. performed experiments; N.W., X.S., and S.A.K. analyzed data; J.N. interpreted results of experiments; J.N. prepared figures; J.N. drafted manuscript; G.L.S., N.R.P., and J.N. edited and revised manuscript; G.L.S., N.R.P., and J.N. approved final version of manuscript.
REFERENCES
- 1.Anvari E, Wikström P, Walum E, Welsh N. The novel NADPH oxidase 4 inhibitor GLX351322 counteracts glucose intolerance in high-fat diet-treated C57BL/6 mice. Free Radic Res 49: 1308–1318, 2015. doi: 10.3109/10715762.2015.1067697. [DOI] [PubMed] [Google Scholar]
- 2.Ban J-J, Ruthenborg RJ, Cho KW, Kim J-w. Regulation of obesity and insulin resistance by hypoxia-inducible factors. Hypoxia (Auckl) 2: 171–183, 2014. doi: 10.2147/HP.S68771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonora E, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L, Saggiani F, Poli M, Perbellini S, Raffaelli A, Cacciatori V, Santi L, Targher G, Bonadonna R, Muggeo M. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona diabetes complications study. Diabetes Care 25: 1135–1141, 2002. doi: 10.2337/diacare.25.7.1135. [DOI] [PubMed] [Google Scholar]
- 4.Brunt JJ, Shi SY, Schroer SA, Sivasubramaniyam T, Cai EP, Woo M. Overexpression of HIF-2α in pancreatic β cells does not alter glucose homeostasis. Islets 6: e1006075, 2014. doi: 10.1080/19382014.2015.1006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantley J, Grey ST, Maxwell PH, Withers DJ. The hypoxia response pathway and β-cell function. Diabetes Obes Metab 12, Suppl 2: 159–167, 2010. doi: 10.1111/j.1463-1326.2010.01276.x. [DOI] [PubMed] [Google Scholar]
- 6.Cantley J, Selman C, Shukla D, Abramov AY, Forstreuter F, Esteban MA, Claret M, Lingard SJ, Clements M, Harten SK, Asare-Anane H, Batterham RL, Herrera PL, Persaud SJ, Duchen MR, Maxwell PH, Withers DJ. Deletion of the von Hippel-Lindau gene in pancreatic beta cells impairs glucose homeostasis in mice. J Clin Invest 119: 125–135, 2009. doi: 10.1172/JCI26934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng K, Ho K, Stokes R, Scott C, Lau SM, Hawthorne WJ, O’Connell PJ, Loudovaris T, Kay TW, Kulkarni RN, Okada T, Wang XL, Yim SH, Shah Y, Grey ST, Biankin AV, Kench JG, Laybutt DR, Gonzalez FJ, Kahn CR, Gunton JE. Hypoxia-inducible factor-1alpha regulates beta cell function in mouse and human islets. J Clin Invest 120: 2171–2183, 2010. doi: 10.1172/JCI35846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diebold I, Petry A, Hess J, Görlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell 21: 2087–2096, 2010. doi: 10.1091/mbc.e09-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunton JE, Kulkarni RN, Yim S, Okada T, Hawthorne WJ, Tseng Y-H, Roberson RS, Ricordi C, O’Connell PJ, Gonzalez FJ, Kahn CR. Loss of ARNT/HIF1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell 122: 337–349, 2005. doi: 10.1016/j.cell.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Ishihara H, Asano T, Tsukuda K, Katagiri H, Inukai K, Anai M, Kikuchi M, Yazaki Y, Miyazaki JI, Oka Y. Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia 36: 1139–1145, 1993. doi: 10.1007/BF00401058. [DOI] [PubMed] [Google Scholar]
- 11.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1alpha. Genes Dev 12: 149–162, 1998. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson DA, Kuznetsov A, Lopez JP, Kash S, Ammälä CE, Philipson LH. Kv2.1 ablation alters glucose-induced islet electrical activity, enhancing insulin secretion. Cell Metab 6: 229–235, 2007. doi: 10.1016/j.cmet.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan SA, Nanduri J, Yuan G, Kinsman B, Kumar GK, Joseph J, Kalyanaraman B, Prabhakar NR. NADPH oxidase 2 mediates intermittent hypoxia-induced mitochondrial complex I inhibition: relevance to blood pressure changes in rats. Antioxid Redox Signal 14: 533–542, 2011. doi: 10.1089/ars.2010.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam S-Y, Tipoe GL, Liong EC, Fung M. Hypoxia-inducible factor (HIF)-1alpha and endothelin-1 expression in the rat carotid body during intermittent hypoxia. Adv Exp Med Biol 580: 21–27, 2006. doi: 10.1007/0-387-31311-7_4. [DOI] [PubMed] [Google Scholar]
- 15.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 20: 463–466, 1996. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 16.Lindberg E, Berne C, Elmasry A, Hedner J, Janson C. CPAP treatment of a population-based sample—What are the benefits and the treatment compliance? Sleep Med 7: 553–560, 2006. doi: 10.1016/j.sleep.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol 24: 1844–1854, 2004. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Muraki I, Wada H, Tanigawa T. Sleep apnea and type 2 diabetes. J Diabetes Investig 9: 991–997, 2018. doi: 10.1111/jdi.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagayoshi M, Punjabi NM, Selvin E, Pankow JS, Shahar E, Iso H, Folsom AR, Lutsey PL. Obstructive sleep apnea and incident type 2 diabetes. Sleep Med 25: 156–161, 2016. doi: 10.1016/j.sleep.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nanduri J, Vaddi DR, Khan SA, Wang N, Makarenko V, Semenza GL, Prabhakar NR. HIF-1α activation by intermittent hypoxia requires NADPH oxidase stimulation by xanthine oxidase. PLoS One 10: e0119762, 2015. doi: 10.1371/journal.pone.0119762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng Y-J, Kumar GK, Garcia JA, Prabhakar NR. Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci USA 106: 1199–1204, 2009. doi: 10.1073/pnas.0811018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ota H, Fujita Y, Yamauchi M, Muro S, Kimura H, Takasawa S. Relationship between intermittent hypoxia and type 2 diabetes in sleep apnea syndrome. Int J Mol Sci 20: 4756, 2019. doi: 10.3390/ijms20194756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng Y-J, Raghuraman G, Khan SA, Kumar GK, Prabhakar NR. Angiotensin II evokes sensory long-term facilitation of the carotid body via NADPH oxidase. J Appl Physiol (1985) 111: 964–970, 2011. doi: 10.1152/japplphysiol.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng Y-J, Yuan G, Khan S, Nanduri J, Makarenko VV, Reddy VD, Vasavda C, Kumar GK, Semenza GL, Prabhakar NR. Regulation of hypoxia-inducible factor-α isoforms and redox state by carotid body neural activity in rats. J Physiol 592: 3841–3858, 2014. doi: 10.1113/jphysiol.2014.273789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng Y-J, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 577: 705–716, 2006. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prabhakar NR, Semenza GL. Regulation of carotid body oxygen sensing by hypoxia-inducible factors. Pflugers Arch 468: 71–75, 2016. doi: 10.1007/s00424-015-1719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell 148: 399–408, 2012. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 24: 97–106, 2009. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 30.Semenza GL, Shimoda LA, and Prabhakar NR. Regulation of gene expression by HIF-1. Novartis Found Symp 272: 2–14, 2006. [PubMed] [Google Scholar]
- 31.Siwasaranond N, Nimitphong H, Manodpitipong A, Saetung S, Chirakalwasan N, Thakkinstian A, Reutrakul S. The relationship between diabetes-related complications and obstructive sleep apnea in type 2 diabetes. J Diabetes Res 2018: 9269170, 2018. doi: 10.1155/2018/9269170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takac I, Schröder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem 286: 13304–13313, 2011. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torrella M, Castells I, Gimenez-Perez G, Recasens A, Miquel M, Simó O, Barbeta E, Sampol G. Intermittent hypoxia is an independent marker of poorer glycaemic control in patients with uncontrolled type 2 diabetes. Diabetes Metab 41: 312–318, 2015. doi: 10.1016/j.diabet.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem 524: 13–30, 2017. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 35.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 27: 1487–1495, 2004. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 36.Wang GL, Semenza GL. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood 82: 3610–3615, 1993. doi: 10.1182/blood.V82.12.3610.3610. [DOI] [PubMed] [Google Scholar]
- 37.Wang N, Khan SA, Prabhakar NR, Nanduri J. Impairment of pancreatic β-cell function by chronic intermittent hypoxia. Exp Physiol 98: 1376–1385, 2013. doi: 10.1113/expphysiol.2013.072454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu X, Williams KJ. NOX4 pathway as a source of selective insulin resistance and responsiveness. Arterioscler Thromb Vasc Biol 32: 1236–1245, 2012. doi: 10.1161/ATVBAHA.111.244525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JSK, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest 103: 691–696, 1999. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan G, Adhikary G, McCormick AA, Holcroft JJ, Kumar GK, Prabhakar NR. Role of oxidative stress in intermittent hypoxia-induced immediate early gene activation in rat PC12 cells. J Physiol 557: 773–783, 2004. doi: 10.1113/jphysiol.2003.058503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan G, Khan SA, Luo W, Nanduri J, Semenza GL, Prabhakar NR. Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J Cell Physiol 226: 2925–2933, 2011. doi: 10.1002/jcp.22640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol 217: 674–685, 2008. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, Rey S, Hammers H, Chang D, Pili R, Dang CV, Liu JO, Semenza GL. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc Natl Acad Sci USA 105: 19579–19586, 2008. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]