Abstract
The prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) quickly reached pandemic proportions, and knowledge about this virus and coronavirus disease 2019 (COVID-19) has expanded rapidly. This review focuses primarily on mechanisms that contribute to acute cardiac injury and dysfunction, which are common in patients with severe disease. The etiology of cardiac injury is multifactorial, and the extent is likely enhanced by preexisting cardiovascular disease. Disruption of homeostatic mechanisms secondary to pulmonary pathology ranks high on the list, and there is growing evidence that direct infection of cardiac cells can occur. Angiotensin-converting enzyme 2 (ACE2) plays a central role in COVID-19 and is a necessary receptor for viral entry into human cells. ACE2 normally not only eliminates angiotensin II (Ang II) by converting it to Ang-(1–7) but also elicits a beneficial response profile counteracting that of Ang II. Molecular analyses of single nuclei from human hearts have shown that ACE2 is most highly expressed by pericytes. Given the important roles that pericytes have in the microvasculature, infection of these cells could compromise myocardial supply to meet metabolic demand. Furthermore, ACE2 activity is crucial for opposing adverse effects of locally generated Ang II, so virus-mediated internalization of ACE2 could exacerbate pathology by this mechanism. While the role of cardiac pericytes in acute heart injury by SARS-CoV-2 requires investigation, expression of ACE2 by these cells has broader implications for cardiac pathophysiology.
Keywords: ACE2, cardiac dysfunction, pericyte, renin-angiotensin-aldosterone system, SARS-CoV-2
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus first identified in December 2019 in Wuhan, China, is the causative agent of the coronavirus disease 2019 (COVID-19) pandemic currently gripping the world. To date, this virus has infected 34.4 million people and caused over a million deaths worldwide (https://covid19.who.int). The primary site for entry of the virus is the airways, and in severe cases this infection can progress to cause substantial pulmonary damage with cascading systemic effects. Although fever, sore throat, cough, and fatigue are common signs of infection in symptomatic individuals, the list of possible symptoms has grown, especially in patients with more severe disease. The expanded list reflects involvement of other organs, including but not limited to the gastrointestinal tract, kidneys and heart (68, 70). Additionally, there is a wealth of evidence documenting that SARS-CoV-2 infections can cause coagulation abnormalities, which can lead to thrombotic complications affecting different organs (i.e., lungs, heart, and brain) and disseminated intravascular coagulation (38, 41, 48)
Cardiac dysfunction with evidence of acute cardiac injury is common in patients with severe COVID-19 (5, 49, 59, 90). It is estimated that ∼10% of hospitalized patients with COVID-19 show some form of cardiovascular pathology, and this increases to ∼30% for those in the intensive care unit. Cardiovascular complications can include acute heart failure, arrhythmias, myocarditis, and acute coronary syndrome (5, 49, 59, 90). Preexisting cardiovascular disease increases the risk for cardiac injury in COVID-19 (12, 45, 73), but some patients present with new cardiac dysfunction (12). Such cases can occur in the absence of coronary artery disease or reflect aggravation of subclinical disease. In any case, acute cardiac injury in COVID-19 is linked to a poor prognosis (5, 49, 59, 90).
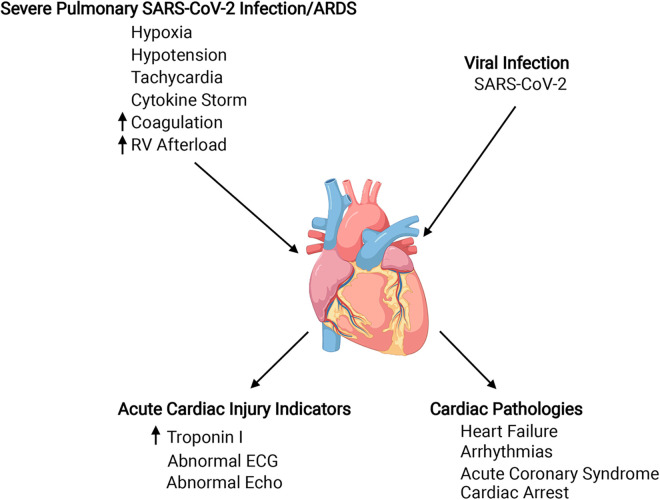
Specific mechanisms that cause cardiac injury and dysfunction in COVID-19 are a topic of intensive, ongoing investigation, but available evidence supports a multiplicity of contributing factors, which can be broadly grouped as effects on the heart that occur consequent to systemic effects of severe pulmonary disease and direct viral infection of the heart (3, 17, 19, 29, 53, 81). We will briefly review how systemic pathophysiology of COVID-19 can affect the heart and discuss evidence for direct infection of the heart by SARS-CoV and SARS-CoV-2 (Fig. 1). Importantly, we will cover the dual, pathogenic and protective, roles of angiotensin-converting enzyme 2 (ACE2) as they apply to COVID-19, focusing especially on the heart. Last, we will review recent evidence implicating ACE2-expressing pericytes in cardiovascular complications of COVID-19.
Fig. 1.
Diagram showing factors that could contribute to acute cardiac injury in coronavirus disease 2019 (COVID-19), some clinical indicators of acute cardiac injury and resulting cardiac pathologies. Known and theoretical factors contributing to cardiac injury are indicated at the top left and top right. Top left: most but not all patients with cardiac complications of COVID-19 experience severe pulmonary infection, which elicits systemic effects that can be detrimental to the heart. Cytokine storm and hypercoagulability can produce direct effects on the heart by fostering cardiac inflammatory responses and microthrombi formation, respectively. Other systemic effects increase cardiac work, increasing oxygen demand while oxygen supply is reduced. Top right: direct infection of cardiac cells by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is possible, given the expression of angiotensin-converting enzyme 2 (ACE2) viral receptor and proteases required for viral entry by cardiac cells. Bottom left: increased levels of high-sensitivity troponin I in the blood are a primary indicator of cardiac injury, and increases in this marker over time are associated with more severe cardiac complications. Electrocardiograms, echocardiographs, and magnetic resonance imaging can identify functional and structural abnormalities. Bottom right: cardiac complications of COVID-19. Patients with preexisting cardiovascular disease are at increased risk for COVID-19 and its cardiac complications. This is likely due to altered immune function and baseline cardiovascular pathology, which are amplified by COVID-19. Created with Biorender.com.
ACUTE CARDIAC INJURY AND POTENTIAL CAUSES
The most widely cited laboratory indicator of acute cardiac injury in COVID-19 is serum levels of troponin I, which is released from damaged cardiomyocytes (5, 49, 59, 90). Elevated serum troponin I is associated with COVID-19 disease severity and cardiac injury. In a retrospective, multicenter cohort study of 191 hospitalized COVID-19 patients in Wuhan, China, Zhou et al. (91) showed that over one-half of the patients who died had elevated high-sensitivity cardiac troponin I levels. Time course of troponin elevation increased over the course of illness in nonsurvivors, with a dramatic upturn after day 16. Other indicators of cardiac injury include biochemical tests for levels of creatine kinase-MB and lactic dehydrogenase and abnormal electrocardiograms and echocardiograms. Clinical manifestations of cardiac damage are acute heart failure, arrhythmias, and myocardial infarction (5, 18, 42, 49, 59, 90).
Severe pulmonary infection and acute respiratory distress syndrome (ARDS) are root causes for cardiac injury that occurs because of the systemic pathology they trigger. Lung infection and the resulting tissue damage cause hypoxia and hypotension, which decrease oxygen supply to the heart, and an overzealous immune response resulting in cytokine storm (5, 29, 53). Elevated levels of proinflammatory cytokines can suppress cardiac function directly, disrupt endothelial barrier function, and trigger or amplify vascular inflammation (29, 53, 60). The latter effect can create a thrombogenic state, evidenced by increased D-dimer levels in the blood and a high incidence of pulmonary embolism. In theory, the inflammatory milieu could also promote the rupture of atherosclerotic plaques in the heart, as can occur with influenza viral pneumonia (44). Surprisingly, the frequency of cardiac catheterization laboratory activations for ST-elevation myocardial infarction (STEMI) decreased 26–38% during the COVID-19 pandemic in China and the United States (28, 87), but this might be attributed to avoidance of seeking medical care for fear of nosocomial infection. Recent STEMI data support the conclusion that COVID-19 patients have a higher thrombus burden and poorer outcomes (16, 20). Additionally, severe lung infection and hypotension trigger reflex tachycardia that increases myocardial oxygen demand at a time when supply is limited, resulting in ischemia. The relative contribution of all these factors to the cardiac complications of COVID-19 is unknown and undoubtedly varies between patients. Beyond these secondary causes of cardiac injury, there is increasing evidence that the heart can be infected by SARS-CoV-2. This evidence will be reviewed after discussion of ACE2.
OPPOSING ROLES OF ACE2 IN THE PATHOPHYSIOLOGY OF COVID-19
ACE2 was discovered only 20 yr ago during searches for additional isoforms of ACE, the enzyme responsible for the synthesis of angiotensin (Ang) II (23, 30, 79). Its unique substrate profile compared with ACE was recognized at that time, and a second function for ACE2 as the receptor for SARS-CoV was elucidated only a few years later (30, 51). Several recent reviews provide detailed accounts of the beneficial effects of ACE2 in attenuating the pathophysiological effects of Ang II and the essential role that ACE2 plays in infection of human cells by SARS-CoV and SARS-CoV-2 (30, 69, 74, 81). Therefore, we will only summarize key points for understanding how ACE2 exerts beneficial and deleterious roles in COVID-19.
ACE2 as a Negative Regulator of the Renin-Angiotensin-Aldosterone System
The renin-angiotensin-aldosterone system (RAAS) plays an important physiological role in regulating fluid and electrolyte balance and blood pressure. Renin, which is secreted from the kidneys in a regulated manner, converts angiotensinogen to Ang I, and ACE hydrolyzes Ang I to form the octapeptide Ang II. Most physiological and pathophysiological effects of Ang II are mediated by the Ang II type 1 receptor (AT1R), and these include release of aldosterone and vasopressin, vasoconstriction, hypertrophy of cells (e.g., cardiomyocytes), tissue fibrosis, and increased oxidative stress, inflammation, and coagulation. The adverse effects of Ang II are facilitated by the presence of a local RAAS in tissues such as the heart. Substantial evidence has established that ACE2 functions as a negative regulatory enzyme in the RAAS by converting Ang II to Ang-(1–7) (30, 69, 74). When Ang-(1–7) binds its G protein-coupled receptor Mas, it has the opposite effects from Ang II binding to the AT1R. Disruption of the dynamic regulatory control in favor of Ang II can contribute to cardiovascular and pulmonary pathophysiology. Conversely, treatments that enhance ACE2 activity (recombinant human ACE2) or Mas agonists have wide-ranging therapeutic potential (30, 61, 69, 74).
ACE2 as a Receptor for Coronaviruses
Shortly after the discovery of ACE2, it was learned that this enzyme also serves as the receptor for SARS-CoV, and recent work has confirmed that ACE2 also plays an essential role in infection by SARS-CoV-2 (82). Specifically, the spike protein of the coronaviruses binds with high affinity to the ectodomain of ACE2 at a region different from the active site for enzymatic hydrolysis of Ang II (10, 30). Subsequent to binding, entry of coronaviruses can occur by an endocytic pathway or by direct membrane fusion, but current evidence favors the former pathway for SARS-CoV-2 (31). Regardless of the specific pathway, entry of the SARS-CoV-2 into the cytosol requires cleavage of the S-glycoprotein of the virus spike protein by cellular proteases such as membrane TMPRSS2 (transmembrane protease serine 2) and/or endosomal cathepsins (30, 31). An important consequence of SARS-CoV-2 infection is loss of ACE2, either by internalization or shedding from the cell membrane, thereby preventing the conversion of pathogenic Ang II to protective Ang-(1–7) (30, 31, 72). In this regard, several recent studies have shown that significant elevation of plasma Ang II levels occurs in patients with severe disease (57, 58, 86).
ACE2 EXPRESSION AND LOCALIZATION IN THE HUMAN HEART
Although there is overwhelming evidence that ACE2 activity counteracts the pathophysiological effects of Ang II (74), our knowledge regarding the expression of this protein by distinct cell types in the heart is incomplete. This is important in the context of the current COVID-19 pandemic and for improved understanding of cellular mechanisms mediating the physiological effects of ACE2 and corresponding consequences of ACE2 deficiency.
Studies conducted early after the discovery of ACE2 established expression by cardiomyocytes and fibroblasts isolated from neonatal rat hearts and demonstrated that Ang II can downregulate Ace2 in these cells (26, 27). Relatively few studies have used immunohistochemistry to examine the cellular localization of ACE2 in the heart, and these show variable results. The earliest report mentions localization of ACE2 to endothelial cells throughout the vasculature of human heart and some staining of smooth muscle (23), but only modest staining is evident in supporting images. This contrasts with the robust staining for ACE2 that has been reported for pneumocytes, intestinal epithelial cells, and renal tubular cells (34, 39, 66). Although ACE2 was not identified in cardiac muscle in the first study, other investigators have reported diffuse staining of cardiomyocytes in rat and human heart (6, 9, 34). However, none of these studies showed distinct labeling of ACE2 on myocyte cell membranes, which would be essential for physiological functions and binding of coronavirus.
Several recent studies have increased our knowledge regarding the cellular expression of Ace2 specifically in human hearts by sequencing RNA from single myocardial nuclei or cells and performing transcriptomic analysis on these data. Chen et al. (11) published the first report using nuclei isolated from normal human hearts not used for transplantation. On the basis of transcriptomic analysis, they identified several large populations of cells that included cardiomyocytes, endothelial cells, fibroblasts, and pericytes. Surprisingly, pericytes had the highest level of Ace2 expression, whereas lower levels were detected in cardiac muscle cells. Relatively few fibroblasts from these normal hearts expressed Ace2. The high expression of Ace2 by pericytes in normal human hearts was verified in several other recent reports (32, 56, 63, 80), and one of these reports suggested that pericytes may be the sole site for expression of Ace2 in normal human and mouse heart (32).
Given the strong molecular evidence for Ace2 gene expression by human cardiac pericytes, we have used immunohistochemistry to reevaluate the localization of ACE2 protein in sections of normal human atrial and ventricular myocardium. We observed that intense staining for ACE2 in this tissue was localized to small cells, which had multiple long, thin processes that emanated from the cell body region (Fig. 2). In some cases, these cells were clearly associated with the microvasculature. These ACE2-positive cells were often located adjacent to myocytes, and their processes traveled between muscle cells. On the basis of their distribution and morphology (4, 33, 40, 47), we believe that these ACE2-positive cells are most likely pericytes, although expression of additional phenotypic markers will need to be confirmed for definitive identification (4, 14, 40, 47). We determined that ACE2 was not expressed by cardiac Schwann cells, which have a similar appearance to pericytes, or by cardiac nerve fibers, which also run parallel to myocytes (Fig. 2). Our stains did not provide convincing evidence for localization of ACE2 to the membrane of cardiomyocytes, although the cytosol of these cells appeared to stain lightly.
Fig. 2.
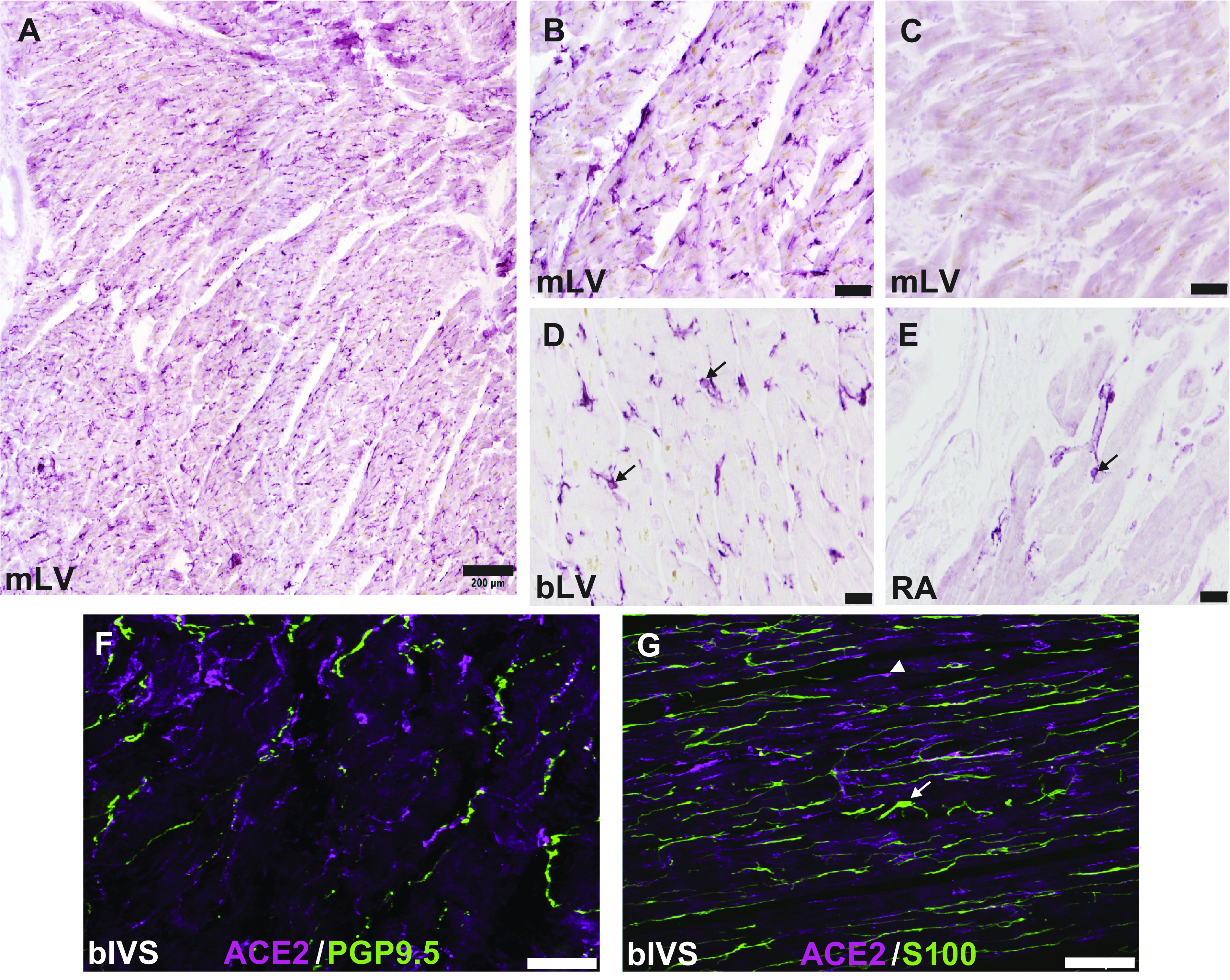
Localization of angiotensin-converting enzyme 2 (ACE2) to pericyte-like cells in the human heart. Human cardiac tissue was provided by Washington Regional Transplant Community as deidentified donor heart tissue, which was rejected for transplantation. The protocol was approved by the George Washington University Institutional Review Board. Aldehyde-fixed sections of normal human heart were evaluated by immunohistochemistry as described in previous publications (1, 8). ACE2 was labeled using an antigen affinity-purified goat anti-human ACE2 antibody at 1:200 dilution (R&D Systems, Minneapolis, MN, cat. no. AF933). Bright-field staining with a Vector Elite ABC kit was used for staining shown in A–E, with Impact VIP used at the chromogen. Staining in A–C, F, and G was performed with 30-μm frozen sections; 5-μm paraffin sections with antigen retrieval were used for staining in D and E. A: photomontage of ×10 images of ACE2 labeling in mid left ventricular myocardium (mLV). B: higher magnification showing labeling of small cells with processes. C: image showing that preabsorption of ACE2 antibody with recombinant human ACE2 (R&D Systems, cat. no. 933-ZN) eliminated staining. D and E: staining of presumed pericytes for ACE2 in paraffin sections of basal left ventricular (bLV) and right atrial (RA) free wall, respectively. Black arrows indicate representative ACE2-positive cells. F and G: merged confocal maximal projection images of sections of basal interventricular septum (bIVS) that were double labeled by fluorescence immunohistochemistry. Images were acquired using a Leica SP8 laser scanning confocal microscope. Rabbit anti-protein gene product (PGP)9.5 (Abcam, cat. no. ab108986) was used at 1:500 and rabbit anti-S100 (Dako, cat. no. GA504) at 1:400. Alexa Fluor-conjugated secondary antibodies were used at 1:200. ACE2 was not colocalized with the pan-neuronal marker PGP9.5 or the Schwann cell marker S100. Representative cells staining for S100 and ACE2 in G are indicated by a white arrow and arrowhead, respectively. Scale bar, 200 μm in A, 50 μm in B and C, 20 μm in D and E, and 100 μm in F and G.
POTENTIAL ROLE OF PERICYTES IN COVID-19 CARDIAC AND VASCULAR PATHOLOGY
Pericytes are mural cells that are present throughout the microvasculature, where they surround capillaries and other small vessels (2, 47, 54). Two-way communication between pericytes and endothelial cells is essential for maintaining microvascular integrity and for local homeostasis of coagulation and immune function (2, 47, 54). Recent studies have established that pericytes are major and possibly exclusive sites for expression of ACE2 in the heart. Furthermore, they coexpress cathepsin B/L protease, which, like TMPRSS2, can cleave the spike protein of SARS-CoV-2, which is required for viral entry (35). Thus, pericytes would be key targets for SARS-CoV-2 in the heart. Infection of pericytes would require the presence of virus in the blood and breakdown of the endothelial barrier, which the virus is too big to cross (32, 61). Both of these conditions would be achieved in patients with severe disease and significant comorbidities (13, 32, 70, 83). Although infection of pericytes by SARS-CoV-2 remains to be proved, recent work has established that the virus can infect human vascular organoids that contain pericytes as a major component (61).
Clues to the potential impact of pericyte dysfunction and damage in COVID-19 pathophysiology are provided by numerous experimental studies. Platelet-derived growth factor B (PDGF-B), which is released from vascular endothelial cells, plays an essential role in the development and maintenance of pericyte coverage of endothelial cells (2, 40, 54). Binding of PDGF-B to matrix proteins in the local microenvironment after release from endothelial cells depends on a retention motif in the factor, and such binding is essential for maintained PDGF signaling to pericytes. Transgenic mice with deletion of this motif still release active PDGF-B, but the factor is not retained locally. As a consequence, these mice lack many pericytes and exhibit leaky microvasculature and hypercoagulability (2, 54). Other investigators found that tyrosine kinase inhibitors like sunitinib produce cardiotoxicity by preventing signaling through PDGF-B receptors on cardiac pericytes (15). Treatment of mice with this agent causes death of pericytes, coronary microvascular dysfunction, increased expression of cardiac hypoxia markers, and impaired left ventricular contractility. Some cardiac pericytes also have the ability to constrict capillaries and precapillary arterioles, and this action has been implicated in causing no-reflow after myocardial ischemia (64). This effect might be triggered by SARS-CoV-2 infection of pericytes and thereby contribute to tissue ischemia. Last, the discovery that cardiac pericytes express ACE2 implies that they are crucial in maintaining the balance between Ang II and Ang-(1–7). Loss of ACE2 would tip this balance in favor of Ang II, which could contribute to new or worsened cardiac injury in COVID-19.
EVIDENCE FOR INFECTION OF THE HEART BY CORONAVIRUSES
Although it is well established that the coronavirus receptor ACE2 is highly expressed by cells within the heart and plays a central role in cardiovascular diseases, the susceptibility of human cardiac cells to infection in situ has not been resolved.
SARS-CoV
The previous outbreak of SARS-CoV produced significant morbidity and mortality, but on a much smaller scale than the current pandemic (67). Nevertheless, the mortality of SARS-CoV was higher than for SARS-CoV-2, and many patients exhibited cardiac symptoms (46, 50, 89). These considerations led to speculation that the heart could be infected by SARS-CoV, and two studies did report detection of virus by real-time PCR in ∼35% of post mortem heart samples (67, 77). One study attempted to culture SARS-CoV from cardiac biopsies but was unsuccessful (77). However, some lung and small bowel samples from the same autopsies did yield positive cultures. This was likely due to higher viral loads in the latter tissues. In another study, histological examination of post mortem cardiac samples from patients who died from SARS-CoV showed evidence of cardiac pathology only when the virus was detected in heart biopsies (67). Specifically, there was evidence of myocyte hypertrophy, fibrosis, and macrophage infiltration, but there was no apoptosis of myocytes and only minimal infiltration of lymphocytes. Parallel studies using transgenic mice showed that pulmonary infection with SARS-CoV could lead to cardiac infection, which required the presence of human ACE2 (67). However, the cellular localization of SARS-CoV within the heart has not been shown in mice or humans. In contrast, there is immunohistochemical and in situ hybridization evidence from a small study showing the presence of SARS-CoV in cells of the lungs and multiple other organs, including the intestines and kidneys (21).
SARS-CoV-2
There is accumulating evidence that SARS-CoV-2 can spread from the primary site of infection to other organs such as the kidneys and heart. The earliest study on multiorgan tropism reported detection of viral RNA in heart samples collected from 17 of 22 autopsies performed on COVID-19 patients (70). Most of those patients were elderly and had multiple preexisting conditions. Only eight blood samples from that series contained viral RNA, and none of the samples in that study were cultured for live virus. However, the cellular localization of viral RNA and protein was established only for the lungs and kidneys. Subsequent studies have specifically evaluated the heart in patients with active COVID-19 and symptoms, suggesting myocarditis by endomyocardial biopsy (24, 78) or in autopsy samples (75, 85). It is notable that one case study detected viral RNA in endomyocardial biopsies of two patients with suspected myocarditis whose nasopharyngeal swabs were negative for SARS-CoV-2 (84). The largest study evaluated endomyocardial biopsies from 104 patients and detected SARS-CoV-2 E-gene-specific RNA in cardiac samples from only five patients (24). All of those patients were diagnosed with acute myocarditis or inflammatory cardiomyopathy, and all survived. Cellular localization of SARS-CoV-2 in the adult heart has been reported for only two studies so far; one was a case report for a COVID-19 patient who recovered from cardiogenic shock (78), and the other was an autopsy study that confirmed SARS-CoV-2 replication in cardiac tissue (55). The first study found virus exclusively in macrophages, which possibly migrated from the lungs (78). Interestingly, ultrastructural images showed evidence of budding, so it is possible that viral infection might spread within the heart. The other study provided in situ hybridization evidence for SARS-CoV-2 within interstitial cells, but their identity was not established (55). Neither study detected virus in cardiomyocytes, but a recent case report found SARS-CoV-2 in myocytes and other cardiac cells in autopsy samples from a child who died from multisystem inflammatory syndrome related to COVID-19 (22). Further evidence that SARS-CoV-2 can infect and replicate in cardiac cells was provided by recent work with human induced pluripotent stem cell-derived cardiomyocytes (76). Thus, there is clear evidence that SARS-CoV-2 can infect cardiac cells, but the extent to which this occurs in patients and contributes to cardiac pathology requires further investigation.
IMPACT OF ALTERED ACE2 EXPRESSION AND DYSFUNCTION
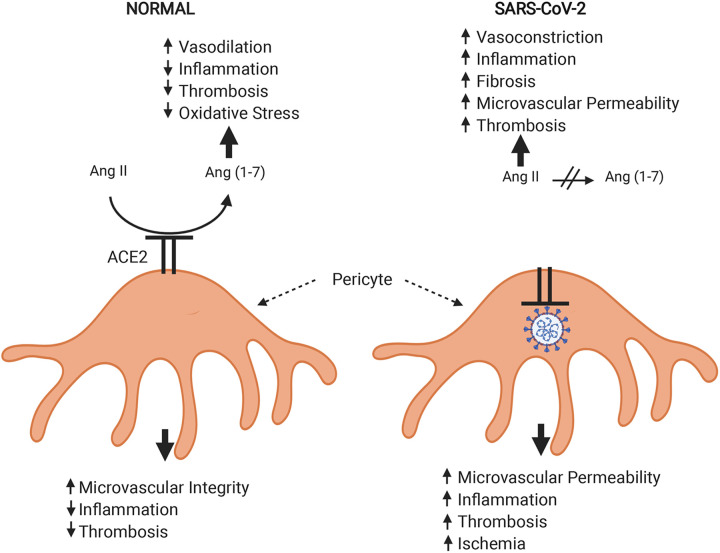
ACE2 has dual roles in SARS-CoV-2 pathophysiology, serving as 1) the essential receptor for cellular infection by this virus and 2) a functional antagonist to the pathophysiological effects of Ang II, which are triggered by viral infections (Fig. 3). The latter effects are most notable in the lungs during COVID-19 but also are likely occurring in other organs such as the heart and kidneys. Therefore, changes in ACE2 expression, whether induced by drug treatments, preexisting diseases, or aging, could have opposing effects relevant to outcome.
Fig. 3.
Diagram depicting the roles of pericytes and angiotensin-converting enzyme 2 (ACE2) in normal vascular and tissue homeostasis and how severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection alters these functions. Pericyte interactions with adjacent vascular endothelial cells (not shown) are crucial to many of these processes. Both pericytes and endothelial cells communicate by release of mediators [e.g., platelet-derived growth factor B (PDGF-B) by endothelial cells]. The recent demonstration that ACE2 is expressed highly by pericytes suggests that these cells likely have another major influence via regulating the balance between angiotensin II (Ang II) and Ang-(1–7). Infection of pericytes by SARS-CoV-2 would cause internalization of ACE2 and eventually cause cell death. Loss of surface ACE2 would shift the humoral balance so that Ang II actions dominate. Pericyte damage would further damage the endothelial barrier, leading to increased microvascular permeability, and would facilitate entry of proinflammatory cells. Thrombosis could be promoted by various mechanisms, including release of tissue factor from pericytes. Contracture of vasoactive pericytes could diminish capillary flow, contributing to ischemia. Created with Biorender.com.
Downregulation of ACE2
Downregulation of ACE2 might decrease the risk for infection through reduced receptor availability for host cell entry but could also increase pathophysiological effects of Ang II. Although total elimination of ACE2 in knockout animal models can prevent lung and heart infection by SARS-CoV (43, 67), it seems unlikely that a reduction of ACE2 would have the same effect, especially given the extremely high affinity of SARS-CoV-2 for ACE2 (65, 82). However, there is good evidence that SARS-CoV-2 infection can cause acute reduction of ACE2 and activation of the RAAS in the lungs (30). The process of viral entry causes internalization and destruction of ACE2, leaving Ang II to function unopposed (30).
Upregulation of ACE2
Upregulation of ACE2 can have diametrically opposed effects on coronavirus infection and organ pathophysiology (36). This was demonstrated by studies of pulmonary disease of mice overexpressing human ACE2. Such mice had more severe lung disease when inoculated with SARS-CoV (88). In contrast, mice and rats that overexpress human ACE2 are resistant to acute lung injury caused by acid (37, 43) or endotoxin (52).
Upregulation of the RAAS occurs in cardiovascular diseases, being a major contributor to hypertension, heart failure, and diabetes-induced pathology. In many cases, there is a corresponding increase in ACE2 expression, which has been suggested as a compensatory response. Recent studies of human heart have shown that ACE2 message and protein are both increased in patients with heart failure (11). Interestingly, results from one analysis suggest that ACE2 expression increases in cardiomyocytes and decreases in pericytes and fibroblasts from failing hearts (80). However, the impact of increased or decreased ACE2 expression by different cardiac cell types on susceptibility to viral infection is unknown.
Attenuation of adverse effects of Ang II has a major role in the treatment of cardiovascular diseases and diabetes. This can be achieved by blockade of AT1Rs or inhibition of ACE. Evidence from animal studies has shown that both approaches can lead to increased expression of ACE2 in the heart, likely due to removal of tonic feedback inhibition by Ang II. This effect of ACE inhibitors and AT1R blockers led to an initial concern that these drugs might increase the risk of infection by SARS-CoV-2. While this issue is still under active study, results from a large retrospective study give evidence that neither class of drug affects the incidence of severity of COVID-19 after adjusting for age, sex, and medical history (25). Given the huge benefit of these drugs in cardiovascular diseases, current recommendations are to continue them in COVID-19 patients (7). A further rationale might be to mitigate contributions of Ang II to the pathophysiological sequelae of COVID-19.
CONCLUSIONS
ACE2 is localized to epithelial cells and other cell types throughout the body, where it normally functions as a counterbalance to the RAAS but also serves as the receptor for infection of cells by SARS-CoV-2. Viral infection causes the loss of surface ACE2 and enables unrestrained actions of Ang II, which contributes to COVID-19 pathology. Severe disease in COVID-19 usually results from primary infection of the lungs, but a significant number of patients also develop acute cardiac injury and dysfunction. The latter might be attributed to systemic consequence of pulmonary infection such as hypoxia, hypotension, metabolic acidosis, thrombosis, and inflammation, but viral infection of the heart is another possibility. Pericytes have been identified recently as a major site of ACE2 expression in the heart and vasculature, and viral infection of these cells might contribute to cardiovascular pathology in COVID-19. Further research is needed to test this hypothesis and to delineate the role of pericytes in cardiac diseases. Another vital area for future study is the long-term cardiovascular consequences of COVID-19, since recent evidence suggests that residual cardiac injury is present in many recovered patients, even those with less severe disease (62, 71).
GRANTS
Funding for this work was provided by the East Tennessee State University(ETSU) Center of Excellence in Inflammation, Infectious Disease and Immunity (D.B.H.), Leducq Foundation Grant RHYTHM (I.R.E.), National Institutes of Health grants OT2 OD-023848 (K.S.), and R01 GM-127584 (B.M.W.), Postdoctoral Fellowship F32 HL152609 (P.H.), and Grant C06 RR-0306551 (ETSU).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.A.R., R.P.M., and D.B.H. conceived and designed research; F.A.R., R.P.M., and D.B.H. interpreted results of experiments; M.D.P. prepared figures; F.A.R., R.P.M., and D.B.H. drafted manuscript; F.A.R., R.P.M., B.M.W., P.H., M.D.P., I.E., K.S., and D.B.H. edited and revised manuscript; F.A.R., R.P.M., B.M.W., P.H., M.D.P., I.E., K.S., and D.B.H. approved final version of manuscript.
REFERENCES
- 1.Ajijola OA, Hoover DB, Simerly TM, Brown TC, Yanagawa J, Biniwale RM, Lee JM, Sadeghi A, Khanlou N, Ardell JL, Shivkumar K. Inflammation, oxidative stress, and glial cell activation characterize stellate ganglia from humans with electrical storm. JCI Insight 2: e94715, 2017. doi: 10.1172/jci.insight.94715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature 468: 557–561, 2010. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 3.Atri D, Siddiqi HK, Lang J, Nauffal V, Morrow DA, Bohula EA. COVID-19 for the cardiologist: basic virology, epidemiology, cardiac manifestations, and potential therapeutic strategies. JACC Basic Transl Sci 5: 518–536, 2020. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avolio E, Madeddu P. Discovering cardiac pericyte biology: from physiopathological mechanisms to potential therapeutic applications in ischemic heart disease. Vascul Pharmacol 86: 53–63, 2016. doi: 10.1016/j.vph.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Bavishi C, Bonow RO, Trivedi V, Abbott JD, Messerli FH, Bhatt DL. Acute myocardial injury in patients hospitalized with COVID-19 infection: a review. Prog Cardiovasc Dis 2020 Jun 6: S0033-0620(20)30123-7, 2020. doi: 10.1016/j.pcad.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertram S, Heurich A, Lavender H, Gierer S, Danisch S, Perin P, Lucas JM, Nelson PS, Pöhlmann S, Soilleux EJ. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS One 7: e35876, 2012. doi: 10.1371/journal.pone.0035876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozkurt B, Kovacs R, Harrington B. Joint HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID-19. J Card Fail 26: 370, 2020. doi: 10.1016/j.cardfail.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown TC, Bond CE, Hoover DB. Variable expression of GFP in different populations of peripheral cholinergic neurons of ChATBAC-eGFP transgenic mice. Auton Neurosci 210: 44–54, 2018. doi: 10.1016/j.autneu.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burrell LM, Risvanis J, Kubota E, Dean RG, MacDonald PS, Lu S, Tikellis C, Grant SL, Lew RA, Smith AI, Cooper ME, Johnston CI. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J 26: 369–375, 2005. doi: 10.1093/eurheartj/ehi114. [DOI] [PubMed] [Google Scholar]
- 10.Chan KK, Dorosky D, Sharma P, Abbasi SA, Dye JM, Kranz DM, Herbert AS, Procko E. Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science 369: 1261–1265, 2020. doi: 10.1126/science.abc0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res 116: 1097–1100, 2020. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 368: m1091, 2020. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W, Lan Y, Yuan X, Deng X, Li Y, Cai X, Li L, He R, Tan Y, Deng X, Gao M, Tang G, Zhao L, Wang J, Fan Q, Wen C, Tong Y, Tang Y, Hu F, Li F, Tang X. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect 9: 469–473, 2020. doi: 10.1080/22221751.2020.1732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen WC, Baily JE, Corselli M, Díaz ME, Sun B, Xiang G, Gray GA, Huard J, Péault B. Human myocardial pericytes: multipotent mesodermal precursors exhibiting cardiac specificity. Stem Cells 33: 557–573, 2015. doi: 10.1002/stem.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chintalgattu V, Rees ML, Culver JC, Goel A, Jiffar T, Zhang J, Dunner K Jr, Pati S, Bankson JA, Pasqualini R, Arap W, Bryan NS, Taegtmeyer H, Langley RR, Yao H, Kupferman ME, Entman ML, Dickinson ME, Khakoo AY. Coronary microvascular pericytes are the cellular target of sunitinib malate-induced cardiotoxicity. Sci Transl Med 5: 187ra69, 2013. doi: 10.1126/scitranslmed.3005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choudry FA, Hamshere SM, Rathod KS, Akhtar MM, Archbold RA, Guttmann OP, Woldman S, Jain AK, Knight CJ, Baumbach A, Mathur A, Jones DA. High thrombus burden in patients with COVID-19 presenting with ST-segment elevation myocardial infarction. J Am Coll Cardiol 76: 1168–1176, 2020. doi: 10.1016/j.jacc.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, Schwartz A, Uriel N. COVID-19 and cardiovascular disease. Circulation 141: 1648–1655, 2020. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 18.Colon CM, Barrios JG, Chiles JW, McElwee SK, Russell DW, Maddox WR, Kay GN. Atrial arrhythmias in COVID-19 patients. JACC Clin Electrophysiol 6: 1189–1190, 2020. doi: 10.1016/j.jacep.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev 53: 25–32, 2020. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dauerman HL. The unbearable thrombus of COVID-19: primary PCI, thrombus, and COVID-19. J Am Coll Cardiol 76: 1177–1180, 2020. doi: 10.1016/j.jacc.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, Wang H, Shen H, Qiu L, Li Z, Geng J, Cai J, Han H, Li X, Kang W, Weng D, Liang P, Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol 203: 622–630, 2004. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolhnikoff M, Ferreira Ferranti J, de Almeida Monteiro RA, Duarte-Neto AN, Soares Gomes-Gouvêa M, Viu Degaspare N, Figueiredo Delgado A, Montanari Fiorita C, Nunes Leal G, Rodrigues RM, Taverna Chaim K, Rebello Pinho JR, Carneiro-Sampaio M, Mauad T, Ferraz da Silva LF, Brunow de Carvalho W, Saldiva PHN, Garcia Caldini E. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc Health 4: 790–794, 2020. doi: 10.1016/S2352-4642(20)30257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 87: E1–E9, 2000. doi: 10.1161/01.RES.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 24.Escher F, Pietsch H, Aleshcheva G, Bock T, Baumeier C, Elsaesser A, Wenzel P, Hamm C, Westenfeld R, Schultheiss M, Gross U, Morawietz L, Schultheiss HP. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail 7: 2440–2447, 2020. doi: 10.1002/ehf2.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fosbøl EL, Butt JH, Østergaard L, Andersson C, Selmer C, Kragholm K, Schou M, Phelps M, Gislason GH, Gerds TA, Torp-Pedersen C, Køber L. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA 324: 168–177, 2020. doi: 10.1001/jama.2020.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallagher PE, Ferrario CM, Tallant EA. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol 295: H2373–H2379, 2008. doi: 10.1152/ajpheart.00426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallagher PE, Ferrario CM, Tallant EA. MAP kinase/phosphatase pathway mediates the regulation of ACE2 by angiotensin peptides. Am J Physiol Cell Physiol 295: C1169–C1174, 2008. doi: 10.1152/ajpcell.00145.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia S, Albaghdadi MS, Meraj PM, Schmidt C, Garberich R, Jaffer FA, Dixon S, Rade JJ, Tannenbaum M, Chambers J, Huang PP, Henry TD. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol 75: 2871–2872, 2020. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geng YJ, Wei ZY, Qian HY, Huang J, Lodato R, Castriotta RJ. Pathophysiological characteristics and therapeutic approaches for pulmonary injury and cardiovascular complications of coronavirus disease 2019. Cardiovasc Pathol 47: 107228, 2020. doi: 10.1016/j.carpath.2020.107228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res 126: 1456–1474, 2020. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartenian E, Nandakumar D, Lari A, Ly M, Tucker JM, Glaunsinger BA. The molecular virology of coronaviruses. J Biol Chem 295: 12910–12934, 2020. doi: 10.1074/jbc.REV120.013930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He L, Mae MA, Sun Y, Muhl L, Nahar K, Liebanas EV. Pericyte-specific vascular expression of SARS-CoV-2 receptor ACE2-implications for microvascular inflammation and hypercoagulopathy in COVID-19 patients. bioRxiv, 2020. . doi: 10.1101/2020.05.11.088500. [DOI]
- 33.Higuchi K, Hashizume H, Aizawa Y, Ushiki T. Scanning electron microscopic studies of the vascular smooth muscle cells and pericytes in the rat heart. Arch Histol Cytol 63: 115–126, 2000. doi: 10.1679/aohc.63.115. [DOI] [PubMed] [Google Scholar]
- 34.Hikmet F, Méar L, Edvinsson Å, Micke P, Uhlén M, Lindskog C. The protein expression profile of ACE2 in human tissues. Mol Syst Biol 16: e9610, 2020. doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280, 2020. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imai Y, Kuba K, Penninger JM. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Exp Physiol 93: 543–548, 2008. doi: 10.1113/expphysiol.2007.040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436: 112–116, 2005. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji HL, Zhao R, Matalon S, Matthay MA. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev 100: 1065–1075, 2020. doi: 10.1152/physrev.00013.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, Farzan M, Wohlford-Lenane C, Perlman S, McCray PB Jr. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol 79: 14614–14621, 2005. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy-Lydon , T. Immune functions and properties of resident cells in the heart and cardiovascular system: pericytes. In: The Immunology of Cardiovascular Homeostasis and Pathology. Basel, Switzerland: Springer, 2017, 93–103. doi: 10.1007/978-3-319-57613-8. [DOI] [PubMed] [Google Scholar]
- 41.Klok FA, Kruip MJ, van der Meer NJ, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MA, Huisman MV, Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res 191: 148–150, 2020. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol 31: 1003–1008, 2020. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11: 875–879, 2005. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwong JC, Schwartz KL, Campitelli MA, Chung H, Crowcroft NS, Karnauchow T, Katz K, Ko DT, McGeer AJ, McNally D, Richardson DC, Rosella LC, Simor A, Smieja M, Zahariadis G, Gubbay JB. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med 378: 345–353, 2018. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 45.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, Chaudhry F, De Freitas JK, Fayad ZA, Pinney SP, Levin M, Charney A, Bagiella E, Narula J, Glicksberg BS, Nadkarni G, Mancini DM, Fuster V, Mount SCOVIDIC; Mount Sinai COVID Informatics Center . Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol 76: 533–546, 2020. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lau ST, Yu WC, Mok NS, Tsui PT, Tong WL, Cheng SW. Tachycardia amongst subjects recovering from severe acute respiratory syndrome (SARS). Int J Cardiol 100: 167–169, 2005. doi: 10.1016/j.ijcard.2004.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee LL, Chintalgattu V. Pericytes in the heart. Adv Exp Med Biol 1122: 187–210, 2019. doi: 10.1007/978-3-030-11093-2_11. [DOI] [PubMed] [Google Scholar]
- 48.Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol 7: e438–e440, 2020. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li JW, Han TW, Woodward M, Anderson CS, Zhou H, Chen YD, Neal B. The impact of 2019 novel coronavirus on heart injury: a systematic review and meta-analysis. Prog Cardiovasc Dis 63: 518–524, 2020. doi: 10.1016/j.pcad.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li SS, Cheng CW, Fu CL, Chan YH, Lee MP, Chan JW, Yiu SF. Left ventricular performance in patients with severe acute respiratory syndrome: a 30-day echocardiographic follow-up study. Circulation 108: 1798–1803, 2003. doi: 10.1161/01.CIR.0000094737.21775.32. [DOI] [PubMed] [Google Scholar]
- 51.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426: 450–454, 2003. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Zeng Z, Cao Y, Liu Y, Ping F, Liang M, Xue Y, Xi C, Zhou M, Jiang W. Angiotensin-converting enzyme 2 prevents lipopolysaccharide-induced rat acute lung injury via suppressing the ERK1/2 and NF-κB signaling pathways. Sci Rep 6: 27911, 2016. doi: 10.1038/srep27911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Libby P. The heart in COVID-19: primary target or secondary bystander? JACC Basic Transl Sci 5: 537–542, 2020. doi: 10.1016/j.jacbts.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S, Landegren U, Nystrom HC, Bergstrom G, Dejana E, Ostman A, Lindahl P, Betsholtz C. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev 17: 1835–1840, 2003. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, Scherschel K, Kirchhof P, Escher F, Schultheiss HP, Blankenberg S, Püschel K, Westermann D. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol 2020 Jul 27: 2020. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Litvinukova M, Talavera-Lopez C, Maatz H, Reichart D, Worth CL, Lindberg EL, Kanda M, Polanski K, Fasouli ES, Samari S. Cells and gene expression programs in the adult human heart. bioRxiv, 2020. doi: 10.1101/2020.04.03.024075. [Preprint.] [DOI]
- 57.Liu N, Hong Y, Chen RG, Zhu HM. High rate of increased level of plasma Angiotensin II and its gender difference in COVID-19: an analysis of 55 hospitalized patients with COVID-19 in a single hospital, WuHan, China. medRxiv, 2020.. doi: 10.1101/2020.04.27.20080432. [Preprint.] [DOI]
- 58.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z, Li J, Li J, Feng C, Zhang Z, Wang L, Peng L, Chen L, Qin Y, Zhao D, Tan S, Yin L, Xu J, Zhou C, Jiang C, Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 63: 364–374, 2020. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med 38: 1504–1507, 2020. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitrani RD, Dabas N, Goldberger JJ. COVID-19 cardiac injury: implications for long-term surveillance and outcomes in survivors. Heart Rhythm 2020 Jun 26: S1547-5271(20)30625-1, 2020. doi: 10.1016/j.hrthm.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del Pozo C, Prosper F, Romero JP, Wirnsberger G, Zhang H, Slutsky AS, Conder R, Montserrat N, Mirazimi A, Penninger JM. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 181: 905–913, 2020. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagel E, Puntmann VO. Errors in statistical numbers and data in study of cardiovascular magnetic resonance imaging in patients recently recovered from COVID-19. JAMA Cardiol 2020 Aug 25: 2020. doi: 10.1001/jamacardio.2020.4661. [DOI] [PubMed] [Google Scholar]
- 63.Nicin L, Abplanalp WT, Mellentin H, Kattih B, Tombor L, John D, Schmitto JD, Heineke J, Emrich F, Arsalan M, Holubec T, Walther T, Zeiher AM, Dimmeler S. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur Heart J 41: 1804–1806, 2020. doi: 10.1093/eurheartj/ehaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Farrell FM, Mastitskaya S, Hammond-Haley M, Freitas F, Wah WR, Attwell D. Capillary pericytes mediate coronary no-reflow after myocardial ischaemia. eLife 6: e29280, 2017. doi: 10.7554/eLife.29280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ortega JT, Serrano ML, Pujol FH, Rangel HR. Role of changes in SARS-CoV-2 spike protein in the interaction with the human ACE2 receptor: an in silico analysis. EXCLI J 19: 410–417, 2020. doi: 10.17179/excli2020-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ortiz ME, Thurman A, Pezzulo AA, Leidinger MR, Klesney-Tait JA, Karp PH, Tan P, Wohlford-Lenane C, McCray PB Jr, Meyerholz DK. Heterogeneous expression of the SARS-Coronavirus-2 receptor ACE2 in the human respiratory tract. EBioMedicine 60: 102976, 2020. doi: 10.1016/j.ebiom.2020.102976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, Butany J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest 39: 618–625, 2009. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parasa S, Desai M, Thoguluva Chandrasekar V, Patel HK, Kennedy KF, Roesch T, Spadaccini M, Colombo M, Gabbiadini R, Artifon ELA, Repici A, Sharma P. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta-analysis. JAMA Netw Open 3: e2011335, 2020. doi: 10.1001/jamanetworkopen.2020.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ Res 118: 1313–1326, 2016. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schröder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Pueschel K, Aepfelbacher M, Huber TB. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 383: 590–592, 2020. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020 Jul 27: e203557, 2020. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Samavati L, Uhal BD. ACE2, Much More Than Just a Receptor for SARS-COV-2. Front Cell Infect Microbiol 10: 317, 2020. doi: 10.3389/fcimb.2020.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sandoval Y, Januzzi JL Jr, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC Review Topic of the Week. J Am Coll Cardiol 76: 1244–1258, 2020. doi: 10.1016/j.jacc.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Santos RA, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, Campagnole-Santos MJ. The ACE2/angiotensin-(1–7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1–7). Physiol Rev 98: 505–553, 2018. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schaller T, Hirschbühl K, Burkhardt K, Braun G, Trepel M, Märkl B, Claus R. Postmortem examination of patients with COVID-19. JAMA 323: 2518–2520, 2020. doi: 10.1001/jama.2020.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sharma A, Garcia G Jr, Wang Y, Plummer JT, Morizono K, Arumugaswami V, Svendsen CN. Human iPSC-derived cardiomyocytes are susceptible to SARS-CoV-2 infection. Cell Rep Med 1: 100052, 2020. doi: 10.1016/j.xcrm.2020.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang JW, To KF, Lo AW, Sung JJ, Ng HK, Chan PK. Quantitative temporal-spatial distribution of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) in post-mortem tissues. J Med Virol 79: 1245–1253, 2007. doi: 10.1002/jmv.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 22: 911–915, 2020. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275: 33238–33243, 2000. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 80.Tucker NR, Chaffin M, Bedi KC Jr, Papangeli I, Akkad AD, Arduini A, Hayat S, Eraslan G, Muus C, Bhattacharyya RP, Stegmann CM, Margulies KB, Ellinor PT; Human Cell Atlas Lung Biological Network .Myocyte specific upregulation of ACE2 in cardiovascular disease: implications for SARS-CoV-2 mediated myocarditis. Circulation 142: 708–710, 2020. doi: 10.1161/CIRCULATIONAHA.120.047911. Epub2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med 76: 14–20, 2020. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181: 281–292, 2020. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 323: 1843–1844, 2020. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wenzel P, Kopp S, Göbel S, Jansen T, Geyer M, Hahn F, Kreitner KF, Escher F, Schultheiss HP, Münzel T. Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovasc Res 116: 1661–1663, 2020. doi: 10.1093/cvr/cvaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med 173: 268–277, 2020. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu Z, Hu R, Zhang C, Ren W, Yu A, Zhou X. Elevation of plasma angiotensin II level is a potential pathogenesis for the critically ill COVID-19 patients. Crit Care 24: 290, 2020. doi: 10.1186/s13054-020-03015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xiang D, Xiang X, Zhang W, Yi S, Zhang J, Gu X, Xu Y, Huang K, Su X, Yu B, Wang Y, Fang W, Huo Y, Ge J. Management and outcomes of patients with STEMI during the COVID-19 pandemic in China. J Am Coll Cardiol 76: 1318–1324, 2020. doi: 10.1016/j.jacc.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoshikawa N, Yoshikawa T, Hill T, Huang C, Watts DM, Makino S, Milligan G, Chan T, Peters CJ, Tseng CT. Differential virological and immunological outcome of severe acute respiratory syndrome coronavirus infection in susceptible and resistant transgenic mice expressing human angiotensin-converting enzyme 2. J Virol 83: 5451–5465, 2009. doi: 10.1128/JVI.02272-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu CM, Wong RS, Wu EB, Kong SL, Wong J, Yip GW, Soo YO, Chiu ML, Chan YS, Hui D, Lee N, Wu A, Leung CB, Sung JJ. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J 82: 140–144, 2006. doi: 10.1136/pgmj.2005.037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol 17: 259–260, 2020. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054–1062, 2020. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]