Abstract
Antiretroviral therapy in HIV patients has lengthened lifespan but led to an increased risk for secondary comorbidities, such as pulmonary complications characterized by vascular dysfunction. In the lung, PDGFRβ+ mesenchymal cells known as pericytes intimately associate with endothelial cells and are key for their survival both structurally and through the secretion of prosurvival factors. We hypothesize that in HIV infection there are functional changes in pericytes that may lead to destabilization of the microvasculature and ultimately to pulmonary abnormalities. Our objective in this study was to determine whether lung pericytes could be directly infected with HIV. We leveraged lung samples from macaque lungs with or without SIV infection and normal human lung for in vitro experiments. Pericytes were isolated based on the marker platelet-derived growth factor receptor-β (PDGFRβ). We determined that lung PDGFRβ-positive (PDGFRβ+) pericytes from both macaques and humans express CD4, the primary receptor for SIV/HIV, as well as the major coreceptors CXCR4 and CCR5. We found cells positive for both PDGFRβ and SIV in lungs from infected macaques. Lung pericytes isolated from these animals also harbored detectable SIV. To confirm relevance to human disease, we demonstrated that human lung pericytes are capable of being productively infected by HIV in vitro, with the time course of infection suggesting development of viral latency. In summary, we show for the first time that SIV/HIV directly infects lung pericytes, implicating these cells as a novel target and potential reservoir for the virus in vivo.
Keywords: HIV, pericytes, pulmonary complications, SIV
INTRODUCTION
The success of combined antiretroviral therapy (cART) has greatly increased survival of HIV-infected (HIV+) patients (14). However, with advancing age of HIV+ populations, chronic lung diseases, including COPD and pulmonary hypertension, are increasingly prevalent (10, 15, 32). Indeed, COPD is now one of the most commonly diagnosed comorbidities in HIV+ patients, observed in up to 20% of HIV+ patients (22, 23), and contributes substantially to morbidity and mortality in virally suppressed HIV+ individuals (10, 11). These findings suggest that the lung is an important site of HIV-induced injury in the aging population (34).
The lung has been implicated as an anatomic viral reservoir based on studies demonstrating different levels and variants of HIV in this organ compared with the peripheral blood (reviewed in Ref. 9). T cells and alveolar macrophages are postulated to be the primary targets of HIV as well as reservoirs of replication-competent virus (4, 8, 9, 26, 33). However, the ability of HIV to infect other cell types in the lung and the impact of infection on the function of these cells are unknown. Nakagawa et al. (27) recently showed that pericytes derived from the brain are susceptible to low-level HIV infection in vitro. Pericytes are mesenchymal cells that wrap around endothelial cells and play a key role in maintenance of endothelial cell barrier function (1). Accordingly, HIV-infected brain pericytes were impaired in their ability to support integrity of an endothelial cell monolayer in vitro (27). If pericytes in the lung are a target for HIV, a similar loss of their functional capabilities could provide one explanation for the vascular abnormalities that characterize the chronic lung diseases seen in HIV+ patients (12, 13, 21).
Therefore, we investigated whether lung pericytes can be infected by HIV. Given the paucity of available tissue from HIV+ patients, we leveraged samples obtained from simian immunodeficiency virus (SIV)-infected nonhuman primates (NHPs) to show in vivo relevance of our findings. We demonstrate for the first time that lung pericytes (defined by PDGFRβ positivity) from humans and macaques express key receptors for HIV/SIV and can be infected. Our data suggest that lung pericytes may function as a potential viral target and thus contribute to the development of chronic pulmonary diseases.
MATERIALS AND METHODS
Infection of macaques with SIV.
Four cynomolgus macaques were inoculated intrarectally with 5000 TCID50 of SIVmac239 viral stock, generously provided by Dr. Thomas Friedrich of the University of Wisconsin School of Veterinary Medicine. The macaques were not virally suppressed and were housed in a biosafety level 2 facility until they were humanely euthanized for blood, bronchoalveolar lavage, and tissue collection. Demographic data for the SIV-negative and SIV-infected macaques, including viral load and CD4+ T lymphocyte counts, are shown in Table 1. This study was approved by the Institutional Animal Care and Use Committee (IACUC) at the Tulane National Primate Research Center.
Table 1.
Macaque demographics
| %CD4+ T Lymphocytes in: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Blood |
Bronchoalveolar lavage |
|||||||||
| Genus and Species | Age, yr | Sex (M/F) | SIV status | Length of Infection, mo | Plasma SIV RNA (log10 copies/mL) at Time of Necropsy | Pre-SIV | At necropsy | Pre-SIV | At necropsy | Lung (at necropsy) |
| Macaca mulatta | 9 | M | Negative | NA | NA | NA | NA | NA | NA | NA |
| Macaca mulatta | 12 | M | Negative | NA | NA | NA | NA | NA | NA | NA |
| Macaca mulatta | 9 | M | Negative | NA | NA | NA | NA | NA | NA | NA |
| Macaca fascicularis | 9 | M | Positive | 12 | 5.56 | 31.8 | 6.2 | 25.8 | 6.2 | 3.9 |
| Macaca fascicularis | 9 | M | Positive | 12 | 6.22 | 49.3 | 15.0 | 32.5 | 1.2 | 1.6 |
| Macaca fascicularis | 9 | M | Positive | 12 | 6.33 | 40.0 | 12.2 | 30.6 | 0.4 | 1.3 |
| Macaca fascicularis | 7 | M | Positive | 12 | 5.72 | 59.3 | 32.7 | 39.3 | 5.5 | 7.3 |
NA, not applicable; SIV, simian immunodeficiency virus.
Isolation and culture of PDGFRβ+ lung pericytes.
Pericytes were isolated from macaque lungs, as previously described (36). Briefly, single-cell lung digests were plated and expanded in Pericyte Medium (ScienCell) to enrich for pericytes (2) and then underwent negative selection for endothelial, epithelial, and leukocyte markers, followed by positive selection for PDGFRβ. Previously isolated pericytes from HIV− human lungs (36) were cultured from frozen stocks. We confirmed absence of α-smooth muscle actin expression by RT-PCR and immunofluorescence. Pericyte purity was assessed by flow cytometry for PDGFRβ positivity. Macaque lung tissue was also fixed in paraffin for histological analysis.
HIV stock.
Peripheral blood leukocytes (PBLs) or H9 cells (25) (NIH AIDS Reagent Program) were activated with PHA-P and infected as previously described (28) with the HIV-1 Ba-L strain (17) (NIH AIDS Reagent Program). For mock-infected controls, PHA-P activated cells were maintained in RPMI-1640 with 10% FBS and IL-2. To verify infection, HIV p24 concentration in supernatants was determined by ELISA (Zeptometrix).
In situ hybridization.
Paraffin-embedded macaque lung tissue and cultured pericytes were analyzed by RNAscope for colocalization of PDGFRβ and SIVmac239 RNA, following the manufacturer’s instructions (ACD Biotechne, Newark, CA). An SIVmac239 sense probe was used as a negative control. Probes were fluorescently tagged with FITC or Cy5 (PerkinElmer) for visualization. Images were acquired using a Zeiss Observer.Z1 microscope with Zen 2 software.
HIV infection of pericytes.
Cultured human lung pericytes were treated with HIV-infected media (equivalent to 10 ng/mL of p24) or mock-infected media for 24 h at 37°C. Cells were washed multiple times to eliminate extracellular virus and then grown in complete Pericyte Medium for the duration of the experiment, with the medium changed every 2–3 days. To confirm active HIV infection, p24 levels in the media were measured by ELISA (Zeptometrix). All samples were run in duplicate, and the concentrations were extrapolated from a standard curve. RT-PCR for the HIV LTR was also performed using total RNA reverse transcribed to cDNA (Bio-Rad iScript cDNA kit). Real-time PCR was performed using a CFX384 real-time system (Bio-Rad) with the following ABI TaqmanGene Expression Assays: HIV-1 (Pa03453409_s1), β2-microglobulin (B2M; Hs00187842_m1), and hypoxanthine-guanine phosphoribosyltransferase (HPRT; Hs00943530_m1). Data are reported as the ratio of gene of interest to reference gene threshold cycle (CT) values.
Incubation of H9 cells with conditioned media from HIV-infected lung pericytes.
Conditioned media were collected 4 days posttreatment from HIV-infected or mock-infected pericytes. H9 cells were stimulated for 3 days with PHA-P (3 µg/mL) and then incubated with pericyte-conditioned media for 24 h, followed by fresh media. After 14 days, H9 cells were washed, fixed, and permeabilized (BD Biosciences). Intracellular staining for p24 (Beckman Coulter) was analyzed using a Guava 8HT flow cytometer and InCyte Analysis Version 3.1 (EMD Millipore, Billerica, MA).
Western blotting.
Cells were lysed in RIPA buffer and proteins separated by SDS-PAGE (10% polyacrylamide). After transfer to nitrocellulose membranes (Bio-Rad), blots were probed with polyclonal antibodies against CD4 (R & D Systems), CXCR4, and CCR5 (AIDS Reagent Program), and bands were visualized by chemiluminescence (ECL2 kit, Pierce).
RESULTS
Characterization of macaque lung PDGFRβ+ cells.
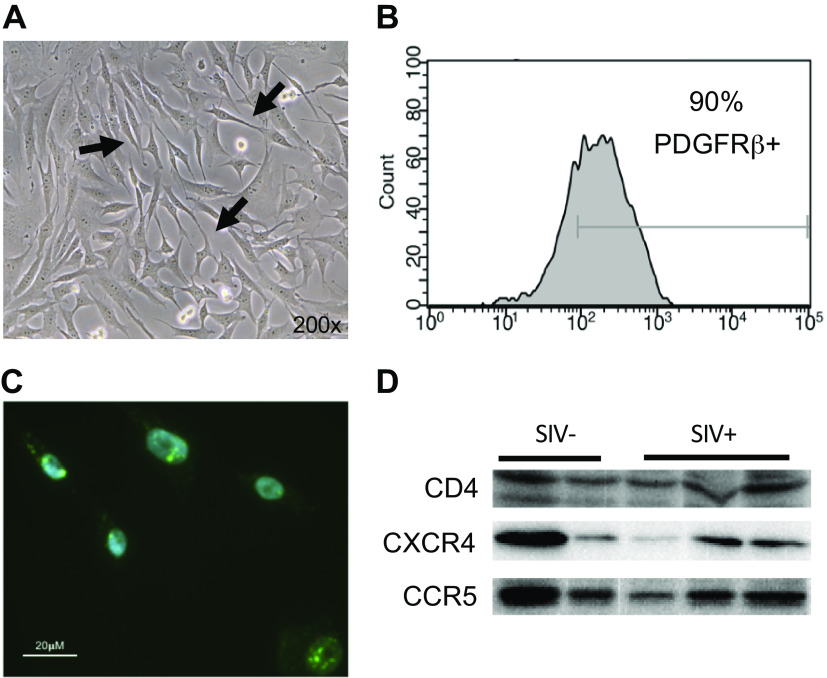
Macaque lung PDGFRβ+ cells in culture (Fig. 1A) are morphologically similar to human lung pericytes (36). We did not detect any gross morphological differences between pericytes isolated from SIV-infected and mock-infected macaques (not shown). Flow cytometry analysis showed that >90% of the cultured cells express PDGFRβ (Fig. 1B). In situ hybridization (RNAscope) confirmed PDGFRβ gene expression (Fig. 1C). Overall, the morphology, localization, and expression pattern of PDGFRβ in these cells are consistent with that of pericytes. We then demonstrated that macaque lung pericytes express the SIV receptor CD4 and the coreceptors CXCR4 and CCR5 (Fig. 1D), indicating that these cells possess the cellular machinery used by SIV for entry into cells and productive infection.
Fig. 1.
Characterization of platelet-derived growth factor receptor-β-positive (PDGFRβ+) pericytes from macaque lungs. A: phase contrast microscopy image of cultured macaque lung pericytes with thin long processes (arrows). B: flow cytometry for PDGFRβ positivity. C: in situ hybridization (RNAscope) for PDGFRβ mRNA (green dots). Representative image of 3 isolates. D: Western blotting of pericytes from simian immunodeficiency (SIV) virus-negative (SIV−; n = 2) and SIV-positive (SIV+; n = 3) macaques for CD4, CXCR4, and CCR5.
SIV RNA is detectable in macaque lung pericytes.
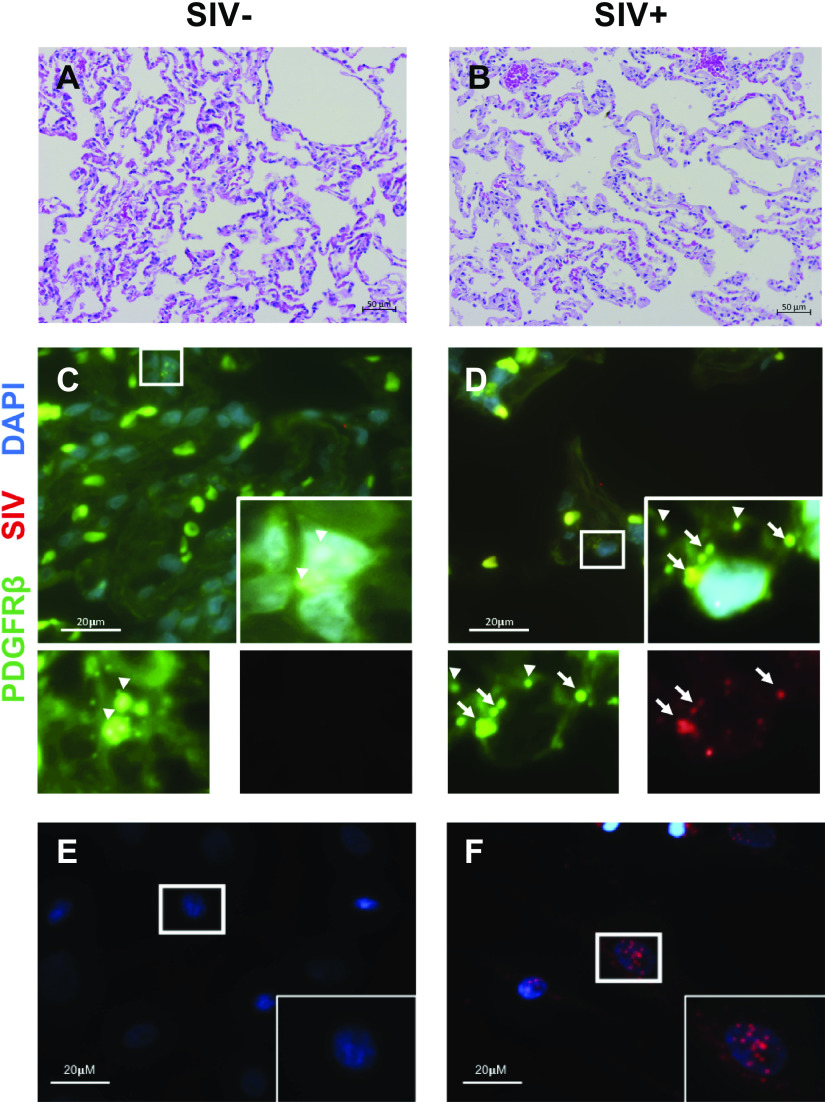
We next asked whether SIV+ pericytes (defined by PDGFRβ positivity) could be detected in lungs from infected macaques. Histologically, we did not note any gross differences in lung architecture between SIV− and SIV+ macaque lungs (Fig. 2, A and B). Lung tissue was assessed by in situ hybridization (RNAscope; Fig. 2, C and D) with probes for PDGFRβ and SIV. PDGFRβ mRNA was present in cells consistent with pericytes based on their localization. In lung tissue from SIV-infected macaques, SIV mRNA was also occasionally seen to coincide with PDGFRβ expression (see Fig. 2D, inset). Not all PDGFRβ+ cells were positive for SIV, and SIV mRNA was clearly observed in macrophage-like cells (not shown). No SIV mRNA was detected in lung tissue from SIV− macaques (Fig. 2C). Additionally, in situ hybridization of cultured PDGFRβ+ lung pericytes (Fig. 2, E and F) from SIV+ macaques showed detectable SIV gene expression (Fig. 2F). These data indicate that SIV is able to infect PDGFRβ+ pericytes in vivo and that these cells retain the virus after isolation.
Fig. 2.
Simian immunodeficiency virus (SIV) gene expression in macaque lung pericytes. Hematoxylin and eosin staining of lung tissue from an SIV-negative (SIV−; A) and SIV-positive (SIV+; B) macaque. C and D: RNAScope for platelet-derived growth factor receptor-β (PDGFRβ; green) and SIV (red) RNA in tissue. Nuclei were counterstained with DAPI (blue). Arrows indicate colocalization of SIV and PDGFRβ signal; arrowheads show PDGFRβ only. E and F: RNAscope for SIV RNA (red) in cultured pericytes. Nuclei were counterstained with DAPI (blue).
Human lung pericytes express HIV-1 receptors and can be infected with HIV In vitro.
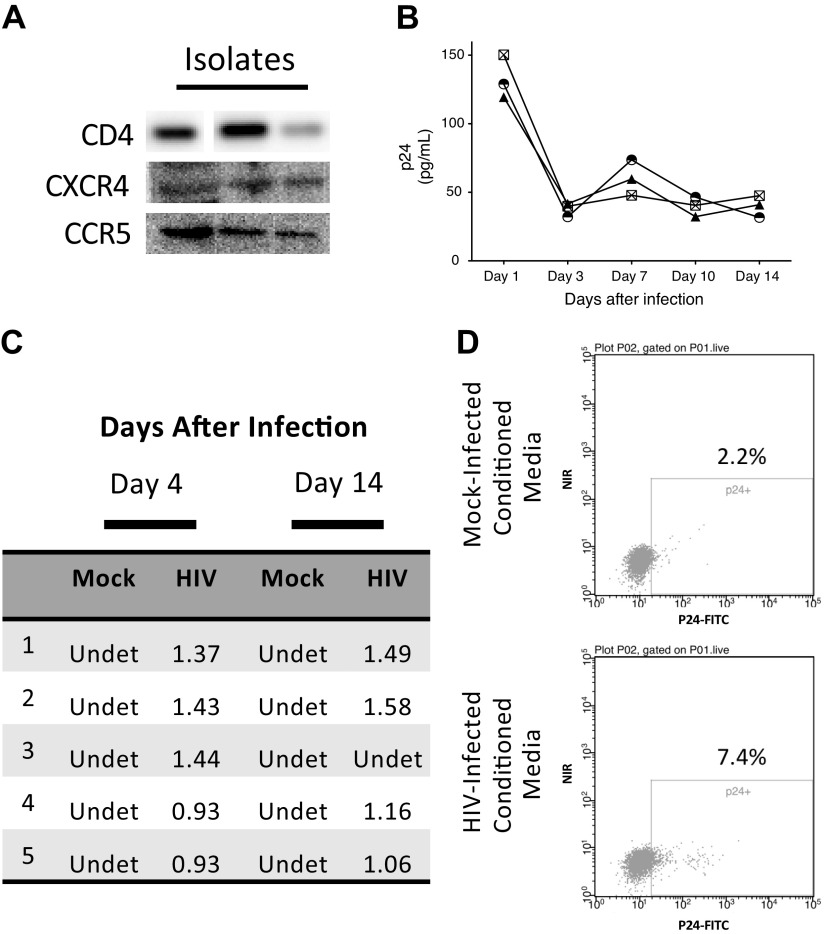
To determine whether human lung pericytes can also be infected by HIV, we first confirmed that the cells express the HIV receptor CD4 and the coreceptors CXCR4 and CCR5 (Fig. 3A). We then exposed pericytes to conditioned media from PBLs or H9 cells in which HIV had been propagated and assessed p24 levels and expression of the HIV LTR up to 14 days later. We found the highest levels of p24 at 24 h after initial infection, with levels declining on day 3 (5). In one of the four isolates tested, p24 was not detectable after day 1 (not shown). No p24 was detected in mock-infected cells (not shown). We also observed persistent expression of the HIV LTR by RT-PCR in four of five isolates (Fig. 3C), which mirrored the p24 data. To determine whether HIV-infected pericytes produce infective viral particles, we incubated H9 cells, a T cell line permissive for HIV infection, with media collected from HIV-infected lung pericytes. After 14 days, we found that ∼5% of H9 cells were positive for intracellular p24 (Fig. 3, D and E), demonstrating that HIV-infected lung pericytes can transmit the virus.
Fig. 3.
Human lung pericytes express CD4 and HIV-1 coreceptors and can be infected by HIV. A: Western blot for CD4, CXCR4, and CCR5 in human lung pericyte isolates. Each lane represents an individual isolate. B: secreted p24 levels were determined by ELISA. C: HIV long terminal repeat (LTR) expression was assessed by real-time PCR. Ratios of HIV LTR to reference gene [β2-microglobulin (B2M) or hypoxanthine-guanine phosphoribosyltransferase (HPRT)] threshold cycle (CT) values are shown. D: H9 cells were analyzed for intracellular p24 by flow cytometry 14 days after incubation with conditioned media from mock-infected (top) or HIV-infected (bottom) pericytes. Shown are representative data from 2 separate experiments.
DISCUSSION
We report for the first time that lung pericytes from both macaques and humans express the receptors necessary for HIV infection. Furthermore, we demonstrate colocalization of SIV and PDGFRβ mRNA in lung sections from infected macaques and show that pericytes isolated from these lungs have detectable SIV expression. Finally, we demonstrate that human lung pericytes can be productively infected with HIV in vitro and are capable of transmitting the virus to susceptible cells.
Our results have several potential implications. First, the demonstration that pericytes can harbor SIV within the lungs suggests that pericytes may be an additional viral reservoir for viral latency. Our findings that p24 levels rapidly fall after in vitro HIV infection of human lung pericytes and the low transmission of virus from these cells to a T cell line suggest development of viral latency, as was proposed for brain pericytes (5). Viral reservoirs are one of the major obstacles to overcome in order to achieve sustainable remission in HIV-infected patients (6). Many different sites have been identified that harbor the virus, including the lungs (26). Therapeutic efforts have focused on eradicating the virus from memory CD4 T cells with complementary therapies to cART, including IL-2 therapy and/or anti-CD3 antibody (7, 31). However, those clinical studies were stopped due to viral re-emergence in patients once cART was discontinued, suggesting that non-T cells also harbor HIV. Our study, together with prior reports that brain pericytes can be infected with HIV, suggest that pericytes in many organs may be capable of being infected. Further work is needed to confirm HIV latency in lung pericytes and to identify conditions that may induce reactivation of the virus in these cells.
Another intriguing possibility is that HIV infection of lung pericytes impacts their function. In patients living with HIV, pulmonary comorbidities contribute substantially to morbidity and mortality (14). COPD is now one of the most commonly diagnosed comorbidities in HIV+ patients, diagnosed in up to 20% of HIV+ patients (22, 23). An early manifestation of emphysema is deterioration of the vascular unit in the lung leading to lung function decline, regardless of cART (13, 30). Pericytes wrapped around endothelial cells provide prosurvival signals to these cells and help maintain vascular barrier integrity. Nakagawa et al. (27) proposed that an impairment in blood-brain barrier function by HIV infection of pericytes may contribute to disease-associated dementia. Thus, it is conceivable that HIV infection of pericytes in the lung could impair endothelial barrier viability and integrity, contributing to the development of emphysema and other lung disorders.
Even on cART, chronic inflammation persists and likely mediates the development of end-organ disease in HIV+ patients (3, 16, 24). There is accumulating evidence that pericytes play an important role in mediating local lung inflammation, as they express multiple TLRs and secrete cytokines in response to specific TLR agonists (20, 36). Whether chronic HIV infection alters pericyte immune function remains to be determined. Finally, pericytes can function as progenitors of myofibroblasts and mesenchymal progenitor cells and contribute to lung repair (18, 19, 36). Disruption of pericyte reparative function by HIV may also contribute to chronic lung dysfunction. Thus, there are multiple pathways by which HIV infection of pericytes may contribute to the development of chronic lung diseases.
Our study has several limitations. Because of the difficulty in obtaining lung samples from HIV+ patients, we used tissues from SIV-infected macaques. However, NHP models of SIV infection recapitulate much of the pulmonary pathology observed in HIV-infected patients, including the development of localized emphysema, with disruption of normal pulmonary architecture (29, 35). The macaques used in our study were not virally suppressed; thus, our findings cannot be directly extrapolated to patients on cART. Nonetheless, our findings provide proof of principle that lung pericytes are a target for HIV in vivo.
Because unequivocal identification of pericytes by electron microscopy is cumbersome, most investigators now identify pericyte-like cells based on localization abutting the microvasculature and expression of pericyte-associated markers. The most common marker used to identify pericytes is PDGFRβ (1). We previously reported the characterization of human lung cells that were isolated based on PDGFRβ and confirmed that the cells expressed many of the common pericyte markers and lacked expression of markers of other cell types, such as macrophages and lymphocytes (36). Although it is possible that other cell types may be present and be responsible for our results, the combination of in vitro and in vivo findings makes this possibility less likely.
In summary, we show for the first time that human lung pericytes can be infected by HIV in vitro and, using an NHP model of SIV infection, that these cells can harbor the virus within tissue and after isolation. Our results suggest that pericytes may act as a novel viral reservoir in the lung. Additional studies are needed to explore the impact of HIV infection on pericyte function and its contribution to chronic lung diseases.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant R01 HL126536 to L.M.S, and NIH Grants R01 AI102693 to A.K., P51 OD011104 to Jay Rappaport, and U42 OD024282 to Rudolf Bohm.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.M.S. conceived and designed research; S.E.S., N.G.B., and C.C.M. performed experiments; S.E.S., C.L.W., X.A., and L.M.S. analyzed data; S.E.S., C.L.W., X.A., and L.M.S. interpreted results of experiments; S.E.S. prepared figures; S.E.S., C.L.W., X.A., and L.M.S. drafted manuscript; S.E.S., C.L.W., N.G.B., A.K., X.A., C.C.M., and L.M.S. edited and revised manuscript; S.E.S., C.L.W., N.G.B., A.K., X.A., C.C.M., and L.M.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The following reagents were obtained through the National Institutes of Health (NIH) AIDS Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), NIH: HIV-1Ba-L, from Drs. Suzanne Gartner, Mikulas Popovic, and Robert Gallo; anti-human CCR5 and anti-human CXCR4 polyclonal (NT) from ProSci; and H9 cells from Dr. Robert Gallo.
We thank Drs. Jay Rappaport and Rudolf Bohm at the Tulane National Primate Research Center for their generous contribution of SIV-negative macaque lung tissue.
Present address of L.M.S. and C.L.W.: Division of Allergy, Pulmonary, and Critical Care Medicine, University of Wisconsin, Madison, WI.
Present address of X.A.: Southwest National Primate Research Center, Texas Biomedical Research Institute, San Antonio, TX.
REFERENCES
- 1.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21: 193–215, 2011. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Bagley RG, Rouleau C, Morgenbesser SD, Weber W, Cook BP, Shankara S, Madden SL, Teicher BA. Pericytes from human non-small cell lung carcinomas: an attractive target for anti-angiogenic therapy. Microvasc Res 71: 163–174, 2006. doi: 10.1016/j.mvr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Baker JV, Neuhaus J, Duprez D, Cooper DA, Hoy J, Kuller L, Lampe FC, Liappis A, Friis-Moller N, Otvos J, Paton NI, Tracy R, Neaton JD; INSIGHT SMART Study Group . Inflammation predicts changes in high-density lipoprotein particles and apolipoprotein A1 following initiation of antiretroviral therapy. AIDS 25: 2133–2142, 2011. doi: 10.1097/QAD.0b013e32834be088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber SA, Gama L, Li M, Voelker T, Anderson JE, Zink MC, Tarwater PM, Carruth LM, Clements JE. Longitudinal analysis of simian immunodeficiency virus (SIV) replication in the lungs: compartmentalized regulation of SIV. J Infect Dis 194: 931–938, 2006. doi: 10.1086/507429. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand L, Cho HJ, Toborek M. Blood-brain barrier pericytes as a target for HIV-1 infection. Brain 142: 502–511, 2019. doi: 10.1093/brain/awy339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chun TW, Davey RT Jr, Engel D, Lane HC, Fauci AS. Re-emergence of HIV after stopping therapy. Nature 401: 874–875, 1999. doi: 10.1038/44755. [DOI] [PubMed] [Google Scholar]
- 7.Chun TW, Engel D, Mizell SB, Hallahan CW, Fischette M, Park S, Davey RT Jr, Dybul M, Kovacs JA, Metcalf JA, Mican JM, Berrey MM, Corey L, Lane HC, Fauci AS. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med 5: 651–655, 1999. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 8.Clarke JR, Gates AJ, Coker RJ, Douglass JA, Williamson JD, Mitchell DM. HIV-1 proviral DNA copy number in peripheral blood leucocytes and bronchoalveolar lavage cells of AIDS patients. Clin Exp Immunol 96: 182–186, 1994. doi: 10.1111/j.1365-2249.1994.tb06539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costiniuk CT, Jenabian MA. HIV reservoir dynamics in the face of highly active antiretroviral therapy. AIDS Patient Care STDS 29: 55–68, 2015. doi: 10.1089/apc.2014.0173. [DOI] [PubMed] [Google Scholar]
- 10.Crothers K. Chronic obstructive pulmonary disease in patients who have HIV infection. Clin Chest Med 28: 575–587, 2007. doi: 10.1016/j.ccm.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC; Veterans Aging Cohort 5 Project Team . Increased COPD among HIV-positive compared to HIV-negative veterans. Chest 130: 1326–1333, 2006. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- 12.Crothers K, Huang L, Goulet JL, Goetz MB, Brown ST, Rodriguez-Barradas MC, Oursler KK, Rimland D, Gibert CL, Butt AA, Justice AC. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med 183: 388–395, 2011. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crothers K, Thompson BW, Burkhardt K, Morris A, Flores SC, Diaz PT, Chaisson RE, Kirk GD, Rom WN, Huang L; Lung HIV Study . HIV-associated lung infections and complications in the era of combination antiretroviral therapy. Proc Am Thorac Soc 8: 275–281, 2011. doi: 10.1513/pats.201009-059WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crum NF, Riffenburgh RH, Wegner S, Agan BK, Tasker SA, Spooner KM, Armstrong AW, Fraser S, Wallace MR; Triservice AIDS Clinical Consortium . Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr 41: 194–200, 2006. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 15.Drummond MB, Merlo CA, Astemborski J, Kalmin MM, Kisalu A, Mcdyer JF, Mehta SH, Brown RH, Wise RA, Kirk GD. The effect of HIV infection on longitudinal lung function decline among IDUs: a prospective cohort. AIDS 27: 1303–1311, 2013. doi: 10.1097/QAD.0b013e32835e395d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubé MP, Sattler FR. Inflammation and complications of HIV disease. J Infect Dis 201: 1783–1785, 2010. doi: 10.1086/652751. [DOI] [PubMed] [Google Scholar]
- 17.Gartner S, Markovits P, Markovitz DM, Kaplan MH, Gallo RC, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 233: 215–219, 1986. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 18.Gaskill CF, Carrier EJ, Kropski JA, Bloodworth NC, Menon S, Foronjy RF, Taketo MM, Hong CC, Austin ED, West JD, Means AL, Loyd JE, Merryman WD, Hemnes AR, De Langhe S, Blackwell TS, Klemm DJ, Majka SM. Disruption of lineage specification in adult pulmonary mesenchymal progenitor cells promotes microvascular dysfunction. J Clin Invest 127: 2262–2276, 2017. doi: 10.1172/JCI88629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, Gharib SA, Schnapp LM, Duffield JS. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 188: 820–830, 2013. doi: 10.1164/rccm.201212-2297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung CF, Mittelsteadt KL, Brauer R, McKinney BL, Hallstrand TS, Parks WC, Chen P, Schnapp LM, Liles WC, Duffield JS, Altemeier WA. Lung pericyte-like cells are functional interstitial immune sentinel cells. Am J Physiol Lung Cell Mol Physiol 312: L556–L567, 2017. doi: 10.1152/ajplung.00349.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, Goulet J, Simberkoff M, Butt AA, Rimland D, Rodriguez-Barradas MC, Gibert CL, Oursler KA, Brown S, Leaf DA, Goetz MB, Bryant K. Veterans Aging Cohort Study (VACS): Overview and description. Med Care 44, Suppl 2: S13–S24, 2006. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Justice AC, Lasky E, McGinnis KA, Skanderson M, Conigliaro J, Fultz SL, Crothers K, Rabeneck L, Rodriguez-Barradas M, Weissman SB, Bryant K; VACS 3 Project Team . Medical disease and alcohol use among veterans with human immunodeficiency infection: A comparison of disease measurement strategies. Med Care 44, Suppl 2: S52–S60, 2006. doi: 10.1097/01.mlr.0000228003.08925.8c. [DOI] [PubMed] [Google Scholar]
- 23.Kim DJ, Westfall AO, Chamot E, Willig AL, Mugavero MJ, Ritchie C, Burkholder GA, Crane HM, Raper JL, Saag MS, Willig JH. Multimorbidity patterns in HIV-infected patients: the role of obesity in chronic disease clustering. J Acquir Immune Defic Syndr 61: 600–605, 2012. doi: 10.1097/QAI.0b013e31827303d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, Paton NI, Neaton JD; INSIGHT SMART Study Group . Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 5: e203, 2008. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann DL, O’Brien SJ, Gilbert DA, Reid Y, Popovic M, Read-Connole E, Gallo RC, Gazdar AF. Origin of the HIV-susceptible human CD4+ cell line H9. AIDS Res Hum Retroviruses 5: 253–255, 1989. doi: 10.1089/aid.1989.5.253. [DOI] [PubMed] [Google Scholar]
- 26.Mzingwane ML, Tiemessen CT. Mechanisms of HIV persistence in HIV reservoirs. Rev Med Virol 27: e1924, 2017. doi: 10.1002/rmv.1924. [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa S, Castro V, Toborek M. Infection of human pericytes by HIV-1 disrupts the integrity of the blood-brain barrier. J Cell Mol Med 16: 2950–2957, 2012. doi: 10.1111/j.1582-4934.2012.01622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pal S, Schnapp LM. HIV-infected lymphocytes regulate fibronectin synthesis by TGF beta 1 secretion. J Immunol 172: 3189–3195, 2004. doi: 10.4049/jimmunol.172.5.3189. [DOI] [PubMed] [Google Scholar]
- 29.Pandrea I, Landay A, Wilson C, Stock J, Tracy R, Apetrei C. Using the pathogenic and nonpathogenic nonhuman primate model for studying non-AIDS comorbidities. Curr HIV/AIDS Rep 12: 54–67, 2015. doi: 10.1007/s11904-014-0245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrache I, Diab K, Knox KS, Twigg HL III, Stephens RS, Flores S, Tuder RM. HIV associated pulmonary emphysema: a review of the literature and inquiry into its mechanism. Thorax 63: 463–469, 2008. doi: 10.1136/thx.2007.079111. [DOI] [PubMed] [Google Scholar]
- 31.Prins JM, Jurriaans S, van Praag RM, Blaak H, van Rij R, Schellekens PT, ten Berge IJ, Yong SL, Fox CH, Roos MT, de Wolf F, Goudsmit J, Schuitemaker H, Lange JM. Immuno-activation with anti-CD3 and recombinant human IL-2 in HIV-1-infected patients on potent antiretroviral therapy. AIDS 13: 2405–2410, 1999. doi: 10.1097/00002030-199912030-00012. [DOI] [PubMed] [Google Scholar]
- 32.Sampériz G, Guerrero D, López M, Valera JL, Iglesias A, Ríos A, Campins A, Sala E, Murillas J, Togores B, Palmer J, Rodriguez M, Soriano JB, Sauleda J, Riera M, Agusti A. Prevalence of and risk factors for pulmonary abnormalities in HIV-infected patients treated with antiretroviral therapy. HIV Med 15: 321–329, 2014. doi: 10.1111/hiv.12117. [DOI] [PubMed] [Google Scholar]
- 33.Sanyal A, Mailliard RB, Rinaldo CR, Ratner D, Ding M, Chen Y, Zerbato JM, Giacobbi NS, Venkatachari NJ, Patterson BK, Chargin A, Sluis-Cremer N, Gupta P. Novel assay reveals a large, inducible, replication-competent HIV-1 reservoir in resting CD4+ T cells. Nat Med 23: 885–889, 2017. doi: 10.1038/nm.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schnapp LM, Crothers K. HIV, the lung and aging. In: The Aging Lungs, edited by Lee P, Bucala R. . London: World Scientific Publishing Co., 2016, p. 257–281. [Google Scholar]
- 35.Van Rompay KK. The use of nonhuman primate models of HIV infection for the evaluation of antiviral strategies. AIDS Res Hum Retroviruses 28: 16–35, 2012. doi: 10.1089/aid.2011.0234. [DOI] [PubMed] [Google Scholar]
- 36.Wilson CL, Stephenson SE, Higuero JP, Feghali-Bostwick C, Hung CF, Schnapp LM. Characterization of human PDGFR-β-positive pericytes from IPF and non-IPF lungs. Am J Physiol Lung Cell Mol Physiol 315: L991–L1002, 2018. doi: 10.1152/ajplung.00289.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]