Keywords: remodeling, sexual dimorphism, sodium-chloride cotransporter, three-dimensional
Abstract
The thiazide-sensitive Na+-Cl− cotransporter (NCC) is more abundant in kidneys of female subjects than of male subjects. Because morphological remodeling of the distal convoluted tubule (DCT) is dependent on NCC activity, it has been generally assumed that there is a corresponding sexual dimorphism in the structure of the DCT, leading to a larger female DCT. Until now, this has never been directly examined. Here, optical clearing techniques were combined with antibody labeling of DCT segment markers, state-of-the-art high-speed volumetric imaging, and analysis tools to visualize and quantify DCT morphology in male and female mice and study the DCT remodeling response to furosemide. We found an unexpected sex difference in the structure of the DCT. Compared with the male mice, female mice had a shorter DCT, a higher cellular density of NCC, and a greater capacity to elongate in response to loop diuretics. Our study revealed a sexual dimorphism of the DCT. Female mice expressed a greater density of NCC transporters in a shorter structure to protect Na+ balance in the face of greater basal distal Na+ delivery yet have a larger reserve and structural remodeling capacity to adapt to unique physiological stresses. These observations provide insight into mechanisms that may drive sex differences in the therapeutic responses to diuretics.
INTRODUCTION
The distal convoluted tubule (DCT) reabsorbs 5–10% of the filtered NaCl load, via Na+-Cl− cotransporter (NCC), encoded by the SLC12a3 gene. This is equivalent to over five times the average dietary Na+ consumption (2, 51). As a consequence, the DCT plays an important role in the control of Na+ and Cl− homeostasis and, by extension, blood pressure (1, 21, 31). Indeed, thiazide diuretics, which specifically inhibit NCC, act as effective first-line antihypertensive drugs (43). Furthermore, inactivating mutations in SLC12a3 lead to Gitelman syndrome, a familial disorder characterized by hypotension, hypokalemia, and metabolic alkalosis (20, 44). In contrast, mutations in the with no lysine kinase (WNK)-SPS1-related proline/alanine-rich kinase (SPAK) signaling pathway, which increase NCC phosphorylation and activity, cause a familial disorder of hyperkalemia and hypertension (aka Gordon’s syndrome, pseudohypoaldosteronism type II) (34).
A sexual dimorphism in NCC has been observed in animal models and in human subjects. Multiple studies in rodents have shown that female animals have more NCC protein and mRNA and a more robust response to thiazides than male animals (6, 25, 26, 47, 48). In humans, phosphorylated (p)NCC and NCC in urinary exosomes is greater in women compared with men, suggesting higher NCC abundance and activity in kidneys of women (40). In female rodents, NCC expression is controlled in part by the action of estrogens, in that ovariectomy decreases NCC expression to the lower levels observed in male animals, and administration of estradiol partially restores NCC expression to nonovariectomized mouse levels (6). The human antihypertensive response of hydrochlorothiazide is greater in postmenopausal women receiving drospirenone-estradiol and estogen-progestin compared with postmenopausal women not receiving hormone replacement therapy (37, 38). The sexual dimorphism may have important clinical implications in certain populations. Indeed, hydrochlorothiazide reduces systolic blood pressure more in women of African descent than men of similar descent (9, 42), although the profound sex difference does not translate to the more diverse population of the ALLHAT trial (3).
It is well appreciated that NCC activity affects DCT structure. However, it is presently unknown if the sex differences in NCC have morphological underpinnings. The DCT is a plastic tubule segment that remodels in response to physiological cues, genetic alterations, and pharmacological perturbations. Indeed, the DCT hypertrophies in response to diets that activate NCC and atrophies when NCC is inhibited (18). Genetic activation of NCC is sufficient to increase DCT mass (12, 22). Additionally, administration of loop diuretics, which increase Na+ delivery to the DCT, increases NCC abundance and activity and also increase the length and volume of the DCT (1, 8, 15). Because female rodents have shorter proximal tubules with less Na+-reabsorbing transporters compared with male rodents, enhanced distal Na+ delivery may stimulate a similar DCT adaptation (14, 27, 47). Here, we combined optical clearing methods, antibody labeling, and three-dimensional (3D) microscopy with volumetric imaging tools to explore potential sexual dimorphism of the DCT in the basal state and in response to remodeling stimuli.
METHODS
Animal experiments.
For these experiments, 8- to 12-wk-old C57/Bl6J male and female mice (The Jackson Laboratory) were used. Mice were fed a defined control pellet diet (catalog no. TD88238, Envigo). Furosemide experiments were performed in metabolic cages (catalog no. 3700D001, Tecniplast Metabolics); after 3 days of acclimation on control diet in metabolic cages, the diet was switched to semisolid paste (20% water, TD88238), and animals were randomized to vehicle or furosemide. Furosemide was mixed (catalog no. F4381, Sigma-Aldrich) with the paste food and was given to test animals, whereas control animals received the same portion of food with no added furosemide. The furosemide dose was 100 mg·day−1·kg body weight−1 of mice (mice finished their portion by the next day). Fresh furosemide was provided in fresh food between 4 and 6 PM, just before the active period when mice begin to eat. At the start of these experiments, we fed control and furosemide-treated mice 80% of their control diet. This ensured that mice ingested all the food and, thus, a full dose of furosemide. Animals in the control and furosemide arms were euthanized on the same day to eliminate any possible effects on differences estrus cycle. Mice were kept on this diet for 7 days until euthanized with ketamine-xylazine intraperitoneal injection. Kidneys were processed as described below. All experiments were approved by the University of Maryland (Baltimore, MD) and Johns Hopkins University Animal Care Und use Committees.
Tissue Clearing and 3D microscopy.
The Clear, Unobstructed Brain/Body Imaging Cocktails and Computational (CUBIC) analysis clearing technique was performed following the Susaki et al. protocol (46) with modifications. Kidneys were fixed by intracardiac perfusion for 2 min with cold PBS and 5 min with cold 4% paraformaldehyde in PBS. Kidneys were kept for 24 h in 4% paraformaldehyde in the cold room. The kidneys were rinsed in PBS before proceeding to sectioning. Kidneys were stabilized in a molded cylinder using low melting temperature agarose, and sagittal or transverse sections through the entire kidney were cut using a Leica VT1200 Vibratome at 300 μm thickness. Early empirical experiments revealed that a tissue thickness of 300 μm provided an ideal balance between the limits of antibody permeation and sufficient area for 3D imaging. Tissue sections were incubated with CUBIC reagent 1 (R-1) diluted 1:1 with water for 1 day while being rotated at 37°C. Tissue sections were then treated for 3 days with undiluted R-1, which was exchanged for fresh R-1 after the first day. Sections were then washed for 1 day with PBS. For antibody labeling, sections were treated for 1 h with 0.3 M glycine in PBS at room temperature and then with blocking/incubation medium (PBS + 0.1% Triton X-100 + 1% BSA + 0.02% sodium azide) for 1 day at 37°C while being rotated. The transverse sections were then incubated for 3 days with chicken anti-NCC primary antibody diluted 1:70 [Welling laboratory (13)] and rabbit anti-parvalbumin diluted 1:400 (catalog no. PV27, Swant) in 300 μL of incubation medium at 37°C while being rotated. Sagittal sections were incubated for 3 days in parvalbumin and aquaporin (AQP)2 (sc-515770, Santa Cruz Biotechnology) antibodies (diluted 1:400 and 1:17, respectively) in 300 μL of incubation medium. After being labeled, tissue sections were washed in PBS for 1 day. Sections were then treated for 3 days with incubation medium containing the secondary antibodies goat anti-chicken-594 (A32932, ThermoFisher) and donkey anti-rabbit-488 (A-21206, ThermoFisher) for transverse sections and donkey anti-rabbit-488 (A-21206, ThermoFisher) and goat anti-mouse (A11004, Thermo-Fisher) for sagittal sections. After the incubation period, sections were washed in PBS for 1 day followed by fresh PBS for another day. To stabilize the antigen-antibody complex, we refined the published methodology (46) by adding a fixation step (2% paraformaldehyde for 30 min at room temperature) followed by a thorough rinse in PBS. Sections were then treated with CUBIC reagent 2 without triethanolamine (R-2) diluted 1:1 with distilled H2O for 1 day, and they were then treated with undiluted R-2 for 3 days. R-2 was changed after the first day. Finally, sections were mounted in R-2 for microscopy imaging. TEA was omitted from the final, refractive index matching solution because, although it decolorizes the tissue, it also attenuates the fluorophore fluorescence.
Images were captured with a Nikon W-1 spinning disk microscope. For quantitative imaging, a ×20 water objective (numerical aperture = 0.95 with CG correction ring) was used to view complete transverse kidney sections (see Fig. 2A), spanning the cortex to papilla. Large images of the cortex were acquired to capture a large number of intact tubules for analysis. In this imaging configuration, DCT tubules rarely transverse imaging plane, and those tubules that did were omitted from analysis. To check for potential z-distortion due to refractive index mismatching between the objective lens and immersion and embedding media (49), we measured Alexa Fluor-labeled beads of various diameters suspended in the final CUBIC reagent. For each bead, the 3-D volume was rendered with IMARIS and was rotated such that the z-axis of the sample was perpendicular to the line-of-sight view of the rotated rendering. The lengths of the Z- and XY-axis diameters were measured and compared. The ratio of Z- to XY-axis length was found to have a mean of 1.02, which represents a maximal 2% overestimation of tubule lengths. Actual measurements would deviate much less because the orientation of the long axes of these convoluted tubules is more random. This provided a refractive index matching to CUBIC. For comparison, image stacks taken with the same microscope except with an air ×20 objective had an ∼30% compression of distance measurements in the Z-direction. Stacks of 664 × 666-μm images were captured at 100-μs exposure (2 μm × ∼150 slices) at optimal laser power. Images were processed initially with Fiji (ImageJ). In the Process menu, Enhance/Contrast submenu, the Normalize tool was chosen to optimize brightness and contrast of each image slice, which uses histogram stretching to recalculate the pixel values of the image so that the intensity range was maximal (0−65,535, 16-bit) over the entire stack of images (ImageJ online instructions) (15a). IMARIS 9 (Bitplane, Oxford) was used to assemble and tile stacked images for 3D rendering, 3D visualization, and analytical measurements of tubule counting, length, and volume.
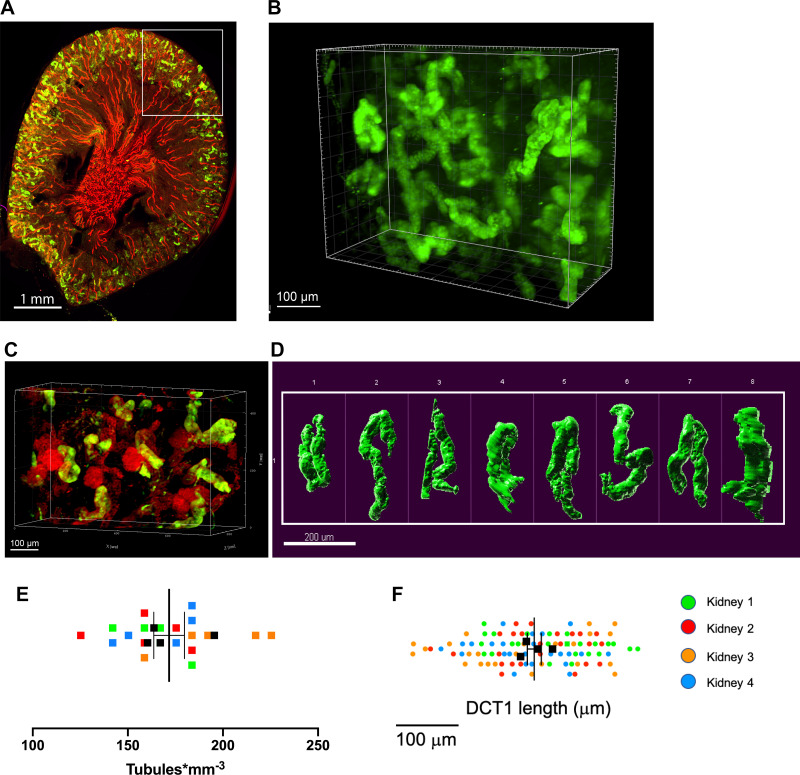
Fig. 2.
Three-dimensional images of the early distal convoluted tubule (DCT1) in Clear, Unobstructed Brain/Body Imaging Cocktails and Computational (CUBIC)-clarified mouse kidneys. A: sagittal section of a male kidney labeled with antibodies to parvalbumin (green) and aquaporin-2 (red) revealing the expected cortical distribution of DCT1 (green) between arrays of connecting tubules and collecting ducts (red). B and C: three-dimensional views of the parvalbumin-positive DCT1 in wild-type mice (B) and in mT/mG reporter mice (C) (33), which express tomato fluorescent protein in all kidney cells but most predominantly in the glomeruli. Notice that DCT1 tubules juxtaposed to glomeruli (red, round structures) D: representative range of different shapes and sizes of the parvalbumin-positive DCT1. E: DCT density measurements. Each square is an independent measurement. Different colors signify different mice. F: relative distribution of DCT1 length (measured by the length of parvalbumin-positive tubules) among male mice revealed similar DCT1 length average values between animals. Data are means ± SE. Each graph represents 4 animals and at least 5 microscope fields of view per animal.
A line tracing tool was used to measure tubule length. Surface rendering tools were used in IMARIS to define the outline of parvalbumin-positive tubules to facilitate measurements of tubule diameter at representative points along the tubule. Using the calculated cross-sectional areas based on each of the measured diameters and the tubule length, the DCT1 volume (V) of each tubule was estimated by the following equation:
where L is the length of DCT1, Dn is the diameter of DCT1 at each measurement point, and N is the number of diameter measurements.
Western blot analysis.
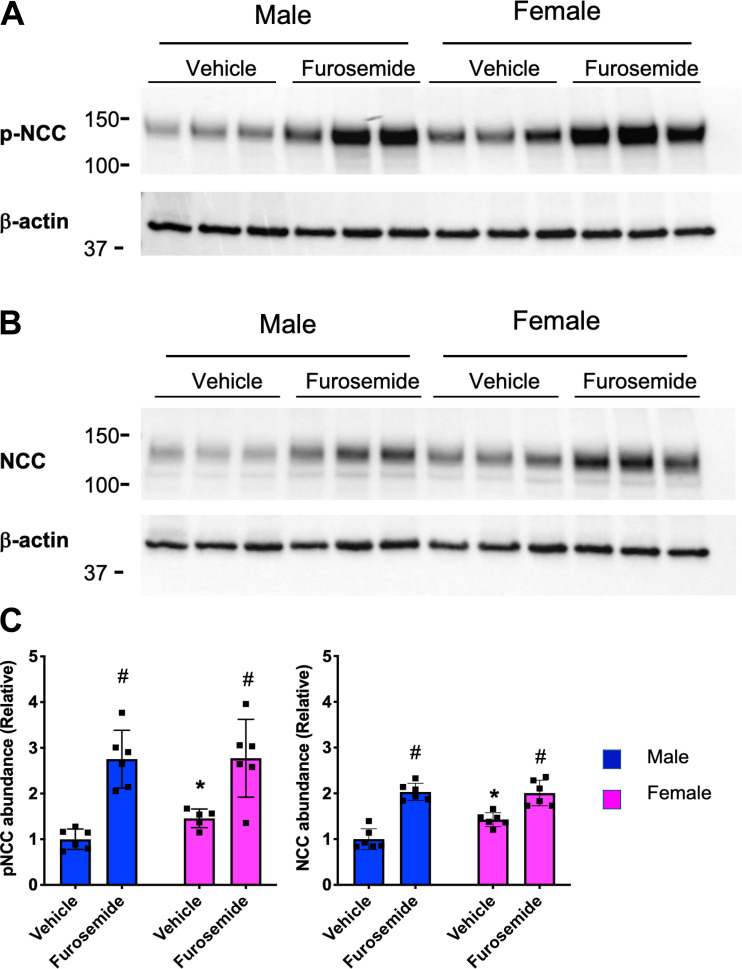
Cortical specimens were dissected from the kidney, snap frozen, and maintain at −80°C until use. The cortex tissue was homogenized in HEENG buffer (10) using zirconium beads (catalog no. Z763799, Sigma-Aldrich), and the homogenate was then lysed using the same buffer supplemented with 10% SDS, 10% Triton X-100, and 10% Tween 20 for 1 h in the cold room with rotation. Protein concentration was measured using a BCA assay (catalog no. 23227, Thermo-Fisher). Total protein (10 μg) was run on a gradient 4–15% SDS-PAGE gel (catalog no. 4568086, Bio-Rad) before transfer to a nitrocellulose membrane using turbo transfer pack (catalog no. 1704158, Bio-Rad). Membranes were blocked by 5% nonfat powdered milk (catalog no. A614-1003, Quality Biology) in Tris-buffered saline-Tween 20 buffer. NCC antibody (catalog no. AB3553, Sigma-Aldrich), phosphorylated (p)S53-NCC (catalog no. p1311-53, Phosphosolutions) developed by the Ellison laboratory at a dilution that specifically detects pNCC (1:2,000) and β-actin (catalog no. A5316, Sigma) were diluted in blocking buffer at 1:1,000, 1:2,000, and 1:5,000 dilutions, respectively, and incubated with the membranes overnight in the cold room. Membranes were washed three time and then incubated at room temperature for 1 h with horseradish peroxidase-conjugated secondary antibodies (goat anti-rabbit, catalog no. 111-035-144 for NCC and pNCC, Jackson Laboratories, and goat anti-mouse catalog no. 115-035-003 for β-actin, Jackson laboratories) diluted in blocking buffer. Membranes were washed and incubated with ECL solution (catalog no. 32106, Thermo-Fisher) for 1 min and then imaged with a Bio-Rad ChemiDoc Imager over the linear range of the detection system.
Statistical analyses.
For comparison between male and female mice at the basal state, a Student’s t test was performed. To compare the effect of treatments between sexes, two-way ANOVA was used to determine whether there was a statistically significant difference. A two-tailed P value of ≤0.05 was considered significant. Statistical analysis was performed using Graph Pad PRISM (v8.0a, La Jolla, CA). Data are presented here as means ± SE.
RESULTS
The distal nephron in CUBIC-clarified mouse kidneys.
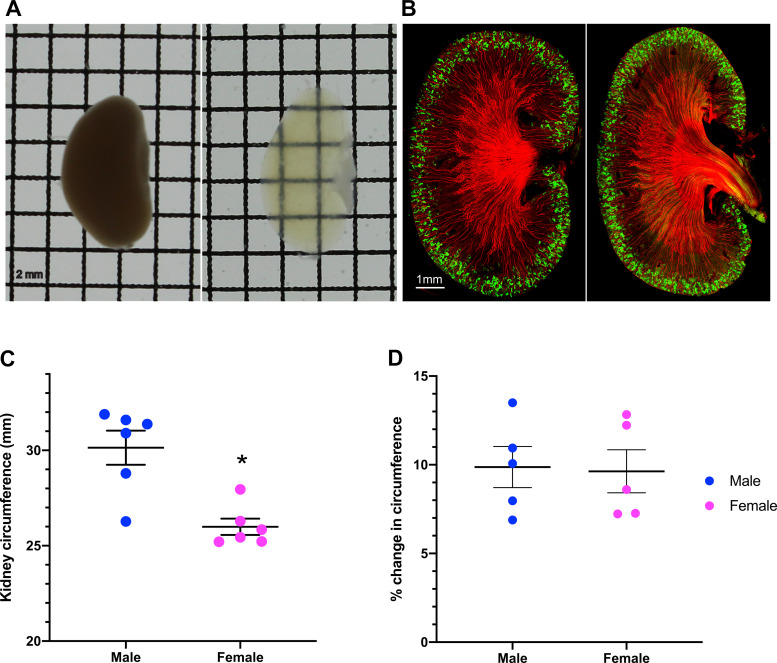
We applied tissue-clearing and 3D imaging techniques to explore if the sex differences in NCC is paralleled by a structural dimorphism of the DCT. The CUBIC pipeline (46) was optimized for visualization and quantitative measurements of DCT length, volume, and density. With our refinements, CUBIC consistently rendered mouse kidneys almost completely transparent (Fig. 1A) while retaining normal structure and antigenicity for antibody labeling (Fig. 1B). In sagittal sections coimmunolabeled with antibodies raised against markers to the early DCT, parvalbumin, and collecting duct (Fig. 1B), we consistently observed AQP2-positive collecting ducts projecting into the cortex, surrounded by arcades of parvalbumin-positive DCTs, indicating that the architecture of the kidney and distal nephron is preserved. We confirmed previous findings that kidneys are bigger in male mice than in female mice (Fig. 1C) (32, 36). Although CUBIC caused a small expansion of the kidney, typical of superhydration clearing methods (39), the extent of expansion did not differ between sexes, so that the sexual dimorphism of the kidney size was maintained in CUBIC (Fig. 1D). The sex difference in the thickness of the cortex (32, 36) was also preserved in CUBIC.
Fig. 1.
Clear, Unobstructed Brain/Body Imaging Cocktails and Computational (CUBIC)-clarified mouse kidneys. A: paraformaldehyde-fixed kidneys were cleared using CUBIC solutions (2-mm grid spacing). B: a representative image of 300-μm-thick sagittal kidney sections from male and female mice labeled with antibodies to parvalbumin (green) and aquaporin 2 (red). Scale bar = 1 mm. C: kidney circumference in male and female mice. D: CUBIC expanded the kidney circumference to the same extent in both sexes. Data are presented here as means ± SE; n = 5–6 animals/group. A Student’s t test was performed, and *P < 0.05 was considered statistically significant.
As shown in Fig. 2A, anti-parvalbumin antibodies specifically labeled a discrete population of short, convoluted tubule segments that associated with glomeruli (Fig. 2, B and C) which are confined to cortical labyrinth-like structures, indicative of the early DCT. At higher power (Fig. 2D and Supplemental Movie S1, available online at https://doi.org/10.6084/m9.figshare.12818675.v1), variable sizes and convoluted shapes of individual DCTs were readily observed. As assessed by parvalbumin-positive object counting, the DCT1 density (∼150 tubules/mm3; Fig. 2E) was comparable to previous estimates of total nephron number in mice, providing confidence that CUBIC, parvalbumin antibody labeling, and spinning disk microscopy provides a means to visualize an all-inclusive population of the DCT. Initial measurements of a large number of individual parvalbumin-positive tubules (n = 24 tubules/animal, n = 4 male animals) revealed that the DCT1 length is highly variable over a range of 344 μm. Nonetheless, the average DCT1 length across animals was relatively uniform (SD 21.51 μm; Fig. 2F).
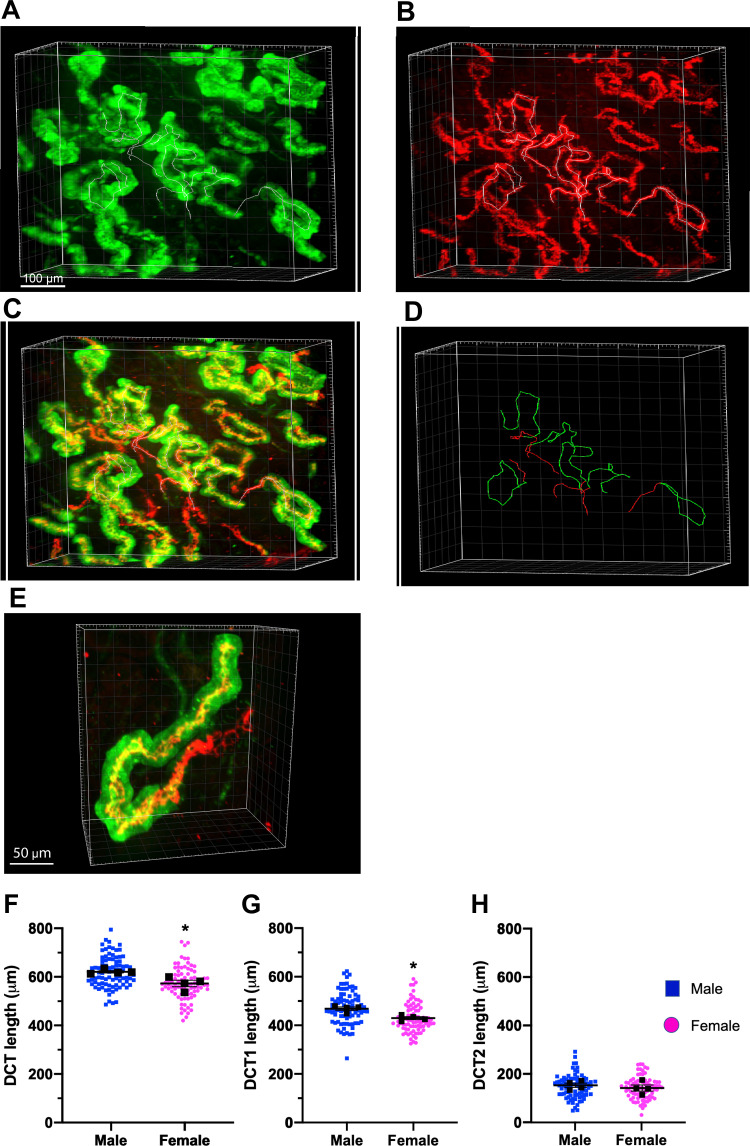
The DCT consists of two anatomically and functionally distinct segments that express NCC. The more distal DCT (DCT2), which uniquely contains NCC, epithelial Na+ channels (ENaC), and renal outer medullary K+ (ROMK) channels, can be distinguished from the early distal convoluted tubule (DCT1) by the absence of parvalbumin. Thus, DCT1 and DCT2 can be easily identified in CUBIC-clarified kidneys by colabeling with antibodies to parvalbumin and NCC (Fig. 3 and Supplemental Movie S2, available online at https://doi.org/10.6084/m9.figshare.12818678.v1). In most tubules, an abrupt transition between NCC and parvalbumin-expressing cells and parvalbumin-negative cells easily defined the boundaries between the cortical thick ascending limb and DCT1 and between DCT1 and DCT2. Some NCC-positive tubules contained short tracks of parvalbumin-expressing cells that mixed with cells that did not contain detectable parvalbumin. In these hybrid cases, we defined the DCT1/DCT2 boundary by the absence of any detectable parvalbumin. The DCT2/connecting tubule boundary was defined as the absence of any NCC. Line tracing measurements of DCT length revealed that male mice had a slightly longer DCT than female mice (620.9 ± 4.7 μm in male mice vs. 572.2 ± 13.2 μm in female mice) and correspondingly longer DCT1 (468.7 ± 8.7 μm in male mice vs. 429.6 ± 6 μm in female mice) despite having less NCC protein (see below). The DCT2 length was similar between sexes (152.2 ± 8.7 μm in male mice vs. 141.0 ± 12.6 μm in female mice).
Fig. 3.
Structural sexual dimorphism of the early distal convoluted tubule (DCT1) and late DCT (DCT2). A−C: three-dimensional views of the DCT in common kidney sections colabeled with antibodies to parvalbumin (A; green, DCT1) and Na+-Cl− cotransporter (NCC; B; DCT1 and DCT2) as well as the merged imaged overlay of both green and red (C). D: line tracing of a representative DCT1 (green) and DCT2 (red only) in a section. E: higher-magnification view of a representative single DCT labeled with parvalbumin (green) and NCC (red). F–H: quantification of DCT (F), DCT1 (G), and DCT2 lengths (H) in male and female mice at the basal state. The NCC-positive labeled section of the tubule represents total DCT length; tubules colabeled with NCC and parvalbumin are DCT1 and single labeling with NCC are DCT2. Colored dots are individual measurement of a signal tubule. Black squares are average measurements per kidney. Data are reported as means ± SE; n = 4 kidneys/group and ∼25 DCTs/kidney). Student t tests were performed, and *P < 0.05 was considered statistically significant.
Differential DCT remodeling responses between sexes.
To test if the structural adaptation of DCT remodeling is different between sexes, we examined the response to furosemide treatment because it activates a robust structural remodeling (8, 17), coincident with the increase in distal Na+ delivery, development of hypokalemia, and intravascular volume depletion, which activates NCC and enhances Na+ transport in the DCT. For these experiments, male and female mice were randomized to vehicle or furosemide treatment for 7 days, and the physiological (Fig. 4), biochemical (Fig. 5), and morphological responses (Figs. 6 and 7) were compared.
Fig. 4.
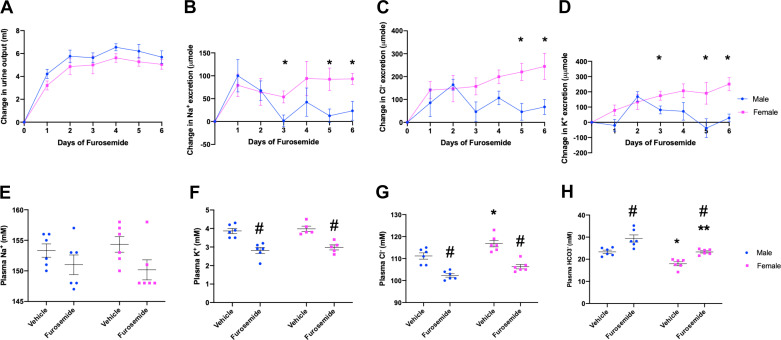
Physiological responses to furosemide treatment. A−D: furosemide- induced diuresis (A) and urinary excretion of Na+ (B), Cl− (C), and K+ (D) in male and female mice. E−H: plasma electrolyte panel postfurosemide treatment [plasma Na+ (E), Cl− (F), K+ (G) and (H)]. P < 0.05 was considered statistically significant *between sexes and #between treatments, respectively. Data are presented as means ± SE; n = 6 animals/group.
Fig. 5.
Biochemical responses in furosemide treatment. A and B: representative Western blot images of phosphorylated Na+-Cl− cotransporter (p-NCC), NCC, and β-actin (as a loading control). C: quantification two-way ANOVA was performed, and P < 0.05 was considered statistically significant (* between sexes and # between treatments, respectively). Data are presented here as means ± SE; 5–6 animals/group.
Fig. 6.
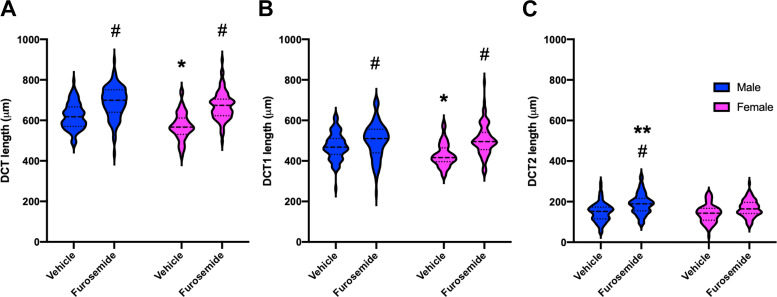
Remodeling of the distal convoluted tubule (DCT) length. A−C: violin plots showing the distribution of individual tubule length measurements and medians (dashed lines) and upper and lower quartiles (dotted lines) of the entire DCT length (A), early DCT (DCT1; B), and late DCT (DCT2; C) for vehicle- and furosemide-treated male mice (blue) and female mice (pink). Four kidneys per group and ∼25 DCTs per kidney were analyzed. Two-way ANOVA tests were performed, and P < 0.05 was considered statistically significant (*between sexes at the basal state, #effect of furosemide in the same sex, and **between sexes after furosemide, respectively).
Fig. 7.
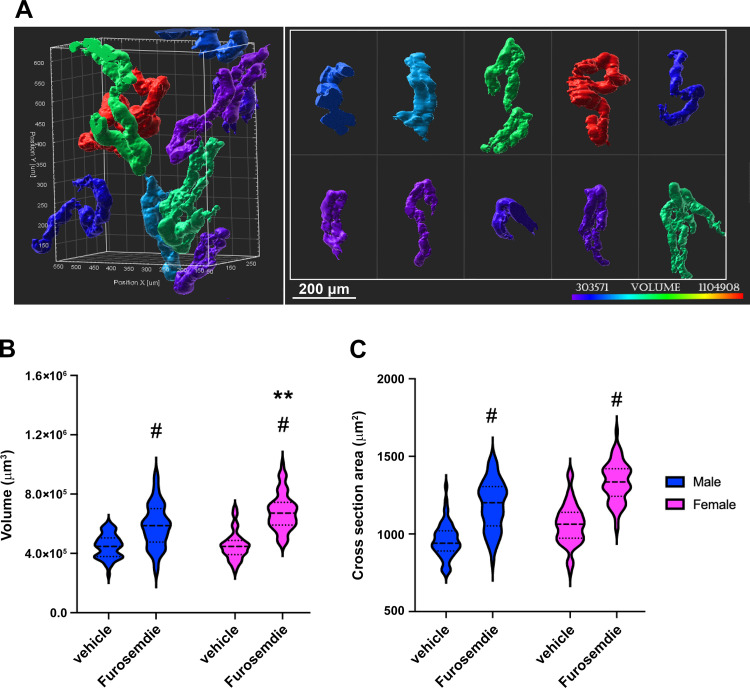
Remodeling of distal convoluted tubule (DCT) volume. A: surface rendering of parvalbumin- expressing DCTs highlighting the variations in size and shape of the DCT. B and C: violin plots showing the distribution of individual tubule measurements and medians (dashed lines) and upper and lower quartiles (dotted lines) of the tubule volume (B) and tubule cross-sectional area (C) of vehicle- and furosemide-treated male mice (blue) and female mice (pink). Four kidneys per group and 15 DCTs per kidney were analyzed. Two-way ANOVA tests were performed to test for interactions, with Tukey post hoc analysis, and P < 0.05 was considered significant (*between sexes in the basal state; #effect of furosemide in the same sex, and **between sexes in the furosemide-treated state, respectively).
As shown in Fig. 4, furosemide rapidly induced a diuresis and urinary Na+ and Cl− wasting, which reached maximum levels within 1–3 days. Initial peak responses were the same in both sexes. Likewise, male and female mice similarly developed hypokalemic alkalosis. After the initial response, male mice exhibited a gradual reduction in salt excretion, whereas female mice did not display a similar diuretic braking response. As measured in Western blots, female mice had higher baseline NCC and pNCC protein abundance compared with male mice (Fig. 5, A and B, vehicle treated), as previously reported (6, 25, 47). Furosemide treatment increased NCC and pNCC protein abundance in both male and female mice significantly. Male mice had a higher percent increase in NCC and pNCC protein abundance after furosemide compared with female mice, so that the basal sex differences disappeared.
Morphometric measurements of the DCT (Fig. 6) from the 3D rendered images of anti-parvalbumin-NCC antibody-labeled kidneys revealed that tubule length increased significantly in both sexes after furosemide treatment. Of note, a more robust elongation response was observed in female mice (20%) than in male mice (8%). As a result, the length difference between sexes disappeared after furosemide treatment. The response was largely rooted in a change in DCT1 in both sexes. In contrast to DCT1, female DCT2 did not increase in a statistically significant manner. A modest increase in male DCT2 was observed.
Surface rendering of anti-parvalbumin antibody-labeled tubules made it possible to visualize and measure the wide distribution of DCT1 volumes (Fig. 7A). No significant difference in DCT1 volume was detected between sexes in the basal state (Fig. 7B) because the smaller tubule length in female mice was offset by a slightly larger cross-sectional area (Fig. 7C). In response to furosemide, the combination of increase in cross-sectional area and tubule length in female mice led to a greater increase in volume than in male mice.
DISCUSSION
New techniques for tissue clearing and 3D microscopy provide unprecedented opportunities to explore complex microscopic structures within whole organs and large tissue specimens (39) for the first time. We refined the methodology to study diverse DCT structures at a kidney-wide scale and to systematically examine the sexual dimorphism of the DCT and its adaptive responses. We found that the female DCT is surprisingly shorter than the male DCT, despite the higher NCC protein abundance. Our data also indicate that the remodeling process is unexpectedly different between sexes. In response to a provoking stimulus of furosemide, female DCTs exhibited a more pronounced increase in tubule length and volume. By having a greater NCC density in a shorter structure, female subjects are able to protect Na+ balance in the face of greater basal distal Na+ delivery (27, 47) and have a larger remodeling reserve to adapt to unique physiological stresses.
Until now, it was broadly assumed that the female DCT might have a greater mass than the male DCT. The idea evolved, in part, from observations from different transport profiles between sexes. Male subjects are more proximal tubule-centric with more abundant proximal tubule Na+ transporters [Na+/H+ exchanger 3 (NHE3) and Na+-phosphate cotransporter 2a (Napi2a)] and AQP1, associated with a greater Na+ reabsorptive capacity and a longer tubular length than female subjects (14, 36). Female subjects have a more distal-centric transport compared with male subjects (27, 47), featuring more NCC, pNCC, and NCC activity (6, 47). Because the DCT structure is dependent on NCC (12, 13, 17, 18, 24, 30), as underscored by the atrophy of the DCT in Gitelman’s syndrome, the hypothesis that female subjects might have a correspondingly larger DCT was especially appealing. The discordant relationship between NCC activity, DCT mass, and sex, as observed here, should lay this concept to rest. Our findings suggest, instead, that sex-specific pathways that are responsible for maintaining larger kidney size and longer tubule length in male subjects may override those that relate NCC activity to DCT mass.
It will be important for future studies to identify what sex-linked processes shape DCT morphogenesis, presumably acting to control the balance between planar polarized cell division, tubule elongation (19), and apoptosis (28), and what sex-linked processes control NCC density and phosphorylation. The remarkable difference in NCC and pNCC between sexes despite similar plasma K+ levels, which is a dominant physiological regulator of NCC (16, 45), indicate that female sex-dependent factors may supersede, perhaps by sensitizing WNK-SPAK signaling that modulates NCC abundance and phosphorylation. Although estrogen is necessary for maintaining the greater NCC activity and abundance (11), classic sex hormone receptors are preferentially expressed in the proximal tubule (41), raising the possibility that these hormones indirectly modulate NCC by primary effects on proximal reabsorption and Na+ delivery to the DCT to affect NCC activity (10, 27). It has been speculated that a coupling between Na+ transport and tubule cell mass might be mediated by autocrine growth factors (42), which, in turn, may be differently controlled by sex.
The stage of the estrogen cycle is unlikely to confound interpretation of the results. Estrogen remains high in mice throughout the cycle, except for the short time of estrus and metestrus (5). Moreover, a recent study by Veiras et al. (47) has shown that there is no correlation between estrus cycle and the abundance/activity of many kidney transporters, including NCC phosphorylation.
Sex also differently affects remodeling responses to furosemide, despite similar initial physiological responses to the diuretic that increase salt delivery, drive NCC activation, and increase Na+ reabsorption in the DCT. Previous studies in male rats have indicated that furosemide treatment increases DCT cell volume, cell height, and basolateral cell membrane area without detectable changes in tubule length in response to furosemide (36). Although it is possible that rats and mice respond differently to furosemide, we think it is more likely that the new imaging and analysis tools, used here, provide a means to detect small changes in length. With the new clearing methods, we found that male and female mice both increase tubule length and volume, but female mice exhibit a more robust elongation response and a more profound cell volume response, indicative of more active hyperplasia and hypertrophy or endocycling, which can replenish damaged nephron cells for postmitotic cell growth (23).
Despite a similar increase in NCC and pNCC compared with male mice, as well as a larger increase in DCT mass, female mice were unable to effectively “brake” the natriuretic response like male mice. We interpret these observations to indicate that male mice rely on the activation of additional Na+ transport pathways, such as their more active proximal tubule processes (11, 27), to achieve Na+ balance in the face of loop diuretic treatment. The powerful structural remodeling response of female mice may be an incomplete response to harness the power of NCC-mediated salt reabsorption to overcome their less avid proximal tubule transport.
These sex-dependent remodeling processes are largely confined to the longer DCT1. Adaptive elongation of DCT2 is only observed in male mice, and, even then, the remodeling response is modest compared with DCT1. DCT2 cells express high levels of ENaC (29) and ROMK (50), in addition to NCC, and are believed to provide a major avenue for urinary K+ excretion. We speculate that hypokalemia, induced by furosemide, may prevent a robust remodeling response of DCT2, as a secondary adaptive mechanism to safeguard against K+ wasting. Alternatively, observations in NCC knockout mouse that the DCT undergoes a selective atrophy of DCT1 (30) suggest that the early DCT may be more predisposed to adaptive remodeling responses than the late DCT.
DCT remodeling is believed to be an important contributor to the development of loop diuretic resistance, a common problem in the management of edematous conditions including heart failure and nephrotic syndrome (25). Indeed, the natriuretic response of loop diuretics wanes over time as NCC becomes activated (2), and treatment with a thiazide-type diuretic can prevent the development of diuretic resistance (7). To the best of our knowledge, there has been no direct study to assess the sex differences in loop diuretic resistance, but the greater NCC density and larger remodeling reserve in female subjects raise the possibility that female subjects may be more susceptible. Additionally, sex hormone-dependent NCC expression might have therapeutic ramifications in the management of postmenopausal women.
The CUBIC technique made it possible to visualize antibody-labeled distal nephron segments for whole kidney tubule-wide quantitative assessments of tubule morphology, which is not feasible with traditional stereology or serial assembly measurements. The all-inclusive sampling approach provided power to detect subtle differences in DCT morphology between sexes and conditions despite the wide individual variation in DCT structure. Use of CUBIC represents a departure from our previous study with CLARITY (41). In our experience, the two techniques are equal in the quality of clearing and preservation of anatomic structures. Indeed, we found that DCT length was the same whether measured in CUBIC- or CLARITY-treated specimens (41) and nearly identical to the benchmark standard, ascertained by assembly of serial sections of fixed tissue (52). Both CUBIC or CLARITY are amenable to antibody labeling and endogenous fluorophores. Some antibodies, like parvalbumin, seem to work better in CUBIC, but CLARITY can provide deeper antibody penetration. Unlike solvent-based clearing techniques, the CUBIC solutions are not toxic or corrosive and thus are easier to use and more practical for multiuser microscopy core facilities. As assessed from measurements of calibration beads (4), a standard water immersion lens collar provided excellent refractive index matching to CUBIC with minimal distortion, making CUBIC easily amendable for conventional high-speed confocal imaging. Although we favored CUBIC for the present study, we recommend that investigators establish the method that works best for their application.
In conclusion, our findings revealed surprising sexual dimorphism of the DCT. Female subjects pack more NCC into a shorter DCT, providing a means to protect Na+ balance in the face of greater basal distal Na+ delivery (10), and still have larger reserve and remodeling capacity to adapt to unique physiological stresses.
GRANTS
This work was funded by financial support from the Leducq Foundation, National Institute of Diabetes and Digestive and Kidney Diseases grants, and a Ben J. Lipps postdoctoral fellowship (to E.T.). T. S. is supported by the START-Program of the Faculty of Medicine, RWTH Aachen (21/20), Else Kröner-Fresenius Stiftung (2015_A197), and Clinician-Scientist program of the German Society of Internal Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.T., T.S., and P.A.W. conceived and designed research; E.T. and R.C. performed experiments; E.T., R.C., T.S., D.H.E., and P.A.W. analyzed data; E.T., D.H.E., and P.A.W. interpreted results of experiments; E.T., R.C., and P.A.W. prepared figures; E.T. and P.A.W. drafted manuscript; E.T., R.C., T.S., D.H.E., and P.A.W. edited and revised manuscript; E.T., R.C., T.S., D.H.E., and P.A.W. approved final version of manuscript.
ACKNOWLEDGMENTS
Authors express their gratitude to Dr. Rick Grimm and Dr. Dimin Li for providing expert technical help with mice. The authors acknowledge the University of Maryland School of Medicine Center for Innovative Biomedical Resources, Confocal Microscopy Core, Baltimore, MD, for help with and use of the facility.
REFERENCES
- 1.Abdallah JG, Schrier RW, Edelstein C, Jennings SD, Wyse B, Ellison DH. Loop diuretic infusion increases thiazide-sensitive Na(+)/Cl(-)-cotransporter abundance: role of aldosterone. J Am Soc Nephrol 12: 1335–1341, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal S, Fulgoni VL III, Spence L, Samuel P. Sodium intake status in United States and potential reduction modeling: an NHANES 2007−2010 analysis. Food Sci Nutr 3: 577–585, 2015. doi: 10.1002/fsn3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 288: 2981–2997, 2002. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 4.Besseling TH, Jose J, Van Blaaderen A. Methods to calibrate and scale axial distances in confocal microscopy as a function of refractive index. J Microsc 257: 142–150, 2015. doi: 10.1111/jmi.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PLoS One 7: e35538, 2012. doi: 10.1371/journal.pone.0035538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z, Vaughn DA, Fanestil DD. Influence of gender on renal thiazide diuretic receptor density and response. J Am Soc Nephrol 5: 1112–1119, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Cox ZL, Hung R, Lenihan DJ, Testani JM. Diuretic strategies for loop diuretic resistance in acute heart failure: the 3T trial. JACC Heart Fail 8: 157–168, 2020. doi: 10.1016/j.jchf.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellison DH, Velázquez H, Wright FS. Adaptation of the distal convoluted tubule of the rat. Structural and functional effects of dietary salt intake and chronic diuretic infusion. J Clin Invest 83: 113–126, 1989. doi: 10.1172/JCI113847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falconnet C, Bochud M, Bovet P, Maillard M, Burnier M. Gender difference in the response to an angiotensin-converting enzyme inhibitor and a diuretic in hypertensive patients of African descent. J Hypertens 22: 1213–1220, 2004. doi: 10.1097/00004872-200406000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Fang L, Garuti R, Kim BY, Wade JB, Welling PA. The ARH adaptor protein regulates endocytosis of the ROMK potassium secretory channel in mouse kidney. J Clin Invest 119: 3278–3289, 2009. doi: 10.1172/JCI37950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher KA, Lee SH, Walker J, Dileto-Fang C, Ginsberg L, Stapleton SR. Regulation of proximal tubule sodium/hydrogen antiporter with chronic volume contraction. Am J Physiol Renal Physiol 280: F922–F926, 2001. doi: 10.1152/ajprenal.2001.280.5.F922. [DOI] [PubMed] [Google Scholar]
- 12.Grimm PR, Coleman R, Delpire E, Welling PA. Constitutively active SPAK causes hyperkalemia by activating NCC and remodeling distal tubules. J Am Soc Nephrol 28: 2597–2606, 2017. doi: 10.1681/ASN.2016090948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimm PR, Taneja TK, Liu J, Coleman R, Chen YY, Delpire E, Wade JB, Welling PA. SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J Biol Chem 287: 37673–37690, 2012. doi: 10.1074/jbc.M112.402800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris AN, Lee HW, Osis G, Fang L, Webster KL, Verlander JW, Weiner ID. Differences in renal ammonia metabolism in male and female kidney. Am J Physiol Renal Physiol 315: F211–F222, 2018. doi: 10.1152/ajprenal.00084.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoorn EJ, Ellison DH. Diuretic resistance. Am J Kidney Dis 69: 136–142, 2017. doi: 10.1053/j.ajkd.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.ImageJ website Process Menu. Enhance Contrast https://imagej.nih.gov/ij/docs/menus/process.html#enhance [21 September 2020].
- 16.Irsik DL, Carmines PK, Lane PH. Classical estrogen receptors and ERα splice variants in the mouse. PLoS One 8: e70926, 2013. doi: 10.1371/journal.pone.0070926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaissling B, Bachmann S, Kriz W. Structural adaptation of the distal convoluted tubule to prolonged furosemide treatment. Am J Physiol Renal Physiol 248: F374–F381, 1985. doi: 10.1152/ajprenal.1985.248.3.F374. [DOI] [PubMed] [Google Scholar]
- 18.Kaissling B, Le Hir M. Distal tubular segments of the rabbit kidney after adaptation to altered Na- and K-intake. I. Structural changes. Cell Tissue Res 224: 469–492, 1982. doi: 10.1007/BF00213746. [DOI] [PubMed] [Google Scholar]
- 19.Karner C, Wharton KA Jr, Carroll TJ. Planar cell polarity and vertebrate organogenesis. Semin Cell Dev Biol 17: 194–203, 2006. doi: 10.1016/j.semcdb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Kunchaparty S, Palcso M, Berkman J, Velázquez H, Desir GV, Bernstein P, Reilly RF, Ellison DH. Defective processing and expression of thiazide-sensitive Na-Cl cotransporter as a cause of Gitelman’s syndrome. Am J Physiol Renal Physiol 277: F643–F649, 1999. doi: 10.1152/ajprenal.1999.277.4.F643. [DOI] [PubMed] [Google Scholar]
- 21.Kurtz TW, Morris RC Jr. Dietary chloride as a determinant of “sodium-dependent” hypertension. Science 222: 1139–1141, 1983. doi: 10.1126/science.6648527. [DOI] [PubMed] [Google Scholar]
- 22.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38: 1124–1132, 2006. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- 23.Lazzeri E, Angelotti ML, Peired A, Conte C, Marschner JA, Maggi L, Mazzinghi B, Lombardi D, Melica ME, Nardi S, Ronconi E, Sisti A, Antonelli G, Becherucci F, De Chiara L, Guevara RR, Burger A, Schaefer B, Annunziato F, Anders HJ, Lasagni L, Romagnani P. Endocycle-related tubular cell hypertrophy and progenitor proliferation recover renal function after acute kidney injury. Nat Commun 9: 1344, 2018. doi: 10.1038/s41467-018-03753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Hir M, Kaissling B, Dubach UC. Distal tubular segments of the rabbit kidney after adaptation to altered Na- and K-intake. II. Changes in Na-K-ATPase activity. Cell Tissue Res 224: 493–504, 1982. doi: 10.1007/BF00213747. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Hatano R, Xu S, Wan L, Yang L, Weinstein AM, Palmer L, Wang T. Gender difference in kidney electrolyte transport. I. Role of AT1a receptor in thiazide-sensitive Na+-Cl− cotransporter activity and expression in male and female mice. Am J Physiol Renal Physiol 313: F505–F513, 2017. doi: 10.1152/ajprenal.00087.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Xu S, Yang L, Yang J, Wang CJ, Weinstein AM, Palmer LG, Wang T. Sex difference in kidney electrolyte transport II: impact of K+ intake on thiazide-sensitive cation excretion in male and female mice. Am J Physiol Renal Physiol 317: F967–F977, 2019. doi: 10.1152/ajprenal.00125.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, McDonough AA, Layton HE, Layton AT. Functional implications of sexual dimorphism of transporter patterns along the rat proximal tubule: modeling and analysis. Am J Physiol Renal Physiol 315: F692–F700, 2018. doi: 10.1152/ajprenal.00171.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loffing J, Loffing-Cueni D, Hegyi I, Kaplan MR, Hebert SC, Le Hir M, Kaissling B. Thiazide treatment of rats provokes apoptosis in distal tubule cells. Kidney Int 50: 1180–1190, 1996. doi: 10.1038/ki.1996.426. [DOI] [PubMed] [Google Scholar]
- 29.Loffing J, Loffing-Cueni D, Valderrabano V, Kläusli L, Hebert SC, Rossier BC, Hoenderop JG, Bindels RJ, Kaissling B. Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am J Physiol Renal Physiol 281: F1021–F1027, 2001. doi: 10.1152/ajprenal.0085.2001. [DOI] [PubMed] [Google Scholar]
- 30.Loffing J, Vallon V, Loffing-Cueni D, Aregger F, Richter K, Pietri L, Bloch-Faure M, Hoenderop JG, Shull GE, Meneton P, Kaissling B. Altered renal distal tubule structure and renal Na+ and Ca2+ handling in a mouse model for Gitelman’s syndrome. J Am Soc Nephrol 15: 2276–2288, 2004. doi: 10.1097/01.ASN.0000138234.18569.63. [DOI] [PubMed] [Google Scholar]
- 31.McCallum L, Lip S, Padmanabhan S. The hidden hand of chloride in hypertension. Pflugers Arch 467: 595–603, 2015. doi: 10.1007/s00424-015-1690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Messow C, Gärtner K, Hackbarth H, Kangaloo M, Lünebrink L. Sex differences in kidney morphology and glomerular filtration rate in mice. Contrib Nephrol 19: 51–55, 1980. doi: 10.1159/000428760. [DOI] [PubMed] [Google Scholar]
- 33.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 34.O’Shaughnessy KM. Gordon syndrome: a continuing story. Pediatr Nephrol 30: 1903–1908, 2015. doi: 10.1007/s00467-014-2956-7. [DOI] [PubMed] [Google Scholar]
- 36.Oudar O, Elger M, Bankir L, Ganten D, Ganten U, Kriz W. Differences in rat kidney morphology between males, females and testosterone-treated females. Ren Physiol Biochem 14: 92–102, 1991. doi: 10.1159/000173392. [DOI] [PubMed] [Google Scholar]
- 37.Posadzy-Malaczynska A, Rajpold K, Woznicka-Leskiewicz L, Marcinkowska J. Hemodynamic and metabolic effects of estrogen plus progestin therapy in hypertensive postmenopausal women treated with an ACE-inhibitor or a diuretic. Clin Res Cardiol 104: 38–50, 2015. doi: 10.1007/s00392-014-0755-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Preston RA, Norris PM, Alonso AB, Ni P, Hanes V, Karara AH. Randomized, placebo-controlled trial of the effects of drospirenone-estradiol on blood pressure and potassium balance in hypertensive postmenopausal women receiving hydrochlorothiazide. Menopause 14: 408–414, 2007. doi: 10.1097/01.gme.0000243572.63322.f7. [DOI] [PubMed] [Google Scholar]
- 39.Richardson DS, Lichtman JW. Clarifying tissue clearing. Cell 162: 246–257, 2015. doi: 10.1016/j.cell.2015.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rojas-Vega L, Reyes-Castro LA, Ramírez V, Bautista-Pérez R, Rafael C, Castañeda-Bueno M, Meade P, de Los Heros P, Arroyo-Garza I, Bernard V, Binart N, Bobadilla NA, Hadchouel J, Zambrano E, Gamba G. Ovarian hormones and prolactin increase renal NaCl cotransporter phosphorylation. Am J Physiol Renal Physiol 308: F799–F808, 2015. doi: 10.1152/ajprenal.00447.2014. [DOI] [PubMed] [Google Scholar]
- 41.Saritas T, Puelles VG, Su XT, McCormick JA, Welling PA, Ellison DH. Optical clearing in the kidney reveals potassium-mediated tubule remodeling. Cell Rep 25: 2668–2675.e3, 2018. doi: 10.1016/j.celrep.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saunders E, Cable G, Neutel J. Predictors of blood pressure response to angiotensin receptor blocker/diuretic combination therapy: a secondary analysis of the irbesartan/hydrochlorothiazide blood pressure reductions in diverse patient populations (INCLUSIVE) study. J Clin Hypertens (Greenwich) 10: 27–33, 2008. doi: 10.1111/j.1524-6175.2007.07195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah SJ, Stafford RS. Current trends of hypertension treatment in the United States. Am J Hypertens 30: 1008–1014, 2017. doi: 10.1093/ajh/hpx085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitleman HJ, Lifton RP. Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12: 24–30, 1996. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 45.Stanton BA, Kaissling B. Regulation of renal ion transport and cell growth by sodium. Am J Physiol 257: F1–F10, 1989. doi: 10.1152/ajprenal.1989.257.1.F1. [DOI] [PubMed] [Google Scholar]
- 46.Susaki EA, Tainaka K, Perrin D, Yukinaga H, Kuno A, Ueda HR. Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nat Protoc 10: 1709–1727, 2015. doi: 10.1038/nprot.2015.085. [DOI] [PubMed] [Google Scholar]
- 47.Veiras LC, Girardi ACC, Curry J, Pei L, Ralph DL, Tran A, Castelo-Branco RC, Pastor-Soler N, Arranz CT, Yu ASL, McDonough AA. Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J Am Soc Nephrol 28: 3504–3517, 2017. doi: 10.1681/ASN.2017030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verlander JW, Tran TM, Zhang L, Kaplan MR, Hebert SC. Estradiol enhances thiazide-sensitive NaCl cotransporter density in the apical plasma membrane of the distal convoluted tubule in ovariectomized rats. J Clin Invest 101: 1661–1669, 1998. doi: 10.1172/JCI601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Visser TD, Oud JL, Brakenhoff GJ. Refractive-index and axial distance measurements in 3-D microscopy. Optik (Stuttg) 90: 17–19, 1992. [Google Scholar]
- 50.Wade JB, Fang L, Coleman RA, Liu J, Grimm PR, Wang T, Welling PA. Differential regulation of ROMK (Kir1.1) in distal nephron segments by dietary potassium. Am J Physiol Renal Physiol 300: F1385–F1393, 2011. doi: 10.1152/ajprenal.00592.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weinstein AM. A mathematical model of rat distal convoluted tubule. I. Cotransporter function in early DCT. Am J Physiol Renal Physiol 289: F699–F720, 2005. doi: 10.1152/ajprenal.00043.2005. [DOI] [PubMed] [Google Scholar]
- 52.Zhai XY, Thomsen JS, Birn H, Kristoffersen IB, Andreasen A, Christensen EI. Three-dimensional reconstruction of the mouse nephron. J Am Soc Nephrol 17: 77–88, 2006. doi: 10.1681/ASN.2005080796. [DOI] [PubMed] [Google Scholar]