Keywords: brain blood flow, cerebral autoregulation, cerebrovascular reactivity, chronic kidney disease, end-stage renal disease
Abstract
Patients with chronic kidney disease (CKD) and end-stage kidney disease (ESKD) experience an increased risk of cerebrovascular disease and cognitive dysfunction. Hemodialysis (HD), a major modality of renal replacement therapy in ESKD, can cause rapid changes in blood pressure, osmolality, and acid-base balance that collectively present a unique stress to the cerebral vasculature. This review presents an update regarding cerebral blood flow (CBF) regulation in CKD and ESKD and how the maintenance of cerebral oxygenation may be compromised during HD. Patients with ESKD exhibit decreased cerebral oxygen delivery due to anemia, despite cerebral hyperperfusion at rest. Cerebral oxygenation further declines during HD due to reductions in CBF, and this may induce cerebral ischemia or “stunning.” Intradialytic reductions in CBF are driven by decreases in cerebral perfusion pressure that may be partially opposed by bicarbonate shifts during dialysis. Intradialytic reductions in CBF have been related to several variables that are routinely measured in clinical practice including ultrafiltration rate and blood pressure. However, the role of compensatory cerebrovascular regulatory mechanisms during HD remains relatively unexplored. In particular, cerebral autoregulation can oppose reductions in CBF driven by reductions in systemic blood pressure, while cerebrovascular reactivity to CO2 may attenuate intradialytic reductions in CBF through promoting cerebral vasodilation. However, whether these mechanisms are effective in ESKD and during HD remain relatively unexplored. Important areas for future work include investigating potential alterations in cerebrovascular regulation in CKD and ESKD and how key regulatory mechanisms are engaged and integrated during HD to modulate intradialytic declines in CBF.
INTRODUCTION
Approximately 15% of the United States population has chronic kidney disease (CKD), which is associated with an elevated risk of cerebrovascular disease (21a). Patients with CKD have ∼43% higher risk of stroke compared with the general population (70), and every 10 mL·min−1·1.73 m−2 decrease in estimated glomerular filtration rate is associated with a 7% increased risk of stroke (79). This risk increases considerably in end-stage kidney disease (ESKD) when renal function deteriorates to the point of requiring renal replacement therapy with either kidney transplantation, peritoneal dialysis, or hemodialysis (HD). The majority (63%) of patients with ESKD are treated with maintenance MD, while 7% receive peritoneal dialysis and ∼30% have a functioning kidney transplant (111). Not only do patients undergoing HD experience an 8- to 10-fold greater risk of stroke (102), but they also exhibit much greater rates of cerebral small vessel disease (33, 35, 90), cognitive dysfunction (35, 40), and cerebral atrophy (33, 35, 40, 63). Recent reviews have highlighted the role of the “kidney-brain axis” in the development and progression of cerebrovascular disease (18, 64, 69). Most of these discussions have focused on the systemic effects of renal disease and how the interaction of traditional and nontraditional (e.g., uremic metabolites and vascular calcification) risk factors augment cerebrovascular risk. The role of comorbidities is also important because diabetes and hypertension, two leading causes of CKD, are independently associated with an elevated risk of cerebrovascular disease (36, 54). Endothelial dysfunction is present in all of these conditions and may be one shared mechanism that augments cerebrovascular risk in CKD (10, 49, 78). Importantly, however, the increased cerebrovascular disease burden experienced in CKD cannot be explained by these comorbidities alone (24, 70), and other renal specific mechanisms likely contribute. One area that has received much less attention are the acute cerebrovascular response to HD and how key cerebrovascular control mechanisms are engaged and integrated during repeated episodes of HD treatment. The goal of this review is to examine potential cerebrovascular mechanisms that contribute to the increased risk of stroke and cognitive dysfunction in ESKD due both to the effects of reduced kidney function and the HD procedure itself.
CEREBRAL PERFUSION IN KIDNEY DISEASE
Patients with ESKD exhibit cerebral hyperperfusion at rest due to the anemia that occurs secondary to renal failure (51, 60, 67, 130, 134). Anemia develops early in the course of renal disease and increases in severity with disease progression (9, 118). The majority of patients with ESKD are anemic (118), and the contributing mechanisms are multifactorial (11). As kidney function declines, erythropioetin production by interstitial fibroblasts in the kidney declines, leading to decreased red blood cell production (83). In addition, iron deficiency and inflammation are highly prevalent with reduced renal function (11), and red blood cell survival time is shortened in ESKD (131), all contributing to chronic anemia.
The cerebrovascular consequences of anemia in ESKD manifest as a decreased blood viscosity and reduced oxygen delivery. Both of these mechanisms lead to increased cerebral blood flow (CBF), and several investigations have reported negative correlations between CBF and hematocrit in ESKD (25, 130, 134). In health, CBF and cerebral oxygen delivery are tightly linked since the oxygen content of delivered blood is sufficient to meet the demands of cerebral metabolism. In anemia, however, oxygen carrying capacity is reduced and cerebral oxygen delivery remains compromised despite chronically elevated CBF. Cerebral hyperperfusion in renal disease is not limited to ESKD, as several investigations have reported CBF elevations in earlier stages of CKD as well. Tamura et al. (126) reported an inverse relationship between estimated glomerular filtration rate (eGFR) and CBF in a cohort of nondiabetic adults with hypertension recruited as part of the Systolic Blood Pressure Intervention Trial. Similarly, Liu et al. (73) observed elevated CBF in a cohort of young patients with CKD at stages II−V that was related to hematocrit. While increased CBF acts as a protective mechanism to increase cerebral oxygenation in the setting of anemia, cerebral oxygen delivery does not fully correct and still remains compromised in ESKD at rest. Zheng et al. (134) compared CBF and cerebral oxygen delivery between individuals with ESKD and age-matched controls by taking into account both CBF, as measured via phase-contrast magnetic resonance imaging, as well as serum hemoglobin concentration. Despite having greater resting CBF (∼76 vs. 59 mL·min−1·100 g−1), cerebral oxygen delivery still remained 15% lower in ESKD due to decreased oxygen carrying capacity. While correction of anemia via erythropoiesis-stimulating agents increases hemoglobin and thereby reduces CBF in ESKD (50, 52, 101), cerebral oxygen delivery still remains compromised (50), likely since hemoglobin levels are not fully corrected with current treatment targets. Specifically, guidelines for anemia management recommend only partial correction of hemoglobin to 10–11.5 g/dL (120), since normalization is associated with an increased risk of adverse cardiovascular events, including myocardial infarction, stroke, and death (115). One small study did explore the effects of complete correction of anemia on CBF in patients with ESKD and observed that correction from 9.8 to 14.2 g/dL normalized CBF to levels observed in health and increased cerebral oxygen delivery (86). Therefore, while it may be physiologically possible to restore cerebral oxygen delivery through aggressive treatment of anemia, the increased cardiovascular risks associated with complete normalization of anemia precludes such an approach as a strategy to increase cerebral oxygenation in ESKD.
Animal models and in vitro studies have also suggested that other mechanisms may impair cerebral oxygen delivery independent of anemia in CKD. These include uremic toxin-induced oxidative stress (17, 121) and chronic inflammation (61), and the reader is referred to several comprehensive reviews on this topic (23, 69). Human data are currently lacking, however, and it appears that anemia is the primary mechanism mediating decreased cerebral oxygenation in humans with ESKD. Overall in the resting state, the brain in ESKD remains overperfused, yet underoxygenated (53, 59, 80, 103), and this may contribute to the increased incidence of cerebrovascular disease and cognitive dysfunction.
CBF DURING HD
Although it may be difficult to isolate the effects of HD from the consequences of ESKD, several studies have suggested that the HD procedure itself may exert deleterious effects on the brain. Most studies have suggested that CBF declines 7–22% during HD (27, 41, 44, 48, 100, 101, 117, 119). In the resting state, compensatory mechanisms can partially oppose the reduction in cerebral oxygenation by increasing oxygen delivery and oxygen extraction. This is accomplished through increased cardiac output (34), a greater resting CBF (51, 60, 67, 130, 134), and a rightward shift in the oxyhemoglobin disassociation curve (88, 89). During HD, there may be limited reserve to tolerate further reductions in cerebral oxygenation that occur with declining CBF, given lower resting values. Additionally, the ability to increase oxygen extraction may be lost with increasing disease severity due to cerebral microcirculatory dysfunction. Cerebral small vessel disease presents in the early stages of CKD (56) and is correlated with dialysis vintage in ESKD (105). Several investigations have reported intradialytic reductions in regional cerebral oxygen saturation (75, 77, 99), which is particularly problematic since cerebral oxygenation is already depressed at rest (53, 59, 80). It has been proposed that repeated bouts of HD may result in cerebral “stunning” (41, 85) similar to the HD-induced myocardial stunning that has been well documented (20, 30, 84). Myocardial stunning is defined as a reversible, postischemic, mechanical dysfunction of the myocardium that persists after reperfusion (16). Cerebral stunning was first described in relation to ischemic stroke and refers to the absence of an immediate clinical improvement following early recanalization followed by delayed improvement in the following months (4). This term has only recently been extended to HD, and a clear definition is currently lacking. In the context of HD and specifically this review, we define “cerebral stunning” as damage to cerebral tissue that occurs with intradialytic reductions in cerebral oxygen delivery. Just as myocardial stunning is associated with mechanical dysfunction of the heart, cerebral stunning has been associated with intradialytic cognitive dysfunction (41). It is currently unclear if cerebral stunning is reversible, but a progressive deterioration of cognitive function is exhibited in patients with ESKD who remain on HD, while renal transplant recipients exhibit improvements in cognition (41). This suggest that some degree of reversibility is present, but it remains unclear what strategies other than renal transplantation may also reverse tissue damage and the resulting deficits. Findlay et al. (41) demonstrated that intradialytic reductions in CBF are related to cognitive impairments. Prohovnik et al. (103) observed significant cerebral atrophy in patients with ESKD that was strongly related to dialysis vintage. Collectively, these studies suggest that frequent occurrences of cerebral stunning with HD may contribute to progressive cerebral injury over time in patients undergoing HD (41).
MECHANISMS CONTRIBUTING TO INTRADIALYTIC DECLINES IN CBF
HD presents a unique stress to cerebrovascular function. The HD procedure provides clearance of uremic toxins, correction of electrolytes and acidosis, and ultrafiltration to remove excess fluid and is usually delivered 3–4 times/wk for 3–4 h/session. CBF is tightly regulated through the integrated response of several key physiological variables, including cerebral perfusion pressure, arterial Pco2 () neuronal activity, and sympathetic outflow (3, 132). Overall, studies have shown that CBF decreases during HD (27, 41, 44, 48, 100, 101, 117, 119), and this is likely due to a reduction in cerebral perfusion pressure. However, the potential effect of intradialytic changes in , the other primary regulator of CBF, on changes in CBF during HD remain unknown but could potentially act to oppose reductions in CBF induced by reduced cerebral perfusion pressure.
Cerebral Perfusion Pressure
Cerebral perfusion pressure is the net pressure gradient that drives oxygen delivery to the brain and is calculated as mean arterial pressure (MAP) – intracranial pressure (ICP). HD acutely induces changes in both MAP and ICP. MAP decreases during HD due to a number of factors including decreased blood volume and cardiac output. The temporal pattern of MAP decline is not linear, with the greatest reductions occurring during the first quarter of treatment followed by a more gradual decline (32). The magnitude of the MAP change is variable and dependent on multiple factors, including predialysis weight (107), antihypertensive medications (32), plasma osmolality (82), and ultrafiltration rate (43). Intradialytic hypotension occurs in ∼17% of HD treatments (110) and is independently associated with mortality (26) and cognitive dysfunction (8).
While ICP monitoring has not been performed during routine, outpatient HD due to its invasive nature, studies have suggested that ICP increases during HD. A retrospective analyses of patients with ESKD with comorbid acute brain injury receiving either HD or continuous renal replacement therapy within an intensive care unit showed that ICP increases by ∼9 mmHg during HD (74). This finding is supported by several case reports of patients with ESKD with various brain injuries that also show intradialytic increases in ICP ranging from 5 to 20 mmHg (15, 37, 74). Although it is difficult to extrapolate these findings to patients with ESKD without preexisting brain injury, one investigation measured changes in intraocular pressure as a surrogate for ICP and reported an intradialytic increases of ∼54% (116). Collectively, these studies suggest that ICP increases during HD and that this may exacerbate reductions in cerebral perfusion pressure.
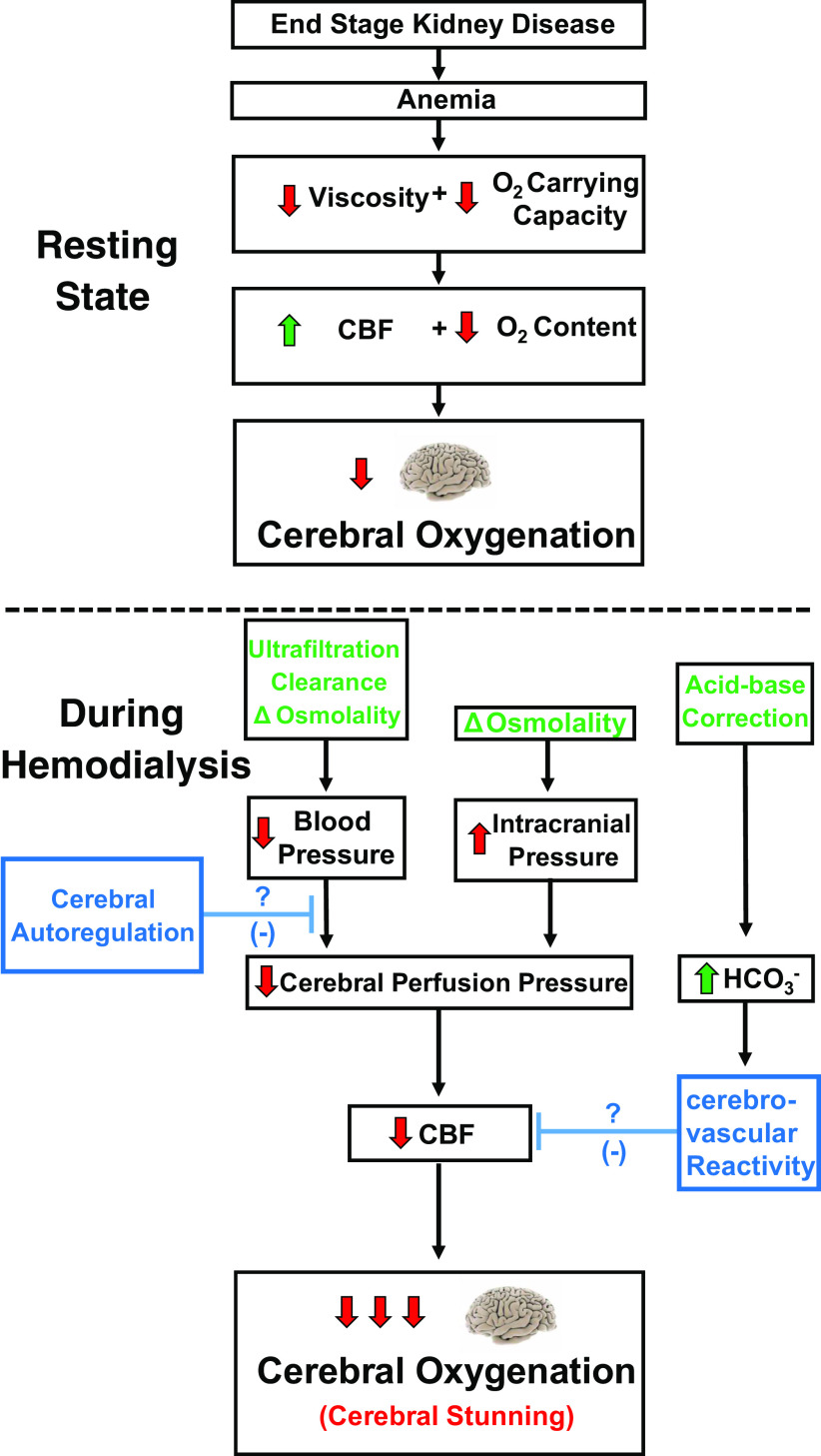
The mechanism through which ICP increases is thought to be related to the rapid osmotic shifts associated with removal of urea and other organic solutes during HD. Historically, two hypotheses have been proposed to explain this phenomenon. The “reverse urea hypothesis” is based on the premise that HD removes urea faster from blood than it does from the central nervous system (e.g., brain and cerebrospinal fluid). This hypothesis is supported by the observation that central nervous system urea concentration is slightly less than blood urea concentration at the beginning of HD but significantly greater than blood urea concentration following HD (113, 114). This shift in concentration gradients facilitates the net movement of water into the brain. Conversely, the “idiogenic osmoles” hypothesis contends that organic osmolytes are produced within the brain and is supported by animal experiments that have reported elevated brain osmolality following HD that cannot be accounted for by changes in urea (7). In either case, the influx of water into the brain increases ICP, and this underscores a key mechanism contributing to the development of cerebral edema and dialysis disequilibrium syndrome, a rare but serious neurological complication of HD (6, 47, 87). In summary, decreases in cerebral perfusion pressure during HD are mediated through reductions in MAP and increases in ICP and this may comprise one key mechanism contributing to intradialytic reductions in CBF (Fig. 1).
Fig. 1.
Mechanisms contributing to intradialytic “cerebral stunning.” Cerebral oxygenation is compromised in end-stage kidney disease at rest due to anemia (top). Cerebral oxygenation is further challenged during hemodialysis through reductions in cerebral blood flow (CBF) mediated through reduced cerebral perfusion pressure (bottom). Both decreases in blood pressure and increases in intracranial pressure promote reductions in cerebral perfusion pressure. Cerebral autoregulation may oppose reductions in cerebral perfusion pressure through attenuating the influence of reduced arterial pressure on CBF. Cerebrovascular reactivity to CO2 may also attenuation intradialytic reductions in CBF through increases in arterial Pco2 associated with the influx of bicarbonate.
Blood Gas Changes
While decreasing cerebral perfusion pressure drives declines in CBF, reductions in cerebral perfusion pressure may be partially offset by increases in that result from bicarbonate influx during HD. Almost all patients with ESKD exhibit some degree of metabolic acidosis (104), and this is corrected during HD via supraphysiological concentrations of bicarbonate in the dialysate (127), typically 32–39 mmol/L (13). The resulting influx of bicarbonate produces a metabolic alkalosis that in turn leads to compensatory respiratory hypoventilation with CO2 retention. In addition, bicarbonate is converted to CO2, leading to more hypercarbia. While individual ion diffusion across the blood-brain barrier is slow, this newly formed CO2 permeates the blood-brain barrier rapidly and decreases pH within the extracellular space surrounding cerebral vessels. Cerebral vessels are extremely sensitive to pH, so this should facilitate cerebral vasodilation (68). While it is unclear how bicarbonate infusion affects CBF in ESKD, the intradialytic bicarbonate load may potentially lead to an increase in CBF that could offset pressure-driven reductions in CBF during HD.
There is conflicting literature regarding whether and to what extent changes during dialysis. Some investigations have reported no change in (48, 50, 100, 103, 124), whereas others have suggested that increases (5, 98, 117) or even decreases (101). One potential explanation for the disparity between studies could be related to the timing of sampling. The majority of studies have only compared values between the start and end of HD. Two studies that sampled 1 h into HD reported an elevation of 3 mmHg that returned to baseline by the end of treatment (5, 98). Although this change may seem negligible, studies performed in healthy humans have suggested that a 1-mmHg increase in above normal levels is associated with a 3–6% increase in CBF (132). Thus, this increase in may be physiologically significant.
Additional considerations that influence responses are related to control of ventilation, specifically chemoreflex sensitivity, and lung function. Chemoreflex sensitivity has been reported to be diminished in ESKD (19), and comorbid conditions may further influence the response. Beecroft et al. (14) observed enhanced chemoreflex sensitivity in patients with ESKD with comorbid sleep apnea. Two studies performed in patients with ESKD with comorbid chronic obstructive pulmonary disease reported intradialytic increases in of roughly twice the magnitude observed in ESKD-matched controls (5, 98). To the best of our knowledge, no investigations have continuously measured directly, end-tidal CO2 (as a surrogate for ) continuously throughout HD, or have sampled at an earlier time point than 1 h from the start of HD, so it is possible that peak increases in may occur even earlier. Collectively, these studies suggest that increases during the first hour of HD and returns to baseline by the end of treatment. This timing coincides with the greatest reductions in MAP, which also occur during the first hour (32). The influence of hypercapnia during HD may be one mechanism through which pressure-driven reductions in CBF are opposed during the dynamic first hour of HD.
RISK FACTORS FOR INTRADIALYTIC CEREBRAL ISCHEMIA
Several studies have related changes in CBF to clinical parameters that are routinely monitored during HD. Ultrafiltration volume (48, 119), ultrafiltration rate (41, 100), arterial pressure (27), intradialytic weight change (48), and blood pH (100) have all been associated with intradialytic decreases in CBF. Many of these observations may be interrelated when considering their underlying cause. For example, patients with greater interdialytic weight gain require more aggressive fluid removal rates. Increasing ultrafiltration volume may lead to greater reductions in blood pressure, all of which have independently been correlated with intradialytic reductions in CBF. Other studies have sought to complement these routine measurements with more direct monitoring of cerebral oxygenation. MacEwen et al. (75) obtained continuous blood pressure and near infrared spectroscopy-derived frontal lobe cerebral oxygenation recordings in a cohort of patients undergoing thrice weekly HD over a period of 4 wk. Cerebral ischemia, defined as a drop in frontal lobe cerebral oxygenation of 15%, occurred in ∼24% of HD sessions, and linear modeling indicated that each 10-mmHg reduction in MAP was associated with a 4% increase in the incidence of cerebral ischemia. Overall, however, the use of MAP thresholds for identifying the presence of cerebral ischemia yielded poor sensitivity and specificity, suggesting that MAP is not the optimal surrogate for cerebral oxygenation in HD. A strength of these approaches is that the predictive variables are readily accessible and routinely monitored during HD (e.g., ultrafiltration rate and blood pressure). They are limited, however, because they do not account for cerebrovascular control mechanisms, notably cerebral autoregulation and cerebrovascular reactivity to CO2 (CVR) that may be altered in ESKD.
CEREBROVASCULAR REGULATORY MECHANISMS ENGAGED DURING HD
While the hemodynamic responses to HD have been well characterized, much less is known in regard to how key cerebrovascular regulatory mechanisms respond to these changes. The two primary mechanisms regulating CBF are cerebral autoregulation and CVR. Autoregulation ensures that CBF remains relatively constant over a wide range of perfusion pressures, while CVR refers to the ability of cerebral vessels to dilate and constrict in response to hyper- and hypocapnia, respectively. Whether cerebral autoregulation or reactivity are impaired in ESKD at rest or during HD is unclear. The function and interaction of these key cerebrovascular regulatory mechanisms in response to intradialytic changes in blood pressure and will ultimately determine the integrated cerebrovascular response (Fig. 1).
Cerebral Autoregulation
Cerebral autoregulation refers to the ability of cerebral vessels to regulate a constant perfusion over a wide range of arterial pressures. Increases in blood pressure above the upper limit of this range will result in cerebral hyperperfusion, while reductions below the lower limit may lead to syncope. Surprisingly, there have been few investigations that have studied cerebral autoregulation in ESKD. Although one animal study demonstrated impaired flow-induced vasoconstriction in cerebral vessels excised from uremic rats (92), cross-sectional human studies comparing cerebral autoregulation in ESKD relative to controls are lacking. Castro et al. (21) reported impairments in autoregulation in patients with CKD following acute stroke, but it remains unclear whether autoregulation was impaired before the stroke onset. The few studies that have been performed in ESKD have focused on the effects of HD on autoregulation and have not studied it in the interdialytic period compared with a healthy control group for baseline comparisons (112, 117). MacEwen et al. (75) quantified cerebral autoregulation from continuous MAP and cerebral oxygenation data by individually modeling the MAP-cerebral oxygenation relationship for each participant in a sample of 635 HD treatments administered to 58 patients with ESKD. This investigation revealed impaired autoregulation in ∼32% of patients and further reported that the lower limits of autoregulation was found to be highly variable (mean = 75 ± 18 mmHg) (75). This variability may be related to duration and severity of hypertension within the population. It has been suggested that the autoregulatory curve is shifted to the right in hypertension, to protect the brain from cerebral hyperperfusion, at the expense of rendering it more susceptible to hypoperfusion (12, 62, 122). Although speculative, it is possible that patients with ESKD may similarly exhibit a rightward shift in the lower limit of autoregulation since many of them also have long-standing hypertension. Alternatively, it is possible that the regular performance of HD itself may induce cerebrovascular adaptations that protect against cerebral hypoperfusion. For instance, one investigation compared intradialytic changes in CBF between patients with ESKD with a dialysis vintage of 2–5 yr to that of patients with acute kidney injury requiring dialysis for the first time (106). Although both groups exhibited similar hemodynamic responses, CBF decreased to a greater extent in the acute kidney injury group relative to ESKD. Collectively, these findings suggest that autoregulation is altered in ESKD and that the lower limits of autoregulation are highly variable within this population.
Cerebrovascular Reactivity to CO2
The few studies that have assessed CVR in ESKD have reported conflicted findings. Ishida et al. (58) observed no difference in CVR between nondiabetic patients with ESKD and controls who were undergoing surgery for non-neurological issues. The use of isoflurane anesthesia may have confounded these findings, however, since this anesthetic dilates cerebral vessels (81) and can influence CVR (93). Conversely, Szpryger et al. (125) observed enhanced CVR in children with advanced CKD who were not yet on HD. Only the hypocapnic response to CO2 was assessed in this investigation, so it remains unclear whether hypercapnia-induced vasodilation (as would be expected to occur during HD) is also affected. Kuwabara et al. (67) reported impaired CVR in ESKD that was related to the degree of anemia and speculated that this may be because cerebral arteries are already near maximal dilation at rest, due to decreased cerebral oxygen delivery. Finally, Skinner et al. (117) reported no change in CVR pre-HD versus post-HD but did not include a control group for baseline comparisons. Based on the available evidence, it currently remains unclear whether, and to what extent, alterations in CVR may influence intradialytic CBF responses. Future work should clarify the role of CVR in mediating CBF changes during HD and how bicarbonate shifts (and subsequent changes in ) may modify this response.
PUTTING IT ALL TOGETHER: INTERACTION BETWEEN REGULATORY MECHANISMS
Many investigations seek to study a single cerebrovascular control mechanism (i.e., autoregulation or reactivity) in isolation. It is likely that both mechanisms rely on the same vascular reserve, so it is equally important to consider their interaction. Specifically, there is a limit to maximal vessel dilation, and autoregulation and CVR both draw from this same reserve (the difference between resting vessel diameter and maximal dilation) to promote increases in CBF in the face of hypotension and hypercapnia, respectively. Considering how these regulatory mechanisms interact may be especially important during HD since multiple parameters change simultaneously. For example, cerebral autoregulation is known to be attenuated in hypercapnia (1, 76, 97), so it is plausible that autoregulation may be blunted during the first hour of HD when is increasing. Additionally, CVR is attenuated during hypotension (46); therefore, rapid MAP reductions may inhibit the ability of intradialytic hypercapnia to oppose pressure-driven reductions in CBF. Finally, decreased MAP and increased promote activation of the sympathetic nervous system via the baro- and chemoreflexes, respectively, and this may independently modulate CBF. The direct role of the sympathetic nervous system on cerebrovascular tone has historically been contentious (123, 129), and few studies have explored this within the context of renal disease. Animal studies have shown decreased sympathetic innervation of cerebral arteries in renal hypertensive rats (109), so it is possible that the direct sympathetic contribution to CBF regulation may be blunted in ESKD. Conversely, CKD is characterized by chronic sympathetic overactivity (45, 66, 95); increased sympathetic tone may extend to the brain and serve to restrain chronic hyperperfusion at the level of pial arteries and arterioles. Additionally, there are variable and significant changes in autonomic function with antihypertensive medication use in CKD; for example, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers reduce sympathetic activity, while dihydropyridine Ca2+ channel blockers and thiazide diuretics increase sympathetic activity (71, 72, 91). It remains unknown how treatments that affect sympathetic tone may in turn modulate cerebrovascular control in CKD. One potential mechanism through which the autonomic nervous system may interact with cerebrovascular control is through the baroreflex, since both mechanisms should oppose intradialytic reductions in CBF. Tzeng et al. (128) observed an inverse relationship between baroreflex sensitivity and cerebral autoregulation in healthy humans. Whether this interaction persists in ESKD is unknown, but it is possible that patients with ESKD with impaired autoregulation may compensate through enhanced baroreflex sensitivity. Whether baroreflex sensitivity is impaired in ESKD and CKD is unclear, and studies have shown conflicting results. Zanotto et al. (133) observed impaired baroreflex function in ESKD with a history of falls, whereas Converse et al. (28) observed intact baroreflex sensitivity in patients with ESKD without comorbid diabetes. Additionally, there may be some degree of plasticity in baroreflex sensitivity that can be modulated by the dialysis modality. For example, one study observed improved baroreflex sensitivity in patients with ESKD who transitioned from traditional HD to nocturnal HD, which is typically performed for longer durations (8 h) and more frequently (6 days/wk) (22). Furthermore, ∼15% of patients undergoing HD exhibit a paradoxical increase in blood pressure during dialysis, termed intradialytic hypertension (57), which is likely due to an enhanced cardiopulmonary reflex sensitivity (96). Based on the variability of baroreflex sensitivity in ESKD, it is possible that this reflex may also interact with cerebral autoregulation, as has been observed in health (128). Future work should aim to clarify the interaction between cerebrovascular and autonomic regulatory mechanisms in ESKD, as this may provide novel insight into the mechanisms underlying cerebrovascular dysregulation.
Although the consideration of these cerebrovascular control mechanisms may aid in the pursuit of predicting intradialytic cerebral ischemia, it is unlikely that their routine assessment will be feasible in the outpatient HD setting. Patients with ESKD exhibit considerable heterogeneity in regard to comorbidities, dialysis vintage, medication use, nutritional status, and etiology of renal disease. Although speculative, it is possible that heterogeneity in the function and sensitivity of key cerebrovascular control mechanisms may also be observed in ESKD. Understanding how cerebrovascular regulation may be influenced by these various patient factors (e.g., comorbid conditions) may allow for the identification of general patterns of dysregulation that are present within specific ESKD subsets. Combining this information with knowledge of which modifiable variables are most closely related to intradialytic declines in CBF could collectively allow for better prediction and perhaps prevention of intradialytic cerebral ischemia. Patients with impaired cerebral autoregulation, for example, may be at an even higher risk of cerebral stunning during HD, and consideration may be made to switching to other dialysis modalities (i.e., peritoneal dialysis) that use lower filtration rates and a longer dialysis duration. Although no studies to date have directly compared intradialytic cerebrovascular responses between these different dialysis modalities, Papadopoulos et al. (94) observed greater resting cerebral oxygenation in patients undergoing peritoneal dialysis compared with patients undergoing HD, so it is possible that peritoneal dialysis may be less detrimental to the brain. Finally, even if cerebrovascular regulatory mechanisms are capable of attenuating intradialytic declines in CBF, it is important to remember that the oxygen content of the delivered blood is still reduced due to anemia. This preservation of blood flow, although desirable, does not completely correct the problem, as evidenced by lower cerebral oxygenation in ESKD at rest (59, 80, 103). Nonetheless, intradialytic reductions in cerebral perfusion have been linked to functional deficits (i.e., cognitive dysfunction) (41), and hemodynamic instability during HD is associated with brain structural abnormalities (i.e., white matter damage) (35). This suggests that intradialytic reductions in cerebral perfusion are maladaptive and contribute to the cerebrovascular disease burden in ESKD. More work is needed to clarify the role of these key cerebrovascular control mechanisms in opposing intradialytic reductions in CBF to inform the development of treatment targets to decrease cerebral ischemia and improve cerebrovascular and cognitive outcomes in patients undergoing HD.
FUTURE DIRECTIONS
There are a number of future directions that should be explored to further understand CBF regulation in renal disease and particularly during HD. First, the potential role of sex differences is currently unknown. Compared with men, women generally have lower hemoglobin and higher CBF (38, 108). These sex differences in hemoglobin persist throughout all stages of CKD (55), and female patients undergoing HD receiving erythropoietin stimulation agents require a higher dose than male patients undergoing HD to maintain target hemoglobin (29). Based on these differences in oxygen carrying capacity, it is possible that women may be more susceptible to intradialytic reductions in cerebral oxygenation and subsequent cerebral stunning. On the other hand, several studies have reported enhanced cerebral autoregulation in women compared with men (31, 39). If so, women may be better equipped to oppose intradialytic reductions in cerebral blood compared with their male counterparts. Future work should clarify the role of sex differences in CBF regulation in ESKD, particularly as it relates to the preservation of cerebral oxygenation during HD. Additionally, the role of CKD-specific pathologies (i.e., uremic toxin-induced oxidative stress) in modulating cerebrovascular regulatory mechanisms should be explored. Although the influence of these factors on resting CBF may be masked by the effects of anemia, which promotes a net increase in CBF, it is possible that the deleterious effects of uremia may impair the ability of vessels to respond to stimuli such as decreased arterial pressure (i.e., autoregulation) and increased (i.e., CVR). One human study demonstrated that infusion of the uremic toxin asymmetric dimethylarginine decreased CBF in healthy humans (65); thus, it is possible that the accumulation of these toxins may similarly mediate cerebrovascular dysfunction in CKD. Finally, the direct relationship between renal disease, cerebrovascular regulatory mechanisms, and the development of dementia and Alzheimer’s disease should be explored, since a dysregulation of CBF may be a shared mechanism common to these conditions.
PERSPECTIVES AND SIGNIFICANCE
HD represents a unique stress to the cerebrovasculature with the net response being a decrease in CBF. Although patients with ESKD exhibit cerebral hyperperfusion, intradialytic reductions in CBF are deleterious since cerebral oxygen delivery is already compromised. Cerebral stunning frequently occurs in HD, and this may be related to the increased cerebrovascular and cognitive disease burden observed in this population. Key cerebrovascular control mechanisms are engaged to oppose intradialytic ischemia; however, the efficacy and interaction of these mechanisms during HD is poorly understood. Understanding how cerebrovascular regulatory mechanisms are engaged in HD may aid in the prediction and prevention of cerebral ischemia.
GRANTS
This work was supported by National Institutes of Health Grants R01HL135183 (PI: J.P.). R61AT10457 (PI: J.P.), and F32HL147547 (PI: J.D.S.) as well as the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Studies Center (Decatur, GA).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.D.S. conceived and designed research; J.D.S. prepared figures; J.D.S. drafted manuscript; J.D.S., J.N., I.H., W.C.O., J.L.B. and J.P. edited and revised manuscript; J.D.S., J.N., I.H., W.C.O., J.L.B. and J.P. approved final version of manuscript.
REFERENCES
- 1.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke 20: 45–52, 1989. doi: 10.1161/01.STR.20.1.45. [DOI] [PubMed] [Google Scholar]
- 3.Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol 296: R1473–R1495, 2009. doi: 10.1152/ajpregu.91008.2008. [DOI] [PubMed] [Google Scholar]
- 4.Alexandrov AV, Hall CE, Labiche LA, Wojner AW, Grotta JC. Ischemic stunning of the brain: early recanalization without immediate clinical improvement in acute ischemic stroke. Stroke 35: 449–452, 2004. doi: 10.1161/01.STR.0000113737.58014.B4. [DOI] [PubMed] [Google Scholar]
- 5.Alfakir M, Moammar MQ, Ali MI, Alhatem E, Curran RD, Saoud RM, Chandran C, Khan MA, Debari VA. Pulmonary gas exchange during hemodialysis: a comparison of subjects with and without COPD on bicarbonate hemodialysis. Ann Clin Lab Sci 41: 315–320, 2011. [PubMed] [Google Scholar]
- 6.Arieff AI. Dialysis disequilibrium syndrome: current concepts on pathogenesis and prevention. Kidney Int 45: 629–635, 1994. doi: 10.1038/ki.1994.84. [DOI] [PubMed] [Google Scholar]
- 7.Arieff AI, Massry SG, Barrientos A, Kleeman CR. Brain water and electrolyte metabolism in uremia: effects of slow and rapid hemodialysis. Kidney Int 4: 177–187, 1973. doi: 10.1038/ki.1973.100. [DOI] [PubMed] [Google Scholar]
- 8.Assimon MM, Wang L, Flythe JE. Cumulative exposure to frequent intradialytic hypotension associates with new-onset dementia among elderly hemodialysis patients. Kidney Int Rep 4: 603–606, 2019. doi: 10.1016/j.ekir.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Astor BC, Muntner P, Levin A, Eustace JA, Coresh J. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988−1994). Arch Intern Med 162: 1401–1408, 2002. doi: 10.1001/archinte.162.12.1401. [DOI] [PubMed] [Google Scholar]
- 10.Avogaro A, Albiero M, Menegazzo L, de Kreutzenberg S, Fadini GP. Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care 34, Suppl 2: S285–S290, 2011. doi: 10.2337/dc11-s239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol 23: 1631–1634, 2012. doi: 10.1681/ASN.2011111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barry DI, Strandgaard S, Graham DI, Braendstrup O, Svendsen UG, Vorstrup S, Hemmingsen R, Bolwig TG. Cerebral blood flow in rats with renal and spontaneous hypertension: resetting of the lower limit of autoregulation. J Cereb Blood Flow Metab 2: 347–353, 1982. doi: 10.1038/jcbfm.1982.35. [DOI] [PubMed] [Google Scholar]
- 13.Basile C, Rossi L, Lomonte C. The choice of dialysate bicarbonate: do different concentrations make a difference? Kidney Int 89: 1008–1015, 2016. doi: 10.1016/j.kint.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Beecroft J, Duffin J, Pierratos A, Chan CT, McFarlane P, Hanly PJ. Enhanced chemo-responsiveness in patients with sleep apnoea and end-stage renal disease. Eur Respir J 28: 151–158, 2006. doi: 10.1183/09031936.06.00075405. [DOI] [PubMed] [Google Scholar]
- 15.Bertrand YM, Hermant A, Mahieu P, Roels J. Intracranial pressure changes in patients with head trauma during haemodialysis. Intensive Care Med 9: 321–323, 1983. doi: 10.1007/BF01692550. [DOI] [PubMed] [Google Scholar]
- 16.Bolli R. Mechanism of myocardial “stunning”. Circulation 82: 723–738, 1990. doi: 10.1161/01.CIR.82.3.723. [DOI] [PubMed] [Google Scholar]
- 17.Bugnicourt JM, Da Silveira C, Bengrine A, Godefroy O, Baumbach G, Sevestre H, Bode-Boeger SM, Kielstein JT, Massy ZA, Chillon JM. Chronic renal failure alters endothelial function in cerebral circulation in mice. Am J Physiol Heart Circ Physiol 301: H1143–H1152, 2011. doi: 10.1152/ajpheart.01237.2010. [DOI] [PubMed] [Google Scholar]
- 18.Bugnicourt JM, Godefroy O, Chillon JM, Choukroun G, Massy ZA. Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. J Am Soc Nephrol 24: 353–363, 2013. doi: 10.1681/ASN.2012050536. [DOI] [PubMed] [Google Scholar]
- 19.Burgess KR, Burgess EE, Whitelaw WA. Impaired ventilatory response to carbon dioxide in patients with chronic renal failure: implications for the intensive care unit. Crit Care Med 22: 413–419, 1994. doi: 10.1097/00003246-199403000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol 4: 914–920, 2009. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castro P, Serrador JM, Rocha I, Sorond F, Azevedo E. Efficacy of cerebral autoregulation in early ischemic stroke predicts smaller infarcts and better outcome. Front Neurol 8: 113, 2017. doi: 10.3389/fneur.2017.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1.Centers for Disease Control and Prevention National Chronic Kidney Disease Fact Sheet, 2017. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2017. [Google Scholar]
- 22.Chan CT, Jain V, Picton P, Pierratos A, Floras JS. Nocturnal hemodialysis increases arterial baroreflex sensitivity and compliance and normalizes blood pressure of hypertensive patients with end-stage renal disease. Kidney Int 68: 338–344, 2005. doi: 10.1111/j.1523-1755.2005.00411.x. [DOI] [PubMed] [Google Scholar]
- 23.Chelluboina B, Vemuganti R. Chronic kidney disease in the pathogenesis of acute ischemic stroke. J Cereb Blood Flow Metab 39: 1893–1905, 2019. doi: 10.1177/0271678X19866733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen YC, Su YC, Lee CC, Huang YS, Hwang SJ. Chronic kidney disease itself is a causal risk factor for stroke beyond traditional cardiovascular risk factors: a nationwide cohort study in Taiwan. PLoS One 7: e36332, 2012. doi: 10.1371/journal.pone.0036332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng BC, Chen PC, Chen PC, Lu CH, Huang YC, Chou KH, Li SH, Lin AN, Lin WC. Decreased cerebral blood flow and improved cognitive function in patients with end-stage renal disease after peritoneal dialysis: An arterial spin-labelling study. Eur Radiol 29: 1415–1424, 2019. doi: 10.1007/s00330-018-5675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou JA, Streja E, Nguyen DV, Rhee CM, Obi Y, Inrig JK, Amin A, Kovesdy CP, Sim JJ, Kalantar-Zadeh K. Intradialytic hypotension, blood pressure changes and mortality risk in incident hemodialysis patients. Nephrol Dial Transplant 33: 149–159, 2018. doi: 10.1093/ndt/gfx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung S, Jeong HS, Choi DE, Song HJ, Lim YG, Ham JY, Na KR, Lee KW. The impact of hemodialysis and arteriovenous access flow on extracranial hemodynamic changes in end-stage renal disease patients. J Korean Med Sci 31: 1239–1245, 2016. doi: 10.3346/jkms.2016.31.8.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Converse RL Jr, Jacobsen TN, Jost CM, Toto RD, Grayburn PA, Obregon TM, Fouad-Tarazi F, Victor RG. Paradoxical withdrawal of reflex vasoconstriction as a cause of hemodialysis-induced hypotension. J Clin Invest 90: 1657–1665, 1992. doi: 10.1172/JCI116037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coronado Daza JA, Cuchi GU. Gender differences in dose of erythropoietin to maintain hemoglobin target in hemodialysis patients. Indian J Nephrol 29: 160–165, 2019. doi: 10.4103/ijn.IJN_124_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dasselaar JJ, Slart RHJA, Knip M, Pruim J, Tio RA, McIntyre CW, de Jong PE, Franssen CFM. Haemodialysis is associated with a pronounced fall in myocardial perfusion. Nephrol Dial Transplant 24: 604–610, 2009. doi: 10.1093/ndt/gfn501. [DOI] [PubMed] [Google Scholar]
- 31.Deegan BM, Sorond FA, Galica A, Lipsitz LA, O’Laighin G, Serrador JM. Elderly women regulate brain blood flow better than men do. Stroke 42: 1988–1993, 2011. doi: 10.1161/STROKEAHA.110.605618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dinesh K, Kunaparaju S, Cape K, Flythe JE, Feldman HI, Brunelli SM. A model of systolic blood pressure during the course of dialysis and clinical factors associated with various blood pressure behaviors. Am J Kidney Dis 58: 794–803, 2011. doi: 10.1053/j.ajkd.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 33.Drew DA, Bhadelia R, Tighiouart H, Novak V, Scott TM, Lou KV, Shaffi K, Weiner DE, Sarnak MJ. Anatomic brain disease in hemodialysis patients: a cross-sectional study. Am J Kidney Dis 61: 271–278, 2013. doi: 10.1053/j.ajkd.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eckardt KU. Managing a fateful alliance: anaemia and cardiovascular outcomes. Nephrol Dial Transplant 20, Suppl 6: vi16–vi20, 2005. doi: 10.1093/ndt/gfh1097. [DOI] [PubMed] [Google Scholar]
- 35.Eldehni MT, Odudu A, Mcintyre CW. Brain white matter microstructure in end-stage kidney disease, cognitive impairment, and circulatory stress. Hemodial Int 23: 356–365, 2019. doi: 10.1111/hdi.12754. [DOI] [PubMed] [Google Scholar]
- 36.Ergul A, Kelly-Cobbs A, Abdalla M, Fagan SC. Cerebrovascular complications of diabetes: focus on stroke. Endocr Metab Immune Disord Drug Targets 12: 148–158, 2012. doi: 10.2174/187153012800493477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esnault P, Lacroix G, Cungi PJ, D’Aranda E, Cotte J, Goutorbe P. Dialysis disequilibrium syndrome in neurointensive care unit: the benefit of intracranial pressure monitoring. Crit Care 16: 472, 2012. doi: 10.1186/cc11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esposito G, Van Horn JD, Weinberger DR, Berman KF. Gender differences in cerebral blood flow as a function of cognitive state with PET. J Nucl Med 37: 559–564, 1996. [PubMed] [Google Scholar]
- 39.Favre ME, Serrador JM. Sex differences in cerebral autoregulation are unaffected by menstrual cycle phase in young, healthy women. Am J Physiol Heart Circ Physiol 316: H920–H933, 2019. doi: 10.1152/ajpheart.00474.2018. [DOI] [PubMed] [Google Scholar]
- 40.Fazekas G, Fazekas F, Schmidt R, Kapeller P, Offenbacher H, Krejs GJ. Brain MRI findings and cognitive impairment in patients undergoing chronic hemodialysis treatment. J Neurol Sci 134: 83–88, 1995. doi: 10.1016/0022-510X(95)00226-7. [DOI] [PubMed] [Google Scholar]
- 41.Findlay MD, Dawson J, Dickie DA, Forbes KP, McGlynn D, Quinn T, Mark PB. Investigating the relationship between cerebral blood flow and cognitive function in hemodialysis patients. J Am Soc Nephrol 30: 147–158, 2019. doi: 10.1681/ASN.2018050462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM. Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol 26: 724–734, 2015. doi: 10.1681/ASN.2014020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gottlieb D, Mildworf B, Rubinger D, Melamed E. The regional cerebral blood flow in patients under chronic hemodialytic treatment. J Cereb Blood Flow Metab 7: 659–661, 1987. doi: 10.1038/jcbfm.1987.119. [DOI] [PubMed] [Google Scholar]
- 45.Grassi G, Quarti-Trevano F, Seravalle G, Arenare F, Volpe M, Furiani S, Dell’Oro R, Mancia G. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension 57: 846–851, 2011. doi: 10.1161/HYPERTENSIONAHA.110.164780. [DOI] [PubMed] [Google Scholar]
- 46.Harper AM, Glass HI. Effect of alterations in the arterial carbon dioxide tension on the blood flow through the cerebral cortex at normal and low arterial blood pressures. J Neurol Neurosurg Psychiatry 28: 449–452, 1965. doi: 10.1136/jnnp.28.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris CP, Townsend JJ. Dialysis disequilibrium syndrome. West J Med 151: 52–55, 1989. [PMC free article] [PubMed] [Google Scholar]
- 48.Hata R, Matsumoto M, Handa N, Terakawa H, Sugitani Y, Kamada T. Effects of hemodialysis on cerebral circulation evaluated by transcranial Doppler ultrasonography. Stroke 25: 408–412, 1994. doi: 10.1161/01.STR.25.2.408. [DOI] [PubMed] [Google Scholar]
- 49.Higashi Y, Kihara Y, Noma K. Endothelial dysfunction and hypertension in aging. Hypertens Res 35: 1039–1047, 2012. doi: 10.1038/hr.2012.138. [DOI] [PubMed] [Google Scholar]
- 50.Hirakata H, Yao H, Osato S, Ibayashi S, Onoyama K, Otsuka M, Ichiya Y, Kuwabara Y, Masuda Y, Fujishima M. CBF and oxygen metabolism in hemodialysis patients: effects of anemia correction with recombinant human EPO. Am J Physiol 262: F737–F743, 1992. doi: 10.1152/ajprenal.1992.262.5.F737. [DOI] [PubMed] [Google Scholar]
- 51.Holzer H, Marguc K, Pogglitsch H, Ott E, Katschnig H. The effects of haemodialysis on cerebral blood flow. Proc Eur Dial Transplant Assoc 18: 126–132, 1981. [PubMed] [Google Scholar]
- 52.Horina JH, Fazekas F, Niederkorn K, Payer F, Valetitsch H, Winkler HM, Horner S, Freidl W, Pogglitsch H, Krejs GJ. Cerebral hemodynamic changes following treatment with erythropoietin. Nephron 58: 407–412, 1991. doi: 10.1159/000186471. [DOI] [PubMed] [Google Scholar]
- 53.Hoshino T, Ookawara S, Goto S, Miyazawa H, Ito K, Ueda Y, Kaku Y, Hirai K, Nabata A, Mori H, Yoshida I, Tabei K. Evaluation of cerebral oxygenation in patients undergoing long-term hemodialysis. Nephron Clin Pract 126: 57–61, 2014. doi: 10.1159/000358432. [DOI] [PubMed] [Google Scholar]
- 54.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab 7: 476–484, 2008. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ifudu O. Patient characteristics determining rHuEPO dose requirements. Nephrol Dial Transplant 17, Suppl 5: 38–41, 2002. doi: 10.1093/ndt/17.suppl_5.38. [DOI] [PubMed] [Google Scholar]
- 56.Ikram MA, Vernooij MW, Hofman A, Niessen WJ, van der Lugt A, Breteler MM. Kidney function is related to cerebral small vessel disease. Stroke 39: 55–61, 2008. doi: 10.1161/STROKEAHA.107.493494. [DOI] [PubMed] [Google Scholar]
- 57.Inrig JK. Intradialytic hypertension: a less-recognized cardiovascular complication of hemodialysis. Am J Kidney Dis 55: 580–589, 2010. doi: 10.1053/j.ajkd.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ishida K, Uchida M, Utada K, Yamashita A, Yamashita S, Fukuda S, Matsumoto M, Sakabe T. Cerebrovascular CO2 reactivity during isoflurane-nitrous oxide anesthesia in patients with chronic renal failure. J Anesth 32: 15–22, 2018. doi: 10.1007/s00540-017-2422-3. [DOI] [PubMed] [Google Scholar]
- 59.Ito K, Ookawara S, Ueda Y, Goto S, Miyazawa H, Yamada H, Kitano T, Shindo M, Kaku Y, Hirai K, Yoshida M, Hoshino T, Nabata A, Mori H, Yoshida I, Kakei M, Tabei K. Factors affecting cerebral oxygenation in hemodialysis patients: cerebral oxygenation associates with pH, hemodialysis duration, serum albumin concentration, and diabetes mellitus. PLoS One 10: e0117474, 2015. doi: 10.1371/journal.pone.0117474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang XL, Wen JQ, Zhang LJ, Zheng G, Li X, Zhang Z, Liu Y, Zheng LJ, Wu L, Chen HJ, Kong X, Luo S, Lu GM, Ji XM, Zhang ZJ. Cerebral blood flow changes in hemodialysis and peritoneal dialysis patients: an arterial-spin labeling MR imaging. Metab Brain Dis 31: 929–936, 2016. doi: 10.1007/s11011-016-9829-7. [DOI] [PubMed] [Google Scholar]
- 61.Jing W, Jabbari B, Vaziri ND. Uremia induces upregulation of cerebral tissue oxidative/inflammatory cascade, down-regulation of Nrf2 pathway and disruption of blood brain barrier. Am J Transl Res 10: 2137–2147, 2018. [PMC free article] [PubMed] [Google Scholar]
- 62.Jones JV, Fitch W, MacKenzie ET, Strandgaard S, Harper AM. Lower limit of cerebral blood flow autoregulation in experimental renovascular hypertension in the baboon. Circ Res 39: 555–557, 1976. doi: 10.1161/01.RES.39.4.555. [DOI] [PubMed] [Google Scholar]
- 63.Kamata T, Hishida A, Takita T, Sawada K, Ikegaya N, Maruyama Y, Miyajima H, Kaneko E. Morphologic abnormalities in the brain of chronically hemodialyzed patients without cerebrovascular disease. Am J Nephrol 20: 27–31, 2000. doi: 10.1159/000013551. [DOI] [PubMed] [Google Scholar]
- 64.Kelly D, Rothwell PM. Disentangling the multiple links between renal dysfunction and cerebrovascular disease. J Neurol Neurosurg Psychiatry 91: 88–97, 2020. doi: 10.1136/jnnp-2019-320526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kielstein JT, Donnerstag F, Gasper S, Menne J, Kielstein A, Martens-Lobenhoffer J, Scalera F, Cooke JP, Fliser D, Bode-Böger SM. ADMA increases arterial stiffness and decreases cerebral blood flow in humans. Stroke 37: 2024–2029, 2006. doi: 10.1161/01.STR.0000231640.32543.11. [DOI] [PubMed] [Google Scholar]
- 66.Klein IH, Ligtenberg G, Neumann J, Oey PL, Koomans HA, Blankestijn PJ. Sympathetic nerve activity is inappropriately increased in chronic renal disease. J Am Soc Nephrol 14: 3239–3244, 2003. doi: 10.1097/01.ASN.0000098687.01005.A5. [DOI] [PubMed] [Google Scholar]
- 67.Kuwabara Y, Sasaki M, Hirakata H, Koga H, Nakagawa M, Chen T, Kaneko K, Masuda K, Fujishima M. Cerebral blood flow and vasodilatory capacity in anemia secondary to chronic renal failure. Kidney Int 61: 564–569, 2002. doi: 10.1046/j.1523-1755.2002.00142.x. [DOI] [PubMed] [Google Scholar]
- 68.Lambertsen CJ, Semple SJ, Smyth MG, Gelfand R. H+ and pCO2 as chemical factors in respiratory and cerebral circulatory control. J Appl Physiol 16: 473−484, 1961. doi: 10.1152/jappl.1961.16.3.473. [DOI] [PubMed] [Google Scholar]
- 69.Lau WL, Huisa BN, Fisher M. The cerebrovascular-chronic kidney disease connection: perspectives and mechanisms. Transl Stroke Res 8: 67–76, 2017. doi: 10.1007/s12975-016-0499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee M, Saver JL, Chang KH, Liao HW, Chang SC, Ovbiagele B. Low glomerular filtration rate and risk of stroke: meta-analysis. BMJ 341: c4249, 2010. doi: 10.1136/bmj.c4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lijnen P, Fagard R, Staessen J, Amery A. Effect of chronic diuretic treatment on the plasma renin-angiotensin-aldosterone system in essential hypertension. Br J Clin Pharmacol 12: 387–392, 1981. doi: 10.1111/j.1365-2125.1981.tb01231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lindqvist M, Kahan T, Melcher A, Hjemdahl P. Acute and chronic calcium antagonist treatment elevates sympathetic activity in primary hypertension. Hypertension 24: 287–296, 1994. doi: 10.1161/01.HYP.24.3.287. [DOI] [PubMed] [Google Scholar]
- 73.Liu HS, Jawad AF, Laney N, Hartung EA, Furth SL, Detre JA. Effect of blood T1 estimation strategy on arterial spin labeled cerebral blood flow quantification in children and young adults with kidney disease. J Neuroradiol 46: 29–35, 2019. doi: 10.1016/j.neurad.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lund A, Damholt MB, Wiis J, Kelsen J, Strange DG, Møller K. Intracranial pressure during hemodialysis in patients with acute brain injury. Acta Anaesthesiol Scand 63: 493–499, 2019. [DOI] [PubMed] [Google Scholar]
- 75.MacEwen C, Sutherland S, Daly J, Pugh C, Tarassenko L. Relationship between hypotension and cerebral ischemia during hemodialysis. J Am Soc Nephrol 28: 2511–2520, 2017. doi: 10.1681/ASN.2016060704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maggio P, Salinet AS, Panerai RB, Robinson TG. Does hypercapnia-induced impairment of cerebral autoregulation affect neurovascular coupling? A functional TCD study. J Appl Physiol 115: 491–497, 2013. doi: 10.1152/japplphysiol.00327.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Malik J, Kudlicka J, Lachmanova J, Valerianova A, Rocinova K, Bartkova M, Tesar V. Tissue ischemia worsens during hemodialysis in end-stage renal disease patients. J Vasc Access 18: 47–51, 2017. doi: 10.5301/jva.5000630. [DOI] [PubMed] [Google Scholar]
- 78.Martens CR, Kirkman DL, Edwards DG. The vascular endothelium in chronic kidney disease: a novel target for aerobic exercise. Exerc Sport Sci Rev 44: 12–19, 2016. doi: 10.1249/JES.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Masson P, Webster AC, Hong M, Turner R, Lindley RI, Craig JC. Chronic kidney disease and the risk of stroke: a systematic review and meta-analysis. Nephrol Dial Transplant 30: 1162–1169, 2015. doi: 10.1093/ndt/gfv009. [DOI] [PubMed] [Google Scholar]
- 80.Matsukawa S, Hamada M, Mizota T. Low preoperative regional cerebral oxygen saturation in hemodialysis patients. JA Clin Rep 3: 13, 2017. doi: 10.1186/s40981-017-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matta BF, Heath KJ, Tipping K, Summors AC. Direct cerebral vasodilatory effects of sevoflurane and isoflurane. Anesthesiology 91: 677–680, 1999. doi: 10.1097/00000542-199909000-00019. [DOI] [PubMed] [Google Scholar]
- 82.Mc Causland FR, Waikar SS. Association of predialysis calculated plasma osmolarity with intradialytic blood pressure decline. Am J Kidney Dis 66: 499–506, 2015. doi: 10.1053/j.ajkd.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McGonigle RJ, Wallin JD, Shadduck RK, Fisher JW. Erythropoietin deficiency and inhibition of erythropoiesis in renal insufficiency. Kidney Int 25: 437–444, 1984. doi: 10.1038/ki.1984.36. [DOI] [PubMed] [Google Scholar]
- 84.McIntyre CW, Burton JO, Selby NM, Leccisotti L, Korsheed S, Baker CS, Camici PG. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol 3: 19–26, 2008. doi: 10.2215/CJN.03170707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McIntyre CW, Goldsmith DJ. Ischemic brain injury in hemodialysis patients: which is more dangerous, hypertension or intradialytic hypotension? Kidney Int 87: 1109–1115, 2015. doi: 10.1038/ki.2015.62. [DOI] [PubMed] [Google Scholar]
- 86.Metry G, Wikström B, Valind S, Sandhagen B, Linde T, Beshara S, Långström B, Danielson BG. Effect of normalization of hematocrit on brain circulation and metabolism in hemodialysis patients. J Am Soc Nephrol 10: 854–863, 1999. [DOI] [PubMed] [Google Scholar]
- 87.Mistry K. Dialysis disequilibrium syndrome prevention and management. Int J Nephrol Renovasc Dis 12: 69–77, 2019. doi: 10.2147/IJNRD.S165925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mitchell TR, Pegrum GD. The oxygen affinity of haemoglobin in chronic renal failure. Br J Haematol 21: 463–472, 1971. doi: 10.1111/j.1365-2141.1971.tb02707.x. [DOI] [PubMed] [Google Scholar]
- 89.Monti JP, Brunet PJ, Berland YF, Vanuxem DC, Vanuxem PA, Crevat AD. Opposite effects of urea on hemoglobin-oxygen affinity in anemia of chronic renal failure. Kidney Int 48: 827–831, 1995. doi: 10.1038/ki.1995.357. [DOI] [PubMed] [Google Scholar]
- 90.Naganuma T, Takemoto Y, Shoji T, Shima H, Ishimura E, Okamura M, Nakatani T. Factors associated with cerebral white matter hyperintensities in haemodialysis patients. Nephrology (Carlton) 17: 561–568, 2012. doi: 10.1111/j.1440-1797.2012.01596.x. [DOI] [PubMed] [Google Scholar]
- 91.Neumann J, Ligtenberg G, Klein IH, Boer P, Oey PL, Koomans HA, Blankestijn PJ. Sympathetic hyperactivity in hypertensive chronic kidney disease patients is reduced during standard treatment. Hypertension 49: 506–510, 2007. doi: 10.1161/01.HYP.0000256530.39695.a3. [DOI] [PubMed] [Google Scholar]
- 92.New DI, Chesser AM, Thuraisingham RC, Yaqoob MM. Cerebral artery responses to pressure and flow in uremic hypertensive and spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 284: H1212–H1216, 2003. doi: 10.1152/ajpheart.00644.2002. [DOI] [PubMed] [Google Scholar]
- 93.Nishiyama T, Matsukawa T, Yokoyama T, Hanaoka K. Cerebrovascular carbon dioxide reactivity during general anesthesia: a comparison between sevoflurane and isoflurane. Anesth Analg 89: 1437–1441, 1999. [DOI] [PubMed] [Google Scholar]
- 94.Papadopoulos G, Dounousi E, Papathanasiou A, Papathanakos G, Tzimas P. Cerebral oximetry values in dialyzed surgical patients: a comparison between hemodialysis and peritoneal dialysis. Ren Fail 35: 855–859, 2013. doi: 10.3109/0886022X.2013.794675. [DOI] [PubMed] [Google Scholar]
- 95.Park J, Campese VM, Nobakht N, Middlekauff HR. Differential distribution of muscle and skin sympathetic nerve activity in patients with end-stage renal disease. J Appl Physiol 105: 1873–1876, 2008. doi: 10.1152/japplphysiol.90849.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park SH, Fonkoue IT, Li Y, DaCosta DR, Middlekauff HR, Park J. Augmented cardiopulmonary baroreflex sensitivity in intradialytic hypertension. Kidney Int Rep 3: 1394–1402, 2018. doi: 10.1016/j.ekir.2018.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Perry BG, Lucas SJ, Thomas KN, Cochrane DJ, Mündel T. The effect of hypercapnia on static cerebral autoregulation. Physiol Rep 2: e12059, 2014. doi: 10.14814/phy2.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pitcher WD, Diamond SM, Henrich WL. Pulmonary gas exchange during dialysis in patients with obstructive lung disease. Chest 96: 1136–1141, 1989. doi: 10.1378/chest.96.5.1136. [DOI] [PubMed] [Google Scholar]
- 99.Polinder-Bos HA, Elting JWJ, Aries MJ, Garcia DV, Willemsen AT, van Laar PJ, Kuipers J, Krijnen WP, Slart RH, Luurtsema G, Westerhuis R, Gansevoort RT, Gaillard CA, Franssen CF. Changes in cerebral oxygenation and cerebral blood flow during hemodialysis - A simultaneous near-infrared spectroscopy and positron emission tomography study. J Cereb Blood Flow Metab 40: 328−340, 2020. doi: 10.1177/0271678X18818652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Polinder-Bos HA, García DV, Kuipers J, Elting JWJ, Aries MJH, Krijnen WP, Groen H, Willemsen ATM, van Laar PJ, Strijkert F, Luurtsema G, Slart RHJA, Westerhuis R, Gansevoort RT, Gaillard CAJM, Franssen CFM. Hemodialysis induces an acute decline in cerebral blood flow in elderly patients. J Am Soc Nephrol 29: 1317–1325, 2018. doi: 10.1681/ASN.2017101088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Postiglione A, Faccenda F, Gallotta G, Rubba P, Federico S. Changes in middle cerebral artery blood velocity in uremic patients after hemodialysis. Stroke 22: 1508–1511, 1991. doi: 10.1161/01.STR.22.12.1508. [DOI] [PubMed] [Google Scholar]
- 102.Power A. Stroke in dialysis and chronic kidney disease. Blood Purif 36: 179–183, 2013. doi: 10.1159/000356086. [DOI] [PubMed] [Google Scholar]
- 103.Prohovnik I, Post J, Uribarri J, Lee H, Sandu O, Langhoff E. Cerebrovascular effects of hemodialysis in chronic kidney disease. J Cereb Blood Flow Metab 27: 1861–1869, 2007. doi: 10.1038/sj.jcbfm.9600478. [DOI] [PubMed] [Google Scholar]
- 104.Qian Q. Acid-base alterations in ESRD and effects of hemodialysis. Semin Dial 31: 226–235, 2018. doi: 10.1111/sdi.12659. [DOI] [PubMed] [Google Scholar]
- 105.Qian Y, Zheng K, Wang H, You H, Han F, Ni J, Hou B, Chen L, Zhu Y, Feng F, Li X. Cerebral microbleeds and their influence on cognitive impairment in dialysis patients. Brain Imaging Behav, 2020. doi: 10.1007/s11682-019-00235-z. [DOI] [PubMed] [Google Scholar]
- 106.Regolisti G, Maggiore U, Cademartiri C, Cabassi A, Caiazza A, Tedeschi S, Antonucci E, Fiaccadori E. Cerebral blood flow decreases during intermittent hemodialysis in patients with acute kidney injury, but not in patients with end-stage renal disease. Nephrol Dial Transplant 28: 79–85, 2013. doi: 10.1093/ndt/gfs182. [DOI] [PubMed] [Google Scholar]
- 107.Rocha A, Sousa C, Teles P, Coelho A, Xavier E. Frequency of intradialytic hypotensive episodes: old problem, new insights. J Am Soc Hypertens 9: 763–768, 2015. doi: 10.1016/j.jash.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 108.Rodriguez G, Warkentin S, Risberg J, Rosadini G. Sex differences in regional cerebral blood flow. J Cereb Blood Flow Metab 8: 783–789, 1988. doi: 10.1038/jcbfm.1988.133. [DOI] [PubMed] [Google Scholar]
- 109.Saito A, Lee TJ. Autonomic innervation of cerebral blood vessels decreases in renal hypertensive rats. Hypertension 7: 514–518, 1985. doi: 10.1161/01.HYP.7.4.514. [DOI] [PubMed] [Google Scholar]
- 110.Sands JJ, Usvyat LA, Sullivan T, Segal JH, Zabetakis P, Kotanko P, Maddux FW, Diaz-Buxo JA. Intradialytic hypotension: frequency, sources of variation and correlation with clinical outcome. Hemodial Int 18: 415–422, 2014. doi: 10.1111/hdi.12138. [DOI] [PubMed] [Google Scholar]
- 111.Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, Gipson D, Gu H, Hirth RA, Hutton D, Jin Y, Kapke A, Kurtz V, Li Y, McCullough K, Modi Z, Morgenstern H, Mukhopadhyay P, Pearson J, Pisoni R, Repeck K, Schaubel DE, Shamraj R, Steffick D, Turf M, Woodside KJ, Xiang J, Yin M, Zhang X, Shahinian V. US Renal Data System 2019 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis 75, Suppl 1: A6–A7, 2020. doi: 10.1053/j.ajkd.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 112.Schramm P, Closhen D, Wojciechowski J, Berres M, Klein KU, Bodenstein M, Werner C, Engelhard K. Cerebrovascular autoregulation in critically ill patients during continuous hemodialysis. Can J Anaesth 60: 564–569, 2013. doi: 10.1007/s12630-013-9912-z. [DOI] [PubMed] [Google Scholar]
- 113.Silver SM. Cerebral edema after rapid dialysis is not caused by an increase in brain organic osmolytes. J Am Soc Nephrol 6: 1600–1606, 1995. [DOI] [PubMed] [Google Scholar]
- 114.Silver SM, DeSimone JA Jr, Smith DA, Sterns RH. Dialysis disequilibrium syndrome (DDS) in the rat: role of the “reverse urea effect”. Kidney Int 42: 161–166, 1992. doi: 10.1038/ki.1992.273. [DOI] [PubMed] [Google Scholar]
- 115.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D; CHOIR Investigators . Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 116.Sitprija V, Holmes JH. Preliminary observations on the change in intracranial pressure and intraocular pressure during hemodialysis. Trans Am Soc Artif Intern Organs 8: 300–308, 1962. doi: 10.1097/00002480-196204000-00061. [DOI] [PubMed] [Google Scholar]
- 117.Skinner H, Mackaness C, Bedforth N, Mahajan R. Cerebral haemodynamics in patients with chronic renal failure: effects of haemodialysis. Br J Anaesth 94: 203–205, 2005. doi: 10.1093/bja/aei016. [DOI] [PubMed] [Google Scholar]
- 118.Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One 9: e84943, 2014. doi: 10.1371/journal.pone.0084943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stefanidis I, Bach R, Mertens PR, Liakopoulos V, Liapi G, Mann H, Heintz B. Influence of hemodialysis on the mean blood flow velocity in the middle cerebral artery. Clin Nephrol 64: 129–137, 2005. doi: 10.5414/CNP64129. [DOI] [PubMed] [Google Scholar]
- 120.Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members . Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158: 825–830, 2013. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 121.Stinghen AE, Chillon JM, Massy ZA, Boullier A. Differential effects of indoxyl sulfate and inorganic phosphate in a murine cerebral endothelial cell line (bEnd.3). Toxins (Basel) 6: 1742–1760, 2014. doi: 10.3390/toxins6061742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Strandgaard S. Autoregulation of cerebral blood flow in hypertensive patients. The modifying influence of prolonged antihypertensive treatment on the tolerance to acute, drug-induced hypotension. Circulation 53: 720–727, 1976. doi: 10.1161/01.CIR.53.4.720. [DOI] [PubMed] [Google Scholar]
- 123.Strandgaard S, Sigurdsson ST. Last Word on Point:Counterpoint: Sympathetic nervous activity does/does not influence cerebral blood flow. J Appl Physiol 105: 1375, 2008. doi: 10.1152/japplphysiol.91088.2008. [DOI] [PubMed] [Google Scholar]
- 124.Symreng T, Flanigan MJ, Lim VS. Ventilatory and metabolic changes during high efficiency hemodialysis. Kidney Int 41: 1064–1069, 1992. doi: 10.1038/ki.1992.162. [DOI] [PubMed] [Google Scholar]
- 125.Szprynger K, Kwieciński J, Szczepańska M, Pierzchała K. Evaluation of cerebrovascular reactivity in children [corrected] with chronic renal failure. Pediatr Nephrol 14: 993–996, 2000. doi: 10.1007/s004670050060. [DOI] [PubMed] [Google Scholar]
- 126.Tamura MK, Pajewski NM, Bryan RN, Weiner DE, Diamond M, Van Buren P, Taylor A, Beddhu S, Rosendorff C, Jahanian H, Zaharchuk G; SPRINT Study Research Group . Chronic kidney disease, cerebral blood flow, and white matter volume in hypertensive adults. Neurology 86: 1208–1216, 2016. doi: 10.1212/WNL.0000000000002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tentori F, Karaboyas A, Robinson BM, Morgenstern H, Zhang J, Sen A, Ikizler TA, Rayner H, Fissell RB, Vanholder R, Tomo T, Port FK. Association of dialysate bicarbonate concentration with mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 62: 738–746, 2013. doi: 10.1053/j.ajkd.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tzeng YC, Lucas SJ, Atkinson G, Willie CK, Ainslie PN. Fundamental relationships between arterial baroreflex sensitivity and dynamic cerebral autoregulation in humans. J Appl Physiol (1985) 108: 1162–1168, 2010. doi: 10.1152/japplphysiol.01390.2009. [DOI] [PubMed] [Google Scholar]
- 129.van Lieshout JJ, Secher NH. Last Word on Point:Counterpoint: Sympathetic activity does/does not influence cerebral blood flow. J Appl Physiol 105: 1374, 2008. doi: 10.1152/japplphysiol.91077.2008. [DOI] [PubMed] [Google Scholar]
- 130.Vorstrup S, Lass P, Waldemar G, Brandi L, Schmidt JF, Johnsen A, Paulson OB. Increased cerebral blood flow in anemic patients on long-term hemodialytic treatment. J Cereb Blood Flow Metab 12: 745–749, 1992. doi: 10.1038/jcbfm.1992.105. [DOI] [PubMed] [Google Scholar]
- 131.Vos FE, Schollum JB, Coulter CV, Doyle TC, Duffull SB, Walker RJ. Red blood cell survival in long-term dialysis patients. Am J Kidney Dis 58: 591–598, 2011. doi: 10.1053/j.ajkd.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 132.Willie CK, Tzeng YC, Fisher JA, Ainslie PN. Integrative regulation of human brain blood flow. J Physiol 592: 841–859, 2014. doi: 10.1113/jphysiol.2013.268953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zanotto T, Mercer TH, van der Linden ML, Traynor JP, Petrie CJ, Doyle A, Chalmers K, Allan N, Price J, Oun H, Shilliday I, Koufaki P. Baroreflex function, haemodynamic responses to an orthostatic challenge, and falls in haemodialysis patients. PLoS One 13: e0208127, 2018. doi: 10.1371/journal.pone.0208127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zheng G, Wen J, Yu W, Li X, Zhang Z, Chen H, Kong X, Luo S, Jiang X, Liu Y, Zhang Z, Zhang LJ, Lu GM. Anemia rather than hypertension contributes to cerebral hyperperfusion in young adults undergoing hemodialysis: A phase contrast MRI study. Sci Rep 6: 22346, 2016. doi: 10.1038/srep22346. [DOI] [PMC free article] [PubMed] [Google Scholar]