Keywords: β-adrenergic receptor, diabetic kidney disease, electron transport chain, mitochondria, mitochondrial dynamics
Abstract
Diabetes is a prevalent metabolic disease that contributes to ∼50% of all end-stage renal disease and has limited treatment options. We previously demonstrated that the β2-adrenergic receptor agonist formoterol induced mitochondrial biogenesis and promoted recovery from acute kidney injury. Here, we assessed the effects of formoterol on mitochondrial dysfunction and dynamics in renal proximal tubule cells (RPTCs) treated with high glucose and in a mouse model of type 2 diabetes. RPTCs exposed to 17 mM glucose exhibited increased electron transport chain (ETC) complex I, II, III, and V protein levels and reduced ATP levels and uncoupled oxygen consumption rate compared with RPTCs cultured in the absence of glucose or osmotic controls after 96 h. ETC proteins, ATP, and oxygen consumption rate were restored in RPTCs treated with formoterol. RPTCs exposed to high glucose had increased phospho-dynamin-related protein 1 (Drp1), a mitochondrial fission protein, and decreased mitofusin 1 (Mfn1), a mitochondrial fusion protein. Formoterol treatment restored phospho-Drp1 and Mfn1 to control levels. Db/db and nondiabetic (db/m) mice (10 wk old) were treated with formoterol or vehicle for 3 wk and euthanized. Db/db mice showed increased renal cortical ETC protein levels in complexes I, III, and V and decreased ATP; these changes were prevented by formoterol. Phospho-Drp1 was increased and Mfn1 was decreased in db/db mice, and formoterol restored both to control levels. Together, these findings demonstrate that hyperglycemic conditions in vivo and exposure of RPTCs to high glucose similarly alter mitochondrial bioenergetic and dynamics profiles and that treatment with formoterol can reverse these effects. Formoterol may be a promising strategy for treating early stages of diabetic kidney disease.
INTRODUCTION
Diabetic kidney disease (DKD) is the leading cause of end-stage renal disease in the United States and other countries (14). DKD occurs through a variety of pathogenic processes that are in part driven by hyperglycemia and glomerular hypertension (11, 20, 23) that leads to gradual loss of kidney function and eventually progresses to end-stage renal disease.
In the early phase of DKD, hyperglycemic conditions affect mitochondrial dynamics and energy generation, leading to mitochondrial dysfunction (21, 29). Mitochondria continuously undergo fission and fusion (mitochondrial dynamics) in response to cellular and environmental signals to maintain a balanced mitochondrial homeostasis (15, 24). Studies have demonstrated that mitochondrial dynamics are altered in mouse models of DKD and that restoring the balance between fission/fusion can prevent the progression of DKD (32). Mitochondrial fission is primarily mediated by dynamin-related protein 1 (Drp1), a dynamin-related GTPase. In response to a stimulus, Drp1 is phosphorylated and translocates to the mitochondria, where it forms ring-like oligomers around the mitochondrial membrane to initiate fission (15). One of the characteristic features of mitochondrial dysfunction in DKD is excess mitochondrial fission (3, 7, 13, 16). In both in vitro and in vivo models of DKD, Drp1 phosphorylation is elevated and pharmacological inhibition of Drp1 phosphorylation results in decreased progression of DKD (3, 15). In contrast, mitochondrial fusion is mainly mediated by mitofusins 1 and 2 (Mfn1/2). During fusion, Mfn1/2 provide a docking function to promote fusion of the outer membranes of adjacent mitochondria through GTP-mediated hydrolysis (27).
The proximal tubule plays an important role in the pathogenesis of DKD (17, 30). Proximal tubules are major energy-consuming cells, are rich in mitochondria (5, 13), and are particularly susceptible to mitochondrial dysfunction. Studies have shown that expression of proteins in electron transport chain (ETC) complexes I−V and the function of these complexes is decreased in models of DKD (18, 28), leading to reduced mitochondrial oxygen consumption rates (OCR) and ATP production (28).
Our laboratory has developed a phenotypic assay to measure mitochondrial biogenesis and identified the β2-adrenergic receptor (AR) agonist formoterol as a potent inducer of mitochondrial biogenesis (4, 19, 31). Activation of the β2-AR by formoterol promotes recovery from acute kidney injury by inducing mitochondrial biogenesis in in vivo and in vitro models of kidney injury (8, 19). Furthermore, our laboratory has shown that formoterol enhances podocyte recovery from injury in a mouse model of focal segmental glomerular sclerosis (2). The goal of the present study was to determine if formoterol treatment in an early stage mouse model of DKD restores the altered expression of mitochondrial dynamics and energetics in renal proximal tubules and in renal proximal tubule cells (RPTCs) treated with high glucose.
METHODS
Animal experiments.
Male BKS.Cg-Dock7m +/+ Leprdb/J mice were obtained from The Jackson Laboratory (Bar Harbor, NE). Mice homozygous (db/db) and heterozygous (db/m) for the Leprdb mutation were housed in a room at a constant temperature of 22 ± 2°C with 12:12-h light-dark cycles. Db/m and db/db mice at 10 wk of age were treated with vehicle (0.1% DMSO) or formoterol (1 mg/kg) via intraperitoneal injection daily for 3 wk. At 13 wk, mice were euthanized and kidneys were harvested. All experiments were approved by The University of Arizona in accordance with the guidelines set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
In vitro experiments.
Female New Zealand White rabbits (1.8–2 kg) were purchased from Charles River (Oakwood, MI). RPTCs were isolated using the iron oxide perfusion method and grown in 35-mm tissue culture dishes under improved culture conditions similar to what is observed in vivo (26). The culture medium was a 1:1 mixture of DMEM-F-12 (without glucose, phenol red, or sodium pyruvate) supplemented with 15 mM HEPES buffer, 2.5 mM l-glutamine, 1 μM pyroxidine HCl, 15 mM sodium bicarbonate, and 6 mM lactate. Hydrocortisone (50 nM), selenium (5 ng/mL), human transferrin (5 μg/mL), bovine insulin (10 nM), and l-ascorbic acid-2-phosphate (50 μM) were added to fresh culture medium. Cells grown in the presence of glucose were supplemented with 17 mM d-glucose or 17 mM d-mannitol (osmotic control). Confluent RPTCs were used for all experiments.
Immunoblot analysis.
Protein was extracted from mouse renal cortical tissue and RPTC cultures using RIPA buffer (50 mM Tris·HCl, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, and 1% Triton X-100, pH 7.4). Protease inhibitor cocktail (1:100), 1 mM sodium fluoride, and 1 mM sodium orthovanadate (Sigma-Aldrich, St. Louis, MO) were added fresh before each extraction. Equal protein quantities (10 μg) were loaded onto 4–15% SDS-PAGE gels, resolved by gel electrophoresis, and transferred onto nitrocellulose or PVDF membranes (Bio-Rad). Membranes were blocked with 5% nonfat milk in Tris-buffered saline with Tween 20 and incubated overnight with primary antibody at 4°C with agitation. The primary antibodies used in these experiments include phosphorylated (p-(Drp1 (1:1,000, ab193216), Mfn1 (1:100, ab104274), Mfn2 (1:1,000, ab56889), and total oxphos rodent WB antibody cocktail (1:1,000, ab110413) (all from Abcam). Histone H3 (1:1,000, no. 4499) and GAPDH (1:1,000, no. 5174) were purchased from Cell Signaling Technology (Danvers, MA). Drp1 (1:1,000, no. 32898) was purchased from Santa Cruz Biotechnology (Dallas, TX). Membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibody before visualization using enhanced chemiluminescence (Thermo Scientific, Waltham, MA) and the GE ImageQuant LAS4000 (GE Life Sciences, Marlborough, MA). Optical density was determined using Bio-Rad Image Laboratory 6.0.
ATP measurements.
ATP levels of confluent RPTC cultures were measured using the CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega, Madison, WI) and the manufacturer’s protocol.
Analysis of oxygen consumption.
Cultured RPTCs were grown on 96-well XF96 extracellular flux analyzer plates (Seahorse Bioscience, Billerica, MA) at a cell density of 1.6 × 104 cells/well and grown in media containing 0 mM glucose, 17 mM mannitol, or 17 mM glucose for 96 h. Basal OCR was measured three times using the Seahorse Bioscience XF96e Analyzer before injection of FCCP (2 μM), rotenone (0.5 μM), or antimycin A (0.5 μM, Sigma-Aldrich) to measure OCR as previously described (4). OCR was reported as picomoles per minute, and the results were normalized as a percentage of the vehicle control (DMSO).
Statistical analysis.
All data are shown as means ± SE. One-way or two-way ANOVA followed by Tukey’s post hoc test was performed for comparisons of multiple groups. P < 0.05 was considered statistically significant. All statistical tests were performed using GraphPad Prism software (GraphPad Software, San Diego, CA).
RESULTS
Formoterol restores mitochondrial energetics and dynamics proteins in high glucose-treated RPTCs.
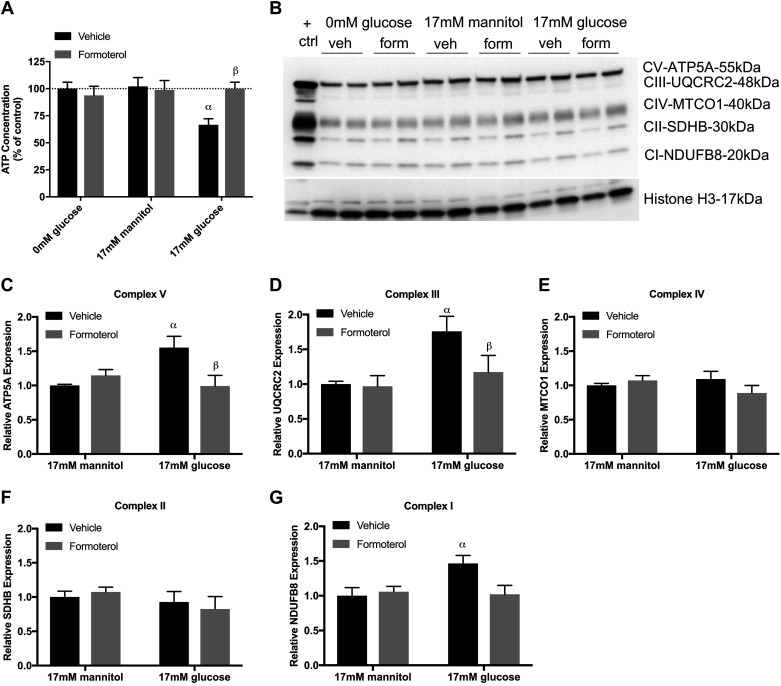
To evaluate the effects of high glucose exposure on RPTCs, we grew primary cultures of RPTCs in defined media without serum and growth factors and in no glucose, 17 mM mannitol, (osmotic control) or 17 mM glucose cotreated with either vehicle or formoterol for 24, 48, 72, or 96 h. No changes in ATP were observed at 24, 48, and 72 h in any group (data not shown) At 96 h, RPTCs grown in the absence of glucose or with mannitol had no effect on ATP levels (Fig. 1A). However, vehicle-treated RPTCs grown in high glucose decreased ATP by 30%, and treatment with formoterol restored ATP to control levels.
Fig. 1.
Formoterol (form) restores mitochondrial energetics in glucose-treated renal proximal tubule cells (RPTCs). RPTCs were grown in 0 mM glucose, 17 mM mannitol, or 17 mM glucose and cotreated with formoterol (30 nM) for 96 h. A: ATP content. B: immunoblot of total oxphos proteins. C−G: densitometry analysis of ATP5A (C), UQCRC2 (D), MTCO1 (E), SDHB (F), and NDUFB8 (G). Data are presented as means ± SE; n = 6–9. Statistical significance was determined by two-way ANOVA and Tukey-Kramer post hoc test. αP < 0.05 compared with 0 mM glucose vehicle (veh); βP < 0.05 compared with 17 mM glucose vehicle. ctrl, control.
Studies evaluating mitochondrial energetics in the context of DKD have shown that high glucose changes expression of ETC proteins (10, 28). To investigate whether high glucose leads to changes in ETC protein expression in RPTCs, we measured ETC subunits representing complexes I−V in high glucose cotreated with vehicle or formoterol for 96 h. RPTCs grown in high glucose exhibited increased ETC complex V, III, and I protein expression (Fig. 1, B–G). Formoterol treatment restored complex V and III and trended to reduce complex I protein expression to control levels.
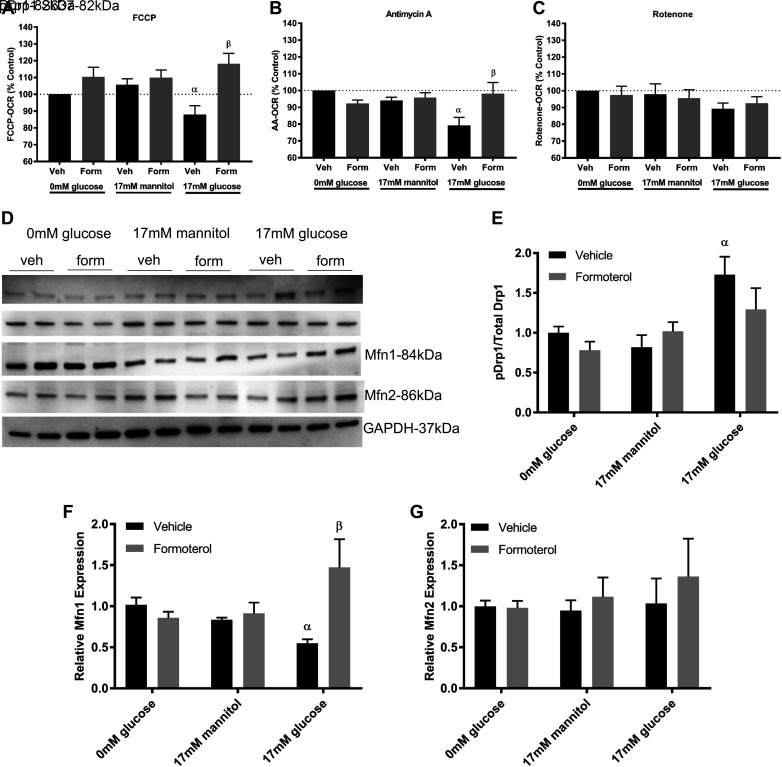
To test whether changes in RPTC ETC proteins lead to functional changes in ETC complex activity, we used the Seahorse XF96e Analyzer to measure parameters of mitochondrial function including maximal respiration and complex I and III function. High glucose decreased FCCP-uncoupled OCR, a measure of maximal ETC activity, compared with controls (Fig. 2A). Treatment with formoterol increased maximal respiration to greater than control levels. To measure complex III function, we injected antimycin A to inhibit complex III activity and measured OCR. RPTCs grown in high glucose had decreased OCR after antimycin A injection compared with controls, and cotreatment with formoterol restored OCR to control levels (Fig. 2B). Injection of the complex I inhibitor rotenone had no effect on OCR in RPTCs (Fig. 2C).
Fig. 2.
Formoterol (form) improves mitochondrial function and dynamics proteins in glucose-treated renal proximal tubule cells (RPTCs). RPTCs were grown in 0 mM glucose, 17 mM mannitol, or 17 mM glucose and cotreated with formoterol (30 nM) for 96 h. The Seahorse XF96e Analyzer was used to measure FCCP-oxygen consumption rate (OCR; A), electron transport chain (ETC) complex III [antimycin A (AA); B], and complex I (rotenone) function (C). D−G: immunoblot (D) and densitometry analysis of phosphorylated (p) dynamin-related protein 1 (Drp1) S637 to total Drp1 (E), mitofusin 1 (Mfn1) expression (F), and mitofusin 2 (Mfn2) expression (G). Data are presented as means ± SE; n = 6–8. Statistical significance was determined by two-way ANOVA and Tukey-Kramer post hoc test. αP < 0.05 compared with 0 mM glucose vehicle (Veh); βP < 0.05 compared with 17 mM glucose vehicle.
To test the effects of glucose and formoterol on mitochondrial dynamics proteins, we grew RPTCs in high glucose cotreated with either vehicle or formoterol for 96 h. High glucose increased phosphorylation of Drp1 at S637 compared with controls (Fig. 2, D and E). RPTCs cotreated with formoterol prevented the increase in Drp1 phosphorylation. In contrast, high glucose decreased expression of the mitochondrial fusion protein Mfn1, and cotreatment with formoterol restored Mfn1 (Fig. 2F). Glucose had no effect on Mfn2 expression levels (Fig. 2G).
Formoterol restores mitochondrial energetics and dynamics proteins in diabetic db/db mice.
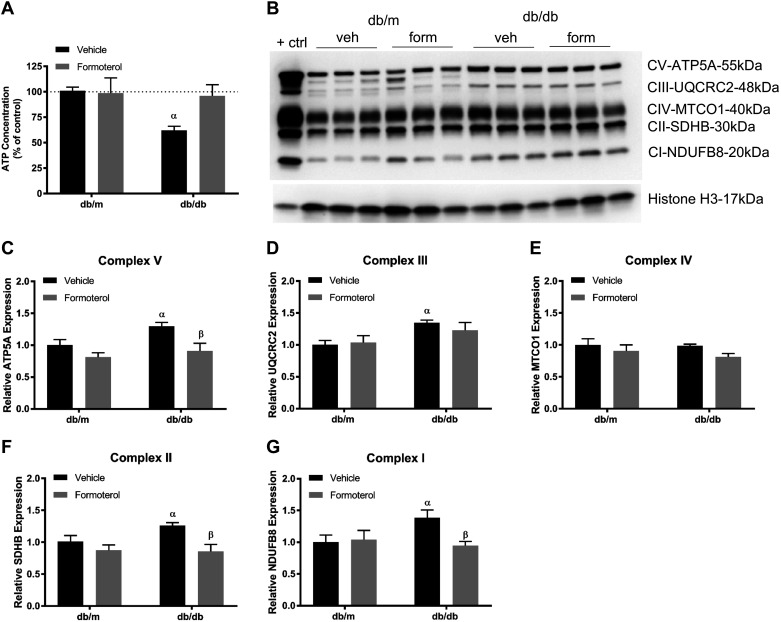
To determine if formoterol induces similar preventative effect under in vivo conditions, we tested its effects on ATP and ETC proteins. Db/m and db/db mice aged 10 wk were treated with vehicle (0.1% DMSO) or formoterol (1 mg/kg) daily for 3 wk. Db/db mice had decreased ATP compared with db/m controls, and formoterol prevented ATP loss (Fig. 3A). Vehicle-treated db/db mice had increased ETC complex subunit proteins representing complexes I, II, III, and V. Formoterol treatment maintained ETC complex I, II, and V subunits at the same level as control db/m mice (Fig. 3, B–G).
Fig. 3.
Formoterol (form) improves glucose-induced alteration of ATP content and electron transport chain proteins in db/db mice. Diabetic db/db and control (ctrl) db/m mice (10 wk of age) were treated with either vehicle (veh; 0.1% DMSO) or formoterol (1 mg/kg) daily for 3 wk. Kidneys were harvested, and renal cortical tissue was analyzed for ATP content (A), immunoblot for total oxphos proteins (B), and densitometry analysis of ATP5A (C), UQCRC2 (D), MTCO1 (E), SDHB (F), and NDUFB8 (G). Data are presented as means ± SE; n = 6. Statistical significance was determined using two-way ANOVA and Tukey-Kramer post hoc test. αP < 0.05 compared with db/m vehicle.
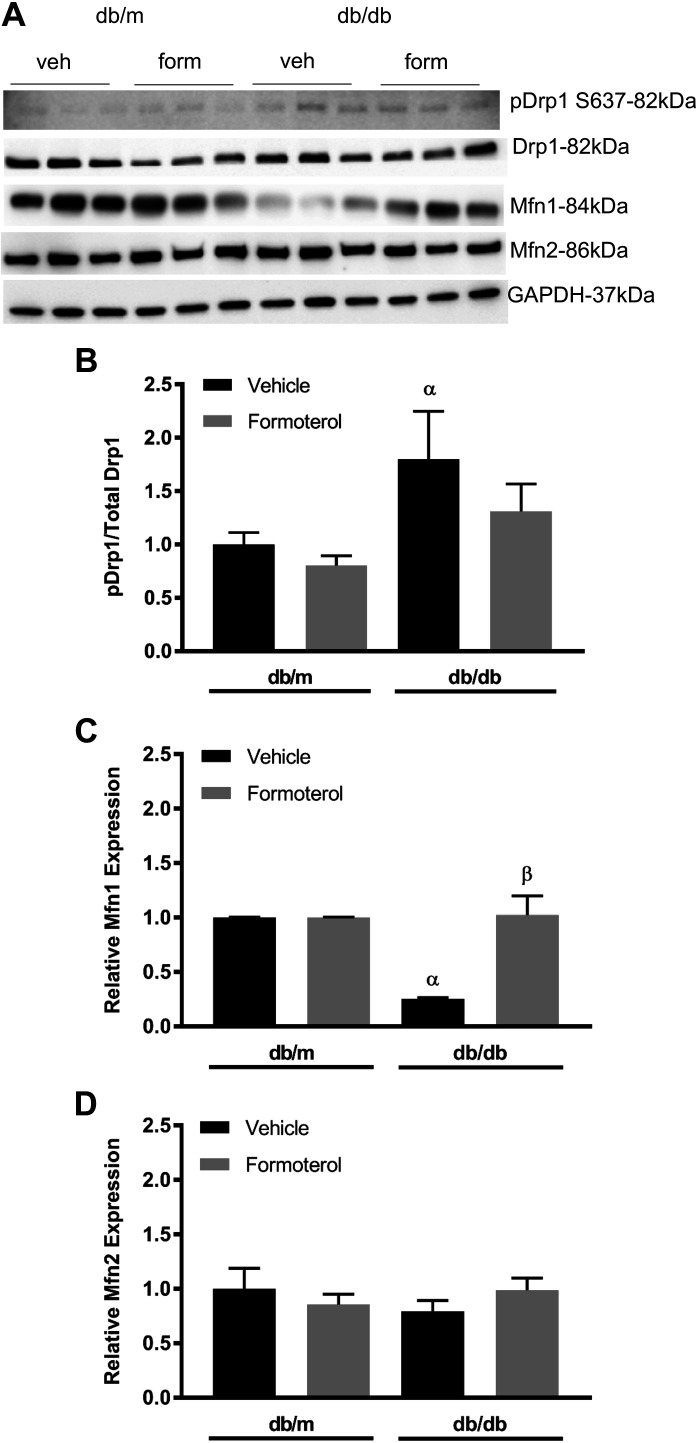
To determine if mitochondrial dynamics are altered in diabetic mice, we harvested kidneys from db/m and db/db mice at 13 wk and measured fission/fusion proteins in the renal cortex. Vehicle-treated db/db mice showed elevated phosphorylation of Drp1 S637 (Fig. 4, A and B). Diabetic mice given formoterol prevented the increase in p-Drp1. Conversely, Mfn1 was decreased compared with db/m controls, and formoterol treatment maintained Mfn1 expression at control levels (Fig. 4C). Mfn2 expression was unchanged under all conditions (Fig. 4D).
Fig. 4.
Formoterol (form) restores glucose-altered mitochondrial fission/fusion proteins in db/db mice. Diabetic db/db and control (ctrl) db/m mice (10 wk of age) were treated with either vehicle (veh; 0.1% DMSO) or formoterol (1 mg/kg) daily for 3 wk. Kidneys were harvested, and protein was extracted from renal cortical tissue. A−D: immunoblot (A) and densitometry analysis of phosphorylated (p) dynamin-related protein 1 (Drp1) S637 to total Drp1 (B), mitofusin 1 (Mfn1; C), and mitofusin 2 (Mfn2; D). Data are presented as means ± SE; n = 6. Statistical significance was determined using one-way ANOVA and Tukey-Kramer post hoc test. αP < 0.05 compared with db/m vehicle; βP < 0.05 compared with db/db vehicle.
DISCUSSION
This study investigated the long-acting β2-AR agonist formoterol as a potential therapeutic for preventing mitochondrial dysfunction in early models of DKD (1). In the kidney, β2-ARs regulate a number of physiological functions (6, 12), and the role of the β2-AR in the proximal tubule has been investigated in disease models such as acute kidney injury, where pharmacological activation of the β2-AR improved renal function after acute injury (8, 31). Prior reports of β2-AR signaling in DKD have focused on its role in inhibition of proinflammatory responses, particularly in monocytes and macrophages (25). Because of the importance of proximal tubular responses and mitochondrial dysfunction early in the pathogenesis of DKD, we investigated whether β2-AR signaling improved mitochondrial function in proximal tubules. Here, we report that formoterol maintains mitochondrial dynamics and energetics in the proximal tubule in in vitro and in vivo models of early DKD.
We have previously reported that RPTCs treated with 17 mM glucose decreased basal and uncoupled respiration after 96 h (9). Therefore, we chose 96 h as the treatment period for all RPTC experiments in this study. In the presence of glucose, phosphorylation of Drp1 at S637 increased, and formoterol prevented phosphorylation. In contrast, glucose decreased Mfn1 expression and was restored by formoterol. The mechanism by which formoterol produces these effects is unknown and will be studied in future experiments. Other studies have shown that formoterol promotes recovery from acute kidney injury by inhibiting mitochondrial fission through Drp1 phosphorylation, as well as studies that have shown that pharmacological inhibition of Drp1 in podocytes prevents the progression of DKD (3, 8). Based on these observations, we suggest that high glucose promotes a profission mitochondrial state in RPTCs that is prevented by formoterol.
RPTCs are particularly susceptible to mitochondrial dysfunction in diabetes. Studies have demonstrated that ETC complex expression and function in the renal cortex are decreased in mouse DKD models at later time points (18, 28). These changes are accompanied by decreased mitochondrial function as measured by OCR, leading to less efficient ATP production (28). However, ETC complex protein expression was elevated in our mouse model of early diabetes (before development of pathological features of DKD) as well as in RPTCs grown in high glucose. These differences can be explained by the differences in stages of DKD, where the early increase in ETC complex expression may be driven by increased glucose metabolic flux that has been demonstrated in the kidney cortex in vivo (28). Together, our results and those in prior publications suggest that there is an initial increase in oxidative phosphorylation proteins in early diabetes that then reverses during the course of DKD pathologic changes.
Despite the increase in ETC complex protein expression in our model, we found that maximal respiration was decreased in the presence of high glucose along with ETC complex III function. FCCP-OCR predicts how well ETC complexes respond to an injury or environmental stressor. Thus, the observed increases in ETC protein levels in this model could reflect a compensatory response to the reduction in ATP generation that resulted from reduced mitochondrial number or efficiency. Conversely, it is possible that the increase in ETC protein expression itself led to injury and mitochondrial dysfunction that was then reflected in the reduced OCR. Future studies will examine these possibilities and identify the mechanisms by which formoterol works to improve mitochondrial dynamics and energetics in the diabetic renal proximal tubule. A potential limitation of using formoterol is that chronic administration could lead to impairment of cardiac relaxation, leading to increased heart rate and blood pressure, as well as reduced mitochondrial protein synthesis and oxidative capacity (22). This is the first report indicating that pharmacological activation of the β2-AR by formoterol improves the disrupted mitochondrial dynamics and energetics to regulate renal mitochondrial homeostasis in two early stage models of DKD. These findings support the clinical relevance for the use of formoterol as a therapeutic agent to prevent the initiation as well as the progression of DKD.
GRANTS
This work was supported by National Institute of Environmental Health Sciences Training Grant 5T32ES00709137.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.H.C. and R.G.S. conceived and designed research; K.H.C. performed experiments; K.H.C. analyzed data; K.H.C., F.C.B., and R.G.S. interpreted results of experiments; K.H.C. prepared figures; K.H.C. drafted manuscript; K.H.C., F.C.B., and R.G.S. edited and revised manuscript; R.G.S. approved final version of manuscript.
REFERENCES
- 1.Arif E, Nihalani D. Beta2-adrenergic receptor in kidney biology: a current prospective. Nephrology (Carlton) 24: 497–503, 2019. doi: 10.1111/nep.13584. [DOI] [PubMed] [Google Scholar]
- 2.Arif E, Solanki AK, Srivastava P, Rahman B, Fitzgibbon WR, Deng P, Budisavljevic MN, Baicu CF, Zile MR, Megyesi J, Janech MG, Kwon SH, Collier J, Schnellmann RG, Nihalani D. Mitochondrial biogenesis induced by the β2-adrenergic receptor agonist formoterol accelerates podocyte recovery from glomerular injury. Kidney Int 96: 656–673, 2019. doi: 10.1016/j.kint.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayanga BA, Badal SS, Wang Y, Galvan DL, Chang BH, Schumacker PT, Danesh FR. Dynamin-related protein 1 deficiency improves mitochondrial fitness and protects against progression of diabetic nephropathy. J Am Soc Nephrol 27: 2733–2747, 2016. doi: 10.1681/ASN.2015101096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beeson CC, Beeson GC, Schnellmann RG. A high-throughput respirometric assay for mitochondrial biogenesis and toxicity. Anal Biochem 404: 75–81, 2010. doi: 10.1016/j.ab.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhargava P, Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrol 13: 629–646, 2017. doi: 10.1038/nrneph.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boivin V, Jahns R, Gambaryan S, Ness W, Boege F, Lohse MJ. Immunofluorescent imaging of beta 1- and beta 2-adrenergic receptors in rat kidney. Kidney Int 59: 515–531, 2001. doi: 10.1046/j.1523-1755.2001.059002515.x. [DOI] [PubMed] [Google Scholar]
- 7.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119: 1275–1285, 2009. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron RB, Gibbs WS, Miller SR, Dupre TV, Megyesi J, Beeson CC, Schnellmann RG. Proximal tubule β2-adrenergic receptor mediates formoterol-induced recovery of mitochondrial and renal function after ischemia-reperfusion injury. J Pharmacol Exp Ther 369: 173–180, 2019. doi: 10.1124/jpet.118.252833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Covington MD, Schnellmann RG. Chronic high glucose downregulates mitochondrial calpain 10 and contributes to renal cell death and diabetes-induced renal injury. Kidney Int 81: 391–400, 2012. doi: 10.1038/ki.2011.356. [DOI] [PubMed] [Google Scholar]
- 10.Czajka A, Malik AN. Hyperglycemia induced damage to mitochondrial respiration in renal mesangial and tubular cells: implications for diabetic nephropathy. Redox Biol 10: 100–107, 2016. doi: 10.1016/j.redox.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eirin A, Lerman A, Lerman LO. The emerging role of mitochondrial targeting in kidney disease. Handb Exp Pharmacol 240: 229–250, 2017. doi: 10.1007/164_2016_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engel G, Maurer R, Perrot K, Richardson BP. β-Adrenoceptor subtypes in sections of rat and guinea-pig kidney. Naunyn Schmiedebergs Arch Pharmacol 328: 354–357, 1985. doi: 10.1007/BF00515567. [DOI] [PubMed] [Google Scholar]
- 13.Forbes JM, Thorburn DR. Mitochondrial dysfunction in diabetic kidney disease. Nat Rev Nephrol 14: 291–312, 2018. doi: 10.1038/nrneph.2018.9. [DOI] [PubMed] [Google Scholar]
- 14.Fu H, Liu S, Bastacky SI, Wang X, Tian XJ, Zhou D. Diabetic kidney diseases revisited: a new perspective for a new era. Mol Metab 30: 250–263, 2019. doi: 10.1016/j.molmet.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galvan DL, Green NH, Danesh FR. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int 92: 1051–1057, 2017. doi: 10.1016/j.kint.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galvan DL, Long J, Green N, Chang BH, Lin JS, Schumacker P, Truong LD, Overbeek P, Danesh FR. Drp1S600 phosphorylation regulates mitochondrial fission and progression of nephropathy in diabetic mice. J Clin Invest 129: 2807–2823, 2019. doi: 10.1172/JCI127277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert RE. Proximal tubulopathy: prime mover and key therapeutic target in diabetic kidney disease. Diabetes 66: 791–800, 2017. doi: 10.2337/db16-0796. [DOI] [PubMed] [Google Scholar]
- 18.Huang C, Kim Y, Caramori ML, Moore JH, Rich SS, Mychaleckyj JC, Walker PC, Mauer M. Diabetic nephropathy is associated with gene expression levels of oxidative phosphorylation and related pathways. Diabetes 55: 1826–1831, 2006. doi: 10.2337/db05-1438. [DOI] [PubMed] [Google Scholar]
- 19.Jesinkey SR, Funk JA, Stallons LJ, Wills LP, Megyesi JK, Beeson CC, Schnellmann RG. Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J Am Soc Nephrol 25: 1157–1162, 2014. doi: 10.1681/ASN.2013090952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang H, Shao X, Jia S, Qu L, Weng C, Shen X, Wang Y, Huang H, Wang Y, Wang C, Feng S, Wang M, Feng H, Geekiyanage S, Davidson AJ, Chen J. The mitochondria-targeted metabolic tubular injury in diabetic kidney disease. Cell Physiol Biochem 52: 156–171, 2019. doi: 10.33594/000000011. [DOI] [PubMed] [Google Scholar]
- 21.Lavandero S, Chiong M, Rothermel BA, Hill JA. Autophagy in cardiovascular biology. J Clin Invest 125: 55–64, 2015. doi: 10.1172/JCI73943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Léger B, Koopman R, Walrand S, Gehrig SM, Murphy KT, Lynch GS. Chronic formoterol administration reduces cardiac mitochondrial protein synthesis and oxidative capacity in mice. Int J Cardiol 146: 270–272, 2011. doi: 10.1016/j.ijcard.2010.10.078. [DOI] [PubMed] [Google Scholar]
- 23.Lin YC, Chang YH, Yang SY, Wu KD, Chu TS. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc 117: 662–675, 2018. doi: 10.1016/j.jfma.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Mishra P, Chan DC. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Biol 15: 634–646, 2014. doi: 10.1038/nrm3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noh H, Yu MR, Kim HJ, Lee JH, Park BW, Wu IH, Matsumoto M, King GL. Beta 2-adrenergic receptor agonists are novel regulators of macrophage activation in diabetic renal and cardiovascular complications. Kidney Int 92: 101–113, 2017. doi: 10.1016/j.kint.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nowak G, Schnellmann RG. Improved culture conditions stimulate gluconeogenesis in primary cultures of renal proximal tubule cells. Am J Physiol Cell Physiol 268: C1053–C1061, 1995. doi: 10.1152/ajpcell.1995.268.4.C1053. [DOI] [PubMed] [Google Scholar]
- 27.Ryan MT, Stojanovski D. Mitofusins ‘bridge’ the gap between oxidative stress and mitochondrial hyperfusion. EMBO Rep 13: 870–871, 2012. doi: 10.1038/embor.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sas KM, Kayampilly P, Byun J, Nair V, Hinder LM, Hur J, Zhang H, Lin C, Qi NR, Michailidis G, Groop PH, Nelson RG, Darshi M, Sharma K, Schelling JR, Sedor JR, Pop-Busui R, Weinberg JM, Soleimanpour SA, Abcouwer SF, Gardner TW, Burant CF, Feldman EL, Kretzler M, Brosius FC III, Pennathur S. Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight 1: e86976, 2016. doi: 10.1172/jci.insight.86976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suárez-Rivero JM, Villanueva-Paz M, de la Cruz-Ojeda P, de la Mata M, Cotán D, Oropesa-Ávila M, de Lavera I, Álvarez-Córdoba M, Luzón-Hidalgo R, Sánchez-Alcázar JA. Mitochondrial dynamics in mitochondrial diseases. Diseases 5: 5, 2016. doi: 10.3390/diseases5010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol 300: R1009–R1022, 2011. doi: 10.1152/ajpregu.00809.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wills LP, Trager RE, Beeson GC, Lindsey CC, Peterson YK, Beeson CC, Schnellmann RG. The β2-adrenoceptor agonist formoterol stimulates mitochondrial biogenesis. J Pharmacol Exp Ther 342: 106–118, 2012. doi: 10.1124/jpet.112.191528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhan M, Usman IM, Sun L, Kanwar YS. Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. J Am Soc Nephrol 26: 1304–1321, 2015. doi: 10.1681/ASN.2014050457. [DOI] [PMC free article] [PubMed] [Google Scholar]