Abstract.
Advances in sepsis resuscitation have significantly improved shock control; however, many patients still die after septic shock reversal. We conducted a retrospective review to examine in-hospital death in whom shock was reversed and vasopressor was discontinued for 72 hours or longer. Factors independently associated with death were determined. Medical records of septic shock survivors from the medical intensive care unit of the Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, during January 2012–January 2019 were analyzed. A total of 350 septic shock patients were enrolled. Of these, 280 survived initial resuscitation. Eighty of 280 patients died, 45 died by 28 days (16.1%), and 35 (12.5%) died thereafter during their hospital stay. Multi-organ failure and hospital-acquired pneumonia (HAP) were the leading causes of death, followed by other infection and noninfectious complication. Although the death group had more laboratory derangement and required more organ support, there were four factors associated with mortality from multivariate analysis. Hospital-acquired pneumonia was the leading factor, followed by sequential organ failure assessment score and serum albumin at 72 hours after discontinuation of vasopressors, and total intravenous fluid during 72 hours after discontinuation of vasopressors. In-hospital mortality after hemodynamic restoration in patients with septic shock was substantial. Causes and contributing factors were identified. Measures to mitigate these risks would be beneficial for rendering more favorable patient outcomes.
INTRODUCTION
Septic shock is the most common cause of intensive care unit (ICU) admission worldwide. Recent guidelines consisting mainly of early diagnosis and effective goal-directed resuscitation bring about better shock control.1 Improvements in the quality of critical care and widespread utilization of new advanced technologies have also enhanced ICU outcomes. However, a significant number of patients still die following septic shock reversal.
The mortality rate during the first 24 hours of septic shock was reported to be 4.9%.2 Those who died had higher initial serum lactate and a higher modified sequential organ failure assessment (SOFA) score, which indicates failure to restore perfusion and reverse severe organ dysfunction. Death in patients who survived initial shock phase was investigated by Prescott et al.3 Nine hundred sixty sepsis patients from the overall U.S. Health and Retirement Study cohort were included. Among those patients, death at 30 days, 90 days, 1 year, and 2 years was 25.4%, 41.3%, 48.5%, and 56.5%, respectively. Mortality in these patients was higher than that in three matched cohorts consisting of adults who were not in the hospital, those who had infection without sepsis, and patients with sterile inflammation. Interestingly, death during the first half of the year after hospitalization was due to sepsis complication, and death during the second half of the year after hospitalization was due to age or preexisting underlying conditions. Long-term outcomes were described in the Cooperative Study of Corticosteroids in Systemic Sepsis.4 The risk of dying from sepsis was higher in sepsis survivors than in controls during the first year, and the risk of dying from non–sepsis-related causes continued to be higher in sepsis survivors than in non-sepsis patients with similar conditions for 5 years. Other important studies revealed significant morbidity following sepsis, including physical disability, cognitive impairment,5 cardiovascular event,6 bronchopulmonary aspiration,7 and hospital readmission with sepsis and other critical problems.8 Using this information, measures to mitigate post-sepsis mortality were proposed.9 However, the factors that influence mortality in this patient population may differ among countries and medical centers.
A study from our center in 2009 reported rates of 28-day and hospital mortality of 34.3% and 52.6%, respectively.10 Sepsis resuscitation guidelines and bundles were implemented thereafter, with substantial improvement in outcomes.11,12 However, no recommendation regarding post-shock management was established. To improve overall hospital outcomes, more information is needed specific to patient clinical status following shock reversal, and the causes of and risk factors for in-hospital death must be identified. Accordingly, the aim of this study was to investigate in-hospital death in early septic shock survivors in whom shock was reversed and vasopressor was discontinued for 72 hours or longer. The secondary objective was to identify factors independently associated with in-hospital mortality.
METHODS
We performed a medical record review of patients with septic shock who were admitted to the medical ICU and medical wards of Siriraj Hospital, which is a 2,300-bed tertiary care hospital that is affiliated with Mahidol University (Bangkok, Thailand). Data of patients who were admitted during the January 2012–January 2019 study period were retrieved and included. Septic shock was diagnosed according to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) criteria.13 Early septic shock survivors in whom shock was successfully resuscitated and vasopressors were discontinued for 72 hours or longer were included. Patients aged less than 18 years and patients who died during first resuscitation were excluded. Those who were referred from our ICU to other hospitals before day 28 and patients with terminal disease or do-not-resuscitate (DNR) order were also excluded. In patients with ICU readmission during a hospital stay, the total course was considered as one visit. The study protocol was reviewed and approved by the Institutional Review Board of the Faculty of Medicine Siriraj Hospital, Mahidol University (SIRB approval no. Si 725/2018). Exemption from obtaining written informed consent was granted because of the retrospective observational nature of our study.
Definitions.
Infection was defined as the presence of a pathogenic microorganism in sterile sites (such as blood, bronchoalveolar lavage, cerebrospinal fluid, or ascitic fluid) and/or clinically suspected infection, plus ameliorating by antibiotic administration. Sepsis was defined according to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) by infection plus organ dysfunction represented by an increase in the SOFA score of two points or more. Septic shock was clinically identified by sepsis patients requiring vasopressor to maintain a mean arterial pressure of 65 mmHg or greater, together with the serum lactate level greater than 2 mmol/L in the absence of hypovolemia.13 Starting time point was when sepsis or septic shock was diagnosed. Dependent status referred to a functional status before admission was categorized into independent, which means not depend on help for activities of daily living; partially dependent and totally dependent, which mean depend on help for activities of daily living less or more than 50%, respectively. Multiple organ dysfunction was defined as the SOFA score of at least one point for two organs or more. Catheter-related bloodstream infection (CRBSI) is defined as the presence of bacteremia originating from an intravenous catheter. The diagnosis of CRBSI is confirmed by positive of two blood cultures, according to Clinical Practice Guidelines for the Diagnosis and Management of Intravascular Catheter-Related Infection: 2009 Update by the Infectious Diseases Society of America,14 obtained before administration of antimicrobial therapy and after exclusion of alternate sources of infection. Hospital-acquired pneumonia (HAP) is defined as pneumonia that occurred 48 hours or more after admission and did not appear to be incubating at the time of admission. Ventilator-associated pneumonia (VAP) was defined as pneumonia that presented more than 48 hours after endotracheal intubation. Inadequate source control was defined as failure to achieve adequate source control by definitive surgical or interventional radiology or administration of inappropriate antibiotics of the presenting infection. Procedural complications included two cases of pneumothorax and air embolism from insertion of central venous catheter, and two cases of severe bleeding from diagnostic procedures.
Data collection.
Information obtained from medical records included age, gender, body weight/body mass index (BMI), nature of infection, dependent status, and comorbid conditions. Illness severity score (Acute Physiology and Chronic Health Evaluation-II [APACHE-II]) and SOFA scores were obtained daily from admission to day 7 after discontinuation of vasopressors for 72 hours. Clinical data including blood pressure, resuscitation fluid, and vasopressor doses on admission, at septic shock onset, at the worst time point of septic shock, and during seven consecutive days after successful discontinuation of vasopressor were recorded. Laboratory data, including white blood cell count, hemoglobin (Hb), platelet (Plt) count, absolute neutrophil count, arterial blood gas analysis, albumin (Alb), prothrombin time, total bilirubin levels, serum creatinine, blood urea nitrogen (BUN), and lactate level, were recorded.
Death in the ICU and in the hospital was reviewed for specific causes. Infection was defined using suggestive history, clinical information, and laboratory findings that led to anti-infective treatment (excluding prophylaxis). We also recorded whether the patient and the family had documented DNR orders or decisions to withhold futile treatments because those patients were excluded from analysis.
Statistical analysis.
Clinical variables were analyzed using descriptive statistics expressed as number and percentage for categorical data, and as mean ± SD or median and interquartile range (IQR) depending on whether the continuous data were normally or non-normally distributed. Comparisons between survivors and non-survivors were made on the day of admission, on the day of septic shock onset, on the worst day of shock, and on each of seven consecutive days after vasopressors were discontinued for 72 hours. Differences between continuous variables were compared using the Mann–Whitney U test for non-normal data distributions, and by independent t-test for normal data distributions. Dichotomous variables were compared using Pearson’s χ2 test or Fisher’s exact test. Factors associated with mortality in septic shock survivors were evaluated by Cox regression analysis and the Kaplan–Meier log-rank test. Univariate logistic regression analysis was used to identify possible predictors of in-hospital mortality. Variables with a P-value less than 0.01 in univariate analysis were included in multivariate logistic regression analysis. In the multivariate model, P-values less than 0.01 were considered statistically significant. The absence of a significant increase in the likelihood value on omission of each of the remaining variables was checked. The results are expressed as adjusted hazard ratio and 95% CI. In addition, the Kaplan–Meier estimate was calculated for overall survival based on the length of in-hospital stay. The log-rank test was used to compare Kaplan–Meier estimates. Significance was accepted for P < 0.01. All statistical tests were performed using Software for Statistics and Data Science for midsized datasets (STATA/IC 14.0; StataCorp LLC, College Station, TX).
RESULTS
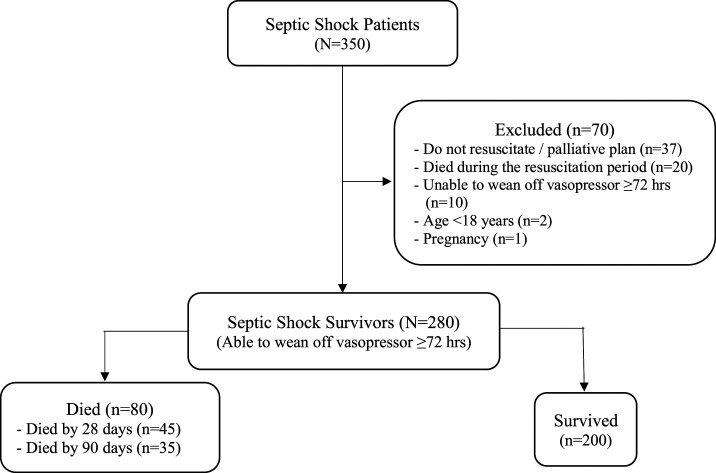
A total of 350 septic shock patients were evaluated for enrollment in this study. As noted in Figure 1, 70 patients were excluded from analysis. Of those, 37 had a DNR order, 20 did not survive first resuscitation, 10 still needed vasopressors after 72 hours and they died thereafter, two were aged less than 18 years, and one patient was pregnant. Among the 280 septic shock survivors, 80 died during their hospital stay, and 200 were discharged alive. Of the 80 patients who died, 45 died by 28 days (16.1%), and 35 (12.5%) died at some point later during their hospital stay.
Figure 1.
Study flow diagram.
Patient demographic and initial physiologic and clinical data are demonstrated in Table 1. The median age of all patients was 66 (IQR: 55–77) years. Fifty percent were male, and the mean BMI was 22.4 ± 4.6 kg/m2. Approximately 35% of patients had dependent status, either total or partial. Severity of illness as judged by the median APACHE-II score and median SOFA score on admission was 21 (IQR: 16–26) and five (IQR: 3–10), respectively. Diabetes mellitus, hypertension, and chronic kidney disease were the most common comorbidities. There was no difference in age, gender, BMI, severity of illness, or comorbidities between the death and survivor groups. Regarding site of infection, the three most frequent diagnoses in both groups were urinary tract infection, intra-abdominal infection, and pneumonia. Initial physiological and clinical data including mean arterial pressure, initial lactate, time to receive antibiotic, total fluid volume during 24 hours after admission, and maximum dose of norepinephrine were not different between the death and survivor groups.
Table 1.
Characteristics of septic shock survivors compared between those who died in-hospital and those who were discharged alive*
| Variable | Total (N = 280) | Hospital outcomes | P-value | |
|---|---|---|---|---|
| Died (n = 80) | Survived (n = 200) | |||
| Age (years) | 66 (55, 77) | 69 (59, 79.5) | 65 (55, 76) | 0.419 |
| Male gender, n (%) | 145 (51.8) | 48 (60) | 97 (48.5) | 0.211 |
| Body mass index (kg/m2) | 22.4 ± 4.6 | 22 ± 4.4 | 22.6 ± 4.7 | 0.374 |
| Acute Physiology and Chronic Health Evaluation-II score on admission | 21 (16, 26) | 21 (17, 27) | 20 (16, 26) | 0.643 |
| Sequential organ failure assessment score upon admission | 5 (3, 10) | 5 (3, 10) | 5 (3, 9) | 0.764 |
| Status before admission, n (%) | ||||
| Independent | 181 (64.6) | 44 (55) | 137 (68.5) | 0.777 |
| Partially dependent | 77 (27.5) | 29 (36.3) | 48 (24) | 0.127 |
| Totally dependent | 22 (7.9) | 7 (8.8) | 15 (7.5) | 0.806 |
| Comorbidities, n (%) | ||||
| Hypertension | 153 (54.6) | 51 (63.8) | 102 (51) | 0.063 |
| Diabetes mellitus | 97 (34.6) | 29 (36.3) | 68 (34) | 0.443 |
| Chronic kidney disease | 80 (28.6) | 37 (46.3) | 43 (21.5) | 0.154 |
| Active malignancy | 76 (27.1) | 24 (30) | 52 (26) | 0.421 |
| Immunosuppressed | 61 (21.8) | 20 (25) | 41 (20.5) | 0.685 |
| Coronary artery disease | 57 (20.4) | 24 (30) | 33 (16.5) | 0.130 |
| Cerebrovascular accident | 42 (15) | 15 (18.8) | 27 (13.5) | 0.825 |
| Chronic liver disease | 40 (14.3) | 12 (15) | 28 (14) | 0.120 |
| Chronic lung disease | 20 (7.1) | 10 (12.5) | 10 (5) | 0.802 |
| Sites of infection, n (%) | ||||
| Urinary tract infection | 77 (27.5) | 11 (13.8) | 66 (33) | 0.140 |
| Intra-abdominal infection: cholecystitis, diverticulitis, and liver abscess | 73 (26.1) | 21 (26.3) | 52 (26) | 0.474 |
| Pneumonia | 72 (25.7) | 29 (36.3) | 43 (21.5) | 0.842 |
| Soft tissue infection: cellulitis, necrotizing fasciitis, and pyomyositis | 23 (8.2) | 9 (11.3) | 14 (7) | 0.684 |
| Septicemia | 12 (4.3) | 4 (5) | 8 (4) | 0.596 |
| Febrile neutropenia | 7 (2.5) | 4 (5) | 3 (1.5) | 0.052 |
| Catheter-related bloodstream infection | 3 (1.1) | 1 (1.3) | 2 (1) | 0.876 |
| Infected peritoneal dialysis | 1 (0.4) | 1 (1.3) | 0 (0) | 0.370 |
| Other source of infection | 12 (4.3) | 4 (5) | 8 (4) | 0.723 |
| Initial physiologic and clinical data | ||||
| Mean arterial blood pressure (mmHg) | 55.6 ± 7 | 54.3 ± 4 | 56.7 ± 8.4 | 0.082 |
| Lactate (mmol/L) | 3 (2, 6.2) | 4 (2.4, 6.2) | 3.8 (2, 4.8) | 0.134 |
| Time to receive antibiotic (minutes) | 48 (24, 81) | 55 (24, 104.5) | 48 (24, 72) | 0.087 |
| Total fluid volume (mL) during 24 hours after admission | 3,750 (1,012, 5,946.5) | 3,847 (1,590, 6,269.5) | 3,710 (1,007, 5,269.5) | 0.081 |
| Maximum dose of norepinephrine (mcg/kg/minute) (for initial resuscitation) | 0.08 (0.05, 0.14) | 0.11 (0.05, 0.17) | 0.08 (0.04, 0.12) | 0.063 |
* Values are presented as median (interquartile range), number, and percentage, or mean ± SD.
The clinical courses of septic shock survivors compared between those who died and those who survived during their hospital stay are shown in Table 2. The duration of ICU stay as well as the length of hospital stay was longer among those who died. During hospital admission, severity of illness as characterized by the SOFA score was significantly higher in the death group. More specifically, the worst SOFA score and the SOFA score at 72 hours after discontinuation of vasopressors were both significantly higher in the death group than in the survivor group. The death group received longer duration of antimicrobial therapy and required significantly more organ support, including renal replacement therapy and mechanical ventilation. In addition, significantly more fluid was given during the 72 hours after discontinuation of vasopressors in the death group. Moreover, major medical complications developed after septic shock reversal. Complications found to be significantly more prevalent in the death group included metabolic acidosis, HAP/VAP, Clostridium difficile–associated diarrhea, CRBSI, and limb ischemia. Laboratory alterations after shock reversal that were more serious in the death group included Hb, serum albumin, bilirubin, pH from arterial blood gas, Plt, the worst PaO2/FiO2, and serum lactate.
Table 2.
Clinical courses of septic shock survivors compared between those who died in-hospital and those who were discharged alive
| Variable | Total (N = 280) | Hospital outcomes | P-value | |
|---|---|---|---|---|
| Died (n = 80) | Survived (n = 200) | |||
| Intensive care unit LOS (days) | 4 (0, 11.5) | 14.5 (7, 26) | 2 (0, 6) | < 0.001 |
| Hospital LOS (days) | 16 (9, 30) | 24.5 (15.5, 40.5) | 12.5 (8, 24) | < 0.001 |
| The worst SOFA score | 8 (6, 15) | 15 (14, 19) | 7 (5, 9) | < 0.001 |
| SOFA score at 72 hours after discontinuation of vasopressor | 3 (0, 5) | 7 (5, 9) | 1 (0, 4) | < 0.001 |
| Support during hospital stay | ||||
| Days on antimicrobial therapy | 11 (7,18) | 16 (11,25) | 9 (6,15) | 0.053 |
| Renal replacement therapy, n (%) | 51 (18.2%) | 37 (46.3%) | 14 (7%) | < 0.001 |
| Days alive without respiratory support | 15 (6, 28) | 3 (0, 12.5) | 22 (8, 28) | < 0.001 |
| Total fluid volume (mL) during 72 hours after discontinuation of vasopressor | 1,000 (346.5, 2,000) | 2,744.5 (2,000, 4,834.5) | 600 (200, 1,135) | < 0.001 |
| Complications during hospital stay, n (%) | ||||
| Acute kidney injury | 189 (67.5) | 63 (78.8) | 126 (63) | 0.333 |
| Metabolic acidosis | 158 (56.4) | 62 (77.5) | 96 (48) | 0.029 |
| Cardiogenic pulmonary edema | 106 (37.9) | 46 (57.5) | 60 (30) | 0.796 |
| Hospital-acquired pneumonia/ventilator-associated pneumonia | 73 (26.1) | 55 (68.8) | 18 (9) | < 0.001 |
| Acute respiratory distress syndrome | 69 (24.6) | 39 (48.8) | 30 (15) | 0.248 |
| New arrhythmia | 48 (17.1) | 22 (27.5) | 26 (13) | 0.785 |
| Upper gastrointestinal bleeding | 26 (9.3) | 15 (18.8) | 11 (5.5) | 0.407 |
| Other hospital-acquired infection | 19 (6.8) | 12 (15) | 7 (3.5) | 0.098 |
| Clostridium difficile–associated diarrhea | 17 (6.1) | 6 (7.5) | 11 (5.5) | 0.037 |
| Catheter-related bloodstream infection | 15 (5.4) | 14 (17.5) | 1 (0.5) | 0.037 |
| Urinary tract infection | 13 (4.6) | 3 (8.6) | 10 (5) | 0.140 |
| Limb ischemia | 9 (3.2) | 6 (7.5) | 3 (1.5) | 0.020 |
| Mesenteric ischemia | 2 (0.7) | 1 (1.3) | 1 (0.5) | 0.715 |
| Laboratory data after discontinuation of vasopressor | ||||
| Hemoglobin (g/dL) | 9.7 (8.5, 11.2) | 8.9 (7.8, 10.3) | 9.95 (8.7, 11.6) | 0.033 |
| Serum albumin (g/dL) | 2.65 (2.2, 3.1) | 2.37 (2, 2.8) | 2.7 (2.3, 3.1) | < 0.001 |
| Bilirubin (g/dL) | 0.8 (0.5, 2.2) | 1.2 (0.6, 5.5) | 0.7 (0.4, 1.94) | < 0.001 |
| Bicarbonate (g/dL) | 18 (14, 21) | 17 (13, 20.5) | 18 (15, 21) | 0.131 |
| pH from arterial blood gas | 7.34 (7.24, 7.4) | 7.27 (7.2, 7.39) | 7.36 (7.27, 7.41) | 0.019 |
| Platelet (cells/µL) | 156,500 (81,000, 247,000) | 97,000 (37,500, 195,000) | 171,500 (96,500, 262,500) | < 0.001 |
| Worst PaO2/FiO2 ratio | 250 (166, 365) | 190 (120, 300) | 307.5 (209, 400) | 0.015 |
| ScvO2, % | 75 (66, 83) | 73 (65, 84) | 76 (70, 83) | 0.455 |
| Lactate (mmol/L) | 1.9 (1, 5.3) | 2.7 (1.4, 6.2) | 1.8 (1, 4.8) | 0.017 |
FiO2 = fractional inspired oxygen; LOS = length of stay; PaO2, = arterial oxygen partial pressure; ScvO2 = central venous oxygen saturation; SOFA = sequential organ failure assessment. Values are presented as number and percentage or median (interquartile range).
Of the 80 patients who died during their hospital stay, 35 died by day 28, and 45 died at some point thereafter during their hospital stay. The causes of death, both in the ICU and during the hospital stay, were quite similar, as shown in Table 3. Most sepsis survivors (71.1% of 28-day mortality and 73.8% of overall in-hospital mortality) died from multi-organ dysfunction. Hospital-acquired pneumonia/VAP was a major contributing factor in 62.2% of 28-day mortality and in 53.8% of overall in-hospital mortality, followed by CRBSI and other infections. The three most commonly isolated pathogens among those with HAP/VAP were Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae. Inadequate infectious source control was found in 6.7% of 28-day death and in 10% of overall in-hospital death. A small proportion of patients died from noninfectious cause, including cardiac cause, malignancy, stroke, procedural complication, and unknown cause.
Table 3.
Causes of death in septic shock reversal patients
| Variable | Death in septic shock survivors | |
|---|---|---|
| 28-day mortality (n = 45), case (%) | In-hospital mortality (n = 80), case (%) | |
| Multi-organ dysfunction | 32 (71.1) | 59 (73.8) |
| Hospital-acquired pneumonia/ventilator-associated pneumonia | 28 (62.2) | 43 (53.8) |
| Inadequate source control | 3 (6.7) | 8 (10) |
| Catheter-related bloodstream infection | 3 (6.7) | 5 (6.3) |
| Other hospital-acquired infection | 4 (8.9) | 7 (8.8) |
| Cardiac cause | 2 (4.4) | 5 (6.3) |
| Malignancy | 2 (4.4) | 3 (3.8) |
| Stroke | 1 (2.2) | 3 (3.8) |
| Procedural complication | 2 (2.2) | 2 (2.5) |
| Unknown | 0 (0) | 4 (5) |
Factors associated with mortality were further examined, as shown in Table 4. Among the variables obtained from univariate analysis, multivariate analysis identified four factors to be independently associated with in-hospital mortality. Those factors included HAP/ventilator-acquired pneumonia, volume of fluid therapy during 72 hours after discontinuation of vasopressors, SOFA score at 72 hours after vasopressor discontinuation, and serum albumin level at 72 hours after vasopressor discontinuation.
Table 4.
Univariate and multivariate analyses for factors independently associated with in-hospital mortality
| Variable | Univariate logistic regression model | Multivariate logistic regression model | ||||
|---|---|---|---|---|---|---|
| Unadjusted hazard ratio | 95% CI | P-value | Adjusted hazard ratio | 95% CI | P-value | |
| Hospital-acquired pneumonia/ventilator-acquired pneumonia | 3.11 | (1.61, 6.01) | < 0.001* | 3.24 | (1.94, 5.43) | < 0.001* |
| SOFA score at 72 hours after discontinuation of vasopressor | 1.2 | (1.14, 1.26) | < 0.001* | 1.12 | (1.04, 1.19) | 0.001* |
| Fluid volume (mL) during 72 hours after discontinuation of vasopressor | 1.01 | (1.01, 1.02) | < 0.001* | 1.01 | (1.01, 1.02) | 0.008* |
| Albumin level at 72 hours after discontinuation of vasopressor | 0.56 | (0.39, 0.81) | 0.002* | 0.57 | (0.39, 0.84) | 0.004* |
| Platelet level at 72 hours after discontinuation of vasopressor | 0.98 | (1.01, 1.02) | < 0.001* | 0.98 | (0.99, 1.01) | 0.181 |
| Bilirubin level at 72 hours after discontinuation of vasopressor | 1.04 | (1.02, 1.06) | < 0.001* | 1.02 | (1, 1.05) | 0.064 |
| Renal replacement therapy | 2.91 | (1.87, 4.53) | < 0.001* | 1.44 | (0.85, 2.43) | 0.177 |
| The worst SOFA score | 1.08 | (1.04, 1.13) | < 0.001* | 1.03 | (0.96, 1.12) | 0.411 |
SOFA = sequential organ failure assessment.
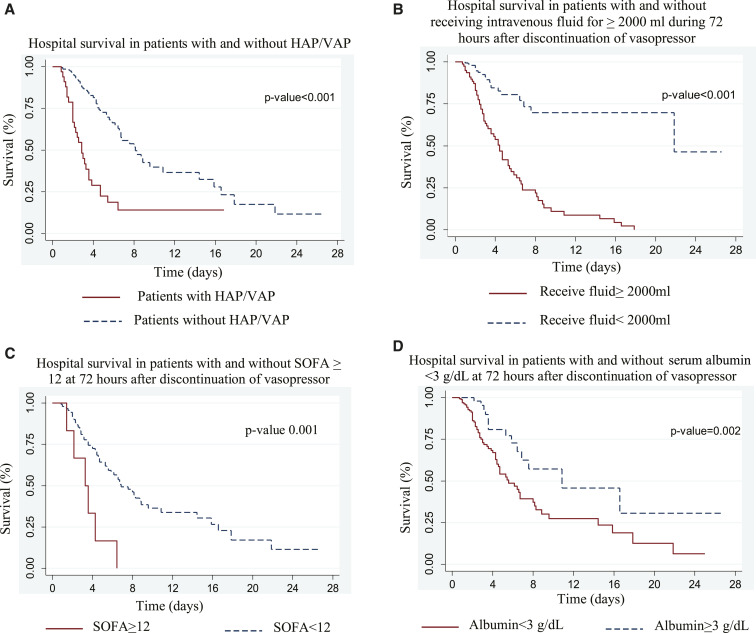
Figure 2 shows the Kaplan–Meier survival curve of four previously identified factors significantly associated with in-hospital mortality in septic shock survivors: presenting of HAP/ventilator-acquired pneumonia, volume of fluid therapy during 72 hours after discontinuation of vasopressors ≥ 2,000 mL, SOFA score at 72 hours after vasopressor discontinuation ≥ 12, and serum albumin level at 72 hours after vasopressor discontinuation < 3 g/dL. P-values were statistically significant in log-rank test comparing each variable (P < 0.01).
Figure 2.
Kaplan–Meier analysis of in-hospital survival of 280 septic shock survivors. Presence of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) (A), total fluid during 72 hours after discontinuation of vasopressor (B), sequential organ failure assessment (SOFA) score at 72 hours after discontinuation of vasopressor (C), and serum albumin level at 72 hours after discontinuation of vasopressor (D) were identified as independent predictors of in-hospital mortality in septic shock survivors. This figure appears in color at www.ajtmh.org.
DISCUSSION
This study determined the prevalence of in-hospital death after septic shock reversal, the causes of death, and the factors independently associated with in-hospital death at Thailand’s largest super-tertiary referral hospital. Of the 350 patients who were evaluated for eligibility, 20 (5.7%) died in early phase, and another 50 were excluded for previously described reasons. The remaining 280 septic shock survivors were included. Eighty of these survivors (28.8%) subsequently died during their stay in the hospital. Major causes of death included multi-organ failure, HAP, inadequacy of source control, and other nosocomial infection, whereas a smaller number died from noninfectious causes. Significant factors associated with these deaths included HAP, volume of fluid therapy during the 72 hours after discontinuation of vasopressors, and SOFA score and serum albumin level at 72 hours after discontinuation of vasopressors.
Our finding that a significant number of patients died after shock reversal was consistent with other studies. A study by Daviaud et al.15 found that 244 (45%) of 543 patients died in-hospital. One-third of these patients died within 72 hours, whereas the remaining died later. Causes of early death were mostly from intractable organ failure related to infection, whereas the late deaths were related to end-of-life decision, nosocomial infection, or mesenteric ischemia. Another retrospective work evaluated outcomes according to duration of stay in the hospital, as follows: phase I (days 1–5), phase II (days 6–15), and phase III (days 16–150).16 Of the 999 patients that they enrolled, 308 died. Overall, 36.7% of those deaths occurred during phase I, and the remaining patients died later. Interestingly, there was a high proportion of positive blood culture results in phase I and a low proportion in phase II, but the proportion of positive results was once again high in phase III, especially relative to opportunistic bacteria and fungi. The phase III finding was suggestive of nosocomial infection as a major cause of morbidity and mortality during the late phase of hospitalization. In addition and as noted earlier, Prescott et al.8 clearly demonstrated higher late-phase mortality in patients with sepsis than in other matching cohorts. The causes of death in these reports were similar to those in our study, with early death being mostly due to an inability to reverse shock, and late mortality mainly due to multi-organ dysfunction and hospital-acquired infection.
The causes of death in our study highlighted certain clues of interest. Multi-organ dysfunction was the major cause of death in our study. This condition is characterized by the development of progressive physiologic dysfunction in two or more organ systems after an acute threat to systemic homeostasis.17 Other previous studies also reported multi-organ failure to be a major cause of death in septic shock patients.18,19 We also found that patients who died in-hospital had a higher SOFA score at 72 hours after discontinuation of vasopressor than those who survived. This is similar to the finding from the SOAP study20 that found that patient mortality increased commensurate with an increase in the number of organs that failed. Interestingly, both groups had the same SOFA score on day 1. Patients who survived the early phase with multiple organ injury, despite having shock reversed and vasopressors tapered, had a higher likelihood of dying later during their hospitalization. This would be a sound explanation for the significantly higher amount of fluid therapy given during the 72-hour period after discontinuation of vasopressor in the death group. These patients were more likely to have depleted intravascular volume from dilated and leaked vascular beds due to multi-organ failure. This would also help to explain the low serum albumin level observed at 72 hours after the discontinuation of vasopressor. Resuscitation with albumin solution should be considered among septic shock patients with a high severity score (APACHE-II score ≥ 20) and low baseline serum albumin level (< 3 g/dL).21 Avoidance of unnecessary fluid administration during early shock recovery may be beneficial.
Consistent with previous study–infection, particularly HAP, was also a major cause of death in our study. Zhao et al.22 reported a series of 476 early sepsis survivors, of whom 39% had secondary infection, and about half of those had pneumonia. They had a higher SOFA score, and certainly had higher mortality. A study in late death among French septic shock patients by Daviaud et al.15 found end-of-life decision and infection to be the main causes. Infection is also an important cause of hospital readmission. Prescott et al.8 reported sepsis to be the leading cause of readmission within 90 days after admission for severe sepsis.
There is currently a plethora of studies in the literature specific to altered immunologic states in sepsis. A study in the postmortem analysis of lung and spleen specimens from sepsis patients revealed evidence of immunosuppression as determined by biochemical tests, flow cytometry, and immunohistochemical findings.23 A multicenter prospective study in circulating biomarkers in sepsis survivors over time for a year after hospital discharge revealed interesting findings.24 Two-thirds of subjects had elevated high sensitivity C-reactive protein (hsCRP) and soluble programmed death ligand 1 (sPD-L1) levels, which reflect inflammation and immunosuppression, respectively. These patients had worse long-term outcomes than the remaining one-third who had normal hsCRP and sPD-L1 levels. In addition to immune alteration, local host defenses are also impaired. Bronchopulmonary aspiration is common in sepsis because of swallowing dysfunction and coughing defect.7 Impairment of consciousness due to delirium is also common in our ICU, which leads to poor airway control and a higher likelihood of aspiration.25 Patients with organ failure requiring artificial support require instrumentation, including endotracheal tube, catheterization, and/or vascular cannulation, to bypass local host defense. In addition to generally recommended infection control bundles, measures to mitigate infection should include strategies that facilitate the early and timely control sepsis, the prevention of organ failure, and the carefully considered use of invasive organ support.
To reduce post-sepsis mortality, not only early and rapid reversal of shock26 that need to be stressed, but also to manage with post-septic shock complications is worth to highlight.
Limitations.
Our study has certain limitations. First, it was conducted in the medical intensive care unit and medical wards of a single-center university-based tertiary hospital, so our results may not be generalizable to patients in surgical wards or in different hospital settings. However, our findings may reflect the current patient composition in tertiary centers in Thailand, with a shift toward chronic, severe underlying medical disorders, such as malignancy, which comprised one-third of our patients. Second, our study was retrospective in nature, which is a study design that is known to have limitations compared with prospective study. Third, the relatively small number of deaths may have given our study insufficient statistical power to identify all existing significant differences and associations. Having acknowledged that, we feel that the findings that we have reported are of value, especially because we only selected independent factors with a P-value less than 0.01. Fourth and last, our study design did not allow for comparison with a validation cohort, which we recommend for future study.
CONCLUSION
In-hospital mortality after septic shock reversal was a high 28.6% at our center. The main causes of death were multi-organ failure and HAP. Factors independently associated with death included HAP/VAP, volume of intravenous fluid given during 72 hours after discontinuation of vasopressor, SOFA score at 72 hours after discontinuation of vasopressors, and serum albumin at 72 hours after discontinuation of vasopressors.
Acknowledgments:
We would like to express gratitude to the patient and the staff of Siriraj Hospital, Mahidol University, Bangkok, Thailand. We gratefully thank Kevin Jones for language editing.
REFERENCES
- 1.Levy MM, Evans LE, Rhodes A, 2018. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med 44: 925–928. [DOI] [PubMed] [Google Scholar]
- 2.Javed A, Guirgis FW, Sterling SA, Puskarich MA, Bowman J, Robinson T, Jones AE, 2017. Clinical predictors of early death from sepsis. J Critical Care 42: 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prescott HC, Osterholzer JJ, Langa KM, Angus DC, Iwashyna TJ, 2016. Late mortality after sepsis: propensity matched cohort study. BMJ 353: i2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quartin AA, Schein RM, Kett DH, Peduzzi PN, 1997. Magnitude and duration of the effect of sepsis on survival. JAMA 277: 1058–1063. [PubMed] [Google Scholar]
- 5.Iwashyna TJ, Ely EW, Smith DM, Langa KM, 2010. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304: 1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah FA, Pike F, Alvarez K, Angus D, Newman AB, Lopez O, Tate J, Kapur V, Wilsdon A, Krishnan JA, 2013. Bidirectional relationship between cognitive function and pneumonia. Am J Respir Crit Care Med 188: 586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zielske J, Bohne S, Brunkhorst FM, Axer H, Guntinas-Lichius O, 2014. Acute and long-term dysphagia in critically ill patients with severe sepsis: results of a prospective controlled observational study. Eur Arch Otorhinolaryngol 271: 3085–3093. [DOI] [PubMed] [Google Scholar]
- 8.Prescott HC, Langa KM, Iwashyna TJ, 2015. Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA 313: 1055–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prescott HC, Angus DC, 2018. Enhancing recovery from sepsis: a review. JAMA 319: 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angkasekwinai N, Rattanaumpawan P, Thamlikitkul V, 2009. Epidemiology of sepsis in Siriraj Hospital 2007. J Med Assoc Thai 92: S68–S78. [PubMed] [Google Scholar]
- 11.Permpikul C, Tongyoo S, Ratanarat R, Wilachone W, Poompichet A, 2010. Impact of septic shock hemodynamic resuscitation guidelines on rapid early volume replacement and reduced mortality. J Med Assoc Thai 93: S102–S109. [PubMed] [Google Scholar]
- 12.Permpikul C, Sringam P, Tongyoo S, 2014. Therapeutic goal achievements during severe sepsis and septic shock resuscitation and their association with patients’ outcomes. J Med Assoc Thai 97: S176–S183. [PubMed] [Google Scholar]
- 13.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, 2016. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad, Rijnders BJ, Sherertz RJ, Warren DK, 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 49: 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daviaud F, Grimaldi D, Dechartres A, Charpentier J, Geri G, Marin N, Chiche JD, Cariou A, Mira JP, Pène F, 2015. Timing and causes of death in septic shock. Ann Intensive Care 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otto GP, Sossdorf M, Claus RA, Rödel J, Menge K, Reinhart K, Bauer M, Riedemann NC, 2011. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care 15: R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ, 1992. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 101: 1644–1655. [DOI] [PubMed] [Google Scholar]
- 18.Bilevicius E, Dragosavac D, Dragosavac S, Araújo S, Falcão AL, Terzi RG, 2001. Multiple organ failure in septic patients. Braz J Infect Dis 5: 103–110. [DOI] [PubMed] [Google Scholar]
- 19.Ruokonen E, Takala J, Kari A, Alhava E, 1991. Septic shock and multiple organ failure. Crit Care Med 19: 1146–1151. [DOI] [PubMed] [Google Scholar]
- 20.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D, 2006. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34: 344–353. [DOI] [PubMed] [Google Scholar]
- 21.Tongyoo S, Chayakul C, Kanoknatsiwattana S, Permpikul C, 2020. Albumin versus gelatin solution for the treatment of refractory septic shock: a patient baseline-matched-cohort study. Siriraj Med J 72: 451–461. [Google Scholar]
- 22.Zhao GJ, Li D, Zhao Q, Song JX, Chen XR, Hong GL, Li MF, Wu B, Lu ZQ, 2016. Incidence, risk factors and impact on outcomes of secondary infection in patients with septic shock: an 8-year retrospective study. Sci Rep 6: 38361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD, Kreisel D, Krupnick AS, 2011. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306: 2594–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yende S, Kellum JA, Talisa VB, Palmer OMP, Chang CCH, Filbin MR, Shapiro NI, Hou PC, Venkat A, LoVecchio F, 2019. Long-term host immune response trajectories among hospitalized patients with sepsis. JAMA Netw Open 2: e198686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jirisant WSV, Permpikul C, 2020. Prevalence and risk factors of delirium in the Medical ICU, Siriraj Hospital during preparation. [Google Scholar]
- 26.Permpikul C, Tongyoo S, Viarasilpa T, Trainarongsakul T, Chakorn T, Udompanturak S, 2019. Early use of norepinephrine in septic shock resuscitation (CENSER). A randomized trial. Am J Respir Crit Care Med 199: 1097–1105. [DOI] [PubMed] [Google Scholar]