Abstract.
Increased levels of guinea worm (GW) disease transmission among dogs in villages along the Chari River in Chad threaten the gains made by the GW Eradication Program. Infected dogs with preemergent worm blisters are difficult to proactively identify. If these dogs are not contained, blisters can burst upon submersion in water, leading to the contamination of the water supply with L1 larvae. Guinea worm antigens previously identified using sera from human dracunculiasis patients were coupled to polystyrene beads for multiplex bead assay analysis of 41 non-endemic (presumed negative) dog sera and 39 sera from GW-positive dogs from Chad. Because commercially available anti-dog IgG secondary antibodies did not perform well in the multiplex assay, dog IgGs were partially purified, and a new anti-dog IgG monoclonal antibody was developed. Using the new 4E3D9 monoclonal secondary antibody, the thioredoxin-like protein 1–glutathione-S-transferase (GST), heat shock protein (HSP1)–GST, and HSP2–GST antigen multiplex assays had sensitivities of 69–74% and specificities of 73–83%. The domain of unknown function protein 148 (DUF148)–GST antigen multiplex assay had a sensitivity of 89.7% and a specificity of 85.4%. When testing samples collected within 1 year of GW emergence (n = 20), the DUF148–GST assay had a sensitivity of 90.0% and a specificity of 97.6% with a receiver-operating characteristic area under the curve of 0.94. Using sera from two experimentally infected dogs, antibodies to GW antigens were detected within 6 months of exposure. Our results suggest that, when used to analyze paired, longitudinal samples collected 1–2 months apart, the DUF148/GST multiplex assay could identify infected dogs 4–8 months before GW emergence.
INTRODUCTION
Dracunculus medinensis, the nematode parasite responsible for guinea worm (GW) disease, was targeted for eradication by the World Health Assembly in 1986.1 The GW Eradication Program (GWEP) succeeded in decreasing the worldwide human case count by > 99% between 1986 and 2019 (from approximately 3.5 million to 54 cases) and narrowed the geographic distribution of the human disease to three countries in Africa: Chad, South Sudan, and Angola.2–4 However, sustained transmission of GW disease in dogs, first recognized in a zone along the Chari River in Chad in 2012, amounted to 1,935 canine cases in 2019 and currently poses a major threat to the ultimate goal of global eradication.4–7
Guinea worm cycles between a human or other mammalian definitive host (e.g., dog, cat, and baboon) and a copepod intermediate host that is found in fresh water.8 Perhaps, because widespread dog infections are a relatively new phenomenon, the identification of fish and frogs as potential transport and paratenic hosts had not previously been recognized as a factor in GW transmission.6,9–11 Difficulties in the application of traditional identification and containment protocols to GW-infected dogs suggest that new tools may be needed to prevent further recrudescence of the disease.11 Given the 10- to 14-month delay between ingestion of infected copepods and the emergence of the gravid female worm from the mammalian host, a serologic assay capable of identifying animals with exposure or prepatent GW infection would have immediate applications in the eradication project.8,11
In a recent report,12 we identified antigens from adult female D. medinensis worms and developed a recombinant protein–based multiplex bead assay for the identification of GW-specific IgG antibody responses in human sera. These responses were directed against the thioredoxin-like protein 1 (TRXL1) and the domain of unknown function protein 148 (DUF148), were dominated by the IgG4 antibody subclass, and were shown to decrease in intensity with time after GW emergence/infection resolution. Human IgG antibody reactivity to two GW heat shock proteins (HSPs) was minimal. The TRXL1–glutathione-S-transferase (GST) multiplex IgG assay had better performance characteristics as measured by the receiver-operating characteristic (ROC) area under the curve (AUC) (AUC = 0.95) than did the DUF148–GST assay (AUC = 0.88) mainly because of the higher cross-reactivity of sera from onchocerciasis-positive donors to the latter antigen. Using only sera collected during or within 1 year of GW emergence, the TRXL1–GST assay was 100% sensitive and 94.7% specific with an ROC AUC of 0.99.
In the current work, we developed a new anti-dog IgG monoclonal antibody reagent so that the multiplex bead assay could be used with canine sera. We validated the multiplex assay using samples from Chad, and we characterized GW-specific antibody responses in two experimentally infected animals with prepatent infections. In the next phase, we expect to use the multiplex assay to examine longitudinal samples from a large-scale epidemiologic survey of dogs from a highly GW-endemic region of Chad, and we will attempt to transition to a lateral flow assay that can be used in the field.
MATERIALS AND METHODS
Study samples from Chad.
The dog samples included in this work were from a collaborative study between the Institut de Recherche en Elevage pour le Développement and the Swiss Tropical and Public Health Institute. Both institutions have a long-standing partnership in rabies research in Chad and a well-experienced local team for studies related to dogs. The research protocol was approved by the Chadian Ministry of Livestock and Pastoral Development. The CDC was not engaged in animal research. Based on survey data collected by the Chadian GWEP, dogs from villages in the Guelengdeng district with documented and confirmed current or recent GW infections within the past 2 years (n = 39) were sampled in January 2017. Dogs had a history of between 1 and 15 emergent worms, and some dogs had worms emerge in both 2015 and 2016. Negative control dog sera (n = 41) were collected in late 2017 from villages north of N’Djamena, where canine GW disease had not yet been reported. Owners were asked to allow one-time only bleeding of their dogs, and the animals were vaccinated against rabies. Blood samples were collected from the saphenous vein (one tube, up to 5 mL), and the sera were separated and stored frozen. Because canine rabies is prevalent throughout Chad, dog owners were contacted 10 days after sample collection to confirm that their dog was still alive and well. This was done to exclude any dogs that might have been infected with rabies at the time of sample collection. Aliquots (1–2 mL) of ascertained rabies-negative sera were shared with the CDC in Atlanta, GA, for serological analysis of GW antibody responses. Sera from negative control dogs were assayed for antigens of Dirofilaria immitis using the SNAP 4Dx Plus Test according to the manufacturer’s directions (IDEXX Reference Laboratories, Westbrook, ME).
Samples from controlled infection studies at the University of Georgia (UGA).
Copepods were exposed to L1 larvae from a D. medinensis female worm from Chad.13,14 The larvae were allowed to mature in the copepods for at least 16 days at which time they were confirmed to have developed to the infectious L3 stage. Two laboratory-raised beagles of the same age (Ridglan Farms, Inc., Mount Horeb, WI) were each exposed per os to 175 copepods infected with GW L3 larvae. The dogs were individually housed in a climate-controlled (21°C) facility and provided food and water ad libitum. Blood samples were collected from the saphenous vein before inoculation and then periodically after inoculation. Blood was allowed to clot, and sera were collected after centrifugation at 1,000 × g for 10 minutes. Serum samples were frozen at −20°C until testing. All experimental methods were conducted at UGA, and their project was approved by the university’s Institutional Animal Care and Use Committee (protocol number A2017 04-005). Aliquots of sera were shipped to the CDC for serological analysis of GW antibody responses.
Canine IgG antibody fractionation.
A 3-mL pool of sera from five dogs from Chad with a history of GW infection (0.5–0.75 mL each) was treated for 1 hour at 4°C with a 45% saturation of ammonium sulfate to precipitate crude IgGs. The precipitate was collected by centrifugation at 17,000 × g for 10 minutes at 4°C. The pellet was washed twice by resuspension in 9 mL of phosphate-buffered saline (PBS; 10 mM Na2HPO4 at pH 7.2 and 0.85% NaCl) saturated to 45% ammonium sulfate and collection by centrifugation. The final washed pellet was dissolved in 0.6 mL PBS and centrifuged at 10,000 × g for 10 minutes at 4°C to remove insoluble particulates. The supernatant was divided into three 200 μL aliquots, and fractions were purified by fast protein liquid chromatography (FPLC) using a Superose 6 10/300 column (GE Healthcare, Piscataway, NJ) with PBS buffer at a flow rate of 0.5 mL/min. A single broad 280-nm absorbance peak with an elution volume between 14.5 and 18.5 mL was collected from each FPLC run, and the elution peaks were combined.
IgGs were further purified by repeated FPLC runs on a 1-mL protein A agarose (Sigma Aldrich Co., St. Louis, MO) column and a 1-mL GammaBind G Sepharose (GE Healthcare) column using the FPLC fractionation scheme described by Mazza et al.15 with the following four modifications. First, although columns were pre-equilibrated in 0.1 M sodium phosphate buffer at pH 8, proteins were loaded onto the columns in PBS buffer at pH 7.2. Second, step gradients (rather than linear gradients) of 40% 0.1 M sodium citrate at pH 2.5/60% sodium phosphate at pH 8 and 100% 0.1 M sodium citrate at pH 2.5 were used to elute the bound antibodies. Third, the peaks eluted by the step gradients were neutralized to pH 7 (determined by pH paper) with 0.5 M Tris at pH 10 and saturated with 45% ammonium sulfate to collect the IgGs. After centrifugation, the precipitated protein pellets were dissolved in PBS and dialyzed overnight at 4°C against 3 L of PBS (Spectrapor 3 membrane, 3,500-Da cutoff; Spectrum Laboratories, Rancho Dominguez, CA) in preparation for the next chromatographic step. Fourth, in an effort to retain antibody function, the number of low pH elution steps during the purification was limited to ≤ 3. The final antibody fractions were ammonium sulfate precipitated, dissolved in PBS, and dialyzed overnight against PBS as described earlier. Protein concentrations were determined using the bicinchoninic acid microassay with bovine serum albumin as the standard (Pierce, Rockford, IL). IgG fractions were analyzed by sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis by the method of Laemmli16 on a 10.5% acrylamide gel loaded with 10.5 μg of protein per lane.
Crude antigen preparation, SDS polyacrylamide gels, and Western blot analysis.
Guinea worm proteins were extracted from dried worm segments into buffer containing 50 mM tris(hydroxymethyl)-aminomethane (Tris) at pH 7.5, 2 mM ethylenediaminetetraacetic acid, 100 mM NaCl, and 1% SDS and reduced with 10% β-mercaptoethanol as previously described.12 Proteins (1.5–2 μg/mm in a 130-mm preparative well) were resolved on a 10–22.5% gradient SDS polyacrylamide gel using the buffer system of Laemmli.16 Following electro-transfer onto polyvinylidene difluoride membrane (PVDF; Immobilon P, Millipore Corp., Bedford, MA), the membrane was cut into 2-mm strips, and strips were incubated overnight at 4°C in 2 mL of 1:100 dilutions of serum in 0.3% Tween-20/PBS. Canine IgG fractions were diluted into 0.3% Tween/PBS buffer for a final concentration of 84 μg/mL. For peak Z, this required a 1:50 dilution. Bound antibodies were detected using a 1:1,000 dilution of biotinylated rabbit anti-dog IgG (Fc specific) secondary antibody (1.5 mg/mL; Sigma Aldrich Co.) and a 1:500 dilution of streptavidin/alkaline phosphatase (Invitrogen, Camarillo, CA), each in 0.3% Tween-20 PBS. Each reagent was incubated with the strips for 1 hour at room temperature, and blots were then developed as previously described.12
Bead coupling and multiplex bead assays.
Guinea worm TRXL1, DUF148, HSP1, and HSP2 proteins were expressed in HB101 Escherichia coli cells (Promega Corp., Madison, WI) with Schistosoma japonicum GST fusion tags (pGEX4T-2 plasmid; GE Healthcare) and purified as previously described.12 Glutathione-S-transferase without any fusion partner was expressed and purified as previously described for use as a negative control protein.17 For the current study, antigens were coupled to magnetic beads (BioRad, Hercules, CA). The GST control protein was coupled at a concentration of 15 μg protein/12.5 × 106 beads in 25 mM 2-(N-morpholino)-ethanesulfonic acid (MES) buffer with 0.85% NaCl at pH 5.0. The domain of unknown function protein 148–GST, HSP1–GST, and HSP2–GST proteins were each coupled using 120 μg protein/12.5 × 106 beads in PBS buffer at pH 7.2, whereas TRXL1–GST was coupled in MES/NaCl buffer at pH 5.0 using 30 μg protein/12.5 × 106 beads.
Sera were diluted 1:400 in buffer containing PBS at pH 7.2, 0.5% polyvinyl alcohol, 0.8% polyvinylpyrrolidone, 0.5% casein, 0.3% Tween-20, 0.02% NaN3, and 3 μg/ml E. coli extract (buffer B).18,19 Canine IgG fractions were diluted into buffer B for a final concentration of 84 μg/mL. For peak Z, this required a 1:50 dilution. The conditions previously described by Priest and Moss18 for serum incubation, secondary antibody binding, streptavidin-coupled R-phycoerythrin binding, and final buffer wash were used for the GW multiplex bead assays. Total IgG antibody assays used a 1:500 dilution of biotinylated rabbit anti-dog IgG (Fc specific; 1.5 mg/mL) secondary antibody (Sigma Aldrich Co.) in PBS buffer with 0.1% Tween-20, 0.5% BSA, and 0.0% sodium azide (buffer A).18 IgG subclass responses were detected with 1:500 dilutions in buffer A of a polyclonal goat anti-dog IgG1 (1 mg/mL; Bio-Rad), a monoclonal mouse anti-dog IgG2 (clone CA4F1 at 1 mg/mL; BioRad),20 or a biotinylated monoclonal mouse anti-dog IgG4 (clone 4E3D9 at 0.5 mg/mL; this work). Because the anti-IgG1 and anti-IgG2 antibodies were not biotinylated, an additional incubation step was required with 1:500 dilutions in buffer A of either a biotinylated monoclonal mouse anti-goat IgG (1.5 mg/mL; Thermo Fisher Scientific, Waltham, MA) or a biotinylated monoclonal rat anti-mouse IgG (1 mg/mL; Invitrogen), respectively. Assays were read using a Bio-Plex 200 instrument with Bio-Plex Manager version 6.2 software build 175 (Bio-Rad). Average results for duplicate well IgG assays are expressed as the median fluorescent intensity value minus the value for the buffer-only background blank (MFI-bg). If ≥ 2 positive responses from a test sample had coefficients of variation > 15% between the duplicate wells, those results were discarded, and the assay was repeated.
Monoclonal antibody generation.
A biotinylated mouse anti-dog monoclonal antibody was generated by Southern Biotech (Birmingham, AL) using canine IgG fraction Z as immunogen. Clones were functionally screened for optimal binding characteristics in the GW multiplex bead assay using sera from GW-positive and GW-negative dogs from Chad as well as the IgG peaks from the serum fractionation procedure described earlier. The selected monoclonal antibody, 4E3D9, was purified and biotinylated by Southern Biotech.
Data analysis.
Cutoff values were determined by ROC analysis (SigmaPlot 13.0; Systat Software, Inc., San Jose, CA) or by Youden’s J-index.21,22 Statistical analyses were conducted using SigmaPlot 13.0 with an alpha level of 0.05 to determine statistical significance.
RESULTS
Canine serum fractionation.
Four IgG peaks designated W, X, Y, and Z were obtained from a crude canine IgG fraction by chromatography on protein A and protein G columns using a modification of the method of Mazza et al.15 Peak W was composed of the flow through material after sequential passage over protein A, protein G, and protein A columns. Yield from 3 mL of canine serum was 11.1 mg. Peak X, composed of material eluted from the initial protein A column at an approximate pH of 6, was passaged over the protein A column a second time. Yield was 4.3 mg. A fraction containing both peak Y and peak Z was eluted from the initial protein A column at pH 2.5. On the protein G column, peak Z was found in the flow through material with minimal binding to the column, whereas peak Y bound to the column and required pH 2.5 for elution. Both fractions were passaged over the protein G column a second time. Peak Y yield was 13.4 mg, and 4.2 mg of protein was recovered in peak Z.
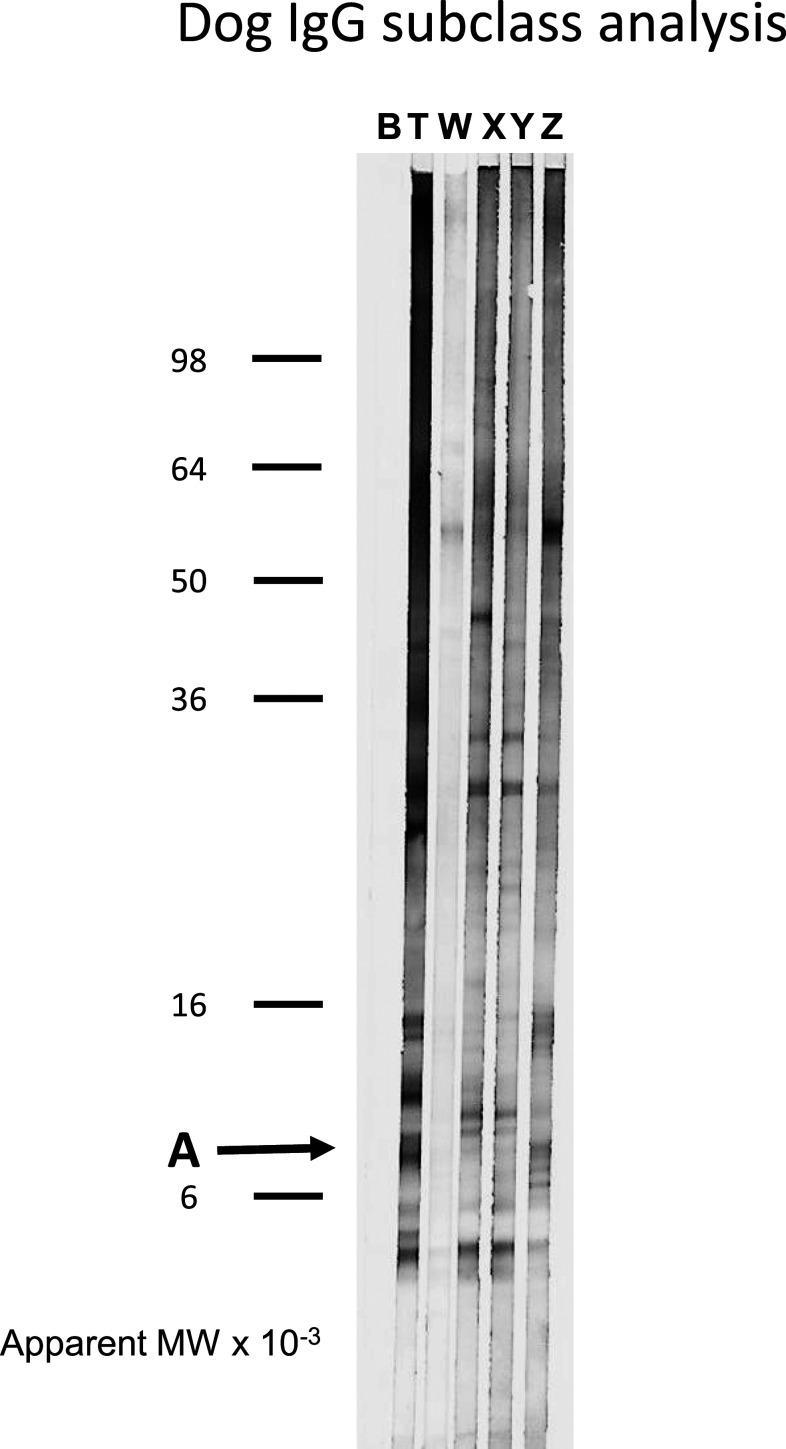
The quality of the IgG fractions was assessed by SDS polyacrylamide electrophoresis and by reactivity with GW proteins by Western blots. As shown in Supplemental Figure 1, the flow through material in peak W that did not bind to either the protein A or protein G columns was grossly contaminated with non-IgG proteins. By contrast, peaks X, Y, and Z were mostly composed of the IgG heavy and light chains at 50–55 kDa and 20–30 kDa, respectively, as previously described.15,23 Because the initial pool of dog serum was generated from dogs with a history of GW infection, we were able to determine which IgG fractions reacted best with GW antigens by Western blot analysis using a polyclonal rabbit anti-dog IgG Fc detection antibody. Canine IgGs that recognize the GW band A antigen in total, unfractionated serum (T) were not evenly distributed between the IgG fractions. Reactivity was concentrated in peak Z and depleted from peaks X and Y (Figure 1). Antibodies to other GW antigens such as the complex at approximately 14 kDa and band at 55 kDa were also concentrated in peak Z, whereas antibodies to two GW proteins just above and below band A were concentrated in peaks X and Y. The relative amount of anti-GW antibodies in peak W was not accurately represented by the Western blot because, as described earlier, this fraction contained a large quantity of non-IgG protein.
Figure 1.
Reactivity of fractionated canine immunoglobulins with guinea worm (GW) proteins by Western blot. Guinea worm proteins extracted into sodium dodecyl sulfate (SDS) buffer as described in Materials and Methods were resolved on 10–22.5% gradient SDS polyacrylamide gels and then electro-blotted onto the polyvinylidene difluoride membrane. Membrane strips were incubated with fractionated canine immunoglobulins or GW-infected dog serum as described in the Materials and Methods section. Bound antibodies were detected using a biotinylated rabbit anti-dog IgG Fc antibody with the streptavidin/alkaline phosphatase system. B indicates a control strip incubated with Tween-phosphate buffered saline buffer alone. T indicates a strip incubated with serum from a dog infected with GW. Immunoglobulin fractions are labeled W, X, Y, and Z according to the convention of Mazza et al.15 The apparent molecular weights of See Blue Plus2 pre-stained markers (ThermoFisher Scientific, Waltham, MA) are indicated on the left. The location of the antigen band of interest (A) is indicated by an arrow.
Monoclonal antibody to canine IgG.
To produce a monoclonal antibody to dog IgG, peak Z was used as immunogen based on its high reactivity to GW band A proteins. Using the GW multiplex bead assay, the functional characteristics of monoclonal antibody 4E3D9 were compared with those of commercially available anti-canine IgG Fc, IgG1, and IgG2 secondary antibody reagents. For the DUF148–GST assay shown in Table 1, the ratio of the positive dog response to the negative dog response was < 2 for the commercial subclass-specific reagents. This ratio was 17.7 for the IgG Fc–specific secondary antibody and > 500 for new monoclonal antibody. Results of similar magnitude were obtained for the TRXL1–GST assay (Supplemental Table 1). For the HSP1–GST and HSP2–GST assays (Supplemental Tables 2 and 3, respectively), only monoclonal antibody 4E3D9 provided good discrimination between the positive and negative dog responses with ratios > 20. The sera from presumed negative dogs and from dogs with confirmed GW infection from Chad exhibited high background reactivity to the GST-negative control protein with the anti-canine IgG1 polyclonal antibody (Supplemental Table 4). The new 4E3D9 monoclonal antibody had the lowest background reactivity with these sera.
Table 1.
Reactivity (in median fluorescent intensity minus background units) of canine serum fractions with guinea worm domain of unknown function protein 148 antigen by multiplex bead assay
| Primary antibody | Rabbit anti-IgG Fc | Goat anti-IgG1 | Anti-IgG2 monoclonal | 4E3D9 monoclonal |
|---|---|---|---|---|
| Chad negative dog serum | 1,488 | 18,333 | 597 | 52 |
| Chad positive dog serum | 26,403 | 30,037 | 868 | 28,240 |
| IgG fraction W | 2,639 | 25,347 | 54 | 7,196 |
| IgG fraction X | 8,447 | 11,688 | 4,001 | 2,318 |
| IgG fraction Y | 17,600 | 16,415 | 11,438 | 3,131 |
| IgG fraction Z | 23,919 | 29,959 | 1,613 | 26,438 |
The reactivities of the fractionated canine IgGs were also measured by multiplex bead assay with the panel of secondary reagents. In this case, the magnitude of the response depended on the amount of antigen-specific antibody in the original dog serum pool, the IgG subclass distribution of that response, the efficiency of the antibody fractionation, and the specificity of the secondary detection reagents. Using the anti-IgG Fc secondary antibody for detection, peak Z contained the highest concentration of antibody to DUF148 (Table 1), peak X contained the highest concentration of antibody to TRXL1 (Supplemental Table 1), and peak Y contained the highest concentrations of antibody to the two HSP proteins (Supplemental Tables 2 and 3). Using the anti-IgG1 secondary antibody for detection, peak antibody concentrations to all four GW antigens were found in peak Z. These results are somewhat suspected, however, because of the high background responses previously noted with the negative dog serum and with the negative control GST-coupled beads. Responses measured with the anti-IgG2 secondary reagent were highest in either peak Y (DUF148) or peak X (HSPs and TRXL1). By contrast, the responses to all four GW antigens using monoclonal antibody 4E3D9 as the detection reagent were always highest in peak Z.
Multiplex bead assays of sera from dogs in endemic and non-endemic communities in Chad.
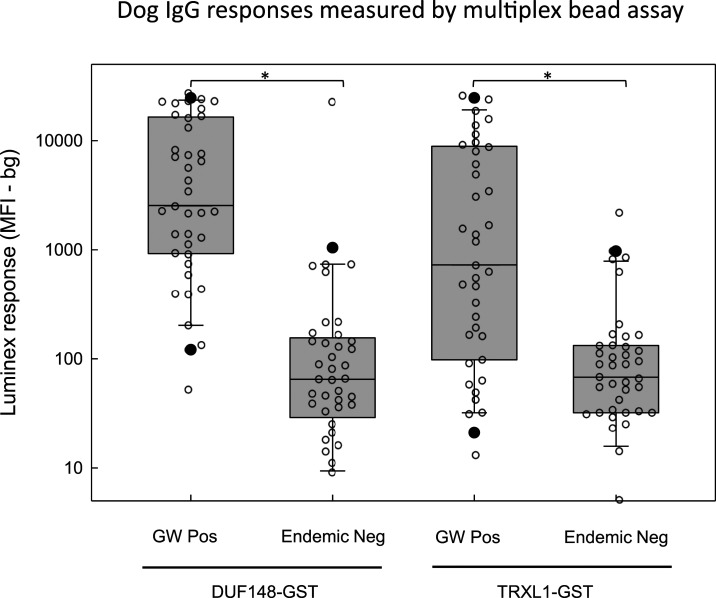
The GW-positive and endemic-negative panels of canine sera from Chad were analyzed by multiplex bead assay using the commercially available and newly developed monoclonal antibodies as secondary detection reagents. All the negative control sera from Chad were negative for D. immitis antigens by the commercial diagnostic test. Box and scatterplots for the GW DUF148–GST and TRXL1–GST assays using monoclonal antibody 4E3D9 as the detection antibody are presented in Figure 2. Antibody levels to the DUF148 antigen were significantly higher (Mann–Whitney rank sum test, P < 0.001) for the 39 GW-positive dogs (median = 2,543 MFI-bg; range = 52–27,883 MFI-bg) than those for the 41 endemic-negative dogs (median = 65 MFI-bg; range = 0–23,163 MFI-bg). Antibody responses to the TRXL1 antigen were also significantly higher (Mann–Whitney rank sum test, P < 0.001) for the positive dogs (median = 728 MFI-bg; range = 13–26,439 MFI-bg) than those for the endemic-negative dogs (median = 68 MFI-bg; range = 0–2,209 MFI-bg). Similarly, significant differences (P < 0.001) were observed for the HSP1 and HSP2 multiplex assays (Supplemental Figure 2); however, the maximum values were much lower for these antigens (HSP1 = 2270 MFI-bg; HSP2 = 3679 MFI-bg) than those for the DUF148 and TRXL1 assays. Responses to the negative control bead coated with GST alone were uniformly low, with a median value of 10.5 MFI-bg (range 0–615 MFI-bg).
Figure 2.
Multiplex bead assay detection of antibodies to recombinant DUF148–GST and TRXL1–GST fusion proteins. Multiplex bead assays were conducted as described in the Materials and Methods using magnetic beads covalently coated with either DUF148–GST (left) or TRXL1–GST (right). Biotinylated monoclonal secondary antibody 4E3D9 was used to detect responses in sera from dogs previously infected with guinea worm (n = 39) or in dog sera from a non-endemic region of Chad (n = 41). Individual values are indicated by open circles. Note that three negative values for the DUF148–GST assay and two negative values for the TRXL1–GST assay are not plotted as they fell below the range of the graph. Boxes include values between the 25th and 75th percentiles. Whiskers and closed circles represent the 10th and 90th and the 5th and 95th percentiles, respectively. Median values are indicated by a horizontal line within the boxes. Distributions that show statistically significant differences (P < 0.05) using the Kruskal–Wallis one-way analysis of variance on ranks are indicated by brackets with asterisks. DUF148 = domain of unknown function protein 148; GST = glutathione-S-transferase; TRXL1 = thioredoxin-like protein 1.
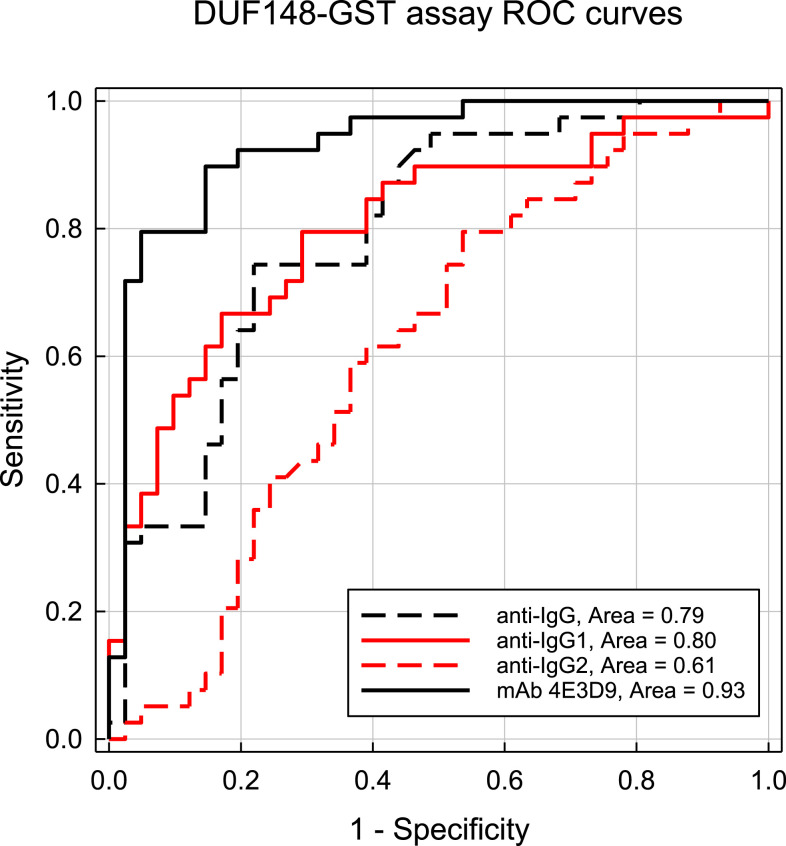
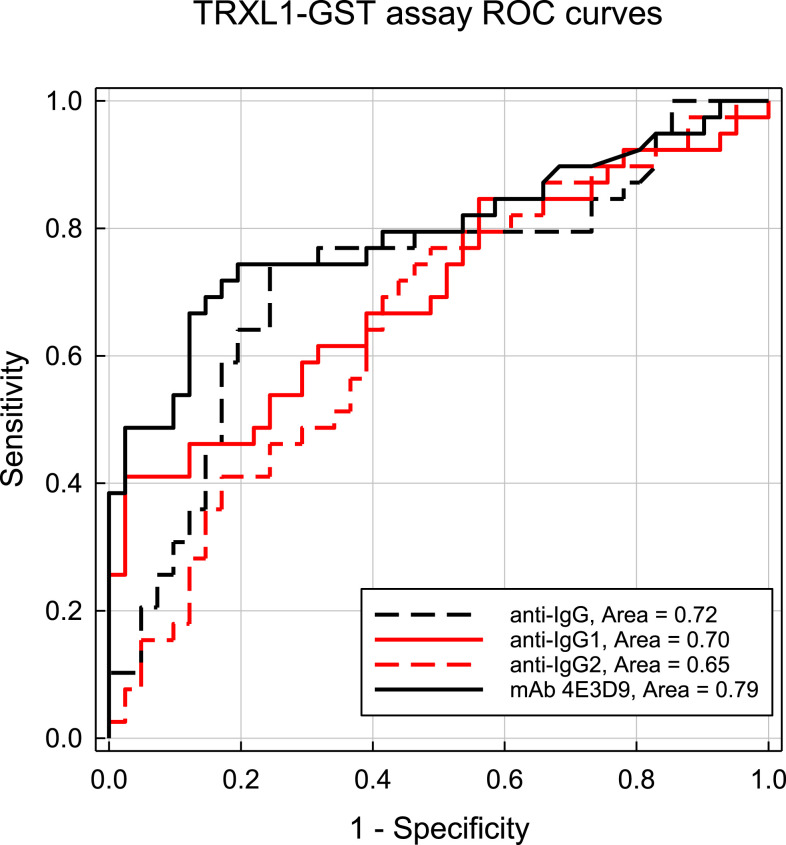
Receiver-operating characteristic curves for the DUF148–GST multiplex assay using the different secondary detection reagents are shown in Figure 3. Receiver-operating characteristic AUCs ranged from 0.93 using the 4E3D9 monoclonal antibody to 0.61 for the anti-IgG2 antibody. At a cutoff value of 305.5 MFI-bg, the DUF148 assay using the 4E3D9 monoclonal antibody reagent had a sensitivity of 89.7% and a specificity of 85.4%. Receiver-operating characteristic AUCs for the TRXL1–GST multiplex assays were lower (Figure 4), ranging from 0.79 for the 4E3D9 monoclonal antibody to 0.65 for the anti-IgG2 antibody. At a cutoff value of 161.5 MFI-bg, the TRXL1 assay using the 4E3D9 monoclonal antibody reagent had a sensitivity of 74.4% and a specificity of 80.5%. An improved specificity of 92.7% can be achieved using a “simultaneously positive by both assays” definition with the 305.5 and 161.5 MFI-bg cutoff values for the DUF148–GST and TRXL1–GST assays, respectively. However, this combined assay definition of positivity reduces the overall sensitivity to 71.8% and results in a Youden’s J-index value (0.645) below that of the DUF148–GST antigen assay alone (J-index = 0.751). For the HSP1–GST and HSP2–GST multiplex assays, the largest ROC AUC (0.78 for both assays) was also observed with the 4E3D9 monoclonal antibody reagent (Supplemental Figures 3 and 4, respectively).
Figure 3.
Receiver-operating characteristic curve results of responses from DUF148–GST multiplex bead assays using different detection reagents. Curves were constructed using MFI-bg responses of samples collected from individuals with previous guinea worm infection (n = 39) or from individuals from a non-endemic region of Chad (n = 41). Antibody responses were detected with an anti-IgG Fc polyclonal antibody (anti-IgG, black dashed line), an anti-IgG1 polyclonal antibody (anti-IgG1, red line), an anti-IgG2 monoclonal antibody (anti-IgG2, red dashed line), or the 4E3D9 monoclonal antibody (anti-IgG4, black line) as described in the Materials and Methods section. The optimal threshold for the DUF148–GST multiplex assay with the rabbit anti-IgG Fc polyclonal antibody was 2,242 MFI-bg with a sensitivity of 74.4% and a specificity of 78%. The optimal threshold with the goat anti-IgG1 polyclonal antibody was 17,369 MFI-bg with a sensitivity of 79.5% and a specificity of 70.7%. The optimal threshold with the anti-IgG2 monoclonal antibody was 716 MFI-bg with a sensitivity of 61.5% and a specificity of 61.0%. The optimal assay threshold for the 4E3D9 monoclonal antibody was 305.5 MFI-bg with a sensitivity of 88.7% and a specificity of 85.4%. DUF148 = domain of unknown function protein 148; GST = glutathione-S-transferase; MFI-bg = median fluorescent intensity minus background. This figure appears in color at www.ajtmh.org.
Figure 4.
Receiver-operating characteristic curve results of responses from TRXL1–GST multiplex bead assays using different detection reagents. Curves were constructed as described in Figure 3. The optimal threshold for the TRXL1–GST multiplex assay with the rabbit anti-IgG Fc polyclonal antibody was 2,283 MFI-bg with a sensitivity of 74.4% and a specificity of 75.6%. The optimal threshold with the goat anti-IgG1 polyclonal antibody was 17,465 MFI-bg with a sensitivity of 61.5% and a specificity of 67.5%. The optimal threshold with the anti-IgG2 monoclonal antibody was 775 MFI-bg with a sensitivity of 64.1% and a specificity of 61.0%. The optimal assay threshold for the with the 4E3D9 monoclonal antibody was 161.5 MFI-bg with a sensitivity of 74.4% and a specificity of 80.5%. MFI-bg = median fluorescent intensity minus background; TRXL1 = thioredoxin-like protein 1. This figure appears in color at www.ajtmh.org.
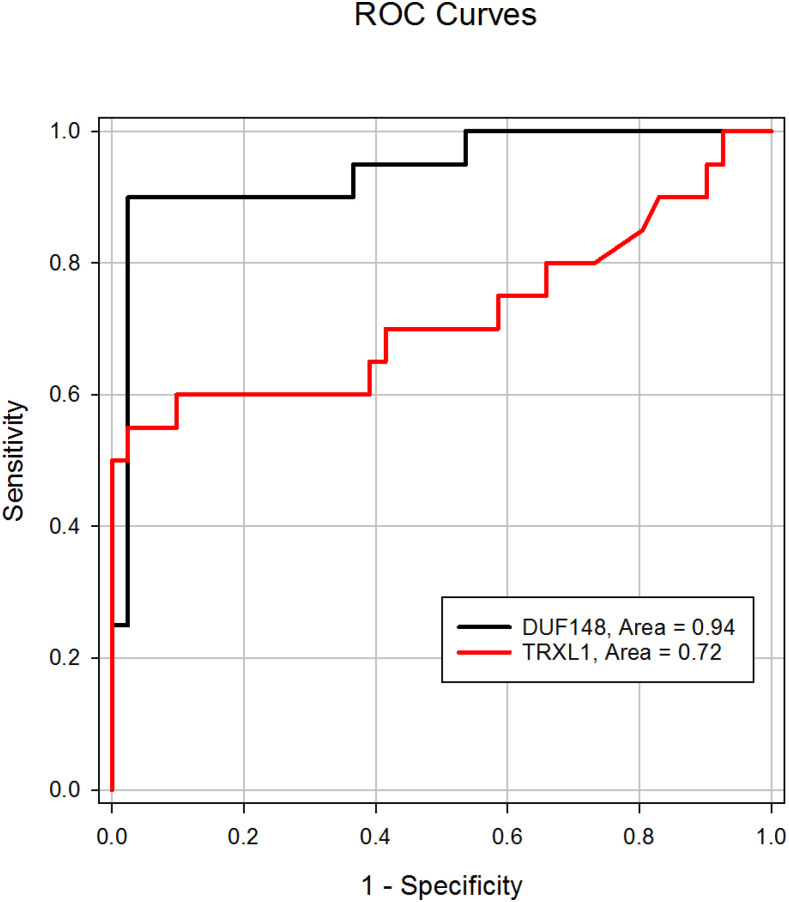
Compared with ROC curves generated with the complete Chad sample set shown previously, curves generated with monoclonal antibody 4E3D9 using the negative samples and samples (n = 20) collected from dogs that had a GW emergence in 2016 (Figure 5) had a slightly greater ROC AUC for the DUF148–GST assay (0.94) but had a smaller area for the TRXL1–GST assay (0.72). At a cutoff of 1107 MFI-bg for the DUF148 assay, the 90.0% sensitivity observed for the 2016 infected dogs was similar to that observed with the complete Chad GW-positive sample set described earlier (89.7%), but the specificity was much greater (97.6% versus 85.4%). Despite the similar ROC AUCs for the two assays (0.94 versus 0.93, respectively), Youden’s J-index for the DUF148 assay using the 2016 infection samples (0.876) reflects this improved specificity and is higher than the J-index for the entire Chad GW-positive sample set (0.751). The TRXL1–GST multiplex assay of the 2016 infection samples demonstrated a similar improvement in specificity (cutoff = 680; 90.2% specificity), but a decrease in the sensitivity to 60% resulted in a decrease in J-index compared with the results from the complete Chad GW-positive sample set (0.502 versus 0.549, respectively).
Figure 5.
Receiver-operating characteristic (ROC) curve results of responses from DUF148–GST and TRXL1–GST multiplex bead assays with sera from individuals with recent guinea worm (GW) infections. Curves were constructed using MFI-bg responses of samples collected from individuals with GW infection in 2016 (n = 20) or from individuals from a non-endemic region of Chad (n = 41). Antibody responses were detected using the 4E3D9 monoclonal antibody as described in Figure 3. The optimal threshold for the DUF148–GST assay was 1107 MFI-bg with a sensitivity of 90.0% and a specificity of 97.6%. The optimal threshold for the TRXL1–GST assay was 680 MFI-bg with a sensitivity of 60.0% and a specificity of 90.2%. DUF148 = domain of unknown function protein 148; GST = glutathione-S-transferase; MFI-bg = median fluorescent intensity minus background; TRXL1 = thioredoxin-like protein 1. This figure appears in color at www.ajtmh.org.
Time course of GW-specific antibody responses in experimentally infected dogs.
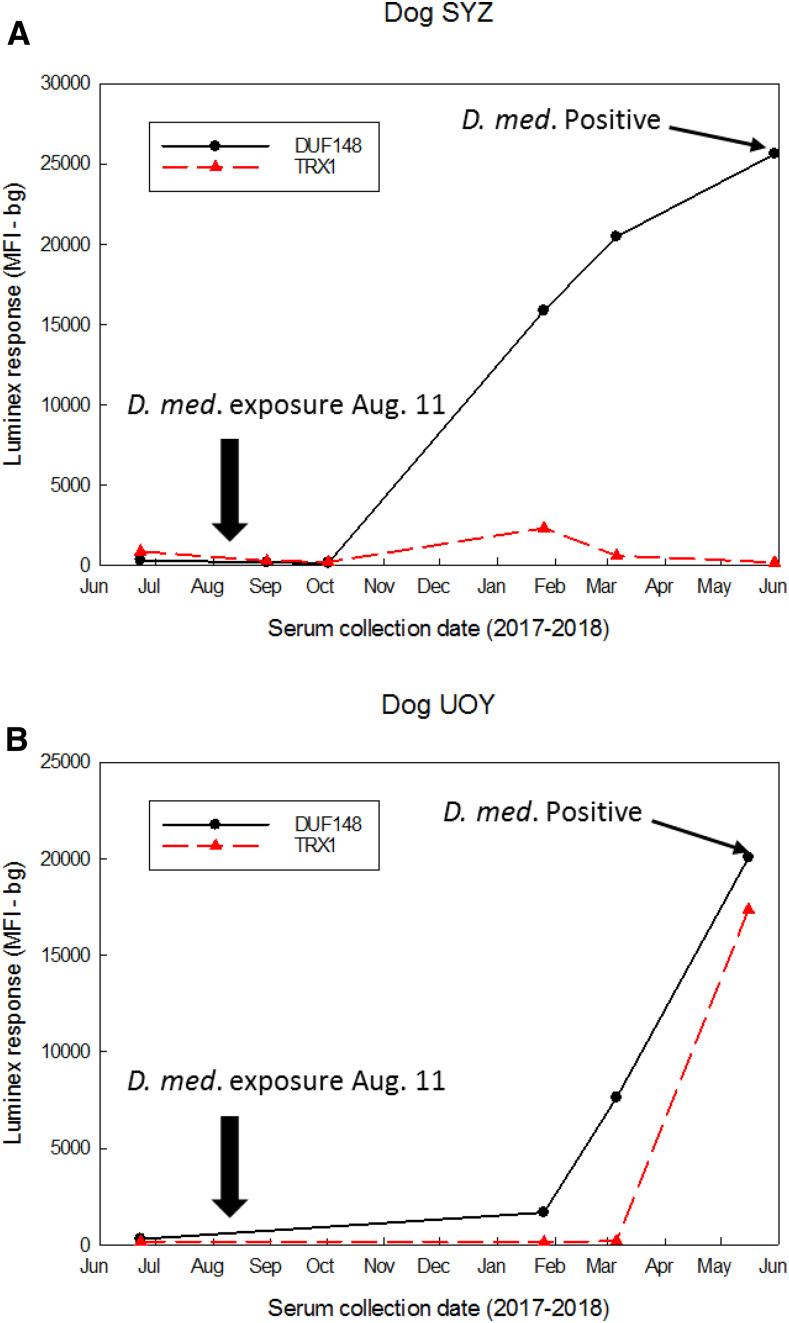
Serum samples collected periodically from two dogs experimentally infected with D. medinensis were used to determine the time course of antibody development during worm growth and maturation. These samples were assayed for GW response by multiplex bead assay with the monoclonal antibody 4E3D9 as secondary detection antibody (Figure 6). Using the more specific, higher cutoffs from the 2016 infection ROC curves, both dogs were negative for antibodies to DUF148 and TRXL1 before copepod exposure. Dog SYZ was negative for antibodies to both antigens at 19 and 71 days postexposure but was positive for antibodies to both antigens by 168 days (24 weeks [∼5.6 months]). Antibody levels to the DUF148 antigen increased at 219 days (31 weeks [∼7.3 months]) and again at 292 days (42 weeks [∼10 months]) postexposure, whereas the response to TRXL1 declined and then reverted to negative status by 292 days. Dog UOY had seroconverted for antibodies to the DUF148 antigen by 168 days postexposure but remained negative for a TRXL1 response until sometime after 219 days postexposure. No responses to HSP1 or HSP2 were detected at any postexposure time point. Subcutaneous adult female worms were confirmed in both dogs when they were necropsied at 292 and 278 days (40 weeks [∼9.3 months]) postexposure, respectively.
Figure 6.
Time course of antibody development to DUF148–GST and TRXL1–GST in dogs experimentally exposed to guinea worm (GW). Curves were constructed using MFI-bg responses of serum samples collected from dog SYZ (A) and dog UOY (B) on the indicated dates. Animals were exposed to copepods containing Dracunculus medinensis L3 larvae at the time point indicated by the black arrow. Multiplex responses to the TRXL1–GST are indicated by the black line, and responses to the DUF148–GST are indicated by the red dashed line. Prepatent GW infection was confirmed at the termination of the study by the detection of subcutaneous gravid female worms. DUF148 = domain of unknown function protein 148; GST = glutathione-S-transferase; MFI-bg = median fluorescent intensity minus background; TRXL1 = thioredoxin-like protein 1. This figure appears in color at www.ajtmh.org.
DISCUSSION
One of the major impediments to the development of a multiplex bead assay for the quantitation of canine antibodies to GW was the absence of a suitable and well-characterized anti-dog IgG secondary antibody reagent. Although we were able to use a rabbit anti-dog IgG Fc polyclonal antibody to detect dog antibodies that bound to GW antigens by Western blot, multiplex bead assays using this detection reagent had ROC AUCs that were classified as “poor” to “fair” (0.65–0.79).24,25 As for IgG subclass detection reagents, the specificities of commercial secondary antibodies against canine IgG1 and IgG2 have recently been called into question.15,23,26 In our hands, multiplex assays using a polyclonal goat anti-dog IgG1 reagent had high background reactivity to the GST-negative control bead, demonstrated consistently high reactivity with presumed negative sera from Chad, and had ROC-defined cutoffs > 17,000 MFI-bg for both the TRXL1–GST and DUF148–GST antigens. Only one anti-dog IgG monoclonal antibody is commercially available, and this antibody was reported to recognize IgG2.20 Perhaps, because of a skewed host IgG subclass response to the GW parasite antigens, multiplex bead assays using this detection reagent consistently had ROC AUCs in the “poor” range (< 0.70).25 Mazza et al.15,27 have reported the generation of a panel of monoclonal antibodies against four canine IgG subclasses, but these reagents were not available for our work.
Given these results, we decided to replicate the dog IgG fractionation of Mazza et al.15 in an effort to generate a monoclonal reagent that could be used for an optimized GW multiplex assay. We used pooled sera from GW-infected dogs as the starting material so that we could monitor the distribution of the GW-specific response during the fractionation procedure. Although our fractionation scheme was not as rigorous as that reported by Mazza et al.15 (their fractions were cycled over protein A and protein G columns a total of three times), we observed that various antibody responses measured by GW Western blot were differentially enriched in our peak fractions X, Y, and Z. Antibodies recognizing GW proteins in the band A region were concentrated in the peak Z fraction. In humans, GW responses are often dominated by the IgG4 subclass of antibodies,12,28–30 and it is interesting to note that Mazza et al.15 have described peak Z as containing the canine equivalent of the human IgG4 antibody. We have not attempted to further classify the IgG proteins in this fraction with respect to the γ chain protein sequence,31 but the strong protein A binding characteristics of peak Z do not match those reported for canine IgGs designated as IgG4 by Bergeron et al.32
Monoclonal antibody 4E3D9 was generated against the dog IgG fraction Z and was used in the GW multiplex bead assays. With this new detection reagent, the ROC AUC of the DUF148–GST improved to 0.93 in the “excellent” range,25 but the ROC areas for TRXL1–GST, HSP1–GST, and HSP2–GST assays only increased to 0.78–0.79. Based on the dog fraction X and fraction Y responses to the TRXL1 and HSP antigens using anti-IgG Fc as the detection reagent (shown in Supplemental Tables 1–3), a monoclonal secondary antibody with a specificity different from antibody 4E3D9 may be required to maximize assay performance for these three antigens. The results of the dog assays contrast with those observed earlier with the human GW multiplex assay using an anti-IgG4 detection reagent12; with the human assay, the TRXL1–GST assay had the largest ROC AUC at 0.90, whereas the DUF148–GST assay had an area of 0.86, and responses to the two HSP were negligible. Whether these observations reflect differences in the capacities of the dog and human immune systems to respond to the various antigens or, perhaps, differences in infection history would require additional human and dog sera to extend and confirm the observations.
Similar to the human GW multiplex assay (Priest et al., submitted), the performance of the dog DUF148–GST multiplex assay was maximized if GW-positive samples collected > 1 year from the date of worm emergence were excluded from the analysis. With this more limited sample set, the DUF148–GST assay ROC AUC was 0.94 with a sensitivity and specificity of 90.0% and 97.6%, respectively. This result is not unexpected, given that circulating antibody concentrations in humans decline following GW emergence and resolution of infection.28,29,33,34 Unfortunately, the number of dog samples and the timing of collection in our study were not sufficient to estimate antibody half-life.
For the purposes of the GWEP, the most important finding of this report was that antibody responses to GW antigens can be detected in infected (but otherwise asymptomatic) dogs within 6 months of exposure to infected copepods which is well before worm emergence. Currently, the only way to know a dog is infected with GW is to wait for a worm to create a swelling or for it to emerge, at which point the dog may have already contaminated a water source. Prepatent antibody responses have previously been detected in humans.29,33 The antibody response curves for two animals infected in a laboratory setting (shown in Figure 6) suggest that paired, longitudinal samples from the same animal 1–2 months apart might allow the differentiation of previously infected dogs with a declining antibody response from dogs with increasing responses caused by prepatent worm development. The presence of detectable IgG responses in dogs 4–8 months before likely GW emergence may be useful in that infected animals could be identified and isolated long before they are capable of contaminating vulnerable water supplies with GW L1-stage larvae.
One weakness of the current study is that the positive serum set used to generate our assay sensitivity estimates did not include sera from dogs with known prepatent infections. Because of the importance of asymptomatically infected dogs to the elimination program, longitudinal studies in an endemic setting will be required to generate a positive sample set that includes substantial numbers of these sera for a more thorough analysis of assay sensitivity. An additional weakness is that we were unable to adequately address the issue of potential cross-reactivity caused by infections with nematode parasites other than D. medinensis. Cross-reactivity is of some concern because sera from patients with Onchocerca volvulus infections reacted with DUF148–GST and, to a lesser extent, with TRXL1–GST in the human multiplex assay (Priest et al., submitted). Onchocerca lupi, Dirofilaria spp., Brugia spp., and Acanthocheilonema sp. are the nematode infections of dogs that are most likely to induce a cross-reactive response, and Dirofilaria spp. and Acanthocheilonema sp. have been reported in parts of Eastern Africa.35–37 Commercial assays are not available for most of these parasites, but dogs from the region near Lake Chad that was not endemic for GW were negative for D. immitis antigens. However, it is unknown if this represents the situation in the remainder of Chad. Also, some data indicate that the commercial D. immitis antigen tests will cross-react with some other parasites (e.g., Dracunculus insignis, Angiostrongylus vasorum, Acanthocheilonema odendhali, and Spirocerca lupi), but the extent of cross-reactivity is unknown.38–41 Future work in Chad will use PCR to amplify nucleic acid target sequences from the microfilaria or from their Wolbachia sp. endosymbionts to identify potential cross-reacting parasitic infections in dogs.42–44
In conclusion, we report the development of a multiplex bead assay that is capable of detecting IgG antibody responses to GW in sera from dogs. The DUF148–GST assay was 89.7% sensitive and 85.4% specific with an ROC AUC of 0.93. Limiting the positive sample set to those sera collected within 1 year of GW emergence allowed the assignment of a higher cutoff value and resulted in a specificity of 97.6%. In laboratory-infected dogs, the antibody responses were detectable 4–8 months before worm emergence. We are now using the assays to monitor longitudinal responses in a large, multiyear study of dogs in a highly endemic region of Chad.
Supplemental figures and tables
Acknowledgments:
We would like to thank the animal resources’ staff of the UGA College of Veterinary Medicine for the excellent husbandry and care of the study animals. We also thank K. Garrett and E. Box for laboratory assistance. We would like to thank DELTAS Africa Initiative (Afrique One-ASPIRE/DEL-15-008) for its financial support during the field data collection. We also thank IRED and the Chadian Ministry of Livestock and Pastoral Development for the support of the study and all the dog owners for allowing us to sample their animals.
Note: Supplemental figures and tables appear at www.ajtmh.org.
REFERENCES
- 1.World Health Assembly , 1986. Elimination of Dracunculiasis: Resolution of the 39th World Health Assembly. Resolution WHA 39.21 Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2.Watts SJ, 1987. Dracunculiasis in Africa in 1986: its geographic extent, incidence, and at-risk population. Am J Trop Med Hyg 37: 119–125. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins DR, Ruiz-Tiben E, Eberhard ML, Roy SL, 2015. Progress toward global eradication of dracunculiasis, January 2014–June 2015. MMWR Morb Mortal Wkly Rep 64: 1161–1165. [DOI] [PubMed] [Google Scholar]
- 4.WHO Collaborating Center for Dracunculiasis Eradication CDC , 2020. Guinea Worm Wrap-Up #266. Available at: https://www.cartercenter.org/resources/pdfs/news/health_publications/guinea_worm/wrap-up/266.pdf. Accessed July 16, 2020. [Google Scholar]
- 5.The Lancet , 2019. Guinea worm disease eradication: a moving target. Lancet 393: 1261. [DOI] [PubMed] [Google Scholar]
- 6.Eberhard ML, et al. 2014. The peculiar epidemiology of dracunculiasis in Chad. Am J Trop Med Hyg 90: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hopkins DR, Weiss AJ, Roy SL, Zingeser J, Guagliardo SAJ, 2019. Progress toward global eradication of dracunculiasis - January 2018–June 2019. MMWR Morb Mortal Wkly Rep 68: 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz-Tiben E, Hopkins DR, 2006. Dracunculiasis (Guinea worm disease) eradication. Adv Parasitol 61: 275–309. [DOI] [PubMed] [Google Scholar]
- 9.Cleveland CA, Eberhard ML, Thompson AT, Smith SJ, Zirimwabagabo H, Bringolf R, Yabsley MJ, 2017. Possible role of fish as transport hosts for Dracunculus spp. larvae. Emerg Infect Dis 23: 1590–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cleveland CA, Eberhard ML, Thompson AT, Garrett KB, Swanepoel L, Zirimwabagabo H, Moundai T, Ouakou PT, Ruiz-Tiben E, Yabsley MJ, 2019. A search for tiny dragons (Dracunculus medinensis third-stage larvae) in aquatic animals in Chad, Africa. Sci Rep 9: 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonald RA, Wilson-Aggarwal JK, Swan GJF, Goodwin CED, Moundai T, Sankara D, Biswas G, Zingeser JA, 2020. Ecology of domestic dogs Canis familiaris as an emerging reservoir of Guinea worm Dracunculus medinensis infection. PLoS Negl Trop Dis 14: e0008170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Priest JW, Stuchlik O, Reed M, Soboslay P, Cama V, Roy SL, 2020. Development of a multiplex bead assay for the detection of IgG antibody responses to guinea worm. Am J Trop Med Hyg (Epub ahead of print, September 8, 2020). Available at: https://doi.org/10.4269/ajtmh.20-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eberhard ML, Yabsley MJ, Zirimwabagabo H, Bishop H, Cleveland CA, Maerz JC, Bringolf R, Ruiz-Tiben E, 2016. Possible role of fish and frogs as paratenic hosts of Dracunculus medinensis, Chad. Emerg Infect Dis 22: 1428–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eberhard ML, Ruiz-Tiben E, Wallace SV, 1988. Dracunculus insignis: experimental infection in the ferret, Mustela putorius furo. J Helminthol 62: 265–270. [DOI] [PubMed] [Google Scholar]
- 15.Mazza G, Duffus WP, Elson CJ, Stokes CR, Wilson AD, Whiting AH, 1993. The separation and identification by monoclonal antibodies of dog IgG fractions. J Immunol Methods 161: 193–203. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli UK, 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 17.Moss DM, Montgomery JM, Newland SV, Priest JW, Lammie PJ, 2004. Detection of cryptosporidium antibodies in sera and oral fluids using multiplex bead assay. J Parasitol 90: 397–404. [DOI] [PubMed] [Google Scholar]
- 18.Priest JW, Moss DM, 2020. Measuring Cryptosporidium serologic responses by multiplex bead assay. Methods Mol Biol 2052: 61–85. [DOI] [PubMed] [Google Scholar]
- 19.Waterboer T, Sehr P, Pawlita M, 2006. Suppression of non-specific binding in serological Luminex assays. J Immunol Methods 309: 200–204. [DOI] [PubMed] [Google Scholar]
- 20.Arce C, Moreno A, Millan Y, Martin de las Mulas J, Llanes D, 2002. Production and characterization of monoclonal antibodies against dog immunoglobulin isotypes. Vet Immunol Immunopathol 88: 31–41. [DOI] [PubMed] [Google Scholar]
- 21.Zweig MH, Campbell G, 1993. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 39: 561–577. [PubMed] [Google Scholar]
- 22.Youden WJ, 1950. Index for rating diagnostic tests. Cancer 3: 32–35. [DOI] [PubMed] [Google Scholar]
- 23.Donaghy D, Moore AR, 2020. Identification of canine IgG and its subclasses, IgG1, IgG2, IgG3 and IgG4, by immunofixation and commercially available antisera. Vet Immunol Immunopathol 221: 110014. [DOI] [PubMed] [Google Scholar]
- 24.Swets JA, 1988. Measuring the accuracy of diagnostic systems. Science 240: 1285–1293. [DOI] [PubMed] [Google Scholar]
- 25.Youngstrom EA, 2014. A primer on receiver operating characteristic analysis and diagnostic efficiency statistics for pediatric psychology: we are ready to ROC. J Pediatr Psychol 39: 204–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Day MJ, 2007. Immunoglobulin G subclass distribution in canine leishmaniosis: a review and analysis of pitfalls in interpretation. Vet Parasitol 147: 2–8. [DOI] [PubMed] [Google Scholar]
- 27.Mazza G, Whiting AH, Day MJ, Duffus WP, 1994. Preparation of monoclonal antibodies specific for the subclasses of canine IgG. Res Vet Sci 57: 140–145. [DOI] [PubMed] [Google Scholar]
- 28.Bloch P, Simonsen PE, Vennervald BJ, 1993. The antibody response to Dracunculus medinensis in an endemic human population of northern Ghana. J Helminthol 67: 37–48. [DOI] [PubMed] [Google Scholar]
- 29.Bloch P, Simonsen PE, 1998. Immunoepidemiology of Dracunculus medinensis infections I. Antibody responses in relation to infection status. Am J Trop Med Hyg 59: 978–984. [DOI] [PubMed] [Google Scholar]
- 30.Garate T, Kliks MM, Cabrera Z, Parkhouse RM, 1990. Specific and cross-reacting antibodies in human responses to Onchocerca volvulus and Dracunculus medinensis infections. Am J Trop Med Hyg 42: 140–147. [DOI] [PubMed] [Google Scholar]
- 31.Tang L, Sampson C, Dreitz MJ, McCall C, 2001. Cloning and characterization of cDNAs encoding four different canine immunoglobulin gamma chains. Vet Immunol Immunopathol 80: 259–270. [DOI] [PubMed] [Google Scholar]
- 32.Bergeron LM, McCandless EE, Dunham S, Dunkle B, Zhu Y, Shelly J, Lightle S, Gonzales A, Bainbridge G, 2014. Comparative functional characterization of canine IgG subclasses. Vet Immunol Immunopathol 157: 31–41. [DOI] [PubMed] [Google Scholar]
- 33.Fagbemi BO, Hillyer GV, 1990. Immunodiagnosis of dracunculiasis by falcon assay screening test-enzyme-linked immunosorbent assay (FAST-ELISA) and by enzyme-linked immunoelectrotransfer blot (EITB) technique. Am J Trop Med Hyg 43: 665–668. [DOI] [PubMed] [Google Scholar]
- 34.Bloch P, Simonsen PE, 1998. Immunoepidemiology of Dracunculus medinensis infections II. variation in antibody responses in relation to transmission season and patency. Am J Trop Med Hyg 59: 985–990. [DOI] [PubMed] [Google Scholar]
- 35.Schwan EV, Durand DT, 2002. Canine filariosis caused by Dirofilaria immitis in Mozambique: a small survey based on the identification of microfilariae. J S Afr Vet Assoc 73: 124–126. [DOI] [PubMed] [Google Scholar]
- 36.Albrechtova K, Sedlak K, Petrzelkova KJ, Hlavac J, Mihalca AD, Lesingirian A, Kanyari PW, Modry D, 2011. Occurrence of filaria in domestic dogs of Samburu pastoralists in northern Kenya and its associations with canine distemper. Vet Parasitol 182: 230–238. [DOI] [PubMed] [Google Scholar]
- 37.Matola YG, 1991. Periodicity of Dirofilaria immitis microfilariae in a dog from Muheza district, Tanzania. J Helminthol 65: 76–78. [PubMed] [Google Scholar]
- 38.Aroch I, Rojas A, Slon P, Lavy E, Segev G, Baneth G, 2015. Serological cross-reactivity of three commercial in-house immunoassays for detection of Dirofilaria immitis antigens with Spirocerca lupi in dogs with benign esophageal spirocercosis. Vet Parasitol 211: 303–305. [DOI] [PubMed] [Google Scholar]
- 39.Schnyder M, Deplazes P, 2012. Cross-reactions of sera from dogs infected with Angiostrongylus vasorum in commercially available Dirofilaria immitis test kits. Parasit Vectors 5: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krucik DD, Van Bonn W, Johnson SP, 2016. Association between positive canine heartworm (Dirofilaria immitis) antigen results and presence of Acanthocheilonema odendhali microfilaria in California sea lions (Zalophus californianus). J Zoo Wildl Med 47: 25–28. [DOI] [PubMed] [Google Scholar]
- 41.Williams BM, et al. 2018. Dracunculus infections in domestic dogs and cats in North America; an under-recognized parasite? Vet Parasitol Reg Stud Rep 13: 148–155. [DOI] [PubMed] [Google Scholar]
- 42.Satjawongvanit H, Phumee A, Tiawsirisup S, Sungpradit S, Brownell N, Siriyasatien P, Preativatanyou K, 2019. Molecular analysis of canine filaria and its Wolbachia endosymbionts in domestic dogs collected from two animal university hospitals in Bangkok Metropolitan region, Thailand. Pathogens 8: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rishniw M, Barr SC, Simpson KW, Frongillo MF, Franz M, Dominguez Alpizar JL, 2006. Discrimination between six species of canine microfilariae by a single polymerase chain reaction. Vet Parasitol 135: 303–314. [DOI] [PubMed] [Google Scholar]
- 44.Slatko BE, Taylor MJ, Foster JM, 2010. The Wolbachia endosymbiont as an anti-filarial nematode target. Symbiosis 51: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.