Abstract.
Bartonella henselae is a zoonotic Gram-negative Bacillus associated with self-limited regional lymphadenopathy. In recent decades, an expanding spectrum of clinical manifestations has been described, in part, due to improved diagnostics. However, updated epidemiological data are sparse. We retrospectively reviewed the clinical features of 31 patients with B. henselae infection over 15 years from 2005 to 2019, in the tropical Top End of Australia. Our annual disease incidence of 1.3 cases per 100,000 population is lower than that in the national database surveillances in the United States, but the hospitalization incidence of 0.9 per 100,000 population in our region is higher than those reported in the literature, with an average length of stay of 9 days. Patients were more commonly male, aboriginal, and aged less than 14 years (median age: 7 years), living in a rural setting with presentation during our monsoon season. The disease spectrum included lymph node disease (74%), organ peliosis, endocarditis, cutaneous lesions, parapharyngeal abscess, parotitis, and neurologic and ocular syndromes. Lymph node disease was far commoner in children than the more serious systemic B. henselae infections associated with adults (P = 0.074). Although no deaths were reported, significant morbidities were observed. Two endocarditis cases presented with glomerulonephritis, and hematological and neurological features mimicking vasculitis, and consequently received immunosuppressants. One case was only diagnosed after representation with serial embolic strokes. Given the heterogeneity of disease manifestations with nonspecific symptoms and significant consequences, a timely and accurate diagnosis is needed to avoid unnecessary treatments or interventions.
INTRODUCTION
Bartonella species primarily affects animals, with humans often infected incidentally. Of the 20 or more described species, three species are responsible for the vast majority of human bartonellosis. This includes Bartonella henselae—a globally endemic slow-growing fastidious Gram-negative Bacillus typically associated with self-limited regional lymphadenopathy (cat scratch disease [CSD]). Bartonella henselae is linked epidemiologically to cats and the cat flea vector Ctenocephalides felis.1 Since the discovery of B. henselae as the causative agent for CSD and availability of serological testing in the early 1990s, the clinical spectrum of B. henselae infection has expanded considerably, especially in the last 20 years.1 Reported manifestations of B. henselae beyond the eponymous lymph node disease include bacillary angiomatosis, visceral organ peliosis, endocarditis, cutaneous lesions, and neurologic and ocular syndromes.1–4 Other than a multicenter study by Tsuneoka and Tsukahara,5 broad epidemiological exploration of the disease spectrum from a geographic region, in particular a tropical region, is sparse. We reviewed the incidence, clinical spectrum, and disease attributed to B. henselae infection in patients presenting to hospitals in the tropical Top End of Australia.
METHODS
The Top End of the Northern Territory (TENT) encompasses the northernmost section of Australia, covering an area in excess of 500,000 km2 with a sparse population of 180,000 and a distinct tropical monsoonal season. The TENT in this study is defined by statistical area three regions of Darwin urban (Darwin city, Darwin suburbs, and Palmerston), Litchfield, Daly-Tiwi-West Arnhem, East Arnhem, and Katherine by the Australian Bureau of Statistics (ABS).6 Patients with probable B. henselae infection were identified retrospectively by review of clinical presentations to the public hospitals in Darwin, Palmerston, Katherine, and Gove with positive B. henselae serology between May 2005 and November 2019 using the centralized laboratory Labtrak (Intersystems, Cambridge, MA) database. Bartonella henselae infection was considered probable if the following two criteria were met—1) seropositivity, B. henselae serology was considered positive if the IgG titer was ≥ 1:64 on indirect fluorescent antibody assay and 2) the presence of at least two of the following four additional criteria: 1) clinical symptoms compatible with bartonellosis, 2) detection of B. henselae DNA in tissue or whole blood by PCR, 3) histopathology findings typical of bartonellosis, or 4) exclusion of other clinical diagnoses on review of investigations, serology, and clinical features. These criteria were chosen as serological results alone can lack some degree of specificity leading to false-positive results.1,7
The annualized disease incidence was calculated using census data from 2011 (study midpoint) obtained from ABS. Areas outside of Darwin urban is considered rural. Incidence rate calculation and simple data analysis were performed using Microsoft Excel. Categorical variables were analyzed with two-tailed Fisher’s exact test using SPSS (IBM Corp., Armonk, NY, released 2017. IBM SPSS Statistics for Windows, version 25.0). The map was created using QGIS 3.10 (Boston, MA).8 This study was approved by the Human Research Ethics Committee of TENT Department of Health and Menzies School of Health Research (Human Research Ethics Committee 2019–3444).
RESULTS
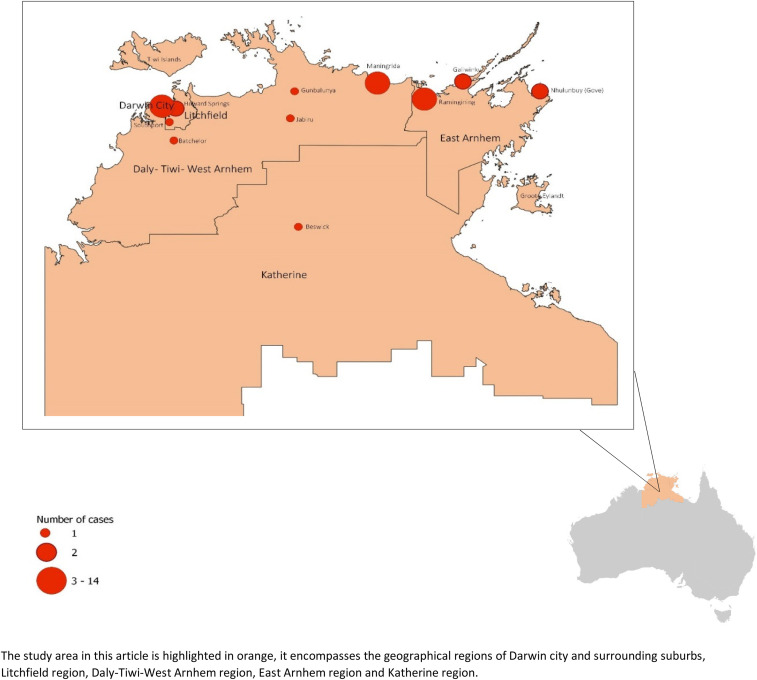
Of the 15-year study period, 363 patients had B. henselae serology requested in TENT. Bartonella henselae serology was positive in 40 patients, and 31 patients fulfilled the criteria for B. henselae infection. Of the 31 patients, the median age was 7 years, and it was apparent that there were two distinct age-groups represented, those younger than 14 years (68% of the patient group); and an older cohort, with a median age of 33 years. The demographic data of the 31 patients are presented in Table 1. There was a slight male preponderance and overrepresentation of rural residence and aboriginal patients, although not statistically significant. Geographical distribution of the place of residence of the 31 patients is depicted in Figure 1.
Table 1.
Demographics of patients with Bartonella henselae infection in the Top End from 2005 to 2019
| Parameter | Age less than 14 years | Age older than 14 years | Total | P-value |
|---|---|---|---|---|
| Number, n (%) | 21 (68) | 10 (32) | 31 (100) | |
| Age, median (interquartile range) (years) | 5 (3–7) | 33 (27–51) | 7 (5–25) | |
| Gender, n (%) | ||||
| Male | 12 (57) | 6 (60) | 18 (58) | 1.00 |
| Female | 9 (43) | 4 (40) | 13 (42) | |
| Ethnicity, n (%) | ||||
| Aboriginal | 14 (67) | 5 (50) | 19 (61) | 0.45 |
| Non-Aboriginal | 7 (33) | 5 (50) | 12 (39) | |
| Residence, n (%) | ||||
| Urban | 8 (38) | 6 (60) | 14 (45) | 0.44 |
| Rural and remote | 13 (62) | 4 (40) | 17 (55) | |
| Alcohol excess and/or homeless | 0 (0) | 5 (55) | 5 (17) | |
| Seasonality, n (%) | ||||
| Monsoon | 12 (57) | 2 (20) | 14 (45) | |
| Dry | 3 (14) | 3 (30) | 6 (19) | |
| Wet season “build-up” | 6 (29) | 5 (50) | 11 (36) |
Monsoon season in the Top End includes the months of January, February, March, and April; dry season in the Top End includes the months of May, June, July, and August; wet season “build-up” months are September, October, November, and December.
Figure 1.
Geographical distribution of Bartonella henselae infections in the Top End from 2005 to 2019. This figure appears in color at www.ajtmh.org.
Incidence.
Overall, annual infection incidence was 1.3 cases per 100,000 population. Incidence in aboriginal children aged less than 14 years was markedly higher than that in non-aboriginal children, with a rate of 7.4 versus 2.0 cases per 100,000 per year, respectively. The annual incidences of rural and urban cases were 1.7 versus 1.0 case per 100,000 per year, respectively. Overall, incidence in children aged less than 14 years compared with adults was 3.8 versus 0.6 cases per 100,000 per year, respectively. The estimated incidence of endocarditis is 0.9 cases per million population per year. The majority (80%) of the cases presented in the monsoon and “build-up” seasons, with a combined RR of 4.2 times, compared with the dry season.
Diagnostics.
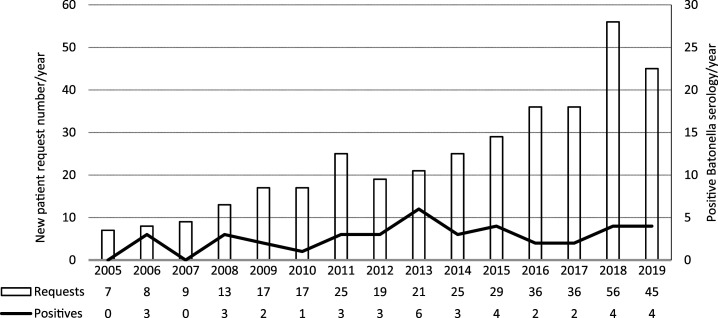
Serological titers ranged from 1:64 to 1:16,384 in our study. Among the 31 patients included in our study, four patients had positive B. henselae DNA PCR (assay performed in seven patients). Two of the three negative PCR were performed on whole blood, and the third PCR was performed on a sample obtained from fine needle aspirate. Given there is a known false-positive rate with B. henselae serology, we analyzed the request numbers per year against the positive results (Figure 2) to ensure that we were not just analyzing false-positive serology. Bartonella henselae serology requests increased markedly over the study period, possibly because of increased recognition of the pathogen; the number of positive results per year remained stable. Cross-reactivity of antibodies was seen in three cases; one patient had probable Q fever endocarditis with phase 1 Q fever IgG of 1:1,024 (corresponding B. henselae serology of 1:1,024; this patient was excluded from the 31 clinical patients), and two other patients had positive Chlamydia pneumoniae serology, including a patient with Bartonella endocarditis.
Figure 2.
Bartonella henselae serology requests in the Top End from 2005 to 2019.
Clinical.
Seventy-four percent (23/31) of patients presented with lymph node disease (Table 2). Among them, three patients also had nonglandular manifestations of hepatic peliosis, optic neuritis, and cutaneous lesions. The disease spectrum in the remaining eight patients included optic neuritis (n = 2), occlusive retinal vasculitis (n = 1), endocarditis (n = 2), splenic abscesses/peliosis (n = 1), parapharyngeal abscess (n = 1), and parotitis (n = 1). The majority of patients (22/30, 71%) were hospitalized for investigation and management, giving an annual hospitalization incidence of 0. 9 per 100,000 population and an average length of stay of 9 days (interquartile range: 4–15 days). One outpatient case with lymphadenopathy was reviewed on a daily basis until diagnosis was made on tissue PCR.
Table 2.
Clinical and laboratory features of patients with Bartonella henselae infection in the Top End from 2005 to 2019
| Parameter | Age less than 14 years (n = 21) | Age older than 14 years (n = 10) | Total (n = 31) | P-value |
|---|---|---|---|---|
| Admission status, n (%) | ||||
| Inpatients | 16 (76) | 6 (60) | 22 (71) | |
| Outpatients | 5 (24) | 4 (40) | 9 (29) | |
| Inpatient length of stay, median (IQR) (days) | 7 (4–11) | 13 (6–17) | 9 (4–15) | |
| Duration of symptoms, median (IQR) (days) | 18 (6–28) | 52 (14–113) | 18 (6–39) | |
| Clinical,* n (%) | ||||
| Lymph node disease | 18 (86) | 5 (55) | 23 (74) | 0.074 |
| Visual loss/impairment | 0 (0) | 4 (44) | 4 (13) | |
| Hepatic or splenic peliosis | 2 (10) | 0 (0) | 2 (6) | |
| Parapharyngeal abscess | 1 (5) | 0 (0) | 1 (3) | |
| Cutaneous lesions | 1 (5) | 0 (0) | 1 (3) | |
| Culture negative endocarditis | 0 (0) | 2 (11) | 2 (6) | |
| Bilateral parotitis | 1 (5) | 0 (0) | 1 (3) | |
| Antimicrobials used, n (%) | 18 (86) | 5 (50) | 23 (74) | |
| Outcomes, n (%) | ||||
| Improved/resolved | 19 (90) | 9 (90) | 28 (90) | |
| Recurrence | 1 (5) | 1 (11) | 2 (7) | |
| Unknown | 1 (5) | 0 (0) | 1 (3) | |
| Initial serological titer, n (%) | ||||
| 1:64 | 6 (29) | 4 (40) | 10 (32) | |
| 1:128 | 7 (33) | 3 (30) | 10 (32) | |
| 1:256 | 2 (10) | 2 (20) | 4 (13) | |
| 1:512 | 3 (14) | 0 (0) | 3 (10) | |
| 1:1,024 | 3 (14) | 0 (0) | 3 (10) | |
| 1:16,384 | 0 (0) | 1 (10) | 1 (3) |
NA = no information available.
Lymph node size on actual radiological measurement.
The median time to presentation was 18, 21, 75, and 21 days for all cases, CSD, endocarditis, and visceral bartonellosis, respectively. The longest incubation was a year in one patient who presented with cervical lymphadenopathy.
Lymphadenopathy tended to be regional (17/23, 74%), multi-nodal on imaging (15/18, 83%), tender (9/13, 69%), and associated with a significant overlying cutaneous swelling (median, 4.5 cm) out of proportion to the size of the underlying predominant lymph node (median, 2.1 cm) as measured on ultrasound or computed tomography (CT) (Table 3). Lymph node disease most commonly occurs in the cervical region. Seven had suppurative lymphadenitis. Ten cases underwent surgical excision or incisional drainage. One case required two incisional drainages and subsequent azithromycin treatment for a necrotizing suppurative granulomatous lymphadenitis. Histological examination of excised lymph nodes showed necrotizing granulomas (4/9, 44%), non-necrotizing granulomas (3/9, 33%; two PCR positive, and the third case had micro-abscesses), and abscess formation (5/9, 56%). One PCR-positive sample with non-necrotizing granulomatous changes also had surrounding lymphocytic vasculitis.
Table 3.
Clinical features of patients with lymph node disease due to Bartonella henselae infection in the Top End from 2005 to 2019
| Parameter | Age less than 14 years (n = 18) | Age older than 14 years (n = 5) | Total (n = 23) |
|---|---|---|---|
| Location of lymphadenopathy, n (%) | |||
| Cervical | 7 (39) | 1 (20) | 8 (35) |
| Submandibular | 7 (39) | 0 (0) | 7 (30) |
| Axillary | 0 (0) | 2 (40) | 2 (9) |
| Groin | 2 (11) | 1 (20) | 3 (13) |
| Multiple sites | 2 (11) | 1 (20) | 3 (13) |
| Cutaneous swelling, median (IQR) (cm) | 4.5 (3–6) | NA | |
| Lymph node size,* median (IQR) (cm) | 2.1 (1.5–2.5) | 2.3 (1.7–3.4) | 2.1 (1.6–2.5) |
| Multiple lymph node involvement per site (yes:no) | 12:3 | 3:0 | 15:3 |
| Associated clinical features (yes:no) | |||
| Tenderness | 8:4 | 1:1 | 9:5 |
| Suppuration/necrotic lymph node | 5:11 | 2:1 | 7:12 |
| Fever | 8:8 | 4:0 | 12:8 |
| Anorexia, diarrhea, vomiting, lethargy, flu-like symptoms | 6:10 | 4:0 | 10:10 |
NA = no information available.
Lymph node size on actual radiological measurement.
Systemic symptoms.
Accompanying fevers (17/25, 68%), lethargy (8/17, 47%), anorexia (6/15, 40%), diarrhea (5/16, 31%), weight loss (5/7, 71%), flu-like illness (4/14, 29%), vomiting (4/15, 27%), sore throat (3/5, 60%), night sweats (3/3, 100%), and nonproductive cough (2/14, 14%) were reported.
Among the nonglandular manifestations, two middle-aged aboriginal patients had aortic valve endocarditis with renal, hematological, and neurological complications. Patient A suffered serial embolic strokes, whereas patient B presented with encephalopathy.
Patient A initially presented with a 4-month history of fatigue, weight loss, acute renal failure, hypocomplementemia, low positive antinuclear antibodies, low-level monoclonal IgM cryoglobulinemia, anemia, and raised inflammatory markers on a background history of known moderate aortic stenosis. Patient B presented with a month history of lethargy, anorexia, and weight loss, and a week history of behavioral change, self-neglect, acute renal impairment, anemia, thrombocytopenia, hypovolemic hyponatremia, hypocomplementemia, and positive serum c-antineutrophil cytoplasmic antibodies. Patient A kept domestic cats and dogs, whereas patient B adopted three feral kittens few months prior.
Renal biopsies from both patients showed prominent diffuse IgM, C3, and C1q deposition predominantly in the mesangium and some capillary loops. No focal proliferative lesions or active crescents were seen. Both patients received intravenous pulsed methylprednisolone and mycophenolate mofetil for an initial diagnosis of atypical lupus nephritis.
However, patient A represented with a right middle-cerebral artery embolic stroke 2 months later, and both patients became febrile during their admissions. On further investigations, both patients were found to have culture-negative aortic valve endocarditis with mild-to-moderate aortic regurgitation (1.4 cm mobile vegetation seen in patient A and 1 cm mobile vegetation in patient B).
Patient A had a B. henselae serological titer of 1:256 and positive B. henselae PCR in blood. Retrospective testing of serum stored 2 months prior when he presented with acute renal impairment revealed an initial titer of 1:1,024. In addition, a review of patient A’s routine echocardiogram performed 6 months earlier showed the presence of the mobile vegetation, suggesting a chronic B. henselae infection. Patient B had a serological titer of 1:16,384 but negative blood PCR.
Patient A underwent a valve replacement because of further cerebral embolization despite 3.5 weeks of ceftriaxone, trimethoprim/sulfamethoxazole, and vancomycin treatment. He was continued on a week of doxycycline, and ceftriaxone with synergistic gentamicin before valve replacement, and is currently completing 6 weeks of doxycycline treatment postoperatively at the time of write-up. Histology and 16S PCR performed on the excised valve confirmed B. henselae endocarditis.
Patient B was managed medically with an initial course of doxycycline and synergistic gentamicin. However, due to further renal impairment, gentamicin was replaced with a 2-week course of rifampicin. Doxycycline was given for a year until a 4-fold reduction in her serological titer was observed (from 1:16,384 to 1:4,096). Transthoracic echocardiogram at 1 year of treatment showed near-resolution of the aortic valve vegetation and a stable aortic regurgitation. Improvement in mental state, renal impairment, and hyponatremia were seen on initial rehydration; however, complete resolution of all symptoms and renal impairment were only observed on commencement of doxycycline treatment.
On retrospective review of their kidney biopsies, it was thought that the features were more consistent with glomerulonephritis associated with chronic infection rather than lupus nephritis.
Visceral peliosis.
There were two cases of probable visceral peliosis in an 11-year-old boy and a 33-year-old female with corresponding serological titers of 1:1,024 and ≥ 1:4,096, respectively. Both presented with fever, anorexia, weight loss, and gastrointestinal symptoms. They are not known to have any immunosuppressive condition. The child presented with splenic peliosis after 2 months of symptoms. Abdominal CT scan revealed a bulky spleen with multiple 5- to 10-mm hypoechoic irregular lesions, with slight wall vascularity on ultrasound. Endocarditis was excluded on echocardiography. He was managed conservatively with 4 months of doxycycline and 10 days of synergistic gentamicin. Posttreatment ultrasound showed resolution of splenic lesions. The adult patient initially presented with a 3.4-cm suppurative inguinal lymphadenitis. Multiple small hypodense liver lesions and a bulky spleen were seen on CT scan. The patient underwent incisional drainage of the lymph node and received a 2-week course of empirical antimicrobials comprising of ceftriaxone and cephalexin.
Ocular syndromes.
Four patients presented with monocular visual loss. Two were diagnosed with optic neuritis, one with ocular retinal vasculitis and one with conjunctivitis. A patient with optic neuritis and conjunctivitis improved spontaneously over a period of weeks to months. The remaining patient with optic neuritis experienced visual improvement on pulsed methylprednisolone followed by a month of oral steroid. No clinical follow-up was recorded for patients with ocular retinal vasculitis.
Antimicrobial use.
Overall, the majority (23/31, 74%) of the patients received antimicrobial treatment. Failure of oral antimicrobial, in particular to flucloxacillin, was a common presenting complaint for patients with lymph node disease. Across the cohort, seven different classes of empirical antimicrobial were prescribed. Seven patients (30%) received more than three classes of antimicrobials. A patient with persistent suppurative lymphadenitis received 28 days of antimicrobials before treatment with azithromycin. Azithromycin and doxycycline were the drugs of choice once bartonellosis was suspected or confirmed, and synergistic gentamicin was given in cases with systemic involvement.
Outcomes.
No deaths occurred during the study period. Two patients experienced symptom recurrence that resolved on repeated surgical drainages in the case of suppurative lymphadenitis and antimicrobials for the case with presumed residual parapharyngeal abscess. One patient with necrotizing granulomatous lymphadenitis continued to have neck swelling for a year, despite an excisional biopsy. The outcomes of the cases with endocarditis, visceral peliosis, and ocular syndromes were discussed earlier
DISCUSSION
Our study revealed that a range of clinical manifestations due to B. henselae infection occur in our tropical population. Similar to a Japanese multicenter study,5 typical CSD predominates (74%), followed by ocular syndromes and hepatosplenic lesions. Juvenile rheumatoid arthritis was not seen in our cohort; however, nonglandular manifestations of endocarditis, encephalopathy, parapharyngeal abscess, cutaneous lesions, and parotitis seen in our cohort were not described in the 2006 Japanese series of 186 cases.5
In our cohort, the commonest presentation was being young aboriginal male children from rural areas presenting with lymph node disease. The overall infection incidence over the 15-year period was 1.3 cases per 100,000 population per year, whereas the infection incidences in all children, aboriginal children, and rural residents were 3.8, 7.1, and 1.7 cases per 100,000 per year, respectively. Lymph node disease was far commoner in children aged less than 14 years in contrast to the more serious systemic B. henselae infections found in older patients (86% versus 55%) with a trend toward statistical significance (P = 0.074). This observation was also noted by Ronen et al.,9 where the differences between those younger than 60s and older than 60s were statistical significant.
Most patients were admitted for investigations and management, requiring a median length of stay of 9 days and multiple antimicrobial prescriptions. Our hospitalization incidence (0.9 per 100,000 per year) and median length of stay are higher than those found on national database surveillances in the United States (hospitalization incidences: 0.19–0.86 per 100,000 per year and average length of stay: 3–4 days)10–12; it may be that the differential diagnoses for a subacute to chronic unilateral lymphadenopathy in our population include tuberculosis and melioidosis.13,14 In a series of 244 cases, concurrent Mycobacterium infection and neoplasm could be found in 5% of PCR-positive CSD.15 Other reasons for the differences could be due to a higher rate of severe lymph node disease observed in our cohort where 37% (7/19) showed evidence of suppuration in comparison to previous studies where the average suppuration rate was 27%,16 and due to poorer medical access, there might have been a lower threshold to observe patients longer prior their return to rural and remote locations. Furthermore, the morbidities associated with CSD can be underappreciated.17,18 In a radiological case series, liver and spleen involvement was found in 30% of the pediatric patients presenting with regional lymphadenopathy.19 These organ involvements and even coexisting vertebrae involvement may not be clinically apparent but can contribute to persistent fevers and illness.20–22
Lymph node disease in our cohort tended to be tender, unilateral, and muti-nodal on imaging with a predominant node and significant overlying cutaneous swelling out in keeping with underlying lymphadenopathy. Cervical and submandibular lymph nodes were most commonly involved in our cohort and as reported in a large case series by Moriarty and Margileth,23 in contrast to the axillary and epitrochlear regions reported in a majority of the 1,200 cases seen by Carithers.16 This difference possibly relates to the site of bacterial inoculation although we were unable to prove in our cohort because of lack of information on the mechanism of disease acquisition.
Among the nonglandular manifestations, all cases were managed conservatively with good clinical outcome, except for one endocarditis case and one parapharyngeal abscess case who required surgical intervention.
Bartonella henselae can be readily found in blood vessel walls during an infection23 and is known to cause angioproliferative lesions such bacillary angiomatosis and organ peliosis.24 Other associated vasculopathies are cutaneous and ocular vasculitis, myocarditis and atherosclerosis formation.1 In our cohort, we report two cases of organ peliosis and one occlusive retinal vasculitis. Similar to our two endocarditis cases, there have been at least 58 case reports documenting B. henselae infection–related glomerulonephritis presenting with a variety of clinical, laboratory, and histological features mimicking vasculitic glomerulonephritis including 12 cases published in the last 5 years.25–36 One notable non-endocarditis case ended up losing a renal allograft as a result.35 Diagnoses of bartonellosis in many of these cases were often made on incidental finding of concurrent culture-negative endocarditis, and in a significant number of cases, an earlier diagnosis could have avoided unnecessary immunosuppression, hence highlighting the need to consider Bartonella or endocarditis-related glomerulonephritis as a differential diagnosis in our population presenting with atypical features of glomerulonephritis or vasculitis.
International series revealed a link between seasons and CSD incidence.10–12,37–40 Infections in the Northern Hemisphere have seasonality, with an increase in autumn and peaks in December and January.10–12,37,39,40 Unlike these locations, TENT experiences a tropical monsoonal climate where 90% of rainfall occurs during the monsoon and “build-up” seasons; temperature and humidity are much higher during the monsoon and “build-up” seasons as than those during the dry season.41 Consistent with the reporting of higher infection incidences and summer peak in the warmer southern states of the United States,10,12 a majority (80%) of our cases presented during the warmer and more humid seasons. An explanation is that fleas survive and reproduce better in warm and humid environment.37,42 Analogous to winter in temperate regions, human and companion animals are more likely to stay indoors during the wet seasons, which may account for an increased transmission rate.
The prevalence of B. henselae infection in our feline population was found comparable to the United States,43 and this is reflected by a similar seroprevalence rate of 5% among healthy individuals in an Australian study.44,45 Despite this, the overall infection incidence in our cohort is much lower than reported incidences in the United States (1.3 versus 3.7–4.7 per 100,000 population).12,46 It is likely that this incidence is an underestimate as diagnoses made in primary health care are not captured in our study. Furthermore, most cases of CSD are pauci-symptomatic and self-limiting, and hence may not require medical attention. Outpatient incidence could potentially be as high as 24 times that of inpatient incidence as estimated by a U.S. surveillance study.12
Transmission and epidemiology of B. henselae infection, and its animal associations are not well characterized in remote Aboriginal communities (RAC) in Australia. The RACs have a lower average domestic cats per 100 people than overall Australia average (7–9 versus 11 cats per 100 people).47 In a point prevalence study in Sydney, 40% of young feral cats were found to have B. henselae bacteremia, whereas only 16% of young domestic cats were bacteremic, suggesting that feral animals may pose a greater infection threat in Australia.43 Of note, Australian feral cat population density appears to fluctuate with rainfall received, with a potential to triple from 1.4 to 5.6 million after periods of high rainfall, especially in arid and semi-arid areas.48 Causative agents of CSD including B. henselae and Bartonella clarridgeiae have also been isolated from fleas,49 foxes,50 and ticks51 in Australia. Bull ant was also implicated in a case of B. henselae neuroretinitis.52 Other clinical cases from Australia are presented in Table 4. Although Bartonella species have yet to be reported in Australian dogs, C. felis has been found on feral dogs.63 Given that the owned dog population in RACs is one of the highest in the world (50–62 dogs per 100 people), canids could potentially serve as the largest reservoir for disease transmission in our population.
Table 4.
Clinical spectrum of Bartonella henselae infections previously reported in Australian cases
| First author | Year/city | n | Description | Treatment | Outcome |
|---|---|---|---|---|---|
| Flexman et al.53 | 1995/Perth | 1 | CSD | Nil | Resolved |
| Wong et al.54 | 1995/Sydney | 1 | Extra-axial mass involving trigeminal nerve, asthma, ulcerated eczematous postauricular lesion, hepatosplenomegaly, thickened nonfunctioning gall bladder, turbid ascites, long bone myeloid hyperplasia | Amoxicillin/clavulanic acid (3 days), intravenous cefotaxime, metronidazole, amikacin, co-trimoxazole (1.5 months), rifampicin (1.5 months) | Resolved |
| Flexman et al.44 | 1997/Perth and Sydney | 98 | CSD | No information (laboratory study) | No information |
| Dondey et al.55 | 1997/Brisbane | 1 | Atypical Parinaud oculoglandular syndrome | Fornix nodule excision, ciprofloxacin (1.5 months) | Resolved |
| Roebuck56 | 1998/Sydney | 1 | Extra-axial mass arising from the left trigeminal nerve | Craniotomy and biopsy, co-trimoxazole, rifampicin | Resolved |
| Robson et al.57 | 1999/Southeast Queensland | 1 | Paravertebral mass and osteomyelitis | Gentamicin (5 days), flucloxacillin, cephalothin (5 weeks) | Resolved |
| Rosen et al58 | 1999/Perth | 1 | Neuroretinitis | Nil | Resolved |
| Grando et al59 | 1999/Melbourne | 1 | Parinaud oculoglandular syndrome | Nil | Resolved |
| Endara et al.60, Oman et al.61 | 2001/Regional northern Queensland | 1 | Endocarditis | Valve replacement, flucloxacillin (1 month), penicillin (1 month), gentamicin (3 weeks), amikacin (2.5 weeks), doxycycline (6 months) | Good |
| Fournier et al.62 | 2002/Queensland | 96 | CSD | No information (laboratory study) | No information |
| Ullrich et al52 | 2012/Adelaide | 1 | Neuroretinitis | Erythromycin (3 months) | Improved |
CSD = cat scratch disease.
Limitations.
As discussed earlier, this is a single regional pathology center study, and data are skewed toward patients with healthcare contact. Primary healthcare facilities in the TENT are also served by other pathology providers.
In addition, this is a retrospective study with small numbers where cases were identified based on available documented information and relied on serological sensitivity. Epidemiological risk factors such as cat or dog contact were not well documented.
Diagnostic assays for B. henselae come with limitations.1 Serological testing has sensitivity as low as 54% depending on accompanying clinical criteria used.44,64 There is also known cross-reactivity with other serology even between Bartonella species; hence, diagnosis of B. henselae infection cannot be based on serology alone. PCR or genetic sequencing will be required to definably differentiate infection between specific Bartonella species,1,65,66 but this is not always feasible, and PCR is not always positive even in proven cases (sensitivity of 72–98% in tissue samples, 58% in blood, and 18% in serum).1,7,67,68 As illustrated by patient A with complicated endocarditis, the timing of serological testing is certainly important, and if we had strictly adhered to the proposed serological cutoff of > 1:800 for endocarditis diagnosis,62 we could potentially have missed his infection.
CONCLUSION
This study reviews clinical features of B. henselae infection from a single tropical Southern Hemisphere location. Although the clinical manifestations of B. henselae infection were typical, the transmission in our cohort and epidemiological aspects remain to be elucidated in detail. The heterogeneity of presentations with nonspecific clinical symptoms and significant morbidities highlights the need for better diagnostic algorithm and testing to allow better documentation of the disease epidemiology, spectrum, and treatment efficacy, and most importantly facilitate timely diagnosis while avoiding unnecessary antimicrobials, immunosuppressants, or interventions.
Acknowledgment:
We thank Stephen Balharrie for his advice on geographic information systems.
REFERENCES
- 1.Okaro U, Addisu A, Casanas B, Anderson B, 2017. Bartonella species, an emerging cause of blood-culture-negative endocarditis. Clin Microbiol Rev 30: 709–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Florin TA, Zaoutis TE, Zaoutis LB, 2008. Beyond cat scratch disease: widening spectrum of Bartonella henselae infection. Pediatrics 121: e1413–e1425. [DOI] [PubMed] [Google Scholar]
- 3.Massei F, Gori L, Macchia P, Maggiore G, 2005. The expanded spectrum of bartonellosis in children. Infect Dis Clin North Am 19: 691–711. [DOI] [PubMed] [Google Scholar]
- 4.Spach DH, Koehler JE, 1998. Bartonella-associated infections. Infect Dis Clin North Am 12: 137–155. [DOI] [PubMed] [Google Scholar]
- 5.Tsuneoka H, Tsukahara M, 2006. Analysis of data in 30 patients with cat scratch disease without lymphadenopathy. J Infect Chemother 12: 224–226. [DOI] [PubMed] [Google Scholar]
- 6.Welfare AIoHa , 2019. Census Advanced Search by Geography. Available at: https://www.abs.gov.au/websitedbs/D3310114.nsf/Home/2016%20search%20by%20geography. Accessed December 1, 2019. [Google Scholar]
- 7.Hansmann Y, DeMartino S, Piemont Y, Meyer N, Mariet P, Heller R, Christmann D, Jaulhac B, 2005. Diagnosis of cat scratch disease with detection of Bartonella henselae by PCR: a study of patients with lymph node enlargement. J Clin Microbiol 43: 3800–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.QGIS Development Team , 2019. QGIS Geographic Information System. Available at: http://qgis.osgeo.org. Accessed January 17, 2020. [Google Scholar]
- 9.Ronen BA, Moshe E, Boaz A, Eugene K, Merav V, Cecilia L, Doron C, Giladi M, 2005. Cat-scratch disease in elderly patients. Clin Infect Dis 41: 969–974. [DOI] [PubMed] [Google Scholar]
- 10.Jackson LA, Perkins BA, Wenger JD, 1993. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health 83: 1707–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds MG, Holman RC, Curns AT, O’Reilly M, McQuiston JH, Steiner CA, 2005. Epidemiology of cat-scratch disease hospitalizations among children in the United States. Pediatr Infect Dis J 24: 700–704. [DOI] [PubMed] [Google Scholar]
- 12.Nelson CA, Saha S, Mead PS, 2016. Cat-scratch disease in the United States, 2005–2013. Emerg Infect Dis 22: 1741–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyd R, Johnston V, Farmer B, Krause VL, 2017. Treatment of latent tuberculosis infections in the Darwin region. Med J Aust 206: 306–307. [DOI] [PubMed] [Google Scholar]
- 14.Currie BJ, et al. 2000. Endemic melioidosis in tropical northern Australia: a 10-year prospective study and review of the literature Clin Infect Dis 31: 981–986. [DOI] [PubMed] [Google Scholar]
- 15.Rolain JM, et al. 2006. Lymph node biopsy specimens and diagnosis of cat-scratch disease. Emerg Infect Dis 12: 1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carithers HA, 1985. Cat-scratch disease. Am J Dis Child 139: 1124–1133. [DOI] [PubMed] [Google Scholar]
- 17.Margileth AM, Wear DJ, English CK, 1987. Systemic cat scratch disease: report of 23 patients with prolonged or recurrent severe bacterial infection. J Infect Dis 155: 390–402. [DOI] [PubMed] [Google Scholar]
- 18.Munson PD, Boyce TG, Salomao DR, Orvidas LJ, 2008. Cat-scratch disease of the head and neck in a pediatric population: surgical indications and outcomes. Otolaryngol Head Neck Surg 139: 358–363. [DOI] [PubMed] [Google Scholar]
- 19.Garcia CJ, Varela C, Abarca K, Ferres M, Prado P, Vial PA, 2000. Regional lymphadenopathy in cat-scratch disease: ultrasonographic findings. Pediatr Radiol 30: 640–643. [DOI] [PubMed] [Google Scholar]
- 20.Fretzayas A, Papadopoulos NG, Moustaki M, Bossios A, Koukoutsakis P, Karpathios T, 2001. Unsuspected extralymphocutaneous dissemination in febrile cat scratch disease. Scand J Infect Dis 33: 599–603. [DOI] [PubMed] [Google Scholar]
- 21.Arisoy ES, Correa AG, Wagner ML, Kaplan SL, 1999. Hepatosplenic cat-scratch disease in children: selected clinical features and treatment. Clin Infect Dis 28: 778–784. [DOI] [PubMed] [Google Scholar]
- 22.Donoso G, Paulsen C, Riquelme P, Lobo G, Gutierrez D, Perez A, Jimenez C, 2013. Bone scintigraphy in children with cat scratch disease. Clin Nucl Med 38: 936–939. [DOI] [PubMed] [Google Scholar]
- 23.Moriarty RA, Margileth AM, 1987. Cat scratch disease. Infect Dis Clin North Am 1: 575–590. [PubMed] [Google Scholar]
- 24.Angelakis E, Raoult D, 2014. Pathogenicity and treatment of Bartonella infections. Int J Antimicrob Agents 44: 16–25. [DOI] [PubMed] [Google Scholar]
- 25.Bannon L, Choshen G, Giladi M, Ablin J, 2019. Bartonella endocarditis masquerading as systemic vasculitis with rapidly progressive glomerulonephritis (aka ‘Lohlein nephritis’). BMJ Case Rep 12: e231413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vercellone J, Cohen L, Mansuri S, Zhang PL, Kellerman PS, 2018. Bartonella endocarditis mimicking crescentic glomerulonephritis with PR3-ANCA positivity. Case Rep Nephrol 2018: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Haare Heijmeijer S, Wilmes D, Aydin S, Clerckx C, Labriola L, 2015. Necrotizing ANCA-positive glomerulonephritis secondary to culture-negative endocarditis. Case Rep Nephrol 2015: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babiker A, El Hag MI, Perez C, 2018. Bartonella infectious endocarditis associated with cryoglobulinemia and multifocal proliferative glomerulonephritis. Open Forum Infect Dis 5: ofy186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams JM, Parimi M, Sutherell J, 2018. Bartonella endocarditis in a child with tetralogy of fallot complicated by PR3-ANCA positive serology, autoimmune hemolytic anemia, and acute kidney injury. Clin Case Rep 6: 1264–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hashemi H, Endicott-Yazdani TR, Oguayo C, Harmon DM, Tran T, Tsai-Nguyen G, Benavides R, Spak CW, Nguyen HL, 2018. Bartonella endocarditis with glomerulonephritis in a patient with complete transposition of the great arteries. Proc (Bayl Univ Med Cent) 31: 102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cervi A, Kelly D, Alexopoulou I, Khalidi N, 2017. ANCA-associated pauci-immune glomerulonephritis in a patient with bacterial endocarditis: a challenging clinical dilemma. Clin Nephrol Case Stud 5: 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouellette CP, Joshi S, Texter K, Jaggi P, 2017. Multiorgan involvement confounding the diagnosis of Bartonella henselae infective endocarditis in children with congenital heart disease. Pediatr Infect Dis J 36: 516–520. [DOI] [PubMed] [Google Scholar]
- 33.Raybould JE, Raybould AL, Morales MK, Zaheer M, Lipkowitz MS, Timpone JG, Kumar PN, 2016. Bartonella endocarditis and pauci-immune glomerulonephritis: a case report and review of the literature. Infect Dis Clin Pract (Baltim Md) 24: 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itoh M, Kann DC, Schwenk HT, Gans HA, 2015. Fever and renal failure in a child with DiGeorge syndrome and tetralogy of fallot. J Pediatr Infect Dis Soc 4: 373–375. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhry AR, Chaudhry MR, Papadimitriou JC, Drachenberg CB, 2015. Bartonella henselae infection-associated vasculitis and crescentic glomerulonephritis leading to renal allograft loss. Transpl Infect Dis 17: 411–417. [DOI] [PubMed] [Google Scholar]
- 36.Singhania G, Singhania N, 2019. Do we care if you have a cat? Bartonella infection related glomerulonephritis with no endocarditis. Infez Med 27: 441–444. [PubMed] [Google Scholar]
- 37.Sanguinetti-Morelli D, Angelakis E, Richet H, Davoust B, Rolain JM, Raoult D, 2011. Seasonality of cat-scratch disease, France, 1999–2009. Emerg Infect Dis 17: 705–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kodama T, Masuda H, Ohira A, 2003. Neuroretinitis associated with cat-scratch disease in Japanese patients. Acta Ophthalmol Scand 81: 653–657. [DOI] [PubMed] [Google Scholar]
- 39.Tsukahara M, 2002. Cat scratch disease in Japan. J Infect Chemother 8: 321–325. [DOI] [PubMed] [Google Scholar]
- 40.Murakami K, Tsukahara M, Tsuneoka H, Iino H, Ishida C, Tsujino K, Umeda A, Furuya T, Kawauchi S, Sasaki K, 2002. Cat scratch disease: analysis of 130 seropositive cases. J Infect Chemother 8: 349–352. [DOI] [PubMed] [Google Scholar]
- 41.Bureau of Meteorology , 2016. All NT Weather Observations. Available at: http://www.bom.gov.au/nt/observations/ntall.shtml. Accessed December 1, 2019. [Google Scholar]
- 42.Dryden MW, Rust MK, 1994. The cat flea: biology, ecology and control. Vet Parasitol 52: 1–19. [DOI] [PubMed] [Google Scholar]
- 43.Branley J, Wolfson C, Waters P, Gottlieb T, Bradbury R, 1996. Prevalence of Bartonella henselae bacteremia, the causative agent of cat scratch disease, in an Australian cat population. Pathology 28: 262–265. [DOI] [PubMed] [Google Scholar]
- 44.Flexman JP, Chen SC, Dickeson DJ, Pearman JW, Gilbert GL, 1997. Detection of antibodies to Bartonella henselae in clinically diagnosed cat scratch disease. Med J Aust 166: 532–535. [DOI] [PubMed] [Google Scholar]
- 45.Regnery RL, Olson JG, Perkins BA, Bibb W, 1992. Serological response to “Rochalimaea henselae” antigen in suspected cat-scratch disease. Lancet 339: 1443–1445. [DOI] [PubMed] [Google Scholar]
- 46.Hamilton DH, Zangwill KM, Hadler JL, Cartter ML, 1995. Cat-scratch disease–Connecticut, 1992–1993. J Infect Dis 172: 570–573. [DOI] [PubMed] [Google Scholar]
- 47.Burleigh A, McMahon S, Kiely S, 2015. Owned dog and cat populations in remote Indigenous communities in the Northern Territory: a retrospective study. Aust Vet J 93: 145–150. [DOI] [PubMed] [Google Scholar]
- 48.Legge S, et al. 2017. Enumerating a continental-scale threat: how many feral cats are in Australia? Biol Conserv 206: 293–303. [Google Scholar]
- 49.Barrs VR, Beatty JA, Wilson BJ, Evans N, Gowan R, Baral RM, Lingard AE, Perkovic G, Hawley JR, Lappin MR, 2010. Prevalence of Bartonella species, Rickettsia felis, haemoplasmas and the Ehrlichia group in the blood of cats and fleas in eastern Australia. Aust Vet J 88: 160–165. [DOI] [PubMed] [Google Scholar]
- 50.Kaewmongkol G, Kaewmongkol S, Fleming PA, Adams PJ, Ryan U, Irwin PJ, Fenwick SG, 2011. Zoonotic Bartonella species in fleas and blood from red foxes in Australia. Vector Borne Zoonotic Dis 11: 1549–1553. [DOI] [PubMed] [Google Scholar]
- 51.Gofton AW, et al. 2015. Inhibition of the endosymbiont “Candidatus Midichloria mitochondrii” during 16S rRNA gene profiling reveals potential pathogens in Ixodes ticks from Australia. Parasit Vectors 8: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ullrich K, Saha N, Lake S, 2012. Neuroretinitis following bull ant sting. BMJ Case Rep 2012: bcr2012006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flexman JP, Lavis NJ, Kay ID, Watson M, Metcalf C, Pearman JW, 1995. Bartonella henselae is a causative agent of cat scratch disease in Australia. J Infect 31: 241–245. [DOI] [PubMed] [Google Scholar]
- 54.Wong M, Isaacs D, Dorney S, 1995. Fever, abdominal pain and an intracranial mass. Pediatr Infect Dis J 14: 725–728. [PubMed] [Google Scholar]
- 55.Dondey JC, Sullivan TJ, Robson JM, Gatto J, 1997. Application of polymerase chain reaction assay in the diagnosis of orbital granuloma complicating atypical oculoglandular cat scratch disease. Ophthalmology 104: 1174–1178. [DOI] [PubMed] [Google Scholar]
- 56.Roebuck DJ, 1998. Cat-scratch disease with an extraaxial mass. Am J Neuroradiol 19: 1294–1295. [PMC free article] [PubMed] [Google Scholar]
- 57.Robson JM, Harte GJ, Osborne DR, McCormack JG, 1999. Cat-scratch disease with paravertebral mass and osteomyelitis. Clin Infect Dis 28: 274–278. [DOI] [PubMed] [Google Scholar]
- 58.Rosen BS, Barry CJ, Nicoll AM, Constable IJ, 1999. Conservative management of documented neuroretinitis in cat scratch disease associated with Bartonella henselae infection. Aust N Z J Ophthalmol 27: 153–156. [DOI] [PubMed] [Google Scholar]
- 59.Grando D, Sullivan LJ, Flexman JP, Watson MW, Andrew JH, 1999. Bartonella henselae associated with Parinaud’s oculoglandular syndrome. Clin Infect Dis 28: 1156–1158. [DOI] [PubMed] [Google Scholar]
- 60.Endara SA, Roati AA, Alizzi AM, Boldery JO, Bidstrup BP, 2001. Aortic valve endocarditis caused by Bartonella henselae: a rare surgical entity. Heart Surg Forum 4: 359–360; discussion 360. [PubMed] [Google Scholar]
- 61.Oman K, Norton R, Gunawardane K, 2003. Bartonella henselae infective endocarditis in north Queensland. Intern Med J 33: 55–56. [DOI] [PubMed] [Google Scholar]
- 62.Fournier PE, Robson J, Zeaiter Z, McDougall R, Byrne S, Raoult D, 2002. Improved culture from lymph nodes of patients with cat scratch disease and genotypic characterization of Bartonella henselae isolates in Australia. J Clin Microbiol 40: 3620–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clark NJ, Seddon JM, Šlapeta J, Wells K, 2018. Parasite spread at the domestic animal - wildlife interface: anthropogenic habitat use, phylogeny and body mass drive risk of cat and dog flea (Ctenocephalides spp.) infestation in wild mammals. Parasit Vectors 11: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zangwill KM, Hamilton DH, Perkins BA, Regnery RL, Plikaytis BD, Hadler JL, Cartter ML, Wenger JD, 1993. Cat scratch disease in Connecticut. Epidemiology, risk factors, and evaluation of a new diagnostic test. N Engl J Med 329: 8–13. [DOI] [PubMed] [Google Scholar]
- 65.Portillo A, Maggi R, Oteo JA, Bradley J, García-Álvarez L, San-Martín M, Roura X, Breitschwerdt E, 2020. Bartonella spp. prevalence (serology, culture, and PCR) in sanitary workers in La Rioja Spain. Pathogens 9: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oteo JA, Maggi R, Portillo A, Bradley J, Garcia-Alvarez L, San-Martin M, Roura X, Breitschwerdt E, 2017. Prevalence of Bartonella spp. by culture, PCR and serology, in veterinary personnel from Spain. Parasit Vectors 10: 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Edouard S, Nabet C, Lepidi H, Fournier PE, Raoult D, 2015. Bartonella, a common cause of endocarditis: a report on 106 cases and review. J Clin Microbiol 53: 824–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vermeulen MJ, Diederen BM, Verbakel H, Peeters MF, 2008. Low sensitivity of Bartonella henselae PCR in serum samples of patients with cat-scratch disease lymphadenitis. J Med Microbiol 57: 1049–1050. [DOI] [PubMed] [Google Scholar]