Abstract.
Protobothrops mucrosquamatus is one of the common venomous snakes in Southeast Asia. This retrospective cohort study conducted in six medical institutions in Taiwan aimed to obtain information on the optimal management strategies for P. mucrosquamatus snakebite envenomation. Data were extracted from the Chang Gung Research Database from January 2006 to December 2016. The association between early antivenom administration and patient demographics, pain requiring treatment with analgesic injections, and hospital length of stay was analyzed. A total of 195 patients were enrolled; 130 were administered antivenom within 1 hour after emergency department arrival (early group), whereas 65 were treated later than 1 hour after arrival (late group). No in-hospital mortality was identified. The difference in surgical intervention rates between the early and late groups was statistically insignificant (P = 0.417). Compared with the early group, the late group showed a higher rate of antivenom skin test performance (46.9% versus 63.1%, respectively, P = 0.033), longer hospital stay (42 ± 62 hours versus 99 ± 70 hours, respectively, P = 0.016), and higher rate of incidences of pain requiring treatment with analgesic injections (29.2% versus 46.2%, respectively, P = 0.019). After adjusting for confounding factors, early antivenom administration was associated with decreased pain requiring treatment with analgesic injections (adjusted odds ratio: 0.51, 95% CI: 0.260–0.985). Antivenom administration within 1 hour of arrival was associated with a decreased likelihood of experiencing pain and hospital length of stay in patients with P. mucrosquamatus snakebites. Antivenom skin testing was associated with delays in antivenom administration.
INTRODUCTION
Snakebite envenomation causes considerable mortality and morbidity in hundreds of thousands of individuals yearly, especially in South and Southeast Asia.1–3 Taiwan is located in Southeast Asia and has six medically important venomous snakes, namely, Protobothrops mucrosquamatus, Viridovipera stejnegeri, Deinagkistrodon acutus, Bungarus multicinctus, Naja atra, and Daboia siamensis.4–7 Protobothrops mucrosquamatus belongs to the Viperidae family and is widely distributed in Asia.8–16 Protobothrops mucrosquamatus and V. stejnegeri are most commonly responsible for venomous snakebites in Taiwan.17–19
The key treatment for P. mucrosquamatus bite is the administration of antivenom extracted from horse serum, which is produced by the Centers for Disease Control, R.O.C (Taiwan). The product, a bivalent antivenom against the venoms of V. stejnegeri and P. mucrosquamatus, is in the form of vacuum freeze-dried F(ab′)2 fragments packaged in vials containing > 1,000 Tanaka units of neutralizing potency.20–22 Treatments such as surgical intervention, bullae aspiration, and antibiotics have also been previously described.23–25
Although there is a consensus among physicians on the recommended prescription of approximately 1–4 vials of antivenom for patients with P. mucrosquamatus snakebites,6,18,24,26 the existing guidelines are insufficient, and the appropriate time of administration is not specified. Faster limb recovery following early administration of Fab antivenom to patients with Viperidae snakebites is reported in a recent study.27 However, the effect of early administration of freeze-dried F(ab′)2 antivenom to patients with P. mucrosquamatus bites is unclear. This study aimed to obtain relevant information from a decade of experience in P. mucrosquamatus snakebite treatment and analyze the effect of early antivenom administration.
METHODS
Ethical considerations.
This retrospective study was approved by the Chang Gung Medical Foundation Institutional Review Board (IRB No.: 201901401B0). All patient data used in the analyses were anonymized.
Study setting.
The data were obtained from the healthcare system of Taiwan, the Chang Gung Memorial Hospital. We used information from the Chang Gung Research Database (CGRD), which includes original medical records from six medical institutions, including Keelung, Taipei, Linkou, Yunlin, Chiayi, and Kaohsiung branches, located along northern to southern Taiwan.
Patients.
Patients who presented at the emergency department (ED) with P. mucrosquamatus snakebites and were administered antivenom at the same visit from January 2006 to December 2016 were identified. A “definite case” was described as the presence of envenoming signs and symptoms and fang marks (if any) in the patient, and the physical identification of the snake. “Suspected cases” were defined as the presence of envenomation signs and symptoms, as well as fang marks (if any) in the patient, with pictorial identification of the snake. “Clinical cases” were defined as patients with wound and history manifestations resembling those seen in P. mucrosquamatus snakebites who were clinically diagnosed by physicians.4 Patients with “definite cases” and “suspected cases,” who were administered antivenom, were enrolled in this study, whereas “clinical cases” and dry-bite cases were excluded.
Measurements.
To reconstitute the antivenom, one vial (20 mL) was diluted with 200–300 mL 0.9% sodium chloride (NaCl) and infused intravenously over 30 minutes. For the skin test, 0.1 mL of 1:100 diluted antivenom was injected intradermally into the forearm. A positive skin test was defined as local redness or indurations at the injection site or any systemic allergic reactions that occurred within 30 minutes following antivenom administration.20,28 Patient data on demographic, age, gender, triage, vital signs, location of envenomation, disposition, hospital length of stay, and surgical interventions such as debridement, fasciotomy, and amputation were extracted from the CGRD database.
Anderson et al.27 discussed the benefit of early antivenom injection and used the median time to treatment as the cutoff time. In this study, the median time from ED arrival to antivenom administration was 0.8667 hours. For better clinical application, early antivenom administration was defined as within 1 hour after ED arrival. Patients who developed skin rashes, redness of the eyes, eyelid swelling, itching, sneezing, wheezing, coughing, or bronchospasm within 3 hours after antivenom administration and received antihistamine, steroid, or bronchodilator treatment were defined as having experienced mild antivenom reactions.9,29,30 Patients who developed 1) hypotension after antivenom administration and were treated with fluid resuscitation or epinephrine injection or 2) experienced respiratory failure with subsequent intubation because of the allergic reaction following antivenom administration were defined as having experienced severe reactions.9,31
There was no standard pain management or discharge protocol for patients with snakebites in the study at these six hospitals. However, emergency medicine in Taiwan is a well-established specialty with standard training and accreditation protocols,32 and the decisions of individual physicians have little bearing on the use of pain control treatments. Under the Taiwanese national health insurance system and Chang Gung medical system, there should be little or no difference in discharge decisions among the six branches of the hospital. Pain requiring treatment with analgesic injections was defined as an episode of severe pain where nonsteroidal anti-inflammatory drugs (NSAIDs) or opioid injections were administered to control pain. Hospital length of stay was defined as the duration from ED arrival to hospital departure.33
Data analysis.
The continuous variable of age is summarized as means ± SDs. The medication doses, length of hospital stay, heart rate, and mean arterial pressure are presented as medians with quartile deviation. The distributions of categorical data are presented as numbers and percentages. Student’s t-test, one-way analysis of variance, Mann–Whitney U test, chi-square test, and Fisher’s exact test were used to analyze data. To determine the associations between the variables and pain requiring treatment with analgesic injections, a binary logistic regression analysis was used to adjust for potential confounding factors.
The rationale for the potential confounding factors used in this study was that they may differentially affect the use of analgesics by the early and late groups, and the factors were age, male gender, the years of ED visit, heart rate and mean arterial pressure during triage, and envenomation location. The effects were estimated using adjusted odds ratios (aORs) and corresponding 95% CIs. Results were considered statistically significant for a two-tailed test at P < 0.05. The IBM statistical package for the social sciences (SPSS) for Windows, version 22.0 (released 2013, IBM Corp., Armonk, NY) was used for all statistical analyses.
RESULTS
The data of 195 patients who presented at the ED emergency with P. mucrosquamatus bites and were administered antivenom were included. No in-hospital mortality was identified in this study, and the difference in surgical intervention rates between the early and late groups was statistically insignificant (P = 0.417). Table 1 shows that 130 patients were administered antivenom within 1 hour after arrival at the ED (early group), whereas the remaining 65 were treated later than 1 hour after arrival (late group). Compared with the early group, the late group had a higher rate of skin tests (46.9% versus 63.1%, respectively, P = 0.033).
Table 1.
Clinical characteristics of patients with Protobothrops mucrosquamatus snakebites in early and late groups
| Early (start antivenom ≤ 1 hour) (n = 130) | Late (start antivenom > 1 hour) (n = 65) | P-value | |
|---|---|---|---|
| Age (years) | 54 ± 17.3 | 53 ± 15.8 | 0.671 |
| Male gender, n (%) | 93 (71.5) | 45 (69.2) | 0.738 |
| Definite cases (n = 100), n (%) | 66 (50.8) | 34 (52.3) | 0.880 |
| Suspected cases (n = 95), n (%) | 64 (49.2) | 31 (47.7) | 0.880 |
| Hypertension, n (%) | 18 (20.0) | 13 (13.8) | 0.301 |
| Diabetes mellitus, n (%) | 5 (3.8) | 5 (7.7) | 0.306 |
| End stage of renal disease, n (%) | 0 (0.0) | 1 (1.5) | 0.333 |
| Obesity, n (%) | 5 (3.8) | 2 (3.1) | 1.000 |
| Malignancy, n (%) | 3 (2.3) | 0 (0.0) | 0.552 |
| The years of emergency department visit, n (%) | 0.175 | ||
| 2006–2009 | 40 (30.8) | 13 (20) | |
| 2010–2013 | 49 (37.7) | 24 (36.9) | |
| 2014–2016 | 41 (31.5) | 28 (43.1) | |
| Urgent triage (triage I and II), n (%) | 63 (48.5) | 24 (36.9) | 0.127 |
| Heart rate at triage (beats/minute) | 87 ± 10.5 | 89 ± 9 | 0.547 |
| Mean blood pressure at triage (mm Hg) | 114 ± 12.5 | 111 ± 11.6 | 0.483 |
| Envenomation location, n (%) | 0.304 | ||
| Upper extremity | 63 (48.5) | 34 (52.3) | |
| Lower extremity | 67 (51.5) | 30 (46.2) | |
| Trunk/other, n (%) | 0 (0) | 1 (1.5) | |
| Initial antivenom dose (vials) | 1 ± 0.5 | 1 ± 0.5 | 0.702 |
| Total antivenom dose (vials) | 2 ± 1.5 | 3 ± 1.5 | 0.070 |
| Perform antivenom skin tests, n (%) | 61 (46.9) | 41 (63.1) | 0.033 |
| Negative, n (%) | 56 (43.1) | 39 (60) | |
| Positive, n (%) | 5 (3.8) | 2 (3.1) | |
| Door to antivenom (minutes) | 43 ± 11.5 | 80 ± 12.5 | < 0.001 |
| Antivenom reactions, n (%) | 39 (30) | 16 (24.6) | 0.431 |
| Severe antivenom reactions, n (%) | 1 (0.8) | 0 (0) | 1.000 |
| Initial laboratory data, n (%) | |||
| Leukocytosis (white blood cells > 12,000/µL) | 10 (16.4) | 16 (12.9) | 0.509 |
| Anemia (hemoglobulin < 12 g/dL) | 4 (6.6) | 9 (7.3) | 1.000 |
| Thrombocytopenia (platelet < 150,000/µL) | 13 (21.3) | 22 (17.7) | 0.556 |
| Prolonged prothrombin time (> 12 seconds) | 10 (16.9) | 16 (13.4) | 0.653 |
| Prolonged activated partial thromboplastin time (> 33.8 seconds) | 0 (0) | 1 (0.9) | 1.000 |
| Renal impairment (creatinine > 1.4 mg/dL) | 1 (1.8) | 3 (2.6) | 1.000 |
| Elevated alanine aminotransferase level (> 40 U/L) | 6 (12) | 10 (11.9) | 1.000 |
Data are presented as number (%), mean ± SD, or median ± quartile deviation.
Patients not subjected to an antivenom skin test were administered antivenom earlier after ED arrival than those who were subjected to an antivenom skin test (0.7 ± 0.34 hours and 0.9 ± 0.23 hours, respectively, P < 0.001). The early group had a shorter hospital length of stay and required less pain requiring treatment with analgesic injections than the late group. In addition, 103 (52.8%) and 82 (42.1%) patients were initially administered one and two vials of antivenom, respectively. Furthermore, 24.6%, 28.2%, 10.3%, 12.8%, 7.7%, 6.2%, and 10.3% of patients received a total of 1, 2, 3, 4, 5, 6, and ≥ 7 vials of antivenom during their in-hospital stay. The difference in initial laboratory data between the early and late groups was statistically insignificant.
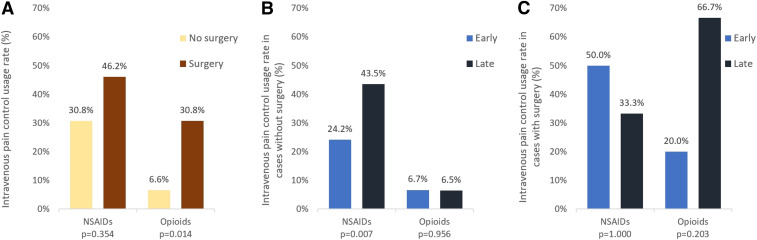
The data presented in Table 2 show that the late group had a longer hospital length of stay (42 ± 62 hours versus 99 ± 70 hours, respectively, P = 0.016) and a higher rate of pain requiring treatment with analgesic injections (29.2% versus 46.2%, respectively, P = 0.019) than the early group. For pain relief, six and 56 patients received NSAIDs intravenously and intramuscularly, respectively, whereas one and 15 patients received opioids intravenously and intramuscularly, respectively. After adjusting for potential confounding factors using logistic regression analysis, early antivenom administration (aOR: 0.51; 95% CI: 0.260–0.985) and surgical intervention (aOR: 6.14; 95% CI: 1.745–21.591) were still associated with pain requiring treatment with analgesic injections (Table 3). After excluding surgery cases, the early group still showed a lower rate of requiring treatment with analgesic injections to alleviate pain than the late group (26.7% versus 45.2%, respectively, P = 0.013). Figure 1 shows the subgroup analysis of medications for pain requiring treatment with analgesic injections for relief in patients with and without surgical intervention. Patients received fewer medications for relieving pain that required treatment with analgesic injections, particularly NSAIDs, in the early than in the late group of those with no surgical intervention (24.2% and 43.5%, respectively, P = 0.007).
Table 2.
Treatment and outcome of patients with Protobothrops mucrosquamatus snakebites in early and late groups
| Early (start antivenom ≤ 1 hour) (n = 130) | Late (start antivenom > 1 hour) (n = 65) | P-value | |
|---|---|---|---|
| Admission, n (%) | 57 (43.8) | 37 (56.9) | 0.085 |
| Intensive care unit admission, n (%) | 0 (0) | 1 (1.5) | 0.333 |
| Length of stay (hours) | 42 ± 62 | 99 ± 70 | 0.016 |
| Developed infection, n (%) | 26 (20) | 16 (24.6) | 0.460 |
| Fever episode | 6 (4.6) | 5 (7.7) | 0.511 |
| Cellulitis | 20 (15.4) | 14 (21.5) | 0.286 |
| Fasciitis | 1 (0.8) | 2 (3.1) | 0.258 |
| Antibiotic use, n (%) | 92 (70.8) | 52 (80) | 0.167 |
| Intravenous antibiotics | 62 (47.7) | 40 (61.5) | 0.068 |
| Penicillin | 30 (23.1) | 25 (38.5) | 0.115 |
| Cephalosporins | 28 (21.5) | 15 (23.1) | |
| Fluoroquinolones | 3 (2.3) | 0 (0) | |
| Others | 1 (0.8) | 0 (0) | |
| Oral antibiotics | 68 (52.3) | 34 (52.3) | 1.000 |
| Penicillin | 62 (47.7) | 31 (47.7) | 0.545 |
| Cephalosporins | 41 (31.5) | 21 (32.3) | |
| Fluoroquinolones | 23 (17.7) | 13 (20) | |
| Others | 4 (3.1) | 0 (0) | |
| Pain score | |||
| Before antivenom (n = 43) | 4.5 ± 3.0 | 3.0 ± 2.0 | 0.768 |
| After antivenom (n = 39) | 0.0 ± 1.0 | 0.5 ± 1.0 | 0.827 |
| Baseline analgesics, n (%) | 104 (80) | 52 (80) | 1.000 |
| Acetaminophen | 77 (59.2) | 38 (58.5) | 0.612 |
| NSAIDs | 22 (16.9) | 13 (20) | |
| Tramadol–acetaminophen | 5 (3.8) | 1 (1.5) | |
| Pain requiring treatment with analgesic injections, n (%) | 38 (29.2) | 30 (46.2) | 0.019 |
| NSAIDs | 34 (26.2) | 28 (43.1) | 0.017 |
| Opioids | 10 (7.7) | 6 (9.2) | 0.712 |
| Surgical intervention, n (%) | 10 (7.7) | 3 (4.6) | 0.417 |
| Debridement | 5 (3.8) | 1 (1.5) | 0.379 |
| Fasciotomy | 8 (6.2) | 3 (4.6) | 0.661 |
NSAID = nonsteroidal anti-inflammatory drug. Data are presented as number (%), mean ± SD, or median ± quartile deviation.
Table 3.
Association of pain requiring treatment with analgesic injections with early antivenom injection, initial dose, and surgery
| Adjusted odds ratio | 95% CI | P-value | |
|---|---|---|---|
| Early antivenom treatment | 0.51 | 0.260–0.985 | 0.045 |
| Initial antivenom dose (vials) | 1.15 | 0.711–1.861 | 0.569 |
| Surgery | 6.14 | 1.745–21.591 | 0.005 |
Confounders adjusted in the model: age, male gender, years of emergency department visit, heart rate during triage, mean arterial pressure during triage, and envenomation location.
Figure 1.
Medications for pain requiring treatment with analgesic injections in (A) all patients, (B) patients without surgery, and (C) patients with surgery. This figure appears in color at www.ajtmh.org.
DISCUSSION
Between January 2006 and December 2016, 195 patients visited the ED because of P. mucrosquamatus bites and were subsequently administered antivenom in the Chang Gung Memorial Hospital System. No in-hospital mortality was identified in this study. The laboratory data showed that 14.1%, 7.0%, 18.9%, 14.6%, 0.6%, 2.4%, and 11.9% of patients had leukocytosis (white blood cells > 12,000/µL), anemia (hemoglobulin < 12 g/dL), thrombocytopenia (platelet < 150,000/µL), prolonged prothrombin time (PT > 12 seconds), prolonged activated partial thromboplastin time (aPTT > 33.8 seconds), renal impairment (creatinine > 1.4 mg/dL), and elevated alanine aminotransferase levels, respectively. Previous studies report that only a few patients with P. mucrosquamatus snakebites had leukocytosis or coagulopathy.17,26
Naja atra snakebites could lead to wound necrosis and infection4 with patients requiring the administration of antibiotics. By contrast, such treatment in patients bitten by P. mucrosquamatus depends on clinical presentation and judgment of the physician.24 In this study, only 5.6% of patients experienced an episode of fever, which was lower than the 32.2% of patients with N. atra snakebite.4 Furthermore, cellulitis and the rate of antibiotic use in this study were similar to those of a previous study evaluating P. mucrosquamatus snakebites.17
Table 2 shows that 13 patients (6.7%) received surgical intervention, which was similar to the findings of a previous study, whereas the hospital length of stay was shorter in this study.17 The hospital length of stay was probably shorter in this study than that previously reported17 because it included some patients who only presented at the ED and were not admitted to the in-patient ward. The hospital length of stay was shorter in the early group than that in the late group (42 ± 62 hours versus 99 ± 70 hours, early and late groups, respectively, P = 0.016). This was likely because a delay in antivenom administration could lead to greater limb swelling and slower recovery of limb function.27
In this study, patients in the late group received more medications for relieving pain that needed analgesic injections than those in the early group, which might explain the longer hospital length of stay. The concerns of using NSAIDs for relieving pain associated with snakebites were its propensity to cause platelet dysfunction and bleeding. A previous study reported that the use of NSAIDs in snakebite patients might be safe.34 In this study, two patients experienced peptic ulcers, including one who used intramuscular NSAIDs for pain relief. No gastrointestinal or intracranial bleeding was identified in this study. In addition, a concern with the use of opioids is the propensity for patients to develop an addiction.35,36 Freiermuth et al.37 reported that snakebite victims would stop opioid use after full limb recovery, but their risk of iatrogenic addiction remains uncertain.
It is noteworthy that the implementation rate of antivenom skin test was lower in the early group than that in the late group (46.9% versus 63.1%, respectively). Snake antivenom skin test is not recommended in many studies,4,26,31 whereas its extremely low sensitivity and possible misinterpretation has been documented in another study.30 The low rate of severe reactions and the likelihood of no value being added to the patient care by skin testing were concerns.31 However, performing an antivenom skin test before administration is still recommended and listed on the product label in Taiwan. Despite considerable evidence against the benefits, many physicians still perform the antivenom skin test before administration because of medicolegal issues. Patients who did not undergo an antivenom skin test were administered antivenom earlier than those who did (0.7 ± 0.34 hours and 0.9 ± 0.23 hours in patients who received and did not receive the skin test, respectively, P < 0.001). A better strategy would be to withhold the antivenom skin test as recommended by the WHO guideline.9
The associations between pain requiring treatment with analgesic injections and early antivenom administration, the initial dose of antivenom administered, and surgical intervention are delineated in Table 3. Early antivenom administration was associated with decreased likelihood of pain requiring treatment with analgesic injections (aOR: 0.51; 95% CI: 0.260–0.985). The implementation of surgical intervention was positively correlated with pain requiring treatment with analgesic injections (aOR: 6.14; 95% CI: 1.745–21.591). Clinically, patients with severe wounds would undergo surgical intervention, and those with more severe injuries would be expected to experience more intense pain.
The results of further analysis of the relationship between the use of medications for pain relief and surgical intervention are shown in Figure 1. More patients who underwent surgical intervention received medication for pain relief than those who did not undergo surgery (61.5% versus 33.0%, respectively), especially opioids (30.8% versus 6.6% in the surgery versus no surgery groups, respectively P = 0.014). More patients in the early group who did not undergo surgical intervention received less medication, particularly NSAIDs, for relieving pain requiring treatment with analgesic injections than those in the late group (24.2% and 43.5%, respectively, P = 0.007). Opioid use was higher in patients in the late group who underwent surgical intervention than that in those in the early group, although the difference was statistically insignificant (P = 0.203).
Most physicians (94.9%) initially administered one to two vials of antivenom. No obvious association was found between the initial antivenom dose administered and pain requiring treatment with analgesic injections (aOR: 1.15; 95% CI: 0.711–1.861). More than half of the patients in this study received one vial of antivenom initially and less than four vials in total during their in-hospital stay. The total dose of antivenom administered was not different from that previously reported.17 Mao and Hung.26 suggested administering two to four vials of antivenom to patients with P. mucrosquamatus snakebites. Most patients in our study received a total of two to four vials of antivenin, and the outcomes (surgical rate and hospital length of stay) were not worse than those previously reported.17,24 In conclusion, the recommended dose of antivenom for P. mucrosquamatus snakebites in this study was one to two vials initially and a total of two to four vials.
Study limitations.
There are a few limitations to our study. First, pain requiring treatment with analgesic injections was defined based on patients who needed pain control with injections of NSAIDs or opioids rather than by the severity of pain using pain scales. The pain scale was poorly recorded in the patient medical history. Only 43 and 39 patients had recorded pain scale assessments before and after antivenom administration, respectively, and the difference between the early and late groups was statistically insignificant (P = 0.768 and P = 0.827, respectively). This result might have been obtained because the number of included cases was too small or the analgesic injection rate differed between the two groups. Further studies would be required to verify the benefits of early antivenom administration in reducing pain intensity. Second, there was no wound severity and swelling scoring in this study. The severity of envenomation was a potential confounding factor associated with the pain, pain management, amount of antivenom used, surgical intervention, and length of hospital stay. Some patients might experience pain from severe wound swelling or inflammation. Although a positive correlation between surgical intervention and pain requiring treatment with analgesic injections was observed in this study, there was no direct evidence to indicate that wound severity was the main reason for surgical interventions. The decision for surgical intervention might have relied on the clinical judgment of surgeons. Furthermore, wound severity scoring may be needed to clarify the relationship between wound severity for surgical intervention in future studies.
Third, early antivenom administration was defined based on the time from ED visit to antivenom administration rather than the time from snakebite to antivenom injection. Benefits of earlier antivenom administration in patients with P. mucrosquamatus snakebite should be further evaluated using the time from snakebite to antivenom administration in future studies, although a recall bias might exist. Fourth, patients who took medications, such as anticoagulants, systemic steroids, or antihistamines, before visiting the ED were not excluded, which could affect laboratory coagulation tests, skin test reliability, and antivenom reactions. Lastly, the limitations of the retrospective design might have introduced some confounding factors that could cause pain requiring treatment with analgesic injections and longer hospital length of stay, which were not considered in this analysis.
CONCLUSION
Antivenom administration within 1 hour of arrival was associated with a decreased likelihood of pain requiring treatment with analgesic injections and hospital length of stay in patients with P. mucrosquamatus snakebites in this study. Antivenom skin testing was also associated with delays in antivenom administration.
Geolocation information.
The study area included the six medical institutions at Keelung, Taipei, Linkou, Yunlin, Chiayi, and Kaohsiung branches located along northern to southern Taiwan.
REFERENCES
- 1.Gutierrez JM, Calvete JJ, Habib AG, Harrison RA, Williams DJ, Warrell DA, 2017. Snakebite envenoming. Nat Rev Dis Primers 3: 17063. [DOI] [PubMed] [Google Scholar]
- 2.Chippaux JP, 1998. Snake-bites: appraisal of the global situation. Bull World Health Organ 76: 515–524. [PMC free article] [PubMed] [Google Scholar]
- 3.Alirol E, Sharma SK, Bawaskar HS, Kuch U, Chappuis F, 2010. Snake bite in South Asia: a review. PLoS Negl Trop Dis 4: e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao YC, Liu PY, Chiang LC, Lai CS, Lai KL, Ho CH, Wang TW, Yang CC, 2018. Naja atra snakebite in Taiwan. Clin Toxicol (Phila) 56: 273–280. [DOI] [PubMed] [Google Scholar]
- 5.Mao YC, Liu PY, Chiang LC, Liao SC, Su HY, Hsieh SY, Yang CC, 2017. Bungarus multicinctus snakebite in Taiwan. Am J Trop Med Hyg 96: 1497–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin CC, Chaou CH, Tseng CY, 2016. An investigation of snakebite antivenom usage in Taiwan. J Formos Med Assoc 115: 672–677. [DOI] [PubMed] [Google Scholar]
- 7.Liu CH, Xie WQ, 2017. Analysis of the Use of Antivenom in 2008–2012 by the Health Insurance Database. Available at: https://www.cdc.gov.tw/File/Get/N_uqcZoh1VZrxEfY1JUigA. Accessed May 9, 2017. [Google Scholar]
- 8.Warrell DA, 2010. Guidelines for the Management of Snake-Bites. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 9.WHO, Staff ROfS-EA , 2016. Guidelines for the Management of Snakebites, 2nd Edition Geneva, Switzerland: World Health Organization. [Google Scholar]
- 10.Vasaruchapong T, Laoungbua P, Tangrattanapibul K, Tawan T, Chanhome L, 2017. Protobothrops mucrosquamatus (Cantor, 1839), a highly venomous species added to the snake fauna of Thailand (Squamata: Viperidae). Trop Nat Hist 17: 111–115. [Google Scholar]
- 11.Leviton AE, Wogan G, Koo M, Zug GR, Lucas R, Vindum J, 2003. The dangerously venomous snakes of Myanmar. Illustrated key and checklist. Proc Calif Acad Sci 54: 407–462. [Google Scholar]
- 12.Aengals R, Kumar VS, Palot MJ, 2011. Updated checklist of Indian reptiles. Occasional Papers, Zoological Survey of India, 24 pp.
- 13.Guo P, Liu Q, Zhu F, Zhong GH, Che J, Wang P, Xie YL, Murphy RW, Malhotra A, 2019. Multilocus phylogeography of the brown-spotted pitviper Protobothrops mucrosquamatus (Reptilia: Serpentes: Viperidae) sheds a new light on the diversification pattern in Asia. Mol Phylogenet Evol 133: 82–91. [DOI] [PubMed] [Google Scholar]
- 14.Zhong G, Liu Q, Li C, Peng P, Guo P, 2017. Sexual dimorphism and geographic variation in the asian lance-headed pitviper Protobothrops mucrosquamatus in the mainland China. Asian Herpetol Res 8: 118–122. [Google Scholar]
- 15.Tan CH, Tan KY, Ng TS, Quah ESH, Ismail AK, Khomvilai S, Sitprija V, Tan NH, 2019. Venomics of Trimeresurus (popeia) nebularis, the cameron highlands pit viper from Malaysia: insights into venom proteome, toxicity and neutralization of antivenom. Toxins (Basel) 11: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orlov N, Ananjeva N, Khalikov R, 2002. Natural history of pitvipers in eastern and southeastern Asia. Biol Vipers 345–361. [Google Scholar]
- 17.Chen YW, Chen MH, Chen YC, Hung DZ, Chen CK, Yen DHT, Huang CI, Lee CH, Wang LM, Yang CC, 2009. Differences in clinical profiles of patients with Protobothrops mucrosquamatus and Viridovipera stejnegeri envenoming in Taiwan. Am J Trop Med Hyg 80: 28–32. [PubMed] [Google Scholar]
- 18.Juan CW, 2012. Venomous snake bites in Taiwan. Taiwan Soc Crit Care Med 23: 93–108. [Google Scholar]
- 19.Hung DZ, 2004. Taiwan’s venomous snakebite: epidemiological, evolution and geographic differences. Trans R Soc Trop Med Hyg 98: 96–101. [DOI] [PubMed] [Google Scholar]
- 20.Available at: https://info.fda.gov.tw/MLMS/ShowFile.aspx?LicId=09000006&Seq=005&Type=9.
- 21.Villalta M, et al. 2012. Snake venomics and antivenomics of Protobothrops mucrosquamatus and Viridovipera stejnegeri from Taiwan: keys to understand the variable immune response in horses. J Proteomics 75: 5628–5645. [DOI] [PubMed] [Google Scholar]
- 22.Liu CH, Li CJ, Li J, 2015. Application research of snake venom protein and anti-venom serum manufacturing technology. Taiwan Epidemiol Bull 31: 76–85. [Google Scholar]
- 23.Tsai YH, Hsu WH, Huang KC, Yu PA, Chen CL, Kuo LT, 2017. Necrotizing fasciitis following venomous snakebites in a tertiary hospital of southwest Taiwan. Int J Infect Dis 63: 30–36. [DOI] [PubMed] [Google Scholar]
- 24.Chen YC, Chen MH, Wang LM, Wu JJJ, Huang CI, Lee CH, Yen DHT, Yang CC, 2007. Antivenom therapy for crotaline snakebites: has the poison control center provided effective guidelines? J Formos Med Assoc 106: 1057–1062. [DOI] [PubMed] [Google Scholar]
- 25.Lin CC, Wang PJ, Liu CC, 2019. Venom concentrations in blisters and hemorrhagic bullae in a patient bitten by a Taiwan habu (Protobothrops mucrosquamatus). Rev Soc Bras Med Trop 52: e20180160. [DOI] [PubMed] [Google Scholar]
- 26.Mao YC, Hung DZ, 2015. Management of snake envenomation in Taiwan. In: Gopalakrishnakone P, Faiz A, Fernando R, Gnanathasan C, Habib A, Yang CC, eds. Clinical Toxinology in Asia Pacific and Africa. Toxinology, vol 2. Dordrecht: Springer. [Google Scholar]
- 27.Anderson VE, et al. 2019. Early administration of Fab antivenom resulted in faster limb recovery in copperhead snake envenomation patients. Clin Toxicol (Phila) 57: 25–30. [DOI] [PubMed] [Google Scholar]
- 28.Chen JC, Bullard MJ, Chiu TF, Ng CJ, Liaw SJ, 2000. Risk of immediate effects from F(ab)2 bivalent antivenin in Taiwan. Wilderness Environ Med 11: 163–167. [DOI] [PubMed] [Google Scholar]
- 29.Sampson HA, et al. 2006. Second symposium on the definition and management of anaphylaxis: summary report--Second national institute of allergy and infectious disease/food allergy and anaphylaxis network symposium. J Allergy Clin Immunol 117: 391–397. [DOI] [PubMed] [Google Scholar]
- 30.Chuang PC, Chang KW, Cheng FJ, Wu MH, Tsai MT, Li CJ, 2019. Risk factors associated with snake antivenom reaction and the role of skin test. Acta Trop 203: 105293. [DOI] [PubMed] [Google Scholar]
- 31.Thiansookon A, Rojnuckarin P, 2008. Low incidence of early reactions to horse-derived F(ab′)(2) antivenom for snakebites in Thailand. Acta Trop 105: 203–205. [DOI] [PubMed] [Google Scholar]
- 32.Seak CJ, Fang CC, 2018. Celebrating the 20th anniversary of emergency medicine specialty in taiwan. J Acute Med 8: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi KJ, Knuesel SJ, White BA, Bravard MA, Chang Y, Metlay JP, Raja AS, Mattison MLp, 2019. Impact on length of stay of a hospital medicine emergency department boarder service. J Hosp Med 14: E1–E7. [DOI] [PubMed] [Google Scholar]
- 34.Pham HX, Mullins ME, 2018. Safety of nonsteroidal anti-inflammatory drugs in copperhead snakebite patients. Clin Toxicol (Phila) 56: 1121–1127. [DOI] [PubMed] [Google Scholar]
- 35.Butler MM, et al. 2016. Emergency department prescription opioids as an initial exposure preceding addiction. Ann Emerg Med 68: 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah A, Hayes CJ, Martin BC, 2017. Factors influencing long-term opioid use among opioid naive patients: an examination of initial prescription characteristics and pain etiologies. J Pain 18: 1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freiermuth CE, Lavonas EJ, Anderson VE, Kleinschmidt KC, Sharma K, Rapp-Olsson M, Gerardo C; Copperhead Recovery Workgroup , 2019. Antivenom treatment is associated with fewer patients using opioids after copperhead envenomation. West J Emerg Med 20: 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]