Abstract
Previous studies have suggested that increases in maternal cortisol or maternal stress in late pregnancy increase the risk of stillbirth at term. In an ovine model with increased maternal cortisol over the last 0.20 of gestation, we have previously found evidence of disruption of fetal serum and cardiac metabolomics and altered expression of genes related to mitochondrial function and metabolism in biceps femoris, diaphragm, and cardiac muscle. The present studies were designed to test for effects of chronically increased maternal cortisol on gene expression and metabolomics in placentomes near term. We hypothesized that changes in placenta might underlie or contribute to the alterations in fetal serum metabolomics and thereby contribute to changes in striated muscle metabolism. Placentomes were collected from pregnancies in early labor (143 ± 1 days gestation) of control ewes (n = 7) or ewes treated with cortisol (1 mg·kg−1·day−1 iv; n = 5) starting at day 115 of gestation. Transcriptomics and metabolomics were performed using an ovine gene expression microarray (Agilent 019921) and HR-MAS NMR, respectively. Multiomic analysis indicates that amino acid metabolism, particularly of branched-chain amino acids and glutamate, occur in placenta; changes in amino acid metabolism, degradation, or biosynthesis in placenta were consistent with changes in valine, isoleucine, leucine, and glycine in fetal serum. The analysis also indicates changes in glycerophospholipid metabolism and suggests changes in endoplasmic reticulum stress and antioxidant status in the placenta. These findings suggest that changes in placental function occurring with excess maternal cortisol in late gestation may contribute to metabolic dysfunction at birth.
Keywords: cortisol, fetus, metabolism
INTRODUCTION
The placenta provides the interface between the mother and the fetus, serving the function of nutrient transport and gas exchange necessary for fetal growth and homeostasis. The placenta also provides the interface between the maternal humoral environment and the fetus, reducing fetal exposures to toxins, but also to maternal hormones. The placenta, through expression of 11β-hydroxysteroid dehydrogenase type II (11βHSD2) provides a relative barrier to free transport of cortisol (58, 74). Although maternal cortisol levels increase in early gestation in humans and several other species, including sheep (6, 36), placental 11βHSD2 activity normally maintains low fetal cortisol concentrations throughout most of gestation before the development of the fetal adrenal in late gestation. Although the rise in fetal cortisol at term is critical for the maturation of fetal organs and viability of the newborn (32, 36), a precocious rise in fetal cortisol initiates premature transition from tissue differentiation (17). Increases in ovine maternal cortisol levels appear to alter maturation of fetal cardiac structure and metabolism (15, 67) but may also alter the morphology and metabolism of the placenta (22, 64).
In previous studies in our laboratory, using an ovine model of chronic maternal stress in late gestation, we found an increased incidence of stillbirth in fetuses of ewes infused with cortisol. In this model, maternal cortisol concentrations are increased to approximately double the normal levels, increasing fetal cortisol concentrations in the period before the normal preparturient surge in cortisol (2, 25). In this cohort, we also found that glucose disappearance was slowed in the cortisol-treated ewes (25), reflecting cortisol effects on glucose metabolism in the ewe. Our subsequent studies in another cohort of animals found that there was an increased incidence of bradycardia and arrhythmias at birth in the fetuses of the cortisol-treated ewes (2), which likely contributed to the high incidence of periparturient stillbirth. Multiomic modeling using transcriptomics and metabolomics of the newborn fetal sheep hearts, and transcriptomics in the term fetal sheep heart suggest changes in cardiac metabolism and mitochondrial function occurring in late gestation or immediately following birth as a consequence of excess maternal cortisol (2, 49, 67). The metabolomic profile in hearts collected immediately following birth in the cortisol-treated pregnancies closely paralleled those in fetal, but not in maternal, serum; this analysis suggested that there are disruptions in placental metabolism of amino acids and TCA cycle metabolites due to excess maternal cortisol (67). Studies by others using a similar dose of cortisol administered to ewes from ∼125–130 days gestation found that uteroplacental glucose utilization and lactate production were both increased (63, 64) and suggested that uteroplacental glucose consumption was increased with a corresponding decrease in the amount of glucose transferred to the fetus (64). Therefore, the present study was undertaken to model the effects of the more chronic excess of maternal cortisol on placental metabolism at term, using transcriptomics and metabolomics. We used placentomes collected in early labor from the previously studied cortisol-treated pregnancies to test the hypothesis that excess cortisol impacts pathways involved in the supply and/or utilization of nutrients in the placentomes, which in turn could contribute to the effects on fetal serum metabolites, cardiac metabolism, and increased stillbirth in this model.
METHODS
Animal studies.
The animal use protocol was approved by the Institutional Animal Care and Use Committee at the University of Florida. Placentomes were collected at necropsy from control ewes and ewes treated with cortisol (CORT: 1 mg·kg−1·day−1 from day 115 of pregnancy) at days 141–144 of gestation. CORT ewes received an intravenous infusion of cortisol (Solu-Cortef, hydrocortisone sodium succinate in sodium phosphate; Pfizer, New York, NY); control animals did not receive any treatment. As previously reported, the incidence of fetal/newborn stillbirth was significantly increased in the group of ewes treated to raise maternal cortisol (25). Because of the increase in the incidence of stillbirth associated with the elevated cortisol, animals were euthanized for collection of tissues at the first signs of labor, as evidenced by changes in uterine blood flow; placentomes were collected from sheep in early stages of labor at between 141 and 144 days and 142–144 days of gestation in the cortisol-treated and control groups, respectively. In this cohort of ewes, there were seven control ewes, of which data were collected from placentomes of seven live fetuses (including one set of twins); there was one lamb born, and that placenta was not collected. In this cohort there were 11 cortisol-treated ewes, from which we collected placentomes from five live fetuses. There was one live lamb born, and five stillborn lambs, from which placentas were not collected. Placentomes were classified as A–D placentomes, snap-frozen in liquid nitrogen, and stored at −80°C until transcriptomic or metabolomic analysis. Although in this study the ewes were chosen randomly for either cortisol or control treatment, there was an imbalance in the distribution of sexes: there were four male and three female fetuses of control ewes and five female fetuses of cortisol-infused ewes (Figs. 1 and 2). This imbalance was not a consistent finding in further cohorts of fetuses studied, and in this cohort, there were two stillborn males and three stillborn females.
Fig. 1.
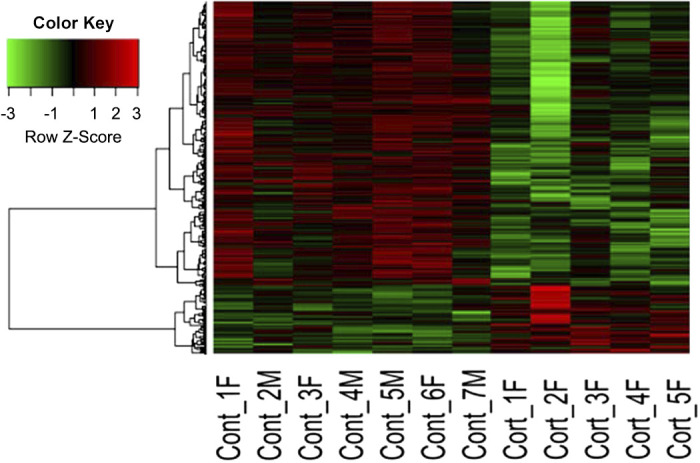
Heat map of the differentially expressed genes (uncorrected P ≤ 0.05) in placentomes of term control (Cont1–Cont7) or cortisol-treated (Cort1–Cort5) ewes. M, male fetus; F, female fetus; Cont-3 and Cont-4 were twin fetuses.
Fig. 2.
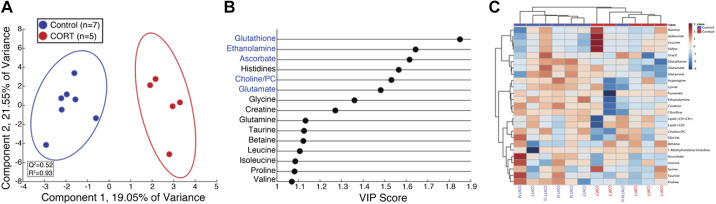
Orthogonal signal corrected partial least squares discriminant analysis (OSC-PLSDA) reveals separation of high-resolution magic angle spinning (HR-MAS) placental tissue specimens from control and cortisol-treated (CORT) fetuses. A: OSC-PLSDA scores plot reveals separation of control (n = 7, blue) and CORT (n = 5, red) placental tissue in the first principal component. B: variable importance of projection (VIP) plot for the first 15 metabolites that contribute to separation in OSC-PLSDA component 1. The more a metabolite contributes to the separation in the scores plot, the greater the VIP score (x-axis). Metabolites colored in blue are significantly reduced (P < 0.05) in CORT placental tissue specimens relative to control. C: heatmap of top 25 metabolites reveals clustering by control (n = 7, blue or purple) and CORT (n = 5, red) placental tissue. Samples are highlighted by sex of fetus as male (blue, n = 4) and female (purple, n = 3 and red, n = 5) at bottom of heatmap. Twins are denoted by (t).
In vivo data in these ewes and transcriptomics of the fetal heart, skeletal muscles, and pericardial fat have been previously reported (24, 48, 49): the average maternal cortisol concentration was 18.4 ± 1.5 in the CORT ewes and 10.6 ± 1.7 ng/mL in the control ewes. Maternal plasma glucose was also increased in the CORT ewes, and the glucose response to intravenous glucose challenge was prolonged (25).
RNA extraction and labeling.
All placentomes used for this study were either type A or B (62); both the maternal caruncular and fetal cotyledonary zones were included in each section and frozen. Placentomes from cortisol-treated (n = 5) and untreated (n = 7) pregnancies were pulverized in liquid nitrogen using a mortar and pestle. RNA was extracted using TRIzol and purified with RNA-easy Plus kits with on-column DNase digestion (Qiagen), as per the manufacturer’s instruction. The quality of the extracted RNA was determined at the Interdisciplinary Center for Biotechnology Research (ICBR) at the University of Florida, using a Bioanalyser. All RNA samples had a RNA integrity number above 8.0. RNA (100 ng) was labeled with Cy3 dye using One Color Low-Input QuickAmp labeling kits (Agilent) and quantified. The labeled cRNA (600 ng) was fragmented and hybridized to an 8 × 15k ovine gene expression microarray (Agilent 019921), and the array was scanned at the Gene Expression Core of the ICBR.
Analysis of microarray data.
The ovine microarray has 15,000 unique probes, for which ∼8,000 unique genes have been annotated and assigned human gene symbols (44). The microarray data were analyzed as previously described (43). Briefly, the raw data were analyzed for outliers, and background correction of the data was performed using the limma package in R (56). Probes at least 10% brighter than the negative controls were used in the analysis. For genes that had multiple probes, the probe with the highest quality was used. Genes differentially regulated by cortisol (P ≤ 0.05) were identified by a moderated t test using the empirical Bayes method (57) and included in subsequent pathway analysis. Raw and normalized data from the R analysis have been submitted to GEO and are available at GSE149402.
The differentially expressed genes (DEG) were modeled with an open platform for complex data analysis, web-based gene enrichment analysis, WEBGESTALT 2019 (31). Gene ontology enrichment analysis (GO) was performed to determine overrepresented biological processes, molecular functions, and cellular components related to DEG in the placentomes after maternal cortisol infusion. Overrepresentation analysis (ORA) was performed to assess the enrichment of pathways on the basis of number of DEG relative to total genes in the pathway. Weighted set cover analysis was used to find the minimum subset of the gene set that could cover all the genes from the enriched sets; this analysis identified a more specific set of processes and functions. Pathway analysis was performed using the Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis to determine pathways differentially regulated by cortisol in these tissues. All analyses were performed using the human genome as the reference set. The significance criteria for the DEG did not include correction for false discovery rate; however, the criterion for the pathway enrichment analyses was P < 0.05, after a multiple test correction using the Benjamini–Hochberg method. The adjusted P, indicating false discovery rate (FDR), is reported for all processes, functions, cell components, and pathways.
Transcription factor analysis.
Regulatory motif matches were identified using g profiler from TRANSFAC (46), a database of eukaryotic transcription factors, their genomic binding sites, and DNA-binding profiles. The overrepresented regulatory motifs were scanned for glucocorticoid response elements (GREs) identified by chromatin immunoprecipitation (ChIP) sequencing (75) as well as for other noncanonical glucocorticoid receptor (GR)-binding sites (41). Genes identified by ChIP sequencing were compared with the annotated genes on the ovine microarray (44); the 1,796 unique genes so identified were then compared with the DEG from the cortisol placentomes to identify potential GR-regulated genes.
Real-time quantitative PCR.
Pathways differently regulated by cortisol were validated by real-time quantitative (q)PCR (Quant Studio, using Taq-Man or SYBR chemistry). Primers (Table 1) were designed from the ovine nucleotide sequences of the respective genes, using Primer Express (Applied Biosystems). The selected amplicon was blasted against the sheep genome to confirm 100% identity. Primer efficiency was tested and the presence of a single peak in the SYBR chemistries was confirmed.
Table 1.
Sequences of probe and primers used in quantitative PCR
| Gene | Forward Primer | Reverse Primer | Probe |
|---|---|---|---|
| IGFII | CTGCCTCTACGACCGTGCTT | TGCTTCCAGGTGTCAGATTGG | TCACAGCATACCCCGTGGGCAAG |
| PLGF | CCCTGGAGACAGCCAACGT | GGCTGGTCCAGAGAGTGGTACT | N/A |
| ACSM1 | TCCCACCCTTTGACATTCAGA | CGTGTTGGGAGGCTGGATAT | N/A |
| SLC7A5 | CTTCCTCAAGCTGTGGATCGA | CGAGCGCCACGATGTACTG | N/A |
N/A, not applicable.
cDNA was generated from 100 ng of RNA using the High Capacity cDNA kit (Applied Biosystems, Thermo Fisher Scientific; catalog no. 4368813). All samples were run in triplicate along with a nontemplate control on each plate. The ΔCT method was used for normalizing the data (33), using 18S abundance, as β-actin expression was altered by the cortisol treatment. Differences in gene expression between the groups were determined by Student’s t test of the ΔCT values. Since the purpose of the qPCR was validation of the microarray, significance was determined by one-tailed t test using the criterion of P ≤ 0.05.
Immunoblotting.
Immunoblotting was done to compare the expression of proteins in the placentomes between the two groups. Briefly, the homogenates were prepared using ∼50 mg of tissue, which was homogenized in a lysis buffer containing phosphatase and protease inhibitors. Expression of total and phosphorylated isoforms of p44/42 mitogen-activated protein kinase (MAPK; mouse mAb, 4696S, and rabbit, 9101S, respectively, Cell Signaling Technologies) and lysosome-associated membrane protein-1 (LAMP1; Rabbit mAb 9091T; Cell Signaling Technologies) were also analyzed by immunoblot. The specificity of the proteins was determined based on the presence of a single band at the appropriate molecular weight. A test of linearity was done by loading increasing amounts of proteins to confirm that the signal intensity was proportional to the amount of protein loaded. Protein concentrations used for the immunoblots were selected for each antibody to be within the linear range. Equal amounts of protein were loaded onto the same gel from all the placentome samples from control and cortisol-treated animals. The relative quantities of protein in each lane were detected and analyzed with Image Studio version 5.2 using the LI-COR Odyssey Clx system (LI-COR, Lincoln, NE), with correction for equal portion of total protein loaded in the lane. Differences in protein expression between placenta from the control and cortisol-treated animals were analyzed by Student’s t test when conditions of equal variance were met, Welch’s t test when conditions of equal variance failed, or by Mann–Whitney nonparametric test when not normally distributed, using a two-tailed hypothesis. The criterion for significance was P ≤ 0.05.
Metabolomic analysis.
High-resolution magic angle spinning (HR-MAS) proton nuclear magnetic resonance (1H-NMR) was used to determine metabolites present in placental tissue as previously described (69). Briefly, 30 μL of deuterium oxide (2H2O) with 3.3 mM 3-(trimethylsilyl)-1-propanesulfonic acid as a reference standard was added to 27.7–52.1 mg (mean 35.3 mg) of placental tissue before being placed into a 4-mm HR-MAS rotor (Bruker Biospin) and spun at 6 kHz with a spectral width of 10 ppm. Data were acquired on an Avance III 600 MHz Bruker NMR spectrometer equipped with a 4-mm HR-MAS probe at the University of Georgia Complex Carbohydrate Research Center. Data were acquired using a one-dimensional (1D) experiment with T2 filter using Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence with water presaturation (CPMGPR1D).
Metabolites identified in 1D NMR experiments were verified with 2D NMR as previously described (69). 2D data were acquired using 13C-1H heteronuclear single quantum correlation (HSQC) and HSQC-TOCSY (HSQC-total correlation spectroscopy) experiments using samples that were extracted using 80% MeOH/20% H2O, ground with a bead homogenizer, and pooled. Metabolites were assigned a confidence scale as previously described (68). The spectra were processed using Bruker Topspin 4.0.06 software and in-house MATLAB scripts. All raw and processed data are available on the metabolomics workbench (http://www.metabolomicsworkbench.org/), along with detailed experimental NMR and analysis methods. The project (Project ID PR00094) can be accessed through http://dx.doi.org/10.21228/M8311J.
Samples were batch corrected before statistical analysis as previously described (67). Metabolomic data were normalized using probabilistic quotient normalization (PQN) and were range scaled before multivariate statistical analysis (12, 14). Multivariate analyses of processed spectra were performed using in-house MATLAB scripts (https://github.com/artedison/Edison_Lab_Shared_Metabolomics_UGA) and the PLS Toolbox for MATLAB (Eigenvector Research, Inc., Manson, WA) (60). Student’s t test was used to identify significantly altered metabolites in placental tissue following PQN normalization and log transformation (12, 14). We utilized an overrepresentation analysis based on a hypergeometric test to identify significantly enriched metabolic pathways in placental tissue (72). Only significantly altered metabolites (P < 0.05) were subjected to ORA, and pathways were defined as significant with FDR-corrected P < 0.10.
Multiomics analysis of the metabolomic and transcriptomic data was performed using MetaboAnalyst (http://www.metaboanalyst.ca) (73), using significantly altered metabolites and genes from placental samples. This analysis was also performed using significantly altered metabolites from fetal serum along with genes from placental samples; the fetal serum results are from a separate cohort of similarly treated pregnant ewes and have been previously reported (67).
RESULTS
Although type C and D cotyledons were more frequent in the cortisol-treated groups, cortisol infusion did not significantly alter total placental weight, numbers of each type of cotyledon, or fetal weight at necropsy (Table 2). This is consistent with the start of this intervention at 115 days of ovine pregnancy, a time at which placental growth is nearly complete (13).
Table 2.
Fetal body weights, placentome weights, and distribution of placentome types (control pregnancy with twin fetuses not included)
| Control (n = 5) |
Cortisol (n = 5) |
|
|---|---|---|
| Fetal body weight, g | 5,686 ± 577 | 5,946 ± 254 |
| Total Placental weight, g | 320 ± 60 | 403 ± 27 |
| Weight (g) and (no. of placentomes) | ||
| Type A | 150.9 ± 48.0 (46 ± 13) | 216.6 ± 47.4 (49 ± 16) |
| Type B | 152.0 ± 68.1 (27 ± 11) | 104.2 ± 45.4 (16 ± 6) |
| Type C* | 13.0 ± 13.0 (2 ± 2) | 40.7 ± 21.4 (16 ± 7) |
| Type D* | 3.6 ± 3.6 (1 ± 1) | 41.9 ± 25.7 (3 ± 3) |
Only 1 Control and 3 cortisol treated had C or D placentomes.
Transcriptomic analysis of the placentome identified 664 DEG, (P ≤ 0.05), of which 539 were downregulated and 125 were upregulated in the placentomes of cortisol-treated ewes (Fig. 1). This small number of upregulated genes resulted in no unique biological processes, molecular functions, or KEGG pathways. The biological processes associated with the most downregulated DEG were metabolic process (343 genes), biological regulation (333 genes), response to stimulus (279 genes), localization (236 genes), cell component organization (216 genes), multicellular organismal process (201 genes), cell communication (190 genes), developmental process (180 genes), and multiorganism process (94 genes). The biological processes overrepresented by the downregulated DEG were more specifically identified by using weighted set cover analysis to find the minimum subset of gene sets that could cover all the genes from the enriched sets; this analysis identified the nonredundant processes as related to immune responses: response to cytokine, immune effector process; processes relating to metabolism: organonitrogen compound catabolic processes; processes relating to cell protein localization: cell macromolecular localization, protein-containing complex assembly, vesicle-mediated transport, and secretion, as well as cell activation and cell cycle (Table 3). When the more specific processes were considered, those with the highest enrichment factor were positive regulation of cellular protein catabolic process (3.4-fold) and endoplasmic reticulum (ER) to Golgi vesicle-mediated transport (2.8-fold).
Table 3.
Significant molecular functions, biological processes, or pathways identified in ORA using Webgestalt 2019
| Function/Process/Pathway | No. of Genes | FDR | ER |
|---|---|---|---|
| Biological process | |||
| Cellular macromolecular localization | 78 | 0.0303 | 1.52 |
| Cell cycle | 76 | 0.0198 | 1.56 |
| Secretion | 74 | 0.0078 | 1.65 |
| Response to cytokine | 50 | 0.0073 | 1.82 |
| Immune effector process | 58 | 0.0073 | 1.82 |
| Protein-containing complex assembly | 78 | 0.0348 | 1.52 |
| Vesicle-mediated transport | 93 | 0.0011 | 1.71 |
| Organonitrogen compound catabolic processes | 60 | 0.0129 | 1.73 |
| Cell activation | 65 | 0.0073 | 1.74 |
| Symbiont process | 42 | 0.0146 | 1.96 |
| Molecular function | |||
| Drug binding | 72 | 0.0089 | 1.57 |
| Identical protein binding | 73 | 0.0043 | 1.61 |
| Purine ribonucleotide binding | 82 | 0.0014 | 1.65 |
| RNA binding | 75 | 0.0012 | 1.74 |
| Structural molecule activity | 40 | 0.0112 | 1.88 |
| Protein complex containing binding | 56 | 0.0012 | 1.97 |
| Cell adhesion molecule binding | 31 | 0.0015 | 2.42 |
| Kinase binding | 40 | 0.0015 | 2.09 |
| Hydrolase activity, acting on glucosyl bonds | 11 | 0.0315 | 3.51 |
| Deacetylase activity | 9 | 0.0043 | 5.59 |
| Cancer wikipathways | |||
| 4-Hydroxytamoxifen, dexamethasone, and retinoic acids regulation of p27 expression | 5 | 0.0400 | 6.87 |
| KEGG pathways | |||
| Lysosome | 18 | 0.0001 | 4.05 |
| Other types of O-glycan biosynthesis | 6 | 0.0159 | 7.54 |
ER, enrichment ratio; FDR, false discovery rate; KEGG, Kyoto Encyclopedia of Genes and Genomes; ORA, overrepresentation analysis.
The molecular functions with the most downregulated genes associated with them were protein binding (389 genes), ion binding (183 genes), nucleic acid binding (117 genes), nucleoside binding (88 genes), hydrolase activity (100 genes), transferase activity (79 genes), enzyme-regulated activity (40 genes), structural molecular activity (40 genes), transporter activity (36 genes), and molecular transducer activity (24 genes). The molecular processes identified by overrepresentation analysis (ORA) of the downregulated DEG in the placentome of cortisol-treated ewes identified by weighted set enrichment analysis were purine ribonucleotide binding, deacetylase activity, cell adhesion molecule binding, RNA binding, kinase binding, protein-containing complex binding, identical protein binding, drug binding, structural molecule activity, and hydrolase activity acting on glycosyl bonds (Table 3). The most highly enriched molecular functions were related to histone and protein deacetylase activity (5.8- and 5.6-fold, respectively), extracellular matrix structural constituents (3.8-fold), and hydrolase activity on glycosyl bond (3.5-fold).
Although transcription factor analysis did not identify overrepresentation of motifs related to GRE in the DEG, a comparison of DEG with genes found by ChIP to have GR binding to the promoter (53) identified 142 DEG. In addition, the transcription factor profiler did identify significant numbers of DEG with known interaction with other transcription factors. Some of these other transcription factors are known to interact with GR including nuclear factor-κB (NF-κB), specificity protein-1 (SP1), and protein C-ets-1 (ETS1) (41). SP1 and ETS1 binding motifs are associated with up to 430 DEG, suggesting that changes in gene expression may be influenced by GR interactions with other transcription factors.
Since genes in cancer pathways are frequently relevant to placental biology, we used Webgestalt 2019 to examine the changes in the cancer wikipathway (54). The cancer wikipathway associated with the downregulated genes was the 4-hydroxytamoxifen, dexamethasone, and retinoic acid regulation of the p27 expression pathway (Table 3) and included five genes: MAPK1, MAPK3, mitogen-activated protein kinase kinase-6 (MAP2K6), MAPK-interacting serine/threonine kinase-1 (MKNK1), and the protooncogene serine/threonine kinase RAF1. These genes were also identified in the cell cycle biological process. However, the protein expressions of MAPK1 (p42 MAPK) and MAPK3 (p44 MAPK) were not significantly altered in the placentomes of cortisol-treated ewes, although the ratio of total MAPK to phosphorylated was increased, consistent with downregulation of activity in this pathway (Table 4).
Table 4.
Expression of proteins in placental homogenates
| Protein | Control | Cortisol | P Value |
|---|---|---|---|
| LAMP1 (nonglycosylated) | 1.00 ± 0.17 | 1.67 ± 0.31 | 0.02 |
| Total p44 | 1.00 ± 0.10 | 0.93 ± 0.10 | 0.68 |
| Total p42 | 1.00 ± 0.07 | 0.96 ± 0.09 | 0.74 |
| Phospho p44 | 1.00 ± 0.26 | 0.54 ± 0.20 | 0.2 |
| Phospho p42 | 1.00 ± 0.29 | 0.53 ± 0.19 | 0.27 |
| Ratio p44 (total/phosphorylated) |
1.00 ± 0.24 | 2.29 ± 0.60 | 0.07 |
| Ratio p42 (total/phosphorylated) |
1.00 ± 0.17 | 2.13 ± 0.51 | 0.12 |
Data are expressed as means ± SE of fold change relative to average level in the Control group. LAMP1, lysosome-associated membrane protein-1.
KEGG pathways associated with downregulated genes were lysosome and other O-glycan biosynthesis (Table 3); included in the genes in the lysosomal pathway are genes encoding for four glycosidases and three lysosomal membrane proteins. The expression of the gene LAMP1, encoding a lysosomal membrane protein, was decreased on the microarray, and the expression of the nonglycosylated form of LAMP1 protein was significantly increased in the placentomes from cortisol-treated ewes (Table 4). When we restricted our ORA KEGG analysis to the 142 DEG known to bind GR as identified by ChIP sequencing (75), these DEG were associated with the advanced glycation end products (AGE) receptor for AGE (RAGE), AGE-RAGE signaling in diabetes complications, relaxin signaling, focal adhesion, and PI3K-Akt pathways (Table 5).
Table 5.
KEGG pathways identified for DEG with GRE
| FDR | ER | No. of Genes | Genes in Pathway | |
|---|---|---|---|---|
| AGE-RAGE signaling pathway in diabetic complications | 0.037 | 6.56 | 6 | CCND1, COL3A1, COL4A1, COL4A6, EDN1, MAPK1 |
| Relaxin signaling pathway | 0.037 | 5.83 | 7 | COL3A1, COL4A1, COL4A6, EDN1, GNAS, GNB1, MAPK1 |
| Focal adhesion | 0.037 | 4.35 | 8 | CCND1, COL4A1, COL4A6, ITGB5, LAMC1, MAPK1, PGF, TLN2 |
| PI3K-Akt signaling pathway | 0.037 | 3.36 | 11 | CCND1, CDC37, COL4A1, COL4A6, EIF4B, GNB1, ITGB5, LAMC1, MAPK1, PGF |
AGE, advanced glycation end products; DEG, differentially expressed genes; ER, enrichment ratio; FDR, false discovery rate; GRE, glucocorticoid response element; KEGG, Kyoto Encyclopedia of Genes and Genomes; RAGE, AGE receptor.
We also identified several metabolites with altered abundance in the placentomes from the pregnancies with maternal cortisol treatment (Fig. 2). Glutathione, ethanolamine, ascorbate, and cholines (choline/phosphocholine), were all significantly decreased (Table 4) in placentomes exposed to excess maternal cortisol in late gestation. Glutamate, a metabolite related to TCA cycle activity, was also decreased (Table 6). Overrepresentation analysis utilizing significantly downregulated metabolites revealed that glutathione and glycerophospholipid metabolism were significantly altered in placental tissue due to cortisol exposure. Genes related to both metabolic pathways were altered; GSTT1 (glutathione S-transferase-θ1) and CDIPT (CDP-diacylglycerol inositol 3-phosphatidyltransferase). When a multiomics approach was used to integrate the transcriptomic and metabolomics analyses, we found that these metabolites were associated with pathways of histidine and glycerophospholipid metabolism (Table 7).
Table 6.
Metabolites and their pathways altered in placentomes with maternal cortisol treatment
| Pathway | Mean ± SE |
|||
|---|---|---|---|---|
| Metabolite | P Value | Control | CORT | Fold Change CORT/Ctrl) |
| Glutathione metabolism | ||||
| Glutathione | 0.01 | 0.27 ± 0.02 | 0.17 ± 0.02 | −0.64 |
| Ascorbate | 0.02 | 0.31 ± 0.02 | 0.25 ± 0.01 | −0.30 |
| Glutamate | 0.05 | 1.17 ± 0.06 | 0.95 ± 0.09 | −0.30 |
| Glycerophospholipid metabolism | ||||
| Ethanolamine | 0.04 | 0.32 ± 0.03 | 0.21 ± 0.03 | −0.65 |
| Cholines (choline/phosphocholine) | 0.04 | 1.09 ± 0.07 | 0.80 ± 0.12 | −0.45 |
Pathways significantly changed by cortisol treatment had a false discovery rate of 0.003. For individual metabolites, an unadjusted P ≤ 0.05 was considered significant. CORT, cortisol treated.
Table 7.
Significant pathways from integration of transcriptomics of placenta with metabolomics of placenta or fetal serum using Metaboanalyst
| Significant Genes | Significant Metabolites | Combined P Value | FDR | |
|---|---|---|---|---|
| Multiomics analysis: Placental transcriptomics with placental metabolomics | ||||
| Glycerophospholipid metabolism | CDIPT | Phosphocholine choline ethanolamine | 0.001 | 0.157 |
| Histidine metabolism | ALDH7A1, CNDP2 | Glutamate | 0.003 | 0.249 |
| Multiomics analysis: Placental transcriptomics with fetal serum metabolomics | ||||
| Valine, leucine, and isoleucine degradation | HSD17B10, ALDH7A1, HADHB | Valine isoleucine 3-Hydroxybutyrate | 6.56E-05 | 0.005 |
| Histidine metabolism | ALDH7A1, CNDP2 | Anserine | 0.007 | 0.256 |
| Glycine, serine, and threonine metabolism | ALDH7A1 | Creatine Glycine Choline |
0.010 | 0.256 |
| β-Alanine metabolism | ALDH7A1, CNDP2 | Anserine | 0.010 | 0.256 |
| Inositol phosphate metabolism | ITPK1, CDIPT | Myoinositol | 0.011 | 0.256 |
| Primary bile acid biosynthesis | HSD17B4, HSD3B7 | Glycine | 0.011 | 0.256 |
| Valine, leucine, and isoleucine biosynthesis | Valine isoleucine | 0.014 | 0.275 | |
| Ascorbate and aldarate metabolism | ALDH7A1 | Myoinositol | 0.037 | 0.597 |
FDR, false discovery rate.
We (67) also used a multiomics approach to integrate the transcriptomics in the placenta with the previously reported changes in fetal serum metabolites in a separate cohort of ewes with the same treatment as in this study. These identified pathways relating to amino acid metabolism, degradation or biosynthesis in placenta with changes in valine, isoleucine, leucine, and glycine in fetal serum (Table 7).
We used PCR analysis to validate the decrease in expression of two other metabolic genes on the array: SLC7A5, encoding the large neutral amino acid transporter small subunit of L-type amino acid transporter 1 (LAT1) (Control: 1.03 ± 0.09, Cortisol: 0.71 ± 0.09, P ≤ 0.02), and ACSM1, encoding acyl-CoA synthetase medium-chain family member 1 (Control:1.21 ± 0.26, Cortisol: 0.54 ± 0.40, P ≤ 0.01).
DISCUSSION
The use of multiomics to examine the placentomes revealed discrete changes in metabolism of amino acids and carbohydrates in the placenta in pregnancies with excess maternal cortisol. The results of the transcriptomic analysis suggest that, although maternal hypercortisolemia also alters the activation of metabolic pathways in the placenta, these are different metabolic pathways and molecular functions than those altered in heart or skeletal muscle in the same fetuses (24, 49). The multiomics analysis suggests that the key metabolic pathways altered by the maternal cortisol excess in the placenta are linked to amino acid, glycerophospholipid, and glutathione metabolism and suggest that ER stress is also altered in placenta during these pregnancies. These pathways may be functionally linked to the altered metabolism in striated muscles and may contribute to the fetal loss in the perinatal period.
Overall, our multiomics model suggests that branched-chain amino acid (BCAA) handling differs in the fetuses of high-cortisol pregnancies. The metabolism of BCAA to ketoacids in placenta appears to be impaired by maternal hypercortisolemia; our previous transcriptomic analysis of the biceps femoris of these same fetuses also modeled a reduction in BCAA metabolism, although the specific genes altered appear to differ between the two tissue types (e.g., the gene for branched-chain dehydrogenase subunit A in placenta, and subunit B in biceps femoris). A reduction in the metabolism of BCAA is consistent with the increase in valine and isoleucine in fetal serum and the increase in valine concentrations in the heart in another cohort of this animal model (67). The present data suggest that placental uptake from the ewe of the BCAA transported by LAT1 (valine, leucine, isoleucine) may be impaired. LAT1 is a sodium-independent transporter on the maternal interface that mediates the active transport BCAA valine, leucine, and isoleucine as well as the large neutral amino acids phenylalanine, tyrosine, histidine, methionine, and tryptophan (18, 26, 37, 42). Taken together, the results suggest that BCAA metabolism is reduced in the placenta and in striated muscle in the hypercortisolemic pregnancy, leading to a net increase in serum BCAA and reduction in fatty acid oxidation. Expression of ASCM1, which encodes a protein that preferentially oxidizes fatty acids, including butyrate, is decreased in placenta; this is consistent with the increased fetal serum concentrations of 3-hydroxybutyrate in serum of fetuses 7–10 days before delivery in the cohort of ewes and fetuses studied through delivery (67).
In this study, there was no effect on glucose transporter expression, although in a study by Vaughn et al. (63), a similar infusion rate of cortisol from 125 to 130 days was found to increase expression of the glucose transporter SLC2A8, increase uteroplacental glucose metabolism, and reduce umbilical glucose uptake. Because these were uncatheterized fetuses, we were not able to measure fetal glucose concentrations; however, in a subsequent cohort of catheterized fetuses (2), we did not find any significant effect of cortisol on maternal-to-fetal glucose ratio. This suggests that glucose transfer across the placenta was not significantly altered by a more chronic infusion of cortisol. In contrast, in other tissues collected from the fetuses in the study reported here, we found that insulin-regulated glucose transporter SLC2A4 mRNA was reduced in biceps femoris (24). We also found several genes associated with insulin resistance to be increased in both the heart and biceps femoris, including thioredoxin-interacting protein (TXNIP) and suppressor of cytokine signaling 3 (SOCS3) (24, 49). In subsequent studies, we have not found an effect on insulin concentrations or glucose-to-insulin ratios in fetuses in this model (2); however, in a study with one-half of this daily dose of cortisol to ewes, we found that the glucose/insulin ratio was increased in the neonates of cortisol-treated ewes (1). BCAA have been implicated in insulin resistance (7, 40), suggesting the possibility that in utero exposure to excess cortisol may influence postnatal insulin sensitivity, and it is possible that the effects on fetal metabolism of BCAA play a role in this postnatal effect.
The metabolomic analysis revealed decreased concentrations of glutamate in the placenta, which may be secondary to changes in amino acid uptake and utilization in the placenta. Glutamate is taken up by the placenta from the fetus and oxidized, with a small percentage converted to glutamine and released into the fetal circulation (38). Although there were no significant changes in fetal serum glutamate or glutamine levels from a cohort of animals that underwent the same treatment, there was also a decreased concentration of glutamate and an increase in the concentration of glutamine in the hearts of the lambs of cortisol-infused ewes at birth (67). Glutamate concentrations in the placenta are also influenced by BCAA transamination (5). In this study, we could not assess whether glutamate concentrations in the placenta followed from reduced amino acid conversion or increased glutamate oxidation. In spontaneous parturition in normal animals, glutamate production and placental uptake of glutamate are both reduced, and this is thought to be an effect of cortisol at term (59). Reductions in glutamate might lead to reduced TCA cycle activity and NADPH and ATP production in the fetuses of cortisol-treated ewes at term, since in human pregnancies glutamine-glutamate are major contributors to placental metabolism (70).
The placental multiomics suggests disruptions in metabolism and transport at the maternal interface. The multiomics does not suggest global effects on metabolism, or effects that severely impact fetal supply of amino acids or glucose, as these were not diminished in the fetal serum. Despite the changes in BCAA metabolism in both striated muscle and placenta, pathways for protein synthesis, and mammalian target of rapamycin (mTOR) pathways were not identified in our transcriptomic modeling as significantly overrepresented by the genes whose expression was changed. The lack of effect of cortisol on amino acid transport and mTOR is consistent with the failure to find differences in amino acid transport or mTOR in human trophoblasts treated with cortisol (65). The failure to adversely impact fetal serum glucose and amino acids, or to impair protein synthesis, is consistent with the lack of impairment of placental and fetal growth in this model. The intervention occurs over the last 20% of ovine gestation; at that time in gestation, placental growth is complete (13), although there is continuing remodeling of the placental vessels to accommodate the needs of the growing fetus (47). In rodents, exposure to excess natural glucocorticoids leads to a reduced volume of fetal capillaries in the placenta, which could reduce transport of nutrients and oxygen to the fetus (66). These changes suggest that vascularization could be altered in the placentomes in pregnancies with excess cortisol, in turn altering substrate and oxygen availability to the fetus during labor. However, our analysis suggests that the effects of cortisol on angiogenesis in our model may be relatively minor. Although in a previous study we found more B-type cotyledons at 130 days of gestation (22), in this study the shift to C- and D-type cotyledons was not significant. We also did not observe changes in gene expression that would suggest alterations in placental growth. Vascular endothelial growth factorwas not significantly altered on the array, and although other genes related to placental growth were differentially expressed on the array, these changes were relatively small and were not significantly different when analyzed by qPCR [fold expression relative to mean control: insulin-like growth factor (IGF) Control: 1.09 ± 0.16, IGF Cortisol: 0.75 ± 0.19, P < 0.10; placental growth factor (PGF) Control: 1.08 ± 0.16, PGF Cortisol: 0.78 ± 0.07, P = 0.11), or transforming growth factor-β3 (TGFB3) (Control: 1.04 ± 0.10, Cortisol: 0.80 ± 0.13, P ≤ 0.10]. Although IGF2 expression was downregulated by higher levels of glucocorticoids in the fetal liver (30), in previous studies in this model of maternal hypercortisolemia we did not find a significant overall change in the expression of IGF2 or other genes in the IGF axis at 130 days of gestation (23). In that previous study, IGF2 expression was increased in the B-type cotyledons, suggesting that a transient increase in IGF2 expression may drive placentome remodeling in hypercortisolemia.
The multiomics analysis also modeled glycerophospholipid metabolism as a downregulated pathway. An effect of cortisol on lipids, in particular phospholipids, was also found in the heart in this model: glycerophophocholines, plasmenyl phophotidylcholines, and cardiolipins were all decreased (67). In contrast, choline was increased in serum of the fetuses of hypercortisolemic ewes. Metabolomic analysis of placenta showed that ethanolamine, phosphocholine, and choline were decreased in the placentomes of the cortisol-infused ewes. Ethanolamine and choline are major contributors to production of membrane lipids and are known to be transported across the brush borders of the placenta at the maternal interface (19). Studies have shown that maternal choline supplementation improves angiogenesis in placentomes and modulates expression of amino acid transporters (29). Ethanolamine also potentiates phosphocholine stimulation of DNA synthesis (27). Phospholipid metabolism appears to be altered in preeclamptic pregnancies; although ethanolamine was decreased, both phosophocholine and glycerophosphocholine were increased in placentas from women with preeclampsia (3). The increase in these two membrane components in preeclampsia suggests catabolism of membranes in placentas of preeclamptic women at term, which is not evident in our model of cortisol-treated ewes. Instead, the metabolomics suggests decreased choline utilization and may reflect reduced membrane phospholipid incorporation.
Our results suggest that ER function may be altered in the placenta from chronic maternal hypercortisolemia. High levels of ER and mitochondrial stress have been implicated in impaired lysosomal production and autophagy in placenta, resulting in decreased LAMP1 and β-galactosidase in serum of women with preeclampsia (39). Placentas collected from patients with gestational diabetes indicate mild activation of ER stress, with in vitro studies in trophoblast-like cells showing that elevated glucose levels can induce ER stress (76). In this study, placental transcriptomics indicate altered ER and lysosomal function, suggesting mild ER stress and activation of lysosomes. With ER stress, protein folding, glycosylation, and lysosomal formation can be altered. Our transcriptomic results also suggest defective glycosylation and folding of proteins in placentomes exposed to excess cortisol. Downregulation of O-glycan biosynthesis and glycosylation of proteins at the hydroxyl group of serine, threonine, or hydroxylysine is consistent with alterations in the ER and protein folding: the glycosylation is important for trafficking of protein targeting to Golgi, ER, lysosomes, plasma membrane, and secretory vesicles (71). Unfolded protein response in the ER is also associated with reductions in cyclin D in the cell cycle pathway (9), a downregulated pathway in the transcriptomic analysis. A decrease in expression of the genes for the β-galactosidase and lysosomal membrane protein LAMP1 and an increase in the levels of the nonglycosylated form of LAMP1 protein is also consistent with lysosomal defects and deficits in folding of proteins, with deglycosylation leading to degradation of LAMP1 (28).
Studies have shown an association between stillbirth and aging of the placenta (55). As gestation progresses, there is an accelerated aging process in placentas, associated with decreased telomere length and increased markers of lipid and DNA oxidation (34). Although the transcriptomic analysis did not suggest changes in pathways relating to DNA and RNA damage, in placenta, as in skeletal muscle and in the heart (24, 49), there appears to be disruption of pathways relating to oxidative stress. Glutathione, which plays a role in protecting cells from oxidative damage and in maintaining redox homeostasis (16), was decreased in the placentomes of the cortisol-treated animals; this may be a reflection of reduced placental glutamate and reduced fetal serum glycine, needed for glutathione production. Ascorbate, an antioxidant molecule that is involved in protecting cellular components from free radicals (50), was also decreased in the placentomes of cortisol-treated ewes. A decrease in glutathione and ascorbate, which protect nucleic acid from oxidative damage, could lead to damage of the placental tissues with insult. In our analysis of transcription factors associated with the DEG, we found that there is a significant overrepresentation of AGE-RAGE signaling by genes without GRE identified by the transcription factor analysis but with indirect GR regulation; these genes were all decreased in expression with maternal hypercortisolemia. The AGE-RAGE pathway is implicated in diseases caused by oxidative stress, including atherosclerosis and diabetes (4). The genes in this pathway are regulated by NF-κB; GR is known to suppress NF-κB stimulation of many inflammatory genes (45, 52). The net effect of cortisol may therefore be protective to the placental vasculature, albeit at the expense of lower remaining levels of antioxidants such as ascorbate and glutathione. The absence of fetal growth retardation or fetal hypoxia are consistent with adequate placental function for most of the pregnancy. The reduced levels of these antioxidants (glutathione and ascorbate) in these placentomes collected in early labor may reflect a lack of ability to compensate for further oxidative stress as labor progresses, consistent with stillbirths that occur in the perinatal period rather than earlier in gestation.
Although the transcriptional effects of cortisol in the placenta do not appear to be by a classical GR interaction with GRE for the affected genes, this is not surprising. In many cell types, GR exerts stimulatory and repressive effects on genes through interactions with other factors. GR tethering to DNA via interaction with other transcription factors has been well known (45, 52, 61). Approximately 20% of all GR-mediated effects on transcription occur through GR tethering to DNA via these other factors, although, in many other cases in which a GRE or half-GRE is present, GR interacts with other transcription factors to cause either gene activation or repression (61).
A major and disappointing limitation is that this cohort of sheep had a maldistribution of male and female fetuses, with only female fetuses in the cortisol-treated group surviving until the study. However, the fetal “losses” were both males (two) and females (three), and in subsequent studies we have not found any greater losses of male vs. female fetuses. Pregnancy outcomes in humans are influenced by fetal sex (8), with more adverse cardiovascular and metabolic outcomes in the mother of male fetuses. In early human placentas, there are differences in metabolomic profile of male vs. female placentas; these differences are primarily in acylcarnitines (51). In a rodent study in which growth retardation was produced with dexamethasone administration, there were different responses in both nutrient transporters and in IGF2 between male and female fetuses, with decreases LAT1 and IGF2 only in the male placentas (20). The sexual dimorphism may include expression of the GR form; intrauterine growth restriction (IUGR) female human fetuses had increased placental expression of the active form of GR protein, GRα, whereas in male IUGR fetuses placental expression of the inactive form, GRβ, was increased (21). We tested whether the apparent effect of cortisol could be explained by the differences between male and female placentas by comparing gene expression and metabolomic profiles in the female vs. male control placentas. The only different KEGG pathway so identified was the HIV-1 infection pathway. There were no molecular functions associated with the genes differing between male and females. The biological processes that were modeled as different were more extensive but included only one biological process in common with those modeled as different in the fetuses of the cortisol-treated ewes compared with the control group. This process was the response to cytokine; there were 3 of the 56 genes associated with this process that were common between the two analyses. In the case of the metabolomics, there were no significant differences between placentomes from male and female controls. However, ascorbate tended to be lower in the placentomes of female fetuses (P = 0.07), and so it is possible that the apparent effect of cortisol on ascorbate was the result of having all female fetuses in this group. However, in the case of glutamate (P = 0.40), glutathione (P = 0.89), and the BCAA (P values of 0.48–0.62) there was no tendency for a difference in males vs. females. Ethanolamine tended to be higher in female fetuses (P = 0.08), a pattern opposite to that in placentomes from the control vs. cortisol groups. Although we cannot rule out any sexual dimorphism in the effects of cortisol on the placenta in this study, the effects of cortisol do not appear to result in excess loss of fetuses of one sex compared with the other or to result in a bias in the overall model, particularly as they relate to metabolic profile of the fetus.
In summary, the results suggest that, although fetal and placental growth are not severely impacted by a modest chronic increase in maternal levels, placental, as well as skeletal and cardiac muscle (24, 49) metabolism, is altered by maternal hypercortisolemia. This is not surprising, given the role of glucocorticoid actions in altering metabolism. The finding of altered gene expression in pathways for BCAA oxidation in placenta as well as in biceps femoris, along with the increase in serum BCAA, merits further investigation. Impaired BCAA metabolism has been implicated in insulin resistance, particularly in individuals with obesity, although it is not clear whether insulin resistance alters BCAA metabolism or whether BCAA excess drives insulin resistance (7, 40). Increased serum BCAA are predictive of increased risk of diabetes, and this suggests that offspring of hypercortisolemic ewes or chronically stressed mothers could have increased risk of metabolic syndrome postnatally. This is consistent with the increased glucose/insulin ratio we found in the newborns of ewes chronically treated with one-half this dose of cortisol. The alterations in gene expression in pathways involved in metabolism in placenta, in addition to those in skeletal muscle and heart, suggest that the stillbirth we observed in cohorts of cortisol-infused pregnant ewes was not simply the effect of cortisol on the maturing heart. Our results suggest that impaired placental amino acid utilization as well as placental ER stress and/or antioxidant status contribute to the overall metabolic dysfunction in the fetus. The link between placental and cardiac function has been suggested by others (10, 11, 35). Further studies are needed to better understand this link.
GRANTS
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development Grants HD-57871 to M. Keller-Wood and HD-87306 to M. Keller-Wood.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.J., J.M.W., and M.K.-W. conceived and designed research; S.J., J.M.W., S.Z., and M.K.-W. performed experiments; S.J., J.M.W., S.Z., and M.K.-W. analyzed data; S.J., J.M.W., S.Z., A.S.E., and M.K.-W. interpreted results of experiments; S.J. and J.M.W. prepared figures; S.J., J.M.W., and M.K.-W. drafted manuscript; S.J., J.M.W., S.Z., A.S.E., and M.K.-W. edited and revised manuscript; S.J., J.M.W., S.Z., A.S.E., and M.K.-W. approved final version of manuscript.
REFERENCES
- 1.Antolic A, Feng X, Wood CE, Richards EM, Keller-Wood M. Increased maternal nighttime cortisol concentrations in late gestation alter glucose and insulin in the neonatal lamb. Physiol Rep 3: e12548, 2015. doi: 10.14814/phy2.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antolic A, Wood CE, Keller-Wood M. Chronic maternal hypercortisolemia in late gestation alters fetal cardiac function at birth. Am J Physiol Regul Integr Comp Physiol 314: R342–R352, 2018. doi: 10.1152/ajpregu.00296.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austdal M, Thomsen LC, Tangerås LH, Skei B, Mathew S, Bjørge L, Austgulen R, Bathen TF, Iversen AC. Metabolic profiles of placenta in preeclampsia using HR-MAS MRS metabolomics. Placenta 36: 1455–1462, 2015. doi: 10.1016/j.placenta.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Barlovic DP, Soro-Paavonen A, Jandeleit-Dahm KA. RAGE biology, atherosclerosis and diabetes. Clin Sci (Lond) 121: 43–55, 2011. doi: 10.1042/CS20100501. [DOI] [PubMed] [Google Scholar]
- 5.Battaglia FC. In vivo characteristics of placental amino acid transport and metabolism in ovine pregnancy–a review. Placenta 23, Suppl A: S3–S8, 2002. doi: 10.1053/plac.2002.0812. [DOI] [PubMed] [Google Scholar]
- 6.Bell ME, Wood CE, Keller-Wood M, Kane C, Kluwe C, Manlove E, Taranovich C, Johnson J. Influence of reproductive state on pituitary-adrenal activity in the ewe. Domest Anim Endocrinol 8: 245–254, 1991. doi: 10.1016/0739-7240(91)90060-W. [DOI] [PubMed] [Google Scholar]
- 7.Bloomgarden Z. Diabetes and branched-chain amino acids: What is the link? J Diabetes 10: 350–352, 2018. doi: 10.1111/1753-0407.12645. [DOI] [PubMed] [Google Scholar]
- 8.Broere-Brown ZA, Adank MC, Benschop L, Tielemans M, Muka T, Gonçalves R, Bramer WM, Schoufour JD, Voortman T, Steegers EAP, Franco OH, Schalekamp-Timmermans S. Fetal sex and maternal pregnancy outcomes: a systematic review and meta-analysis. Biol Sex Differ 11: 26, 2020. doi: 10.1186/s13293-020-00299-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burton GJ, Yung HW, Cindrova-Davies T, Charnock-Jones DS. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta 30, Suppl A: 43–48, 2009. doi: 10.1016/j.placenta.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camm EJ, Botting KJ, Sferruzzi-Perri AN. Near to one’s heart: the intimate relationship between the placenta and fetal heart. Front Physiol 9: 629, 2018. doi: 10.3389/fphys.2018.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courtney JA, Cnota JF, Jones HN. The role of abnormal placentation in congenital heart disease; cause, correlate, or consequence? Front Physiol 9: 1045, 2018. doi: 10.3389/fphys.2018.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dieterle F, Ross A, Schlotterbeck G, Senn H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal Chem 78: 4281–4290, 2006. doi: 10.1021/ac051632c. [DOI] [PubMed] [Google Scholar]
- 13.Ehrhardt RA, Bella AW. Growth and metabolism of the ovine placenta during mid-gestation. Placenta 16: 727–741, 1995. doi: 10.1016/0143-4004(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson L, Johansson E, Kettaneh-Wold N, Wold S. Multi- and Megavariate Data Analysis. Principles and Applications. Umea, Sweden: Umetrics AB, 2001. [Google Scholar]
- 15.Feng X, Reini SA, Richards E, Wood CE, Keller-Wood M. Cortisol stimulates proliferation and apoptosis in the late gestation fetal heart: differential effects of mineralocorticoid and glucocorticoid receptors. Am J Physiol Regul Integr Comp Physiol 305: R343–R350, 2013. doi: 10.1152/ajpregu.00112.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med 30: 1–12, 2009. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowden AL, Valenzuela OA, Vaughan OR, Jellyman JK, Forhead AJ. Glucocorticoid programming of intrauterine development. Domest Anim Endocrinol 56, Suppl: S121–S132, 2016. doi: 10.1016/j.domaniend.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Gaccioli F, Aye IL, Roos S, Lager S, Ramirez VI, Kanai Y, Powell TL, Jansson T. Expression and functional characterisation of System L amino acid transporters in the human term placenta. Reprod Biol Endocrinol 13: 57, 2015. doi: 10.1186/s12958-015-0054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grassl SM. Ethanolamine transport in human placental brush-border membrane vesicles. J Pharmacol Exp Ther 298: 695–702, 2001. [PubMed] [Google Scholar]
- 20.Guo J, Fang M, Zhuang S, Qiao Y, Huang W, Gong Q, Xu D, Zhang Y, Wang H. Prenatal dexamethasone exposure exerts sex-specific effect on placental oxygen and nutrient transport ascribed to the differential expression of IGF2. Ann Transl Med 8: 233, 2020. doi: 10.21037/atm.2019.12.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutter S, Hepp P, Hofmann S, Kuhn C, Messner J, Andergassen U, Mayr D, Emilia Solano M, Obermeier V, Mahner S, Arck P, Jeschke U. Glucocorticoid receptors α and β are modulated sex specifically in human placentas of intrauterine growth restriction (IUGR). Arch Gynecol Obstet 300: 323–335, 2019. doi: 10.1007/s00404-019-05189-7. [DOI] [PubMed] [Google Scholar]
- 22.Jensen E, Wood CE, Keller-Wood M. Chronic alterations in ovine maternal corticosteroid levels influence uterine blood flow and placental and fetal growth. Am J Physiol Regul Integr Comp Physiol 288: R54–R61, 2005. doi: 10.1152/ajpregu.00149.2004. [DOI] [PubMed] [Google Scholar]
- 23.Jensen EC, Rochette M, Bennet L, Wood CE, Gunn AJ, Keller-Wood M. Physiological changes in maternal cortisol do not alter expression of growth-related genes in the ovine placenta. Placenta 31: 1064–1069, 2010. doi: 10.1016/j.placenta.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joseph S, Alava B, Antolic A, Richards EM, Wood CE, Keller-Wood M. Fetal ovine skeletal and cardiac muscle transcriptomics are differentially altered by increased maternal cortisol during gestation. Physiol Genomics 52: 178–190, 2020. doi: 10.1152/physiolgenomics.00096.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller-Wood M, Feng X, Wood CE, Richards E, Anthony RV, Dahl GE, Tao S. Elevated maternal cortisol leads to relative maternal hyperglycemia and increased stillbirth in ovine pregnancy. Am J Physiol Regul Integr Comp Physiol 307: R405–R413, 2014. doi: 10.1152/ajpregu.00530.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DK, Kanai Y, Choi HW, Tangtrongsup S, Chairoungdua A, Babu E, Tachampa K, Anzai N, Iribe Y, Endou H. Characterization of the system L amino acid transporter in T24 human bladder carcinoma cells. Biochim Biophys Acta 1565: 112–122, 2002. doi: 10.1016/S0005-2736(02)00516-3. [DOI] [PubMed] [Google Scholar]
- 27.Kiss Z, Mukherjee JJ, Crilly KS, Chung T. Ethanolamine, but not phosphoethanolamine, potentiates the effects of insulin, phosphocholine, and ATP on DNA synthesis in NIH 3T3 cells–role of mitogen-activated protein-kinase-dependent and protein-kinase-independent mechanisms. Eur J Biochem 250: 395–402, 1997. doi: 10.1111/j.1432-1033.1997.0395a.x. [DOI] [PubMed] [Google Scholar]
- 28.Kundra R, Kornfeld S. Asparagine-linked oligosaccharides protect Lamp-1 and Lamp-2 from intracellular proteolysis. J Biol Chem 274: 31039–31046, 1999. doi: 10.1074/jbc.274.43.31039. [DOI] [PubMed] [Google Scholar]
- 29.Kwan STC, King JH, Yan J, Wang Z, Jiang X, Hutzler JS, Klein HR, Brenna JT, Roberson MS, Caudill MA. Maternal choline supplementation modulates placental nutrient transport and metabolism in late gestation of mouse pregnancy. J Nutr 147: 2083–2092, 2017. doi: 10.3945/jn.117.256107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Saunders JC, Fowden AL, Dauncey MJ, Gilmour RS. Transcriptional regulation of insulin-like growth factor-II gene expression by cortisol in fetal sheep during late gestation. J Biol Chem 273: 10586–10593, 1998. doi: 10.1074/jbc.273.17.10586. [DOI] [PubMed] [Google Scholar]
- 31.Liao Y, Wang J, Jaehnig EJ, Shi Z, Zhang B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res 47: W199–W205, 2019. doi: 10.1093/nar/gkz401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liggins GC. The role of cortisol in preparing the fetus for birth. Reprod Fertil Dev 6: 141–150, 1994. doi: 10.1071/RD9940141. [DOI] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Maiti K, Sultana Z, Aitken RJ, Morris J, Park F, Andrew B, Riley SC, Smith R. Evidence that fetal death is associated with placental aging. Am J Obstet Gynecol 217: 441.e1–441.e14, 2017. doi: 10.1016/j.ajog.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Maslen CL. Recent advances in placenta-heart interactions. Front Physiol 9: 735, 2018. doi: 10.3389/fphys.2018.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci 997: 136–149, 2003. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- 37.Mastroberardino L, Spindler B, Pfeiffer R, Skelly PJ, Loffing J, Shoemaker CB, Verrey F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature 395: 288–291, 1998. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]
- 38.Moores RR Jr, Vaughn PR, Battaglia FC, Fennessey PV, Wilkening RB, Meschia G. Glutamate metabolism in fetus and placenta of late-gestation sheep. Am J Physiol Regul Integr Comp Physiol 267: R89–R96, 1994. doi: 10.1152/ajpregu.1994.267.1.R89. [DOI] [PubMed] [Google Scholar]
- 39.Nakashima A, Cheng SB, Kusabiraki T, Motomura K, Aoki A, Ushijima A, Ono Y, Tsuda S, Shima T, Yoshino O, Sago H, Matsumoto K, Sharma S, Saito S. Endoplasmic reticulum stress disrupts lysosomal homeostasis and induces blockade of autophagic flux in human trophoblasts. Sci Rep 9: 11466, 2019. doi: 10.1038/s41598-019-47607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neinast M, Murashige D, Arany Z. Branched chain amino acids. Annu Rev Physiol 81: 139–164, 2019. doi: 10.1146/annurev-physiol-020518-114455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polman JAE, Welten JE, Bosch DS, de Jonge RT, Balog J, van der Maarel SM, de Kloet ER, Datson NA. A genome-wide signature of glucocorticoid receptor binding in neuronal PC12 cells. BMC Neurosci 13: 118, 2012. doi: 10.1186/1471-2202-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prasad PD, Wang H, Huang W, Kekuda R, Rajan DP, Leibach FH, Ganapathy V. Human LAT1, a subunit of system L amino acid transporter: molecular cloning and transport function. Biochem Biophys Res Commun 255: 283–288, 1999. doi: 10.1006/bbrc.1999.0206. [DOI] [PubMed] [Google Scholar]
- 43.Rabaglino MB, Chang EI, Richards EM, James MO, Keller-Wood M, Wood CE. Genomic effect of triclosan on the fetal hypothalamus: evidence for altered neuropeptide regulation. Endocrinology 157: 2686–2697, 2016. doi: 10.1210/en.2016-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rabaglino MB, Richards E, Denslow N, Keller-Wood M, Wood CE. Genomics of estradiol-3-sulfate action in the ovine fetal hypothalamus. Physiol Genomics 44: 669–677, 2012. doi: 10.1152/physiolgenomics.00127.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao NA, McCalman MT, Moulos P, Francoijs KJ, Chatziioannou A, Kolisis FN, Alexis MN, Mitsiou DJ, Stunnenberg HG. Coactivation of GR and NFKB alters the repertoire of their binding sites and target genes. Genome Res 21: 1404–1416, 2011. doi: 10.1101/gr.118042.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reimand J, Kull M, Peterson H, Hansen J, Vilo J. g:Profiler—a web-based toolset for functional profiling of gene lists from large-scale experiments. Nucleic Acids Res 35, Suppl 2: W193–W200, 2007. doi: 10.1093/nar/gkm226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reynolds LP, Borowicz PP, Vonnahme KA, Johnson ML, Grazul-Bilska AT, Redmer DA, Caton JS. Placental angiogenesis in sheep models of compromised pregnancy. J Physiol 565: 43–58, 2005. doi: 10.1113/jphysiol.2004.081745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richards EM, McElhaney E, Zeringue K, Joseph S, Keller-Wood M. Transcriptomic evidence that cortisol alters perinatal epicardial adipose tissue maturation. Am J Physiol Endocrinol Metab 317: E573–E585, 2019. doi: 10.1152/ajpendo.00007.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richards EM, Wood CE, Rabaglino MB, Antolic A, Keller-Wood M. Mechanisms for the adverse effects of late gestational increases in maternal cortisol on the heart revealed by transcriptomic analyses of the fetal septum. Physiol Genomics 46: 547–559, 2014. doi: 10.1152/physiolgenomics.00009.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rose RC, Bode AM. Biology of free radical scavengers: an evaluation of ascorbate. FASEB J 7: 1135–1142, 1993. doi: 10.1096/fasebj.7.12.8375611. [DOI] [PubMed] [Google Scholar]
- 51.Saoi M, Kennedy KM, Gohir W, Sloboda DM, Britz-McKibbin P. Placental metabolomics for assessment of sex-specific differences in fetal development during normal gestation. Sci Rep 10: 9399, 2020. doi: 10.1038/s41598-020-66222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scheinman RI, Gualberto A, Jewell CM, Cidlowski JA, Baldwin AS Jr. Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol Cell Biol 15: 943–953, 1995. doi: 10.1128/MCB.15.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slater EP, Anderson T, Cattini P, Isaacs R, Birnbaum MJ, Gardner DG, Eberhardt NL, Baxter JD. Mechanisms of glucocorticoid hormone action. Adv Exp Med Biol 196: 67–80, 1986. doi: 10.1007/978-1-4684-5101-6_5. [DOI] [PubMed] [Google Scholar]
- 54.Slenter DN, Kutmon M, Hanspers K, Riutta A, Windsor J, Nunes N, Mélius J, Cirillo E, Coort SL, Digles D, Ehrhart F, Giesbertz P, Kalafati M, Martens M, Miller R, Nishida K, Rieswijk L, Waagmeester A, Eijssen LMT, Evelo CT, Pico AR, Willighagen EL. WikiPathways: a multifaceted pathway database bridging metabolomics to other omics research. Nucleic Acids Res 46: D661–D667, 2018. doi: 10.1093/nar/gkx1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith R, Maiti K, Aitken RJ. Unexplained antepartum stillbirth: a consequence of placental aging? Placenta 34: 310–313, 2013. doi: 10.1016/j.placenta.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 56.Smyth GK. Limma: linear models for microarray data In: Bioinformatics and Computational Biology Solutions Using R and Bioconductor, edited by Gentleman R, Carey V, Huber W, Irizarry R, Dudoit S. New York: Springer, 2005. [Google Scholar]
- 57.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: e3, 2004. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 58.Sun K, Yang K, Challis JR. Differential expression of 11 beta-hydroxysteroid dehydrogenase types 1 and 2 in human placenta and fetal membranes. J Clin Endocrinol Metab 82: 300–305, 1997. doi: 10.1210/jcem.82.1.3681. [DOI] [PubMed] [Google Scholar]
- 59.Timmerman M, Teng C, Wilkening RB, Chung M, Battaglia FC. Net amino acid flux across the fetal liver and placenta during spontaneous ovine parturition. Biol Neonate 79: 54–60, 2001. doi: 10.1159/000047066. [DOI] [PubMed] [Google Scholar]
- 60.Trygg J, Wold S. Orthogonal projections to latent structures (O-PLS). J Chemomr 16: 119–128, 2002. doi: 10.1002/cem.695. [DOI] [Google Scholar]
- 61.Uhlenhaut NH, Barish GD, Yu RT, Downes M, Karunasiri M, Liddle C, Schwalie P, Hübner N, Evans RM. Insights into negative regulation by the glucocorticoid receptor from genome-wide profiling of inflammatory cistromes. Mol Cell 49: 158–171, 2013. doi: 10.1016/j.molcel.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vatnick I, Schoknecht PA, Darrigrand R, Bell AW. Growth and metabolism of the placenta after unilateral fetectomy in twin pregnant ewes. J Dev Physiol 15: 351–356, 1991. [PubMed] [Google Scholar]
- 63.Vaughan OR, Davies KL, Ward JW, de Blasio MJ, Fowden AL. A physiological increase in maternal cortisol alters uteroplacental metabolism in the pregnant ewe. J Physiol 594: 6407–6418, 2016. doi: 10.1113/JP272301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vaughan OR, De Blasio MJ, Fowden AL. Ovine uteroplacental and fetal metabolism during and after fetal cortisol overexposure in late gestation. Am J Physiol Regul Integr Comp Physiol 314: R791–R801, 2018. doi: 10.1152/ajpregu.00194.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vaughan OR, Powell TL, Jansson T. Glucocorticoid regulation of amino acid transport in primary human trophoblast cells. J Mol Endocrinol 63: 239–248, 2019. doi: 10.1530/JME-19-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vaughan OR, Sferruzzi-Perri AN, Fowden AL. Maternal corticosterone regulates nutrient allocation to fetal growth in mice. J Physiol 590: 5529–5540, 2012. doi: 10.1113/jphysiol.2012.239426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walejko JM, Antolic A, Koelmel JP, Garrett TJ, Edison AS, Keller-Wood M. Chronic maternal cortisol excess during late gestation leads to metabolic alterations in the newborn heart. Am J Physiol Endocrinol Metab 316: E546–E556, 2019. doi: 10.1152/ajpendo.00386.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walejko JM, Chelliah A, Keller-Wood M, Gregg A, Edison AS. Global metabolomics of the placenta reveals distinct metabolic profiles between maternal and fetal placental tissues following delivery in non-labored women. Metabolites 8: 10, 2018. doi: 10.3390/metabo8010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walejko JM, Koelmel JP, Garrett TJ, Edison AS, Keller-Wood M. Multiomics approach reveals metabolic changes in the heart at birth. Am J Physiol Endocrinol Metab 315: E1212–E1223, 2018. doi: 10.1152/ajpendo.00297.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Bucher M, Myatt L. Use of glucose, glutamine and fatty acids for trophoblast respiration in lean women, women with obesity, and women with gestational diabetes. J Clin Endocrinol Metab 104: 4178–4187, 2019. doi: 10.1210/jc.2019-00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wopereis S, Lefeber DJ, Morava E, Wevers RA. Mechanisms in protein O-glycan biosynthesis and clinical and molecular aspects of protein O-glycan biosynthesis defects: a review. Clin Chem 52: 574–600, 2006. doi: 10.1373/clinchem.2005.063040. [DOI] [PubMed] [Google Scholar]
- 72.Xia J, Wishart DS. MetPA: a web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 26: 2342–2344, 2010. doi: 10.1093/bioinformatics/btq418. [DOI] [PubMed] [Google Scholar]
- 73.Xia J, Wishart DS. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr Protoc Bioinformatics 55: 14.10.1–14.10.91, 2016. doi: 10.1002/cpbi.11. [DOI] [PubMed] [Google Scholar]
- 74.Yang K. Ovine 11 beta-hydroxysteroid dehydrogenase: from gene to function. Endocr Res 21: 367–377, 1995. doi: 10.3109/07435809509030453. [DOI] [PubMed] [Google Scholar]
- 75.Yu CY, Mayba O, Lee JV, Tran J, Harris C, Speed TP, Wang JC. Genome-wide analysis of glucocorticoid receptor binding regions in adipocytes reveal gene network involved in triglyceride homeostasis. PLoS One 5: e15188, 2010. doi: 10.1371/journal.pone.0015188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yung HW, Alnæs-Katjavivi P, Jones CJ, El-Bacha T, Golic M, Staff AC, Burton GJ. Placental endoplasmic reticulum stress in gestational diabetes: the potential for therapeutic intervention with chemical chaperones and antioxidants. Diabetologia 59: 2240–2250, 2016. doi: 10.1007/s00125-016-4040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]