Abstract
Purpose of review:
Opioids remain the most potent form of pain relief currently available, yet have a high abuse liability. Here we discuss underlying neurobiological changes in Opioid Use Disorder (OUD) that likely contribute to drug craving, which in turn drives continued drug use and relapse.
Recent findings:
Craving has emerged as a strong indicator in drug-seeking and relapse. Studies have demonstrated a number of allostatic changes in circuitry that facilitate learning of drug-stimuli relationships, thereby augmenting cue-triggered drug use and relapse.
Summary:
This review will focus on key neurobiological changes in underlying circuitry observed during the initial and continued exposure to opioids that result in an increase in neural-reactivity to drug-related intrinsic and extrinsic drug cues, and to enhanced learning of drug-context correlations. This sensitized learning state may be an indication of the underlying framework that drives craving and ultimately, motivates increased salience of drug cues and drives drug-seeking.
Keywords: Opioid Use Disorder, Drug craving, Incentive sensitization, Negative reinforcement, Stress, Negative affect
Introduction
While opioid prescriptions in the United States declined from 2012 to 2018 [1], partially due to increased awareness of risks for both patients and physicians and measures implemented by the Center for Disease Control, opioid-related overdoses and deaths continued to rise [2]. Also worrying is the increasing availability of illicit, low cost and very potent alternatives, including etorphine, fentanyl and fentanyl derivatives such as carfentanyl and sufentanil [3-5]. Despite decades of research on the underlying mechanisms leading to Opioid Use Disorder (OUD), there has been limited progress in stemming its impact.
Though it is likely that a number of genetic, biological, and environmental factors play a role in the development of OUD, ultimately, it is the allostatic changes within the brain that continue to support many OUD behaviors, including drug-seeking and relapse. In this review, we will focus on the role of craving, as craving has been strongly correlated with ongoing OUD, as well as relapse [6-10]. For those vulnerable to addiction, we argue that the neurocircuitry changes that occur during the initial exposure to the drug allow for a potentiation of learning of drug related cues and contexts that drive craving [7,10-13]. This enhanced craving network in turn drives drug-seeking behavior.
Historically, in the context of addiction research, craving has often been overlooked as merely a subjective feeling of desire to take a drug. This limited definition has stunted research with a focus on craving as a primary driver in the development, continuance, and relapse of substance use disorders. However, in more recent years, many studies have embraced craving as a major component of OUD, acknowledging a broader definition that includes a complex and dynamic interplay between three main behavioral-affective domains: reward & motivation, stress & negative affect, and learning, memory & executive function [7,10,14-16]. Dysregulation of the balance within and between the circuitries that underlie these behavioral domains can contribute to the manifestation of craving, and each domain plays a different role in the continuance and relapse of drug use. Recent extensive reviews have described underlying mechanisms of this dysregulation precipitated by opioid use [17-23], including in the context of pain and pain treatment [8,24-26].
Etiology of OUD
Though there are numerous theories and models of addiction, in this review we focus on aspects of two popular models that emphasize the neurobiological and behavioral changes that occur during the addiction cycle that we believe best support the role of craving in OUD. One model, originally proposed by Robinson and Berridge in 1993 [27], describes the neurobiological changes following escalating drug use that results in a hyper-reactive dopaminergic state that facilitates learned associations between drugs and drug related cues, also known as incentive sensitization. Though this model has been updated over the years [11], the central idea is that the allostatic changes occurring in the brain during the development of substance use disorders facilitate learning between the drug and internal and external cues, thus enhancing craving, while potentially driving drug seeking and relapse. And in fact, there is much evidence that this sensitized state is able to give salience to otherwise neutral stimuli, and these cues can in turn stimulate desire for the drug, consciously or subconsciously [28-32]. However, while this model primarily focuses on positive reinforcement as a driver for aberrant drug use, in OUD it is thought that the neural adaptive changes that occur after repeated drug use result in a switch to negative reinforcement, rather than due to any positively rewarding aspects of the drug [17].
A second model by Koob and colleagues addresses the impact of negative reinforcement in OUD and focuses on the idea of negative reinforcement as the main driver of continued drug use and relapse, arguing that the development of the “antireward” state motivates users to seek drugs as a means of relieving the negative affect and withdrawal states [17,19,33-35]. This model is especially relevant in OUD, as studies show that the majority of individuals that misuse and go on to abuse opioids had experienced pain or chronic pain prior to their first exposure to developing OUD [36,37]. The alleviation of pain and negative affect from chronic pain may be a significant reinforcing factor in the initial development of OUD in these populations, rather than reinforcement purely from the hedonic effects of the drugs, and perhaps the etiology of OUD differs between these groups. In the context of these models, we will discuss the most relevant allostatic changes in the underlying circuitry of OUD, across the domains of reward and motivation, stress and negative affect, and cognitive function, that most likely drive craving (Figure 1).
Figure 1.
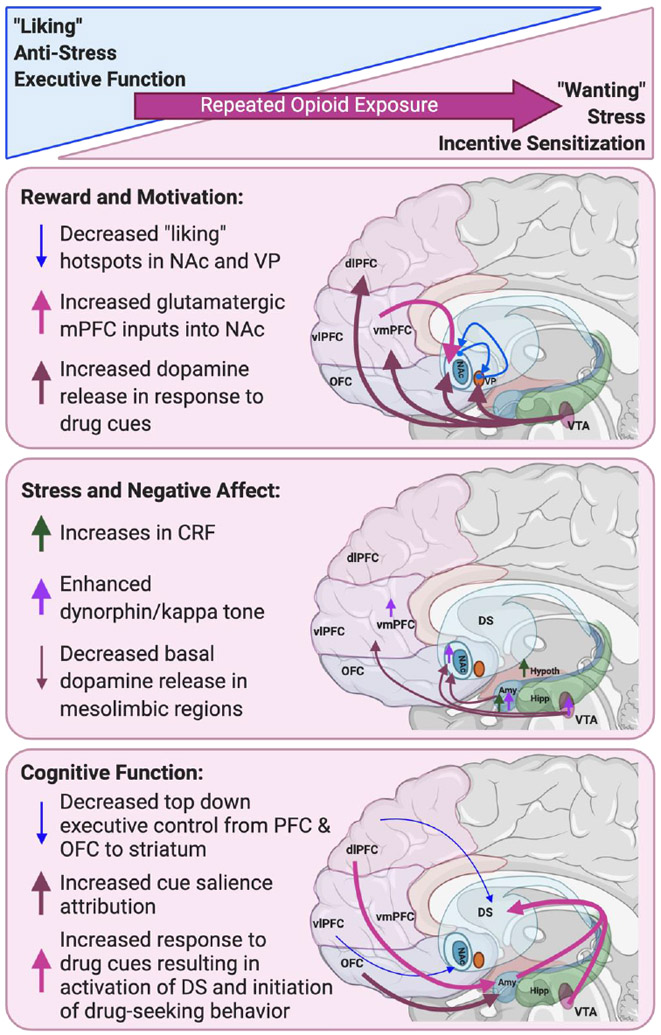
Allostatic changes in neurocircuitry following repeated opioid exposure in OUD. Following repeated opioid use, allostatic changes are observed in circuitry that mediates reward and motivation (a switch from “liking” to “wanting”), stress and negative affect (decreases in anti-stress; increases in stress), as well as cognitive function (decreases in executive function, and increased incentive sensitization to drug cues); Amy, amygdala; dlPFC, dorsal lateral PFC; DS, Dorsal striatum; Hipp, hippocampus; Hypothal, hypothalamus; NAc, nucleus accumbens; OFC, orbitofrontal cortex; vlPFC, ventral lateral PFC; vmPFC, ventral medial PFC; VP, ventral pallidum; VTA, ventral tegmental area. Figure created with Biorender.com.
Reward and Motivation
For decades researchers have pinned the pathophysiology of addiction on changes in the mesolimbic, dopaminergic pathways in the brain [38]. This classical pathway involves projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) in the ventral striatum. However, while the dopamine release in these regions was originally thought to correlate with the hedonic value of drugs, or ‘liking’, studies have since shown a much stronger correlation with ‘wanting’, or craving of the drug [11]. ‘Liking’ is defined as the pleasurable experience associated with a drug while ‘wanting’ is the preoccupation of craving and desire to take more drug [11]. Even though conceptually these terms overlap, the circuitry responsible for each differs, both in terms of initial activation and response to repeated drug exposure. Recent research suggests that the ‘liking’ system is made up of small, neurochemically-stimulated hotspots that are found within subregions of mesolimbic structures, whereas general sensitization and stimulation of the mesolimbic pathways is correlated with enhanced ‘wanting’ [11,39-41]. For example, one such hotspot in the posterior ventral pallidum (VP) is critical for experiencing “liking”, and lesioning of this spot is sufficient enough to cause a loss of hedonic value [40]. Furthermore, reciprocal connections between hotspots, such as the NAc and VP, can work to enhance or decrease ‘liking’ [39]. Over time and with repeated drug use, it appears that while the ‘wanting’ circuitry becomes more sensitized, the ‘liking’ hotspots may actually shrink or become desensitized (Figure 1 [41]). And in fact, a central tenet of incentive sensitization theory is that focus is narrowed to drug-wanting and seeking, as well as to drug relevant cues, often at the expense of devaluing other available non-drug rewards, including natural rewards necessary for survival such as food and sex. Rather than a distinct switch from ‘liking’ to ‘wanting’, this sensitization is more a change in ‘potency’ or strength of circuitry between and within each system, with the mesolimbic dopamine system ramping up incentive salience, while reducing engagement of circuits responsible for the ‘liking’. Such changes in sensitization of the mesolimbic subregions can also be correlated to the differing contributions of positive versus negative reinforcement.
It is important to note that hyper-reactivity does not mean an overall increase in dopamine activity, but instead an altered dopaminergic state that has been sensitized to respond to drug cues [11]. Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) studies in humans have demonstrated this sensitization by showing increased dopamine release following the presentation of drug cues [38,42], while functional MRI (fMRI) studies have also shown increased reactivity of mesolimbic circuitry in response to drug cues [43,44]. However, in contrast to this enhanced cue-reactivity, individuals with OUD have shown blunted dopamine release in response to opioid administration [38,42,45]. One explanation for apparent divergence is the extent of tolerance versus sensitization within the same circuitry, and in fact, similar studies have shown that while drug-induced dopamine is blunted, users show robust dopamine hyper-reactivity to drug cues [11,44,46-48]. Considering the relatively fast and robust onset of tolerance (i.e., a decline in pharmacological effect with equivalent doses) from repeated opioid use, this is likely an important distinction. Furthermore, studies have shown that the rewarding and reinforcing properties of opioids do not always require dopamine to drive drug-seeking behavior, likely due to the opioid system itself, as well as other neurotransmitter systems activated by opioids [49,50]. However, it is now generally accepted that dopamine release is involved in modulation of cue salience, and while some drug seeking may continue to be driven through other relevant neurotransmitter systems, cue-encoding and cue-induced drug seeking is likely driven mainly via dopamine [35,51]. Furthermore, emerging evidence demonstrates that this reward and motivation circuitry is inextricably interconnected with affective and cognitive circuitry that also plays a role in driving drug craving, as discussed below.
Stress and Negative Affect
In addition to sensitized cue reactivity, individuals experiencing OUD often present with altered affective states [16,52,53] and sensitized stress reactivity [17,18,23,54-56]. Allostatic changes observed in many brain regions have been implicated in mediating the negative affective components of OUD, both as a result of chronic opioid use, and especially in withdrawal states. More specifically, the extended amygdala, which includes a transition portion of the NAc-shell, bed nucleus of the stria terminalis (BNST) and central nucleus of the amygdala (CeA), is a central brain region involved in stress and negative affect [17]. Within the extended amygdala, the corticotropin-releasing factor (CRF) system has been consistently implicated [57]. Changes in CRF expression correlated with escalation of heroin intake [58], and CRF antagonists were able to attenuate compulsive-like drug taking in rats [59]. CRF is upregulated in the amygdala during opioid withdrawal, and CRF antagonists blocked withdrawal-induced anxiogenic-like behaviors in rats, which is thought to be a potential driver of relapse [57]. Concurrently, while CRF levels increased with repeated opioid exposure [58,60], neuropeptide Y (NPY), which has been shown to exert opposing effects to CRF, appears to be downregulated in the extended amygdala, and this imbalance is a potential mechanism for the sensitized stress response in OUD [17,20,23,33].
The dynorphin-kappa opioid receptor system has also been strongly linked to stress induced opioid seeking behavior. Stressors induce release of dynorphin, and kappa opioid antagonists have been shown to block the development of both pain and stress-induced negative affect in mice [61-63]. Kappa opioid antagonists have also been shown to block compulsive-like responding for heroin in rodent self-administration, as well as decrease anxiety-like behavior observed during withdrawal [64]. Moreover, stress-induced reinstatement of heroin self-administration is blocked by kappa opioid antagonists [65,66], and more specifically antagonist administration directly into the NAc shell blocked stress-induced reinstatement and escalation of drug taking [19].
Chronic kappa opioid receptor activation results in decreases in dopamine release in mesolimbic brain regions and presents a potential mechanism for the intersection of opioid use and pain- and stress-induced negative affect. One study demonstrated that inflammatory pain decreased dopamine release as a result of disinhibition of dynorphin releasing neurons and subsequent activation of kappa opioid receptors in the NAc shell, and this was sufficient to drive a negative affective state [63]. Stress-induced negative affect has implicated both CRF and kappa opioid receptors, where the CRF system is shown to mediate kappa/dynorphin activation, which in turns drive the dysphoric component of stress [62,67]. Currently, there is much promise in a kappa opioid antagonist that recently entered clinical trials under the National Institute of Mental Health (NIMH) ‘fast-fail’ approach showing the compound to be well tolerated, and to lower anhedonia in patients suffering from mood or anxiety disorders [68,69].
Learning, Memory, and Executive Function
The crux of incentive sensitization is the facilitation of learning and memory for drug related cues. Within the mesolimbic circuitry, conditioned reinforcement, which is the driver of learned cue-drug relationships, has been strongly correlated with activation of the basolateral amygdala (BLA) and dorsal and ventral hippocampal brain regions, as well as prefrontal cortical regions [22]. While it may seem obvious that cues are generated by conditioned reinforcement of external stimuli and contexts, this circuitry is also robust at encoding internal emotional changes as cues, such as the alleviation of negative affective states following drug use during acute or protracted withdrawal [14,22]. The learned association between taking a drug and relief of symptoms is likely a strong component of continued opioid use, and the salience of these learned associations appears to persist and often grow over time, even under protracted abstinence, a phenomenon referred to as incubation of craving [11,18]. And in fact, conditioned opioid withdrawal cues, including things as seemingly innocuous as smells, have been shown to produce craving in humans and induce drug seeking in rodents [19]. In terms of underlying circuitry, during withdrawal, the BLA specifically has been implicated in altered reward processing, with generalized enhanced reward value encoding early on [70,71], and skewed reward value encoding towards drug related cues after many weeks [71]. More generally, the BLA has been heavily implicated in encoding both the salience of reward [72], as well as reward-related cues, and mediating reward expectations [73,74].
While conditioned reinforcement and reward learning may be enhanced in the mesolimbic structures of the brain to facilitate learning and memory of rewards and affective states, layered over these adaptations are changes in connectivity with important cortical structures, often resulting in decreased top-down control. In order to encode the proper salience for any given cue following a drug stimulus, and thus modulate appropriate behavioral output, a certain level of executive control is needed. For example, frontal cortical lesions enhance morphine self-administration in rats [75], and medial prefrontal cortical (mPFC) lesions enhance acquisition of cocaine self-administration [22], likely due to loss of inhibitory control. Human studies report that OUD results in impairment of working memory, increased impulsivity, and poor decision making, all behaviors dependent on frontal cortical function [19]. These findings are consistent with results from imaging studies, where OUD patients show decreased cerebral perfusion and resting state activity in the frontal cortex [76]. Researchers have also found altered function of reciprocal glutamatergic and GABAergic connectivity between regions of the cortex, including mPFC and orbitofrontal cortex (OFC) with mesolimbic structures (See [77] for extensive review of glutamate homeostasis on cortico-limbic function in addiction). Taken together, the mesolimbic incentive sensitization to internal and external drug cues, combined with a lack of appropriate top-down control, results in neurocircuitry that is strongly primed for continued drug use and/or potential relapse.
A focus on craving as a driver of OUD
Prior to 1990, research pertaining to craving was very limited with much of the early craving research centered around alcohol use disorder. Craving was historically thought of as a “secondary outcome”, and in fact, it was only added to the DSM-5 criteria for substance use disorders in 2013 [78]. Under this criteria, craving is defined as the “strong desire or urge to use the substance” where craving “makes it difficult to think of anything else” and “often results in the onset” of use or relapse of use [78]. More recent studies show that craving is one of the most strongly correlated symptoms with likelihood of relapse, often independent of other factors such as pain and even negative affect [7,9,19,79,80], though these states, along with stress, can trigger craving [23,55,81].
While the inclusion of craving in the DSM-5 is a step in the right direction, in this review, and in agreement with other previously published work, we support the argument that the definition of craving should be expanded to encompass a neurocognitive state beyond just the conscious, subjective experience [14,15]. While the subjective state of excessive “wanting” is central to most definitions of craving, a comprehensive definition of craving includes subconscious elements such as increased activity in attention networks involving drug cues, physiological, psychological, and emotional reactions to drug-related cues, episodic memories related to drug use, and impaired concentration due to intrusive or craving-related thoughts [14,15]. A drug cue could be any number of external physical objects, locations, or people, as well as internal physiological states, or any number of affective states that becomes a learned association with drug use. Within this definition, we suggest that the learned associations of negative reinforcement associated with the relief of stress or anxiogenic states that contributed to the development of an OUD is generalizable to future stressful or anxiogenic states (independent of the original learned association), and thereby will precipitate craving. Here, we argue for the broadened definition of both craving and drug cues, which means that any form of craving discussed is a form of cue-induced craving, regardless of whether that cue is an internal emotional, psychological, or physiological change, or a context. Furthermore, by broadening the definition of craving, research has begun to more concisely identify underlying neurocircuitry as well as its role in numerous aspects of the pathophysiology of OUD.
Rodent and human studies on craving-Current findings
Very recently, researchers have used functional near-infrared spectroscopy (fNIRS) to quantify activation of PFC regions in response to drug-cues in an attempt to develop a measurement predictive of the likelihood of current and future opioid use. This study showed that left lateral PFC activation upon drug cue presentation was correlated with a lower percentage of current opioid negative urine drug screens, and interestingly was independent of self-reported or cue-induced craving [31]. Furthermore, activity of the left lateral PFC was highly predictive of abstinence or relapse in the 90-day follow-up period. This highlights the necessity for a broader definition of craving, as self-reported, subjective craving may not accurately reflect underlying, subconscious neurophysiology. And in fact, numerous studies have correlated neurophysiological responses with cue presentation in the absence of self-reported craving [28,31,82-85].
In patients that report high levels of craving, stimulation of regions of the dorsal lateral PFC using transcranial magnetic stimulation was very effective at reducing cue-induced craving in heroin users [82,86]. Rodent studies also show that the mPFC to NAc drive cue-induced reinstatement, whereas the ventromedial PFC and subiculum to NAc connections drive context-induced reinstatement [19,23]. Other cortical projections have also been implicated in cue encoding and cue-induced drug seeking, including OFC projections to the NAc and BLA [87,88]. Both the mPFC- and OFC connectivity has been strongly implicated in incubation of craving during abstinence [23,89], while OFC to BLA projections have been shown to be involved in encoding and retrieval of reward value, an important component of salience attribution for encoding drug cues [74].
Stress and negative affect are strong drivers of drug craving, especially in OUD individuals [14,15,87]. Studies have shown that OUD patients often have a dysregulated hypothalamic-pituitary-adrenal (HPA) axis, which is an indicator of chronic stress [15,19], as well as increases in dynorphin and CRF within the extended amygdala, which contribute to OUD stress and dysphoria, especially during withdrawal periods [34]. Acute heroin administration has been shown to diminish anxiety levels, decrease HPA-axis related hormones, and decrease activation of the left amygdala [56], likely contributing to the incentive value of alleviating acute and conditioned withdrawal states. In rodents, drug cues paired with naloxone-precipitated withdrawal increase heroin consumption and elevated intracranial self-stimulation thresholds [90], and even naloxone (an opioid receptor antagonist) itself can induce drug-seeking [23]. Many stressors reinstate drug-seeking behavior, where extended amygdala, and more specifically, the BLA has been strongly implicated in stress-induced drug seeking and reinstatement [21,23]. In rats, silencing the BLA induced reinstatement of drug seeking produced by either a conditioned stimulus- or drug-priming with heroin [91,92]. Studies have also shown that reversible inhibition of other extended amygdala regions, including CeA and BNST, decrease both cue- and footshock stress-induced reinstatement for heroin seeking [23].
While few preclinical studies have examined sex differences, women have been shown to be more sensitive to stress-induced craving in response to both stress- and drug- related cues [21,93,94], though when controlling for drug access, women and men show similar rates of drug use [21]. Retrospective studies have shown that women tend to initiate drug use as a means for coping with negative affective states [21], and women also report more negative affect during withdrawal compared to men [21,94]. This is consistent with rodent studies showing females acquire heroin self-administration more quickly than males [95,96] and females showed much higher break points (work effort) for heroin in progressive ratio tests [96].
How the etiology of OUD contributes to the development of craving
Though specific cortico-limbic circuitry has been correlated with craving, the precise underlying circuitry and mechanisms are still be fully understood. In agreement with previously reviewed research [7,8,10,13,14,41,87,97], we argue here that drug craving results from enhanced state of incentive salience to drug-related stimuli, which results in aberrant learned associations between the drug and drug cues and drives motivation to drug-seeking. We also argue that craving is more than just a “subjective urge” to consume a drug and is involved in both conscious and subconscious desire, making it an integral component of each phase of the addiction cycle. While changes to reward and motivation circuitry may be the most obvious allostatic changes driving craving, other domains of behavior and neurocircuitry must also be considered. Executive function is often compromised, while certain drug-related learning and memory functions are enhanced, and associations between the negative affective states of withdrawal and the alleviating effects of opioid use will also be learned [7,15,17,19].
In terms of development of OUD, it seems likely that both positive and negative reinforcement play a role in its etiology, and perhaps the contribution and timing of each is dependent on the drug involved, as well as the baseline level of stress, physical or affective, prior to opioid exposure. In the context of opioids, we suggest that the initial exposure likely exploits a neurocircuitry primed to react to enhanced dopaminergic tone, that then quickly adapts to a new baseline functioning, ultimately dampening down the positively reinforcing aspects of the drug, and instead enhancing learning of drug cues that alleviate the negative allostatic load that has been created. A salient example of this potential difference in circuitry primed for cue encoding is found in rodent studies. Rats trained to press a lever for a reward (including opioids) in the presence of a cue can be grouped according to one of two distinct behaviors. Some rats quickly engaging with the cue that predicts a reward, and are willing and motivated to work for the reward (i.e., sign-trackers), while others instead are more interested in the area where the reward is dispensed (i.e., goal-trackers) [11,12]. The sign-tracker rodents show higher incentive-salience towards the cues and appear more vulnerable to cue-induced relapse.
Furthermore, while anxiety and stress, and even chronic pain have been correlated with higher levels of craving, studies show that the strength of craving, and not the affective or pain state, is most predictive of drug use [15]. It could also be argued that negative reinforcement model does not necessarily account for periods of prolonged abstinence when withdrawal symptoms have dissipated, so perhaps negative reinforcement is facilitating incentive sensitization/salience, which contributes to the incubation of craving that in turn drives relapse during protracted abstinence [22]. Therefore, in describing the etiology and continuance of OUD, especially in chronic pain states [26], perhaps neither “incentive sensitization” via positive reinforcement nor “antireward” involving negative reinforcement are fully encompassing of the processes involved in OUD, and might better be summarized as a “reward processing imbalance”, involving dysregulation of the neurocircuitry between reward & motivation, stress & negative affect, and learning, memory & executive function.
Inclusion of craving in future OUD studies
In human studies, we need to broaden the metrics used to identify craving in iatrogenic-induced OUD in patients prescribed opioid medications. While some individuals experiencing OUD may readily and accurately report persistent cravings, other individuals may use different language, such as urges or intrusive thoughts and memories correlated with previous drug use. Additionally, more subtle indicators such as physiological measures and brain imaging responses to drug related cues may provide further support and insight into how active a “craving network” may be in an individual. This more complete picture of craving can more accurately identify the role of craving in continued drug use, and assist researchers and clinicians in finding useful treatments that may help reverse or modify the allostatic changes and neuroplasticity within circuitry that led to enhancement and strengthening of craving [97].
If craving is indeed a strong driver of continued drug use and relapse, and craving is a result of enhanced learning of drug related cues, perhaps the key to breaking the cycle involves leveraging the learning and memory system in a way that overrides past associations with new positive associations, rather than simply attempting to block them [98]. This might be similar to approaches taken in PTSD cognitive behavioral therapy, which involve reimagining a traumatic event in order to reprocess and reappraise negative narratives that might be trigger reexperience of the traumatic experience [99]. In the same way, if strong memories are attached to past drug use, and the cues continue to be triggering, rather than focusing on the negative consequences of drug use, individuals reimage a drug-free future and focus on positive outcomes as rewards, which are inconsistent with drug use, and in fact, this approach has shown some efficacy with other drugs of abuse [98].
In pre-clinical studies, researchers must carefully consider how to accurately model and measure the various domains of craving observed in human populations of OUD. A large majority of publications regarding craving are from contingent self-administration studies that utilized cue-induced, drug-induced, and stress-induced reinstatement models following extinction [81]. Although these models have been effective for studying neural circuitry and behavior involved in craving, abstinence through extinction training is not a realistic representation of human behavior, since human abstinence is usually forced, due to incarceration or inpatient treatment, or voluntary, due to a cognitive recognition of the negative consequences of continued drug use. Rather than using extinction-based models, newer research models have focused on forced abstinence or abstinence induced by negative consequences [23,81]. Punishment-based model employ the use of foot-shock after operant responding for the drug [23]. These studies are then able to assess the impact of incubation of craving and cue-induced relapse following abstinence [81]. Another method for inducing voluntary abstinence in rodents is by offering alternative rewards, such as food or social interaction, which studies have shown decrease opioid drug-seeking and even lower cue-induced relapse [23]. However, when using sweetened water as a palatable alternative to heroin, extended access to heroin actually increased choice in heroin over a nondrug alternative [100], which might suggest these models are not ideal for opioid specific abstinence.
Conclusions
Neurocircuitry changes that occur following repeated exposure to an opioid result in incentive salience state that facilitates learning of drug-relevant cues, both internal and external, while blunting circuitry related to non-drug related rewards. Chronic opioid use results in rapid allostatic changes that lead to the development of tolerance, resulting in periods of acute withdrawal that provide additional salience to cues via negative reinforcement. The long-term adaptations result in altered executive function and increased stress and negative affect, which in turn continue to support a strong craving network, formed from these learned associations, which continue to drive drug-seeking or produce vulnerability in protracted abstinence.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
Papers of particular interest, published recently, have been highlighted as:
* Of importance
** Of major importance
- 1.U.S. Opioid Prescribing Rate Maps ∣ Drug Overdose ∣ CDC Injury Center; 2020. [Google Scholar]
- 2.Board INC. Narcotic Drugs: Estimated world requirements for 2018, statistics for 2016. 2017. [Google Scholar]
- 3.Prekupec MP, Mansky PA, Baumann MH. Misuse of Novel Synthetic Opioids: A Deadly New Trend. J Addict Med 2017:11:256–65. Doi: 10.1097/ADM.0000000000000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryson EO, Silverstein JH. Addiction and substance abuse in anesthesiology. Anesthesiology 2008:109:905–17. Doi: 10.1097/ALN.0b013e3181895bc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochalek TA, Parker MA, Higgins ST, Sigmon SC. Fentanyl exposure among patients seeking opioid treatment. J Subst Abuse Treat 2019:96:23–5. Doi: 10.1016/j.jsat.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unnithan S, Gossop M, Strang J. Factors associated with relapse among opiate addicts in an out-patient detoxification programme. Br J Psychiatry 1992:161:654–7. Doi: 10.1097/ALN.0b013e3181895bc. [DOI] [PubMed] [Google Scholar]
- 7.Tiffany ST, Wray JM. The clinical significance of drug craving. Ann N Y Acad Sci 2012:1248:1–17. Doi: 10.1097/ALN.0b013e3181895bc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kakko J, Alho H, Baldacchino A, Molina R, Nava FA, Shaya G. Craving in opioid use disorder: From neurobiology to clinical practice. Front Psychiatry 2019:10:1–12. Doi: 10.3389/fpsyt.2019.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martel MO, Jamison RN, Wasan AD, Edwards RR. The association between catastrophizing and craving in patients with chronic pain prescribed opioid therapy: A preliminary analysis. Pain Med 2014:15:1757–64. Doi: 10.1111/pme.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kober H, Mell MM. Neural mechanisms underlying craving and the regulation of craving In: Wilson SJ, editor. Wiley Handb Cogn Neurosci Addict. John Wiley & Sons, Ltd; 2015. [Google Scholar]
- 11.Berridge KC, Robinson TE. Liking, wanting, and the incentive-sensitization theory of addiction. Am Psychol 2016:71:670–9. Doi: 10.1037/amp0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: Individual differences. Neuropharmacology 2014:76:450–9. Doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinz A, Beck A, Halil MG, Pilhatsch M, Smolka MN, Liu S. Addiction as learned behavior patterns. J Clin Med 2019:8:1–9. Doi: 10.3390/jcm8081086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekhtiari H, Nasseri P, Yavari F, Mokri A, Monterosso J. Neuroscience of drug craving for addiction medicine: From circuits to therapies In: Ekhtiari H, Paulus MP, editors. Prog Brain Res. Elsevier B.V.; 2016. Doi: 10.1016/bs.pbr.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Sinha R The clinical neurobiology of drug craving. Curr Opin Neurobiol 2013:23:649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kober H Emotion regulation in substance use disorders In: Gross J, editor. Handb Emot Regul. The Guilford Press; 2014. [Google Scholar]
- 17.Koob GF. Neurobiology of opioid addiction: Opponent process, hyperkatifeia and negative reinforcement. Biol Psychiatry 2019:44–53. Doi: 10.1016/j.biopsych.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 18.Evans CJ, Cahill CM. Neurobiology of opioid dependence in creating addiction vulnerability. F1000Research 2016:5:1748 Doi: 10.12688/f1000research.8369.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **19.Strang J, Volkow ND, Degenhardt L, Hickman M, Johnson K, Koob GF, et al. Opioid use disorder. Nat Rev Dis Prim 2020:6:1–28. Doi: 10.1038/s41572-019-0137-5.This is a comprehensive review consisting of the most current data on the epidemiology, pathphysiology, and underlying circuitry associated with OUD. The review covers the socio-economic burden of OUD around the world, both preclinical and human studies on the current understanding of OUD, as well as a public health approach for detection, prevention, and treatment of OUD.
- 20.Koob GF, Volkow ND. Neurobiology of addiction: A neurocircuitry analysis. The Lancet Psychiatry 2016:3:760–73. Doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *21.Becker JB, Chartoff E. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 2019:44:166–83. Doi: 10.1038/s41386-018-0125-6.This review covers the most recent research on sex differences observed in reward and addiction, for both pre-clinical and human studies.
- 22.Feltenstein MW, See RE, Fuchs RA. Neural substrates and circuits of drug addiction. Cold Spring Harb Perspect Med 2020:a039628 Doi: 10.1101/cshperspect.a039628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **23.Reiner DJ, Fredriksson I, Lofaro OM, Bossert JM, Shaham Y. Relapse to opioid seeking in rat models: Behavior, pharmacology and circuits. Neuropsychopharmacology 2019:44:465–77. Doi: 10.1038/s41386-018-0234-2.This review is a comprehensive analysis of the most relevant models for researching opioid seeking and relapse in rodents. In addition to the behavioral assays, it reviews the underlying neurobiology and circuitry mediating drug seeking, abstience, and relapse in rodents.
- 24.Volkow ND, McLellan AT. Opioid abuse in chronic pain — Misconceptions and mitigation strategies. N Engl J Med 2016:374:1253–63. Doi: 10.1056/NEJMra1507771. [DOI] [PubMed] [Google Scholar]
- 25.Navratilova E, Porreca F. Reward and motivation in pain and pain relief. Nat Neurosci 2014:17:1304–12. Doi: 10.1038/nn.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron 2016:89:11–36. Doi: 10.1016/j.neuron.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 27.Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Rev 1993:18:247–91. Doi: 10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- 28.Parvaz MA, Moeller SJ, Goldstein RZ. Incubation of cue-induced craving in adults addicted to cocaine measured by electroencephalography. JAMA Psychiatry 2016:73:1127–34. Doi: 10.1001/jamapsychiatry.2016.2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawa AB, Bentzley BS, Robinson TE. Less is more: Prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology 2016:233:3587–602. Doi: 10.1007/s00213-016-4393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao M, Fan C, Du J, Jiang H, Chen H, Sun H. Cue-induced craving and physiological reactions in recently and long-abstinent heroin-dependent patients. Addict Behav 2012:37:393–8. Doi: 10.1016/j.addbeh.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. *.Huhn AS, Sweeney MM, Brooner RK, Kidorf MS, Tompkins DA, Ayaz H, et al. Prefrontal cortex response to drug cues, craving, and current depressive symptoms are associated with treatment outcomes in methadone-maintained patients. Neuropsychopharmacology 2019:44:826–33. Doi: 10.1038/s41386-018-0252-0.This research article used a non-invasive imaging technique to identify activity levels in the llPFC that correlated with illicit opioid use in methadone-maintained patients. This technique, in combination with craving scores and depressive symptoms, was highly predictive of relapse in the following months and could be used as an aid in treatment and monitoring of patients at a higher risk of relapse.
- 32.Steketee JD, Kalivas PW. Drug wanting: Behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev 2011:63:348–65. Doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology 2010:35:217–38. Doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volkow ND, Koob GF, McLellan AT. Neurobiologic advances from the brain disease model of addiction Longo DL, editor. N Engl J Med 2016:374:363–71. Doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volkow ND, Michaelides M, Baler R. The neuroscience of drug reward and addiction. Physiol Rev 2019:99:2115–40. Doi: 10.1152/physrev.00014.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hser YI, Mooney LJ, Saxon AJ, Miotto K, Bell DS, Huang D. Chronic pain among patients with opioid use disorder: Results from electronic health records data. J Subst Abuse Treat 2017:77:26–30. Doi: 10.1016/j.jsat.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins C, Smith BH, Matthews K. Comparison of psychiatric comorbidity in treatment-seeking, opioid-dependent patients with versus without chronic pain. Addiction 2020:115:249–58. Doi: 10.1111/add.14768. [DOI] [PubMed] [Google Scholar]
- 38.Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PRA. The dopamine theory of addiction: 40 years of highs and lows. Nat Rev Neurosci 2015:16:305–12. Doi: 10.1038/nrn3939. [DOI] [PubMed] [Google Scholar]
- 39.Smith KS, Berridge KC. Opioid limbic circuit for reward: Interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci 2007:27:1594–605. Doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron 2015:86:646–64. Doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carmichael O, Robinson MJF, Fischer AM, Ahuja A, Lesser EN, Maniates H. Roles of “wanting” and “liking” in motivated behavior: Gambling, food, and drug addictions. Curr Top Behav Neurosci 2016:27:105–36. Doi: 10.1007/7854. [DOI] [PubMed] [Google Scholar]
- 42.Watson BJ, Taylor LG, Reid AG, Wilson SJ, Stokes PR, Brooks DJ, et al. Investigating expectation and reward in human opioid addiction with [11C]raclopride PET. Addict Biol 2014:19:1032–40. Doi: 10.1111/adb.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Courtney KE, Schacht JP, Hutchison K, Roche DJO, Ray LA. Neural substrates of cue reactivity: Association with treatment outcomes and relapse. Addict Biol 2016:21:3–22. Doi: 10.1111/adb.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: A survey of human neuroimaging studies. Neurosci Biobehav Rev 2014:38:1–16. Doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daglish MRC, Williams TM, Wilson SJ, Taylor LG, Eap CB, Augsburger M, et al. Brain dopamine response in human opioid addiction. Br J Psychiatry 2008:193:65–72. Doi: 10.1192/bjp.bp.107.041228. [DOI] [PubMed] [Google Scholar]
- 46.Smith KS, Mahler SV., Peciña S, Berridge KC. Hedonic hotspots: Generating sensory pleasure in the brain In: Kringelbach ML, Berridge KC, editors. Pleasures of the brain. Oxford University Press; 2010. [Google Scholar]
- 47.Zijlstra F, Booij J, van den Brink W, Franken IHA. Striatal dopamine D2 receptor binding and dopamine release during cue-elicited craving in recently abstinent opiate-dependent males. Eur Neuropsychopharmacol 2008:18:262–70. Doi: 10.1016/j.euroneuro.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Yang Z, Xie J, Shao Y-C, Xie C-M, Fu L-P, Li D-J, et al. Dynamic neural responses to cue- reactivity paradigms in heroin-dependent users: An fMRI study. Hum Brain Mapp 2009:30:766–75. Doi: 10.1002/hbm.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lüscher C, Robbins TW, Everitt BJ. The transition to compulsion in addiction. Nat Rev Neurosci 2020. Doi: 10.1038/s41583-020-0289-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ting-A-Kee R, van der Kooy D. The neurobiology of opiate motivation. Cold Spring Harb Perspect Med 2012:2:1–16. Doi: 10.1101/cshperspect.a012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halbout B, Marshall AT, Azimi A, Liljeholm M, Mahler SV., Wassum KM, et al. Mesolimbic dopamine projections mediate cue-motivated reward seeking but not reward retrieval in rats. Elife 2019:8:1–21. Doi: 10.7554/eLife.43551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martel MO, Dolman AJ, Edwards RR, Jamison RN, Wasan AD. The association between negative affect and prescription opioid misuse in patients with chronic pain: The mediating role of opioid craving. J Pain 2014:15:90–100. Doi: 10.1016/j.jpain.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garland EL, Bryan CJ, Nakamura Y, Froeliger B, Howard MO. Deficits in autonomic indices of emotion regulation and reward processing associated with prescription opioid use and misuse. Psychopharmacology 2017:234:621–9. Doi: 10.1007/s00213-016-4494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: An effect mimicking heroin, not withdrawal. Psychopharmacology 1995:119:334–41. Doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- 55.Hyman SM, Fox H, Hong KIA, Doebrick C, Sinha R. Stress and drug-cue-induced craving in opioid-dependent individuals in naltrexone treatment. Exp Clin Psychopharmacol 2007:15:134–43. Doi: 10.1037/1064-1297.15.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmidt A, Borgwardt S, Gerber H, Wiesbeck GA, Schmid O, Riecher-Rössler A, et al. Acute effects of heroin on negative emotional processing: Relation of amygdala activity and stress-related responses. Biol Psychiatry 2014:76:289–96. Doi: 10.1016/j.biopsych.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 57.Roberto M, Spierling SR, Kirson D, Zorrilla EP. Corticotropin-releasing factor (CRF) and addictive behaviors Int Rev Neurobiol. Academic Press Inc; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McFalls AJ, Imperio CG, Bixler G, Freeman WM, Grigson PS, Vrana KE. Reward devaluation and heroin escalation is associated with differential expression of CRF signaling genes. Brain Res Bull 2016:123:81–93. Doi: 10.1016/j.brainresbull.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 59.Park PE, Schlosburg JE, Vendruscolo LF, Schulteis G, Edwards S, Koob GF. Chronic CRF1 receptor blockade reduces heroin intake escalation and dependence-induced hyperalgesia. Addict Biol 2015:20:275–84. Doi: 10.1111/adb.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev 2001:53:209–43. [PubMed] [Google Scholar]
- 61.Liu SS, Pickens S, Burma NE, Ibarra-Lecue I, Yang H, Xue L, et al. Kappa opioid receptors drive a tonic aversive component of chronic pain. J Neurosci 2019:39:4162–78. Doi: 10.1523/JNEUROSCI.0274-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin κ-opioid system. J Neurosci 2008:28:407–14. Doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Massaly N, Copits BA, Wilson-Poe AR, Hipólito L, Markovic T, Yoon HJ, et al. Pain-induced negative affect is mediated via recruitment of the nucleus accumbens kappa opioid system. Neuron 2019:102:564–573.e6. Doi: 10.1016/j.neuron.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schlosburg JE, Whitfield TW, Park PE, Crawford EF, George O, Vendruscolo LF, et al. Long-term antagonism of κ opioid receptors prevents escalation of and increased motivation for heroin intake. J Neurosci 2013:33:19384–92. Doi: 10.1523/JNEUROSCI.1979-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sedki F, Eigenmann K, Gelinas J, Schouela N, Courchesne S, Shalev U. A role for kappa-, but not mu-opioid, receptor activation in acute food deprivation-induced reinstatement of heroin seeking in rats. Addict Biol 2015:20:423–32. Doi: 10.1111/adb.12133. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Y, Leri F, Grella SL, Aldrich J V., Kreek MJ. Involvement of dynorphin and kappa opioid receptor in yohimbine-induced reinstatement of heroin seeking in rats. Synapse 2013:67:358–61. Doi: 10.1002/syn.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res 2010:1314:44–55. Doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pizzagalli DA, Smoski M, Ang Y-S, Whitton AE, Sanacora G, Mathew SJ, et al. Selective kappa-opioid antagonism ameliorates anhedonic behavior: evidence from the Fast-fail Trial in Mood and Anxiety Spectrum Disorders (FAST-MAS). Neuropsychopharmacology 2020:0:1–8. Doi: 10.1038/s41386-020-0738-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krystal AD, Pizzagalli DA, Smoski M, Mathew SJ, Nurnberger J, Lisanby SH, et al. A randomized proof-of-mechanism trial applying the ‘fast-fail’ approach to evaluating κ-opioid antagonism as a treatment for anhedonia. Nat Med 2020:26:760–8. Doi: 10.1038/s41591-020-0806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wassum KM, Greenfield VY, Linker KE, Maidment NT, Ostlund SB. Inflated reward value in early opiate withdrawal. Addict Biol 2016:21:221–33. Doi: 10.1111/adb.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harris GC, Aston-Jones G. Altered motivation and learning following opiate withdrawal: Evidence for prolonged dysregulation of rewardprocessing. Neuropsychopharmacology 2003:28:865–71. Doi: 10.1038/sj.npp.1300122. [DOI] [PubMed] [Google Scholar]
- 72.Wassum KM, Ostlund SB, Maidment NT, Balleine BW. Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc Natl Acad Sci 2009:106:12512–7. Doi: 10.1073/pnas.0905874106. Doi: 10.1073/pnas.0905874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lichtenberg NT, Pennington ZT, Holley SM, Greenfield VY, Cepeda C, Levine MS, et al. Basolateral amygdala to orbitofrontal cortex projections enable cue-triggered reward expectations. J Neurosci 2017:37:8374–84. Doi: 10.1523/JNEUROSCI.0486-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Malvaez M, Shieh C, Murphy MD, Greenfield VY, Wassum KM. Distinct cortical–amygdala projections drive reward value encoding and retrieval. Nat Neurosci 2019:22:762–9. Doi: 10.1038/s41593-019-0374-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Glick SD, Cox RD. Changes in Morphine Self-Administration After Tel-Diencephalic Lesions in Rats. Psychopharmacology 1978. [DOI] [PubMed] [Google Scholar]
- 76.Roberts D, Wolfarth A, Sanchez C, Pehrson AL. Frontal cortex dysfunction as a target for remediation in opiate use disorder: Role in cognitive dysfunction and disordered reward systems Prog Brain Res. Elsevier B.V; 2018. Doi: 10.1016/bs.pbr.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 77.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 2009:10:561–72. Doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 78.American Psychiatric Association Task Force On DSM-V. Diagnostic and statistical manual of mental disorders: DSM-V 5th ed. Diagnostic Stat. Man. Ment. Disord 2013. Doi: 10.1176/appi.books.9780890425596. [DOI] [Google Scholar]
- 79.Martel MO, Finan PH, McHugh RK, Issa M, Edwards RR, Jamison RN, et al. Day-to-day pain symptoms are only weakly associated with opioid craving among patients with chronic pain prescribed opioid therapy. Drug Alcohol Depend 2016:162:130–6. Doi: 10.1016/j.drugalcdep.2016.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsui JI, Anderson BJ, Strong DR, Stein MD. Craving predicts opioid use in opioid-dependent patients initiating buprenorphine treatment: A longitudinal study. Am J Drug Alcohol Abuse 2014:40:163–9. Doi: 10.3109/00952990.2013.848875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Venniro M, Caprioli D, Shaham Y. Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence Prog Brain Res. Elsevier B.V; 2016. Doi: 10.1016/bs.pbr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Liu X, Zhao X, Liu T, Liu Q, Tang L, Zhang H, et al. The effects of repetitive transcranial magnetic stimulation on cue-induced craving in male patients with heroin use disorder. EBioMedicine 2020:56 Doi: 10.1016/j.ebiom.2020.102809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Back SE, Gros DF, McCauley JL, Flanagan JC, Cox E, Barth KS, et al. Laboratory-induced cue reactivity among individuals with prescription opioid dependence. Addict Behav 2014:39:1217–23. Doi: 10.1016/j.addbeh.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: An activation likelihood estimation meta-analysis. Biol Psychiatry 2011:70:785–93. Doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murphy A, Lubman DI, McKie S, Bijral PS, Peters LA, Faiz Q, et al. Time-dependent neuronal changes associated with craving in opioid dependence: an fMRI study. Addict Biol 2018:23:1168–78. Doi: 10.1111/adb.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mahoney JJ, Marshalek PJ, Rezai AR, Lander LR, Berry JH, Haut MW. A case report illustrating the effects of repetitive transcranial magnetic stimulation on cue-induced craving in an individual with opioid and cocaine use disorder. Exp Clin Psychopharmacol 2020:28:1–5. Doi: 10.1037/pha0000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ray LA, Roche DJO. Neurobiology of Craving: Current Findings and New Directions. Curr Addict Reports 2018:5:102–9. Doi: 10.1007/s40429-018-0202-2. [DOI] [Google Scholar]
- 88.Reiner DJ, Lofaro OM, Applebey S V., Korah H, Venniro M, Cifani C, et al. Role of projections between piriform cortex and orbitofrontal cortex in relapse to fentanyl seeking after palatable food choice-induced voluntary abstinence. J Neurosci 2020:40:2485–97. Doi: 10.1523/JNEUROSCI.2693-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Altshuler RD, Yang ES, Garcia KT, Davis IR, Olaniran A, Haile M, et al. Role of orbitofrontal cortex in incubation of oxycodone craving in male rats. Addict Biol 2020:e12927 Doi: 10.1111/adb.12927. [DOI] [PubMed] [Google Scholar]
- 90.Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci 2006:26:5894–900. Doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology 2002:160:425–33. Doi: 10.1007/s00213-001-0997-7. [DOI] [PubMed] [Google Scholar]
- 92.Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 2005:30:296–309. Doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- 93.Moran LM, Kowalczyk WJ, Phillips KA, Vahabzadeh M, Lin JL, Mezghanni M, et al. Sex differences in daily life stress and craving in opioid-dependent patients. Am J Drug Alcohol Abuse 2018:44:512–23. Doi: 10.1080/00952990.2018.1454934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Becker JB, Koob GF. Sex differences in animal models: Focus on addiction. Pharmacol Rev 2016:68:242–63. Doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology 1999:144:77–82. Doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- 96.Cicero TJ, Aylward SC, Meyer ER. Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol Biochem Behav 2003:74:541–9. Doi: 10.1016/S0091-3057(02)01039-0. [DOI] [PubMed] [Google Scholar]
- *97.Goodyear K, Haass-Koffler CL. Opioid craving in human laboratory settings: A review of the challenges and limitations. Neurotherapeutics 2020:17:100–4. Doi: 10.1007/s13311-019-00791-8.This review offers a thoughtful overview and discussion on the most effective ways in which to elicit and measure craving in the human laboratory.
- 98.Bornstein AM, Pickard H. “Chasing the first high”: Memory sampling in drug choice. Neuropsychopharmacology 2020:45:907–15. Doi: 10.1038/s41386-019-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Watkins LE, Sprang KR, Rothbaum BO. Treating PTSD: A review of evidence-based psychotherapy interventions. Front Behav Neurosci 2018:12:258 Doi: 10.3389/fnbeh.2018.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology 2013:38:1209–20. Doi: 10.1038/npp.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]


