Abstract
Rationale:
Cognitive impairment is a primary feature of many neuropsychiatric disorders and there is a need for new therapeutic options. Catechol-O-methyltransferase (COMT) inhibitors modulate cortical dopaminergic function and have been proposed as potential cognitive enhancers. Unfortunately, currently available COMT inhibitors are not good candidates due to either poor blood-brain barrier penetration or severe toxicity.
Objectives:
To address the need for safe, brain-penetrant COMT inhibitors, we tested multiple novel compounds in a set of preclinical in vivo efficacy assays in rats to determine their ability to inhibit COMT function and viability as potential clinical candidates.
Methods:
We measured the change in concentration of dopamine (DA) metabolites in cerebrospinal fluid (CSF) from the cisterna magna and extracellular fluid (ECF) from the frontal cortex produced by our novel compounds. Additionally, we tested the effects of our brain-penetrant COMT inhibitors in an attentional set-shifting assay (ASST). We benchmarked the performance of the novel COMT inhibitors to the effects produced by the known COMT inhibitor tolcapone.
Results:
We found that multiple COMT inhibitors, exemplified by LIBD-1 and LIBD-3, significantly modulated dopaminergic function measured as decreases in homovanillic acid (HVA) and increases in 3,4-Dihydroxyphenylacetic acid (DOPAC), two DA metabolites, in CSF and the frontal cortex. Additionally, we found the LIBD-1 significantly improved cognitive flexibility in the ASST, an effect previously reported following tolcapone administration.
Conclusions:
These results demonstrate that LIBD-1 is a novel COMT inhibitor with promising in vivo activity and the potential to serve as a new therapy for cognitive impairment.
Keywords: catechol-o-methyltransferase, dopamine, tolcapone, cortex, executive function, microdialysis, rat, schizophrenia, Parkinson’s disease, cognitive impairment
Introduction
Cognitive impairment is a core feature of many neurological and psychiatric disorders and the severity of the impairment is directly correlated with functional outcomes for patients (Gratwicke et al. 2015; Keefe 2014; Lane et al. 2018; Muslimovic et al. 2005; Wanneveich et al. 2018). Currently, there are very few therapeutic options for patients with cognitive impairment. The options that are available have limited efficacy and in some cases, including psychostimulants in schizophrenia, are contraindicated due to potential exacerbation of other symptoms (Hsu et al. 2018).
The neuromodulator dopamine (DA) plays a critical role in cognitive function across species and strategies that augment or refine DA activity in the brain have been shown to improve cognitive function (Nieoullon 2002). The enzyme catechol-O-methyltransferase (COMT) is a key component of the DA metabolic pathway (Axelrod and Tomchick 1958) and inhibition of the enzyme has been proposed as a cognitive enhancement strategy (Apud and Weinberger 2007). COMT is located throughout the body with the highest expression in the liver and brain (Tenhunen et al. 1994). There is a soluble (S-COMT) and a membrane-bound (MB-COMT) form of the enzyme. S-COMT has a higher Vmax but lower affinity for DA compared to MB-COMT (Lotta et al. 1995). It is thought that S-COMT activity predominates in the periphery where it metabolizes many substrates including catecholestrogens and exogenous catechols (Tenhunen and Ulmanen 1993). In contrast, MB-COMT accounts for most of the enzyme activity in the brain, especially in the primate brain, where the major substrate is dopamine, which is present at relatively low concentrations (Matsumoto et al. 2003).
The major routes for terminating DA signaling in the brain are reuptake and metabolic conversion (Meiser et al. 2013). In the striatum, the dopamine transporter (DAT) is the major termination mechanism (Yavich et al. 2007). In the frontal cortex and hippocampus, there are relatively small amounts of DAT, most of which is extrasynaptic (Lewis et al. 2001). There is evidence that the norepinephrine transporter (NET) can clear DA from the extracellular space in cortex and hippocampus (Moron et al. 2002), but NETs are also outside of DA synapses and the majority of functional DA signal termination in these regions appears to depend on COMT activity, with some contribution from monoamine oxidase (Kaenmaki et al. 2010; Paterson et al. 1995). COMT directly converts DA to 3-Methoxytyramine (3-MT) and also converts the DA metabolite 3,4-Dihydroxyphenylacetic acid (DOPAC) to homovanillic acid (HVA). The fact that COMT has an outsized role in DA signaling in the cortex and hippocampus compared to the striatum has led to the hypothesis that selective targeting of COMT activity may modulate cognitive function without the psychostimulant effects (e.g. abuse liability) seen with other DA modulators (Apud and Weinberger 2007).
There is a large body of evidence showing that COMT activity has a dynamic relationship with cognitive function in humans and animal models. There is a highly-studied functional polymorphism in the COMT gene (Val158Met) that produces a “high” activity enzyme (Val158) and a “low” activity enzyme (Met158). In general, the Val form of COMT is associated with lower cognitive function compared to the Met form (Egan et al. 2001; Malhotra et al. 2002; Miskowiak et al. 2017). However, this effect is labile and interactions with other genetic and environmental factors can modify or even reverse the relationship (Papaleo et al. 2012; Zhang et al. 2016). Administration of COMT inhibitors also has variable effects on cognitive function based on the individual status of the Val/Met polymorphism and baseline performance, particularly in studies of people without a diagnosis of cognitive impairment (Cameron et al. 2018; Valomon et al. 2018). Overall, there have been a number of studies of patients across multiple indications that suggest that COMT inhibition may be a viable strategy for improving cognitive function (Gasparini et al. 1997; Grant et al. 2013).
Currently, COMT inhibitors are utilized as an adjunctive therapy alongside L-DOPA administration in patients with Parkinson’s disease (PD) to improve motor symptoms. COMT metabolizes L-DOPA to 3-O-Methyldopa (3-OMD) in the periphery and decreases the amount of L-DOPA available to cross the blood-brain barrier (BBB). Therefore, COMT inhibition serves to augment the CNS availability and effects of L-DOPA without having to increase the dosage of L-DOPA (Limousin et al. 1993; Reches and Fahn 1984). Since the target enzyme pool is in the periphery, a brain-penetrant COMT inhibitor is not necessary for this indication. However, for cognitive enhancing effects, there is evidence that a brain-penetrant or central COMT inhibitor is required. A comparison of the effects of tolcopone (brain-penetrant) to those of entacapone (peripherally-restricted) in patients with PD showed that only those taking tolcapone demonstrated improvement in the cognitive symptoms associated with PD (Gasparini et al. 1997).
Tolcapone is not a viable, long-term option as a cognitive enhancer due to safety concerns. Tolcapone is associated with potentially fatal liver toxicity that makes the drug a poor choice for a chronic cognitive enhancement therapy (Watkins 2000). Given the relative safety of the other COMT inhibitors on the market, the toxicity is thought to be compound-specific as opposed to mechanism-based (Haasio 2010). Therefore, a potent, brain-penetrant, and safe COMT inhibitor is needed to fully explore this mechanism as a potential avenue for cognitive enhancement.
Here we describe the in vivo activity of novel COMT inhibitors. Our goal was to identify a COMT inhibitor with good oral bioavailability and significant effects on neurochemical biomarkers and behavior in rats. We used tolcapone as our efficacy benchmark. We identified LIBD-1 as a candidate molecule for future studies. LIBD-1 has high selectivity for MB-COMT over S-COMT, which we believe will produce an outsized effect on central COMT activity while having a smaller impact on liver COMT function compared to nonselective inhibitors. The evidence presented here indicates that LIBD-1 is a viable lead compound with favorable properties that support continued development.
Materials and Methods
Animals
Male CD® (Strain code: 001; Sprague Dawley) IGS rats (8–11 weeks old; Charles River Laboratories, Wilmington, MA, USA) were used for all experiments. Age-matched female CD® IGS rats were also used for DA metabolite biomarker studies. Rats used for experiments at the Lieber Institute for Brain Development were housed in a temperature (22°C) and humidity-controlled facility on a 12h/12h light/dark cycle (lights on at 0600 hours). Rats used for experiments at RenaSci were housed in a temperature (21 ± 4°C) and humidity-controlled (55 ± 15%) room on a 12h/12h light/dark cycle (lights on at 0700 hours) facility. Rats used in behavioral experiments were handled for one minute at least three times in the week prior to testing. All procedures were approved by the local Animal Care and Use Committee and were in compliance with the Guide for the Care and Use of Laboratory Animals.
Drugs
Novel COMT inhibitors and tolcapone (Figure 1) were synthesized in-house. The compounds were suspended in vehicle (0.1% Tween80, 0.1% 1510 silicone antifoam, 1% methylcellulose in water) and administered either by oral gavage (po) or through intraperitoneal (ip) injection (tolcapone). Tolcapone was administered ip because in pilot studies we found that oral administration produced behavioral side effects not seen with other routes of administration and not correlated with plasma exposure levels. Administration volumes were 10 mL/kg and 5 mL/kg for po and ip dosing, respectively. In the microdialysis experiment, a dose volume of 2 mL/kg was used for tolcapone administration.
Figure 1. COMT inhibitors.
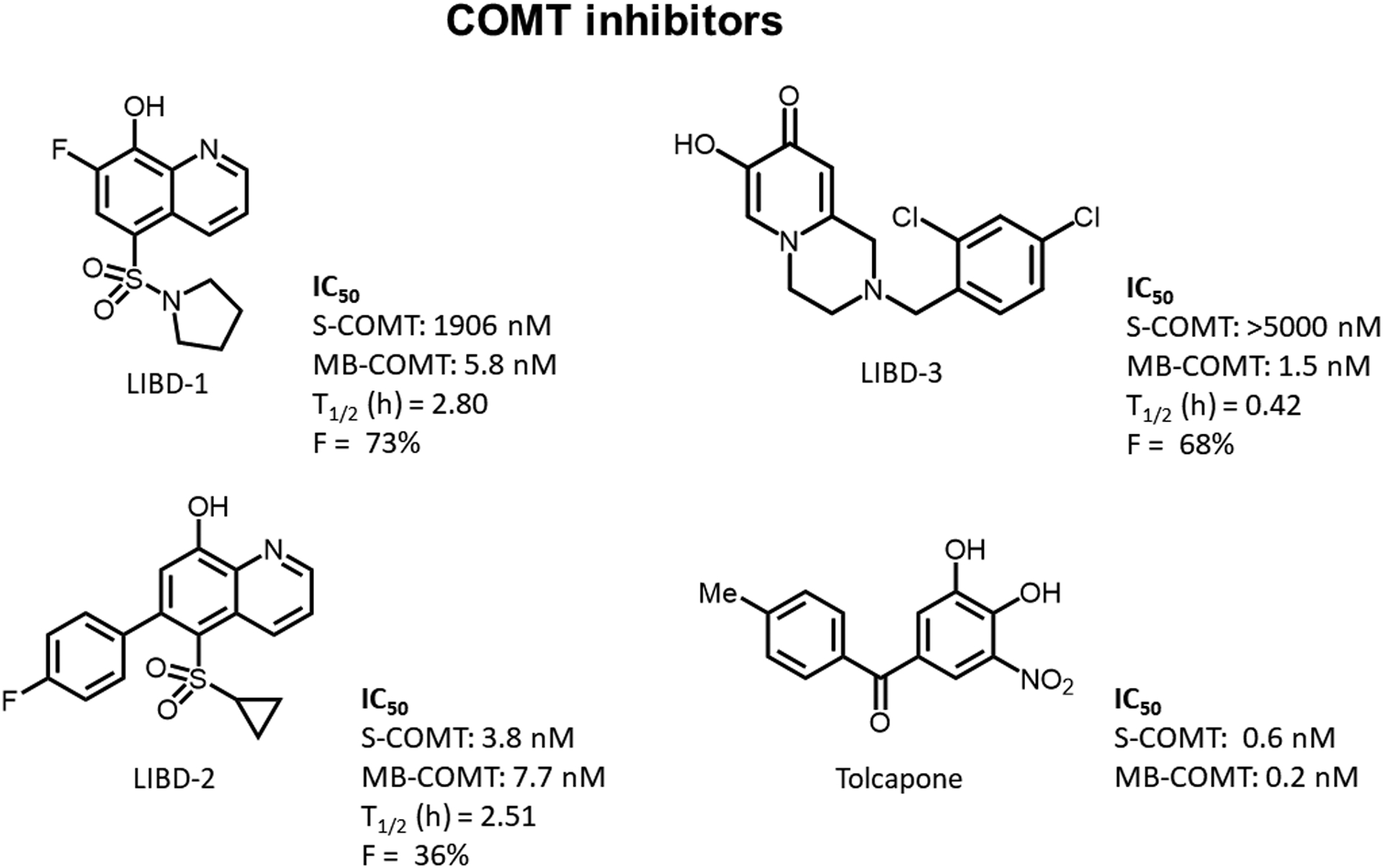
The structures and in vitro potency values (rat COMT) for LIBD-1, LIBD-2, LIBD-3, and tolcapone. Additional pharmacokinetic information iv half-life (T1/2) and oral bioavailability (F) is included for LIBD-2. These data for LIBD-1 and LIBD-2 are included in the Buchler et al., 2018 (compound 28) and Ernst et al., 2019 (compound 38), respectively. PK parameters were calculated based on 1 mg/kg iv (n = 3) and 10 mg/kg po (n = 3) doses.
COMT enzyme activity assay
COMT activity was measured using the MTase Glo methyltransferase assay (Promega, Madison, WI, USA) according to manufacturer’s instructions. Assays were carried out in Corning low volume 384-well white flat-bottom polystyrene NBS microplates with a final volume of 5 μL containing approximately 4 ng of rat MB-COMT or 1 ng of Rat S-COMT as estimated by the Bradford Lowry method from the membrane homogenate. All reactions contained 20 μM high purity S-adenosyl methionine (SAM, CisBio, Bedford, MA, USA) in COMT assay buffer (50 mM Tris, 5–10 mM MgCl2, 2.5 mM DTT, pH 6.9). For MB-COMT, the catechol substrate was 7 μM norepinephrine (MilliporeSigma, St. Louis, MO,USA) and for S-COMT the substrate was 10 μM 7,8-dihydroxy-4-methylcoumarin(32) (MilliporeSigma, St. Louis, MO, USA).
Reactions were performed in a 37 °C incubator for 1 h. The plate was removed from the incubator and allowed to cool to room temperature for 15 min. MTase reagent A (Promega) was first diluted 1:5 into RO water, and 1 μL was then added to the well. The plate was spun down, shaken, and allowed to incubate for 30 min at room temperature avoiding light. Then 5 μL of MTase reagent B (Promega) were added to all of the wells. The plate was spun down, shaken, and allowed to incubate for 30 min at room temperature avoiding light. Luminescence was detected with a Tecan Infinite M100 Pro plate reader.
Standard Curve
A standard curve was run on every plate. The amount of S-adenosyl homocysteine (SAH; CisBio) produced was determined using a standard curve and a linear back-calculation method. The standard curve comprised of varying concentrations of SAH from 500 nM down to 0 nM while maintaining a final SAM/SAH concentration of 50 μM. To correct for background levels present in the enzymatic lysate (MB-COMT), enzyme at assay concentration was added to the standard curve as well.
Determination of Inhibition
Percentage inhibition was calculated by using 10 μM tolcapone value as 100% inhibition value and the DMSO control as the 0% inhibition value. The dose response curves were constrained at 0% inhibition while keeping the percentage inhibition of the highest compound concentration floating. IC50 was determined by nonlinear regressions and curve fitting using a four-parameter fit with a variable slope in the Dotmatics studies program (Dotmatics, Bishops Stortford, UK).
Potency data presented is an average of three separate experiments in which each data point was run in triplicate and reported as the IC50.
DA metabolite biomarker protocol
On the day of testing, rats were transferred to a holding room and weighed. After an hour acclimation period, rats received either vehicle or a COMT inhibitor. At the appropriate time point (1 or 4 hours) following vehicle or drug administration, rats were moved to a separate procedure room where they were anaesthetized via isoflurane. Once the rats were determined to be unresponsive, their heads were shaved using electric clippers. The rats were positioned in a stereotaxic frame, with their heads pointed down at a 45-degree angle. To collect cerebrospinal fluid (CSF), previously published protocols (Mahat et al. 2012; Nirogi et al. 2009) were adapted. Briefly, a 23 gauge needle, connected via PE50 tubing to a collection syringe, was used to access the cisterna magna. Slight negative pressure was used to ensure the CSF flowed evenly. The CSF was collected in previously chilled (dry ice) Eppendorf tubes containing 0.05M perchloric acid (4:1 CSF:perchloric acid ratio). The tubes were put back on dry ice until the end of the procedure. CSF samples with visible blood contamination were not used in subsequent bioanalytical analyses. Next, the chest cavity was opened, and blood was collected by cardiac puncture. The blood was collected in Lithium-Heparin 1.3 mL microtubes (Sarstadt, Numbrecht, Germany) and stored on ice. Blood was then centrifuged at 2000 g at 4°C for 10 minutes to separate the plasma. Plasma was then transferred into Thermo Scientific Matrix tubes (Thermo Fisher Scientific, Waltham, MA, USA) for storage. CSF and plasma were stored at −80°C until analysis. DA metabolite and COMT inhibitor concentrations were measured by LC-MS/MS as previously described (Buchler et al. 2018).
Frontal cortex microdialysis
Surgery
Rats were anaesthetized with isoflurane (5% to induce, 2% to maintain) in O2 (1 L/min) delivered via an anaesthetic unit (Burtons Medical Equipment Ltd, UK). A concentric microdialysis probe (CMA 12 Elite, with an exposed polyarylethersulphone (PAES) membrane tip; Linton Instrumentation, Norfolk, UK) was stereotaxically implanted into the frontal cortex (2 mm of active membrane, coordinates: AP: +3.2 mm; L: +/-2.5 mm relative to bregma; V: –4.0 mm relative to the skull surface). Coordinates were taken from the stereotaxic atlas of Paxinos and Watson (1986). The upper incisor bar was set at 3.3 mm below the interaural line so that the skull surface between bregma and lambda was horizontal. Additional burr holes were made for skull screws (stainless steel) and the probes were secured using dental cement. Carprofen (Carprieve, Norbrook Laboratories (GB) Ltd, Newry, UK) was administered for pain relief at least 30 min prior to animals regaining consciousness following surgery (5 mg/kg sc). Following surgery, animals were individually housed in the dialysis bowls (245 mm internal diameter at base of bowl, 360 mm wall height, Harvard Apparatus, Cambridge, UK), with the microdialysis probes connected to swivels and a counter-balanced arm to allow unrestricted movement. Rats were allowed a recovery period of at least 16 h with standard rodent chow and filtered water available ad libitum. During this time, the probes were continuously perfused at a flow rate of 1.2 μL/min with an artificial cerebrospinal fluid (aCSF; Harvard Apparatus, UK) of the following electrolyte composition (in mM): sodium 150; potassium 3.0; magnesium 0.8; calcium 1.4; phosphate 1.0; chloride 155.0, pH 7.2.
Dialysate sample collection
Dialysate samples were collected at 20 min intervals for a baseline period of 80 min prior to the onset of administration of vehicle, tolcapone, LIBD-1, or LIBD-3. Sampling continued for an additional 2 h. Samples were collected into Eppendorf vials (300 μl, Microbiotech, Stockholm, Sweden) containing 5.0 μL of 0.1 M perchloric acid to prevent oxidation of DA and its metabolites. Samples were stored at −80°C until assayed by HPLC with electrochemical detection.
HPLC analysis of microdialysis samples
Detection and subsequent quantification of DA, DOPAC and HVA in the dialysis samples was achieved by reverse-phase, ion-pair HPLC coupled with electrochemical detection and involved the use of an ALEXYS monoamine analyser (Antec Scientific, Zoeterwoude, The Netherlands). The system consisted of two separate analytical columns (ALF-115, 150 mm × 1 mm internal diameter) that shared a dual-loop autosampler, allowing for one sample to be simultaneously analysed by two systems optimized for different neurotransmitters. One column separated DOPAC and HVA, while the other separated DA. Two solvent delivery pumps (LC110) were used to circulate the respective mobile phases (DOPAC and HVA: 50 mM citric acid, 50 mM phosphoric acid, 8 mM NaCl, 0.1 mM EDTA, 6.9 mM 1-octanesulfonic acid, 10% methanol, pH 3.25; DA: 50 mM phosphoric acid, 8 mM NaCl, 0.1 mM EDTA, 10.4 mM 1-octane sulphonic acid, 20% methanol, pH 6.0) at a flow rate of 50 μL/min. An Antec in-line degassing unit was used to remove air. Samples (10 μl) were injected onto the columns via an autosampler (AS 110) with a cooling tray set at 4°C. Antec DECADE II electrochemical detectors were used comprising Antec micro VT03 cells employing a high density, glassy carbon working electrode (+0.59 V for DOPAC and HVA; +0.3 V for DA) combined with an Antec ISAAC reference electrode. The electrode signal was integrated using Antec’s CLARITY data acquisition system. Individual stock solutions of DA, DOPAC and HVA (1.0 mM) were prepared by their dissolution in a mixture of equal quantities of deionized water and 0.1 M perchloric acid and stored at 4°C. A working solution containing all the transmitters and metabolites was prepared daily by dilution in aCSF.
Attentional set-shifting task (ASST)
This version of the ASST was adapted from previously described protocols (Birrell and Brown 2000; Popik and Nikiforuk 2015), and was performed over a four-day period. Rats were food restricted to 90% of free-feeding weight during the week before testing and stabilized at 90% of free-feeding weight during testing.
Apparatus
The apparatus is a modified version of an arena previous described (Birrell and Brown 2000). Briefly, the apparatus was a customized clear Plexiglas arena (70 × 40 × 18 cm (L × W × H); K and J Fabrications, Aberdeen, MD, USA) with an opaque Plexiglas panel dividing one third of the cage into two equally sized choice areas. The choice areas are separated from the start box via a removable opaque Plexiglas panel. Water was provided for rats in the start box.
Odor/Digging exemplars
Rats dug in porcelain, 3-ounce ramekin bowls (Norpro, Everett, WA, USA). Blotting paper (Maiko, Kyoto, Japan) was attached to the inner rim of the bowls with adhesive tape. The rewards used in this protocol were fresh Honey Nut Cheerio® halves (General Mills, Minneapolis, MN, USA). Cheerios® were stored in their original bag and held in resealable, plastic storage containers. The supply of Cheerios® was replaced every two weeks as digging behavior greatly decreased when the Cheerio® rewards became stale. Scented oils (Bakto Flavors, North Brunswick, NJ, USA) were applied to the blotting paper. Digging media consisted of small materials used to cover rewards in the bowls. Bowls were filled with the designated medium and blotting paper was affixed to each bowl’s interior rim prior to the beginning of each attentional set-shifting session, minimizing inter-stage breaks as much as possible. Following the conclusion of any habituation, training or testing, blotting paper was removed and bowls were cleaned thoroughly.
Pre-habituation
Rats were given a bowl of corn cob bedding with three buried rewards in their home cage the day before habituation. This ensured that rats were familiar with digging for and eating rewards before testing began.
Habituation (Day 1)
Habituation was performed one week following the start of food restriction. Rats were allowed to acclimate to the testing room for one hour. An unscented bowl baited with three rewards was placed in the home cage and the rat was allowed to eat all rewards from the bowl. Next, an unscented bowl with one reward was placed in the home cage. Following retrieval, the bowl was re-baited with a reward and with increasing amounts of digging medium (corn cob bedding). This process was repeated six times in the home cage allowing the rat to eat the reward each trial. These steps were repeated in the testing apparatus, beginning with a baited bowl with no digging media present in either choice area and ending with deeply buried rewards. After rats recovered buried rewards in each choice area they were ushered back into the starting box by gently rattling the removable divider. If a rat failed to complete habituation criterion in its first day, re-habituation was performed for up to two extra days.
Training (Day 2)
On the day following habituation, rats were trained on a series of two simple discriminations based on an odor cue (vanilla/banana) or a digging medium cue (black tea/sphagnum moss) to a criterion of six consecutive correct trials. The digging medium for the odor discrimination training consisted of home cage corn cob bedding. The bowl marked with the ‘positive’ cue (i.e. vanilla+, black tea+) always contained a reward, while the bowl marked with the other cue (banana−, sphagnum moss−) never contained a reward. The same discriminations were presented to rats in the same order and were not used again during testing. The negative bowl was masked by the addition of a small amount of fresh Honey Nut Cheerio® powder before each third trial. Placement of positive and negative bowls was balanced across the two choice areas during training. Digging media and odor cues are listed in Table 1.
Table 1.
Digging media and odor cues
| Phase | Odors | Media | Media Suppliers |
|---|---|---|---|
| Training | Vanilla/Banana | Black Tea/Long Fibered Sphagnum Moss | Positively Tea Company, Sellinsgrove, PA, USA/Mosser Lee Company, Millston, WI, USA |
| SD | Rum/Irish Crème | Home Bedding (corn cob) | Shepherd Specialty Papers, Milford, NJ, USA |
| CD | Rum/Irish Crème | Living World® Pine Shavings/Carefresh® Animal Bedding | Rolf C. Hagen, Mansfield, MA, USA/Healthy Pet, Ferndale, WA, USA |
| CDR | Rum/Irish Crème | Living World® Pine Shavings/Carefresh® Animal Bedding | Rolf C. Hagen, Mansfield, MA, USA/Healthy Pet, Ferndale, WA, USA |
| ID | Almond/Lemon | Blue Shredded Confetti/Pure Comfort® Animal Bedding | Amscan/Oxbow Animal Health, Omaha, NE, USA |
| IDR | Almond/Lemon | Blue Shredded Confetti/Pure Comfort® Animal Bedding | Amscan/Oxbow Animal Health, Omaha, NE, USA |
| ED | Orange/Anise | Red Gravel/White Gravel | PetSmart, Phoenix, AZ, USA |
| EDR | Orange/Anise | Red Gravel/White Gravel | PetSmart, Phoenix, AZ, USA |
Bold represents correct exemplar for each stage
A trial was initiated by raising the starting divider giving the rat access to both choice areas. The first four trials in each discrimination stage were discovery trials where rats were allowed to dig in both choice areas without recording response accuracy. Discovery trials ended when they consumed the reward and explored the unbaited bowl. Trials continued beyond the discovery trials until a criterion of six consecutive correct trials was reached. Latency to dig and response accuracy were recorded for each trial following the discovery phase. An incorrect dig signaled the end of the trial- the rewarded bowl was removed and rats were ushered back to the starting box. Rats were allowed to climb over and investigate the bowls; a dig was only scored if the rat broke the surface by purposefully displacing digging medium with either its paws or nose. Occasionally, rats would mark the bowls with urine, possibly in attempts to use a mediating behavior to identify reward- containing bowls. In this situation, digging media for both bowls were discarded and replaced with fresh media before continuing the session. Once again, if a rat failed to complete training in its first day, re-training was performed for up to two extra days before that rat was removed from the study.
Testing (Day 3)
Rats performed a single attentional set shifting test the day following training. Testing was conducted in the same manner as training. Briefly, trials were initiated by the raising of the divider, the first four trials being unscored discovery trials, and a criterion of six consecutive correct responses was necessary to pass a stage. Rats were dosed with vehicle, tolcapone, LIBD-1, or LIBD-3 and returned to their home cage for one hour until testing.
Rats performed a series of seven discriminations in the following order: simple discrimination (SD), compound discrimination (CD), compound reversal (CDR), intradimensional discrimination (ID), intradimensional reversal (IDR), extradimensional discrimination (ED) and extradimensional reversal (EDR) (Table 1). The simple discrimination (SD) consisted of bowls differing by one of two dimensions (odor or digging medium). The compound discrimination stage (CD) introduced the second irrelevant dimension while the correct and incorrect exemplars were the same. The relevant dimension remained consistent during the compound discrimination reversal stage (CDR) but the correct and incorrect exemplars were swapped so that the previously rewarded exemplar was no longer rewarded and vice versa. Following completion of the CDR the relevant dimension remained consistent in the intradimensional discrimination stage (ID) while novel sets of odor and digging medium exemplars were used. As with the CDR stage, in the IDR stage the rewarded and non-rewarded exemplars were swapped. The previously relevant and irrelevant dimensions were swapped during the extradimensional discrimination (ED) with novel sets of odor and digging medium exemplars. Finally, the EDR stage, where correct and incorrect exemplars were swapped, was initiated following the completion of the ED. Once a rat reached a criterion of 6 correct trials in a row during the EDR the task was terminated and rats were returned to their home cages.
Data analyses
In our CSF neurochemical experiments, we compared drug-treated rats to vehicle-rats using either one-way ANOVAs or t-tests. For ANOVAs significant main effects were followed up with post hoc Tukey’s tests. Data are represented by the mean value as a percentage of the vehicle-treated group. For the microdialysis experiment, data were log transformed and analyzed by ANCOVA with log(baseline) as a covariate. Baseline was defined as the geometric mean of the four pretreatment samples. Comparisons to the vehicle-treated group were by the multiple t-test. Data are presented as the mean (% of vehicle-treated) ± SEM. The ASST data were analyzed using two-way ANOVA with repeated measures. Drug treatment was the between-subjects variable and ASST stage was the within-subjects variable. Significant main effects were further probed with post hoc Tukey’s tests to determine significant pairwise comparisons. ASST data are presented as the mean ± SEM.
Results
Novel, non-nitrocatechol compounds are potent COMT inhibitors
In these experiments, we tested the in vivo effects of three novel compounds that are potent COMT inhibitors (Figure 1). LIBD-1 [compound 28 in (Buchler et al. 2018)] and LIBD-3 [compound 38 in (Ernst et al., 2019) have been previously described in depth . Briefly, LIBD-1 is a COMT inhibitor with over 300-fold selectivity for MB-COMT over S-COMT. LIBD-2 is a potent nonselective inhibitor with poor brain penetration and LIBD-3 is a highly MB-COMT preferring inhibitor (>3000-fold) with good bioavailability. These novel compounds are all roughly an order of magnitude less potent than the nonselective nitrocatechol COMT inhibitor tolcapone.
COMT inhibitors modulate HVA and DOPAC concentrations in CSF
To determine if our novel COMT inhibitors modulate COMT activity in vivo, we measured the concentrations of the dopamine metabolites HVA and DOPAC in cerebrospinal fluid (CSF) following compound dosing. Previous research has shown that potent brain-penetrant COMT inhibitors reliably decrease HVA concentrations while increasing DOPAC concentrations in CSF (Robinson et al. 2012). Here, we benchmarked our COMT inhibitors to the prototypical COMT inhibitor tolcapone (Table 2). Tolcapone (15 mg/kg) significantly decreased HVA (1 h: t9 = 8.730, p < 0.0001; 4 h: t13 = 13.090, p < 0.0001) and increased DOPAC (1 h: t9 = 8.528, p < 0.0001; 4 h: t13 = 9.866, p < 0.0001) at both time points sampled (1 and 4 h). All doses of our MB-COMT selective inhibitors LIBD-1 and LIBD-3 also significantly decreased HVA (LIBD-1 1 h: F3,30 = 26.450, p < 0.0001; 4 h: F3,33 = 29.470, p < 0.0001; LIBD-3 1 h: F3,32 = 42.700, p < 0.0001; 4 h: F2,19 = 126.200, p < 0.0001) and increased DOPAC (LIBD-1 1 h: F3,30 = 46.850, p < 0.0001; 4 h: F3,33 = 42.670, p < 0.0001; LIBD-3 1 h: F3,32 = 32.730, p < 0.0001; 4 h: F2,19 = 41.510, p < 0.0001) at both time points tested. The highest doses of LIBD-1 and LIBD-3 were unable to modulate the DA metabolites to the same degree as tolcapone despite reaching concentrations that would be expected to completely block MB-COMT, suggesting some role for S-COMT in regulating DA metabolism in rats. Previous research has shown sexually dimorphic effects related to COMT activity (Laatikainen et al. 2013; Sannino et al. 2015), so we tested both tolcapone (HVA: t10 = 9.505, p < 0.0001; DOPAC: t10 = 6.307, p < 0.0001) and LIBD-1 (HVA: t11 = 6.696, p < 0.0001; DOPAC: t11 = 5.478, p = 0.0003) in female rats and found similar changes in DA metabolites. In contrast, LIBD-2, a potent, isoform nonselective COMT inhibitor, with extremely low brain penetrance, does not significantly alter CSF DA metabolites (HVA: t13 = 0.1482, p = 0.8845; DOPAC: t13 = 1.793, p = 0.0962), indicating that our biomarker assay is a sensitive measure of in vivo target engagement.
Table 2.
Effects of COMT inhibitors on DA metabolite concentrations in CSF
| Drug Concentration | |||||||
|---|---|---|---|---|---|---|---|
| Compound | Dose | Sex | Timepoint (h) | Free Plasma (nM) | CSF (nM) | CSF HVA (%vehicle) | CSF DOPAC (%vehicle) |
| Tolcapone | 15 mg/kg | Male | 1 | 35 | BLQ | 7**** | 291**** |
| 15 mg/kg | Male | 4 | 4 | BLQ | 28**** | 304**** | |
| 15 mg/kg | Female | 4 | 10 | BLQ | 31**** | 233**** | |
| LIBD-1 | 10 mg/kg | Male | 1 | 69 | 132 | 63**** | 234**** |
| 30 mg/kg | Male | 1 | 98 | 352 | 49**** | 229**** | |
| 100 mg/kg | Male | 1 | 211 | 1220 | 36**** | 273**** | |
| 10 mg/kg | Male | 4 | 35 | 157 | 72**** | 140**** | |
| 30 mg/kg | Male | 4 | 64 | 176 | 66**** | 210**** | |
| 100 mg/kg | Male | 4 | 175 | 423 | 49**** | 251**** | |
| 100 mg/kg | Female | 4 | 142 | 349 | 42**** | 238*** | |
| LIBD-2 | 100 mg/kg | Male | 4 | 41 | BLQ | 101 | 119 |
| LIBD-3 | 10 mg/kg | Male | 1 | N/A | N/A | 59**** | 201**** |
| 30 mg/kg | Male | 1 | 145 | 84 | 39**** | 197**** | |
| 100 mg/kg | Male | 1 | 496 | 328 | 35**** | 276**** | |
| 30 mg/kg | Male | 4 | 46 | 15 | 48**** | 182**** | |
| 100 mg/kg | Male | 4 | 290 | 78 | 22**** | 207**** | |
BLQ = below limit of quantitation
Limits of quantitation: Tolcapone (74 nM in CSF and 144 nM in brain). LIBD-1 (66 nM in brain and CSF).
LIBD-2 (58 nM in CSF and 233 nM in brain).
N/A = no values
p <0.001 and
p <0.0001 compared to the vehicle-treated group at the same time point
COMT inhibitors modulate HVA and DOPAC concentrations in the frontal cortex
The CSF biomarker assay is a good measure of target engagement, but we also wanted to confirm the activity of our compounds directly in the brain. Therefore, we also tested the effects of our novel COMT inhibitors on DA, HVA, and DOPAC concentrations in extracellular fluid (ECF) sampled from the cortex by microdialysis (Figure 2). As previously reported, none of the COMT inhibitors altered extracellular DA concentrations (Acquas et al. 1992; Lapish et al. 2009; Tunbridge et al. 2004). Here, both LIBD-1 and LIBD-3 significantly decrease HVA and increase DOPAC as expected (Figure 2b–c). As seen in the CSF experiment, LIBD-1 effects do not quite reach the same magnitude as our positive control tolcapone. In contrast, the effects of LIBD-3 were not significantly different from tolcapone. These microdialysis results support the effects we see in CSF and further demonstate that our MB-selective compounds may have similar utility when compared to tolcapone.
Figure 2. Effects of COMT inhibitors on DA and metabolites in the frontal cortex measured by microdialysis.
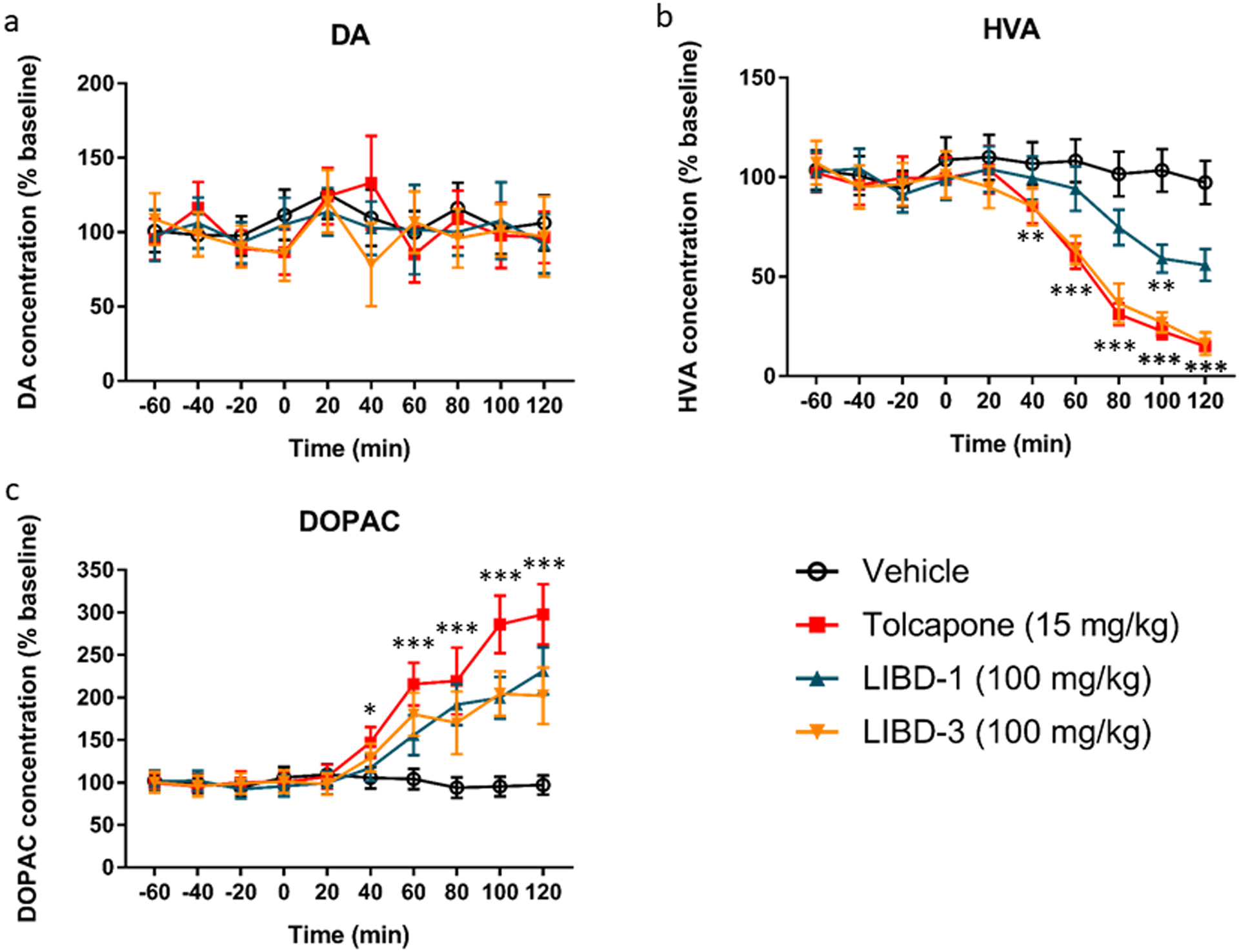
a DA concentration. None of the COMT inhibitors altered extracellular DA concentration. b HVA concentration. All of the COMT inhibitors decreased the extracellular HVA concentration. Tolcapone and LIBD-3 significantly decreased HVA for the 40–120-min time points while LIBD-1 significantly decreased HVA at the 100-min time point. c DOPAC concentration. All of the COMT inhibitors increased the extracellular DOPAC concentration. Tolcapone significantly increased DOPAC for the 40–120-min time points while LIBD-1 and LIBD-3 significantly increased DOPAC for the 60–120-min time points. n = 8 for vehicle, 7 for tolcapone, 8 for LIBD-1 and 8 for LIBD-3. Drug or vehicle was administered at t = 0. *p <0.05, **p <0.01, ***p < 0.001 compared to the vehicle-treated group at that time point.
LIBD-1 improves cognitive flexibility in the set shifting assay
Both LIBD-1 and LIBD-3 demonstrated good in vivo COMT inhibitor activity. The next step was to test whether LIBD-1 and LIBD-3 would be able to modulate cognitive function, measured as performance in the ASST. The rat digging version of the ASST is a validated assay for measuring cognitive flexibility and the task is dependent on an intact frontal cortex (Birrell and Brown 2000). Moreover, COMT inhibition has been shown to improve performance during the extradimensional shift (ED) stage of the task (Tunbridge et al. 2004), analogous to an ID/ED shift task in monkeys that has been shown to be a dopamine-mediated prefrontal cognitive task (Dias et al. 1996). To establish the validity of our in-house protocol, we first measured the effects of tolcapone (15 mg/kg) as a positive control. As expected, there were significant differences in the number of trials to criterion between the stages (F6,126 = 4.729, p = 0.0002) and tolcapone improved performance (F1,21 = 5.075, p = 0.0351); Figure 3a). Post hoc analyses revealed that the ED stage required more trials to criterion than the compound discrimination stage (CD; p = 0.0104) and the intradimensional shift stage (ID; p = 0.0003) in vehicle-treated rats. Tolcapone improved ED stage performance (p = 0.0177) without significantly altering performance on any of the other stages.
Figure 3. Effects of COMT inhibitors on behavior in the ASST.

a Tolcapone (n = 12/vehicle group and n = 11/tolcapone group b LIBD-1 (n = 24/vehicle group, n = 15/LIBD-1 30 mg/kg group, and n = 14/LIBD-1 100 mg/kg group). c LIBD-3 (n = 21/vehicle group, n = 14/LIBD-3 30 mg/kg group, and n = 10/ LIBD-3 100 mg/kg group). d Performance of select groups in the ED stage across independent experiments. These data were not analyzed together and are presented here only for visual comparison. The vehicle-treated group represents a pooled sample from the LIBD-1 and tolcapone cohorts. *p <0.05 and **p <0.01 compared to the vehicle-treated group in the stage. Stage abbreviations: SD = simple discrimination; CD= compound discrimination; CDr = compound discrimination reversal; ID = intradimensional discrimination; IDr = intradimensional discrimination reversal; ED = extradimensional discrimination; EDr = extradimensional discrimination reversal.
Next, we tested the effects of LIBD-1 (30 and 100 mg/kg) in the ASST. Again, there was a significant difference in the number of trials to criterion between stages (F6,300 = 31.8, p < 0.0001). In the vehicle-treated rats, there was a significant difference in trials to criterion between the ED stage and the CD (p < 0.0001) and ID (p < 0.0001) stages. Like tolcapone, LIBD-1 improved performance in the ASST compared to vehicle (F2,50 = 7.52, p = 0.0014; Figure 3b). LIBD-1 (100 mg/kg) significantly improved performance in the ED stage of the task (p = 0.0087). Additionally, the 30 mg/kg group performed worse than the vehicle group on the compound discrimination reversal (CDr; p = 0.0123) and worse than both the vehicle and 100 mg/kg groups on the intradimensional shift reversal stage (IDr; p = 0.0296 and p = 0.0142, respectively). These data indicate that LIBD-1 modulates cognitive function and the 100 mg/kg dose produces effects similar to the FDA-approved COMT inhibitor tolcapone.
Finally, we tested the effects of LIBD-3 in the ASST (Figure 3c). There was a significant difference in trials to criterion between the stages (F6,252 = 12.83, p = 0.0001). The ED stage required more trials than the CD and ID stages, although only the differences with the CD stage reached the significance threshold (p = 0.0019 and p = 0.0674. respectively). Unlike both tolcapone and LIBD-1, LIBD-3 did not affect performance (F2,42 = 0.4392, p = 0.6475).
Discussion
Research suggests that COMT inhibition is a viable strategy for improving executive function, with potential utility across many clinical indications (Apud et al. 2007; Gallagher et al. 2015). Data from patients with Parkinson’s disease indicates that the cognitive benefits of COMT inhibition require a brain-penetrant compound (Gasparini et al. 1997). Of the FDA-approved COMT inhibitors, tolcapone has marginal brain penetration. Unfortunately, long-term use of tolcapone is associated with potentially fatal liver toxicity in some patients, therefore, novel, safe, and brain-penetrant COMT inhibitors are needed to explore the utility of the mechanism for cognitive enhancement. Here we describe the in vivo activity of novel, non-nitrocatechol, brain-penetrant COMT inhibitors in rats and identify our current lead drug candidate.
In the CNS, COMT inhibitors are known to alter the concentrations of the DA metabolites HVA and DOPAC. Specifically, COMT inhibition decreases HVA and increases DOPAC concentrations. Previous research has used post-administration measurement of CSF HVA and DOPAC as in vivo markers of COMT inhibition (Robinson et al. 2012). We found that two of our novel compounds, LIBD-1 and LIBD-3, produced sustained modulation of HVA and DOPAC concentrations in CSF. Additionally, we showed that the changes in CSF HVA and DOPAC were due to central COMT inhibition by testing the effects of a third compound, LIBD-2. LIBD-2 is extremely potent in vitro, but its brain-penetrance is negligible and it did not significantly alter central DA metabolism.
While CSF DA metabolites are translational biomarkers for central COMT inhibition, we believe the ultimate target for the use of COMT inhibitors as cognitive enhancers is the frontal cortex. To determine if our novel COMT inhibitors could modulate COMT function directly within the cortex, we conducted microdialysis experiments with probes sampling from the frontal cortex. We found that both LIBD-1 and LIBD-3 significantly altered HVA and DOPAC concentrations within the cortex similar to the effect produced by tolcapone. Our results in primary motor cortex are similar to previously reported findings for tolcapone in the medial prefrontal cortex (Tunbridge et al. 2004) and show that non-nitrocatechol COMT inhibitors produce relevant changes in DA metabolism in the cortex.
The experiments discussed so far showed that our novel COMT inhibitors produce qualitatively similar effects compared to the COMT inhibitor tolcapone. We also confirmed that central measures of COMT inhibition require a brain-penetrant inhibitor. The final characteristic we wanted to test was the ability to modulate behavior in an assay of cognitive function. In the next series of experiments, we showed that LIBD-1 produced behavioral changes at relevant central exposure levels. LIBD-1 improves cognitive flexibility in the ED phase of the task comparable to the effects of tolcapone. Interestingly, the lower dose of LIBD-1 increased the number of trials required to complete the CDr and ID stages. Although it is not clear what caused this change in performance, one hypothesis generated from the clinical literature suggests that tolcapone can impair performance by increasing cognitive control at times when more automatic responding is advantageous (Cameron et al. 2018). This may be a particular problem for healthy subjects, like the rats in our experiments, compared to those with cognitive impairment. Taken together, these results indicate that our novel non-nitrocatechol COMT inhibitor possesses many of the in vivo properties required to test its utility as a cognitive enhancer in a clinical trial.
LIBD-3 produced similar, and arguably superior, results in the neurochemical assays compared to LIBD-1, but did not significantly improve cognitive flexibility in the ASST. At this time, it is not clear what is responsible for the discrepancy in performance. In a cell-based assay, LIBD-3 produces significant cellular toxicity and maximum changes in DOPAC concentrations much lower than LIBD-1 and tolcapone (Zhang et al. 2019). These data suggest that there may be compound-specific effects that could impede performance in the ASST, but are not evident given the limited temporal and spatial resolution of our in vivo neurochemical measures.
A large body of evidence suggests that COMT inhibition improves cognitive function across multiple clinical domains (Gasparini et al. 1997; Grant et al. 2013; Muller and Investigators 2014). Although there are multiple FDA-approved COMT inhibitors, none of them are suitable for long-term use as cognitive enhancers. Entacapone and opicapone (approved in Europe) do not cross the blood-brain barrier. Tolcapone, which is brain-penetrant, has a black-box warning due to potentially fatal liver toxicity. The toxicity associated with tolcapone appears to be compound-specific, as no other marketed COMT inhibitor shares this liability (Haasio 2010). The lack of an approved, brain-penetrant, and safe COMT inhibitor has prevented the full exploration of the utility of the mechanism for cognitive disorders. Because our compounds are less potent than tolcapone, we believe their key potential advantage would be an improved safety profile. Empirical evidence of larger safety margins is a prerequisite for continued development of these novel compounds. To this end, we are currently testing LIBD-1 in a battery of preclinical toxicological assays.
In contrast with tolcapone, which is a nonselective inhibitor, two of our novel COMT inhibitors are selective for MB-COMT compared with S-COMT. In the rat brain, MB-COMT and S-COMT protein levels are approximately equal (Tenhunen et al. 1993). We believe the lack of S-COMT activity of our inhibitors contributes to the lower in vivo potency in the rat when compared to tolcapone. In clinical usage, MB-COMT selectivity may actually be an advantage for our compounds. In humans, and nonhuman primates, roughly 85% of total brain COMT is the MB-COMT form (Chen et al. 2004). Moreover, given the enzyme kinetics and endogenous dopamine concentrations in the brain, MB-COMT is most likely responsible for more than 85% of total brain COMT activity in humans (Lotta et al. 1995; Tammimaki et al. 2010).
An additional consideration when evaluating COMT function is the documented sex differences in endogenous COMT activity and response to COMT inhibitors. In both preclinical and clinical studies, modulation of COMT activity produces different effects in males and females (Lee and Prescott 2014; McCane et al. 2018; Papaleo et al. 2015; Risbrough et al. 2014; Sannino et al. 2015; Sannino et al. 2017). In these series of experiments, we showed that LIBD-1 produced the expected change in CSF DA metabolites in female rats, but we did not test our compounds in a behavioral assay in female rats. Our goal with these experiments was to benchmark our novel compounds to tolcapone. We believe more comprehensive pharmacodynamic evaluation of these compounds should be done in primate behavioral models, where total COMT enzyme activity and relative MB-COMT levels are more similar to human biology.
A critical outstanding question concerning the utility of COMT inhibitors is which clinical indication is most likely to be improved by treatment. Small, short-duration pilot studies have suggested potential efficacy in multiple disorders (Gasparini et al. 1997; Grant et al. 2013; Muller and Investigators 2014). In contrast, other studies have shown that in certain circumstances COMT inhibition has no effect on cognitive function and can actually impair cognition (Cameron et al. 2018; Valomon et al. 2018). However, there is a convergence of data suggesting that disorders characterized by impaired executive function and/or impulse control, including traumatic brain injury (TBI), behavioral variant frontotemporal dementia (bvFTD), and ADHD may be good candidates for a COMT inhibitor clinical trial. To be clear, we do not believe that a COMT inhibitor strategy would be disease-modifying in these indications. However, there are currently no treatments available to significantly improve the chronic symptoms of TBI or bvFTD and there is a still a need for effective ADHD treatments without stimulant effects and abuse liability (Beeldman et al. 2018; Jain and Katic 2016; Wood and Worthington 2017). LIBD-1 has shown significant efficacy in our preclinical models that indicate it may be able to serve as the COMT inhibitor to test these hypotheses.
Acknowledgments
The authors thank Michael Poslusney and Noelle White for technical assistance, Richard Brammer for statistical analysis of the microdialysis data, Elizabeth Tunbridge for advice on the ASST assay, and Daniel R. Weinberger for constructive comments on a draft of this manuscript.
Funding and Disclosure
This work was funded by US National Institutes of Health grant R01MH107126 and The Lieber Institute for Brain Development. IPB and JCB are inventors on patents that include the novel COMT inhibitors (WO2016123576 and WO2017091818). HLR, RSK, LP, and SCC are employees of RenaSci Ltd. The remaining authors have nothing to disclose.
References
- Acquas E, Carboni E, de Ree RH, Da Prada M, Di Chiara G (1992) Extracellular concentrations of dopamine and metabolites in the rat caudate after oral administration of a novel catechol-O-methyltransferase inhibitor Ro 40–7592. J Neurochem 59: 326–30. [DOI] [PubMed] [Google Scholar]
- Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, Alce G, Iudicello JE, Akbar N, Egan MF, Goldberg TE, Weinberger DR (2007) Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology 32: 1011–20. [DOI] [PubMed] [Google Scholar]
- Apud JA, Weinberger DR (2007) Treatment of cognitive deficits associated with schizophrenia: potential role of catechol-O-methyltransferase inhibitors. CNS Drugs 21: 535–57. [DOI] [PubMed] [Google Scholar]
- Axelrod J, Tomchick R (1958) Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem 233: 702–5. [PubMed] [Google Scholar]
- Beeldman E, Raaphorst J, Klein Twennaar M, Govaarts R, Pijnenburg YAL, de Haan RJ, de Visser M, Schmand BA (2018) The cognitive profile of behavioural variant FTD and its similarities with ALS: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 89: 995–1002. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ (2000) Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci 20: 4320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchler I, Akuma D, Au VQ, Carr G, de Leon P, DePasquale M, Ernst G, Huang Y, Kimos M, Kolobova A, Poslusney MS, Wei H, Swinnen D, Montel F, Moureau F, Jigorel E, Schulze MS, Wood M, Barrow JC (2018) Optimization of 8-Hydroxyquinolines as Inhibitors of Catechol O-Methyltransferase. J Med Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron IGM, Wallace DL, Al-Zughoul A, Kayser AS, D’Esposito M (2018) Effects of tolcapone and bromocriptine on cognitive stability and flexibility. Psychopharmacology (Berl) 235: 1295–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR (2004) Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 75: 807–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC (1996) Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci 110: 872–86. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR (2001) Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A 98: 6917–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher CL, Bell B, Palotti M, Oh J, Christian BT, Okonkwo O, Sojkova J, Buyan-Dent L, Nickles RJ, Harding SJ, Stone CK, Johnson SC, Holden JE (2015) Anterior cingulate dopamine turnover and behavior change in Parkinson’s disease. Brain Imaging Behav 9: 821–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini M, Fabrizio E, Bonifati V, Meco G (1997) Cognitive improvement during Tolcapone treatment in Parkinson’s disease. J Neural Transm (Vienna) 104: 887–94. [DOI] [PubMed] [Google Scholar]
- Grant JE, Odlaug BL, Chamberlain SR, Hampshire A, Schreiber LR, Kim SW (2013) A proof of concept study of tolcapone for pathological gambling: relationships with COMT genotype and brain activation. Eur Neuropsychopharmacol 23: 1587–96. [DOI] [PubMed] [Google Scholar]
- Gratwicke J, Jahanshahi M, Foltynie T (2015) Parkinson’s disease dementia: a neural networks perspective. Brain 138: 1454–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haasio K (2010) Toxicology and safety of COMT inhibitors. Int Rev Neurobiol 95: 163–89. [DOI] [PubMed] [Google Scholar]
- Hsu WY, Lane HY, Lin CH (2018) Medications Used for Cognitive Enhancement in Patients With Schizophrenia, Bipolar Disorder, Alzheimer’s Disease, and Parkinson’s Disease. Front Psychiatry 9: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R, Katic A (2016) Current and Investigational Medication Delivery Systems for Treating Attention-Deficit/Hyperactivity Disorder. Prim Care Companion CNS Disord 18. [DOI] [PubMed] [Google Scholar]
- Kaenmaki M, Tammimaki A, Myohanen T, Pakarinen K, Amberg C, Karayiorgou M, Gogos JA, Mannisto PT (2010) Quantitative role of COMT in dopamine clearance in the prefrontal cortex of freely moving mice. J Neurochem 114: 1745–55. [DOI] [PubMed] [Google Scholar]
- Keefe RS (2014) Cognition and motivation as treatment targets in schizophrenia. JAMA Psychiatry 71: 987–8. [DOI] [PubMed] [Google Scholar]
- Laatikainen LM, Sharp T, Harrison PJ, Tunbridge EM (2013) Sexually dimorphic effects of catechol-O-methyltransferase (COMT) inhibition on dopamine metabolism in multiple brain regions. PLoS One 8: e61839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane CA, Hardy J, Schott JM (2018) Alzheimer’s disease. Eur J Neurol 25: 59–70. [DOI] [PubMed] [Google Scholar]
- Lapish CC, Ahn S, Evangelista LM, So K, Seamans JK, Phillips AG (2009) Tolcapone enhances food-evoked dopamine efflux and executive memory processes mediated by the rat prefrontal cortex. Psychopharmacology (Berl) 202: 521–30. [DOI] [PubMed] [Google Scholar]
- Lee LO, Prescott CA (2014) Association of the catechol-O-methyltransferase val158met polymorphism and anxiety-related traits: a meta-analysis. Psychiatr Genet 24: 52–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A (2001) Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol 432: 119–36. [DOI] [PubMed] [Google Scholar]
- Limousin P, Pollak P, Gervason-Tournier CL, Hommel M, Perret JE (1993) Ro 40–7592, a COMT inhibitor, plus levodopa in Parkinson’s disease. Lancet 341: 1605. [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J (1995) Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 34: 4202–10. [DOI] [PubMed] [Google Scholar]
- Mahat MY, Fakrudeen Ali Ahamed N, Chandrasekaran S, Rajagopal S, Narayanan S, Surendran N (2012) An improved method of transcutaneous cisterna magna puncture for cerebrospinal fluid sampling in rats. J Neurosci Methods 211: 272–9. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D (2002) A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry 159: 652–4. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Akil M, Lipska BK, Hyde TM, Herman MM, Kleinman JE, Weinberger DR (2003) Catechol O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience 116: 127–37. [DOI] [PubMed] [Google Scholar]
- McCane AM, DeLory MJ, Timm MM, Janetsian-Fritz SS, Lapish CC, Czachowski CL (2018) Differential COMT expression and behavioral effects of COMT inhibition in male and female Wistar and alcohol preferring rats. Alcohol 67: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiser J, Weindl D, Hiller K (2013) Complexity of dopamine metabolism. Cell Commun Signal 11: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskowiak KW, Kjaerstad HL, Stottrup MM, Svendsen AM, Demant KM, Hoeffding LK, Werge TM, Burdick KE, Domschke K, Carvalho AF, Vieta E, Vinberg M, Kessing LV, Siebner HR, Macoveanu J (2017) The catechol-O-methyltransferase (COMT) Val158Met genotype modulates working memory-related dorsolateral prefrontal response and performance in bipolar disorder. Bipolar Disord 19: 214–224. [DOI] [PubMed] [Google Scholar]
- Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT (2002) Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci 22: 389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T, Investigators TS (2014) Tolcapone addition improves Parkinson’s disease associated nonmotor symptoms. Ther Adv Neurol Disord 7: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, Schmand B (2005) Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 65: 1239–45. [DOI] [PubMed] [Google Scholar]
- Nieoullon A (2002) Dopamine and the regulation of cognition and attention. Prog Neurobiol 67: 53–83. [DOI] [PubMed] [Google Scholar]
- Nirogi R, Kandikere V, Mudigonda K, Bhyrapuneni G, Muddana N, Saralaya R, Benade V (2009) A simple and rapid method to collect the cerebrospinal fluid of rats and its application for the assessment of drug penetration into the central nervous system. J Neurosci Methods 178: 116–9. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Lipska BK, Weinberger DR (2012) Mouse models of genetic effects on cognition: relevance to schizophrenia. Neuropharmacology 62: 1204–20. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Sannino S, Piras F, Spalletta G (2015) Sex-dichotomous effects of functional COMT genetic variations on cognitive functions disappear after menopause in both health and schizophrenia. Eur Neuropsychopharmacol 25: 2349–63. [DOI] [PubMed] [Google Scholar]
- Paterson IA, Davis BA, Durden DA, Juorio AV, Yu PH, Ivy G, Milgram W, Mendonca A, Wu P, Boulton AA (1995) Inhibition of MAO-B by (−)-deprenyl alters dopamine metabolism in the macaque (Macaca facicularis) brain. Neurochem Res 20: 1503–10. [DOI] [PubMed] [Google Scholar]
- Popik P, Nikiforuk A (2015) Attentional Set-Shifting Paradigm in the Rat. Curr Protoc Neurosci 72: 9 51 1–13. [DOI] [PubMed] [Google Scholar]
- Reches A, Fahn S (1984) Catechol-O-methyltransferase and Parkinson’s disease. Adv Neurol 40: 171–9. [PubMed] [Google Scholar]
- Risbrough V, Ji B, Hauger R, Zhou X (2014) Generation and characterization of humanized mice carrying COMT158 Met/Val alleles. Neuropsychopharmacology 39: 1823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RG, Smith SM, Wolkenberg SE, Kandebo M, Yao L, Gibson CR, Harrison ST, Polsky-Fisher S, Barrow JC, Manley PJ, Mulhearn JJ, Nanda KK, Schubert JW, Trotter BW, Zhao Z, Sanders JM, Smith RF, McLoughlin D, Sharma S, Hall DL, Walker TL, Kershner JL, Bhandari N, Hutson PH, Sachs NA (2012) Characterization of non-nitrocatechol pan and isoform specific catechol-O-methyltransferase inhibitors and substrates. ACS Chem Neurosci 3: 129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannino S, Gozzi A, Cerasa A, Piras F, Scheggia D, Manago F, Damiano M, Galbusera A, Erickson LC, De Pietri Tonelli D, Bifone A, Tsaftaris SA, Caltagirone C, Weinberger DR, Spalletta G, Papaleo F (2015) COMT Genetic Reduction Produces Sexually Divergent Effects on Cortical Anatomy and Working Memory in Mice and Humans. Cereb Cortex 25: 2529–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannino S, Padula MC, Manago F, Schaer M, Schneider M, Armando M, Scariati E, Sloan-Bena F, Mereu M, Pontillo M, Vicari S, Contarini G, Chiabrera C, Pagani M, Gozzi A, Eliez S, Papaleo F (2017) Adolescence is the starting point of sex-dichotomous COMT genetic effects. Transl Psychiatry 7: e1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammimaki A, Kaenmaki M, Kambur O, Kulesskaya N, Keisala T, Karvonen E, Garcia-Horsman JA, Rauvala H, Mannisto PT (2010) Effect of S-COMT deficiency on behavior and extracellular brain dopamine concentrations in mice. Psychopharmacology (Berl) 211: 389–401. [DOI] [PubMed] [Google Scholar]
- Tenhunen J, Salminen M, Jalanko A, Ukkonen S, Ulmanen I (1993) Structure of the rat catechol-O-methyltransferase gene: separate promoters are used to produce mRNAs for soluble and membrane-bound forms of the enzyme. DNA Cell Biol 12: 253–63. [DOI] [PubMed] [Google Scholar]
- Tenhunen J, Salminen M, Lundstrom K, Kiviluoto T, Savolainen R, Ulmanen I (1994) Genomic organization of the human catechol O-methyltransferase gene and its expression from two distinct promoters. Eur J Biochem 223: 1049–59. [DOI] [PubMed] [Google Scholar]
- Tenhunen J, Ulmanen I (1993) Production of rat soluble and membrane-bound catechol O-methyltransferase forms from bifunctional mRNAs. Biochem J 296 ( Pt 3): 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ (2004) Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci 24: 5331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valomon A, Holst SC, Borrello A, Weigend S, Muller T, Berger W, Sommerauer M, Baumann CR, Landolt HP (2018) Effects of COMT genotype and tolcapone on lapses of sustained attention after sleep deprivation in healthy young men. Neuropsychopharmacology 43: 1599–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanneveich M, Jacqmin-Gadda H, Dartigues JF, Joly P (2018) Projections of health indicators for chronic disease under a semi-Markov assumption. Theor Popul Biol 119: 83–90. [DOI] [PubMed] [Google Scholar]
- Watkins P (2000) COMT inhibitors and liver toxicity. Neurology 55: S51–2; discussion S53–6. [PubMed] [Google Scholar]
- Wood RL, Worthington A (2017) Neurobehavioral Abnormalities Associated with Executive Dysfunction after Traumatic Brain Injury. Front Behav Neurosci 11: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Mannisto PT (2007) Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J Neurosci 27: 10196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Buchler IP, DePasquale M, Wormald M, Liao G, Wei H, Barrow JC, Carr GV (2019) Development of a PC12 Cell Based Assay for Screening Catechol-O-methyltransferase Inhibitors. ACS Chem Neurosci 10: 4221–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Feng S, Nie K, Zhao X, Gan R, Wang L, Zhao J, Tang H, Gao L, Zhu R, Wang L, Zhang Y (2016) Catechol-O-methyltransferase Val158Met polymorphism influences prefrontal executive function in early Parkinson’s disease. J Neurol Sci 369: 347–353. [DOI] [PubMed] [Google Scholar]


