Abstract
Cell-free secretory products (secretome) of human induced pluripotent stem cells (iPSCs) have been shown to attenuate tissue injury and facilitate repair and recovery. To examine whether iPSC secretome facilitates mechanically induced compensatory responses following unilateral pneumonectomy (PNX), litter-matched young adult female hounds underwent right PNX (removing 55%–58% of lung units), followed by inhalational delivery of either the nebulized-conditioned media containing induced pluripotent stem cell secretome (iPSC CM) or control cell-free media (CFM); inhalation was repeated every 5 days for 10 treatments. Lung function was measured under anesthesia pre-PNX and 10 days after the last treatment (8 wk post-PNX); detailed quantitative analysis of lung ultrastructure was performed postmortem. Pre-PNX lung function was similar between groups. Compared with CFM control, treatment with iPSC CM attenuated the post-PNX decline in lung diffusing capacity for carbon monoxide and membrane diffusing capacity, accompanied by a 24% larger postmortem lobar volume and distal air spaces. Alveolar double-capillary profiles were 39% more prevalent consistent with enhanced intussusceptive angiogenesis. Frequency distribution of the harmonic mean thickness of alveolar blood-gas barrier shifted toward the lowest values, whereas alveolar septal tissue volume and arithmetic septal thickness were similar, indicating septal remodeling and reduced diffusive resistance of the blood-gas barrier. Thus, repetitive inhalational delivery of iPSC secretome enhanced post-PNX alveolar angiogenesis and septal remodeling that are associated with improved gas exchange compensation. Results highlight the plasticity of the remaining lung units following major loss of lung mass that are responsive to broad-based modulation provided by the iPSC secretome.
NEW & NOTEWORTHY To examine whether the secreted products of human induced pluripotent stem cells (iPSCs) facilitate innate adaptive responses following loss of lung tissue, adult dogs underwent surgical removal of one lung, then received repeated administration of iPSC secretory products via inhalational delivery compared with control treatment. Inhalation of iPSC secretory products enhanced capillary formation and beneficial structural remodeling in the remaining lung, leading to improved lung function.
Keywords: alveolar remodeling, compensatory lung growth, induced pluripotent stem cells, lung diffusing capacity, secretome
INTRODUCTION
Major lung resection by pneumonectomy (PNX) mimics the consequences of destructive lung disease regardless of specific etiology and is a useful model for studying the mechanisms and adaptive potential of the remaining functioning units. Following right PNX, the markedly increased suprathreshold mechanical stresses on the remaining lung units result in vigorous expansion and compensatory alveolar tissue-capillary growth (5, 7, 37, 38), leading to balanced generation of new acinar structural components, progressive remodeling of existing structure, and eventually augmentation of lung function. Robust post-PNX responses have been documented in multiple species and in both young and adult animals (18); compensation is more complete in young than adult animals, suggesting a need for exploring interventions to amplify the innate response and fully harness the potential plasticity in the adult lung. In the presence of sufficient mechanical signals, active compensatory responses may be pharmacologically augmented; supplementation with individual growth promoters in adult canines enhanced selected aspects of post-PNX structural growth, angiogenesis, and acinar and alveolar septal remodeling but not global lung function (4, 6, 34, 51). This “structure-function gap” in response may be the result of supraphysiological and/or skewed pharmacological stimulation, inadequate tissue protection from mechanically induced oxidative stress damage, and inadequate architectural remodeling. These considerations suggest a need for a broad panel of factors such as those produced by stem cells capable of modulating the myriad homeostatic pathways involved in a balanced interactive response, to maximize the innate potential for compensation and improve lung function.
The role of stem cells during post-PNX compensation remains poorly understood (26, 30). In addition to alveolar type-2 epithelial cells, classically considered to be progenitors, putative distal airway progenitor cells also increase in number post-PNX (9) and exhibit age-related proliferative and reparative potentials (31). Delivery of exogenous stem cells including induced pluripotent stem cells (iPSCs) has been reported to alleviate acute lung injury and facilitate repair and regrowth (3, 11, 15, 29, 42, 47, 53). However, in vivo delivery of intact stem cells has been limited by a low rate of engraftment and retention, and risks of immunogenicity and tumorigenicity (1, 16, 45). Instead, the beneficial effects of stem cell delivery are thought to be mediated mainly through the production of growth factors, cytokines, exosomes, and microvesicles that constitute the secretome (2); therefore, an alternative approach is targeted local delivery of cell-free conditioned media (CM) containing the stem cell secretome. We have shown that tracheal delivery of iPSC CM containing the secretome alleviated bleomycin- and hyperoxia-induced acute lung injury (10, 11), treatment activated endogenous antioxidant proteins, enhanced antioxidant capacity, and ameliorated oxidative damage to DNA, lipid, and protein (11).
Based on the above-mentioned observations, we hypothesized that pulmonary delivery of cell-free iPSC secretome protects lung tissue from damage arising from post-PNX mechanical stress and facilitates compensatory growth and remodeling, leading to enhanced function of the remaining lung. To test this hypothesis and establish the feasibility of pulmonary delivery of stem cell products in a large animal model, we nebulized the CM-containing well-characterized iPSC secretome (10, 11) for repeated inhalational delivery into the lungs of adult canines over ∼8 wk following right PNX. Control animals received cell-free media (CFM) in a similar fashion. Blood oxidative damage markers and lung function were measured before and after PNX, and detailed postmortem lung morphometry was performed. Results show that inhalation of iPSC secretome enhanced post-PNX alveolar angiogenesis and septal remodeling, leading to reduced alveolar-capillary diffusion resistance and improved diffusing capacity in the remaining lung.
METHODS
Animals and experiments.
The Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center approved all procedures. Litter-matched young adult mixed-breed female hounds (total n = 13, 10 mo old, body weight = 18.3 ± 1.1 kg, means ± SD) were obtained from approved vendor (Marshall Farms, Rose, NY). A flowchart of experimental design is shown in Fig. 1.
Fig. 1.
Timeline of the studies. CFM, cell-free media; iPSC CM, induced pluripotent stem cell-conditioned media; PNX, pneumonectomy.
Right pneumonectomy.
Following completion of baseline (pre-PNX) measurements, the animal underwent right PNX following established procedures (5, 7). Briefly, the animal was premedicated, anesthetized, intubated, and mechanically ventilated. Rectal temperature, heart rate, blood pressure, and transcutaneous O2 saturation were continuously monitored. Through a small right-lateral fifth intercostal space thoracotomy, individual hilar vessel ligation and stapling of the bronchus was performed, covering the bronchus by oversewing adjacent healthy regional tissue. The bronchial stump was checked for leaks and then oversewn with loose hilar tissue for added protection. Lidocaine (1%) was applied to the intercostal nerves, and the chest wall was closed in layers. Residual thoracic air was evacuated to underwater seal. Supplemental O2 was administered as needed. Intraoperative fluid administration was minimized (<50 mL). Analgesia (buprenorphine) was administered postoperatively for 48 h and then as needed. The animal was monitored daily; skin stitches were removed in 7–10 days. The remaining left lobes were estimated to comprise on average 42% of the pre-PNX total lung volume (38).
iPSC conditioned media.
Production of iPSC CM followed established procedures (10, 11). Human foreskin dermal fibroblasts were reprogrammed into iPSCs using established protocols (10). For producing the cell-free iPSC conditioned media (CM), iPSCs (1 × 106) were grown in Corning ultra-low attachment flask (75 cm2); once the cells formed spheres, the culture media were changed to a serum-free media without additional supplements and the cells were grown for 24 h. Cell-free CM containing the iPSC secretome was harvested and characterized, then kept deeply frozen (−80°C) until use. An aliquot (10 mL) was defrosted (4°C) overnight and gently vortexed before use. Control cell-free media (CFM) were similarly processed.
Inhalational delivery.
Following PNX and chest wall closure, animals received the nebulized compound (iPSC CM or CFM) before recovery from anesthesia; subsequent treatments were given every 5 days for 10 treatments. For each treatment (6), the animal was fasted overnight and premedicated with acepromazine (0.05 mg/kg im) and atropine (0.04 mg/kg im). Anesthesia was induced with propofol (4 mg/kg iv) and maintained with propofol as needed (∼0.5–1.0 mL/min). Each animal was intubated and mechanically ventilated in the supine position (16–18 breaths/min, 50/50 inspiration/expiration ratio, and tidal volume = 10–12 mL/kg). Mouth pressure, O2 saturation, and heart rate were monitored. The animal prebreathed 100% oxygen in an open circuit for 1–2 min and then was switched to a closed circuit connected to a reservoir bag and a nebulizer (Aerogen Aeroneb Pro, Tri-Anim, Sylmar, CA). The desired media (10 mL) was nebulized (4 μm droplets) into the inspiratory limb of the breathing circuit and delivered via the tracheal tube (average flow rate = 0.4 mL/min, minimum flow rate = 0.2 mL/min), followed by nebulization of two 1 mL saline rinses to ensure complete delivery. Oxygen was added to the circuit as needed to keep O2 saturation above 90%. The entire procedure was complete in 20–30 min.
Biochemical assays.
Peripheral venous blood (2 mL each) was collected before, during, and after the first two treatments and then at every other treatment. Plasma was used for measurement of oxidative stress markers 8-hydroxy-2′-deoxyguanosine (8-OHdG, Cell Biolabs, San Diego, CA) and 8-isoprostane (Cayman Chemical, Ann Arbor, MI). Serum was used for measurement of total antioxidant capacity (OxiSelect STA-360, Cell Biolabs, San Diego, CA). Complete blood counts and biochemical panels (Superchem + CBC) were measured pre-PNX and at 1 h, 5 days, 30 days, and 55 days post-PNX.
Physiological studies.
Lung function was measured pre-PNX and 55 days post-PNX (10 days after completion of inhalation treatments) (6, 7). The animal was fasted overnight, premedicated with acepromazine (0.05 mg/kg im) and atropine (0.04 mg/kg im). Anesthesia was induced with propofol (4 mg/kg iv) and maintained with intravenous ketamine and diazepam infusion at a dose titrated to effect. Animal was intubated with a cuffed endotracheal tube and mechanically ventilated supine (tidal volume = 10–12 mL/kg, 16–18 breaths/min) to eliminate spontaneous breathing effort. Rectal temperature, heart rate, transcutaneous O2 saturation, and mouth and esophageal pressures were monitored. Static transpulmonary pressure-lung volume (PV) curves were measured using a calibrated syringe inflating the lungs to 15, 30, 45, and 60 mL/kg above end-expiratory lung volume (EELV), or up to a transpulmonary pressure of 30 cmH2O, in increasing and then decreasing order. End-inspiratory lung volume (EILV) and end-expiratory lung volume, pulmonary blood flow, lung diffusing capacity for carbon monoxide (DLCO) and nitric oxide (DLNO), and septal tissue (including microvascular blood) volume were measured simultaneously using an established rebreathing technique (4, 23) at two inspired O2 concentrations (21% and 99%) and a lung volume of 45 mL/kg above EELV. The components of DLCO, i.e., membrane diffusing capacity (DMCO) and pulmonary capillary blood volume (Vc) were calculated from DLCO measurements obtained at the two alveolar O2 levels using established methods (19, 39). Duplicate measures under each condition were averaged. PV curves were analyzed using established methods (32, 41). Specific lung compliance was calculated from the changes in lung volume and transpulmonary pressure between 10 and 30 cmH2O transpulmonary pressure and normalized by the lung volume at 10 cmH2O.
Lung fixation.
Under deep anesthesia, a tracheostomy was performed; a cuffed endotracheal tube was inserted and tied securely. The chest was opened via left lateral thoracotomy. An overdose of pentobarbital (120 mg/kg iv) was administered, and the remaining lung was reinflated within the thorax by tracheal instillation of 2.5% buffered glutaraldehyde at a hydrostatic pressure of 25 cmH2O above the sternum. After the flow of fixative ceased, the tracheal tube was closed to maintain airway pressure. The lungs were removed intact, immersed in 2.5% buffered glutaraldehyde, floated on a water bath, and stored at 4°C for at least 4 wk before processing.
Lung morphometry.
Volume of the left caudal lobe was measured by saline immersion; then, the lobe was serially sectioned (2 cm thickness). Volume of the sectioned stress-free lobe was measured using the Cavalieri principle, an established method of measuring the volume of irregularly shaped objects where the total area of each slice is estimated by point counting, multiplied by slice thickness and summed over all slices to yield lobe volume (52). An unbiased systematic random sampling scheme was used to select eight blocks per lobe (21). Sectioned slices were arranged on a tray in the same orientation with a grid overlay. From a random start, tissue samples (1.5 cm in each dimension) were systematically selected at a fixed interval along the grid points (4 each at subpleural and interior locations). Tissue blocks were postfixed with 1% osmium tetroxide in 0.1 M cacodylate buffer, treated with 2% uranyl acetate, dehydrated through graded alcohol, and embedded in Spurr resin (Electron Microscopy Sciences, Hartfield, PA). The other remaining lobes were processed separately for other studies.
An established stratified analytical scheme (21) was used under low- and high-power light microscopy (LM; ×275 and ×550) and transmission electron microscopy (TEM; approximately ×16,000). For LM, each block was sectioned (1 µm) and stained (toluidine blue). One section per block was overlaid with a test grid. At ×275, at least 20 nonoverlapping fields were systematically sampled from a random start. Excluding the structures between 20 µm and 1 mm in diameter, the volume densities of fine parenchyma, alveolar sac, and alveolar duct were estimated using point counting. At ×550, at least 20 nonoverlapping fields were systematically sampled to estimate the volume density of alveolar septa. For TEM, each block was sectioned (70 nm) and mounted on copper grids. Each grid was examined at ×16,000 (JEOL EXII). At least 30 nonoverlapping fields per grid were systematically sampled. Volume densities of epithelium type I and II, interstitium, endothelium, and capillaries were estimated using point counting with alveolar septum as the reference space. Surface densities of alveolar epithelium and capillaries were estimated using intersection counting (21). At least 300 points or intersections were counted per grid. Harmonic mean barrier thickness of the blood-gas barrier (τhb) was measured from the lengths of intercept lines between alveolar surface and erythrocyte membrane.
Absolute volumes and surface areas of individual structures were calculated from the products of fractional quantities estimated at each level. Prevalence of double capillary profiles, an index of intussusceptive capillary formation (8, 22, 38), was calculated by completely sampling two grids under TEM (approximately ×2,500) and the results expressed as a ratio of (double capillaries)/(total number of capillaries).
Statistical analysis.
Results (means ± SD) were normalized by body weight where appropriate. Pressure-volume curves and temporal changes (pre- to post-PNX) between treatment groups were compared by factorial and/or repeated-measures ANOVA with post hoc test by Fisher’s protected least significant difference (StatView v.5.0). Morphometric parameters were compared between groups by an unpaired t test. A P value of ≤0.05 was considered significant.
RESULTS
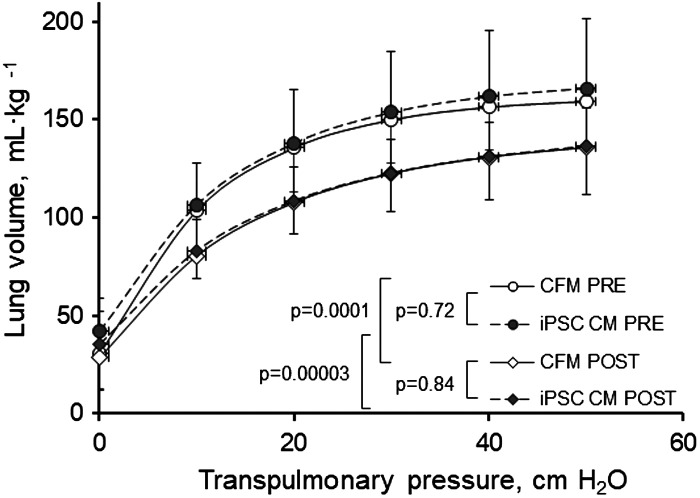
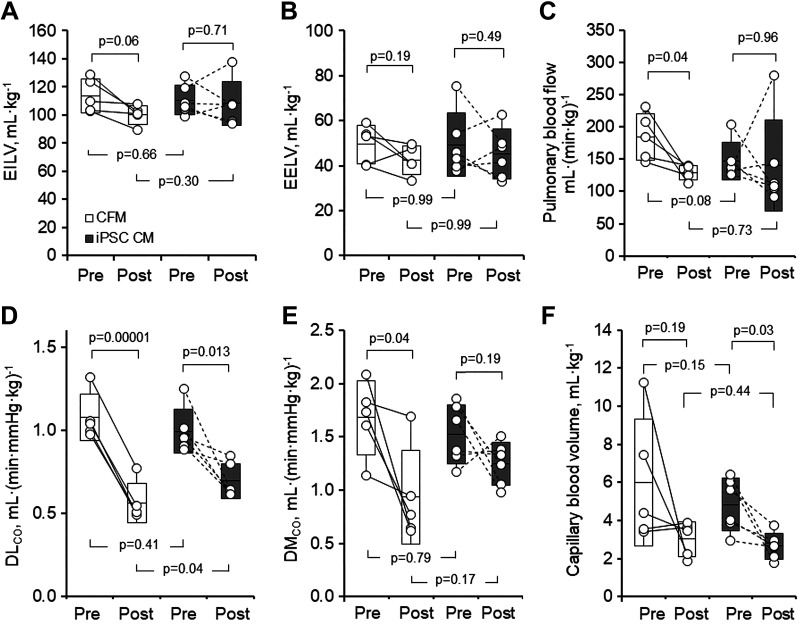
One animal in the iPSC CM group died from acute postoperative pulmonary bleeding; one animal in CFM group was terminated due to postoperative vomiting and weight loss. The remaining 11 animals (6 iPSC CM, 5 CFM) completed the study without complications. Physiological data are summarized in Table 1 and Figs. 2 and 3. Body weight, systemic hematocrit, and pre-PNX lung function were similar between groups (Table 1). In both the groups, post-PNX static lung volume at a given transpulmonary pressure was lower (Fig. 2) while specific lung compliance was similarly unchanged compared with pre-PNX (Table 1). During rebreathing, mean alveolar O2 tension and septal tissue volume were unchanged from pre- to post-PNX. Mean lung volumes were similarly maintained post-PNX in both the groups (Fig. 3, A and B). Pulmonary blood flow decreased post-PNX in the CFM group and changed variably in the iPSC CM group (Fig. 3C). Post-PNX, DLCO declined in both the groups; the magnitude of decline from pre-PNX in the iPSC CM group (29%) was attenuated compared with that in the CFM group (48%, P = 0.026) by paired analysis (Fig. 3D). In a similar pattern, DMCO declined from pre- to post-PNX in the CFM group (42%, P = 0.04), whereas the post-PNX decline in the iPSC CM group was less and not significantly different from pre-PNX (14%, P = 0.19) (Fig. 3E). Baseline Vc was highly variable in the CFM group and pre-to-post-PNX paired comparison did not reach significance (P = 0.19) (Fig. 3F). In the iPSC group, pre-PNX Vc was less variable and declined consistently post-PNX (P = 0.03). The average Vc magnitude did not differ significantly between pre- or post-PNX groups (P = 0.15 and 0.44, respectively), and the average decline (post-/pre-PNX ratio) also did not differ (0.68 vs. 0.60 in the CFM and iPSC groups, respectively, P = 0.69).
Table 1.
Lung function
| Group (PNX) | CFM |
iPSC CM |
P Value |
||||
|---|---|---|---|---|---|---|---|
| Pre-PNX | Post-PNX | Pre-PNX | Post-PNX | vs. Group | vs. PNX | Group × PNX | |
| n | 5 | 5 | 6 | 6 | |||
| Body weight, kg | 18.4 ± 1.6 | 18.5 ± 2.1 | 18.2 ± 0.3 | 18.5 ± 1.8 | 0.87 | 0.78 | 0.83 |
| Hematocrit, %, sedated | 42.8 ± 1.8 | 39.9 ± 3.2 | 42.0 ± 2.7 | 40.0 ± 2.8 | 0.78 | 0.048 | 0.68 |
| Specific lung compliance, mL·(cmH2O·L)−1 | 23.0 ± 5.8 | 27.7 ± 5.9 | 22.0 ± 2.4 | 24.5 ± 5.1 | 0.32 | 0.13 | 0.63 |
| Alveolar Po2 breathing 21% O2, mmHg | 125.3 ± 36.4 | 129.3 ± 19.5 | 128.8 ± 30.9 | 135.9 ± 18.5 | 0.70 | 0.60 | 0.88 |
| Alveolar Po2 breathing 99% O2, mmHg | 664.7 ± 14.3 | 669.9 ± 4.0 | 663.1 ± 4.8 | 670.1 ± 4.4 | 0.86 | 0.12 | 0.77 |
| End-expiratory lung volume, mL/kg | 55.7 ± 11.9 | 49.0 ± 9.1 | 52.5 ± 10.6 | 50.1 ± 16.4 | 0.85 | 0.38 | 0.67 |
| End-inspiratory lung volume, mL/kg | 113.8 ± 12.0 | 100.2 ± 6.8# | 110.7 ± 10.6 | 108.4 ± 15.6 | 0.68 | 0.08 | 0.19 |
| Pulmonary blood flow, mL·(min·kg)−1 | 184.5 ± 35.8 | 129.2 ± 11.6† | 146.8 ± 28.8 | 140.4 ± 70.8 | 0.45 | 0.17 | 0.26 |
| DLNO, mL·(min·mmHg·kg)−1 | 3.46 ± 1.25 | 2.61 ± 0.46 | 3.22 ± 0.54 | 2.58 ± 0.64 | 0.65 | 0.07 | 0.78 |
| DLCO measured, mL·(min·mmHg·kg)−1 | 1.01 ± 0.16 | 0.50 ± 0.06† | 0.87 ± 0.04 | 0.58 ± 0.11† | 0.60 | <0.0001† | 0.004‡ |
| DLCO-std, mL·(min·mmHg·kg)−1 | 1.08 ± 0.14 | 0.56 ± 0.12† | 0.99 ± 0.13 | 0.69 ± 0.10*† | 0.71 | <0.0001† | 0.04‡ |
| DMCO, mL·(min·mmHg·kg)−1 | 1.68 ± 0.35 | 0.93 ± 0.44† | 1.53 ± 0.28 | 1.25 ± 0.20 | 0.51 | 0.01† | 0.17 |
| Capillary blood volume, mL/kg | 6.00 ± 3.34 | 3.03 ± 0.91 | 4.82 ± 1.38 | 2.65 ± 0.69† | 0.22 | 0.02† | 0.68 |
| Septal tissue volume, mL/kg | 6.62 ± 1.70 | 6.60 ± 2.65 | 5.96 ± 3.01 | 6.14 ± 3.85 | 0.66 | 0.95 | 0.94 |
Values are means ± SD. Repeated-measures ANOVA: specific lung compliance was measured at transpulmonary pressures between 10 and 30 cmH2O, normalized by lung volume at 10 cmH2O. DLCO was measured at 45 mL/kg inflation volume. DLCO-std: results were expressed at standardized conditions (hematocrit = 0.45 and alveolar Po2 = 120 mmHg). PNX, pneumonectomy.
P < 0.05 vs. CFM group post-PNX.
P < 0.05,
P = 0.06 vs. corresponding pre-PNX group.
P < 0.05 group × PNX interaction.
Fig. 2.
Lung volume-transpulmonary pressure relationships pre- and post-PNX. Lung volume at a given transpulmonary pressure was similarly lower post-PNX compared with pre-PNX in animals treated with cell-free media (CFM) or induced pluripotent stem cell-conditioned media (iPSC CM). Means ± SD. Repeated-measures ANOVA. Number of animals = 5 CFM and 6 iPSC CM. PNX, pneumonectomy.
Fig. 3.
Lung function pre- and post-PNX in animals treated with cell-free media (CFM) or induced pluripotent stem cell conditioned media (iPSC CM). A: end-inspiratory lung volume (EILV). B: end-expiratory lung volume (EELV). C: pulmonary blood flow. D: lung diffusing capacity for carbon monoxide (DLCO) measured at an inflation volume of 45 mL/kg and inspired O2 concentration of 21% was expressed under standard conditions (hematocrit = 0.45, alveolar Po2 = 120 mmHg). E: membrane diffusing capacity (DMCO). F: pulmonary capillary blood volume. Box: means ± SD; whiskers extend to maximum and minimum values. Factorial and repeated-measures ANOVA. Number of animals = 5 CFM and 6 iPSC CM. PNX, pneumonectomy.
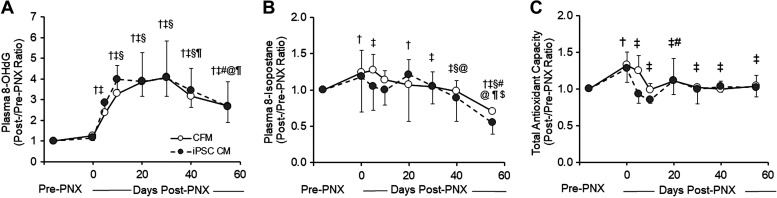
Plasma oxidative damage markers and total antioxidant capacity (Fig. 4, A–C) increased post-PNX and then declined at different rates. Plasma 8-OHdG, a marker of DNA oxidative damage, steadily increased up to fourfold post-PNX, peaking around day 10, then slowly declined but remained elevated at day 55 (Fig. 4A). Plasma 8-isoprostane, a marker of lipid oxidation, increased only mildly post-PNX, and then declined below pre-PNX baseline (Fig. 4B). Total antioxidant capacity also increased modestly post-PNX and then returned to baseline in ∼10 days. At this arbitrary secretome dose, these profiles did not differ significantly between the treatment groups.
Fig. 4.
Changes (post-/pre-PNX ratio) in plasma biomarkers.8-Hydroxy-2′-deoxyguanosine (8-OHdG) (A), 8-isoprostane (B), and total antioxidant capacity (copper-reducing equivalents) (C) in animals treated with cell-free media (CFM) or induced pluripotent stem cell-conditioned media (iPSC CM). Means ± SD. Symbols for P < 0.05 with respect to time post-PNX: † vs. Pre-PNX, ‡ vs. 1 h post-PNX (0 days), § vs. 5 days, # vs. 10 days, @ vs. 20 days, ¶ vs. 30 days, $ vs. 40 days by factorial ANOVA. Overall comparison between the treatment groups by repeated-measures ANOVA: P = 0.69 (A), P = 0.39 (B), and P = 0.25 (C). Number of animals = 5 CFM and 6 iPSC CM. PNX, pneumonectomy.
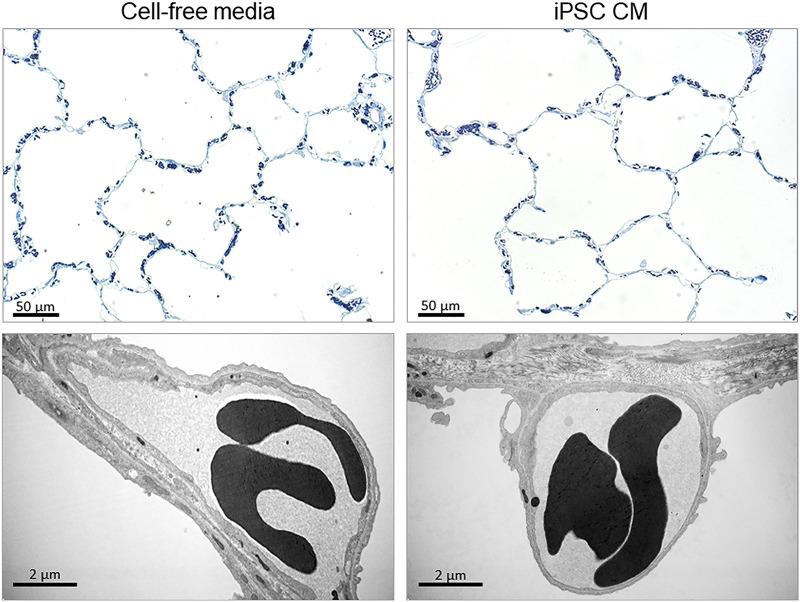
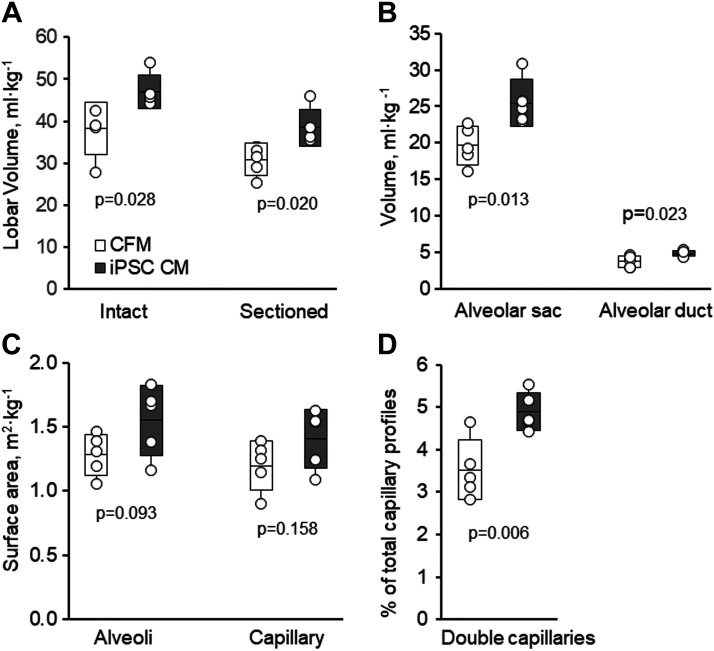
Morphometric results are available in five animals per group (Tables 2–4; Figs. 5–7). The lung from one animal (iPSC CM group) was excluded due to inadequate inflation and fixation. In the iPSC CM group, volume of the remaining left caudal lobe was 26% larger compared with the control group (P = 0.02) due to larger alveolar air spaces (Tables 2 and 4; Fig. 6, A and B), as shown in representative micrographs (Fig. 5). Volume and surface densities (Table 3) and absolute volumes and surface areas (Table 4) of septal tissue and capillary components did not significantly differ between the groups. With iPSC CM, the alveolar surface area was 21% higher but did not reach statistical significance (P = 0.09) (Table 4; Fig. 6C). The prevalence of double capillary profiles was significantly (39%) higher in the iPSC CM group (P = 0.006) (Table 2; Fig. 6D), consistent with enhanced intussusceptive capillary formation. The frequency distribution of harmonic mean blood-gas barrier thickness (τhb) shifted significantly toward the lowest value category (Fig. 7), suggesting remodeling and rearrangement of septal constituents and reduced barrier resistance to diffusion, whereas the arithmetic thickness of alveolar septum was similar between groups (Table 2). The combined results indicate larger volume and air spaces, enhanced intussusceptive alveolar capillary formation, and septal remodeling that reduced barrier resistance to diffusion in secretome-treated lungs. These changes are associated with a 25% higher estimated morphometric diffusing capacity of the tissue-plasma barrier (DbO2) (P = 0.068) (Table 4) and correspond to the better preservation of post-PNX whole lung DLCO measured by a physiological method (Fig. 3D).
Table 2.
Morphometric data
| CFM | iPSC CM | P Value | |
|---|---|---|---|
| Number of animals | 5 | 5 | |
| Terminal body weight, kg | 18.5 ± 2.1 | 18.6 ± 1.9 | 0.902 |
| Total lobar volume, mL/kg | |||
| Intact (immersion method) | 38.2 ± 6.2 | 47.0 ± 4.0* | 0.028 |
| Sectioned (Cavalieri method) | 30.9 ± 3.9 | 38.5 ± 4.3* | 0.020 |
| Morphometric hematocrit, % | 43.0 ± 2.4 | 44.4 ± 2.4 | 0.357 |
| Arithmetic mean septal thickness, µm | 4.73 ± 0.56 | 4.41 ± 0.49 | 0.356 |
| Harmonic mean barrier thickness (τhb), µm | 0.97 ± 0.03 | 0.92 ± 0.03* | 0.046 |
| Double capillary profiles, % | 3.52 ± 0.70 | 4.90 ± 0.44* | 0.006 |
Values are means ± SD. CFM, cell-free media; iPSC CM, induced pluripotent stem cell-conditioned media.
P < 0.05 iPSC CM vs. CFM by unpaired t test.
Table 4.
Absolute volumes, surface areas, and conductance for oxygen
| CFM | iPSC CM | P Value | |
|---|---|---|---|
| Volume, mL/kg | |||
| Coarse parenchyma | 27.41 ± 3.87 | 34.80 ± 4.16* | 0.020 |
| Fine parenchyma | 26.96 ± 3.80 | 34.32 ± 4.09* | 0.018 |
| Alveolar sac | 19.67 ± 2.64 | 25.49 ± 3.16* | 0.013 |
| Alveolar duct | 3.75 ± 0.78 | 4.86 ± 0.41* | 0.023 |
| Respiratory bronchioles | 0.89 ± 0.31 | 1.13 ± 0.24 | 0.212 |
| Septum | 3.04 ± 0.58 | 3.40 ± 0.54 | 0.348 |
| Total epithelium | 0.51 ± 0.08 | 0.61 ± 0.15 | 0.253 |
| Type I | 0.31 ± 0.06 | 0.36 ± 0.10 | 0.369 |
| Type II | 0.20 ± 0.02 | 0.24 ± 0.08 | 0.260 |
| Interstitium | 0.72 ± 0.18 | 0.81 ± 0.14 | 0.409 |
| Collagen fibers | 0.57 ± 0.13 | 0.62 ± 0.12 | 0.496 |
| Cells and matrix | 0.15 ± 0.07 | 0.18 ± 0.04 | 0.385 |
| Endothelium | 0.36 ± 0.06 | 0.47 ± 0.14 | 0.155 |
| Extravascular tissue | 1.59 ± 0.28 | 1.88 ± 0.41 | 0.224 |
| Capillaries | 1.45 ± 0.54 | 1.51 ± 0.29 | 0.825 |
| Surface area, m2/kg | |||
| Alveolar surface | 1.28 ± 0.16 | 1.55 ± 0.27 | 0.093 |
| Capillary surface | 1.20 ± 0.19 | 1.41 ± 0.23 | 0.158 |
| O2 conductance of tissue-plasma barrier (DbO2), mL·(min·mmHg·kg)−1 | 1.95 ± 0.29 | 2.46 ± 0.46 | 0.068 |
Values are means ± SD. CFM, cell-free media; iPSC CM, induced pluripotent stem cell-conditioned media.
P < 0.05 iPSC CM vs. CFM by unpaired t test.
Fig. 5.
Representative distal lung morphology under light and electron microscopy in post-PNX animals treated with cell-free media (CFM) or induced pluripotent stem cell-conditioned media (iPSC CM), illustrating enlarged air spaces and a thinner septal tissue layer in the iPSC CM group on the “thin side” of the blood-gas barrier where the bulk of alveolar gas exchange takes place. Top: bar = 50 µm. Bottom: bar = 2 µm. PNX, pneumonectomy.
Fig. 7.
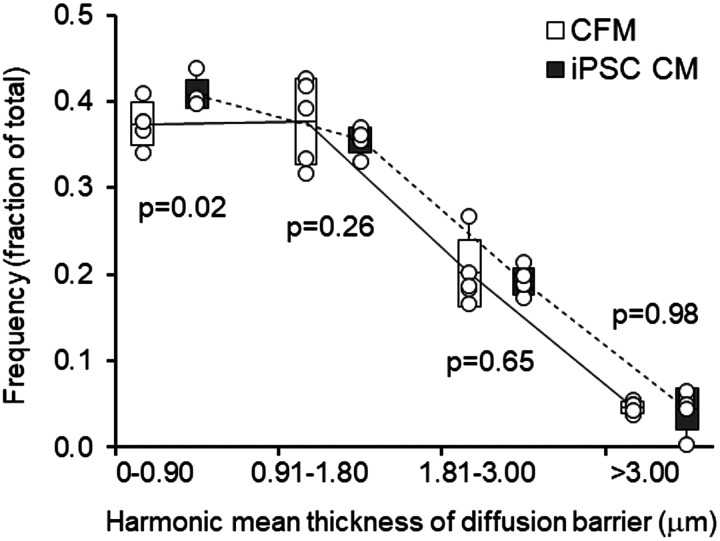
Frequency distribution of harmonic mean thickness of the tissue-plasma barrier in post-PNX animals treated with cell-free media (CFM) or induced pluripotent stem cell-conditioned media (iPSC CM). Box: Means ± SD; whiskers extend to maximum and minimum values. P values indicate iPSC CM versus CFM in each barrier thickness category by unpaired t test. Overall comparison between the treatment groups by repeated-measures ANOVA (P = 0.16). n = 5 animals per group. PNX, pneumonectomy.
Fig. 6.
Morphometric results in the left caudal lobe in post-PNX animals treated with cell-free media (CFM) or induced pluripotent stem cell-conditioned media (iPSC CM). A: total lobe volume (intact or serial sectioned). B: volume of alveolar sacs and ducts. C: alveolar surface area. D: prevalence of alveolar double capillary profiles. Box: Means ± SD; whiskers extend to maximum and minimum values. P values indicate iPSC CM versus CFM by unpaired t test. n = 5 animals per group. PNX, pneumonectomy.
Table 3.
Volume-to-volume and surface-to-volume ratios of alveolar structures
| CFM | iPSC CM | P Value | |
|---|---|---|---|
| Volume per unit lung volume | |||
| Coarse parenchyma | 0.8870 ± 0.0234 | 0.9039 ± 0.0154 | 0.213 |
| Fine parenchyma | 0.8723 ± 0.0225 | 0.8914 ± 0.0133 | 0.141 |
| Respiratory bronchioles | 0.0286 ± 0.0096 | 0.0292 ± 0.0049 | 0.904 |
| Alveolar sac | 0.6370 ± 0.0165 | 0.6620 ± 0.0148* | 0.035 |
| Alveolar duct | 0.1211 ± 0.0175 | 0.1267 ± 0.0079 | 0.536 |
| Septum (tissue + blood) | 0.0982 ± 0.0095 | 0.0886 ± 0.0141 | 0.246 |
| Total epithelium | 0.0166 ± 0.0011 | 0.0156 ± 0.0027 | 0.467 |
| Type I epithelium | 0.0101 ± 0.0012 | 0.0094 ± 0.0022 | 0.555 |
| Type II epithelium | 0.0065 ± 0.0005 | 0.0062 ± 0.0015 | 0.677 |
| Interstitium | 0.0232 ± 0.0048 | 0.0210 ± 0.0037 | 0.438 |
| Collagen fibers | 0.0184 ± 0.0031 | 0.0163 ± 0.0032 | 0.320 |
| Cells and matrix | 0.0049 ± 0.0022 | 0.0048 ± 0.0011 | 0.924 |
| Endothelium | 0.0118 ± 0.0022 | 0.0122 ± 0.0031 | 0.828 |
| Septal extravascular tissue | 0.0517 ± 0.0068 | 0.0489 ± 0.0087 | 0.583 |
| Capillaries | 0.0465 ± 0.0136 | 0.0398 ± 0.0093 | 0.391 |
| Surface area per unit lung volume, cm−1 | |||
| Alveolar surface | 416 ± 18 | 403 ± 57 | 0.643 |
| Capillary surface | 387 ± 24 | 366 ± 51 | 0.428 |
Values are means ± SD. CFM, cell-free media; iPSC CM, induced pluripotent stem cell-conditioned media.
P < 0.05 iPSC CM vs. CFM by unpaired t test.
DISCUSSION
Summary of the main findings.
This is the first report to establish the feasibility and efficacy of inhaled iPSC-derived cell-free secretome for enhancing post-PNX compensation in a large animal model. Pre-PNX lung function was similar between the groups. Plasma 8-OHdG level remained elevated for 55 days post-PNX, indicating persistent DNA oxidative stress, whereas plasma 8-isoprostane and total antioxidant capacity levels increased mildly and transiently; the temporal profiles did not differ between the groups. In secretome-treated animals compared with control animals, physiological whole lung volume measured at near total lung capacity was similar. However, postmortem stress-free volume of the caudal lobe was 26%–30% larger in secretome-treated animals. Differences between antemortem and postmortem volumes suggest dynamic extra-pulmonary factors, e.g., thoracic and/or diaphragmatic restriction that limited lung expansion in the living animal. Secretome-treated animals exhibited increased intussusceptive alveolar angiogenesis (double capillaries) and septal remodeling with enlarged terminal airspaces and reduced barrier resistance to diffusion; absolute alveolar surface area was also higher without reaching statistical significance. These structural changes resulted in a 25% higher conductance of the blood-gas barrier (P = 0.068), which is consistent with the physiological findings of better preservation of DMCO and a 23% higher physiological DLCO in post-PNX animals treated with iPSC CM. We conclude that repetitive inhalational delivery of iPSC secretome enhanced endogenous post-PNX angiogenesis and acinar and alveolar septal remodeling leading to modest and significant improvement in gas exchange compensation. These findings highlight the plasticity of adult lung units that remain following destructive processes, and the responsiveness of these units to broad-based modulation provided by iPSC secretome.
Critique of the methods.
Both iPSCs (10, 11, 33, 44) and mesenchymal stem cells (MSCs) (12, 27–29, 49) or their secretome possess injury-alleviating and tissue regenerative potentials. A Phase I/II trial of systemic infusion of allogenic MSCs on aging frailty showed promising results compared with placebo (13); however, more studies are needed in this and other conditions. Here, we chose to test a cell-free iPSC secretome preparation for enhancing post-PNX compensation; the preparation has been characterized and shown to alleviate experimental lung injury (10, 11, 44). The iPSCs may be derived from readily available sources, e.g., human dermal fibroblasts, and can differentiate into a variety of cell types including organ-specific MSCs (43). Other cell types, e.g., fibroblasts, may secrete some of the same ingredients but lack the overall injury-alleviating capacity of iPSCs (10, 11). There are no direct comparisons between iPSCs and MSCs for post-PNX compensation; this comparison is beyond the scope of our report but may be pursued in the future. The inhalational approach (6) and physiological (19) and morphometric assessments (21) are established. An earlier canine study of post-PNX inhalation of exogenous erythropoietin documented the delivery of exogenous protein to the caudal lobe (6) and showed that alveolar septal changes and the magnitude of enhancement of angiogenesis were comparable among all post-PNX remaining lobes; structural response in the caudal lobe was representative of that of the remaining lung (6, 7, 38). In the current study, the largest remaining lobe (left caudal, ∼55% of total left lung volume) was sampled for morphometry. Both subpleural and central lobar regions were sampled.
The derived components of DLCO (DMCO and Vc) are interdependent quantities that typically exhibit larger variability than DLCO. In the CFM group, post-PNX DMCO declined by 42% (P = 0.04 vs. pre-PNX), whereas in the iPSC CM group, the decline was less (14%) and the pre-to-post-PNX paired values were not significantly different (P = 0.19), suggesting that iPSC CM minimized post-PNX decline in DMCO, i.e., a similar pattern as DLCO. Vc is sensitive to hemodynamic fluctuations, exhibiting a variable baseline in the CFM group that hampered intergroup comparisons. Nevertheless, post-PNX Vc was less variable and reached similar absolute levels in both the groups, consistent with morphometric results where the average postmortem alveolar capillary blood volume also did not differ significantly between the groups (P = 0.82) (Table 4). Variability in baseline Vc does not alter the conclusion that DLCO, the primary measure of diffusive gas exchange, was better preserved in post-PNX animals treated with iPSC CM.
Two plasma biomarkers, 8-isoprostane and 8-OHdG, that reflect oxidative stress damage to lipid and DNA, respectively, and are known to increase post-PNX (6), were measured along with total antioxidant capacity. Plasma 8-OHdG was persistently elevated at day 55 post-PNX (Fig. 4A), indicating ongoing DNA oxidative stress most likely related to the still increased mechanical stresses on the remaining lung. Pulmonary delivery of iPSC secretome attenuates oxidative damage in acute hyperoxic lung injury (11); a higher dose or more frequent dosing may be needed to attenuate persistent post-PNX mechanically induced oxidative stress. The optimal dose-response relationships remain to be determined. Treatment duration (∼8 wk) spanned the early post-PNX period marked by active cell proliferation and matrix deposition; progressive architectural remodeling and further gains in lung function continue well beyond this period (20, 55, 56). Therefore, a longer period of therapy and monitoring will be needed to assess the ultimate outcome. Physiological assessment was made under anesthesia; the responses while awake or during exercise may differ. This study used female animals; sex differences in response to PNX are largely explained by body size (Dane, Kernstine, Hsia, unpublished data). Further studies will be required to determine any sex differences in response to iPSC secretome treatment.
Mechanically induced post-PNX lung growth and remodeling.
Post-PNX increases in lung volume, perfusion, and blood volume of the remaining lobes transduce compensatory responses (5, 7, 37, 59). We previously documented a threshold, optimal range and upper limit of post-PNX compensatory response (for summary, see Ref. 38). Following left PNX (42% of lung units removed), most of the remaining lobes compensated via recruitment of existing alveolar-capillary reserves without new tissue growth, except the right infracardiac lobe that underwent the largest expansion across the midline anterior and caudal to the cardiac fossa with nearly twofold increases in tissue-capillary volumes. Following right PNX (58% resection), mechanical stress on the remaining lobes exceeded a growth-stimulating threshold and all the remaining lobes exhibited a 2- to 2.5-fold increase in alveolar septal components. Following bilateral resection removing up to 70% of lung units (∼35% each side), the remaining lobes exhibited significant though diminished alveolar-capillary growth than that after 58% resection, suggesting that an optimal stimulus-response range was exceeded.
Interventions to augment compensatory lung growth.
Attempts to enhance innate post-PNX responses using individual growth promoters in rodent models led to mixed cellular and structural effects (14, 24, 25, 40, 57), and few studies assessed the functional outcome on gas exchange. In our earlier canine studies (4, 34, 50, 51), oral all-trans retinoic acid significantly enhanced active alveolar regrowth after right PNX (34, 51) but had no effect after left PNX (50), suggesting that pharmacological agents modify active mechanically induced lung growth but cannot reinitiate growth de novo in the absence of sufficient mechanical stimuli. Delivery of retinoic acid or recombinant erythropoietin (6, 54) enhanced post-PNX alveolar double-capillary formation, consistent with the notion of intussusceptive angiogenesis as an essential event in compensatory lung growth (22). Retinoic acid augmented post-PNX volumes of alveolar type-1 epithelium, interstitial collagen and matrix, and endothelium and pulmonary capillary blood, but the volume increase in type-2 epithelium lagged behind; structural distortion developed with thicker alveolar septa and basement membrane and smaller air spaces indicating inadequate remodeling (4, 34, 51). Erythropoietin possesses potent cytoprotective and proangiogenic properties; inhaled recombinant human erythropoietin improved post-PNX in vivo distribution of pulmonary blood flow (54) and abrogated oxidative stress damage (6), but exerted only minor effects on extravascular alveolar tissue compartments. Despite enhancing certain aspects of the post-PNX response, neither of the above agents augmented lung function above that in vehicle-treated post-PNX control animals. This “structure-function gap” in response to pharmacological intervention is not surprising, as each agent can stimulate only a subset of interacting growth-related pathways, and may elicit supraphysiologic responses causing distortion at microscopic and macroscopic levels. Furthermore, the selective structural alterations were not accompanied by appropriate acinar or alveolar septal remodeling to optimize gas exchange efficiency. This structure-function gap provides rationale for a cocktail approach such as that offered by the iPSC secretome consisting of a broad panel of counteracting mediators capable of supporting balanced modulation of post-PNX adaptation without distortion to ultimately attain functional benefit.
The iPSC secretome.
Delivery of stem cell secretome has been called an empirical “shotgun” approach; yet this approach directly and mechanistically addresses the prerequisite for useful lung growth and remodeling leading to functional compensation, i.e., the need for balanced physiological modulation of all relevant mediators and pathways and gas exchange structures with minimal distortion. Few studies focused on iPSC secretome; our preparation has been characterized by proteomic analysis, and found to contain >1,200 proteins (10, 11) including a markedly enriched αKlotho content (10- to 25-fold that in normal serum). αKlotho is an essential cell maintenance and cytoprotective protein with pleiotropic actions including antiapoptosis and potent antioxidation via activation of the nuclear factor (erythroid 2)-related factor 2 (Nrf2) network of endogenous antioxidant proteins (11, 35, 36). The lung normally does not express αKlotho (58) but depends on kidney-derived circulating αKlotho for cytoprotection (35, 36). Immunodepletion of αKlotho from iPSC CM reduced in vitro cytoprotective effects of iPSC CM by ∼50% (11). Tracheal delivery of this secretome preparation alleviated bleomycin- and hyperoxia-induced acute lung injury compared with fibroblast-conditioned media (10, 11), enhanced total antioxidant capacity, ameliorated oxidative damage to DNA, lipid, and protein, and broadly activated endogenous antioxidant proteins (11). Others reported that iPSCs or their secretory products reduced pro-inflammatory and profibrotic cytokines and chemokines (17), and iPSC-derived exosomes/microvesicles protected against cardiac ischemia/reperfusion injury (46). Gene network analysis of lung interstitial macrophages from bleomycin-injured rats treated with this secretome preparation demonstrated modulation of multiple pathways involved in immunomodulation, branching morphogenesis, and canonical Wnt signaling (44). Our data support the benefit of intact iPSC secretome in modulating the manifold adaptive mechanisms required for compensatory alveolar angiogenesis and remodeling to facilitate more efficient diffusive gas exchange. iPSC-derived cytoprotective factors such as αKlotho may have permitted fuller expression of the innate compensatory potential of the remaining lung.
Comparisons between the single-agent and our broad-based interventional approaches offer several useful insights:
-
1.
Pharmacological augmentation of the formation of new alveolar tissue-capillary elements alone is insufficient for achieving functional benefit unless the increase in these elements is accompanied by appropriate architectural remodeling to optimize gas exchange.
-
2.
As lung growth and regeneration involve multiple dynamic processes, a broad-based cocktail approach is superior to single or a few agents for bridging the structure-function gap in attempts to amplify innate adaptive responses. This general concept is analogous to the “cocktail” therapy routinely used in cancer chemotherapy to broadly target the myriad factors that promote tumor growth and spread.
-
3.
Individual secretome-induced structural modulations may show modest or borderline statistical significance but assume cumulative biological significance with respect to global lung function. For example, the 5% lower (P < 0.05) average harmonic mean barrier thickness (τhb) in secretome-treated lungs was due to a preferential shift in the frequency distribution of τhb to the thinnest part of the barrier (a 9% increase, P = 0.02, Fig. 7). This modest shift disproportionally increases gas transfer efficiency, because alveolar gas conductance is proportional to the reciprocal of τhb (48) and >90% of alveolar gas exchange takes place across the thinnest part of the septa while the thicker parts mainly provide physical support. Combined with the 17%–20% higher (P = 0.09) alveolar-capillary surface areas, these changes could account for a 25% higher morphometric estimate of barrier conductance (P = 0.068) and a 23% higher physiological DLCO (P = 0.04) in animals treated with iPSC CM compared with CFM over ∼8 wk. These are very reasonable rates of improvement considering the need to maintain balanced structure-function responses and minimize distortion throughout all regions of a large stratified lung.
Conclusions.
Previous attempts to amplify post-PNX compensatory responses were partially successful but identified an important challenge of structure-function dissociation, i.e., individual exogenous growth promoters augmented selective aspects of tissue-capillary growth but not architectural remodeling or functional outcome. We report here that inhalation of a well-characterized cell-free iPSC secretome preparation overcame this dissociation by enhancing post-PNX angiogenesis and alveolar remodeling leading to more efficient gas exchange. These novel findings reinforce the concept that post-PNX compensation is a highly orchestrated multiphasic process involving myriad pathways and mediators, many of which are not classically considered to be growth promoters. In addition to mechanically stimulated cell proliferation and tissue-capillary growth, progressive structural remodeling minimized resistance of the diffusion barrier, a critical requirement for achieving functional compensation. Innate post-PNX compensation may be augmented by supplementing with iPSC secretome composed of broad-based mediators in a physiologically relevant cocktail. These results established the feasibility and efficacy of inhalational delivery of iPSC secretome in the canine model.
Many aspects of this emergent approach require further investigation, including characterization and optimization of secretome composition and bioactivity, elucidation of the mechanisms of action and interaction among the components, and determination of the degrees to which the major components contribute to the observed effects. Our premise is that the entire secretome is responsible for coordinated enhancement of post-PNX angiogenesis and remodeling required for translating post-PNX tissue growth into functional gain; future studies may compare intact secretome with subfractions such as exosomes and microvesicles. Unlike the short-term use of secretome in acute lung injury, the post-PNX remaining lung undergoes progressive remodeling over many months with gradual functional improvement (56), and may be susceptible to secretome action throughout this period as the existing acinar scaffold is slowly modified. Therefore, sustained modest and balanced modulation is preferable to acute supraphysiological skewed stimulation. Prolonged secretome therapy may be needed to maximize long-term structural and functional gain and facilitate realization of the full innate compensatory potential. Measurement of lung function during exercise may accentuate treatment effects. Impact of the secretome delivery approach extends beyond the PNX model to regenerative therapy for parenchymal lung diseases irrespective of the specific etiology.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01‐HL134373 and U01‐HL111146 (to C. C. W. Hsia) and the Swiss National Science Foundation Grant SNF 310030_141102 (to T. Geiser).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.G., T.G., and C.C.W.H. conceived and designed research; D.M.D., K.C., Y-A.Z., K.H.K., A.G., and C.C.W.H. performed experiments; D.M.D., K.C., Y-A.Z., A.G., and C.C.W.H. analyzed data; D.M.D., K.C., A.G., T.G., and C.C.W.H. interpreted results of experiments; D.M.D., K.C., and C.C.W.H. prepared figures; D.M.D., K.C., and C.C.W.H. drafted manuscript; K.H.K., A.G., T.G., and C.C.W.H. edited and revised manuscript; D.M.D., K.C., Y-A.Z., K.H.K., A.G., T.G., and C.C.W.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Matthew Riegel, DVM, and the staff of the Animal Resources Center at UT Southwestern for veterinary assistance and Anna-Barbara Tschirren at University of Bern for technical assistance.
REFERENCES
- 1.Abad M, Mosteiro L, Pantoja C, Cañamero M, Rayon T, Ors I, Graña O, Megías D, Domínguez O, Martínez D, Manzanares M, Ortega S, Serrano M. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature 502: 340–345, 2013. doi: 10.1038/nature12586. [DOI] [PubMed] [Google Scholar]
- 2.Abman SH, Matthay MA. Mesenchymal stem cells for the prevention of bronchopulmonary dysplasia: delivering the secretome. Am J Respir Crit Care Med 180: 1039–1041, 2009. doi: 10.1164/rccm.200909-1330ED. [DOI] [PubMed] [Google Scholar]
- 3.Asmussen S, Ito H, Traber DL, Lee JW, Cox RA, Hawkins HK, McAuley DF, McKenna DH, Traber LD, Zhuo H, Wilson J, Herndon DN, Prough DS, Liu KD, Matthay MA, Enkhbaatar P. Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia. Thorax 69: 819–825, 2014. doi: 10.1136/thoraxjnl-2013-204980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dane DM, Yan X, Tamhane RM, Johnson RL Jr, Estrera AS, Hogg DC, Hogg RT, Hsia CCW. Retinoic acid-induced alveolar cellular growth does not improve function after right pneumonectomy. J Appl Physiol (1985) 96: 1090–1096, 2004. doi: 10.1152/japplphysiol.00900.2002. [DOI] [PubMed] [Google Scholar]
- 5.Dane DM, Yilmaz C, Estrera AS, Hsia CCW. Separating in vivo mechanical stimuli for postpneumonectomy compensation: physiological assessment. J Appl Physiol (1985) 114: 99–106, 2013. doi: 10.1152/japplphysiol.01213.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dane DM, Yilmaz C, Gyawali D, Iyer R, Menon J, Nguyen KT, Ravikumar P, Estrera AS, Hsia CCW. Erythropoietin inhalation enhances adult canine alveolar-capillary formation following pneumonectomy. Am J Physiol Lung Cell Mol Physiol 316: L936–L945, 2019. doi: 10.1152/ajplung.00504.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dane DM, Yilmaz C, Gyawali D, Iyer R, Ravikumar P, Estrera AS, Hsia CCW. Perfusion-related stimuli for compensatory lung growth following pneumonectomy. J Appl Physiol (1985) 121: 312–323, 2016. doi: 10.1152/japplphysiol.00297.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Djonov V, Baum O, Burri PH. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res 314: 107–117, 2003. doi: 10.1007/s00441-003-0784-3. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhauer P, Earle B, Loi R, Sueblinvong V, Goodwin M, Allen GB, Lundblad L, Mazan MR, Hoffman AM, Weiss DJ. Endogenous distal airway progenitor cells, lung mechanics, and disproportionate lobar growth following long-term postpneumonectomy in mice. Stem Cells 31: 1330–1339, 2013. doi: 10.1002/stem.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gazdhar A, Grad I, Tamò L, Gugger M, Feki A, Geiser T. The secretome of induced pluripotent stem cells reduces lung fibrosis in part by hepatocyte growth factor. Stem Cell Res Ther 5: 123, 2014. doi: 10.1186/scrt513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gazdhar A, Ravikumar P, Pastor J, Heller M, Ye J, Zhang J, Moe OW, Geiser T, Hsia CCW. Alpha-klotho enrichment in induced pluripotent stem cell secretome contributes to antioxidative protection in acute lung injury. Stem Cells 36: 616–625, 2018. doi: 10.1002/stem.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gennai S, Monsel A, Hao Q, Park J, Matthay MA, Lee JW. Microvesicles derived from human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am J Transplant 15: 2404–2412, 2015. doi: 10.1111/ajt.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golpanian S, DiFede DL, Pujol MV, Lowery MH, Levis-Dusseau S, Goldstein BJ, Schulman IH, Longsomboon B, Wolf A, Khan A, Heldman AW, Goldschmidt-Clermont PJ, Hare JM. Rationale and design of the allogeneiC human mesenchymal stem cells (hMSC) in patients with aging fRAilTy via intravenoUS delivery (CRATUS) study: A phase I/II, randomized, blinded and placebo controlled trial to evaluate the safety and potential efficacy of allogeneic human mesenchymal stem cell infusion in patients with aging frailty. Oncotarget 7: 11899–11912, 2016. doi: 10.18632/oncotarget.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guimarães-Fernandes F, Samano MN, Vieira RP, Carvalho CR, Pazetti R, Moreira LFP, Pêgo-Fernandes PM, Jatene FB. Effect of methylprednisolone on perivascular pulmonary edema, inflammatory infiltrate, VEGF and TGF-beta immunoexpression in the remaining lungs of rats after left pneumonectomy. Braz J Med Biol Res 44: 647–651, 2011. doi: 10.1590/s0100-879x2011007500061. [DOI] [PubMed] [Google Scholar]
- 15.Hao Q, Zhu Y-G, Monsel A, Gennai S, Lee T, Xu F, Lee J-W. Study of bone marrow and embryonic stem cell-derived human mesenchymal stem cells for treatment of escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells Transl Med 4: 832–840, 2015. doi: 10.5966/sctm.2015-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogan BLM, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CCW, Niklason L, Calle E, Le A, Randell SH, Rock J, Snitow M, Krummel M, Stripp BR, Vu T, White ES, Whitsett JA, Morrisey EE. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 15: 123–138, 2014. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.How C-K, Chien Y, Yang K-Y, Shih H-C, Juan C-C, Yang Y-P, Chiou G-Y, Huang P-I, Chang Y-L, Chen L-K, Wang C-Y, Hsu H-S, Chiou S-H, Lee C-H. Induced pluripotent stem cells mediate the release of interferon gamma-induced protein 10 and alleviate bleomycin-induced lung inflammation and fibrosis. Shock 39: 261–270, 2013. doi: 10.1097/SHK.0b013e318285f2e2. [DOI] [PubMed] [Google Scholar]
- 18.Hsia CCW. Comparative analysis of the mechanical signals in lung development and compensatory growth. Cell Tissue Res 367: 687–705, 2017. doi: 10.1007/s00441-016-2558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsia CC, Herazo LF, Ramanathan M, Johnson RL Jr. Cardiopulmonary adaptations to pneumonectomy in dogs. IV. Membrane diffusing capacity and capillary blood volume. J Appl Physiol (1985) 77: 998–1005, 1994. doi: 10.1152/jappl.1994.77.2.998. [DOI] [PubMed] [Google Scholar]
- 20.Hsia CC, Herazo LF, Ramanathan M, Johnson RL Jr, Wagner PD. Cardiopulmonary adaptations to pneumonectomy in dogs. II. VA/Q relationships and microvascular recruitment. J Appl Physiol (1985) 74: 1299–1309, 1993. doi: 10.1152/jappl.1993.74.3.1299. [DOI] [PubMed] [Google Scholar]
- 21.Hsia CCW, Hyde DM, Ochs M, Weibel ER; ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure . An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 181: 394–418, 2010. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsia CC, Ravikumar P. Role of mechanical stress in lung repair and regeneration In: Stem Cells in the Lung, edited by Bertoncello I. Cham, Switzerland: Springer International, 2015, chapt 12, p. 191–210. [Google Scholar]
- 23.Hsia CCW, Wagner PD, Dane DM, Wagner HE, Johnson RL Jr. Predicting diffusive alveolar oxygen transfer from carbon monoxide-diffusing capacity in exercising foxhounds. J Appl Physiol (1985) 105: 1441–1447, 2008. doi: 10.1152/japplphysiol.01328.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaza AK, Kron IL, Kern JA, Long SM, Fiser SM, Nguyen RP, Tribble CG, Laubach VE. Retinoic acid enhances lung growth after pneumonectomy. Ann Thorac Surg 71: 1645–1650, 2001. doi: 10.1016/S0003-4975(01)02478-X. [DOI] [PubMed] [Google Scholar]
- 25.Kaza AK, Kron IL, Leuwerke SM, Tribble CG, Laubach VE. Keratinocyte growth factor enhances post-pneumonectomy lung growth by alveolar proliferation. Circulation 106, Suppl 1: I120–I124, 2002. [PubMed] [Google Scholar]
- 26.Krause A, Xu Y, Joh J, Hubner R, Gess A, Ilic T, Worgall S. Overexpression of sonic Hedgehog in the lung mimics the effect of lung injury and compensatory lung growth on pulmonary Sca-1 and CD34 positive cells. Mol Ther 18: 404–412, 2010. doi: 10.1038/mt.2009.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA 106: 16357–16362, 2009. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammadipoor A, Antebi B, Batchinsky AI, Cancio LC. Therapeutic potential of products derived from mesenchymal stem/stromal cells in pulmonary disease. Respir Res 19: 218, 2018. doi: 10.1186/s12931-018-0921-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monsel A, Zhu Y-G, Gudapati V, Lim H, Lee JW. Mesenchymal stem cell derived secretome and extracellular vesicles for acute lung injury and other inflammatory lung diseases. Expert Opin Biol Ther 16: 859–871, 2016. doi: 10.1517/14712598.2016.1170804. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nolen-Walston RD, Kim CF, Mazan MR, Ingenito EP, Gruntman AM, Tsai L, Boston R, Woolfenden AE, Jacks T, Hoffman AM. Cellular kinetics and modeling of bronchioalveolar stem cell response during lung regeneration. Am J Physiol Lung Cell Mol Physiol 294: L1158–L1165, 2008. doi: 10.1152/ajplung.00298.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paxson JA, Gruntman AM, Davis AM, Parkin CM, Ingenito EP, Hoffman AM. Age dependence of lung mesenchymal stromal cell dynamics following pneumonectomy. Stem Cells Dev 22: 3214–3225, 2013. doi: 10.1089/scd.2012.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pengelly LD. Curve-fitting analysis of pressure-volume characteristics of the lungs. J Appl Physiol Respir Environ Exerc Physiol 42: 111–116, 1977. doi: 10.1152/jappl.1977.42.1.111. [DOI] [PubMed] [Google Scholar]
- 33.Pieróg J, Fytianos K, Tamò L, Simillion C, Taddeo A, Kocher G, Gugger M, Grodzki T, Heller M, Blank F, Geiser T, Schmid RA, Gazdhar A. Stem cell secretome attenuates acute rejection in rat lung allotransplant. Interact Cardiovasc Thorac Surg 28: 812–818, 2019. doi: 10.1093/icvts/ivy306. [DOI] [PubMed] [Google Scholar]
- 34.Ravikumar P, Dane DM, McDonough P, Yilmaz C, Estrera AS, Hsia CCW. Long-term post-pneumonectomy pulmonary adaptation following all-trans-retinoic acid supplementation. J Appl Physiol (1985) 110: 764–773, 2011. doi: 10.1152/japplphysiol.00994.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravikumar P, Li L, Ye J, Shi M, Taniguchi M, Zhang J, Kuro-o M, Hu MC, Moe OW, Hsia CCW. αKlotho deficiency in acute kidney injury contributes to lung damage. J Appl Physiol (1985) 120: 723–732, 2016. doi: 10.1152/japplphysiol.00792.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravikumar P, Ye J, Zhang J, Pinch SN, Hu MC, Kuro-o M, Hsia CCW, Moe OW. α-Klotho protects against oxidative damage in pulmonary epithelia. Am J Physiol Lung Cell Mol Physiol 307: L566–L575, 2014. doi: 10.1152/ajplung.00306.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ravikumar P, Yilmaz C, Bellotto DJ, Dane DM, Estrera AS, Hsia CCW. Separating in vivo mechanical stimuli for postpneumonectomy compensation: imaging and ultrastructural assessment. J Appl Physiol (1985) 114: 961–970, 2013. doi: 10.1152/japplphysiol.01394.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ravikumar P, Yilmaz C, Dane DM, Bellotto DJ, Estrera AS, Hsia CCW. Defining a stimuli-response relationship in compensatory lung growth following major resection. J Appl Physiol (1985) 116: 816–824, 2014. doi: 10.1152/japplphysiol.01291.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roughton FJ, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol 11: 290–302, 1957. doi: 10.1152/jappl.1957.11.2.290. [DOI] [PubMed] [Google Scholar]
- 40.Sakurai MK, Lee S, Arsenault DA, Nose V, Wilson JM, Heymach JV, Puder M. Vascular endothelial growth factor accelerates compensatory lung growth after unilateral pneumonectomy. Am J Physiol Lung Cell Mol Physiol 292: L742–L747, 2007. doi: 10.1152/ajplung.00064.2006. [DOI] [PubMed] [Google Scholar]
- 41.Salazar E, Knowles JH. An analysis of pressure-volume characteristics of the lungs. J Appl Physiol 19: 97–104, 1964. doi: 10.1152/jappl.1964.19.1.97. [DOI] [PubMed] [Google Scholar]
- 42.Song L, Xu J, Qu J, Sai Y, Chen C, Yu L, Li D, Guo X. A therapeutic role for mesenchymal stem cells in acute lung injury independent of hypoxia-induced mitogenic factor. J Cell Mol Med 16: 376–385, 2012. doi: 10.1111/j.1582-4934.2011.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steens J, Klein D. Current strategies to generate human mesenchymal stem cells in vitro. Stem Cells Int 2018: 6726185, 2018. doi: 10.1155/2018/6726185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamò L, Simillion C, Hibaoui Y, Feki A, Gugger M, Prasse A, Jäger B, Goldmann T, Geiser T, Gazdhar A. Gene network analysis of interstitial macrophages after treatment with induced pluripotent stem cells secretome (iPSC-cm) in the bleomycin injured rat lung. Stem Cell Rev Rep 14: 412–424, 2018. doi: 10.1007/s12015-017-9790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan Y, Ooi S, Wang L. Immunogenicity and tumorigenicity of pluripotent stem cells and their derivatives: genetic and epigenetic perspectives. Curr Stem Cell Res Ther 9: 63–72, 2014. doi: 10.2174/1574888X113086660068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo W, Zhang X, Qin G, He S-H, Zimmerman A, Liu Y, Kim I-M, Weintraub NL, Tang Y. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol 192: 61–69, 2015. doi: 10.1016/j.ijcard.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y-Y, Li X-Z, Wang L-B. Therapeutic implications of mesenchymal stem cells in acute lung injury/acute respiratory distress syndrome. Stem Cell Res Ther 4: 45, 2013. doi: 10.1186/scrt193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weibel ER, Federspiel WJ, Fryder-Doffey F, Hsia CC, König M, Stalder-Navarro V, Vock R. Morphometric model for pulmonary diffusing capacity. I. Membrane diffusing capacity. Respir Physiol 93: 125–149, 1993. doi: 10.1016/0034-5687(93)90001-Q. [DOI] [PubMed] [Google Scholar]
- 49.Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, Kwong A, Mitsialis SA, Kourembanas S. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med 197: 104–116, 2018. doi: 10.1164/rccm.201705-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan X, Bellotto DJ, Dane DM, Elmore RG, Johnson RL Jr, Estrera AS, Hsia CCW. Lack of response to all-trans retinoic acid supplementation in adult dogs following left pneumonectomy. J Appl Physiol (1985) 99: 1681–1688, 2005. doi: 10.1152/japplphysiol.00553.2005. [DOI] [PubMed] [Google Scholar]
- 51.Yan X, Bellotto DJ, Foster DJ, Johnson RL Jr, Hagler HK, Estrera AS, Hsia CCW. Retinoic acid induces nonuniform alveolar septal growth after right pneumonectomy. J Appl Physiol (1985) 96: 1080–1089, 2004. doi: 10.1152/japplphysiol.00771.2003. [DOI] [PubMed] [Google Scholar]
- 52.Yan X, Polo Carbayo JJ, Weibel ER, Hsia CCW. Variation of lung volume after fixation when measured by immersion or Cavalieri method. Am J Physiol Lung Cell Mol Physiol 284: L242–L245, 2003. doi: 10.1152/ajplung.00184.2002. [DOI] [PubMed] [Google Scholar]
- 53.Yang K-Y, Shih H-C, How C-K, Chen C-Y, Hsu H-S, Yang C-W, Lee Y-C, Perng R-P, Peng C-H, Li H-Y, Chang C-M, Mou C-Y, Chiou S-H. IV delivery of induced pluripotent stem cells attenuates endotoxin-induced acute lung injury in mice. Chest 140: 1243–1253, 2011. doi: 10.1378/chest.11-0539. [DOI] [PubMed] [Google Scholar]
- 54.Yilmaz C, Dane DM, Tustison NJ, Song G, Gee JC, Hsia CCW. In vivo imaging of canine lung deformation: effects of posture, pneumonectomy, and inhaled erythropoietin. J Appl Physiol (1985) 128: 1093–1105, 2020. doi: 10.1152/japplphysiol.00647.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yilmaz C, Ravikumar P, Dane DM, Bellotto DJ, Johnson RL Jr, Hsia CC. Noninvasive quantification of heterogeneous lung growth following extensive lung resection by high-resolution computed tomography. J Appl Physiol (1985) 107: 1569–1578, 2009. doi: 10.1152/japplphysiol.00503.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yilmaz C, Tustison NJ, Dane DM, Ravikumar P, Takahashi M, Gee JC, Hsia CCW. Progressive adaptation in regional parenchyma mechanics following extensive lung resection assessed by functional computed tomography. J Appl Physiol (1985) 111: 1150–1158, 2011. doi: 10.1152/japplphysiol.00527.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan S, Hannam V, Belcastro R, Cartel N, Cabacungan J, Wang J, Diambomba Y, Johnstone L, Post M, Tanswell AK. A role for platelet-derived growth factor-BB in rat postpneumonectomy compensatory lung growth. Pediatr Res 52: 25–33, 2002. doi: 10.1203/00006450-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 58.Zhang J, Cao K, Pastor JV, Li L, Moe OW, Hsia CCW. Alpha-Klotho, a critical protein for lung health, is not expressed in normal lung. FASEB Bioadv 1: 675–687, 2019. doi: 10.1096/fba.2019-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Q, Bellotto DJ, Ravikumar P, Moe OW, Hogg RT, Hogg DC, Estrera AS, Johnson RL Jr, Hsia CCW. Postpneumonectomy lung expansion elicits hypoxia-inducible factor-1alpha signaling. Am J Physiol Lung Cell Mol Physiol 293: L497–L504, 2007. doi: 10.1152/ajplung.00393.2006. [DOI] [PubMed] [Google Scholar]