Abstract
Excessive blood pressure variation is linked to the development of hypertension and other diseases. This study assesses the relative role of respiratory sinus arrhythmia (RSA) and pulse pressure (PP) on the amplitude and timing of blood pressure variability with respiration [Traube–Hering (TH) waves]. We analyzed respiratory, electrocardiogram, and blood pressure traces from healthy, supine male subjects (n = 10, mean age = 26.7 ± 1.4) during 20-min epochs of resting, slow deep breathing (SDB), and recovery. Across all epochs, blood pressure and heart rate (HR) were modulated with respiration and the magnitude of RSA; TH waves increased during SDB. The data were deconstructed using a simple mathematical model of blood pressure to dissect the relative roles of RSA and PP on TH waves. We constructed the time series of the R-wave peaks and compared the recorded TH waves with that predicted by the model. Given that cardiac output is determined by both heart rate and stroke volume, it was surprising that the magnitude of the TH waves could be captured by only HR modulation. However, RSA alone did not accurately predict the timing of TH waves relative to the respiratory cycle. Adding respiratory modulation of PP to the model corrected the phase shift showing the expected pattern of BP rising during inspiration with the peak of the TH wave during early expiration. We conclude that short-term variability of blood pressure referred to as TH waves has at least two independent mechanisms whose interaction forms their pattern: RSA and respiratory-driven changes in PP.
NEW & NOTEWORTHY Variability in blood pressure has become an important metric to consider as more is learned about the link between excessive blood pressure variability and adverse health outcomes. In this study using slow deep breathing in human subjects, we found that heart rate and pulse pressure variations have comparable effects on the amplitude of blood pressure waves, and it is the common action of the two that defines the phase relationship between respiration and blood pressure oscillations.
Keywords: blood pressure, cardiorespiratory coupling, heart rate variability, pulse pressure
INTRODUCTION
Blood pressure (BP) varies in different time frames in healthy humans: within a cardiac cycle, beat to beat, minute to minute, morning to evening, and day to day. Normal “very short-term BP variations” (36) include the following: 1) arterial pulse pressure (PP), which refers to the peak and nadir of BP associated with systolic and diastolic phases of cardiac cycle; 2) Traube–Hering (TH) waves, which refer to the BP oscillations associated with the respiratory cycle; and 3) Mayer waves, which are ∼0.1 Hz oscillations occurring over multiple respiratory cycles. Short-term variations in BP are those associated with the circadian rhythm and with the sleep-wake cycle.
Interest in BP variability has grown as a potential biometric because it has been linked in pathogenesis and mortality in animal models and humans (6, 22, 38). BP variability has both pathological and prognostic importance (5, 36). Excessive variation in BP is related to end-organ damage, adverse cardiovascular events, and hypertension. In hypertensive patients, respiratory-modulated BP variation (TH waves) rather than mean BP is a risk factor for organ damage (44). There is a higher mortality risk in patients with excessive BP variation across a range of conditions (diabetes mellitus, kidney disease, and hypertension, for example). Unlike heart rate variability (HRV) for which a lower variability is related to high risk in patients, it is excessive variation in BP that is problematic. For example, the magnitude of the TH waves was greater in spontaneously hypertensive compared with normotensive rats (31). Similarly, the magnitude of TH waves differed between robust (>185 mmHg) or weak (<140 mmHg) elevations in systolic BP during mild exercise. The robust responders had amplified TH waves compared with the weak responders; robust responders have a greater risk of developing hypertension later in life (31). In summary, TH wave amplification is associated with adverse health outcomes (5).
In this emerging paradigm, ongoing research aims to establish the mechanisms of BP variability and the amplification of TH waves to design better targeted therapies. BP variability is largely defined by variable cardiac output, which is determined by heart rate and stroke volume (SV). Therefore, one seemingly obvious mechanism for TH waves is the respiratory variation in heart rate (a.k.a. respiratory sinus arrhythmia or RSA). One the other hand, RSA may act to buffer BP variability (17, 18, 43). Therefore, perturbation of the relationship between the two respiratory coupled outcomes, heart rate and SV, could underlie the shift toward excessive BP variability.
One of the oldest problems in mammalian physiology concerns the interaction of breathing and the heart. A manifestation of this interaction is HRV on the timescale of respiration, which historically has been referred to as RSA even though it is not an arrhythmia (30). The magnitude of RSA is an easily measured biometric for human health (30a, 16). For example, a low RSA following a myocardial infarction (28) or in utero (23, 24) leads to poor prognosis. In contrast, an increase in RSA is a target metric for athlete training performance (39) and is sought by slow deep breathing therapy (41). A number of underlying mechanisms have been studied to determine the source of heart rate oscillations in RSA: 1) central respiratory-modulated input to the heart via the vagus nerve, 2) other neural components (e.g., respiratory-modulated sympathetic nerve activity, afferent vagal nerve activity, and the baroreflex), and 3) mechanical coupling (e.g., changes in intrathoracic pressure due to ventilation affecting venous return and hence stroke volume, which triggers differences in baroreflex afferent input) (11, 29, 42). The physiological function of RSA may be to increase ventilation-perfusion efficiency (21, 50). Mathematical models also indicate that it improves the energy efficiency of the heart (3, 4). As stated earlier, RSA may paradoxically buffer excessive variation in BP (17, 18, 43).
Previously, we reported that a major component of BP variability occurred at the respiratory frequency and that this variability increases during slow deep breathing (13). A priori we expected that RSA would be the primary determinant of the BP variation observed in TH waves. Even though after brain death, “respiratory” oscillations in BP persist because of mechanical ventilation, these were absent in heart rate (9), indicating that mechanisms for HR and BP variation may be differentially balanced. In particular, mechanical coupling may play a greater role in TH waves than RSA. Other neurally controlled variables, such as total peripheral resistance, vascular compliance, and stroke volume, may play a role in TH waves. In this study, we revisited the data set obtained from supine male subjects generated by Wehrwein and colleagues (27, 33, 49) to address this unresolved question in physiology.
Despite data to the contrary showing a poor predicative relationship between heart rate and blood pressure variation, we observe that RSA and blood pressure variation change in tandem with similar magnitude in healthy young people during slow deep breathing. To investigate the contributing factors to the amplitude and timing of blood pressure variation, our data set of slow deep breathing in human subjects was then deconstructed using a simple mathematical model to assess the relative importance of RSA and/or PP on TH wave timing and amplitude. This model is based on human subjects’ electrocardiogram (ECG) and continuous arterial BP data during normal and slow deep breathing. Using this approach, we tested the hypothesis that TH waves can be explained entirely by RSA (i.e., respiratory modulation of the RR interval). We observed that respiratory modulation of the RR interval alone could explain the magnitude of respiratory modulation in BP (i.e., amount of BP variation in mmHg) but not the phase (i.e., the time lag between TH waves and the respiratory cycle). A combination of both heart rate and pulse pressure variations throughout the respiratory cycle appeared to be necessary to explain a specific pattern of TH waves. Therefore, we conclude that the respiratory-related variability in the human BP has at least two independent components: a component that emerges from RSA and a component that emerges from respiratory modulation of the arterial pulse pressure (PP).
METHODS
Human recordings.
Data were recorded from young, healthy, yoga-naive men (N = 10, mean age = 26.7 ± 1.4). Supine subjects completed three consecutive 20-min epochs of breathing: baseline, a slow deep breathing (SDB), and recovery. During recovery, the participant returned to the baseline pattern. Subjects were screened and instrumented as described previously (13). Briefly, continuous monitoring of BP was done through a brachial arterial catheter with pressure transducer, heart rate was recorded using standard three-lead ECG, and respiratory rate and pattern were monitored using a calibrated double pneumobelt attached to the ribcage and abdomen. SDB did not involve any devices, pacing, and feedback. Subjects were asked to breath in and out through their nose at a slower rate than normal to their comfort level. The experiments and procedures were approved by the Institutional Review Board at the Mayo Clinic and conformed to the Declaration of Helsinki. All subjects signed an approved informed consent form. The data were deidentified to comply with Health Insurance Portability And Accountability Act (HIPAA) rules and regulations for data analysis. Previously, this data set was used by Dick et al. (13) to demonstrate an increase in the spectral power of BP in the frequency band of respiration during SDB. Here, we specifically investigate the respiratory modulation of BP.
Statistical comparisons.
We did statistical comparisons among experimental groups using the R computing environment including the CAR (Companion to Applied Regression) and PMCMRplus libraries (20, 40). For comparisons among experimental epochs, we performed a repeated-measures ANOVA followed by paired t tests for parametric comparisons. If necessary, we performed a log transformation of data to attain a normal distribution before performing an ANOVA (not depicted in bar plots). In other cases, the Friedman rank sum test and the Wilcoxon signed-rank test were used for nonparametric comparisons. For the comparisons depicted in Fig. 3, we only performed pairwise comparisons between the baseline and SDB epochs. For the data depicted in Fig. 5, we performed one-way repeated-measures or Friedman rank sum tests within the human participant, Model 1 and Model 2 data sets for each epoch. We specifically compared Model 1 groups and Model 2 groups to the human data sets in our post hoc analysis. For the data depicted in Fig. 6, we performed one-way repeated-measures or Friedman rank sum tests within Model 2, Model 2 ØRRI, and Model 2 ØPP for each epoch. As each post hoc test included two comparisons, we adjusted our alpha level using the Bonferroni correction to P = 0.025. We specifically compared Model 2 ØRRI group and Model 2 ØPP group with the Model 1 data sets in our post hoc analysis. As each post hoc test included two comparisons, we adjusted our alpha level using the Bonferroni correction to P = 0.025.
Fig. 3.
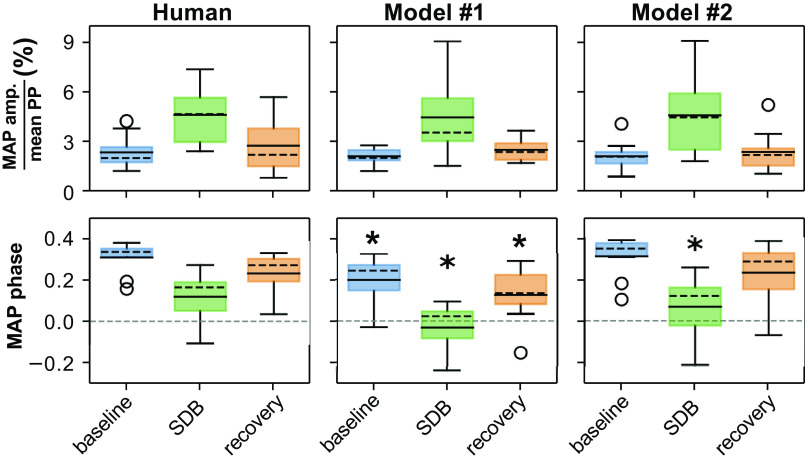
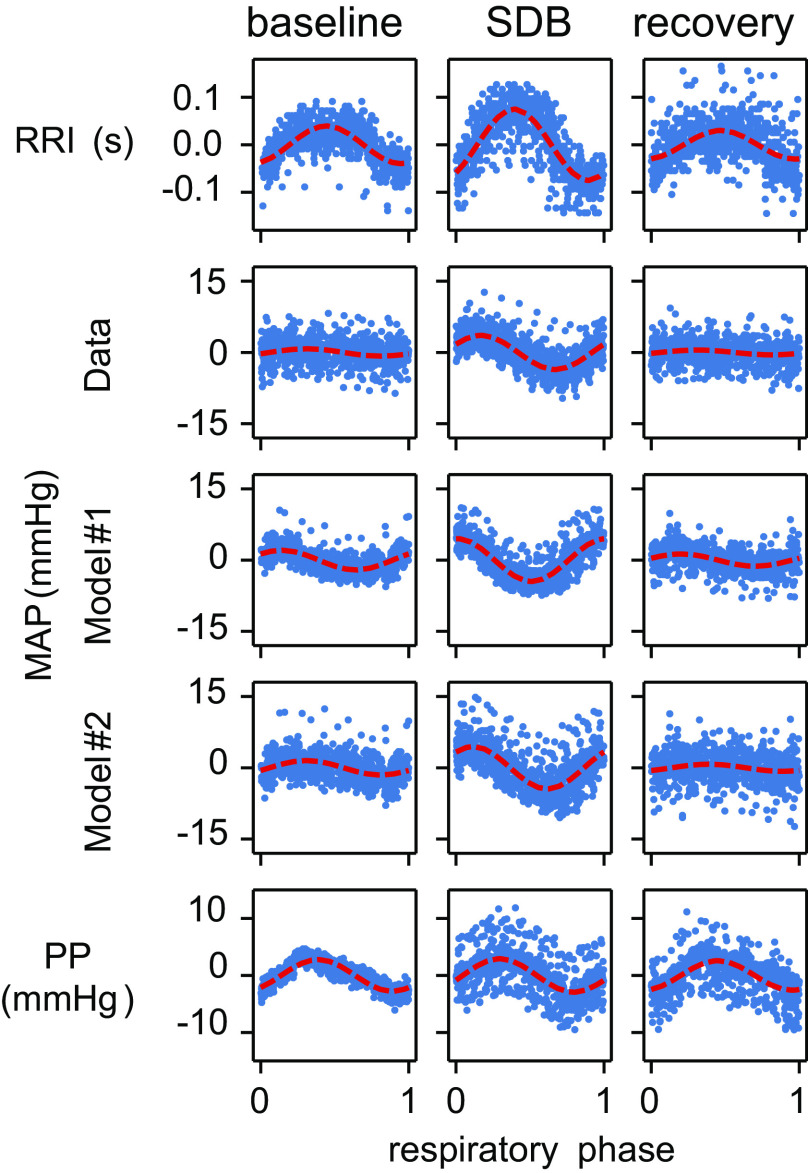
The epoch mean, the modulation amplitude, and the modulation phase characterize the respiratory modulation of the RR interval (RRI), the mean arterial pressure (MAP), and the pulse pressure (PP) by experimental epoch in human participants. These quantities are determined by the three coefficients of the cosine tuning curve (see methods). Data are depicted as box-and-whisker plots with both mean (solid black horizontal line) and median (dashed black horizontal line). *Significant difference from baseline (P < 0.05).
Fig. 5.
The amplitude of modulation of mean arterial pressure (MAP) as a percent of the pulse pressure (PP) (upper row) and the respiratory phase of MAP modulation (lower row) is used to evaluate model performance against human participant data. Data are depicted as box-and-whisker plots with both mean (solid black horizontal line) and median (dashed black horizontal line). *Significant difference compared with the corresponding epoch in the human data (P < 0.025).
Fig. 6.
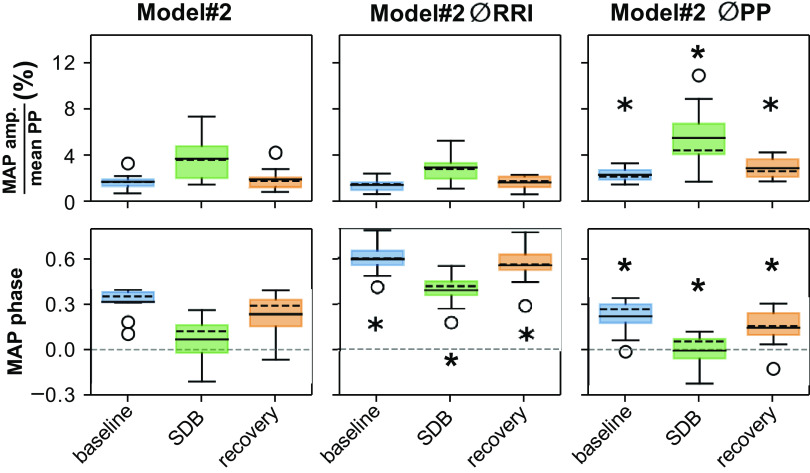
Modulation amplitude and modulation phase of MAP in Model #2 without respiratory modulation of the RR interval (Model #2 ØRRI) and without respiratory modulation of arterial pulse pressure (Model #2 ØPP). Data are depicted as box-and-whisker plots with both mean (solid black horizontal line) and median (dashed black horizontal line). *Significant difference compared with the corresponding epoch in the Model #2 (P < 0.025).
We depicted the cardiovascular response to slow deep breathing in human data and model outcomes as box-and-whisker plots. In these plots, the box indicates the extent from the lower quartile to the upper quartile. The whiskers indicate 1.5 times the extent of the interquartile range. Empty circles indicate flier points that lay outside of the range of the whiskers. These plots include both mean (solid black horizontal line) and median (dashed black horizontal line).
Analysis of ventilatory activity.
We produced a smoothed derivative of ventilatory activity by differentiating the time series with a timestep of 200 ms and then median filtering this derivative with a 200-ms window. Respiratory phase changes were characterized by detecting events where the smoothed derivatives crossed from negative to positive (putatively the beginning of inspiration) and where the smoothed derivative crossed from positive to negative (putatively the beginning of expiration). If the change in ventilation between these two time points was greater than some threshold (between 50 mL and 200 mL depending on the subject), we accepted the pair of events as a transition from expiration to inspiration and a transition from inspiration to expiration.
Analysis of cardiovascular activity.
We characterized the respiratory modulation of cardiovascular metrics with a cosine tuning curve: f(φ) = a0 + a1cos(2π(φ − a2)). The coefficient a0 quantified the mean value of the metric. The coefficient a1 quantified the amplitude of respiratory modulation of the metric. The coefficient a2 quantified the phase offset of the metric from the inspiratory-to-expiratory phase transition. For each respiratory cycle in an experimental epoch, we computed the respiratory phase of cardiovascular events. Then, the cosine tuning curve was fitted to these data to characterize the respiratory modulation of the metric in that experimental epoch. The metrics analyzed in this manner were RR interval (RRI; the difference between successive peaks of the R-wave in the ECG), the mean arterial pressure (MAP), and the PP (the difference between end-systolic and end-diastolic pressures).
Mathematical model.
We used a simple model of human BP dynamics that directly incorporates biological variability from processed participant physiological data. We modeled the BP of individual human participants by feeding heartbeat times (Model 1) or a combination of heartbeat times and the coefficients resulting from our analysis of arterial PP (Model 2) to the differential equations mentioned below. In both models, heartbeat times for each participant were produced by adding 300 ms to the time of R peaks to account for the lag between each R peak and the subsequent peak pressure event at the end of systole.
In Model 1, the BP, p, was defined by an ordinary differential equation:
The parameters p0 and τp = 750 ms determined BP dynamics between heartbeats. At the time of the heartbeat (ti), the BP was incremented by the PP0, which was fixed. dp/dt is the time derivative of BP (p). Specific values of p0 and PP0 did not matter for the analysis of model output.
In Model 2, we extended the equation for BP to include respiratory modulation of the arterial PP:
In this model, the PP fPP was a function of the respiratory phase of each R peak: fPP(φ) = PP0 + PP1cos[2π(φ − PP2)]. The coefficients PP0, PP1, and PP2 were directly drawn from our analysis of the corresponding participants’ arterial PP. Here, τp = 1,200 ms. The respiratory phase φ was defined for each R peak over the duration of the respiratory cycle: φi = [ti − Ta]/[Tb − Ta], where Ta and Tb were the beginning and end of the respiratory cycle contemporaneous with the ith R peak. The onset of each respiratory cycle was the time of the transition from inspiration to expiration.
As our model relied upon participant data, each model was instantiated for each of the available experimental epochs: three epochs for each of the 10 participants. So, each model was run for a total of 30 trials. We developed simulation software in Python using NumPy, SciPy, and pandas. The code is available in a GitHub repository at https://github.com/whbdupree/japplBloodPressureProcessing. All simulations were run on an off-the-shelf consumer desktop and laptop computers.
RESULTS
Physiological recordings during slow deep breathing.
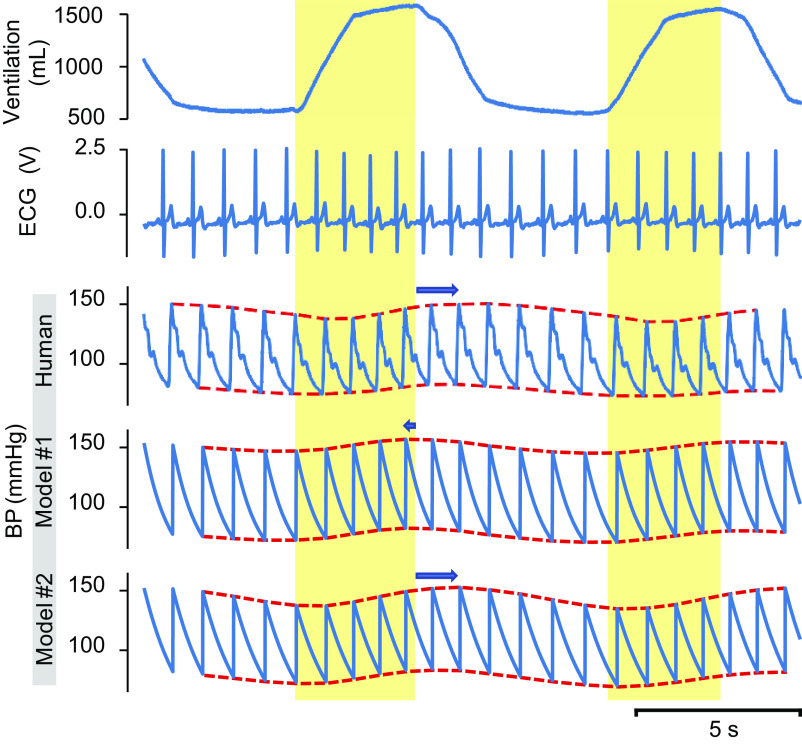
Figure 1 presents representative data from a single subject showing respiratory modulation of BP during SDB (emphasized by red dashed lines). Example recordings of tidal volume (VT; Fig. 1, 1st (top) trace), ECG (Fig. 1, 2nd trace), and BP (Fig. 1, 3rd trace) from the subject are displayed. Inspiration is noted in the yellow-shaded intervals matched to ventilation in the top tracing. Human BP is compared with two different models. The peak of the systolic BP occurs in early expiration/late post-inspiration in humans during SDB (3rd trace), but in Model 1, it occurs in late inspiration/early expiration. BP is rising during inspiration in the human subject and in Model 2. This pattern shifts in Model 1, where the peak of the TH wave is at the end of inspiration rather than during expiration, and the rising phase for BP starts with the onset of inspiration. This shift is quantified as the “modulation phase.” The amplitude and phase of BP variation are averaged and presented in Fig. 3.
Fig. 1.
Physiological recordings from a volunteer participant during slow deep breathing. Recordings include tidal volume [VT, 1st (top) trace], electrocardiogram (ECG, 2nd trace), and arterial blood pressure (BP, 3rd trace). Simulations of blood pressure from Model 1 (4th trace) and Model 2 [5th (bottom) trace] use features of the participant’s cardiovascular data as an input. The yellow-shaded intervals correspond to inspiration. The red dotted lines superimpose on the human BP tracing, and model BP traces delineate the modulation of the end-systolic and end-diastolic BP. The blue arrows show a shift of the BP modulation envelope relative to the transition time from inspiration to expiration.
Respiratory characteristics of slow deep breathing.
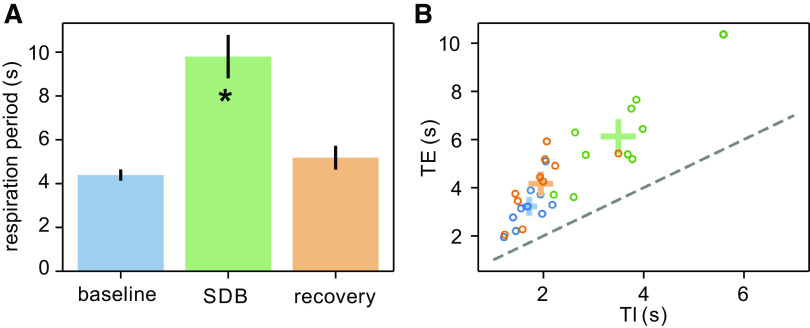
Previously, we quantified the change in respiratory frequency during SDB (13). Here, we quantified respiratory duration (seconds, TTOT), inspiration duration (TI), and expiration duration (TE). Average respiratory duration (TTOT) increased from 4.4 ± 0.3 s during baseline to 9.8 ± 1.0 s during SDB and then decreased to 5.2 ± 0.5 s in the recovery epoch (SDB vs. baseline P < 0.01; Fig. 2A). The increase in TTOT during SDB resulted from increases in both TE and TI, but TE remained greater than TI (Fig. 2B). TE increased from 3.2 ± 0.3 s during baseline to 6.1 ± 0.6 s during SDB and decreased to 4.2 ± 0.4 s during the recovery epoch (SDB vs. baseline P < 0.01; Fig. 2B). Similarly, TI increased from 1.7 ± 0.1 s during baseline to 3.5 ± 0.3 s during SDB; TI was 2.0 ± 0.2 s during the recovery epoch (SDB vs. baseline P < 0.01; Fig. 2B). So, during SDB, there was a twofold increase in duration of both inspiration and expiration as well as their variabilities.
Fig. 2.
The blue, green, and orange data, respectively, correspond to the baseline, slow deep breathing (SDB), and recovery epochs. A: respiratory duration increases during SDB. B: expiratory durations (TE) and inspiratory durations (TI) for each participant are depicted as points color coded for each epoch. The standard error for TE and TI of participants in each epoch is depicted as a colored cross at the mean TE and mean TI for each epoch. The dashed line indicates a line of slope one emerging from the origin. *Significant difference from baseline (P < 0.05).
Cardiovascular response to slow deep breathing.
In each experimental epoch, we analyzed three cardiovascular metrics: RR interval (RRI; Fig. 3, top row), MAP (Fig. 3, middle row), and PP (Fig. 3, bottom row). The respiratory modulation of each metric was quantified by its cosine tuning curve fit to the respiratory phase (see example data in Fig. 4). Respiratory phase was defined as the normalized time from one inspiration-to-expiration phase transition to the next inspiration-to-expiration phase transition. Based on the fit of the tuning curves, the respiratory modulation of each metric was characterized by three coefficients: mean value, modulation amplitude, and respiratory modulation phase (i.e., the time shift in the oscillation relative to the respiratory cycle). The first coefficient characterized the mean of each metric over the experimental epoch (Fig. 3, left column): RRI (seconds), MAP (mmHg), and PP (mmHg). The second coefficient characterized modulation amplitude over the experimental epoch (Fig. 3, middle column). For an example of modulation amplitude, see the red dashed line in Fig. 4 where the amplitude of the curve from baseline represents the amplitude of modulation. The third coefficient characterized the respiratory phase offset of the peak of modulation as a fraction of the respiratory cycle duration (modulation phase; Fig. 3, right column). Comparing baseline with SDB, we observed that the mean RRI over the entire epoch did not significantly differ among conditions, but importantly, Fig. 4 shows that in fact subjects displayed the expected increase in HR with inspiration and decrease with expiration that is amplified with SDB within a given respiratory cycle. That is to say that the modulation amplitude of RRI (the magnitude of the variation) differed between the baseline and SDB epochs (Fig. 3, top row). Also, the respiratory modulation phase of RRI differed between the baseline and SDB epochs (Fig. 3, top row). The mean, modulation amplitude, and modulation phase of MAP differed between the baseline and SDB epochs (Fig. 3, middle row). We did not detect statistically significant differences in the mean or modulation amplitude of PP between baseline and SDB, but the PP modulation phase differed between baseline and SDB (Fig. 3, bottom row). In summary, SDB enhanced TH waves and RSA. In summary, during SDB, RSA and TH waves amplified significantly, whereas the PP did not undergo statistically significant changes in terms of either its mean value or the amplitude of its respiratory modulation. However, there is a striking individual variation in PP during SDB where four of 10 subjects showed a <5 mmHg reduction (mean = −7.1 mmHg), five of 10 showed minimal change of <1 mmHg (mean = 0.1 mmHg), and one of 10 showed moderate elevation. These changes carried into the recovery period where four of 10 subjects had PP of <6 mmHg lower than their baseline values.
Fig. 4.
Exemplar scatter plots depicting model-dependent respiratory modulation of the RR interval (RRI), mean arterial pressure (MAP), and pulse pressure (PP) for a single human participant as well as the respiratory modulation of MAP in two computational models of blood pressure. In all panels, the mean metric value for that epoch is represented by 0 on the vertical axis. The dashed red curves represent the cosine tuning curve fit to the data set depicted in each panel. Note that the difference between the modulatory phase of human MAP and Model 1 MAP is strikingly apparent during the slow deep breathing (SDB) epoch.
A simple blood pressure model suggests that modulation of RRI is not sufficient to explain modulation pattern of the mean arterial pressure.
To test our hypothesis that the respiratory modulation of RRI is sufficient to produce respiratory modulation in MAP, we used a simple model of BP (Model 1, Fig. 4 and shown compared with raw human data tracing in Fig. 1). For each participant, we captured the R peak times. By feeding the time of each heartbeat to the model and incrementing the BP at the heartbeat times a constant PP, we produced a surrogate BP for that participant which depended solely on the timing of the heartbeats (see 4th trace in Fig. 1). As the difference in time between two successive R peaks was modulated by the respiratory phase, we anticipated that the MAP in the surrogate BP traces produced by Model 1 would exhibit respiratory modulation. Using the same technique performed on the human participant BP, we analyzed the surrogate BP traces produced by Model 1 to characterize the respiratory modulation of MAP (Fig. 4). We used two performance metrics to compare model activity to the experimental outcomes. The first performance metric (PM1) was the quotient of the amplitude of MAP and the epoch mean PP—in other words, the amplitude of modulation of MAP in units of PP. For the three experimental epochs, we were able to fit the model by varying the BP adaptation time constant to produce a difference in PM1 between the human participant data and the surrogate BP traces produced by Model 1 (Fig. 5) that was not significant. The second performance metric (PM2) was the modulation phase of MAP. In each of the three epochs, PM2 significantly differed between the human participant data and the surrogate BP traces produced by Model 1 (Fig. 5). This difference in the modulation phase between the human participant MAP and Model 1 MAP is visible in the BP trace during slow deep breathing (middle row in Fig. 1) and in the plot depicting MAP respiratory modulation (Fig. 4). Note that the difference between the modulatory phase of human MAP and Model 1 MAP is strikingly apparent during the slow deep breathing (SDB) epoch. There is a misalignment in the BP response at a given part of the respiratory cycle, where, for example, the lowest MAP occurs earlier in the respiratory cycle in Model 1 as compared with the human data.
We hypothesized that the respiratory modulation of PP was responsible for the difference in PM2 between human participant data and the surrogate BP traces produced by Model 1. To validate this hypothesis, we implemented a second model of BP in which PP was modulated by the respiratory phase (Model 2). In this model, the human participant R peak times provided the timing of systole events in the model. However, the respiratory phase of the R peak events was used to adjust the amplitude of the incremental changes in BP from diastole to systole (see methods). The respiratory phase of each R peak event was transformed by the cosine tuning curve using the modulation amplitude and modulation phase coefficients as measured in that human participant. In this way, the respiratory modulation of each participant’s PP was realized in the surrogate BP trace corresponding to that participant’s ECG and respiratory data. Model 2 produced a surrogate BP with phasic PP (exemplar trajectory in Fig. 1, bottom trace) and MAP modulation (Fig. 4, Model 2 MAP). We were not able to detect statistically significant differences in PM1 between the human participant data and the surrogate BP produced by Model 2 [compare BP (3rd–5th) traces in Fig. 1 and the scatterplots depicting respiratory modulation of MAP in Fig. 4]. In Model 2, PM2 only differed significantly from the human data during the SDB epoch. However, PM2 became positive—following the inspiration-to-expiration transition rather than leading it. Therefore, considering respiratory modulation of the RRI and PP together appears sufficient to reproduce both metrics of the respiratory modulation of BP as well as their changes during SDB.
We further investigated the contributions of the two sources of respiratory modulation—modulation of RRI and modulation of PP—to the modulation of BP by removing one of the putative sources. We achieved this by setting in the model each participant’s RRI or PP to the mean value for the corresponding experimental epoch (Fig. 6). By doing so, in this model, the elimination of respiratory modulation in RRI (Model 2 ØRRI) did not significantly reduce PM1 (the amplitude of respiratory modulation of MAP expressed as a percent of mean PP) in any of the three experimental epochs. However, the elimination of respiratory modulation in PP (Model 2 ØPP) significantly increased PM1 in all three experimental epochs. PM2 (the phase shift of the respiratory modulation of MAP) significantly increased in Model 2 ØRRI across all three epochs and significantly decreased in Model 2 ØPP across all three epochs.
DISCUSSION
Variation in BP has become a metric of interest in the clinic because, in contrast to HRV, increased variation has been linked to disease and mortality in animals and humans. Understanding the mechanisms of BP variation in healthy humans is important if we are to improve monitoring and treatment of patients with exaggerated TH waves associated with disease progression. Using a robust data set of 20-min epochs of baseline breathing, SDB, and recovery breathing, we characterized the respiratory modulation of RRI (RSA), BP (TH waves), and PP in supine human data and tested the hypothesis that RSA was responsible for TH waves.
After implementing a mathematical model that used human participant RRI as the only input to produce surrogate BP traces (Model 1), our simulations captured one aspect of respiratory modulation of MAP (modulation amplitude; i.e., the magnitude of the variation in mmHg) but not another (modulation phase; i.e., the time lag in the BP oscillation relative to the respiratory phase). Although we were initially impressed that RSA alone could account for the magnitude of TH waves, we realized that that RSA was not the sole mechanism of TH waves, as the timing of the BP oscillations to the respiratory cycle was temporally offset from that observed in the human data. Thus, we implemented a second model that incorporated the respiratory modulation of PP as well as RRI (Model 2), and this model succeeded in capturing both the magnitude of variation (i.e., modulation amplitude) and alignment of BP oscillations with the respiratory cycle (i.e., modulation phase).
Role of respiratory sinus arrhythmia in blood pressure variation.
Although RSA results from redundant mechanisms, respiratory-modulated vagal activity is the primary one. Vagal preganglionic neuronal activity that originates in the brainstem modulates heart rate via inhibition of the sinoatrial node (14) and may be modulated by inputs from neurons of the respiratory central pattern generator. On the other hand, RSA may emerge from the contribution of the baroreflex because of changes in BP (26). Physiological mechanisms may differ as breathing modalities change; for instance, the recruitment of slowly adapting pulmonary receptors may act to increase HRV during slow deep breathing (35).
The role of RSA in the respiratory modulation of BP is persistently controversial (37). Several experiments have sought to eliminate or otherwise manipulate RSA with a view of investigating its role in BP homeostasis. Understanding the factors that control MAP, especially those related to cardiorespiratory coupling, may be critical for dissecting mechanisms of hypertension (34). For instance, applying intermittent hypoxia over 30 days can induce sustained hypertension not by increased RSA but increased sympathorespiratory coupling (19). However, 10 exposures acutely can augment sympathorespiratory coupling without increasing BP (12).
Vagal modulation of heart rate acts by enhancing or relieving inhibitory tone and during different phases of respiration. When vagal inhibition of sinoatrial (SA) node pacing is weakest (i.e., low suppression of SA node), the RR interval is shortest (i.e., faster heart rate). Vagal modulation of heart rate can be altered by systemic application of atropine. Toska and Eriksen (47) systemically blocked cholinergic receptors in supine humans; this manipulation eliminated respiratory modulation of the RR interval, but the variance of MAP increased on the timescale of respiration. Elstad et al. (17) also eliminated respiratory modulation in the RR interval with atropine; they observed an increase in the variance of MAP and a decrease in the variance of systolic pressure on the timescale of respiration following the atropine dose. However, Tan and Taylor (43) administered separate vagotonic and vagolytic doses of atropine to supine humans. The change in respiratory fluctuation strength of diastolic pressure correlated positively with the change in respiratory fluctuation strength of the RR interval; in other words, the strength of respiratory fluctuations in diastolic pressure decreased with a decrease in RSA (43).
Atrial pacing can be used to initiate a heartbeat before its naturally paced timing from the SA node. Using atrial pacing, respiratory modulation of HRV can be eliminated by forcing all RR intervals to be less than or equal to the shortest RR intervals produced during restful inhalation. This manipulation is experimentally attractive because it is targeted to direct changes in heart rate while altering the underlying physiological state to a substantially lesser extent than systemic pharmacological inhibition does. When HRV is abolished in this manner, the spectral power of systolic and diastolic pressure decreases in supine humans (45).
The magnitude of RSA can be reduced by intermittent positive pressure ventilation (IPPV) versus paced breathing at the same respiratory frequency (18). IPPV attenuates the mechanical aspects of negative pleural pressure increasing venous return and the central respiratory drive, whereas these mechanisms remain intact in paced breathing. In the 10 subjects, IPPV decreased RSA and increased the magnitude of TH waves, whereas paced breathing retained RSA and decreased the magnitude of the TH wave to 50% of that during IPPV. Although the MAP was not significantly different, it trended to be lower during paced compared with ventilated breathing (P = 0.11 with 10 subjects). Thus, paradoxically, RSA acted to buffer TH waves rather than to produce them (18).
Finally, previous models of HRV and MAP have incorporated multiple factors, such as vagal feedback from pulmonary stretch receptors, central medullary coupling between respiratory and cardiovagal neurons, and arterial baroreceptor feedback (25), which contribute over other timescales. We adopted a more straightforward approach accepting the integrated physiological signals to feed our model.
Role of pulse pressure in blood pressure variability.
As noted above, we show that RSA is involved in the regulation of TH waves, but additional mechanisms other than parasympathetic input to the heart modulate BP oscillation on the timescale of respiration. Indeed, lung transplant patients do not experience substantial respiratory modulation of their heart rate but do exhibit respiratory modulation of the end-systolic BP (42). In our Model 2, we implement a heart rate-independent mechanism—respiratory modulation of arterial PP—to capture this phenomenon. As compared with MAP, which is very well described by cardiac output and total peripheral resistance, PP (the difference between systolic and diastolic arterial pressures) has more nuance. In general, PP is related to stroke volume in healthy young people; however, one must also take into consideration the vascular characteristics such as arterial stiffness, vessel compliance, and wave reflection. In particular, the stiffness of vessels is related to overall arterial compliance and impacts left ventricular afterload and pulse wave velocities.
PP is an interesting metric to consider in the context of disease progression associated with BP variability, as increased PP itself has been associated with a number of adverse outcomes with aging and disease (10, 48). Transient alterations in PP can be because of changes in systolic pressure, diastolic pressure, stroke volume, or vascular compliance. PP varies among individuals based on sex, age, height, body position, and genetics.
Our subjects had normal ranges of PP (40–60 mmHg), which, despite lack of statistical significance, showed a mean reduction of 5 mmHg during SDB that was sustained into recovery. This magnitude of change of PP in a given individual would be considered clinically relevant and cannot be ignored. Therefore, additional work is needed to determine why some subjects show substantial changes in PP during SDB that are sustained in recovery, yet others do not. This modulation may be attributed to phasic respiratory-related baroreflex-mediated changes to vascular tone. Alternately, this modulation may be related to the physical expansion of the lungs because of negative pleural pressure during inspiration, which would increase venous return and hence cardiac stroke volume. Despite the underlying complexities, the use of PP in Model 2 allows for a matching of the model output to human BP data in terms of alignment of the modulation phase (the time lag between BP oscillations and the respiratory phase). Our data, in combination with the recognition that increases in PP are predictive of adverse outcomes in a range of conditions, support additional exploration into the role of PP in BP variability.
The phase shifts of respiratory modulation in RRI and PP are similar in each epoch (Figs. 3 and 4); that is, the modulation of PP reaches its peak at roughly the same respiratory phase as the RRI. When RR intervals are longest (which corresponds to the lowest heart rate), the MAP is declining from beat to beat, but PP is increasing and near its modulatory peak (Fig. 4). Similarly, the respiratory phase where RRI is shortest is near the respiratory phase where PP is smallest. In this case, the rapid beating of the heart is more likely to lead to an increase in BP by way of temporal summation, but the arterial PP is smaller, thus damping the effect of higher heart rate. However, the effects of these two mechanisms are almost aligned in antiphase, and the tuning of the phase shifts of respiratory modulation in RRI and PP could be used to produce specific outcomes in the modulation of MAP. Moreover, the discrepancies in BP response to manipulation of RSA could result from differential phase shifts of RRI and PP because of differential experimental conditions (17, 18, 43, 45, 47). For example, for humans lying in the supine position, Elstad et al. (17) determined the phase relationship of heart rate and MAP to respiratory cycle (set to 0° phase angle), before and after attenuating RSA by administering propranolol and atropine. The phase angle of MAP remained ∼225° out of phase (180° is the inverse phase) before and after attenuating RSA, whereas HR shifted its phase angle with respiration from 15° to 110°. Subsequently, Elstad et al. (18) compared these phase angles with intermittent positive pressure ventilation (IPPV) and breathing timed by a metronome. In this case, the phase angle for HR remained close to that for respiration, but the phase angle for MAP changed from nearly aligned with inflation during IPPV to 250° out of phase with respiration. These data indicate independence in MAP and HR in aligning with respiration, but both studies supported the interpretation that RSA buffers TH waves and stabilizes BP.
Limitations.
Given our observation that it was necessary to consider both RSA and the respiratory modulation of PP to explain the amplitude of TH waves and the timing of BP peak relative to the respiratory cycle, it is natural to delve deeper into the specific role for stroke volume or cardiac output. We did not directly assess either parameter in this study, as one can argue that stroke volume and pulse pressure are related but not the same thing. This study was designed to go after the simplest model to predict and understand blood pressure variation, and in fact, it comes down to an interesting interplay between different components of the cardiac output (HR and stroke volume). Either of them can produce BP oscillations of observed or even higher magnitude; however, both are required to form a wave with the observed phase relative to the breathing cycle. The authors recognize, as already noted, that PP is a complex parameter, yet its use in this study is a foundation for further studies to delve into which components of PP are most relevant to BP variability, with a particular interest on stroke volume and vascular stiffness.
Given important sex differences in blood pressure regulation, it is important to address the limitation that this data set was collected from men only. This is particularly relevant because it relates to the presence of β-adrenergic receptor-mediated vasodilation, which offsets sympathetic vasoconstriction in young women (1). This balancing effect of vasodilation diminishes with age, and so, these data in men are more relevant to postmenopausal rather than young women. In terms of overall blood pressure variations across sexes, several studies have addressed this in Mayer waves. Mayer waves appear to be similar in young women, young men, and older men, despite vastly different sympathetic outflow patterns (8). Further, the variability of Mayer waves is similar across sexes and age, indicating that variability alone may not reflect sympathetically mediated vasoconstriction (46). For other blood pressure parameters, no sex difference exists in response of blood pressure to exercise between young men and young women; specifically, systolic, diastolic, mean, and pulse pressures were consistent across the sexes even considering high- and low-responder binning of the data set (31).
The relatively small sample size of 10 healthy male participants could be viewed as too few for the conclusions in the human data as well as for being the basis for the models. However, this sample size resulted in an adequate effect size for key observations in heart rate and blood pressure respiratory modulation as well as for discrimination between mathematical models used for their interpretation. The authors recognize that this sample size limits further interpretation of data.
We used a 20-min epoch of data to calculate parameters of interest because we are focused on events occurring over a short timescale and slow deep breathing over 20 min is doable for an untrained, naive individual. A key task-force paper has suggested that the analysis of time series into meaningful clinical data may need up to 24 h of recording (43a), but this is for an analysis of relevant but intermittent events and thus related to screening rather than assessing short-term response to a perturbation. We used nonoverlapping epochs of an individual’s recording and found that within-subject variability of the measures was negligibly smaller than subject-to-subject variability. Therefore, in terms of statistical power, our analysis would not benefit from longer recordings.
Slow deep breathing reduces the respiratory rate to such a level that in terms of frequency, TH wave blood pressure oscillations become aligned with the slower Mayer waves related to sympathetic nerve-mediated oscillations. This is akin to vagotomy studies that likewise slow breathing resulting in apparent synchrony between TH and Mayer waves (7, 32). If synchronization between TH and Mayer waves occurred during slow deep breathing in our experiments, it could significantly affect the pattern of blood pressure modulation. However, our analysis shows that this effect (if any) is within variability limits because surrogate blood pressure traces generated by the model are statistically indistinguishable from the recorded ones in terms of respiratory modulation.
Conclusion.
Variability in BP has become an increasingly important metric to consider as more is learned about the link between excessive BP variability and adverse health outcomes. The use of data from healthy human subjects is a strong foundation for developing an understanding of the mechanisms of variability of BP and can offer insight to the pathways that go awry in disease progression. This study uses a combination of human subject data and mathematical modeling to test mechanisms of TH waves. We conclude that BP variability has at least two independent mechanisms: RSA and respiratory modulation of PP.
GRANTS
The study was supported by NIH grants R01 AT008632 (to Y. I. Molkov) and U01 EB021960 (to T. E. Dick and Y. I. Molkov). The human data were collected by E. A. Wehrwein. in collaboration with Michael Joyner under support of the NIH grant HL 083947.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.H.B., T.E.D., E.A.W., and Y.I.M. conceived and designed research; E.A.W. performed experiments; W.H.B. and Y.I.M. analyzed data; W.H.B., T.E.D., E.A.W., and Y.I.M. interpreted results of experiments; W.H.B. and Y.I.M. prepared figures; W.H.B., T.E.D., E.A.W., and Y.I.M. drafted manuscript; W.H.B., E.M.L., R.A.C., T.E.D., E.A.W., and Y.I.M. edited and revised manuscript; W.H.B., E.M.L., R.A.C., T.E.D., E.A.W., and Y.I.M. approved final version of manuscript.
REFERENCES
- 1.Baker SE, Limberg JK, Ranadive SM, Joyner MJ. Neurovascular control of blood pressure is influenced by aging, sex, and sex hormones. Am J Physiol Regul Integr Comp Physiol 311: R1271–R1275, 2016. doi: 10.1152/ajpregu.00288.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Tal A, Shamailov SS, Paton JF. Central regulation of heart rate and the appearance of respiratory sinus arrhythmia: new insights from mathematical modeling. Math Biosci 255: 71–82, 2014. doi: 10.1016/j.mbs.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Tal A, Shamailov SS, Paton JF. Evaluating the physiological significance of respiratory sinus arrhythmia: looking beyond ventilation-perfusion efficiency. J Physiol 590: 1989–2008, 2012. doi: 10.1113/jphysiol.2011.222422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chadachan VM, Ye MT, Tay JC, Subramaniam K, Setia S. Understanding short-term blood-pressure-variability phenotypes: from concept to clinical practice. Int J Gen Med 11: 241–254, 2018. doi: 10.2147/IJGM.S164903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chenniappan M. Blood pressure variability: assessment, prognostic significance and management. J Assoc Physicians India 63: 47–53, 2015. [PubMed] [Google Scholar]
- 7.Cherniack NS, Edelman NH, Fishman AP. Pattern of discharge of respiratory neurons during systemic vasomotor waves. Am J Physiol 217: 1375–1383, 1969. doi: 10.1152/ajplegacy.1969.217.5.1375. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MA, Taylor JA. Short-term cardiovascular oscillations in man: measuring and modelling the physiologies. J Physiol 542: 669–683, 2002. doi: 10.1113/jphysiol.2002.017483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conci F, Di Rienzo M, Castiglioni P. Blood pressure and heart rate variability and baroreflex sensitivity before and after brain death. J Neurol Neurosurg Psychiatry 71: 621–631, 2001. doi: 10.1136/jnnp.71.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dart AM, Kingwell BA. Pulse pressure--a review of mechanisms and clinical relevance. J Am Coll Cardiol 37: 975–984, 2001. doi: 10.1016/S0735-1097(01)01108-1. [DOI] [PubMed] [Google Scholar]
- 11.Di Rienzo M, Parati G, Radaelli A, Castiglioni P. Baroreflex contribution to blood pressure and heart rate oscillations: time scales, time-variant characteristics and nonlinearities. Philos Trans A Math Phys Eng Sci 367: 1301–1318, 2009. doi: 10.1098/rsta.2008.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dick TE, Hsieh YH, Wang N, Prabhakar N. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp Physiol 92: 87–97, 2007. doi: 10.1113/expphysiol.2006.035758. [DOI] [PubMed] [Google Scholar]
- 13.Dick TE, Mims JR, Hsieh YH, Morris KF, Wehrwein EA. Increased cardio-respiratory coupling evoked by slow deep breathing can persist in normal humans. Respir Physiol Neurobiol 204: 99–111, 2014. doi: 10.1016/j.resp.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckberg DL. Point:counterpoint: respiratory sinus arrhythmia is due to a central mechanism vs. respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol (1985) 106: 1740–1742, 2009. doi: 10.1152/japplphysiol.91107.2008. [DOI] [PubMed] [Google Scholar]
- 16.Elstad M, O’Callaghan EL, Smith AJ, Ben-Tal A, Ramchandra R. Cardiorespiratory interactions in humans and animals: rhythms for life. Am J Physiol Heart Circ Physiol 315: H6–H17, 2018. doi: 10.1152/ajpheart.00701.2017. [DOI] [PubMed] [Google Scholar]
- 17.Elstad M, Toska K, Chon KH, Raeder EA, Cohen RJ. Respiratory sinus arrhythmia: opposite effects on systolic and mean arterial pressure in supine humans. J Physiol 536: 251–259, 2001. doi: 10.1111/j.1469-7793.2001.t01-1-00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elstad M, Walløe L, Holme NL, Maes E, Thoresen M. Respiratory sinus arrhythmia stabilizes mean arterial blood pressure at high-frequency interval in healthy humans. Eur J Appl Physiol 115: 521–530, 2015. doi: 10.1007/s00421-014-3042-3. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher EC. Invited review: physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol (1985) 90: 1600–1605, 2001. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- 20.Fox J, Weisberg S. An R companion to applied regression. Los Angeles, CA: SAGE, 2019, p. 577. [Google Scholar]
- 21.Hayano J, Yasuma F, Okada A, Mukai S, Fujinami T. Respiratory sinus arrhythmia. A phenomenon improving pulmonary gas exchange and circulatory efficiency. Circulation 94: 842–847, 1996. doi: 10.1161/01.CIR.94.4.842. [DOI] [PubMed] [Google Scholar]
- 22.Höcht C. Blood pressure variability: prognostic value and therapeutic implications. Int Sch Res Notices 2013: 398485, 2013. doi: 10.5402/2013/398485. [DOI] [Google Scholar]
- 23.Hon EH, Lee ST. Electronic evaluation of the fetal heart rate. Viii. Patterns preceding fetal death, further observations. Am J Obstet Gynecol 87: 814–826, 1963. [PubMed] [Google Scholar]
- 24.Hon EH, Lee ST. The fetal electrocardiogram. I. The electrocardiogram of the dying fetus. Am J Obstet Gynecol 87: 804–813, 1963. [PubMed] [Google Scholar]
- 25.Jo JA, Blasi A, Valladares E, Juarez R, Baydur A, Khoo MC. Determinants of heart rate variability in obstructive sleep apnea syndrome during wakefulness and sleep. Am J Physiol Heart Circ Physiol 288: H1103–H1112, 2005. doi: 10.1152/ajpheart.01065.2003. [DOI] [PubMed] [Google Scholar]
- 26.Karemaker JM. Counterpoint: respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol (1985) 106: 1742–1743, 2009. doi: 10.1152/japplphysiol.91107.2008a. [DOI] [PubMed] [Google Scholar]
- 27.Kelly K, Dick T, Lin J, Moser J, Wehrwein E. Self-paced slow deep breathing: persistence of effects on vascular function (Abstract). FASEB J 29: 652.617, 2015. [Google Scholar]
- 28.Lanza GA, Guido V, Galeazzi MM, Mustilli M, Natali R, Ierardi C, Milici C, Burzotta F, Pasceri V, Tomassini F, Lupi A, Maseri A. Prognostic role of heart rate variability in patients with a recent acute myocardial infarction. Am J Cardiol 82: 1323–1328, 1998. doi: 10.1016/S0002-9149(98)00635-3. [DOI] [PubMed] [Google Scholar]
- 29.Larsen PD, Tzeng YC, Sin PY, Galletly DC. Respiratory sinus arrhythmia in conscious humans during spontaneous respiration. Respir Physiol Neurobiol 174: 111–118, 2010. doi: 10.1016/j.resp.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Ludwig C. Beitrage zur Kenntnis des Einflusses der Respirationsbewegung auf den Blutlauf im Aortensystem. Arch Anat Physiol Wiss Med 242–302, 1847. [Google Scholar]
- 30a.Malik M, Bigger JT, Camm AJ, Kleiger RE, Malliani A, Moss AJ, Schwartz PJ; Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology . Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J 17: 354–381, 1996. doi: 10.1093/oxfordjournals.eurheartj.a014868. [DOI] [PubMed] [Google Scholar]
- 31.Menuet C, Le S, Dempsey B, Connelly AA, Kamar JL, Jancovski N, Bassi JK, Walters K, Simms AE, Hammond A, Fong AY, Goodchild AK, McMullan S, Allen AM. Excessive respiratory modulation of blood pressure triggers hypertension. Cell Metab 25: 739–748, 2017. doi: 10.1016/j.cmet.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Morris KF, Nuding SC, Segers LS, Baekey DM, Shannon R, Lindsey BG, Dick TE. Respiratory and Mayer wave-related discharge patterns of raphé and pontine neurons change with vagotomy. J Appl Physiol (1985) 109: 189–202, 2010. doi: 10.1152/japplphysiol.01324.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mozer M, Fadel P, Johnson C, Wallin B, Charkoudian N, Drobish J, Joyner M, Wehrwein E. Acute slow-paced breathing increases periods of sympathetic nervous system quiescence (Abstract). FASEB J 28: 1170.1112, 2014. [Google Scholar]
- 34.Mussalo H, Vanninen E, Ikäheimo R, Laitinen T, Laakso M, Länsimies E, Hartikainen J. Heart rate variability and its determinants in patients with severe or mild essential hypertension. Clin Physiol 21: 594–604, 2001. doi: 10.1046/j.1365-2281.2001.00359.x. [DOI] [PubMed] [Google Scholar]
- 35.Noble DJ, Hochman S. Hypothesis: pulmonary afferent activity patterns during slow, deep breathing contribute to the neural induction of physiological relaxation. Front Physiol 10: 1176, 2019. doi: 10.3389/fphys.2019.01176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parati G, Luscher TF, Ochoa JE. Short-Term Blood Pressure Variability, edited by Mancia G, Grassi G, Tsioufis K, Dominiczak A, Rosei E. Boca Raton, FL: CRC Press, 2019, p. 209–216. [Google Scholar]
- 37.Parati G, Mancia G, Di Rienzo M, Castiglioni P. Point: cardiovascular variability is/is not an index of autonomic control of circulation. J Appl Physiol (1985) 101: 676–678, 2006. doi: 10.1152/japplphysiol.00446.2006. [DOI] [PubMed] [Google Scholar]
- 38.Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol 10: 143–155, 2013. [Erratum in Nat Rev Cardiol 11: 314, 2014]. doi: 10.1038/nrcardio.2013.1. [DOI] [PubMed] [Google Scholar]
- 39.Plews DJ, Laursen PB, Stanley J, Kilding AE, Buchheit M. Training adaptation and heart rate variability in elite endurance athletes: opening the door to effective monitoring. Sports Med 43: 773–781, 2013. doi: 10.1007/s40279-013-0071-8. [DOI] [PubMed] [Google Scholar]
- 40.Pohlert T. PMCMRplus: Calculate Pairwise Multiple Comparisons of Mean Rank Sums Extended. R Package Version 1 2018. https://cran.r-project.org/web/packages/PMCMRplus/index.html.
- 41.Russo MA, Santarelli DM, O’Rourke D. The physiological effects of slow breathing in the healthy human. Breathe (Sheff) 13: 298–309, 2017. doi: 10.1183/20734735.009817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taha BH, Simon PM, Dempsey JA, Skatrud JB, Iber C. Respiratory sinus arrhythmia in humans: an obligatory role for vagal feedback from the lungs. J Appl Physiol (1985) 78: 638–645, 1995. doi: 10.1152/jappl.1995.78.2.638. [DOI] [PubMed] [Google Scholar]
- 43.Tan CO, Taylor JA. Does respiratory sinus arrhythmia serve a buffering role for diastolic pressure fluctuations? Am J Physiol Heart Circ Physiol 298: H1492–H1498, 2010. doi: 10.1152/ajpheart.00974.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43a.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93: 1043–1065, 1996. doi: 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- 44.Tatasciore A, Renda G, Zimarino M, Soccio M, Bilo G, Parati G, Schillaci G, De Caterina R. Awake systolic blood pressure variability correlates with target-organ damage in hypertensive subjects. Hypertension 50: 325–332, 2007. doi: 10.1161/HYPERTENSIONAHA.107.090084. [DOI] [PubMed] [Google Scholar]
- 45.Taylor JA, Eckberg DL. Fundamental relations between short-term RR interval and arterial pressure oscillations in humans. Circulation 93: 1527–1532, 1996. doi: 10.1161/01.CIR.93.8.1527. [DOI] [PubMed] [Google Scholar]
- 46.Taylor JA, Williams TD, Seals DR, Davy KP. Low-frequency arterial pressure fluctuations do not reflect sympathetic outflow: gender and age differences. Am J Physiol Heart Circ Physiol 274: H1194–H1201, 1998. doi: 10.1152/ajpheart.1998.274.4.H1194. [DOI] [PubMed] [Google Scholar]
- 47.Toska K, Eriksen M. Respiration-synchronous fluctuations in stroke volume, heart rate and arterial pressure in humans. J Physiol 472: 501–512, 1993. doi: 10.1113/jphysiol.1993.sp019958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on Hypertension . Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 66: 698–722, 2015. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vianna LC, Fadel PJ, Joyner MJ, Wehrwein EA. Acute slow-paced breathing modulates the pressor and depressor responses following spontaneous oscillations in muscle sympathetic nerve activity (Abstract). FASEB J 30: 752.758, 2016. [Google Scholar]
- 50.Yasuma F, Hayano J. Respiratory sinus arrhythmia: why does the heartbeat synchronize with respiratory rhythm? Chest 125: 683–690, 2004. doi: 10.1378/chest.125.2.683. [DOI] [PubMed] [Google Scholar]