To the Editor:
The factors that facilitate and impact transmission of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are not well understood, particularly in children and young adults. It has been established that the S (Spike) protein of SARS-CoV-2 binds to ACE2 (angiotensin-converting enzyme 2) as the entry receptor and employs the serine protease TMPRSS2 for proteolytic separation of the S protein subunits, termed “S protein priming,” which is necessary for membrane fusion (1). ACE2 and TMPRSS2 are expressed in ciliated epithelial cells of the upper and lower airway where the initial transmission of the virus likely occurs and are expressed at high concentrations on pneumocytes in the distal lung (2). Although the upregulation of this system serves as a potential mechanism to facilitate viral entry and thus transmission, there is also evidence that upregulation of ACE2 may decrease the severity of the disease (3, 4). Thus, we questioned whether asthma, which is the most prevalent chronic medical condition in children and young adults, or respiratory viral infection, which serves as a common trigger for asthma in this population, alters the expression of either ACE2 or TMPRSS2. Infections with major serotype rhinovirus, including human rhinovirus-A16 (HRV-A16) are responsible for the majority of HRV infections in children and young adults. For other respiratory viruses, it has been established that heterologous viral infection can alter the susceptibility and immune response to a second respiratory viral insult (5).
We initially examined the expression of the ACE2 and TMPRSS2 genes in epithelial brushings from a previously described cohort of individuals with and without asthma who were extensively characterized for airway hyperresponsiveness (AHR) and were not using controller therapy (6). We did not see any evidence of differential expression between subjects with asthma and healthy control subjects (Figures 1A and 1C), which is consistent with other emerging data (7). We did not detect any correlation between ACE2 expression and baseline lung function or direct AHR (data not shown). However, subjects with asthma defined by both a positive methacholine challenge (i.e., direct AHR) and indirect or endogenous AHR as determined by a dry air exercise challenge to assess exercise-induced bronchoconstriction (EIB+) were found to have higher concentrations of ACE2 expression in comparison with subjects with asthma without EIB (EIB−, Figure 1A). There was also a modest association between ACE2 expression with the severity of EIB as determined by the area under the forced expiratory volume in 1 second (FEV1) versus 30 minutes postexercise challenge time curve (AUC30) (Figure 1B). In ptcontrast, the expression of TMPRSS2 correlated with lower baseline FEV1 (Figure 1D) and baseline FEV1/forced vital capacity (FVC) values (r2 = 0.31; P = 0.006) but not direct or indirect AHR. We acknowledge the inherent limitations of this study and their impacts on our findings, particularly the small number of control subjects and the analysis of multiple subgroups.
Figure 1.
Epithelial brushings were obtained via bronchoscopy from the fourth- and fifth- generation airways from a cohort of individuals with and without asthma, and for those with asthma, with and without exercise-induced bronchoconstriction (EIB) (control, n = 3; EIB−, n = 9; EIB+, n = 11). (A and C) ACE2 (angiotensin-converting enzyme 2) expression (A) and TMPRSS2 expression (C) were quantified by normalized log2 counts. Median values with interquartile ranges are shown. P values represent the result of a one-way ANOVA. There was a trend toward correlation between ACE2 expression and indirect or endogenous airway hyperresponsiveness as determined by the severity of EIB, defined by the area under the forced expiratory volume in 1 second (FEV1) versus 30 minutes postexercise challenge time curve (B). (D) TMPRSS2 expression correlated with baseline FEV1. Linear regression lines are shown with the 95% confidence intervals as dotted lines. AUC30 = 30 minutes postexercise challenge time curve; TMPRSS2 = transmembrane serine protease 2.
We also examined the expression of ACE2 and TMPRSS2 in ex vivo cultured lower airway epithelial cells (AECs) from children with and without asthma both at baseline and during infection with HRV-A16 for 48 hours (6). There were no significant differences in the age, sex, baseline FEV1, and fraction of exhaled nitric oxide between the groups, but the children with asthma had more airflow obstruction measured by the FEV1/FVC ratio (P = 0.02), significantly higher total serum IgE concentrations (mean values of 381 vs. 16 kU/L; P = 0.01), and higher number of positive allergen-specific IgE titers on radioallergosorbent testing (mean values, 2.7 vs. 0; P < 0.01) relative to healthy control children. We did not identify any difference in the baseline expression amount of ACE2 and TMPRSS2 between AECs cultured from children with asthma compared with healthy control children; however, there was upregulation of both ACE2 and TMPRSS2 expression in response to infection with HRV-A16 in AECs from children with asthma and healthy control children (Figures 2A and 2D). We further confirmed these results using primary tracheal AECs from nondiseased donors to demonstrate increased expression of ACE2 (Figure 2B) and TMPRSS2 (Figure 2E) after infection with HRV-A16. Interestingly, the expression pattern of these genes differs after HRV-A16 infection, as TMPRSS2 expression increased at 4 hours postinfection and remained elevated at 72 hours (Figure 2E), whereas ACE2 expression was decreased at 4 hours but then increased at 48 and 72 hours after infection (Figure 2B). This suggests that changes in ACE2 and TMPRSS2 expression after HRV infection are regulated by different mechanisms. Rhinovirus infection is known to induce a type 1 and type III IFN response in airway epithelium (8), which prompted us to stimulate pediatric AECs with IFNβ1 and IFNλ2 and evaluate their effects on ACE2 and TMPRSS2 expression. We found IFNβ1 stimulation resulted in increased ACE2 expression, but IFNλ2 stimulation did not induce the expression of ACE2 (Figure 2C). There was no effect of either IFN on TMPRSS2 expression (Figure 2F).
Figure 2.
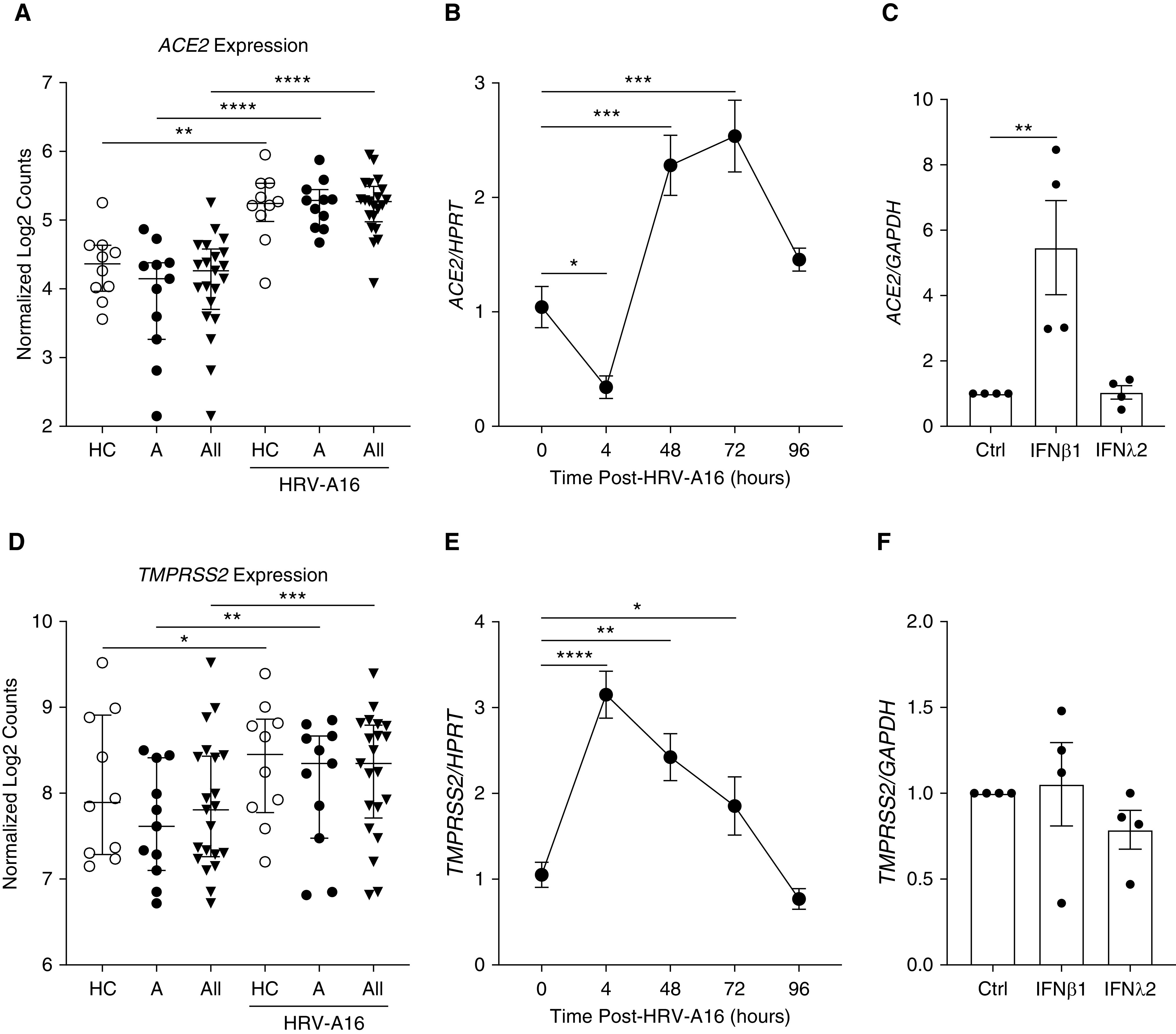
(A and D) RNA sequencing was performed on airway epithelial cells (AECs) obtained via blind bronchial epithelial brushings from intubated pediatric study subjects (healthy control subjects [HC], subjects with asthma [A], and all subjects [All]) and differentiated in organotypic cultures. ACE2 expression (A) and TMPRSS2 expression (D) were quantified by normalized log2 counts both at baseline and after infection with human rhinovirus-A16 (HRV-A16) at 48 hours. Median values with interquartile ranges are shown. P values comparing HC to A ± HRV-A16 represent the results of a two-way ANOVA. P values comparing All ± HRV-A16 represent the results of paired t tests. (B and E) PCR analysis was performed on nondiseased lung transplant donor AECs differentiated in air–liquid interface organotypic cultures and exposed to HRV-A16. ACE2 (B) and TMPRSS2 (E) expression levels in comparison with the housekeeping gene HPRT were assessed at various time points after infection (n = 4, each data point represents the mean of two PCR reactions). Mean values are shown with error bars representing the SE of the mean. P values represent the result of one-way ANOVA. (C and F) PCR analysis was performed on AECs from healthy control pediatric study subjects who were stimulated with IFNβ1 (1 ng/ml) and IFNλ2 (1 ng/ml) for 72 hours. ACE2 (C) and TMPRSS2 (F) expression levels in comparison with the housekeeping gene GAPDH (n = 4, each data point represents the mean of three PCR reactions) are shown. Mean values are shown with error bars representing the SE of the mean. The P value in C is the result of a one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Ctrl = control; HPRT = hypoxanthine guanine phosphoribosyltransferase.
Our findings demonstrate that HRV-A16 infection significantly upregulates the expression of the ACE2 and TMPRSS2 in epithelial cells and indicates ACE2 expression is regulated by IFNβ1. Major serotype rhinoviruses are among the most common respiratory viral infections in children and young adults (9, 10), and upregulation of ACE2 and TMPRSS2 on AECs with symptomatic infection or asymptomatic harboring of rhinovirus could impact transmission of SARS-CoV-2 through our communities. Studies of the original SARS coronavirus suggested that the virus itself decreased ACE2 expression and that the upregulation of ACE2 may be protective for severe disease in mice (3, 4). We expand on recently published studies that have demonstrated ACE2 is an IFN-stimulated gene (11–13) by specifically exploring the roles of IFNβ1 and IFNλ2, which are known to be induced in the airway epithelium in response to rhinovirus infection and evaluated effects on both ACE2 and TMPRSS2. These results suggest that infection with major serotype HRVs could facilitate SARS-CoV-2 transmission and modulate disease severity through IFNβ1.
Our results further refine the relationship between asthma and the expression of the ACE2 and TMPRSS2 in the airway epithelium by identifying relationships to AHR and lung function. Prior studies have found that individuals with asthma may have heightened susceptibility to HRV infection, and although ex vivo infection with HRV-A16 upregulated ACE2 and TMPRSS2 expression, there was no specific effect of asthma status. Our findings are concordant with initial reports suggesting that asthma is not a major risk factor for severe disease (14), but little is known about how these factors affect transmission. Our results should spur additional studies that focus on risk factors involved in SARS-CoV-2 transmission, including the prevalence of asthma and the frequency of infection or coinfection with HRVs, particularly the timing of these factors in relation to community surges of coronavirus disease (COVID-19).
Supplementary Material
Footnotes
Supported by U.S. National Institutes of Health Grants U19AI125378 (S.F.Z.), K24AI150991 (J.S.D.), R01HL089215, and K24AI130263 (T.S.H.).
Originally Published in Press as DOI: 10.1165/rcmb.2020-0394LE on September 18, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280, e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, et al. HCA Lung Biological Network. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen HD, Fraire AE, Joris I, Welsh RM, Selin LK. Specific history of heterologous virus infections determines anti-viral immunity and immunopathology in the lung. Am J Pathol. 2003;163:1341–1355. doi: 10.1016/S0002-9440(10)63493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altman MC, Lai Y, Nolin JD, Long S, Chen CC, Piliponsky AM, et al. Airway epithelium-shifted mast cell infiltration regulates asthmatic inflammation via IL-33 signaling. J Clin Invest. 2019;129:4979–4991. doi: 10.1172/JCI126402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters MC, Sajuthi S, Deford P, Christenson S, Rios CL, Montgomery MT, et al. COVID-19-related genes in sputum cells in asthma: relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020;202:83–90. doi: 10.1164/rccm.202003-0821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khaitov MR, Laza-Stanca V, Edwards MR, Walton RP, Rohde G, Contoli M, et al. Respiratory virus induction of alpha-, beta- and lambda-interferons in bronchial epithelial cells and peripheral blood mononuclear cells. Allergy. 2009;64:375–386. doi: 10.1111/j.1398-9995.2008.01826.x. [DOI] [PubMed] [Google Scholar]
- 9.Mäkelä MJ, Puhakka T, Ruuskanen O, Leinonen M, Saikku P, Kimpimäki M, et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010;23:74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. HCA Lung Biological Network. Electronic address: lung-network@humancellatlas.orgHCA Lung Biological Network. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chua RL, Lukassen S, Trump S, Hennig BP, Wendisch D, Pott F, et al. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol. 2020;38:970–979. doi: 10.1038/s41587-020-0602-4. [DOI] [PubMed] [Google Scholar]
- 13.Sajuthi SP, DeFord P, Jackson ND, Montgomery MT, Everman JL, Rios CL, et al. Type 2 and interferon inflammation strongly regulate SARS-CoV-2 related gene expression in the airway epithelium bioRxiv 2020[accessed 2020 Apr 10]. Available from: https://www.biorxiv.org/content/10.1101/2020.04.09.034454v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



