Abstract
Smoking remains a leading cause of preventable morbidity and mortality worldwide. Despite a downward trend in cigarette use, less-regulated tobacco products, such as cigarillos, which are often flavored to appeal to specific demographics, such as younger people, are becoming increasingly popular. Cigar/cigarillo smoking has been considered a safer alternative to cigarettes; however, the health risks associated with cigar in comparison with cigarette smoking are not well understood. To address this knowledge gap, we characterized the effects of multiple brands of cigarillos on the airway epithelium using ex vivo and in vivo models. To analyze these effects, we assessed the cellular viability and integrity of smoke-exposed primary airway cell cultures. We also investigated the protein compositions of apical secretions from cigarillo-exposed airway epithelial cultures and BAL fluid of cigarillo-exposed mice through label-free quantitative proteomics and determined the chemical composition of smoke collected from the investigated cigarillo products. We found that cigarillo smoke exerts similar or greater effects than cigarette smoke in terms of reduced cell viability; altered protein levels, including those of innate immune proteins; induced oxidative-stress markers; and greater nicotine delivery to cells. The analysis of the chemical composition of the investigated cigarillo products revealed differences that might be linked to the differential effects of these products on cell viability and protein abundance profiles, which have been associated with a range of health risks in the context of airway biology. These findings contradict the assumption that cigarillos might be safer and less harmful than cigarettes. Instead, our results indicate that cigarillo smoke is associated with equal or greater health risks and the same or increased airway toxicity compared with cigarette smoke.
Keywords: cigarillo, airways, smoking harm
Clinical Relevance
Less-regulated tobacco products, such as cigarillos, are becoming increasingly popular, but their health risks in comparison with cigarettes are not well understood. Our findings indicate that cigarillo smoke is associated with equal or greater health risks and the same or increased airway toxicity compared with cigarette smoke.
Tobacco smoking is a significant cause of morbidity and mortality worldwide and has been linked to severe respiratory tract diseases, such as chronic obstructive pulmonary disease (COPD) and lung cancer. Despite a decrease in cigarette smoking in the United States, cigar and cigarillo smoking has recently become increasingly popular among middle- and high-school students (1, 2). The 2011–2017 NYTS (National Youth Tobacco Surveys) data showed that 3.62 million U.S. middle- and high-school students were users of tobacco products and that more than 1.74 million of these students were using at least two types of tobacco products (3). In fact, ∼7.7% and 1.5% of high-school and middle-school students, respectively, are cigar users (3). The prevalence of cigar smoking is higher among black non-Hispanic high-school students than among white, Hispanic, and other minority students (9.5% vs. 7.9%, 7.2%, and 3.7%, respectively) (2). Youths who have tried a tobacco product are at high risk of being tobacco users in adulthood (4). Although some people assume that cigar smoking is safer and is associated with fewer health risks than traditional cigarette smoking (5), the effects of cigars or cigarillos on human lung health have not been sufficiently studied.
A cigar is a roll of tobacco cured for smoking that is usually wrapped in a tobacco leaf, whereas cigarettes consist of tobacco wrapped in paper or another material that does not contain tobacco. Cigars are categorized by size and include small, filtered cigars or cigarillos and larger cigars, such as large premium cigars. Cigarillos are smaller than regular cigars but are usually larger than cigarettes. In this study, we investigated the effects of cigarillo smoke on the airway epithelial surface and its mucus layer.
Airway mucus is a structured, multilayer protective gel matrix that acts as a foundation for the innate defense mechanism of the respiratory pathway (6). The mechanism of mucociliary clearance uses mucus and its movement via the cilia to facilitate the removal of obstructions, pathogens, and toxins from the airway tract (6, 7). In chronic airway diseases, such as COPD, asthma, and other hypersecretory conditions, airway mucus develops qualitative and quantitative abnormalities, and these abnormalities contribute to disease progression and thereby lead to morbidity and mortality (7–9). Among tobacco users, COPD is characterized by mucus hypersecretion/hyperconcentration and dysfunctional mucociliary clearance (9, 10). Continuous exposure to tobacco smoke results in goblet-cell metaplasia, mucus hypersecretion, and mucus dehydration, and these effects lead to abnormal mucus composition and properties (10). Because airway mucus serves as the front line of defense against inhaled toxicants, such as tobacco smoke, the present study assessed the changes in airway mucus and markers associated with smoke exposure to obtain a better understanding of the airway epithelial responses to cigarillo smoking.
We previously demonstrated that little cigars are more toxic than cigarettes, and in vitro studies have revealed that the adaptation of cells to the noxious effects of smoking results in stronger airway biological responses (11). In the present study, we characterized the effects of common/popular cigarillo brands (12, 13) on the airway epithelial mucosal barrier. Specifically, we analyzed primary, well-differentiated human bronchial epithelial-cell cultures and BAL fluid (BALF) from murine lungs that were exposed to cigarillo smoke to determine the health effects of cigarillos on the airways.
Methods
Cell Culture
Human tracheobronchial epithelial (HTBE) cells were cultured on Transwell column supports (24 mm in diameter) on an air–liquid interface for 4–6 weeks. To obtain mucus secretions, the culture’s apical surface on each Transwell was washed with 500 μl of PBS solution (14). Each wash was collected after incubation for 1 hour after exposure at a temperature of 37°C.
In Vitro Cigarillo-Smoke Exposure
An LM1 smoke engine (Borgwaldt) was used to generate smoke according to the manufacturer’s protocol (15). The cultured HTBE cells were transferred to a smoke apparatus and exposed apically to cigarillo and cigarette smoke. The exposure paradigm involved a whole cigarette or cigarillo per exposure and thus involved 14 × 35-ml puffs of conventional cigarettes (Kentucky research cigarette [KCS] 3R4F, class A cigarette) and 30 × 35-ml puffs of cigarillos at a rate of one puff every 30 seconds. To compare the effects of cigarillo and cigarette smoke at a product size–independent level, additional exposure experiments were conducted using 14 cigarillo puffs and 14 cigarette puffs. Three cigarillo tobacco products were used in this study: Swisher Sweets cigarillos (SSWs; Swisher International, Inc.), Garcia y Vega Game black cigarillos (GBKs; Swedish Match, Inc.) and Hi-Fi Tropical Tango cigarillos (HTTs; Unitabac LLC). The control-cell cultures were exposed to air using a paradigm that matched the number of puffs obtained from the tobacco products. The HTBE cells were perfused basolaterally with sterile Ringer’s solution during exposure.
In Vivo Exposure of Mice to Cigarette and Cigarillo Smoke
Six-week-old male mice (C57BL/6J) were exposed to smoke from unfiltered 3R4F reference cigarettes (referred to as KCSs), SSWs with a natural flavor, or room air (sham) using the SCIREQ inExpose whole-body exposure system. The sham mice were placed in the inExpose system and exposed to room air for 20 minutes. BAL was performed on the right lung a total of three times using 0.5 ml of calcium- and magnesium-free Dulbecco’s PBS (Life Technologies) for each lavage. The BALF was maintained on ice as it was collected. All three lavages were pooled and immediately frozen in liquid nitrogen.
Label-Free Quantitative Proteomic Analysis of Apical Secretions from HTBE Cells and BALF from Mice after Exposure
A Q-Exactive Hybrid Quadrupole-Orbitrap mass spectrometer coupled to an Ultimate 3000 nano-HPLC system (liquid chromatography–tandem mass-spectrometry system; Thermo Fisher Scientific, Thermo Electron North America, LLC) was used for the label-free quantitative proteomic analysis. The filter-aided sample preparation method was used to prepare the samples for proteomic analysis (16). Proteome Discoverer 1.4 software (Thermo Fisher Scientific) was used to process the raw data and to search the Universal Protein Resource protein-sequence database. The tandem mass-spectrometry–based proteomics data were validated using Scaffold 4.4.8 (Proteome Software, Inc.).
One-way ANOVA was then performed, and multiple-comparison tests were conducted with representative graphs using GraphPad Prism version 6.00 for Windows (GraphPad Software). The STRING (Search Tool for the Retrieval of Interacting Genes) algorithm was used for the functional-enrichment pathway analysis of proteins (STRING version 10.0) (17).
For additional details on the methods, see the data supplement.
Results
To assess the toxicity of cigarillo smoke on the airway epithelia, we exposed primary human bronchial epithelium cultures to different brands of cigarillos and compared the results with those of the cigarette-smoke and air controls. In parallel, mice were exposed to SSWs, cigarette smoke, or air to investigate the in vivo airway effects. The physical characteristics (including the weight [grams], length [centimeters], and thickness [centimeters]) and differences among the three investigated brands of cigarillo tobacco products, namely SSWs, GBKs, and HTTs, and KCS are depicted in Table 1.
Table 1.
Physical Characteristics of KCSs and Three Different Cigarillo Tobacco Products
| Tobacco Product | Weight (g) | Length (cm) | Thickness (cm) |
|---|---|---|---|
| KCS | 1.02 | 8.5 | 0.7 |
| SSW | 2.83 | 11 | 1.2 (first 1.5-cm length was 1) |
| GBK | 2.89 | 10.5 | 1 |
| HTT | 2.97 | 10.5 | 1 |
Definition of abbreviations: GBK = Garcia y Vega Game black cigarillo; HTT = Hi-Fi Tropical Tango cigarillo; KCS = Kentucky research cigarette; SSW = Swisher Sweets cigarillo.
Cigarillo-Smoke Toxicity in the Airway Epithelium
Cigarillo-smoke exposure decreased the cellular viability and airway epithelial-cell-layer integrity of apically exposed HTBE cultures (Figure 1). Cigarillo smoke–exposed cultures showed significantly increased numbers of dead cells compared with the air controls (Figures 1A and 1B), with GBK smoke also significantly exceeding cigarette smoke–induced dead cell numbers. To assess the airway epithelial-layer integrity, we measured the transepithelial electrical resistance (TEER). The TEER of cigarillo smoke–exposed HTBE cultures was significantly reduced compared with the air- and cigarette smoke–exposed HTBE cultures (P < 0.05, Figure 1C). Assuming that one (whole) cigarillo serves as a tobacco-product use unit (30 puffs), the data revealed that exposure to smoke from a whole cigarillo had more potential to cause epithelial-cell death than exposure to smoke from one (whole) cigarette or air. In addition, when an equal number of cigarette and cigarillo puffs (14 puffs) were compared, the exposure of HTBE cells to the smoke from 14 cigarillo puffs resulted in the same or greater potential to cause epithelial-cell death and a similarly reduced TEER (see Figure E1 in the data supplement).
Figure 1.

Effects of cigarillo (CLLO) smoke on human tracheobronchial epithelial (HTBE) cell viability and layer integrity. (A) Representative images of propidium iodide (red) uptake by chronic smoke–exposed HTBE cells showing calcein acetoxymethyl (AM) staining (green) for live cells after CLLO-smoke exposure. (B) Quantitation of the number of propidium iodide–positive cells per image (n = 3 donors, 5–10 random images/culture were captured and quantitated). (C) Transepithelial electrical resistance (n = 6 donors, approximately six individual culture inserts per donor code were measured) after CLLO-product (Swisher Sweets CLLO [SSW], Garcia y Vega Game black CLLO [GBK], and Hi-Fi Tropical Tango CLLO [HTT]) and Kentucky research cigarette (KCS) smoke exposure. Data are expressed as the mean + SEM. *P ≤ 0.05, ***P ≤ 0.005, and ****P ≤ 0.001 by one-way ANOVA. TEER = transepithelial electrical resistance.
Cigarillo smoke changes the protein composition of airway epithelial apical secretions
The proteomic analysis of secretions from HTBE cells exposed to smoke of whole cigarillos revealed qualitative and quantitative changes compared with air or the smoke from whole cigarettes. Across all samples, ∼118,725 spectra were obtained during the mass-spectrometry data acquisition, which led to ∼5,200 identified peptides that were assigned to 727 proteins. These proteins were identified on the basis of at least two peptide assignments and quantified on the basis of their total peptide precursor intensities, which were calculated as the sum of the individual precursor peak areas. We observed significant changes (P ≤ 0.05) in the abundance of ∼389 of the 727 quantified proteins (Figure 2A1). Overall, numerous protein-level changes were shared across the different groups, and unique change profiles were identified among the groups exposed to smoke from different cigarillo brands (Figure 2A2). A principle-component analysis (PCA) showed clustering of samples belonging to the same exposure group for each investigated tobacco product compared with the air-exposed cultures (Figure 2B). Furthermore, the PCA revealed segregation between the KCS data cluster and those resulting from cigarillo exposure. Importantly, unique clustering patterns were also found between cigarillo brands, such as HTTs versus SSWs and HTTs versus GBKs, and some overlap was found between the cigarillo brands GBK and SSW.
Figure 2.
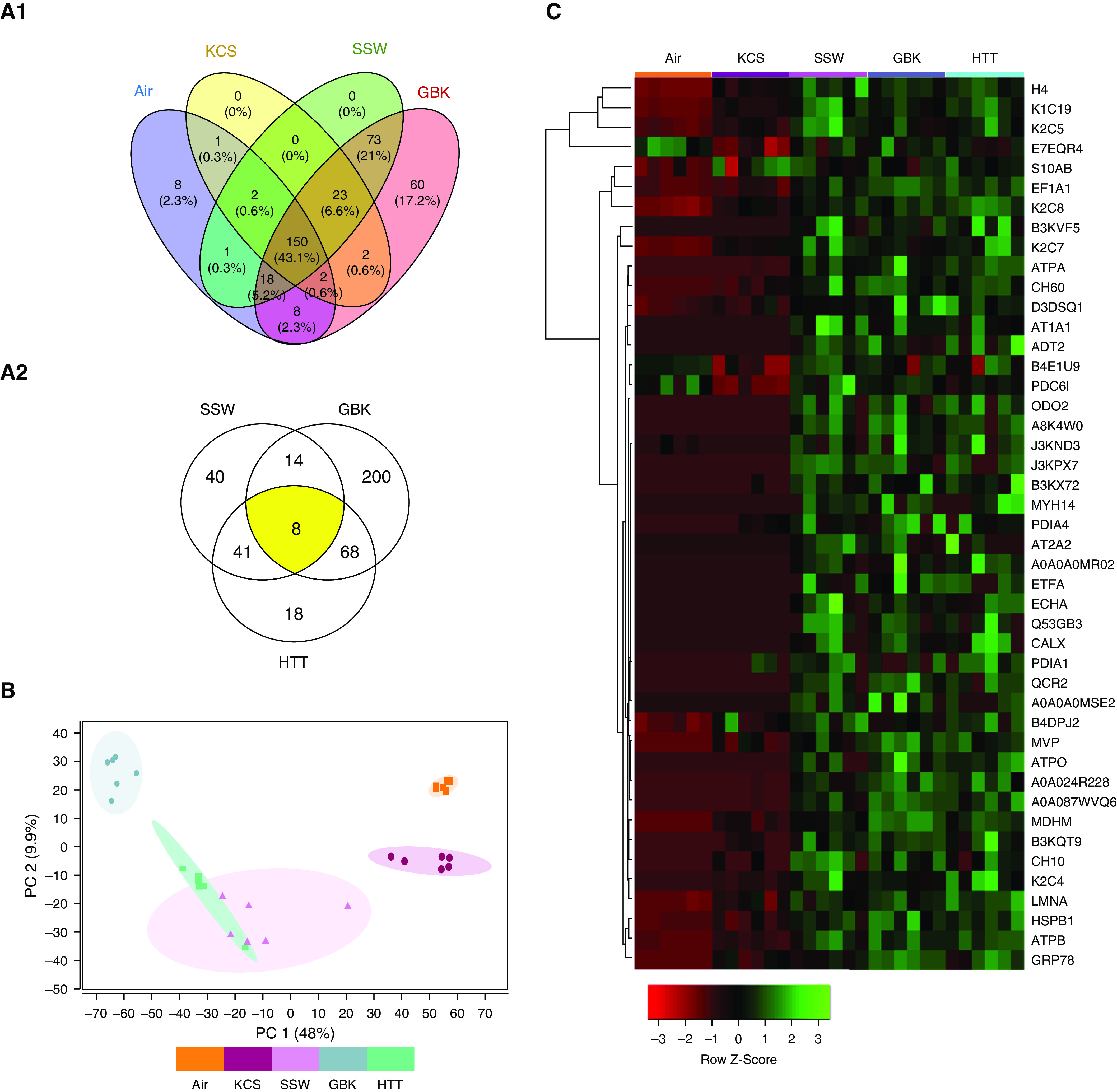
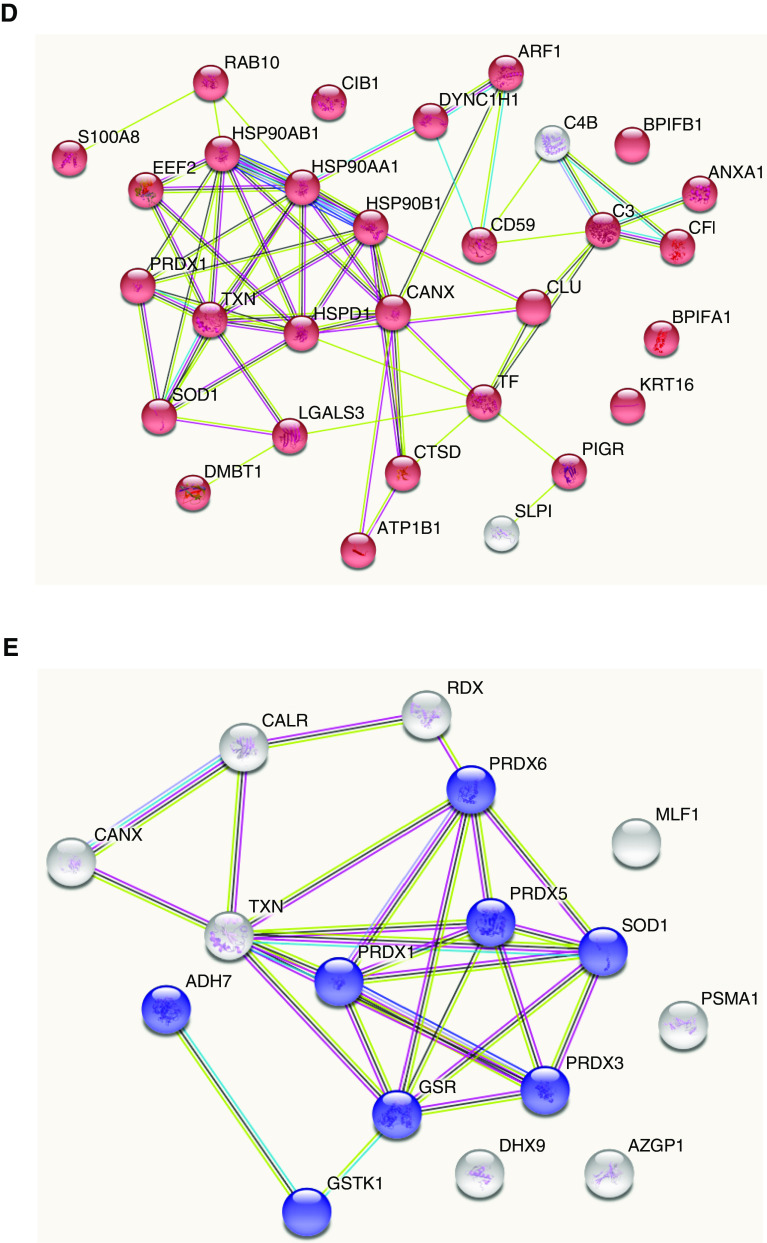
Proteomic analysis of the apical secretions of CLLO smoke–exposed HTBE cells (n = 6/group). (A1) Venn diagrams show (a) significant differentially expressed proteins shared across groups and unique to each exposure group (air, KCSs, and CLLOs [SSWs and GBKs]) and (A2) quantitative profile analysis of significant proteins across the CLLO groups (SSWs, GBKs, and HTTs). (B) Principal-component (PC) analysis of protein expression reveals clustering of the CLLO- (SSW, GBK, and HTT), KCS-, and air-exposed groups. A hierarchical heatmap displays the clustering of protein expression after KCS- and CLLO-smoke exposure. (C) CLLOs resulted in a clustering pattern different from those found for cigarettes and air. A functional-enrichment pathway analysis of the differentially upregulated proteins after CLLO exposure revealed exposure-induced changes in a number of proteins related to (D) the innate immune pathways (red) and (E) oxidative-stress/oxidative stress–induced cell-death pathways (blue).
The hierarchical clustering of the top 44 differentially expressed proteins (based on P value) revealed differential clustering patterns for cigarette- and cigarillo smoke–exposed groups, as shown in the heatmap (Figure 2C). This heatmap further illustrates that changes resulting from the smoke of the different cigarillo brands were more similar to each other than to the changes resulting from cigarette smoke or air. The full list of quantified proteins with significantly altered levels is presented in Excel File E1.
An enrichment analysis using the Gene Ontology Consortium terms for the proteins that showed altered levels in the apical secretions from smoke-exposed HTBE cells revealed that cigarillo smoke affected many biological processes, including the innate immune response, the response to stimuli, and signaling (Figure E2). The proteins that presented differential expression in the cigarillo groups were subjected to pathway analysis using the STRING protein-interactome analysis. A functional-enrichment analysis identified surfaced pathways that affect various biological processes, including innate immunity, immune-response elements (Figure 2D), oxidative stress, and cell killing (Figure 2E). Among the innate immune processes altered by cigarillo smoke, we observed changes in the mucus protein profiles, antimicrobial-response proteins, and complement-system members, and they included decreases in BPI fold–containing family members A1 (BPIFA1 [bactericidal/permeability-increasing-fold-containing family A, member 1], SPLUNC1 [short palate, lung, and nasal epithelial clone 1]) and B1 (BPIFB1, LPLUNC1), neutrophil gelatinase–associated lipocalin, complement C3, (Figures 3A–3D), and polymeric immunoglobulin receptor (Figure 3E). A Western blot analysis of the apical secretions using BPIFA1 and BPIFB1 antibodies replicated the observed decreases in response to cigarette and cigarillo-smoke exposure, thus confirming the quantitative proteomics data (Figure E3).
Figure 3.
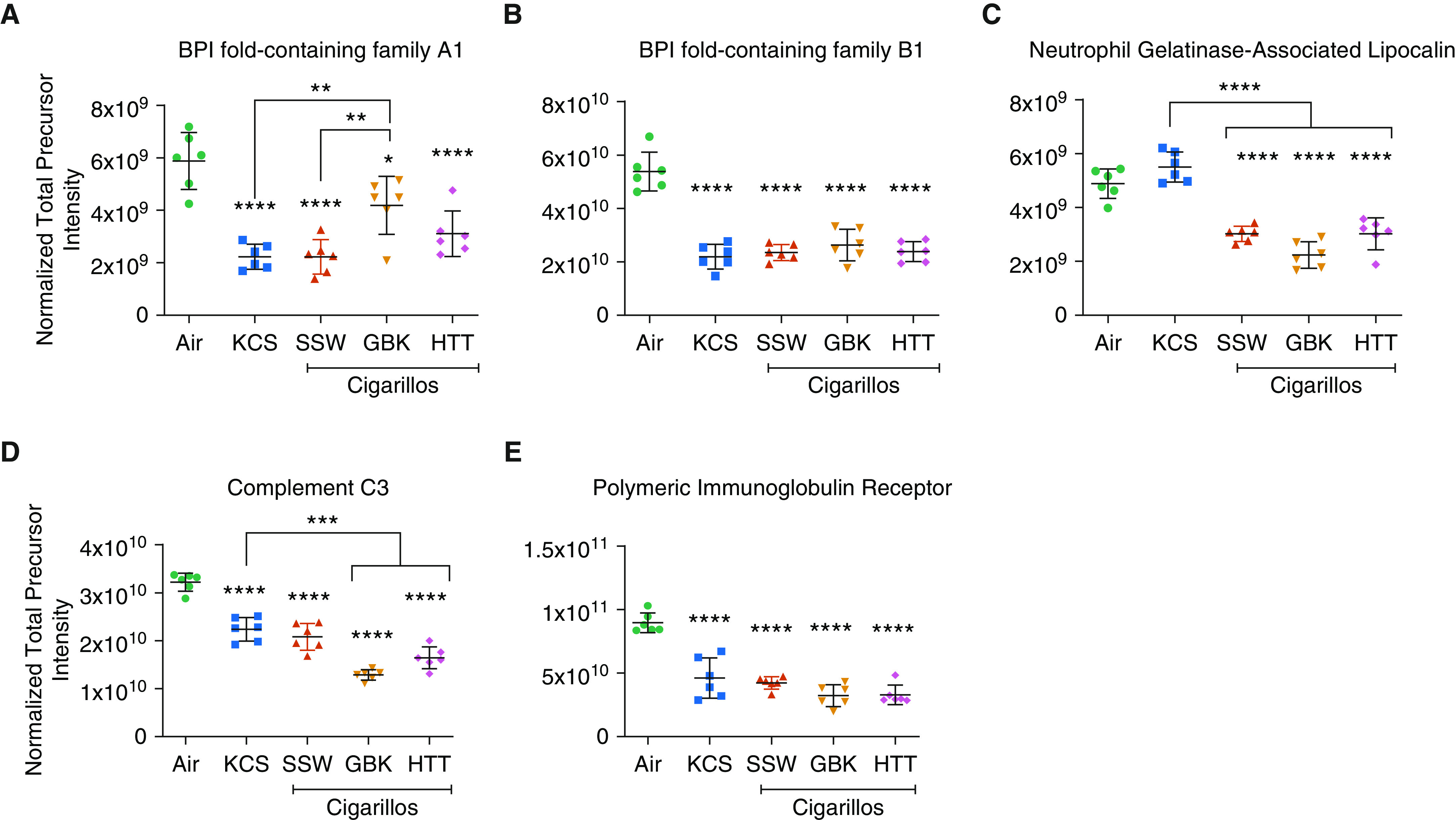
CLLO exposure changes the expression of proteins related to the immune response. (A) BPI fold–containing family A1, (B) BPI fold–containing family B1, (C) neutrophil gelatinase–associated lipocalin, (D) complement C3, and (E) polymeric immunoglobulin receptor. *Significantly different from air exposure, unless indicated with bracket. Data are expressed as the mean ± SEM. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005, and ****P ≤ 0.001 by one-way ANOVA.
In addition, cigarillo smoke also led to alterations in mucin expression. Significant decreases in the membrane-bound airway mucins MUC1, MUC4, and MUC16 were observed (Figures 4A–4C). Surprisingly, although MUC5B was decreased after cigarillo-smoke exposure, it was significantly increased in the KCS smoke–exposed group (Figure 4D).
Figure 4.
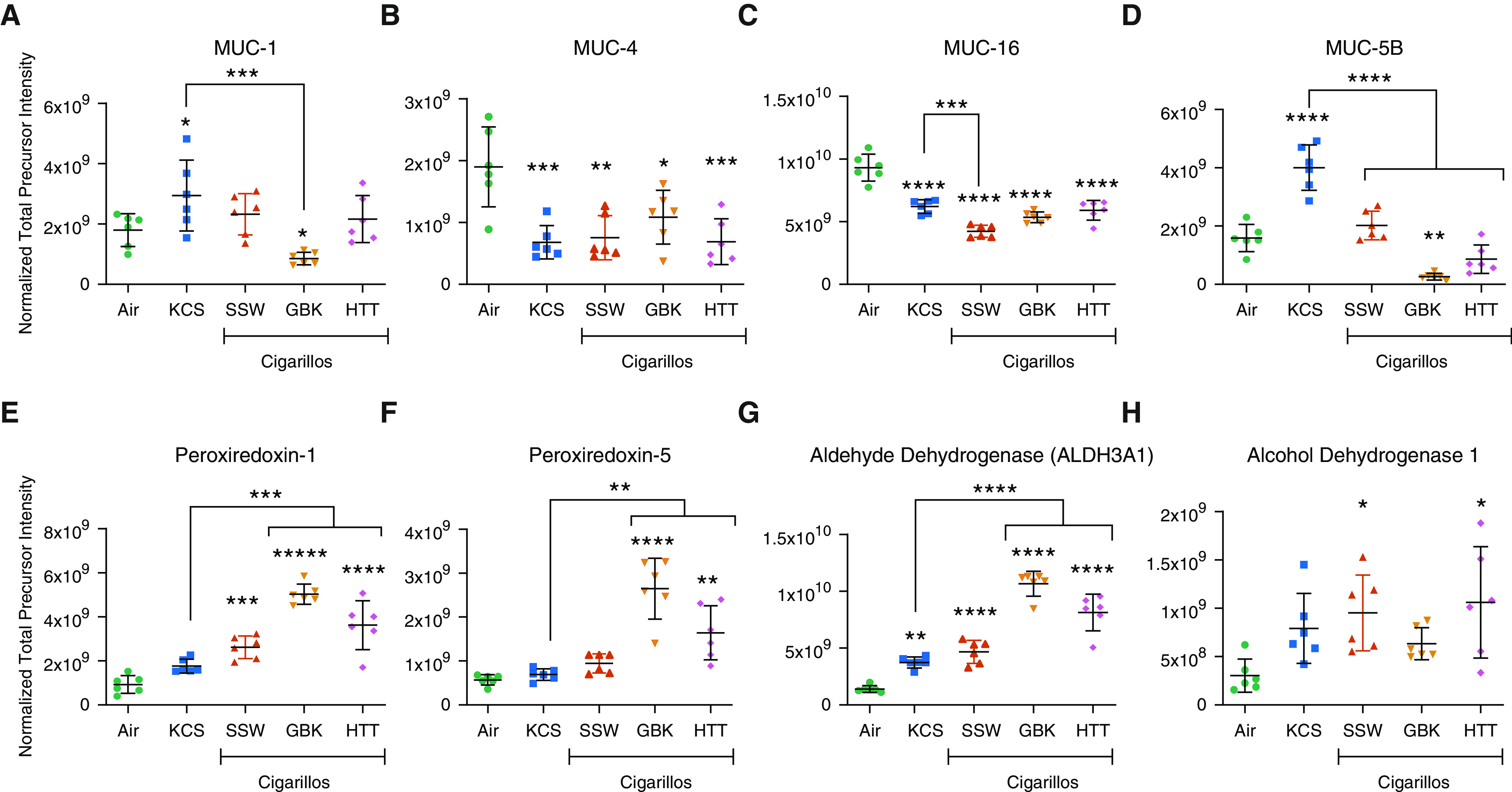
CLLO-smoke exposure alters the expression of the mucins (A) MUC1, (B) MUC4, (C) MUC16, and (D) MUC5B in the apical secretions of smoke-exposed HTBE cells. Exposure to CLLO smoke increases the expression of proteins in the oxidative-stress pathway, namely, (E) PRDX1 (peroxiredoxin-1) and (F) peroxiredoxin-5. Exposure to CLLO smoke also upregulated the levels of the (G) aldehyde dehydrogenase-3A1 and (H) alcohol dehydrogenase-1 enzymes involved in the detoxification process. *Significantly different from air exposure, unless indicated with bracket. Data are expressed as the mean ± SEM. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005, and ****P ≤ 0.001 by one-way ANOVA.
The ability of cigarillo smoke to promote oxidative stress became apparent through the increases in peroxiredoxin-1 and peroxiredoxin-5 (Figures 4E and 4F), aldehyde dehydrogenase-3A1 (Figure 4G), and alcohol dehydrogenase-1 (Figure 4H).
The toxicity effects of the analyzed tobacco products on cell viability and cell-layer integrity raised the question of whether increases or decreases in the quantity of innate-immunity and oxidative-stress proteins might be due to cell death rather than to specific differential expression or secretion patterns in response to the exposure treatments. To address this question, we compared the quantitative signal of a typically nonsecreted and abundant intracellular protein, RS2 (40S ribosomal protein), to a known secreted oxidative stress–/hypoxia-induced protein, PRDX1 (peroxiredoxin-1). This comparison revealed that the intensity of PRDX1 is multiple orders of magnitude higher than that of intracellular ribosomal proteins in the cell secretions after cigarillo exposure (Figure E4). Although a number of proteins were observed in the secretion samples after tobacco-product exposure that represented extracellular markers of toxicity, we focused on characterizing the changes observed in secreted proteins that described the toxic impacts of cigarillo smoke on the airway mucus layer.
Cigarillo-smoke exposure changes the airway mucus composition in vivo
To understand the effect of cigarillo-smoke toxicity in vivo, we analyzed the BALF samples obtained from the lungs of mice exposed to cigarillo smoke, cigarette smoke, or air for 5 days. The label-free quantitative proteomic analysis of the BALF samples revealed similar alterations in the protein-expression patterns in response to cigarillo smoke as observed in human ex vivo epithelial cultures. Our analysis identified ∼746 proteins at a confidence interval of 95.0%, with at least two peptides per protein. Similar to the cell-culture data, the in vivo results showed that both cigarette and cigarillo smoke quantitatively and qualitatively altered the airway mucus composition, particularly in terms of redox stress response, innate immunity, and inflammation (Figure 5). The levels of 135 proteins were significantly altered across the exposure groups (Figure 5B), and ∼38 of these proteins were increased after cigarillo-smoke exposure, as shown in the heatmap (Figure 5A). The complete list of proteins with significantly altered levels is presented in Excel File E2. The PCA revealed significant segregation among the sample group clusters, and the cigarillo smoke–exposed group overlapped with the cigarette smoke–exposed group but clustered separately from the air-sham group (Figure 5C).
Figure 5.

In vivo proteomic analysis of BAL fluid (BALF) from mice exposed to CLLO or cigarette smoke daily for 5 days. (A) A hierarchical heatmap displays the clustering of protein expression after exposure to KCSs, CLLOs, or air. The clustering patterns of protein expression after CLLO-smoke exposure differ from those observed after cigarette or air-sham exposure. (B) Venn diagrams show the number of significantly increased proteins in BALF from animals exposed to tobacco-product smoke compared with air. (C) PC analysis of the protein-expression levels in mouse BALF illustrates the clustering of samples after CLLO, cigarette, or air-sham exposure.
Our proteomic analysis of BALF samples showed that cigarillo smoke upregulated the expression of oxidative-stress proteins, such as thioredoxin reductase (Figure 6A), and enzymes associated with cellular response to stress, including oxoglutarate dehydrogenase (Figure 6B). Cigarillo smoke also significantly decreased the expression of proteins related to immune responses, such as BPI fold–containing family A1 (Figure 6C) and secretoglobin 1a1 (Figure 6D), but upregulated ceruloplasmin (Figure 6F). In addition, cigarillo smoke induced the downregulation of pulmonary SP-D (surfactant-associated protein D) and SP-A (surfactant-associated protein A) (Figures 6E and 6G). The biological processes, molecular functions, and cell-component pathways affected by cigarillo smoke in our in vivo experiments are summarized in Figure E5.
Figure 6.

In vivo proteomic analysis of BALF from mice exposed to CLLO smoke showed that CLLO smoke induced the expression of oxidative-stress markers, such as (A) thioredoxin reductase, and enzymes associated with the cellular response to stress, such as (B) oxoglutarate dehydrogenase. CLLO smoke caused dysregulated expression of proteins that function in the immune response, such as (C) BPI fold–containing family A1, (D) secretoglobin 1A1, and (E) ceruloplasmin. CLLO smoke decreased the expression of pulmonary (F) SP-D (surfactant-associated protein D) and (G) SP-A (surfactant-associated protein A). *Significantly different from air exposure, unless indicated with bracket. Data are expressed as the mean ± SEM. *P ≤ 0.05, **P ≤ 0.01, and ****P ≤ 0.001 by one-way ANOVA.
Chemical-Compound Analysis of Cigarillo Smoke versus Cigarette Smoke
To investigate the potential connections between the effects of the analyzed tobacco products and the toxicity of their chemical components, we analyzed the chemical composition of the gas and mainstream smoke extracts generated by the smoking machine from the investigated cigarillo and cigarette tobacco products. A gas chromatography–mass-spectrometry analysis revealed that similar sets of chemical compounds were shared by different cigarillos brands (SSW, GBK, and HTT) and KCSs. However, the analysis also demonstrated that the chemical profiles of extracts from cigarillo tar particles were different from those of KCSs (Figures 7A and 7B). Taken together, 22 (29.7%) of the identified chemical compounds were found in all of the investigated tobacco products, including KCSs, whereas 12 compounds (16.2%) were found in the SSW, GBK, and HTT groups but not in the KCS group (Figure 7C). The list of all compounds identified in this analysis is provided in Excel File E3. Moreover, the chemical analysis identified marked differences in chemical profiles among the different cigarillo brands. For example, 3-ethoxy-4-hydroxybenzaldehyde, 4-methoxybenzaldehyde, 2,2-dimethyl-2-sila-1,3-dioxacyclohexane, ethyl propanoic acid, and 2-ethylhexyl hexyl ester sulfurous acid were exclusively detected in the GBK brand, whereas dihydro-5-pentyl-2(3H)-furanone, 3-ethoxybenzaldehyde, and hexanoic acid were identified only in the HTT brand. 2-(2-[2-ethoxyethoxy]ethoxy) ethanol and mannitol were detected only in SSWs.
Figure 7.
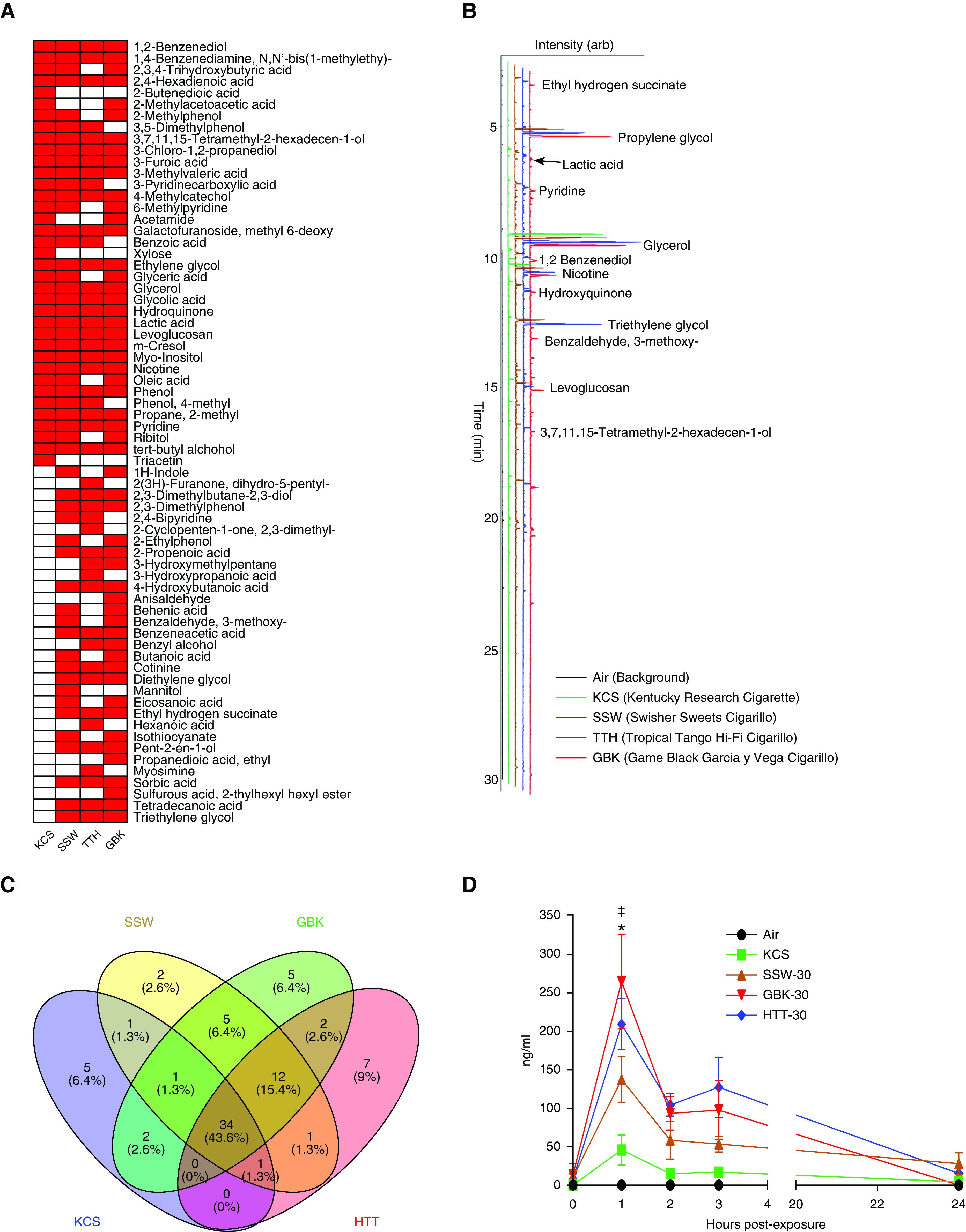
Analysis of chemical compounds. (A) Presence (red) and absence (white) of compounds identified in the particulate phase of smoke from tobacco products by gas chromatography–mass-spectrometry. (B) Stacked chromatograms of trimethylsilyl-derivatized filter extracts collected from mainstream KCS and CLLO smoke. (C) Unique and shared chemical entities were identified in the smoke particles from different CLLO products and KCSs. (D) Time course of the nicotine concentrations in smoke-exposed HTBE-cell apical secretions after exposure to tobacco products. Significant differences were observed in mainstream smoke and *air or ‡KCSs compared with CLLOs. Data are expressed as the mean ± SEM. *P < 0.05 by two-way ANOVA with repeated measurements and ‡P < 0.05 by Sidak’s multiple-comparison test.
Cigarillos Deliver More Nicotine to Airway Epithelial Cells Than Cigarettes
Nicotine is the primary addictive compound in tobacco products. We measured the nicotine levels in the apical secretion layer of smoke-exposed HTBE cells to estimate the amount of nicotine delivered to the epithelial surface by each of the tobacco products. The peak nicotine levels in the apical secretions of smoke-exposed HTBE cells were detected at 1 hour after exposure, and the secretions of cigarillo smoke–exposed groups (30 puffs) contained the highest nicotine levels (average, 203.6 ng/ml) compared with the cigarette smoke–exposed group (45.8 ± 20 ng/ml) (Figure 7D). Notably, the different investigated brands of cigarillo products delivered different nicotine concentrations to the cell-layer surfaces. The GBK brand showed the highest nicotine levels (264.9 ± 61 ng/ml), the SSW brand delivered the lowest nicotine levels (137.4 ± 30 mg/ml), and the HTT brand delivered a nicotine concentration of 207.0 ± 32 ng/ml. Overall, this analysis showed that the cigarillo brands SSW and GBK delivered more nicotine to the airway epithelial surface than cigarettes (equal amounts, 14 puffs) (Table E1, E2, and Figure E6).
Discussion
Cigarette smoking exerts adverse effects on the respiratory and mucociliary airway systems, including but not limited to alterations in mucus clearance (18–20), inflammation (21), increased oxidative-stress elements (22–24), apoptosis (25), and dysregulation of the innate immune response (26, 27). However, the toxic effects of cigarillo smoking on the airway have not been sufficiently studied in comparison with those of regular cigarettes. Cigar and cigarillo smoking have seen an increase in popularity, particularly among youth, which emphasizes the importance of better understanding the effects and associated risks.
In this study, we attempted to address this information gap by characterizing the effects of cigarillos on the airway mucosal barrier at multiple levels using ex vivo and in vivo models. Several novel observations were obtained from these studies. First, the effects of whole cigarillo smoke on primary epithelial cultures were greater than those of cigarette smoke, as determined on the basis of measurements of cytotoxicity, cell integrity, and protein expression. Second, in vivo experiments indicated that cigarillo-smoke exposure exerted a somewhat similar effect on the airway epithelial response in mice. Third, cigarillo smoke contained more potentially toxic chemicals than cigarette smoke, and this increase could be linked to the higher cytotoxicity and alterations in cell integrity and protein expression. Overall, these findings suggest that cigarillos should not be considered a safer tobacco product associated with fewer health risks than cigarettes.
We previously demonstrated that compared with cigarettes, little cigars that are smaller in size than typical cigarillos result in decreased cell viability and increased effects on the airways (11). Our data collectively indicate that cigarillos have effects similar to those of little cigars and cause significantly greater adverse effects on airway epithelial function than cigarettes by affecting multiple important pathways, including oxidative stress–related “response to stimulus” and “innate immune-response” pathways. Comparatively, HTBE cells were exposed in vitro to the cigarillo-smoke protocol for 5 days, and the propidium iodide–positive cells were correlated with dead/damaged epithelia; these results provide evidence showing that cigarillos are associated with more toxic effects than cigarettes. Furthermore, the different cigarillo products exhibited a range of cytotoxicity. Although cigarillos are categorized as one tobacco-product entity, our studies demonstrate that cigarillo products show variations in physical characteristics, chemical components, and delivered nicotine levels. Accordingly, variations in the magnitude of the toxic effects associated with different cigarillo brands or tobacco products were observed. For example, the analysis showed that greater quantitative protein-expression dysregulation was associated with the cigarillo group, particularly for the GBK brand, which delivered the greatest amount of nicotine to the airway epithelial surface. Notably, the chemical analysis revealed that the GBK brand contains five (6.4%) unique chemical compounds that were not identified in the other cigarillo brands.
Cigarillos and Oxidative Stress
The accumulation of oxidative damage has been implicated in both acute and chronic cell injury (28), and oxidative stress might participate in the pathogenesis of some airway diseases, such as COPD (22). The oxidant/antioxidant imbalance caused by cigarette smoke is implicated in the inflammatory process (29). Our investigation of protein-level changes in the secretions of HTBE cells showed that cigarillos induced more oxidative stress and caused more profound alterations in the lung innate immune response than cigarette smoke. The redox burden in the lungs produced by cigarillo smoke can be worsened by the dysregulated expression of immune-response proteins and activation of inflammatory leukocytes (21). With chronic exposure, these effects may increase and prolong the insult to the lungs. Various lung diseases have been associated with and are mediated by chronic inflammation (30).
Cigarillos and Innate Immune Response
Cigarette smoking induces changes in innate immune responses (27). Smoking has been implicated in the production of many immune or inflammatory mediators, including both proinflammatory and antiinflammatory cytokines. Cigarette smoking exerts adverse effects on the respiratory system, the immune system, and innate host-defense mechanisms, such as mucociliary clearance and antimicrobial activity (26, 31, 32). The dysregulation of immune proteins alters immunological homeostasis, restores immune tolerance, and can therefore contribute to the development of diseases and increase the susceptibility to secondary microbial infections (33–36). The exposure to cigarillo smoke altered proteins that play important roles in innate immune processes, quantitative mucus profiles, antimicrobial responses, and the complement system. For instance, cigarette and cigarillo smoke decreased the expression of the SPLUNC1 protein, which is also known as BPIFA1. BPIFA1 is a protein secreted into the airway lumen, where it maintains airway hydration via interactions with the epithelial sodium channel and contributes to airway surface liquid homeostasis and proper mucus clearance (37, 38). Other crucial innate defense proteins that were decreased in response to cigarillo smoke included neutrophil gelatinase–associated lipocalin, which is associated with bacterial overgrowth (39).
Alteration of Receptor-mediated Pathways Responsible for Airway Immunity
The levels of polymeric immunoglobulin-receptor proteins were decreased, which suggested alterations in the transcytosis of soluble dimeric IgA antibodies, the first line of antigen‐specific immune defense at mucosal surfaces in epithelial cells (40). Secretory IgA antibodies represent the dysregulation of polymeric immunoglobulin-receptor protein expression, and subsequent alterations in transcytosis and immune complexes from the basolateral to the apical mucosal epithelial-cell surface have profound consequences on the pathogenesis of infections and inflammations (40–42). Secretoglobin 1a1 is an important innate defense/immunomodulatory protein that is highly expressed in the lower airways, particularly in nonciliated club cells (also known as CC10-positive cells) (43). CC10 plays an antiinflammatory role in the lungs (44, 45). The expression of this protein was highly suppressed after cigarillo exposure in mice, and this finding suggests one mechanism through which immunosuppressive effects might occur in the airways. The data from our mouse exposure experiments showed that chitinase-like protein 3, which is involved in inflammation and tissue remodeling (46, 47), was significantly increased in response to cigarette smoke but not after cigarillo exposure. Cigarillo smoke increased ceruloplasmin, and this change is associated with experimentally induced inflammation (48), which suggests a mechanism through which cigarillo smoke could trigger inflammation.
Alterations in Pulmonary Surfactants, Which Promote Pathogen Recognition and Regulate Inflammatory Responses
Pulmonary surfactants, which constitute another class of molecules important to lung health that is affected by smoke, are composed of complex phospholipids and proteins that reduce surface tension at the air–liquid interface of the lung and regulate immune-cell function in the lung. Pulmonary SP-A and SP-D are also known as “collectins” (49) and function in opsonization to promote the recognition of pathogens for uptake by phagocytes. These molecules also regulate inflammatory responses and interact with the adaptive immune response (50, 51). Cigarillo smoke decreased the pulmonary SP-A and SP-D levels, and these decreases could contribute to the enhanced susceptibility to lung inflammation and infection (51, 52). Further studies are warranted to further understand the impacts of cigarillo smoking on the function of these immune proteins, particularly in humans. Elucidating the functions of these sets of immune proteins in lung immunological homeostasis will contribute to our understanding of the pathogenesis of airway diseases, including COPD.
Altered Mucin Profile
Multiple studies have demonstrated that cigarette smoke has the potential to induce respiratory mucins via proinflammatory stimuli that are relevant to COPD pathogenesis and thus contribute to the mucin hyperproduction status in patients with COPD (19, 20, 53, 54). Similarly, the effects of cigarillo smoke involve alterations in mucin homeostasis and subsequent mucociliary clearance in the airways. In the present study, we measured relevant tissue responses to evaluate the adverse health effects of tobacco products. Our findings were consistent with those of previous studies, which revealed that respiratory mucins, such as mucins MUC5B and MUC1, were elevated after exposure to cigarette smoke. However, the mucins MUC5B, MUC16, MUC4, and MUC1 were decreased after exposure to cigarillo smoke. Because membrane mucins, such as MUC4 and MUC16, are associated with ciliary cells, a decrease in these mucins could be associated with airway remodeling in response to exposure. Alterations in the MUC5B concentrations have been linked to various pathologies of the lung. Increased MUC5B is related to chronic bronchitis/airway obstruction, whereas decreased MUC5B has been associated with impaired mucociliary clearance in aged mice (55) and T-helper cell type 2 inflammation (56). The decreased mucin concentrations after exposure to cigarillo smoke might be associated with toxicity levels observed after cigarillo smoke being higher than those observed with cigarette smoke because these higher levels reduced the secretion and impacted the clearance of these molecules. Future studies are needed to further clarify these findings.
Taken together, our findings show that exposure to the same number of puffs (14 puffs) and to whole cigarillos (30 puffs) exerts equal or greater health effects than those of regular cigarettes. In particular, cigarillos were able to reduce cell viability, alter protein-expression patterns, cause oxidative stress, and deliver higher nicotine levels to cells. Therefore, the findings indicate that exposure to cigarillo smoke leads to potential health risks and causes damage to the airways. These results could help inform the regulation of tobacco and cigarillo products, increase the awareness of their health risks, and change the public perception of these products. However, this study is somewhat limited by the fact that only ∼38.2% of adolescent cigarillo users use cigarillos as marketed; thus, the majority of users alter the product before use. For example, 40.3% of cigarillo smokers mix cigarillo tobacco with marijuana, and an additional 28% use other manipulation methods, such as adding or removing tobacco from the wrapper (57–59). In addition, because many cigarillo users are users of multiple tobacco products, there is a need to further evaluate the health risks associated with cigarillo use, use of multiple tobacco products, and concurrent cannabis use. There are wide variations among the cigarillo tobacco flavors/brands on the market, and these different products are expected to exert varying health effects on the airways. The clustering or categorizing of these tobacco products might be needed to achieve a more comprehensive analysis of their short-term and long-term health effects.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Dr. Agathe Ceppe for assisting with the statistical analysis of TEER data. They also thank the Marsico Lung Institute/University of North Carolina Cystic Fibrosis Center Tissue Culture Core for providing the HTBE cells and medium.
Footnotes
Supported by U.S. National Institutes of Health (NIH)/U.S. Food and Drug Administration Grant P50 HL120100. The research reported in this publication was supported by the NIH and the Family Smoking Prevention and Tobacco Control Act. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the U.S. Food and Drug Administration.
Author Contributions: S.H.A., G.L.G., R.T., C.M.D., and M.K. designed the research studies. S.H.A., P.H., and J.C. performed the ex vivo exposure experiments. J.E.K. and G.L.G. performed the chemical analysis of cigarettes and cigarillos. J.R.M. and C.M.D. designed and performed the in vivo exposure experiments. S.H.A. and B.R. analyzed the proteomics data. S.H.A. and A.G. performed the cell toxicity and transepithelial electrical resistance measurements. S.H.A., G.L.G., R.T., C.M.D., and M.K. wrote the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0085OC on September 2, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Singh T, Arrazola RA, Corey CG, Husten CG, Neff LJ, Homa DM, et al. Tobacco use among middle and high school students: United States, 2011-2015. MMWR Morb Mortal Wkly Rep. 2016;65:361–367. doi: 10.15585/mmwr.mm6514a1. [DOI] [PubMed] [Google Scholar]
- 2.Jamal A, Gentzke A, Hu SS, Cullen KA, Apelberg BJ, Homa DM, et al. Tobacco use among middle and high school students: United States, 2011-2016. MMWR Morb Mortal Wkly Rep. 2017;66:597–603. doi: 10.15585/mmwr.mm6623a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang TW, Gentzke A, Sharapova S, Cullen KA, Ambrose BK, Jamal A. Tobacco product use among middle and high school students: United States, 2011-2017. MMWR Morb Mortal Wkly Rep. 2018;67:629–633. doi: 10.15585/mmwr.mm6722a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reducing tobacco use: a report of the Surgeon General. Executive summary. MMWR Recomm Rep. 2000;49:1–27. [PubMed] [Google Scholar]
- 5.Cohn A, Cobb CO, Niaura RS, Richardson A. The other combustible products: prevalence and correlates of little cigar/cigarillo use among cigarette smokers. Nicotine Tob Res. 2015;17:1473–1481. doi: 10.1093/ntr/ntv022. [DOI] [PubMed] [Google Scholar]
- 6.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickey BF, Fahy JV, Kesimer M, Boucher RC, Evans CM, Thornton D. Measuring airway mucin 2 in patients with severe chronic obstructive pulmonary disease with bacterial colonization. Ann Am Thorac Soc. 2016;13:2103–2104. doi: 10.1513/AnnalsATS.201607-532LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonser LR, Erle DJ. Airway mucus and asthma: the role of MUC5AC and MUC5B. J Clin Med. 2017;6:112. doi: 10.3390/jcm6120112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kesimer M, Ford AA, Ceppe A, Radicioni G, Cao R, Davis CW, et al. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med. 2017;377:911–922. doi: 10.1056/NEJMoa1701632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson WH, Coakley RD, Button B, Henderson AG, Zeman KL, Alexis NE, et al. The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am J Respir Crit Care Med. 2015;192:182–190. doi: 10.1164/rccm.201412-2230OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh A, Abdelwahab SH, Reeber SL, Reidel B, Marklew AJ, Garrison AJ, et al. Little cigars are more toxic than cigarettes and uniquely change the airway gene and protein expression. Sci Rep. 2017;7:46239. doi: 10.1038/srep46239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maxwell JC., Jr . Richmond, VA: John C. Maxwell, Jr.; 2016. The Maxwell report: cigar industry in 2015. [Google Scholar]
- 13.Centers for Disease Control and PreventionTobacco brand preferences. Atlanta, GA: Centers for Disease Control and Prevention; 2015[accessed 2020 May 18]. Available from: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/tobacco_industry/brand_preference/ [Google Scholar]
- 14.Holmén JM, Karlsson NG, Abdullah LH, Randell SH, Sheehan JK, Hansson GC, et al. Mucins and their O-glycans from human bronchial epithelial cell cultures. Am J Physiol Lung Cell Mol Physiol. 2004;287:L824–L834. doi: 10.1152/ajplung.00108.2004. [DOI] [PubMed] [Google Scholar]
- 15.Clunes LA, Davies CM, Coakley RD, Aleksandrov AA, Henderson AG, Zeman KL, et al. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J. 2012;26:533–545. doi: 10.1096/fj.11-192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 17.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aufderheide M, Scheffler S, Ito S, Ishikawa S, Emura M. Ciliatoxicity in human primary bronchiolar epithelial cells after repeated exposure at the air-liquid interface with native mainstream smoke of K3R4F cigarettes with and without charcoal filter. Exp Toxicol Pathol. 2015;67:407–411. doi: 10.1016/j.etp.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev. 2007;87:1047–1082. doi: 10.1152/physrev.00048.2006. [DOI] [PubMed] [Google Scholar]
- 20.Ramos FL, Krahnke JS, Kim V. Clinical issues of mucus accumulation in COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:139–150. doi: 10.2147/COPD.S38938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J Dent Res. 2012;91:142–149. doi: 10.1177/0022034511421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tavilani H, Nadi E, Karimi J, Goodarzi MT. Oxidative stress in COPD patients, smokers, and non-smokers. Respir Care. 2012;57:2090–2094. doi: 10.4187/respcare.01809. [DOI] [PubMed] [Google Scholar]
- 23.Reidel B, Radicioni G, Clapp PW, Ford AA, Abdelwahab S, Rebuli ME, et al. E-cigarette use causes a unique innate immune response in the lung, involving increased neutrophilic activation and altered mucin secretion. Am J Respir Crit Care Med. 2018;197:492–501. doi: 10.1164/rccm.201708-1590OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isik B, Ceylan A, Isik R. Oxidative stress in smokers and non-smokers. Inhal Toxicol. 2007;19:767–769. doi: 10.1080/08958370701401418. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh A, Nethery RC, Herring AH, Tarran R. Flavored little cigar smoke induces cytotoxicity and apoptosis in airway epithelia. Cell Death Discov. 2017;3:17019. doi: 10.1038/cddiscovery.2017.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaspers I. Cigarette smoke effects on innate immune mechanisms in the nasal mucosa: potential effects on the microbiome. Ann Am Thorac Soc. 2014;11:S38–S42. doi: 10.1513/AnnalsATS.201306-154MG. [DOI] [PubMed] [Google Scholar]
- 27.Qiu F, Liang CL, Liu H, Zeng YQ, Hou S, Huang S, et al. Impacts of cigarette smoking on immune responsiveness: up and down or upside down? Oncotarget. 2017;8:268–284. doi: 10.18632/oncotarget.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Eeden SF, Sin DD. Oxidative stress in chronic obstructive pulmonary disease: a lung and systemic process. Can Respir J. 2013;20:27–29. doi: 10.1155/2013/509130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macnee W, Rahman I. Oxidants and antioxidants as therapeutic targets in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:S58–S65. doi: 10.1164/ajrccm.160.supplement_1.15. [DOI] [PubMed] [Google Scholar]
- 30.Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017;9:7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radicioni G, Cao R, Carpenter J, Ford AA, Wang T, Li L, et al. The innate immune properties of airway mucosal surfaces are regulated by dynamic interactions between mucins and interacting proteins: the mucin interactome. Mucosal Immunol. 2016;9:1442–1454. doi: 10.1038/mi.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mall MA. Role of cilia, mucus, and airway surface liquid in mucociliary dysfunction: lessons from mouse models. J Aerosol Med Pulm Drug Deliv. 2008;21:13–24. doi: 10.1089/jamp.2007.0659. [DOI] [PubMed] [Google Scholar]
- 33.Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest. 2009;135:505–512. doi: 10.1378/chest.08-0412. [DOI] [PubMed] [Google Scholar]
- 34.Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58:157–170. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- 35.Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9:377–384. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 36.Bals R, Weiner DJ, Wilson JM. The innate immune system in cystic fibrosis lung disease. J Clin Invest. 1999;103:303–307. doi: 10.1172/JCI6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Caballero A, Rasmussen JE, Gaillard E, Watson MJ, Olsen JC, Donaldson SH, et al. SPLUNC1 regulates airway surface liquid volume by protecting ENaC from proteolytic cleavage. Proc Natl Acad Sci USA. 2009;106:11412–11417. doi: 10.1073/pnas.0903609106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore PJ, Reidel B, Ghosh A, Sesma J, Kesimer M, Tarran R. Cigarette smoke modifies and inactivates SPLUNC1, leading to airway dehydration. FASEB J. 2018;32:fj201800345R. doi: 10.1096/fj.201800345R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartlett JA, Fischer AJ, McCray PBJ., Jr Innate immune functions of the airway epithelium. Contrib Microbiol. 2008;15:147–163. doi: 10.1159/000136349. [DOI] [PubMed] [Google Scholar]
- 40.Kaetzel CS. Els. Hoboken, NJ: John Wiley & Sons; 2013. The polymeric immunoglobulin receptor. [Google Scholar]
- 41.Hunziker W, Kraehenbuhl JP. Epithelial transcytosis of immunoglobulins. J Mammary Gland Biol Neoplasia. 1998;3:287–302. doi: 10.1023/a:1018715511178. [DOI] [PubMed] [Google Scholar]
- 42.Ohlmeier S, Mazur W, Linja-Aho A, Louhelainen N, Rönty M, Toljamo T, et al. Sputum proteomics identifies elevated PIGR levels in smokers and mild-to-moderate COPD. J Proteome Res. 2012;11:599–608. doi: 10.1021/pr2006395. [DOI] [PubMed] [Google Scholar]
- 43.Rokicki W, Rokicki M, Wojtacha J, Dżeljijli A. The role and importance of club cells (Clara cells) in the pathogenesis of some respiratory diseases. Kardiochir Torakochirurgia Pol. 2016;13:26–30. doi: 10.5114/kitp.2016.58961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hay JG, Danel C, Chu CS, Crystal RG. Human CC10 gene expression in airway epithelium and subchromosomal locus suggest linkage to airway disease. Am J Physiol. 1995;268:L565–L575. doi: 10.1152/ajplung.1995.268.4.L565. [DOI] [PubMed] [Google Scholar]
- 45.Mantile G, Miele L, Cordella-Miele E, Singh G, Katyal SL, Mukherjee AB. Human Clara cell 10-kDa protein is the counterpart of rabbit uteroglobin. J Biol Chem. 1993;268:20343–20351. [PubMed] [Google Scholar]
- 46.Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med. 2009;206:1149–1166. doi: 10.1084/jem.20081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sohn MH, Kang MJ, Matsuura H, Bhandari V, Chen NY, Lee CG, et al. The chitinase-like proteins breast regression protein-39 and YKL-40 regulate hyperoxia-induced acute lung injury. Am J Respir Crit Care Med. 2010;182:918–928. doi: 10.1164/rccm.200912-1793OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deshmukh VK, Raman PH, Dhuley JN, Naik SR. Role of ceruloplasmin in inflammation: increased serum ceruloplasmin levels during inflammatory conditions and its possible relationship with anti-inflammatory agents. Pharmacol Res Commun. 1985;17:633–642. doi: 10.1016/0031-6989(85)90070-0. [DOI] [PubMed] [Google Scholar]
- 49.Weaver TE. Pulmonary surfactant-associated proteins. Gen Pharmacol. 1988;19:361–368. doi: 10.1016/0306-3623(88)90029-8. [DOI] [PubMed] [Google Scholar]
- 50.Weaver TE, Whitsett JA. Function and regulation of expression of pulmonary surfactant-associated proteins. Biochem J. 1991;273:249–264. doi: 10.1042/bj2730249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wright JR. Host defense functions of pulmonary surfactant. Biol Neonate. 2004;85:326–332. doi: 10.1159/000078172. [DOI] [PubMed] [Google Scholar]
- 52.Scott JE. The pulmonary surfactant: impact of tobacco smoke and related compounds on surfactant and lung development. Tob Induc Dis. 2004;2:3–25. doi: 10.1186/1617-9625-2-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nikota JK, Stämpfli MR. Cigarette smoke-induced inflammation and respiratory host defense: insights from animal models. Pulm Pharmacol Ther. 2012;25:257–262. doi: 10.1016/j.pupt.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Seys LJ, Verhamme FM, Dupont LL, Desauter E, Duerr J, Seyhan Agircan A, et al. Airway surface dehydration aggravates cigarette smoke-induced hallmarks of COPD in mice. PLoS One. 2015;10:e0129897. doi: 10.1371/journal.pone.0129897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grubb BR, Livraghi-Butrico A, Rogers TD, Yin W, Button B, Ostrowski LE. Reduced mucociliary clearance in old mice is associated with a decrease in Muc5b mucin. Am J Physiol Lung Cell Mol Physiol. 2016;310:L860–L867. doi: 10.1152/ajplung.00015.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Livraghi A, Grubb BR, Hudson EJ, Wilkinson KJ, Sheehan JK, Mall MA, et al. Airway and lung pathology due to mucosal surface dehydration in β-epithelial NA+ channel-overexpressing mice: Role of TNF-α and IL-4Rα signaling, influence of neonatal development, and limited efficacy of glucocorticoid treatment. J Immunol. 2009;182:4357–4367. doi: 10.4049/jimmunol.0802557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blank MD, Cobb CO, Eissenberg T, Nasim A. Acute effects of “hyping” a Black&Mild cigarillo. Nicotine Tob Res. 2016;18:460–469. doi: 10.1093/ntr/ntv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blank MD, Nasim A, Hart A, Jr, Eissenberg T. Acute effects of cigarillo smoking. Nicotine Tob Res. 2011;13:874–879. doi: 10.1093/ntr/ntr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kostygina G, Huang J, Emery S. TrendBlendz: how Splitarillos use marijuana flavours to promote cigarillo use. Tob Control. 2017;26:235–236. doi: 10.1136/tobaccocontrol-2015-052710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.