Abstract
Electronic-cigarette (e-cig) vaping is a serious concern, as many pregnant women who vape consider it safe. However, little is known about the harmful effects of prenatal e-cig exposure on adult offspring, especially on extracellular-matrix (ECM) deposition and myogenesis in the lungs of offspring. We evaluated the biochemical and molecular implications of maternal exposure during pregnancy to e-cig aerosols on the adult offspring of both sexes, with a particular focus on pulmonary ECM remodeling and myogenesis. Pregnant CD-1 mice were exposed to e-cig aerosols with or without nicotine, throughout gestation, and lungs were collected from adult male and female offspring. Compared with the air-exposed control group, female mice exposed to e-cig aerosols, with or without nicotine, demonstrated increased lung protein abundance of LEF-1 (lymphoid enhancer–binding factor 1), fibronectin, and E-cadherin, whereas altered E-cadherin and PPARγ (peroxisome proliferator–activated receptor γ) levels were observed only in males exposed to e-cig aerosols with nicotine. Moreover, lipogenic and myogenic mRNAs were dysregulated in adult offspring in a sex-dependent manner. PAI-1 (plasminogen activator inhibitor-1), one of the ECM regulators, was significantly increased in females exposed prenatally to e-cig aerosols with nicotine and in males exposed to e-cig aerosols compared with control animals exposed to air. MMP9 (matrix metalloproteinase 9), a downstream target of PAI-1, was downregulated in both sexes exposed to e-cig aerosols with nicotine. No differences in lung histology were observed among any of the treatment groups. Overall, adult mice exposed prenatally to e-cig aerosols could be predisposed to developing pulmonary disease later in life. Thus, these findings suggest that vaping during pregnancy is unsafe and increases the propensity for later-life interstitial lung diseases.
Keywords: e-cigs, prenatal, ECM remodeling, plasminogen activator inhibitor-1, myogenesis
Clinical Relevance
Little is known about the harmful effects of prenatal electronic-cigarette (e-cig) exposure on adult offspring, especially on extracellular-matrix deposition and myogenesis in the lungs of offspring. E-cig exposure led to dysregulated lipogenic/myogenic pathways and dysregulated extracellular-matrix remodeling in adult offspring in a sex-dependent manner. Hence, adult mice exposed prenatally to e-cig aerosols could be predisposed to developing pulmonary disease later in life, suggesting that e-cig vaping during pregnancy increases the propensity for later-life interstitial lung diseases.
The rapid growth of electronic-cigarette (e-cig) use is raising serious health concerns globally, particularly because of the public perception of their safety (1). Electronic nicotine-delivery systems vaporize e-cig liquids that are loaded into cartridges, which are then delivered as an e-cig aerosol. The e-cig liquid usually contains nicotine in propylene glycol (PG) and/or vegetable glycerin (VG), often with added chemicals as flavors. Studies show that some e-cig devices deliver nicotine to the user in amounts equal to or higher than those delivered by conventional tobacco cigarettes (2–4). Despite increasing numbers of studies demonstrating the toxic effects of e-cig humectants (PG/VG), nicotine, and flavorings, information concerning the health effects of e-cigs on pregnant women and the unborn fetus is still lacking (5–7).
Evidence is emerging from human studies that e-cig use during pregnancy is associated with decreased fertility in adults, poor embryotic growth, and impaired memory in the e-cig aerosol–exposed offspring (8, 9). Because of germline epigenetic effects and potential transgenerational consequences, nicotine exposure during pregnancy is particularly concerning (10). Using both in vivo and in vitro studies, we and others have shown that e-cig aerosols induce oxidative stress and proinflammatory responses of human alveolar macrophages after exposure (11–14). Relatively few studies have addressed the molecular mechanisms involved in e-cig toxicity responses in the developing fetus or in later life.
The effects of e-cig aerosol exposure on developing lungs are unknown. Using a murine model, we have recently shown sex-specific proinflammatory and dysregulated repair effects from e-cig aerosols with and without nicotine (15). Because dysregulated repair is a hallmark for pulmonary conditions such as idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease (COPD), and asthma, it is important to examine the effects of e-cig exposure on such repair mechanisms in the developing lung.
PAI-1 (plasminogen activator inhibitor-1), produced mainly by the endothelium, is the primary inhibitor of plasminogen activator. Through modulation of extracellular-matrix (ECM) degradation, it is critically involved in the process of thrombosis and hemostasis (16). Recent studies have linked PAI-1 to pulmonary fibrosis, and higher serum PAI-1 levels have been observed in patients with COPD (17, 18); moreover, MMP9 (matrix metalloproteinase 9), a downstream target of PAI-1, is also considered a critical factor for COPD.
Here, using an in vivo mouse model, we report the effects of maternal e-cig aerosol exposure throughout gestation on lung ECM remodeling. Findings suggest that e-cig aerosol exposure during pregnancy, with or without nicotine, dysregulates ECM remodeling, which could potentially contribute to fibrogenic effects mediated via PAI-1/MMP dysregulation.
Methods
For a description of the mouse model and exposure details, see the data supplement. All experimental details for molecular analysis, including protein extraction, Western blotting, RNA extraction, quantitative real-time PCR, and gelatin zymography, are presented in the data supplement and are described previously (15, 19).
Animal Model
Beginning 1 day after arrival, the estrous cycle was monitored daily in female CD-1 mice for at least two complete normal estrous phases. In the third proestrous cycle, each female mouse was paired overnight with one male, and a total of 30 female mice were mated (14, 20). Confirmation of successful mating was determined by the presence of a copulatory plug (gestational Day [GD] 0.5). Males were then removed and females (two per cage), were exposed to e-cig aerosols (PG/VG or PG/VG + nicotine) or filtered air for 3 weeks (3 h/d; 5 d/wk) (14, 20). Dams were separated into single cages and housed individually at or around GD 15, and daily exposures continued until just before parturition (∼3 wk) (14, 20). Pregnancy incidence for this mating paradigm was ∼90%, and e-cig aerosol exposure had no effect on pregnancy incidence, litter size, or male-to-female offspring sex ratio.
E-Cig Aerosol Generation and Exposure
E-cig aerosols (50% PG, 50% VG, and 16% nicotine, prepared in-house) were generated using a commercially available three-port e-cig generation system (CH Technologies) used in our previous studies (13, 14, 20). Urinary cotinine levels in dams were measured on GD5.5, GD10.5, and GD15.5 using a commercially available ELISA kit to assure e-cig exposure in the appropriate treatment group (20). Aerosol concentration measurements of particulate matter were taken every 15–30 minutes using the portable Data-Logging Real-Time Aerosol Monitor 4 (Thermo Fisher Scientific) (20).
Hematoxylin and Eosin Staining
Lung sections (5 μm) were deparaffinized, rehydrated, and then rinsed with tap water. Rehydrated sections were soaked in hematoxylin stain for 2 minutes and rinsed in cold tap water until the water runoff was colorless. Slides were then incubated in 7% ammonia–93% water for 30 seconds for bluing the tissues. This was followed by washing in tap water for 1 minute and staining with eosin (hematoxylin and eosin [H&E]) for 30 seconds. After staining, slides were dehydrated with two 10-second rinses each in increasing ethanol concentrations (i.e., 80%, 95%, and 100%) and were then rinsed three times in xylene for a total of 30 seconds; all slides were mounted with Permount and cover-slipped for evaluation by light microscopy (10× and 40×).
Lung Morphometry
Lung sections stained with H&E were used to measure the mean linear intercept of airspace by using MetaMorph software (Molecular Devices). A total of 7–14 pictures were randomly selected per slide in a blinded manner, and analysis was based on a manual threshold described previously (21).
Statistical Analysis
One-way ANOVA and the Student’s t test were used, as appropriate, to determine the statistical significance among and between conditions by using GraphPad Prism Software version 8.0 (GraphPad) (19). Results were expressed as the mean ± SEM, and P < 0.05 was considered statistically significant (19).
Results
Effects of E-Cig Prenatal Exposure in Lung Development
To study the effect of in utero e-cig exposure on key lung developmental processes, the expression of well-established lipogenic and myogenic markers, including PPARγ (peroxisome proliferator–activated receptor γ), β-catenin (catenin β-1), HDAC-1 (histone deacetylase 1), and LEF-1 (lymphoid enhancer–binding factor 1), were determined (Figure 1). Upregulated protein abundance of LEF-1 and HDAC-1 in aerosol-exposed animals compared with air-exposed control animals was demonstrated in female pups prenatally exposed to PG/VG only, with no change in β-catenin and PPARγ levels (Figures 1A and 1B). There was no effect on β-catenin, LEF-1, or HDAC-1 protein levels in male pups exposed in utero to e-cig aerosols with or without nicotine. However, lung PPARγ levels increased in adult male pups exposed to e-cig aerosols containing PG/VG + nicotine compared with control animals exposed to air and sex- and age-matched counterparts exposed to PG/VG alone (Figures 1A and 1C).
Figure 1.
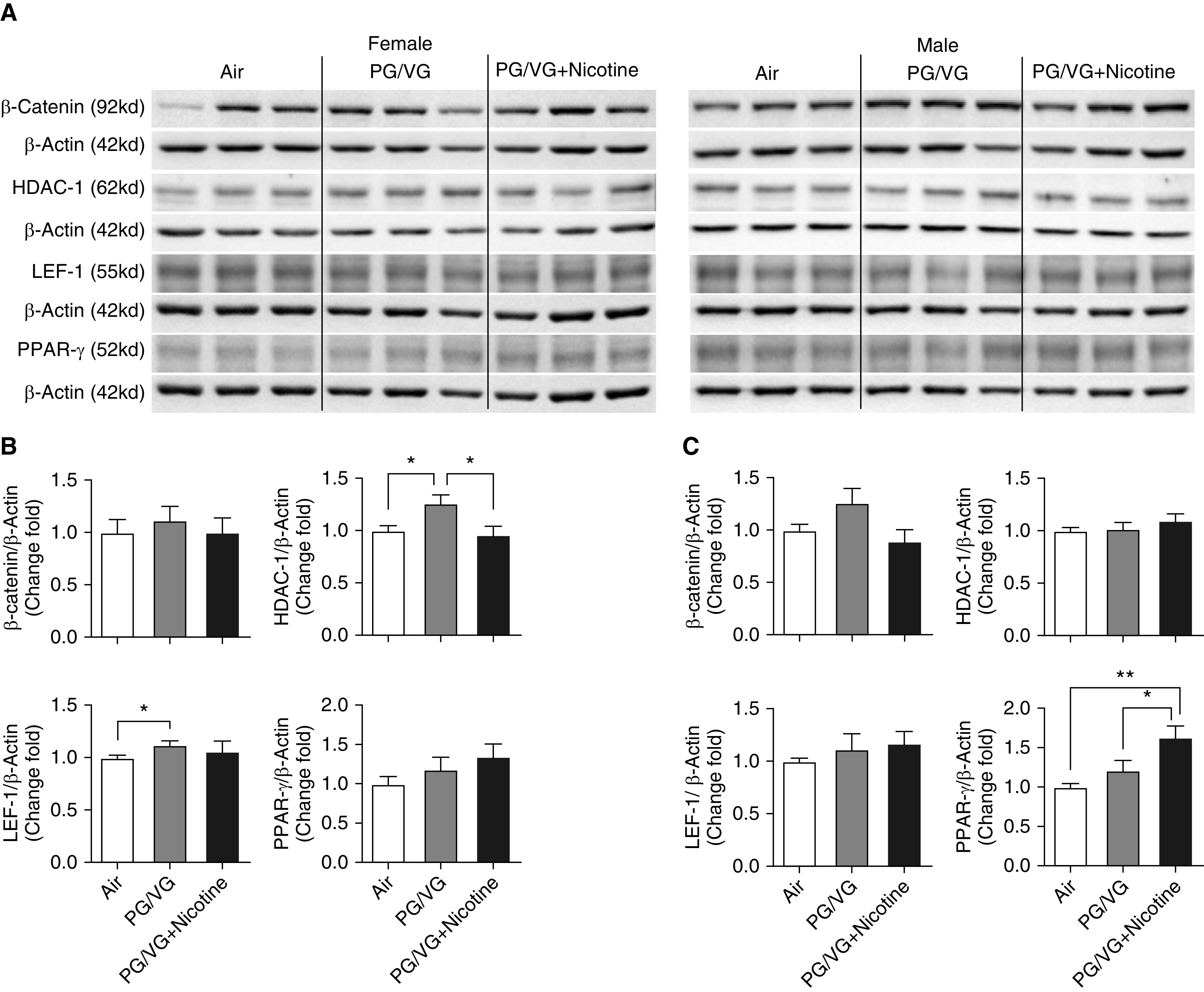
Lungs from both 6-week-old female and male offspring from mouse dams exposed throughout pregnancy to electronic-cigarette (e-cig) aerosols with or without nicotine, were homogenized, and related developmental protein markers were analyzed by Western blot. (A) Blot image of proteins included β-catenin (catenin β-1), HDAC-1 (histone deacetylase 1), LEF-1 (lymphoid enhancer–binding factor 1), and PPAR-γ (peroxisome proliferator–activated receptor γ). The fold changes of protein expression in (B) female and (C) male offspring were analyzed on the basis of densitometry, with β-actin serving as an endogenous control for normalization (n = 6–8 for both female and male mice). *P < 0.05 and **P < 0.01. PG = propylene glycol; VG = vegetable glycerin.
Effects of E-Cig Prenatal Exposure on ECM Remodeling
As shown in Figure 2, pulmonary fibronectin, one of the key ECM proteins, was significantly increased in female mice exposed in utero to PG/VG alone compared with mice exposed to air and mice exposed to PG/VG + nicotine (Figures 2A and 2B). Fibronectin levels decreased significantly in male offspring exposed to e-cig aerosols with or without nicotine compared with control animals exposed to air (Figures 2A and 2C). E-cadherin levels were decreased significantly in PG/VG + nicotine–exposed male pups compared with air-exposed control animals (Figures 2A and 2C). Although both male and female offspring exposed to PG/VG + nicotine revealed a decrease in pulmonary COL1A1 (type-1 collagen) levels, statistical significance was reached only in female offspring, as compared with the sex-matched PG/VG-only exposure group (Figure 2).
Figure 2.

Lungs from both 6-week-old female and male offspring from mouse dams exposed throughout pregnancy to e-cig aerosols with or without nicotine were homogenized, and extracellular matrix (ECM)-related protein markers were analyzed by Western blot. (A) Blot image of proteins included fibronectin, E-cadherin, and Col1A1 (type-1 collagen). The fold changes of protein expression in (B) female and (C) male offspring were analyzed on the basis of densitometry, with β-actin serving as endogenous control for normalization. (n = 6–8 for both female and male mice). *P < 0.05 and ***P < 0.001.
In addition to the key ECM proteins discussed above, PAI-1, a known ECM deposition modulator, was examined. Compared with air-exposed control animals, both female and male pups exposed in utero to PG/VG + nicotine exhibited higher PAI-1 levels (Figure 3). Although male pups did not show any significant change in phospho-p53 levels after maternal exposure to PG/VG with or without nicotine (Figures 3A and 3C), compared with their sex-matched air-exposed counterparts and age-matched female pups exposed in utero to PG/VG + nicotine, a significant increase in phospho-p53 expression was observed when it was normalized to β-actin, and a significant decrease in phospho-p53 expression was observed when it was normalized to total-p53 levels (Figures 3A and 3B).
Figure 3.
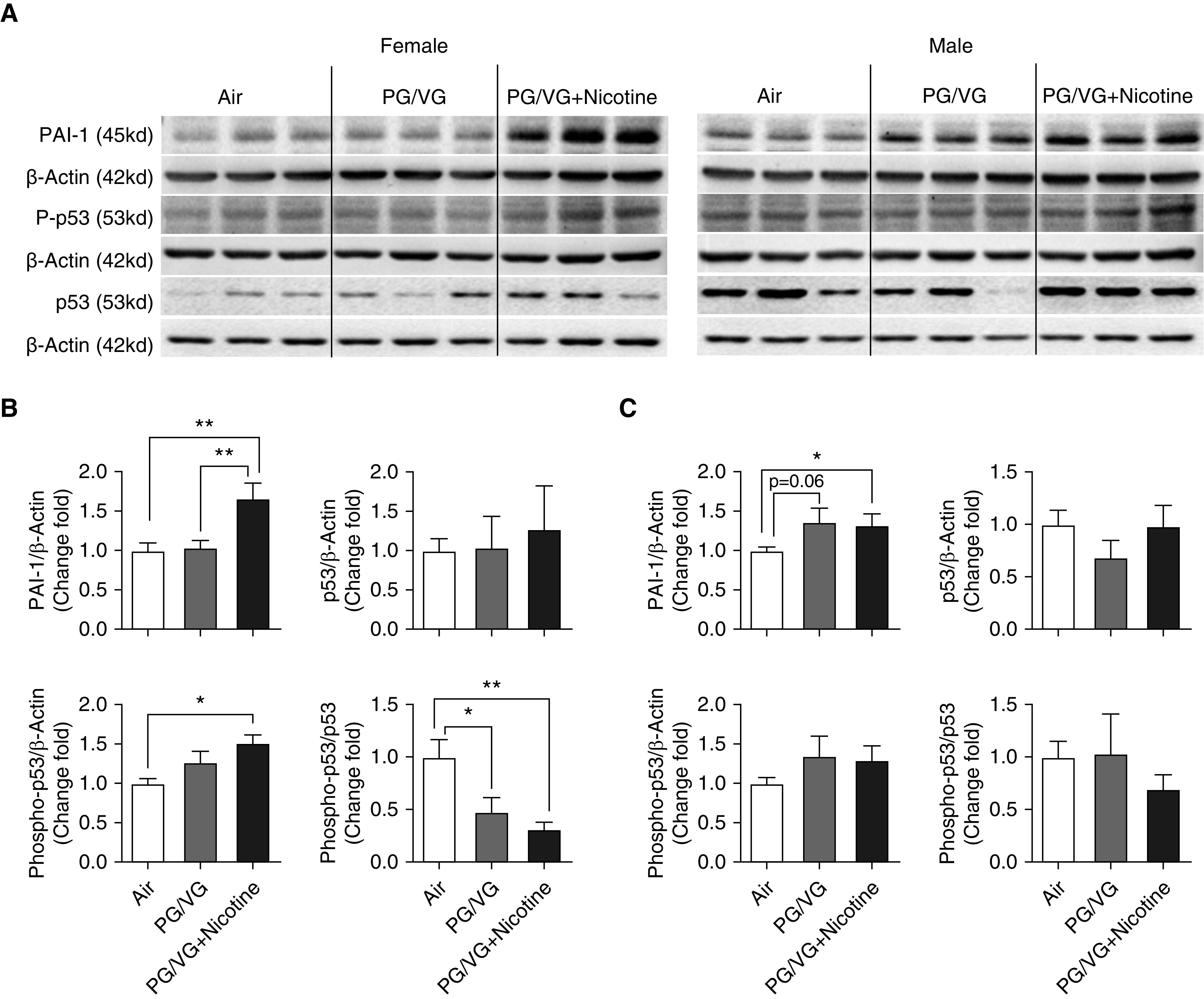
Lungs from both 6-week-old female and male offspring from mouse dams exposed throughout pregnancy to e-cig aerosols with or without nicotine were homogenized, and ECM-related protein markers were analyzed by Western blot. (A) Blot image of proteins included PAI-1 (plasminogen activator inhibitor-1), phospho-p53, and p53. Fold changes of protein expression in (B) female and (C) male offspring were analyzed on the basis of densitometry, with β-actin serving as endogenous control for normalization. (n = 6–8 for both female and male mice). *P < 0.05 and **P < 0.01.
MMP9 and MMP2, two downstream targets of PAI-1 and key determinants of ECM deposition, were also examined. As shown in Figure 4, pulmonary MMP9 levels were significantly decreased in both female and male offspring exposed prenatally to PG/VG + nicotine compared with mice exposed to either PG/VG alone or air control. MMP2 levels were significantly increased in female pups exposed to PG/VG with and without nicotine compared with mice exposed to air control (Figures 4A and 4B), whereas no significant changes were observed in age-matched male offspring (Figures 4A and 4C). In addition, gelatin zymography of activated MMP9/MMP2 revealed decreased MMP9 activity in the PG/VG + nicotine–exposed male and female offspring compared with the air-exposed control group (see Figure E1 in the data supplement). In contrast, Timp1 (TIMP metallopeptidase inhibitor 1), an inhibitor of MMP 2 and 9, was significantly upregulated in females exposed to PG/VG with or without nicotine compared with control animals exposed to air (Figures 4A and 4B) but was not altered in age-matched males after in utero exposure to PG/VG with or without nicotine (Figures 4A and 4C).
Figure 4.
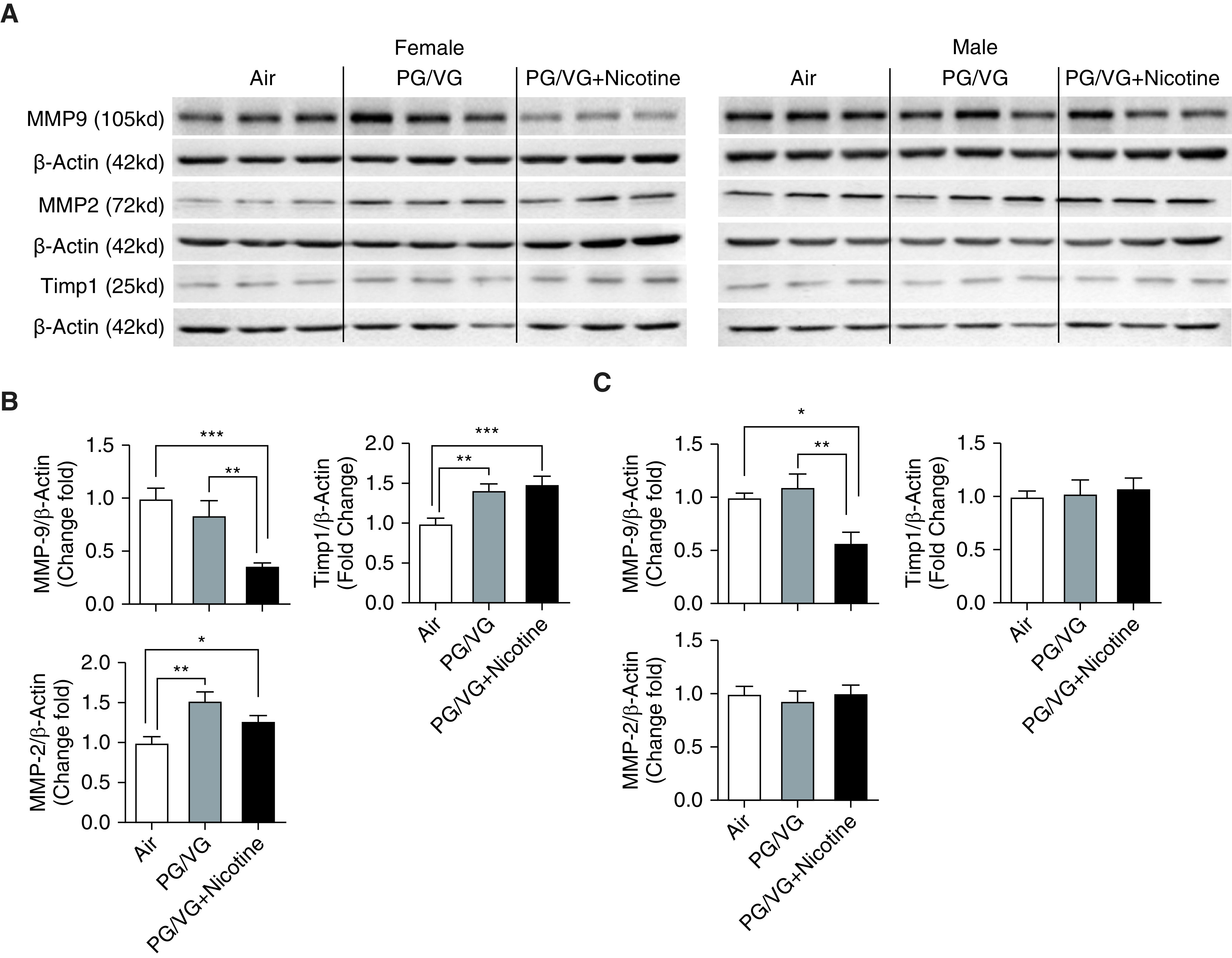
Lungs from both female and male mouse offspring (6-wk old) from mothers prenatally exposed to e-cig aerosols with or without nicotine were homogenized, and ECM-related protein markers were analyzed by Western blot. (A) Blot image of proteins included MMP-9 (matrix metalloproteinase 9), MMP-2, and Timp1 (TIMP metallopeptidase inhibitor 1). Fold changes of protein expression in (B) female and (C) male mice were analyzed on the basis of densitometry, with β-actin serving as an endogenous control for normalization (n = 6–8 for both female and male mice). *P < 0.05, **P < 0.01, and ***P < 0.001.
The abundance of TGF-β and pSmad2 (phospho-Smad2) in the lung was determined by immunohistochemistry staining (Figure 5). There was a nonsignificant increase in pSmad2 levels when mice were exposed to PG/VG alone compared with female offspring exposed to air or PG/VG + nicotine. A nonsignificant increase in TGF-β was observed in female offspring exposed to e-cig aerosols with or without nicotine (Figures 5A and 5B) compared with control animals, whereas expression of TGF-β was increased in male offspring exposed to PG/VG alone compared with the group exposed to PG/VG + nicotine (Figure 5B). As expected, pSmad2 showed similar trends with TGF-β in male offspring’s lung sections (Figures 5C and 5D). The protein abundance of pSmad2 was significantly increased in male offspring exposed to PG/VG only compared with groups exposed to PG/VG + nicotine or air; no significant changes in pSmad2 were observed in female offspring of any group (Figure 5D). However, when examined for site- and sex-specific changes in these same markers in the PG/VG-alone group, the expression of both TGF-β and pSmad2 were specifically upregulated in the alveolar area in males, whereas no changes in the tracheal/bronchial region were observed (Figures 5A and 5C).
Figure 5.
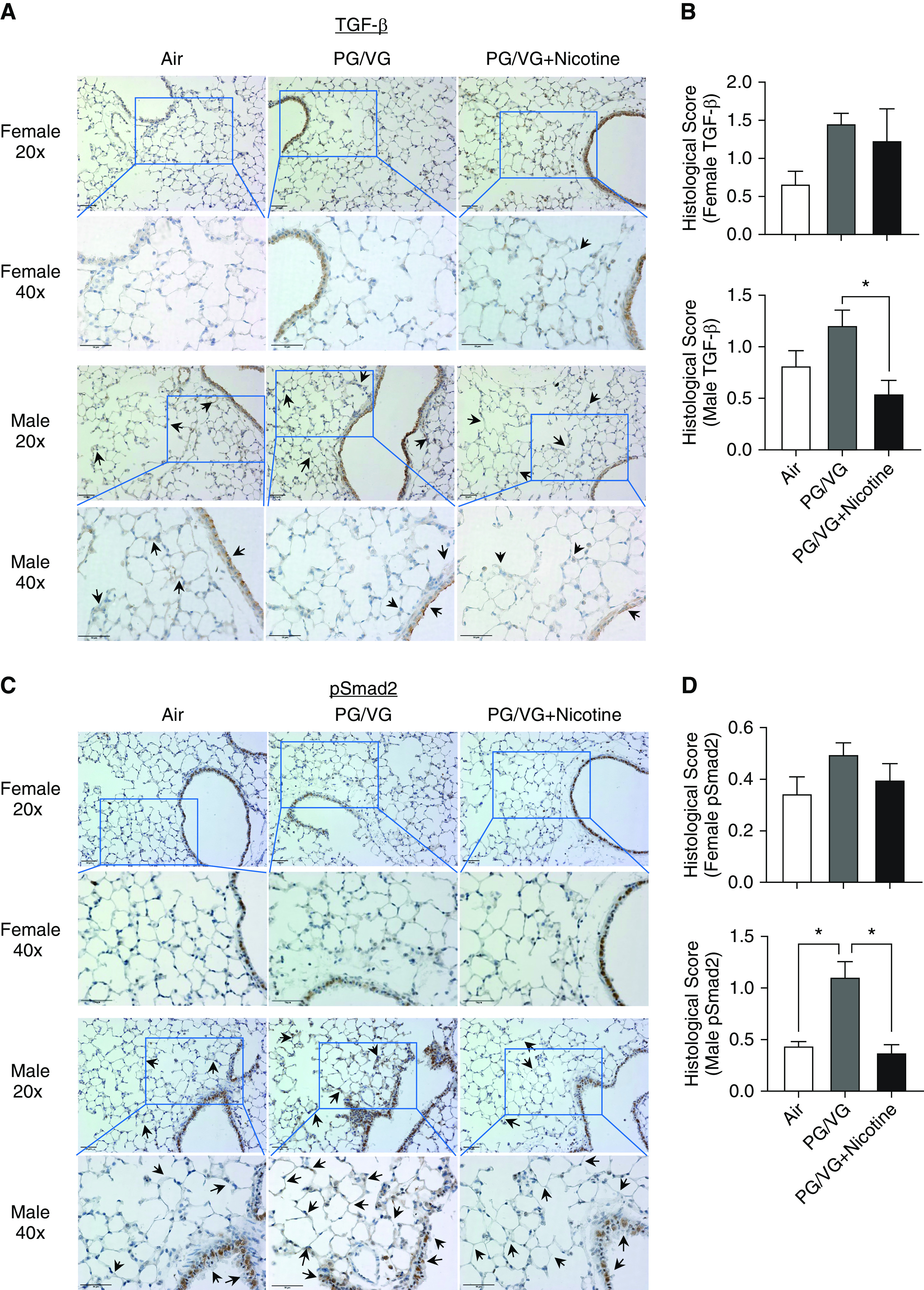
Lungs from female and male offspring (6-wk old) prenatally exposed to e-cig aerosols with or without nicotine were paraffin-embedded and sectioned for immunohistochemistry staining: (A and B) TGF-β and (C and D) pSmad2 (phospho-Smad2). Scale bars, 50 μm. The brown color represent the abundance of targets of interest in different regions. Regions of interest are indicated by black arrows, 40x pictures were the views from blue boxes in 20x pictures. Histological scores were applied in a blinded manner. (n = 3–4 for both female and male mice). *P < 0.05. TGF = transforming growth factor.
Expressions of Lipogenic/Myogenic Genes after Prenatal E-Cig Exposure
Key lipogenic/myogenic genes, including ADRP (adipose differentiation-related protein), CNN1 (calponin 1), ACTA2 (α- smooth muscle actin), FN1 (fibronectin), SERPINE1 (plasminogen activator inhibitor-1), and MMP9 were examined in offspring of both sexes to investigate the effects of prenatal exposure of PG/VG with or without nicotine on lipogenic/myogenic pathways using quantitative real-time-PCR (Figure 6). The lipogenic marker ADRP was decreased significantly, in comparison with the air-exposed control animals, in females exposed in utero to PG/VG with or without nicotine (Figure 6A), and there was an absence of observed effects in the age-matched male counterparts (Figure 6B). In contrast, the myogenic marker CNN1 was increased in male offspring exposed to PG/VG + nicotine compared with offspring exposed to air control (Figure 6B), whereas age-matched female counterparts were unaffected by maternal exposure to e-cig aerosols during pregnancy (Figure 6A). Expression of the myogenic marker ACTA2 was increased in male offspring exposed in utero to PG/VG + nicotine compared offspring exposed to air. A significant upregulation of ACTA2 was also seen in female offspring exposed to PG/VG alone compared with the group exposed to air. In addition, adult female offspring whose mothers were exposed to PG/VG + nicotine during pregnancy had increased lung SERPINE1 and decreased MMP9 (albeit nonsignificant) expression as compared with offspring whose mothers were exposed to PG/VG alone or air. Expression of other lipogenic/myogenic markers, including PPARg (peroxisome proliferator activated receptor gamma), CEBPA (CCAAT enhancer–binding protein α), and FN1 (fibronectin), was unaffected by maternal exposure during pregnancy to e-cig aerosols compared with that observed in the air-exposed control mice of either sex.
Figure 6.

Lungs from both female and male mouse offspring (6-wk old) from mothers prenatally exposed to e-cig aerosols with or without nicotine were homogenized, and lipogenic/myogenic markers were analyzed by quantitative real-time-PCR. The mRNA expression levels of PPARg (peroxisome proliferator–activated receptor γ), ADRP (adipose differentiation related protein), CEBPA (CCAAT enhancer–binding protein α), CNN1 (calponin 1), ACTA2 (α-smooth muscle actin), FN1 (fibronectin), SERPINE1 (plasminogen activator inhibitor-1), and MMP9 were measured in both (A) female and (B) male pup lungs. β-tubulin/GAPDH was used as an endogenous control for normalization (n = 4–6 for both female and male mice). *P < 0.05 and **P < 0.01.
Consequences of E-Cig Prenatal Exposure on Lung Morphology
The effects of maternal e-cig exposure during pregnancy on adult-offspring lung morphometry, with particular emphasis on alveolar destruction/airspace enlargement and collagen deposition, were observed using H&E staining and Gomori’s Trichrome staining (Figure 7). As shown in Figures 7A and 7C, lung structure in both male and female offspring was unaffected by in utero exposure to air, PG/VG, and PG/VG + nicotine compared with air-exposed control mice. However, an abnormal collagen deposition in male offspring prenatally exposed to PG/VG compared with male offspring prenatally exposed to PG/VG + nicotine or air was observed (Figure 7B). As shown in Figure 7B, only male offspring exposed to PG/VG alone exhibited more collagen deposition around the tracheal/bronchial area, compared with offspring exposed to PG/VG + nicotine or air. There was no difference in collagen distribution in the alveolar region among any of the exposure groups. No significant difference in collagen deposition was revealed in female offspring among the groups, whereas male offspring showed higher fibrotic scoring in the PG/VG-alone exposure group than in the other treatment groups (Figures 7B and 7D).
Figure 7.

Lungs from female and male offspring (6-wk old) prenatally exposed to e-cig aerosols with or without nicotine were paraffin-embedded and sectioned for (A) hematoxylin and eosin (H&E) and (B) Gomori’s Trichrome staining. Scale bars: A, 20 μm; B, 50 μm. (C) Lm of H&E-stained sections was conducted using MetaMorph software (Molecular Devices), and (D) visualized collagen accumulation/deposition (red arrows) was quantified on the basis of the Ashcroft fibrosis score (n = 4 for both female and male mice). *P < 0.05. Lm = lung morphometry analysis.
Nicotinic-Receptor Expression after Prenatal E-Cig Exposure
Because there is strong evidence to suggest that pulmonary effects of nicotine are predominantly mediated via nAChR α7 (nicotinic acetylcholine receptor α7) and nAChR α3, these receptors were also investigated in prenatally e-cig–exposed offspring (Figure E2). Only female offspring prenatally exposed to PG/VG + nicotine demonstrated a significant decrease in nAChR α7 protein abundance, compared with sex-matched offspring exposed to PG/VG alone (Figures E2B and E2C).
Discussion
Given the overwhelming evidence of the harmful effects of cigarette/tobacco smoking during pregnancy, it is not surprising that alternate presumably safer approaches such as e-cigs have been sought. However, to the contrary, increasing evidence of adverse health outcomes of e-cig use is emerging, with only limited information available on the effects of e-cig use on pregnancy and the developing fetus. In particular, data are extremely limited on the effects of prenatal exposure to e-cig aerosols on the developing lungs (22–25). Here, we show that maternal e-cig aerosol exposure during pregnancy, especially with PG/VG alone, induces sex-dependent pulmonary ECM accumulation and a myogenic lung profile. Moreover, as evidenced by increased pulmonary PAI-1 and decreased MMP9 levels, lungs from both adult male and female offspring appear particularly vulnerable to increased ECM deposition.
After in utero e-cig aerosol exposure, fibronectin levels were upregulated in adult female pups exposed to PG/VG alone. Interestingly, other dysregulated myogenesis/lipogenesis markers also showed sex-dependent differences. This corroborates our previous finding that prenatal nicotine exposure causes myogenic effects (i.e., increased pulmonary fibronectin, α-SMA, and calponin levels) (24). We observed decreased E-cadherin levels only in the lungs of male offspring exposed to PG/VG + nicotine, compared with control offspring exposed to air control. This is in agreement with a previous study that showed that maternal nicotine exposure during pregnancy led to epithelial–mesenchymal transition in the lungs of male offspring, with increased N-cadherin and α-SMA protein levels and decreased E-cadherin levels (23). A recent study has reported that prenatal exposure to e-cig aerosols decreased body weight and length and dysregulated gene expression associated with the Wnt signaling pathway in comparison with exposure to air control (26). Here, we observed increased fibronectin protein levels in adult females exposed prenatally to only PG/VG, whereas calponin and α-SMA values were upregulated (at the transcription level) in male offspring exposed to PG/VG, and PG/VG + nicotine compared with offspring exposed to air control.
The increased transcription levels of ACTA2 and CNN1 in male offspring exposed to PG/VG + nicotine compared with levels in control animals exposed to air indicate the likely transition of lipofibroblasts to myofibroblasts, a key process in the development of pulmonary fibrosis. However, the upregulated PPARγ in male offspring prenatally exposed to PG/VG + nicotine (compared with the air control) could be protective, as characterized by decreased fibronectin levels in this group (27).
Altered global DNA methylation and an increased tendency toward inflammation in offspring lungs after prenatal nicotine exposure has also recently been described (28). In this study, we observed that after prenatal exposure to e-cig aerosols with nicotine, HDAC-1 was unaffected in female pups, whereas levels of ADRP decreased compared with levels in male pups. These findings are in partial agreement with those of Nguyen and colleagues, who showed decreased activating transcription factor-2 in the absence of any significant effect on HDAC-1 after maternal e-cig exposure (with or without nicotine) in male pups (25). In contrast, we showed increased HDAC-1 protein abundance in female pups exposed prenatally to PG/VG alone, suggesting that effects may be independent of nicotine. Notably, decreased ADRP expression supports our previous work in a prenatal nicotine–exposed rat model (29). Although sexual dimorphism was not considered in our previous rat model (30), e-cig–induced changes in ADRP expression in this study were observed only in females exposed to e-cig aerosol with or without nicotine.
Apart from the dysregulated myogenesis/lipogenesis noted above, the PAI-1/MMP9 pathway was dysregulated after prenatal e-cig aerosol exposure. Both female and male offspring showed increased PAI-1 and decreased MMP9 protein levels after in utero exposure to PG/VG + nicotine, whereas male offspring also showed increased PAI-1 protein levels after in utero exposure to PG/VG without nicotine. In support of our findings, other human and animal studies have demonstrated increased PAI-1 protein in fibrotic lung conditions (e.g., in human samples and in a bleomycin-induced lung fibrosis mouse model), together with other increased fibrogenic markers, such as fibronectin and α-SMA (31–33). Similarly, Jiang and colleagues demonstrated increased PAI-1/SERPINE1 levels in lungs affected by idiopathic pulmonary fibrosis. PAI-1 also plays an essential role in cell-cycle regulation and cellular senescence through the p53-p21-Rb pathway (34). Corroborating these data, significantly decreased phospho-53, normalized to total p53, was observed in female offspring exposed to both PG/VG alone and PG/VG + nicotine. In addition to the known direct effects of cigarette-smoke exposure on PAI-1, this study shows that prenatal exposure to e-cig aerosols can also result in dysregulation of PAI-1 protein abundance in offspring lungs.
A decrease in pulmonary MMP protein levels usually accompanies increased PAI-1 protein levels, which ultimately leads to increased ECM deposition and chronic fibrosis (35–38). Because MMP2 and MMP9 share similar substrates, the observed increase in MMP2 levels accompanying decreased MMP9 in female pups exposed prenatally to PG/VG + nicotine could suggest a compensatory mechanism (39). Increased PAI-1 together with decreased MMP9 could set the stage for enhanced airway inflammation on future allergen exposure (40). Offspring exposed to e-cig aerosols with or without nicotine could face a worsened lung situation when challenged with environmental toxicants later in life. Taken together, our data suggest long-term pulmonary consequences of prenatal e-cig exposure that are independent of nicotine and result from downregulated MMP9 and increased PAI-1 and MMP2 levels, leading to ECM remodeling, dysregulated myogenesis, and predisposition to fibrosis in adult offspring.
The trend toward increased TGF-β and pSmad2 levels in female offspring exposed to PG/VG alone could be one of the triggers for the upregulated fibronectin and collagen levels observed in this study. The upregulated PAI-1 in our model could be independent of TGF-β/pSmad2, as has been demonstrated previously (41). The observed collagen deposition was also shown in offspring rat lungs after maternal nicotine administration for 21 days (42). Furthermore, in our model, TGF-β/pSmad2 upregulation appeared to be site specific (i.e., in the alveolar region vs. the tracheobronchial region), whereas, paradoxically, increased collagen deposition was mainly restricted to the tracheal or bronchial region in male offspring exposed prenatally to PG/VG alone. These intriguing observations obviously need further detailed investigation.
Pulmonary ECM remodeling in adult offspring associated with prenatal exposure to e-cig aerosols appears to be sex-dependent. Differences between the sexes in e-cig–induced PAI-1 dysregulation could be related to alterations in hormone levels, as the expression of PAI-1 is inhibited by testosterone (43) as well as by estrogen in endothelial cells (44). PAI-1 levels are affected by the testosterone/estrogen balance (45), with a decrease in PAI-1 activity observed in postmenopausal females (46). Other ECM-related proteins, such as fibronectin, COL1A1, and E-cadherin, are also associated with effects that are sex-dependent (47), as is the T-cell activity response to PPARγ modulation, an important determinant of ECM deposition (48). Additional research examining the relationship between prenatal e-cig exposure and sex-specific pulmonary outcomes is needed. Although the harmful effects of e-cig use during pregnancy are rapidly emerging (13, 14), a considerable number of women still “vape” during pregnancy.
Conclusions
Recently, it has been shown that ∼39% of pregnant women (out of ∼1,000) use e-cigs during pregnancy (49). According to a CDC report (42), roughly 7% of women in certain U.S. regions reported that they used e-cigs before, during, or after pregnancy. This translational study reveals that maternal exposure during pregnancy to e-cig aerosols, with or without nicotine, induces myogenesis with dysregulated ECM remodeling in a sex-dependent manner that could arise from e-cig–induced dysregulation of PAI-1/MMP-9 expression, which can in turn result in an increased predisposition to lung fibrosis/intestinal lung diseases in adult offspring. These findings add substantially to the emerging body of literature that concludes that e-cig vaping during pregnancy is not a safe alternative to cigarette/tobacco smoking and increases the propensity for later-life lung complications.
Supplementary Material
Footnotes
Supported by the U.S. National Institutes of Health grants 1R01HL135613 (I.R.) and HL127237, Tobacco-related Disease Research Program grants T29IR0737 and 27IP-0050 (V.K.R.), and NIH P30 grant ES000260 (J.T.Z).
Author Contributions: Q.W., J.T.Z., and I.R. conceived and designed the experiments. J.L.B., J.R.R., and J.T.Z. performed all timed pregnancies and electronic-cigarette exposures. Q.W., I.K.S., J.L.B., J.R.R., J.H.L., T.-D.C., Y.W., and J.L. conducted the pulmonary experiments. Q.W., I.K.S., and Y.W. analyzed the data. Q.W., V.K.R., J.T.Z., and I.R. wrote and revised/edited the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2020-0036OC on August 27, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.McCubbin A, Fallin-Bennett A, Barnett J, Ashford K. Perceptions and use of electronic cigarettes in pregnancy. Health Educ Res. 2017;32:22–32. doi: 10.1093/her/cyw059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15:158–166. doi: 10.1093/ntr/nts103. [DOI] [PubMed] [Google Scholar]
- 3.Grana RA, Popova L, Ling PM. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Intern Med. 2014;174:812–813. doi: 10.1001/jamainternmed.2014.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res. 2013;15:267–270. doi: 10.1093/ntr/ntr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King BA, Alam S, Promoff G, Arrazola R, Dube SR. Awareness and ever-use of electronic cigarettes among U.S. adults, 2010–2011. Nicotine Tob Res. 2013;15:1623–1627. doi: 10.1093/ntr/ntt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tegin G, Mekala HM, Sarai SK, Lippmann S. E-cigarette toxicity? South Med J. 2018;111:35–38. doi: 10.14423/SMJ.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 7.Chun LF, Moazed F, Calfee CS, Matthay MA, Gotts JE. Pulmonary toxicity of e-cigarettes. Am J Physiol Lung Cell Mol Physiol. 2017;313:L193–L206. doi: 10.1152/ajplung.00071.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith KW, Carter JW, Okimoto R, Christine C, Stenlund K, Swanson J. In vivo mouse embryo growth with exposure to e-cig vapor. FASEB. 2019;33:lb143. [Google Scholar]
- 9.O’Reilly JM, Aboaziza E, Moore J, Johnson A, Chantler PD, Engler-Chiurazzi E, et al. Memory and learning in offspring exposed to maternal vaping. FASEB J. 2019;33:737.737–737.737. [Google Scholar]
- 10.Rehan VK, Liu J, Naeem E, Tian J, Sakurai R, Kwong K, et al. Perinatal nicotine exposure induces asthma in second generation offspring. BMC Med. 2012;10:129. doi: 10.1186/1741-7015-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10:e0116732. doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott A, Lugg ST, Aldridge K, Lewis KE, Bowden A, Mahida RY, et al. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax. 2018;73:1161–1169. doi: 10.1136/thoraxjnl-2018-211663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauterstein DE, Tijerina PB, Corbett K, Akgol Oksuz B, Shen SS, Gordon T, et al. Frontal cortex transcriptome analysis of mice exposed to electronic cigarettes during early life stages. Int J Environ Res Public Health. 2016;13:417. doi: 10.3390/ijerph13040417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zelikoff JT, Parmalee NL, Corbett K, Gordon T, Klein CB, Aschner M. Microglia activation and gene expression alteration of neurotrophins in the hippocampus following early-life exposure to e-cigarette aerosols in a murine model. Toxicol Sci. 2018;162:276–286. doi: 10.1093/toxsci/kfx257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, Khan NA, Muthumalage T, Lawyer GR, McDonough SR, Chuang T-D, et al. Dysregulated repair and inflammatory responses by e-cigarette-derived inhaled nicotine and humectant propylene glycol in a sex-dependent manner in mouse lung. FASEB Bioadv. 2019;1:609–623. doi: 10.1096/fba.2019-00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Browder T, Folkman J, Pirie-Shepherd S. The hemostatic system as a regulator of angiogenesis. J Biol Chem. 2000;275:1521–1524. doi: 10.1074/jbc.275.3.1521. [DOI] [PubMed] [Google Scholar]
- 17.Flevaris P, Vaughan D. The role of plasminogen activator inhibitor type-1 in fibrosis. Semin Thromb Hemost. 2017;43:169–177. doi: 10.1055/s-0036-1586228. [DOI] [PubMed] [Google Scholar]
- 18.Waschki B, Watz H, Holz O, Magnussen H, Olejnicka B, Welte T, et al. Plasminogen activator inhibitor-1 is elevated in patients with COPD independent of metabolic and cardiovascular function. Int J Chron Obstruct Pulmon Dis. 2017;12:981–987. doi: 10.2147/COPD.S128689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q, Sundar IK, Li D, Lucas JH, Muthumalage T, McDonough SR, et al. E-cigarette-induced pulmonary inflammation and dysregulated repair are mediated by nAChR α7 receptor: role of nAChR α7 in SARS-CoV-2 COVID-19 ACE2 receptor regulation. Respir Res. 2020;21:154. doi: 10.1186/s12931-020-01396-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Church JS, Chace-Donahue F, Blum JL, Ratner JR, Zelikoff JT, Schwartzer JJ. Neuroinflammatory and behavioral outcomes measured in adult offspring of mice exposed prenatally to e-cigarette aerosols. Environ Health Perspect. 2020;128:47006. doi: 10.1289/EHP6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundar IK, Rashid K, Gerloff J, Rangel-Moreno J, Li D, Rahman I. Genetic ablation of histone deacetylase 2 leads to lung cellular senescence and lymphoid follicle formation in COPD/emphysema. FASEB J. 2018;32:4955–4971. doi: 10.1096/fj.201701518R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zakarya R, Adcock I, Oliver BG. Epigenetic impacts of maternal tobacco and e-vapour exposure on the offspring lung. Clin Epigenetics. 2019;11:32. doi: 10.1186/s13148-019-0631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H, Li G, Chan YL, Chapman DG, Sukjamnong S, Nguyen T, et al. Maternal e-cigarette exposure in mice alters DNA methylation and lung cytokine expression in offspring. Am J Respir Cell Mol Biol. 2018;58:366–377. doi: 10.1165/rcmb.2017-0206RC. [DOI] [PubMed] [Google Scholar]
- 24.Sakurai R, Liu J, Gong M, Bo J, Rehan VK. Perinatal nicotine exposure induces myogenic differentiation, but not epithelial-mesenchymal transition in rat offspring lung. Pediatr Pulmonol. 2016;51:1142–1150. doi: 10.1002/ppul.23458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen T, Li GE, Chen H, Cranfield CG, McGrath KC, Gorrie CA. Maternal e-cigarette exposure results in cognitive and epigenetic alterations in offspring in a mouse model. Chem Res Toxicol. 2018;31:601–611. doi: 10.1021/acs.chemrestox.8b00084. [DOI] [PubMed] [Google Scholar]
- 26.Noël A, Hansen S, Zaman A, Perveen Z, Pinkston R, Hossain E, et al. In utero exposures to electronic-cigarette aerosols impair the Wnt signaling during mouse lung development. Am J Physiol Lung Cell Mol Physiol. 2020;318:L705–L722. doi: 10.1152/ajplung.00408.2019. [DOI] [PubMed] [Google Scholar]
- 27.El Agha E, Moiseenko A, Kheirollahi V, De Langhe S, Crnkovic S, Kwapiszewska G, et al. Two-way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis Cell Stem Cell 201720261–273, e3.[Published erratum appears in Cell Stem Cell 20:571.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beauval N, Verrièle M, Garat A, Fronval I, Dusautoir R, Anthérieu S, et al. Influence of puffing conditions on the carbonyl composition of e-cigarette aerosols. Int J Hyg Environ Health. 2019;222:136–146. doi: 10.1016/j.ijheh.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Sakurai R, Liu J, Wang Y, Torday JS, Rehan VK. Prevention of perinatal nicotine-induced bone marrow mesenchymal stem cell myofibroblast differentiation by augmenting the lipofibroblast phenotype. Clin Sci (Lond) 2018;132:2357–2368. doi: 10.1042/CS20180749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krebs M, Sakurai R, Torday JS, Rehan VK. Evidence for in vivo nicotine-induced alveolar interstitial fibroblast-to-myofibroblast transdifferentiation. Exp Lung Res. 2010;36:390–398. doi: 10.3109/01902141003714023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marudamuthu AS, Shetty SK, Bhandary YP, Karandashova S, Thompson M, Sathish V, et al. Plasminogen activator inhibitor-1 suppresses profibrotic responses in fibroblasts from fibrotic lungs. J Biol Chem. 2015;290:9428–9441. doi: 10.1074/jbc.M114.601815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shioya S, Masuda T, Senoo T, Horimasu Y, Miyamoto S, Nakashima T, et al. Plasminogen activator inhibitor-1 serves an important role in radiation-induced pulmonary fibrosis. Exp Ther Med. 2018;16:3070–3076. doi: 10.3892/etm.2018.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omori K, Hattori N, Senoo T, Takayama Y, Masuda T, Nakashima T, et al. Inhibition of plasminogen activator inhibitor-1 attenuates transforming growth factor-β-dependent epithelial mesenchymal transition and differentiation of fibroblasts to myofibroblasts. PLoS One. 2016;11:e0148969. doi: 10.1371/journal.pone.0148969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang C, Liu G, Luckhardt T, Antony V, Zhou Y, Carter AB, et al. Serpine 1 induces alveolar type II cell senescence through activating p53-p21-Rb pathway in fibrotic lung disease. Aging Cell. 2017;16:1114–1124. doi: 10.1111/acel.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh CK, Ariue B, Alban RF, Shaw B, Cho SH. PAI-1 promotes extracellular matrix deposition in the airways of a murine asthma model. Biochem Biophys Res Commun. 2002;294:1155–1160. doi: 10.1016/S0006-291X(02)00577-6. [DOI] [PubMed] [Google Scholar]
- 36.Rerolle JP, Hertig A, Nguyen G, Sraer JD, Rondeau EP. Plasminogen activator inhibitor type 1 is a potential target in renal fibrogenesis. Kidney Int. 2000;58:1841–1850. doi: 10.1111/j.1523-1755.2000.00355.x. [DOI] [PubMed] [Google Scholar]
- 37.Bergheim I, Guo L, Davis MA, Duveau I, Arteel GE. Critical role of plasminogen activator inhibitor-1 in cholestatic liver injury and fibrosis. J Pharmacol Exp Ther. 2006;316:592–600. doi: 10.1124/jpet.105.095042. [DOI] [PubMed] [Google Scholar]
- 38.Simone TM, Higgins SP, Higgins CE, Lennartz MR, Higgins PJ. Chemical antagonists of plasminogen activator inhibitor-1: mechanisms of action and therapeutic potential in vascular disease. J Mol Genet Med. 2014;8:125. doi: 10.4172/1747-0862.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kato H, Duarte S, Liu D, Busuttil RW, Coito AJ. Matrix metalloproteinase-2 (MMP-2) gene deletion enhances MMP-9 activity, impairs PARP-1 degradation, and exacerbates hepatic ischemia and reperfusion injury in mice. PLoS One. 2015;10:e0137642. doi: 10.1371/journal.pone.0137642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMillan SJ, Kearley J, Campbell JD, Zhu XW, Larbi KY, Shipley JM, et al. Matrix metalloproteinase-9 deficiency results in enhanced allergen-induced airway inflammation. J Immunol. 2004;172:2586–2594. doi: 10.4049/jimmunol.172.4.2586. [DOI] [PubMed] [Google Scholar]
- 41.Zidovetzki R, Chen P, Fisher M, Hofman FM, Faraci FM. Nicotine increases plasminogen activator inhibitor-1 production by human brain endothelial cells via protein kinase C-associated pathway. Stroke. 1999;30:651–655. doi: 10.1161/01.str.30.3.651. [DOI] [PubMed] [Google Scholar]
- 42.Huang LT, Chou HC, Lin CM, Yeh TF, Chen CM. Maternal nicotine exposure exacerbates neonatal hyperoxia-induced lung fibrosis in rats. Neonatology. 2014;106:94–101. doi: 10.1159/000362153. [DOI] [PubMed] [Google Scholar]
- 43.Jin H, Lin J, Fu L, Mei YF, Peng G, Tan X, et al. Physiological testosterone stimulates tissue plasminogen activator and tissue factor pathway inhibitor and inhibits plasminogen activator inhibitor type 1 release in endothelial cells. Biochem Cell Biol. 2007;85:246–251. doi: 10.1139/O07-011. [DOI] [PubMed] [Google Scholar]
- 44.Gopal S, Garibaldi S, Goglia L, Polak K, Palla G, Spina S, et al. Estrogen regulates endothelial migration via plasminogen activator inhibitor (PAI-1) Mol Hum Reprod. 2012;18:410–416. doi: 10.1093/molehr/gas011. [DOI] [PubMed] [Google Scholar]
- 45.De Pergola G, De Mitrio V, Perricci A, Cignarelli M, Garruti G, Lomuscio S, et al. Influence of free testosterone on antigen levels of plasminogen activator inhibitor-1 in premenopausal women with central obesity. Metabolism. 1992;41:131–134. doi: 10.1016/0026-0495(92)90139-2. [DOI] [PubMed] [Google Scholar]
- 46.van Wersch JW, Ubachs JM, van den Ende A, van Enk A. The effect of two regimens of hormone replacement therapy on the haemostatic profile in postmenopausal women. Eur J Clin Chem Clin Biochem. 1994;32:449–453. doi: 10.1515/cclm.1994.32.6.449. [DOI] [PubMed] [Google Scholar]
- 47.Sarver DC, Kharaz YA, Sugg KB, Gumucio JP, Comerford E, Mendias CL. Sex differences in tendon structure and function. J Orthop Res. 2017;35:2117–2126. doi: 10.1002/jor.23516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park HJ, Choi JM. Sex-specific regulation of immune responses by PPARs. Exp Mol Med. 2017;49:e364. doi: 10.1038/emm.2017.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu B, Xu G, Rong S, Santillan DA, Santillan MK, Snetselaar LG, et al. National estimates of e-cigarette use among pregnant and nonpregnant women of reproductive age in the United States, 2014–2017. JAMA Pediatr. 2019;173:600–602. doi: 10.1001/jamapediatrics.2019.0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


