A patient presents to the Emergency Department (ED) with chest pain and is diagnosed with an acute myocardial infarction (AMI). In accordance with treatment protocols and core measures, aspirin is administered, which prevents up to one death for every 40 patients treated.1 If the patient survives the initial presentation, they have a one-year mortality between 5% and 10%. As a result, they are continued on aspirin and closely followed by outpatient specialists to ensure progress and compliance.1
If a patient presents to the ED with an opioid overdose and survives, their one-year mortality is also 5%.2 Similar to aspirin for AMI, medications for opioid use disorder (MOUD), including methadone and buprenorphine, prevent one death for every 40 patients treated, reducing annual mortality from 5% to 2%.2,3 However, while nearly all AMI patients receive aspirin and follow up, in this edition of JAMANetworkOpen Kilaru et al. found that few patients have any prescriptions or treatment encounters for Opioid Use Disorder (OUD) in the 90 days following an ED visit for an opioid overdose.4
Kilaru et al. included patients who presented to an ED from 2011 to 2016 with an index non-fatal opioid overdose, a cohort drawn from a commercially insured database of 15 million participants. The outcome was receipt of treatment for OUD in the 3 months following the index ED visit for overdose, defined as any single occurrence of a prescription for buprenorphine or at least one outpatient encounter for OUD.4 They found that overall, only 17% of patients had any claims for treatment at 3 months. After excluding patients who had received treatment services prior to the index overdose, the proportion of patients who received any OUD treatment 90 days post-overdose dropped to 11%. Females, patients who self-identified as black or Hispanic, and patients who overdosed on prescription opioids rather than heroin were less likely to receive OUD treatment. Overdose patterns differed by region, and though overdoses increased steadily for the 6 years included, the proportion receiving MOUD did not.4
The Kilaru et al. study was limited to patients that maintained continuous insurance enrollment, likely creating a bias towards a population with greater resources; the data also did not include patients on methadone. Despite these limitations, this study is consistent with a growing body of literature demonstrating a low prevalence of treatment for patients with OUD. Mojtabai et al. found that 75% of addiction treatment facilities did not offer buprenorphine as of 2016.5 Wakeman et al. found only 12% of patients received buprenorphine within 90 days of an index inpatient or outpatient visits for OUD. Those patients maintained on buprenorphine for 6 months or longer had annual an overdose rate of 1.1% compared to 3.6% for patients not on MOUD.6 Larochelle et al. examined treatment after non-fatal opioid overdoses in Massachusetts from 2012–2014 and found that, at 12 months, 11% received methadone and 17% received buprenorphine.3 Receipt of methadone and buprenorphine were again associated with a decreased risk of opioid-related death (AHR, 0.41 [CI, 0.24 to 0.70] and AHR, 0.62 [CI, 0.41 to 0.92], respectively) and a decreased risk of all-cause mortality.3
If the mortality for patients after an overdose is similar to that of patients after an AMI and evidence-based treatments are effective in reducing future death, why do rates of treatment for OUD remain low? What are the barriers that contribute to the poor implementation of MOUD? Implementation science frameworks such as the Consolidated Framework for Implementation Research (CFIR), provide insight into the barriers to implementation of evidence-based practices such as MOUD.7 The CFIR organizes barriers and facilitators into 5 domains: individual characteristics, intervention characteristics, inner setting, outer setting, and implementation process. Targeting efforts on these barriers and facilitators may improve the likelihood of implementation success.
For both clinicians and patients, many individual-level barriers to MOUD implementation exist, including lack of knowledge, beliefs about MOUD, and self-efficacy. Unlike aspirin for AMI, which has been in clinical guidelines for decades, clinicians may lack knowledge about the effectiveness of MOUD, how to screen patients, and how to initiate treatment. Additionally, stigma regarding OUD remains pervasive. Unlike AMI, addiction has often been viewed as a “choice” or moral failing rather than a disease process. In turn, although clinicians may be aware of evidence supporting MOUD, it is still falsely considered by some to be “trading one addiction for another.” This stigma undermines the consistent agreement in the medical literature that medications are effective in treating OUD. Clinicians may also lack self-efficacy – that is, although they may believe the evidence regarding MOUD, they may doubt their ability to start a patient on MOUD. Likewise, patients, may not believe in their own ability to ‘get clean.’
Other barriers – structural, logistical, and economical – also exist at the local or ‘inner setting.’ Clinicians may not have adequate resources to implement OUD treatment in their practice setting or may have concerns about the impact that prescribing MOUD may have on patient flow and volume. A lack of screening mechanisms to identify patients with OUD, a shortage of addiction specialists with whom to discuss difficult cases, and a lack of community resources to support patients with OUD may further inhibit incorporation into practice in many local settings. Although buprenorphine was developed and promoted based on the premise that barriers would be minor compared to methadone, substantial inner-setting challenges remain.
The larger context or ‘outer setting’ presents additional impediments to treatment. For example, while aspirin for AMI is reported as a core quality measure, offering treatment for patients with OUD is not measured as part of healthcare practice. Few incentives exist to encourage clinicians, clinics, or health systems to systematically provide MOUD. The burden, then, often falls to the individual clinician to consistently identify and treat OUD patients with little structural support. Additionally, regulatory barriers, including the requirement that buprenorphine prescribers undergo special training and obtain a DEA-X waiver, deter clinicians from offering or even being able to prescribe buprenorphine.
Lastly, MOUD can be a complex intervention both for clinicians to provide and for patients to receive. Clinicians must not only screen, identify, and initiate MOUD but also must either provide long-term treatment or find a means to providing long-term treatment for the patient. This is a current challenge for EDs across the country – prescribing 5 days of buprenorphine presents one set of challenges, bridging patients to long-term care poses yet a different set. While Kilaru et al. examined an insured population, many individuals with OUD may struggle with cost of treatment – as both buprenorphine and methadone may be required indefinitely for maximum effectiveness.
In categorizing the obstacles to implementation, it becomes evident that there is a complex web of clinician, patient, logistic, regulatory, and structural barriers that serve to impede the broad implementation of treatment for patients with OUD. Future research must focus on addressing these barriers to implementation, but must also address the acceptance and implementation of MOUD in a variety of practice settings – EDs, addiction treatment facilities, community clinics, and primary care offices. The low prevalence of treatment after overdose highlighted by Kilaru et al. means that – at the height of the opioid epidemic – we as a healthcare community are missing opportunities to save lives. Increasing knowledge, reducing stigma, addressing logistics, decreasing regulations, and providing incentives to treat patients with OUD must all be considered if we are to truly make an impact.
Figure.
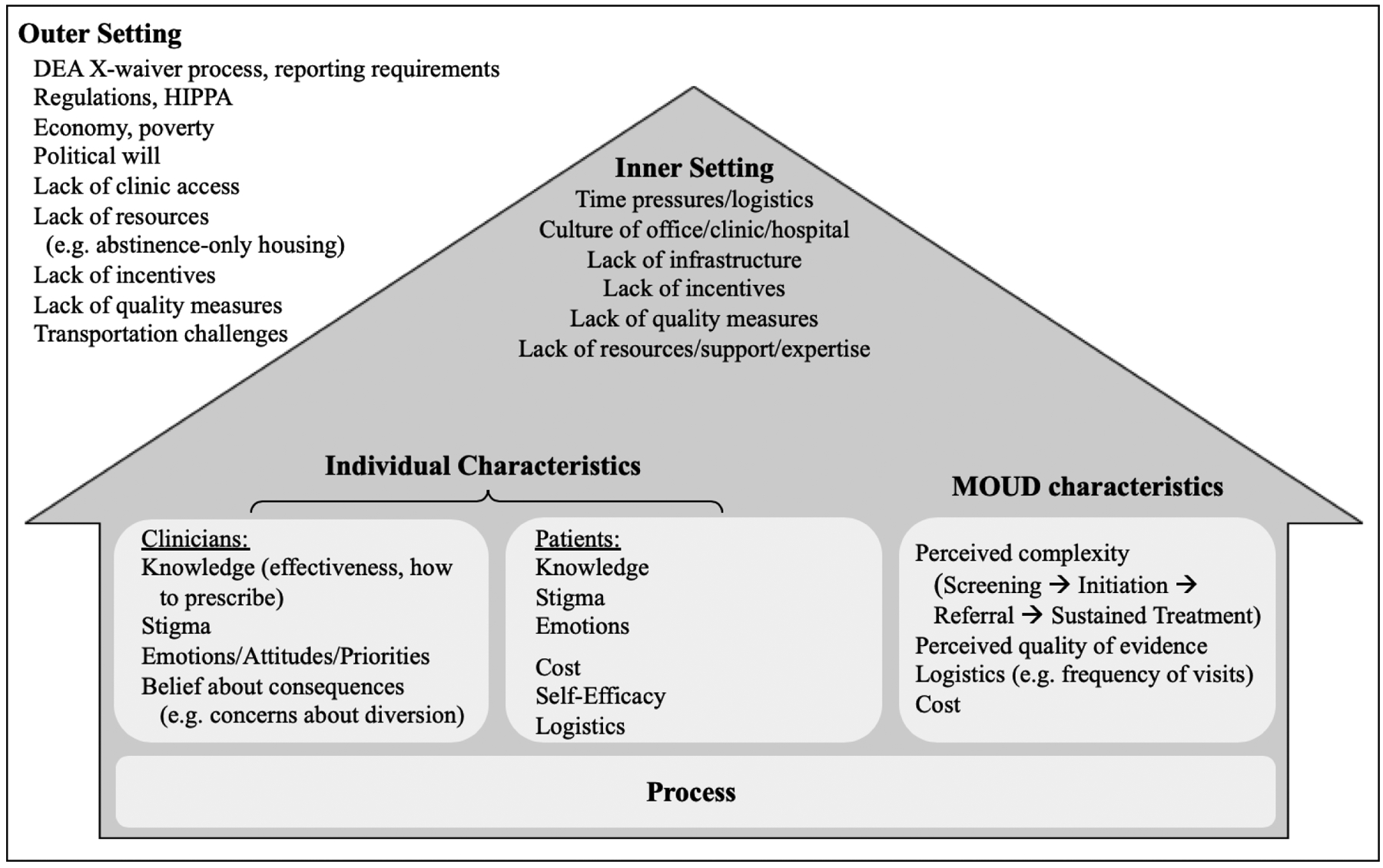
Known barriers to implementation, as mapped to CFIR domains.
Acknowledgements:
The Authors would like to acknowledge Peter Lindenauer, MD, MSc, MHM for his contributions.
Dr. Soares is supported by a K08 from NIDA
Dr. Schoenfeld is supported by a K08 from AHRQ (5K08HS025701-02)
Dr. Westafer is supported by a KL2 from the UMMS MA Consortium for Cardiopulmonary Implementation Science Scholars
Footnotes
No Conflicts of Interest.
References:
- 1.Randomized trial of intravenous streptokinase, oral aspirin, both, or neither among 17187 cases of suspected acute myocardial infarction. Lancet. 1988. August 13;2(8607):349–60. And https://www.thennt.com/nnt/aspirin-for-major-heart-attack/ (Accessed February 20, 2020) [PubMed] [Google Scholar]
- 2.Weiner SG, Baker O, Bernson D, Schuur JD. One-Year Mortality of Patients After Emergency Department Treatment for Nonfatal Opioid Overdose. Annals of Emergency Medicine. June 2019. doi: 10.1016/j.annemergmed.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larochelle MR, Bernson D, Land T, et al. Medication for Opioid Use Disorder After Nonfatal Opioid Overdose and Association With Mortality. Ann Intern Med. 2018;169(3):137–18. doi: 10.7326/M17-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kilaru AS, Xiong A, Lowenstein M, et al. Incidence of treatment for opioid use disorder following nonfatal overdose in commercially insured patients. JAMA Netw Open. 2020;3(5):e205852. doi: 10.1001/jamanetworkopen.2020.5852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mojtabai R, Mauro C, Wall MM, Barry CL, Olfson M. Medication Treatment For Opioid Use Disorders In Substance Use Treatment Facilities. Health Affairs. 2019;38(1):14–23. doi: 10.1377/hlthaff.2018.05162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wakeman SE, Larochelle MR, Ameli O, et al. Comparative Effectiveness of Different Treatment Pathways for Opioid Use Disorder. JAMA Network Open. 2020;3(2):e1920622–12. doi: 10.1001/jamanetworkopen.2019.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement Sci 2009;4(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]


