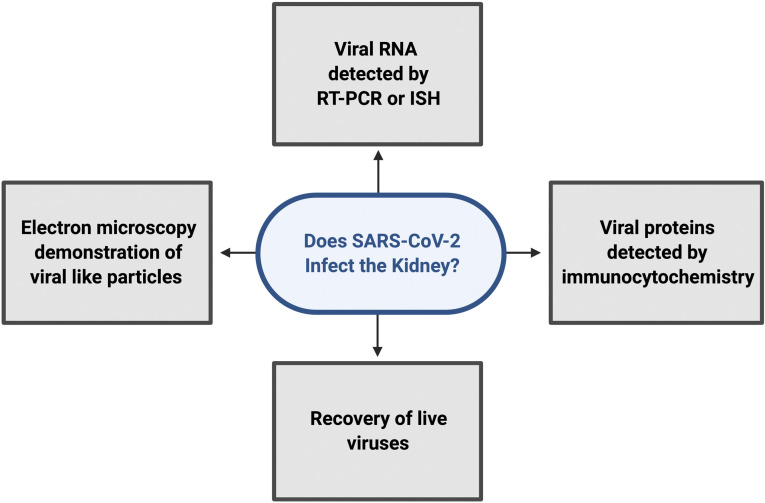
Six recent publications have provided evidence compatible with the conclusion that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can directly infect the kidney (Figure 1).1–6 The objective of this short treatise is to evaluate this evidence and the validity of the conclusion. This matters because as discussed below, infection of renal epithelial cells is likely to be associated with the presence of SARS-CoV-2 virions in the urine, affecting strategies for preventing transmission in the community and in the hospital environment. Also, AKI has been found to be a frequent concomitant of coronavirus disease 2019 (COVID-19),7 and direct infection of the kidney could be a contributing factor.
Figure 1.
Types of evidence supporting claims that SARS-CoV-2 can infect the kidney. ISH, in situ hybridization.
SARS-CoV-2 enters cells by binding to an endogenous viral receptor, namely angiotensin-converting enzyme 2 (ACE2).8 Thus, we would expect that vulnerable cells in the kidney would be those that express ACE2. The COVID-19 pandemic has come at a time when researchers have begun to use next generation sequencing techniques, namely RNA-Seq, to profile gene expression in many, if not most, cell types of the body. Such data provide an unbiased data source that can tell us what cells express ACE2. Several kidney-specific RNA-Seq datasets provide detailed information about ACE2 gene expression in kidney cell types. The data indicate that ACE2 mRNA is strongly expressed in proximal tubule epithelial cells9–11 but not in more distal renal tubule cells,10,11 podocytes,9,12 mesangial cells,12 or glomerular endothelial cells.9,12 Of course, absence of an mRNA signal does not necessarily imply absence of the corresponding protein. In this regard, immunohistochemistry shows strong ACE2 protein labeling in the apical plasma membrane of proximal tubule cells,13,14 with little or no labeling of the glomerulus with one antibody13 and glomerular labeling with another.14 Overall, because ACE2 is expressed chiefly in the proximal tubule in kidney, the expectation is that renal infection, if it occurs, would be largely in proximal tubule cells. There could be some skepticism about this possibility because the route that a virion must take to encounter the lumen of the proximal tubule, where ACE2 is expressed, is much more complicated than the route to the primary sites of infection in the lung or small intestine. Viremia must happen first, followed by penetration of the glomerular barrier or of the proximal tubule tight junction. It is also possible that the virus can enter cells other than proximal tubule cells via receptors other than ACE2 or via nonreceptor-mediated mechanisms, but proximal tubule cells seem to be the most likely site of infection.
When SARS-CoV-2 infects cells, it hijacks the machinery of the host cell for replication. One of the functions that is hijacked is the endosomal/lysosomal pathway, which results in accumulation of SARS-CoV-2 virions in late endosomes (multivesicular bodies) and lysosomes (Ghosh S, Dellibovi-Ragheb TA, Pak E, Qiu Q, Fisher M, Takvorian P, et al.: β-Coronaviruses use lysosomal organelles for cellular egress. bioRxiv 2020.07.25.192310, 2020), much in the same way as exosomes.15 The viral particles are secreted from the cell by fusion of the outer membrane of multivesicular bodies or lysosomes with the plasma membrane, following the same pathway as urinary exosomes.15 Like exosomes, secretion would be expected to be on the apical side of cells into the urinary space. Thus, infection of proximal tubules with SARS-CoV-2 would likely be accompanied by the presence of virions in urine.
Recent brief publications in New England Journal of Medicine,1 Kidney International,2,3 JASN,4 The Lancet Microbe,5 and The Lancet6 argue for direct infection of kidney epithelial cells and suggest direct viral tissue damage. They marshal four types of evidence: (1) presence of viral RNA in kidneys of patients with COVID-19 detected by RT-PCR and/or in situ hybridization,1,5,6 (2) presence of viral protein in kidneys of patients with COVID-19 detected by immunocytochemistry,1,2 (3) recovery of live virus from kidney tissue of affected patients,6 and (4) identification of coronavirus-like structures in kidney tissue of patients with COVID-19 by electron microscopy.2–4 The last type of evidence, the electron microscopy demonstration of viral particles in renal tissue, has been called into question because normally occurring structures (e.g., clathrin-coated vesicles or the internal vesicles of multivesicular bodies) can be mistaken for virions.16 The other three types of data, nevertheless, provide more direct evidence for the presence of SARS-CoV-2 in kidney tissue in some patients with COVID-19. The evidence presented by Puelles et al.1 focused mainly on the presence of SARS-CoV-2 in the glomerulus, where immunocytochemistry, in situ hybridization, and RT-PCR observations were made. Limited immunocytochemical evidence using an anti-spike protein antibody was also consistent with tubular infection, although the tubule epithelial cell type was not identified. If ACE2 is not expressed in any glomerular cell as discussed above, what could be the origin of the glomerular labeling? The kidney receives about 20% of the cardiac output, and with viremia, it would not be surprising to have some nonspecific accumulation of virions, potentially associated with non–ACE2-dependent uptake into cells (e.g., via the clathrin- and caveolae-independent endocytic pathway).17 The paper by Su et al.2 also described immunocytochemical evidence for infection of tubular cells using an antibody to the SARS-CoV-2 nucleocapsid protein, although the affected tubule segment was not investigated. Hanley et al.5 showed detection of subgenomic viral RNA transcripts in postmortem samples from patients with COVID-19, which is evidence for active replication of SARS-CoV-2 in kidney tissue. Braun et al.6 isolated SARS-CoV-2 from an autopsied kidney and were able to infect nonhuman primate kidney tubular epithelial cells with patient-derived SARS-CoV-2, providing perhaps the strongest evidence for kidney infection. Of the six papers, five analyzed necropsy kidneys representing patients with severe cases of COVID-19, so it is probable that, in cases with severe multisystemic involvement, the kidney can become infected.1,2,4–6 One of the six papers reported electron microscopy evidence in a biopsy specimen3 from a less severe case, although as mentioned above, this evidence has been challenged. Several studies found no evidence of SARS-CoV-2 in kidney biopsies from patients with COVID-19.18–20
Overall, although all of the four types of evidence have limitations and represent anecdotal observations, it seems reasonable to believe that some severely affected patients with COVID-19 can develop secondary infections in the kidney. However, the important question is, “how frequently does renal SARS-CoV-2 infection occur in the general population of hospitalized patients with COVID-19?” As noted above, infection at the main site of ACE2 expression, the proximal tubule, is likely to be associated with SARS-CoV-2 in urine. We can, therefore, look at published natural history studies to assess the frequency of viruria and hence, renal infection. In one study, among 205 patients hospitalized with the clinical diagnosis of COVID-19 in January and February 2020, none of the 72 urine specimens obtained tested positive for viral RNA by RT-PCR.21 In contrast, positive results were obtained in 72 of 104 sputum samples. Another study in 74 hospitalized patients with COVID-19 reported positive results in two of 247 urine samples (0.8%).22 These studies support the idea that renal infection in hospitalized patients with COVID-19 is not common, but the sensitivity of the method for detection of viral RNA may not be sufficient to rule out low-level viruria, especially because ribonucleases in urine may degrade viral RNA. Hence, a focused study may be in order in which urine samples are concentrated, for example, by ultracentrifugation and analyzed not only by RT-PCR but also, through detection of viral proteins (e.g., by protein mass spectrometry) and infectious particles (via culture methods) to assess the prevalence of renal infection among patients with COVID-19.
In summary, several types of evidence seem to support the idea that SARS-CoV-2 can infect the kidney, at least in severe cases that result in death of the patient. Whether patients with less severe COVID-19 also commonly experience renal infection is not yet established. Statistically based trials to identify the virus in urine samples of patients with COVID-19 are recommended as a means of addressing the frequency of kidney infection in the general population of hospitalized patients with COVID-19.
Disclosures
M. Knepper is a Deputy Editor of JASN. All remaining authors have nothing to disclose.
Funding
This work was funded by Division of Intramural Research, National Heart, Lung, and Blood Institute project ZIA-HL006253 “Urinary Exosomes and COVID-19” (to M. Knepper).
Acknowledgments
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendations. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or JASN. Responsibility for the information and views expressed herein lies entirely with the author(s).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al.: Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 383: 590–592, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al.: Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219–227, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kissling S, Rotman S, Gerber C, Halfon M, Lamoth F, Comte D, et al.: Collapsing glomerulopathy in a COVID-19 patient. Kidney Int 98: 228–231, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farkash EA, Wilson AM, Jentzen JM: Ultrastructural evidence for direct renal infection with SARS-CoV-2. J Am Soc Nephrol 31: 1683–1687, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, et al. : Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: A post-mortem study [published online ahead of print August 20, 2020] Lancet Microbe, 10.1016/s2666-5247(20)30115-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun F, Lütgehetmann M, Pfefferle S, Wong MN, Carsten A, Lindenmeyer MT, et al.: SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet 396: 597–598, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al.: Extrapulmonary manifestations of COVID-19. Nat Med 26: 1017–1032, 2020. [DOI] [PubMed] [Google Scholar]
- 8.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al.: A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579: 270–273, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart BJ, Ferdinand JR, Young MD, Mitchell TJ, Loudon KW, Riding AM, et al.: Spatiotemporal immune zonation of the human kidney. Science 365: 1461–1466, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JW, Chou CL, Knepper MA: Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Limbutara K, Chou CL, Knepper MA: Quantitative proteomics of all 14 renal tubule segments in rat. J Am Soc Nephrol 31: 1255–1266, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung JJ, Goldstein L, Chen YJ, Lee J, Webster JD, Roose-Girma M, et al.: Single-cell transcriptome profiling of the kidney glomerulus identifies key cell types and reactions to injury [published online ahead of print July 10, 2020]. J Am Soc Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Human Protein Atlas : ACE2. Available at: https://www.proteinatlas.org/ENSG00000130234-ACE2. Accessed August 25, 2020
- 14.Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D: Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: Implications for albuminuria in diabetes. J Am Soc Nephrol 17: 3067–3075, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Pisitkun T, Shen RF, Knepper MA: Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 101: 13368–13373, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller SE, Goldsmith CS: Caution in identifying coronaviruses by electron microscopy. J Am Soc Nephrol 31: 2223–2224, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Yang P, Liu K, Guo F, Zhang Y, Zhang G, et al.: SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res 18: 290–301, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu H, Larsen CP, Hernandez-Arroyo CF, Mohamed MMB, Caza T, Sharshir M, et al.: AKI and collapsing glomerulopathy associated with COVID-19 and APOL1 high-risk genotype. J Am Soc Nephrol 31: 1688–1695, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kudose S, Batal I, Santoriello D, Xu K, Barasch J, Peleg Y, et al.: Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol 31: 1959–1968, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma P, Uppal NN, Wanchoo R, Shah HH, Yang Y, Parikh R, et al.: Northwell Nephrology COVID-19 Research Consortium : COVID-19-Associated kidney injury: A case series of kidney biopsy findings. J Am Soc Nephrol 31: 1948–1958, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al.: Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 323: 1843–1844, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JM, Kim HM, Lee EJ, Jo HJ, Yoon Y, Lee NJ, et al.: Detection and isolation of SARS-CoV-2 in serum, urine, and stool specimens of COVID-19 patients from the Republic of Korea. Osong Public Health Res Perspect 11: 112–117, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]