Significance Statement
ATP depletion is central in kidney disease pathogenesis, but technology has not effectively monitored in vivo ATP dynamics. Experiments using a novel mouse strain that incorporates an ATP biosensor to visualize spatiotemporal ATP dynamics at single-cell resolution revealed the crucial difference between cells of the proximal tubules (PTs) and those of the distal tubules (DTs). Mitochondrial changes were consistent with the differing ATP dynamics in cells of the two regions, explaining the different sensitivity to ischemic reperfusion injury. A strong correlation emerged between ATP recovery of PT cells in acute phase and renal fibrosis in the chronic phase. Cold ischemia enhanced ATP recovery, providing a proof of concept for the possible protective value of renal hypothermia.
Keywords: ATP, energy dynamics, imaging, AKI, fibrosis, hypothermia
Visual Abstract
Abstract
Background
Depletion of ATP in renal tubular cells plays the central role in the pathogenesis of kidney diseases. Nevertheless, inability to visualize spatiotemporal in vivo ATP distribution and dynamics has hindered further analysis.
Methods
A novel mouse line systemically expressing an ATP biosensor (an ATP synthase subunit and two fluorophores) revealed spatiotemporal ATP dynamics at single-cell resolution during warm and cold ischemic reperfusion (IR) with two-photon microscopy. This experimental system enabled quantification of fibrosis 2 weeks after IR and assessment of the relationship between the ATP recovery in acute phase and fibrosis in chronic phase.
Results
Upon ischemia induction, the ATP levels of proximal tubule (PT) cells decreased to the nadir within a few minutes, whereas those of distal tubule (DT) cells decreased gradually up to 1 hour. Upon reperfusion, the recovery rate of ATP in PTs was slower with longer ischemia. In stark contrast, ATP in DTs was quickly rebounded irrespective of ischemia duration. Morphologic changes of mitochondria in the acute phase support the observation of different ATP dynamics in the two segments. Furthermore, slow and incomplete ATP recovery of PTs in the acute phase inversely correlated with fibrosis in the chronic phase. Ischemia under conditions of hypothermia resulted in more rapid and complete ATP recovery with less fibrosis, providing a proof of concept for use of hypothermia to protect kidney tissues.
Conclusions
Visualizing spatiotemporal ATP dynamics during IR injury revealed higher sensitivity of PT cells to ischemia compared with DT cells in terms of energy metabolism. The ATP dynamics of PTs in AKI might provide prognostic information.
AKI is a common clinical condition associated with high morbidity and mortality.1,2 Previous clinical studies demonstrate that AKI predisposes to the development and progression of CKD,3,4 and the concept of AKI to CKD transition has been established; however, the underlying mechanisms of AKI to CKD transition remain unclear.5 Recent studies indicate that the impaired renal energy metabolism and mitochondrial dysfunction are linked with AKI to CKD transition.6–10
Because of its key roles in energy metabolism, adenosine 5’ triphosphate (ATP) is often referred to as the “molecular unit of currency” of intracellular energy transfer.11 The kidneys constantly require a large amount of ATP to meet the demands for their intricate functions. Alteration of cellular ATP levels during ischemia and reperfusion was previously demonstrated utilizing luciferase assays and nuclear magnetic resonance spectroscopy of whole kidneys.12–15 Several lines of evidence utilizing dissected nephrons indicate that ATP source in the kidney might be cell-type specific.16–19 The proximal tubules (PTs), which consume ATP for reabsorption and are the vulnerable segment during ischemia,20 are considered to depend mostly on mitochondrial oxidative phosphorylation for energy supply, whereas the distal tubules (DTs) are considered to have the potential to produce ATP by glycolysis.
Although this cell-type specific ATP metabolism suggested by ex vivo analysis might be one of the reasons explaining the vulnerability of PTs to ischemic insults than DTs,21 ATP dynamics in each nephron segment during kidney injuries have been unknown because of lack of technology to visualize in vivo spatiotemporal ATP dynamics at a single-cell level noninvasively. The conventional ATP quantification method utilizing luciferase assay does not allow us to monitor the spatiotemporal ATP dynamics in the tissues. Mass imaging technique provides us spatial distribution of ATP in the kidney sections but not ATP dynamics during injury in native microenvironment, especially in the point of oxygen and substrate supply. To visualize ATP dynamics in vivo, we generated a novel mouse line, which expresses the Förster resonance energy transfer (FRET)–based ATP biosensor22 in all tissues.23
Here, we demonstrate, for the first time, in vivo spatiotemporal ATP dynamics in the kidney at a single-cell resolution in pathophysiologic conditions utilizing the novel mouse strain and in vivo imaging technique with a two-photon microscope.24,25
Methods
Animals
GO-ATeam2 mice were generated in our laboratory, and they are described in a separate paper23,26 (M. Yamamoto, manuscript in preparation). They were housed in a specific pathogen-free facility and received a routine diet. Mice used in vivo imaging were 10–14 weeks old. All animal experiments were approved by the Animal Research Committee, Graduate School of Kyoto University and performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health).
Mice Treatment
Mice were anesthetized with 2% isoflurane inhalation. The left kidney was exteriorized through a small incision. Ischemic reperfusion (IR) injury was induced by clamping the unilateral renal pedicle for each length of time.27 We analyzed four mice in each group, as previously described in kidney intravital imaging reports.28,29 We kept both the core body temperature and the kidney surface temperature at 36°C during warm IR by using two heater systems as described below in In Vivo Two-Photon Imaging Settings. During cold IR experiment, the temperatures of core body and the kidney surface were kept at 33°C and 24°C, respectively. The heart rate was kept at >450 bpm and breathing rate was kept at >100/min by adjusting the dose of anesthesia continuously during imaging studies.
Cell Culture and Treatment
Primary tubular cells were isolated and cultured from 1- to 2-week-old GO-ATeam2 mice. The kidneys were minced and digested in 1.25 mg/ml dispase for 30 minutes at 37°C. After removal of undigested fragments, the digested tissues were washed with PBS and were suspended in DMEM supplemented with 10% bovine calf serum, 50 U/ml penicillin, and 50 μg/ml streptomycin. Cells were grown in cell culture dishes for 4 days.
Microscopy and Image Processing
For two-photon excitation microscopy, we used an FV1200MPE-BX61W1 upright microscope equipped with a 25×/1.05-NA water-immersion objective lens (XLPLN25XW-MP; Olympus, Tokyo, Japan) and an Insight DeepSee Ultrafast Laser (Spectra Physics, Mountain View, CA). The excitation wavelength for green fluorescent protein (GFP) was 930 nm. We used an IR-cut filter (BA750RXD), two dichronic mirrors (DM505 and DM690), and two emission filters (BA495–540 [Olympus] for GFP and BA562–596 [Olympus] for Kusabira orange fluorescent protein [OFP], respectively). Images were analyzed with MetaMorph software (Universal Imaging, West Chester, PA). Our microscopy allows us to detect tubules within 40 μm from the surface of the kidney in high-enough resolution to differentiate between PTs and DTs. We took images at 20 μm from the surface of the kidney to analyze images without the effect of light scattering and absorption.
In Vivo Two-Photon Imaging Settings
Anesthetized mice were placed on an electric adjustable heater pad. The kidney was attached to the vacuum-stabilized cup with imaging window,30 which was also equipped with heater function. Using two heater systems, we controlled both the core body and the kidney temperature during all experimental procedures. The bowl of the imaging window is designed to be filled with water for the water immersion. Detailed schemes of the vacuum-stabilized imaging window were described in the previous study.30
Analysis of Ratio Changes in Tubules
Quantification of ratio was performed using MetaMorph software. The ratio in each tubular section was calculated in the area described in Supplemental Figure 1A. Averages of ratios in five PT sections were utilized as the mean ratios of PTs in the mouse, and the averages from three to four mice are shown in the figures (n=4 in Figures 1E, 3B, and 6B; n=3 in Figure 2C and Supplemental Figure 2C). As for DTs, because the numbers of DT sections in one view are limited, averages of around three DT sections were utilized as the mean ratios of DTs in the mouse, and the averages from four mice are shown in the figures (n=4 in Figures 1E, 2D, and 3C). The ATP recovery slopes in S1 PTs were calculated as the slope between 0 and 2 minutes after the induction of reperfusion. The ATP percentage recoveries were calculated in comparison between the ratios before ischemia and the ratios 60 minutes after the induction of reperfusion.
Figure 1.
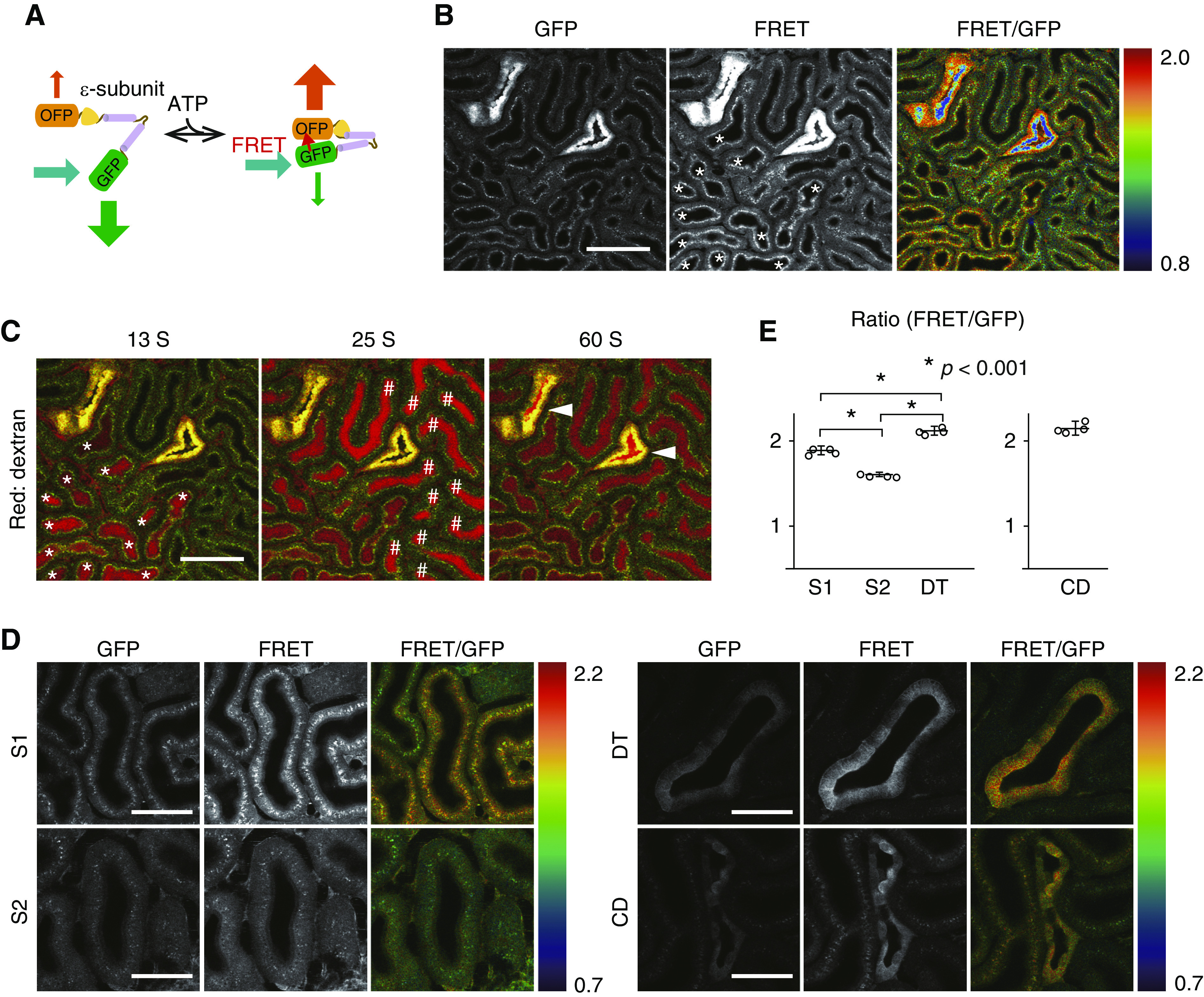
Visualization of ATP levels in the kidney with GO-ATeam2 mice revealed ATP distribution. (A) Schematic drawing of an FRET-based fluorescent ATP probe, termed GO-Ateam. GFP and OFP sandwich the ε-subunit of B. subtilis FoF1-ATP synthase. (B) Images of GFP and FRET signal and the emission ratio image of FRET to GFP emission. Warm colors indicate high FRET ratios (high ATP levels), and cool colors indicate low FRET ratios (low ATP levels). (C) Identification of S1 (*) and S2 (#) PTs and DTs. In order to identify the segment of these tubules, we administered 3-kD Texas red dextran to visualize tubular flow. According to the sequences of the flow, PTs with higher apical signals (*) were considered as S1 segments, PTs with lower apical signals were considered as S2 segments (#), and the tubules with high-intensity signals were DTs (arrowheads). Note that the ATP levels are not correlated with the signal intensity but with the ratio of FRET to GFP emission. (D) Visualization of ATP levels in each segment. Although the ATP levels in PTs were homogeneous, the ATP levels of DTs and CDs were heterogeneous. Scale bars: 100 μm in B and C; 50 μm in D. (E) Ratios in PT S1, PT S2, DTs, and principal cell in CDs (n=4 mice per group). We presented graphs of CDs separately from those of PTs and DTs because the ratios in PTs and DTs are the average in each segment and the ratio of CDs is the average in principal cells. Statistical significance among S1 PTs, S2 PTs, and DTs was assessed by one-way ANOVA with Bonferroni post hoc tests for comparisons. *P<0.001.
Figure 3.
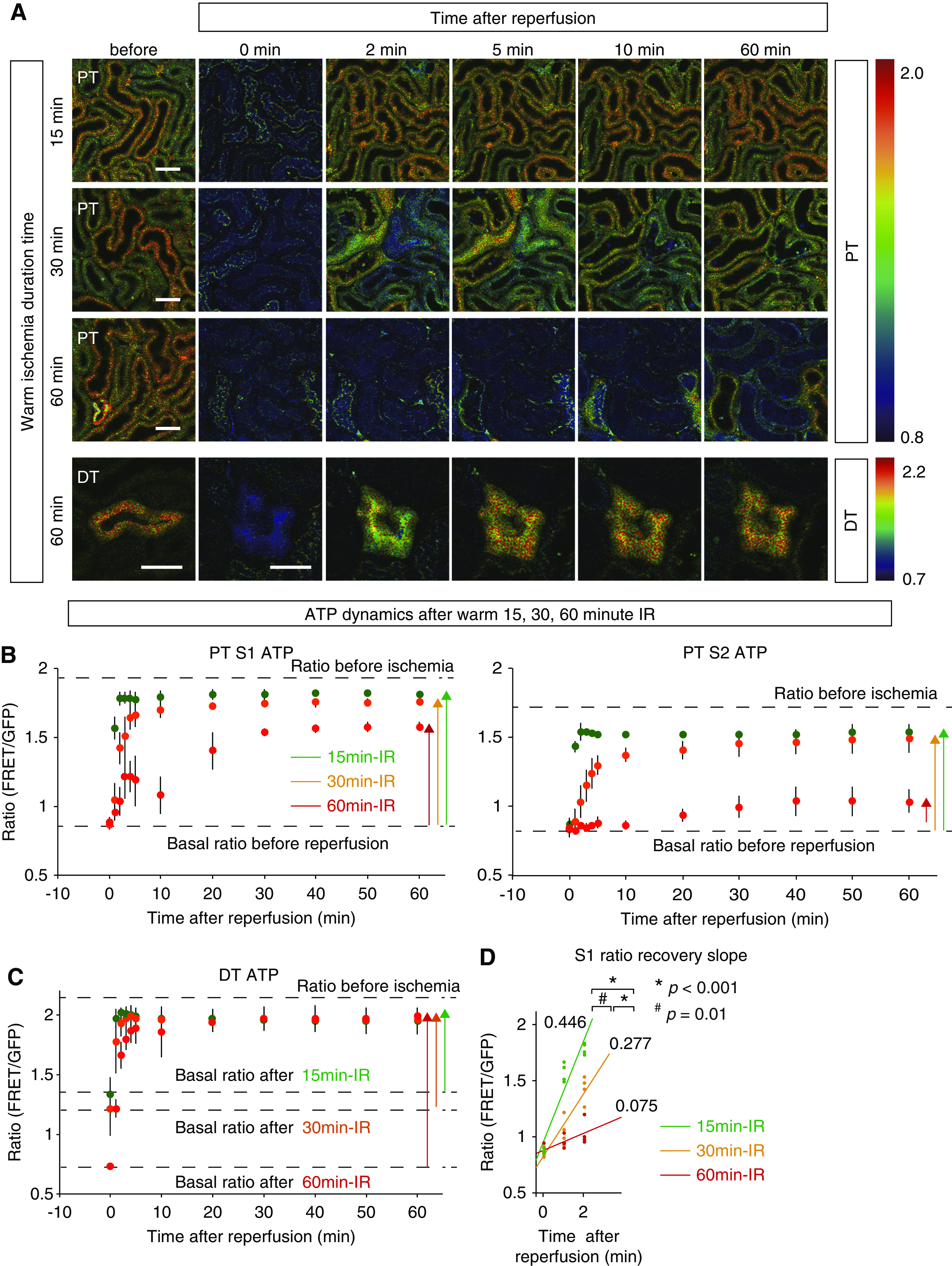
Although ATP recovery in PTs after reperfusion varied depending on the length of ischemia, ATP recovery in DTs was quickly restored irrespective of ischemia duration. (A) Ratio images of PTs during reperfusion after warm 15-, 30-, and 60-minute ischemia and those of DTs after warm 60-minute ischemia. Scale bars: 50 μm. (B) Ratio graphs of S1 and S2 PTs during reperfusion after 15-, 30-, and 60-minute ischemia (green, orange, and red, respectively; n=4 mice per group). The longer ischemic time resulted in slower and poorer recovery of ATP levels in PTs. (C) Ratio graphs of DTs during reperfusion after 15-, 30-, and 60-minute ischemia (green, orange, and red, respectively; n=4 mice per group). Note that the basal ratios after ischemia varied depending on ischemia time as shown in Figure 2D. Compared with those of PTs, the ratios in DTs recovered more quickly and almost completely even after 60-minute IR. (D) The graph of S1 ratio recovery slopes after warm 15-, 30-, and 60-minute IR. The longer ischemic times resulted in slower recovery slopes. Multiple group comparisons were performed using analysis of covariance. Warm 15-minute versus warm 60-minute, P<0.001; warm 15-minute versus warm 30-minute, P=0.01; warm 30-minute versus warm 60-minute, P<0.001.
Figure 6.
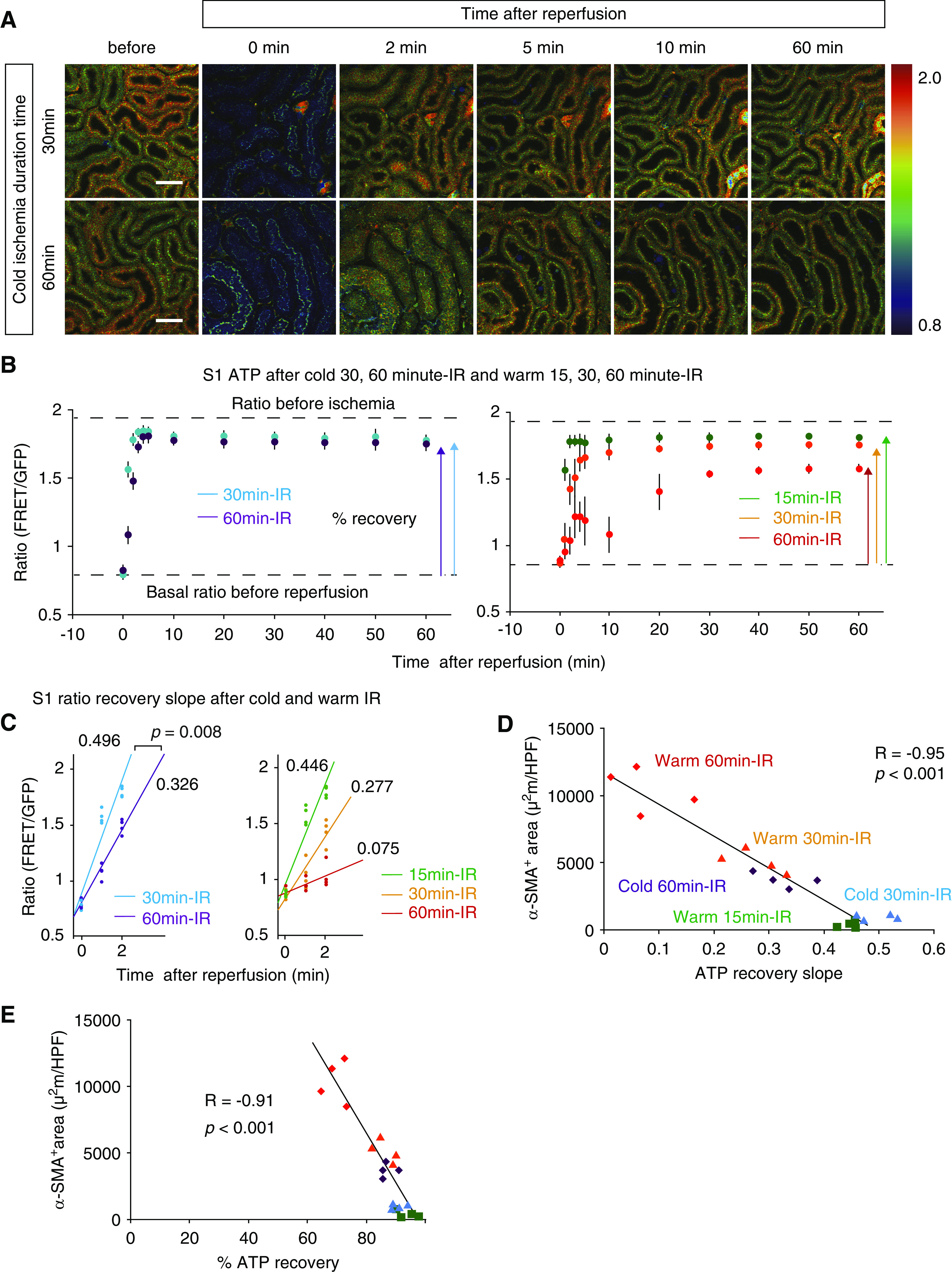
Renal hypothermia improved the ATP recoveries in acute phase and the fibrosis in chronic phase after IR injury. (A) Ratio images of PTs during cold 30- and 60-minute IR. Scale bars: 50 μm. (B) Ratio graphs of PTs S1 during cold 30- and 60-minute IR (blue and purple, respectively; n=4 per group). Cold ischemia improved the ATP recovery dramatically. Ratio graphs during warm IR (Figure 3B) are presented again for comparison. (C) The graph of ratio recovery slopes in cold 30- and 60-minute IR. Two group comparisons were performed using analysis of covariance. P=0.008. The graph of ratio recovery slopes in warm IR (Figure 3D) is presented again for comparison. (D and E) Graphs demonstrated that the fibrosis area was inversely correlated well with the ratio recovery slope and percentage ratio recovery in acute phase. The correlation was determined by Pearson correlation analysis. Graphs demonstrated that the fibrosis area was inversely correlated well with the (D) ratio recovery slopes and (E) percentage ratio recovery in acute phase. The data of warm IR in (D) are the same with those shown in Figure 5C, but they were analyzed again with the data of cold IR to show that the correlation was well maintained even when kidney samples treated with warm and cold ischemia were evaluated together. The correlation was determined by Pearson correlation analysis.
Figure 2.
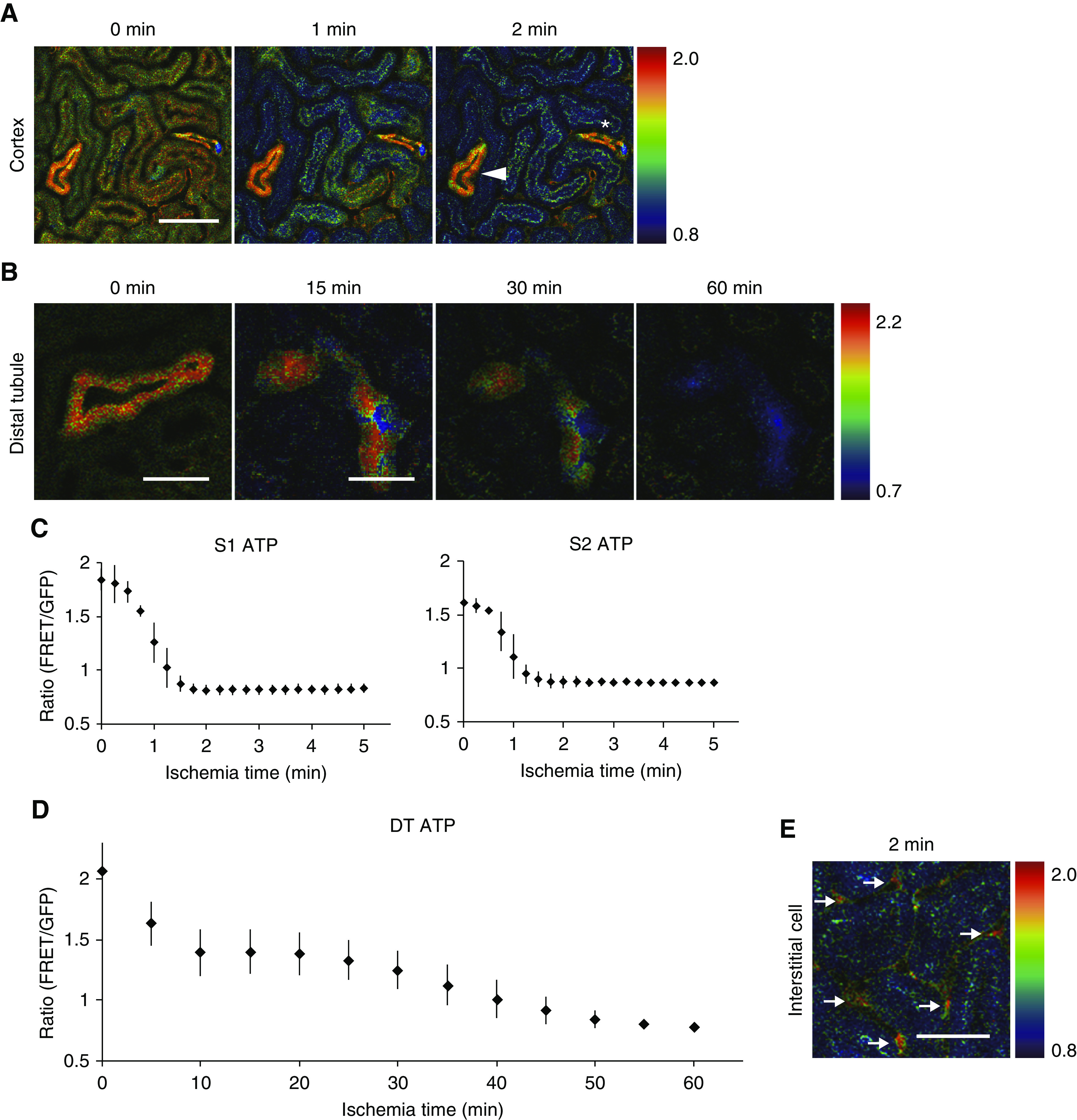
Rapid ATP reduction in PTs and slow ATP reduction in DTs after ischemia. (A) Ratio images of PTs, DT, and CDs during ischemia. Although the ATP levels of PTs decreased very rapidly, in contrast the ATP levels in DT (arrowhead) and CD (asterisk) were maintained well. (B) Ratio images of DT during ischemia. (C and D) Ratio graphs of S1 and S2 PTs and DTs during ischemia. The ATP levels in both S1 and S2 PTs reached the minimum plateau in 2 minutes (C: n=3 mice per group), although the ATP levels in DTs were maintained even 30 minutes after induction of ischemia and decreased gradually up to 1 hour (D: n=4 mice per group). (E) The ATP levels in interstitial cells (arrows) were well maintained. Scale bars: 100 μm in A and E; 50 μm in B.
Renal Histochemistry
The harvested kidney samples were fixed in Carnoy solution, embedded in paraffin, sectioned (2.0 μm), and stained with periodic acid–Schiff. All of the periodic acid–Schiff samples were analyzed with a Zeiss Axio Imager A2 microscope using Zeiss Axio Vision 4.8 software.
Renal Immunofluorescence
The harvested kidney samples were fixed in 4% paraformaldehyde, incubated in 20% sucrose in PBS at 4°C overnight, and incubated in 30% sucrose overnight. OCT-embedded kidneys were cryosectioned into 6.0-μm sections. The following primary antibodies were used for immunostaining: anti–α-SMA (catalog no. C 6198; Sigma-Aldrich) and anti–Tim-1/Kim-1 (catalog no. 14–5861–82; eBioscience).
Quantitative Assessment of Fibrosis
Quantification of fibrosis was performed by measuring the α–smooth muscle actin (α-SMA)–positive area in the interstitium, as described previously.31 Eight images of each kidney section at cortical fields (×200 magnification) were taken in the whole circumference with a confocal microscope (n=4 in each group). All images were obtained using the same laser power and gain intensity with a confocal microscope (FV1000D; Olympus). The α-SMA–positive areas in the vascular smooth muscle cells was excluded. The α-SMA–positive areas were automatically calculated by MetaMorph software.
Quantification of mRNA by Real-Time PCR
RNA extraction and real-time RT-PCR were performed, as described previously (n=4 in each group). 32Specific primers utilized in our previous work27 were utilized in this study.
Compounds Administration
NO donor (catalog 345–06941; Fuji Film Wako; 3 mg/kg, n=3) was administered to mice by intravenous injection 5 minutes before IR; 5-aminoimidazole-4-carboxamide 1-β-d-ribofuranoside (AICAR; catalog no. 011–22533; Fuji Film Wako; 500 mg/kg, n=3) was administered to mice by intraperitoneal injection for 3 consecutive days and 2 hours before IR. Nicotinamide mononucleotide (NMN; gifted from Yo-ichi Nabeshima; 500 mg/kg, n=3) was administered to mice by subcutaneous injection for 6 consecutive days and 3 hours before IR.
Statistical Analyses
Results are presented as means ± SD (Figures 2, C and D, 3, B and C, and 6B, Supplemental Figure 2C). Some data are reported as box and whisker plots (Figures 4, B and C and 5B, Supplemental Figure 2B). Statistical significance was assessed by a two-tailed t test for comparisons between two groups (Figure 5B, Supplemental Figure 3A); one-way ANOVA with Bonferroni post hoc tests for comparisons among more than two groups (Figure 1E); one-way ANOVA (Figure 4, B and C, Supplemental Figure 2B); and the nonparametric test for trend across 15-, 30-, and 60-minute warm IR groups developed by Cuzick, which is an extension of the Wilcoxon rank-sum test (Figures 4, B and C and 5B). Comparisons between the ATP recovery slopes were assessed by analysis of covariance (Figures 3D and 6C). Correlations were determined by Pearson correlation analysis (Figures 5C and 6, D and E). We conducted statistical analyses by Stata (StataCorp, College Station, TX; version 15.1), with values of P<0.05 considered statistically significant.
Figure 4.
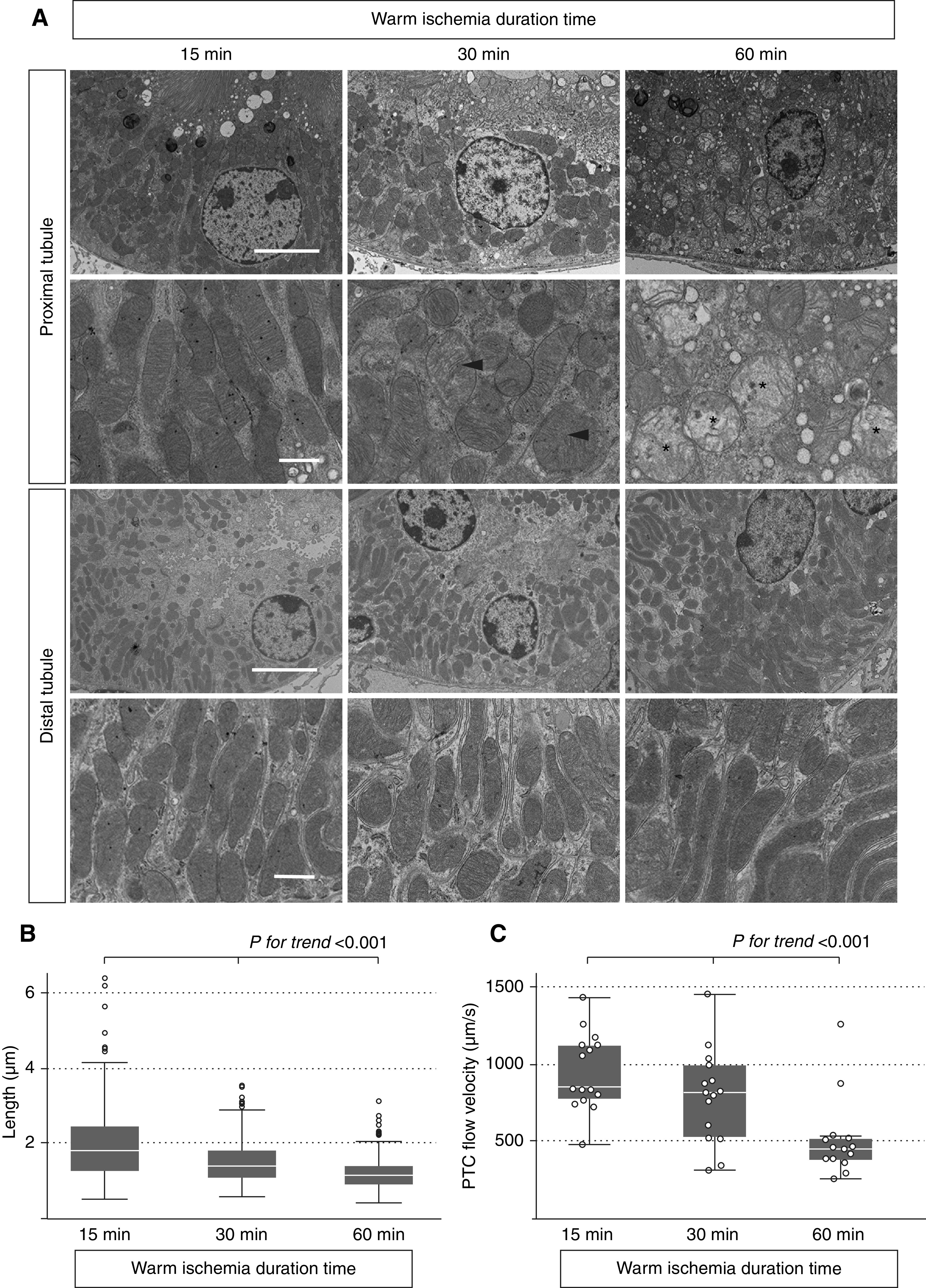
Mitochondrial structural changes and PTC flow velocities after warm 15-, 30-, and 60-minute IR. (A) Electron microscopic analysis of the kidney 5 minutes after the induction of reperfusion after 15-, 30-, and 60-minute ischemia. Mitochondrial swelling (arrowheads) and fragmentation were observed in PTs after 30- and 60-minute IR, and the loss of cristae was additionally observed after 60-minute IR (asterisks). In contrast, no obvious fragmentation of mitochondria was observed in DTs even after 60-minute IR. Scale bars: 5 μm in upper panels of both PTs and DTs; 1 μm in lower panels of both PTs and DTs. (B) The longer ischemic time resulted in the decrease of length of mitochondria (n=3 mice, nine tubules, 300 mitochondria). (C) The longer ischemic time resulted in the slower PTC flow velocities (n=3 mice, 15 areas). Statistical significance was assessed in B and C by the nonparametric test for trend across 15-, 30-, and 60-minute warm IR groups and ANOVA. P value for trend <0.001.
Figure 5.
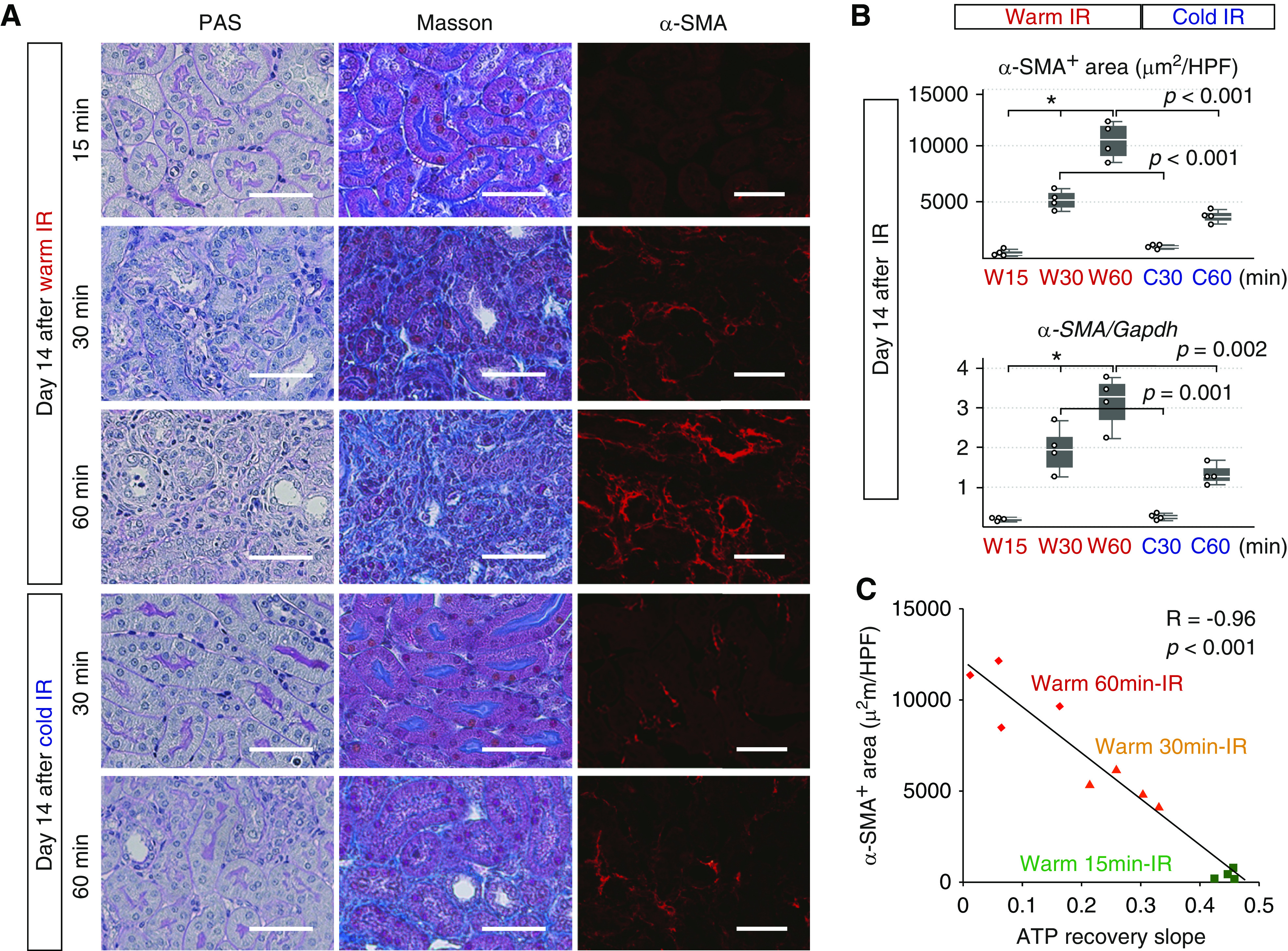
Fibrosis in chronic phase was inversely correlated well with the ATP recoveries in acute phase. (A) Periodic acid–Schiff (PAS) and Masson staining of the kidneys 14 days after warm 15-, 30-, and 60-minute IR and cold 30- and 60-minute IR and immunofluorescence analysis of α-SMA. Scale bars: 50 μm. (B) The quantitative analysis of α-SMA–positive area and the expression of α-SMA mRNA in the kidneys harvested 14 days after IR (n=4 mice per group). The expression levels of α-SMA mRNA were normalized to those of Gapdh mRNA. Statistical significance was assessed by the nonparametric test for trend across 15-, 30-, and 60-minute warm IR groups. *P value for trend =0.002. Differences between the two groups (warm 30-minute IR versus cold 30-minute IR and warm 60-minute IR versus cold 60-minute IR) were compared using t test. These graphs showed the progression of renal fibrosis after longer warm ischemia as well as the amelioration of renal fibrosis after cold IR. (C) Graph showing that the fibrosis area in chronic phase was inversely correlated with S1 PTs ratio recovery slopes in acute phase. The correlation was determined by Pearson correlation analysis. HPF, high power field.
Study Approval
All animal studies were approved by the Animal Research Committee, Graduate School of Medicine, Kyoto University and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Results
Visualization of ATP Levels at a Single-Cell Resolution in the Kidney with GO-ATeam2 Mice
An FRET-based fluorescent ATP probe, termed GO-ATeam, has been developed for ATP imaging at the single-cell resolution,22,33 in which variants of GFP and OFP sandwich the ε-subunit of Bacillus subtilis FoF1-ATP synthase (Figure 1A).33 The ε-subunit binds to ATP dose dependently and specifically but not to ADP, dATP, or GTP.22 Therefore, the ratio of OFP to GFP emission reflects intracellular ATP levels.22,33 The probe is almost insensitive to pH within the physiologic ranges.33
Recently, we generated a novel mouse line systemically expressing GO-ATeam by inserting CAG promoter-driven GO-ATeam in the ROSA26 locus (GO-ATeam2 mice).23,26 The expression of GO-ATeam is ubiquitous, allowing ATP visualization in various tissues. Utilizing this mouse line named GO-ATeam2 mice, we visualized renal ATP of living mice with a two-photon microscope. In the FRET ratio image (Figure 1B, right panel), warm colors indicate high FRET ratios (high ATP levels), and cool colors indicate low FRET ratios (low ATP levels). There were also tubules with high GO-ATeam expression (Figure 1B, center panel). In order to identify the segments of these tubules, we administered 3-kD Texas red dextran to visualize tubular flow (Figure 1C) as previously reported.34,35 The sequences of the flow indicated that these tubules with high-intensity signals were DTs. The laser power was reduced to take images of DT without saturation of OFP signal (Figure 1D, right panels). Among PTs, apical signals in some tubules (asterisks) were relatively higher than others (Figure 1B). According to the sequences of the flow (Figure 1C), PTs with higher apical signals were considered as S1 segments, and PTs with lower apical signals were considered as S2 segments. This result is in accordance with the previous study demonstrating high autofluorescence in apical sides of S1 segments of wild-type mice.36 Because of the high autofluorescence, apical sides of S1 segments of PTs were excluded during monitoring FRET ratios (Supplemental Figure 1A). S3 segments of PTs were not observed from the surface of the kidney.
Although the ATP levels in PTs were homogeneous (Figure 1D, left panels), the ATP levels of DTs and collecting ducts (CDs) were heterogeneous (Figure 1D, right panels). As for the evaluation of the ratios in DTs, we averaged the results of all cells in each DT section, whereas we selected principal cells in the evaluation of CDs because we could not obtain required signal in intercalated cells (Supplemental Figure 1A). Immunostaining revealed higher expression of GO-ATeam in principal cells and lower expression in intercalated cells (Supplemental Figure 1B). We assume that the different expression of biosensor between two cell types is due to the characteristics of CAG promoter driving FRET probe.
The FRET ratios in S1 and S2 segments of PTs, DTs, and CDs (principle cells) were higher in DTs and CDs than in PTs (Figure 1E).
Rapid ATP Reduction in PTs and Slow ATP Reduction in DTs after Ischemia
In addition to the heterogeneity in basal ATP concentrations between cell types in kidney, we expected that ATP dynamics upon AKI would vary between cell types because of the different ATP demands and energy metabolism. We first confirmed that renal dysfunction as well as histologic injury of GO-ATeam2 mice was comparable with those of wild-type mice after IR injury (Supplemental Figure 3).
To analyze spatiotemporal ATP dynamics during AKI, we next performed ATP imaging on the kidneys of GO-ATeam2 mice upon induction of IR injury. S1 and S2 PTs and DTs were identified by the autofluorescence features and FRET patterns described in the previous section.
As expected, we noticed the crucial difference of ATP dynamics between PTs and DTs during ischemia (Figure 2, Supplemental Videos 1 and 2). Although the ATP levels in both S1 and S2 segments of PTs decreased rapidly and homogenously, reaching the minimum plateau within 2 minutes after the induction of ischemia (Figure 2, A and C), the ATP levels in DTs and CDs decreased very slowly, reaching the minimum plateau after 60 minutes (Figure 2, B and D). ATP reduction rates were heterogeneous even within DTs and CDs (Figure 2, A and B, Supplemental Video 2). In contrast, the ATP levels in interstitial cells were well maintained (Figure 2E).
ATP Recovery in PTs after Reperfusion Varies Depending on the Length of Ischemia
Next, we analyzed the recovery of ATP after IR in GO-Ateam2 mice. We induced 15-, 30-, and 60-minute warm ischemia and monitored ATP dynamics in each segment after reperfusion (Supplemental Videos 3–5). Although the minimum plateau ATP levels in PTs after warm 15-, 30-, and 60-minute ischemia were similar, longer ischemia resulted in slower recovery of ATP in PTs (Figure 3A). The ATP recovery in S1 PTs during reperfusion after warm 15-, 30-, and 60-minute ischemia took 2, 5, and 30 minutes, respectively, to reach a peak plateau, and the percentage ATP recovery rates were 92.3%±4.1%, 86.2%±3.8%, and 69.6%±4.1%, respectively (Figure 3B). The ATP recovery slopes in S1 PTs between 0 and 2 minutes after 15-, 30-, and 60-minute IR were 0.446±0.016, 0.277±0.052, and 0.075±0.064, respectively, showing by analysis of covariance that longer ischemia resulted in lower recovery slopes (Figure 3D). The percentage ATP recovery rates in S2 PTs after warm 15-, 30-, and 60-minute ischemia were 92.6%±3.6%, 84.0%±10.9%, and 25.5%±10.6%, respectively, indicating that S2 PTs were more vulnerable than S1 PTs in the severe IR injury (Figure 3B). In addition, significant vacuolization was observed in S2 PTs but not in S1 PTs (Supplemental Figure 4).
ATP Recovery in DTs Is Rapid and Almost Complete, Even after Long Ischemia
The ATP dynamics in DTs were strikingly different from those of PTs. First, the ATP level continued to decrease until 60 minutes (Figure 2D). Second, the ATP levels in DTs recovered quickly and almost completely, even after 60-minute ischemia (Figure 3, A and C). ATP recovery in DTs after warm 15-, 30-, and 60-minute ischemia only took 1, 2, and 4 minutes, respectively, to reach a peak plateau, and the percentage ATP recovery rates were 87.5%±7.4%, 89.8%±7.0%, and 89.2%±5.0%, respectively, indicating the resistance of DTs to IR injury (Figure 3C).
Mitochondrial Damage and Peritubular Capillary Flow after Reperfusion Might Be the Determinants of ATP Recovery
To explore the determinants of the ATP recovery after reperfusion, we next examined mitochondrial injury 5 minutes after reperfusion. Mitochondrial fragmentation was obvious in PTs after reperfusion (Figure 4A), and by the nonparametric trend test,37 the longer ischemic time resulted in the increase of fragmented mitochondria in PTs (Figure 4B). On the contrary, no obvious mitochondrial fragmentation was observed in DTs even after long ischemia (Figure 4A). We also visualized peritubular capillary (PTC) flow 5 minutes after reperfusion utilizing two-photon microscopy38 (Supplemental Videos 6–8) and found that the longer ischemia time resulted in decreased PTC flow by the nonparametric trend test (Figure 4C). Red blood cell rouleaux formation leading to the instability of PTC flow was also confirmed after 60-minute ischemia (Supplemental Video 9). Statistical differences between groups in Figure 4, B and C were also confirmed by ANOVA.
Fibrosis in Chronic Phase Is Inversely Correlated with the ATP Recovery in Acute Phase
Fibrosis in chronic phase after AKI is often regarded as the sign of poor renal prognosis.39 Histologic analysis of fibrosis, measurement of α-SMA–positive area, and real-time RT-PCR of α-SMA mRNA on day 14 after IR revealed the progression of renal fibrosis after longer warm ischemia by the nonparametric test for trend (Figure 5, A and B). We next analyzed the correlation between the ATP recovery slopes in acute phase and α-SMA–positive area in chronic phase by Pearson correlation analysis, and surprisingly, we found that α-SMA–positive area in chronic phase was inversely well correlated with the ATP recovery of PTs in acute phase (Figure 5C), indicating that the ATP recovery of PTs in acute phase is an important predictor of renal prognosis.
Renal Hypothermia Improves ATP Recovery in Acute Phase and Fibrosis in Chronic Phase after IR Injury
Renal hypothermia may decrease the metabolic demand of nephrons and is expected to protect the kidney from IR injury.40,41 The efficacy of renal hypothermia has been tested in clinical practice of kidney transplantation from diseased donors42 and partial nephrectomy.43 However, the mechanism of its protective effects remained unclear. We hypothesized that hypothermia protects kidney from ischemia by suppressing depletion of ATP.
We examined the effect of hypothermia on ATP dynamics during IR by holding the core body temperature at 33°C. The ATP recovery after 30- and 60-minute ischemia was dramatically improved in the cold ischemia group (Figure 6A). The ATP recovery in S1 PTs after cold 30- and 60-minute ischemia took only 2 and 4 minutes, respectively, to reach a peak plateau (Figure 6B), whereas the ATP recovery after warm 30- and 60-minute ischemia took 5 and 30 minutes, respectively (Figure 3B). The percentage ATP recovery rates after cold 30- and 60-minute ischemia were 90.6%±2.5% and 87.1%±2.6%, respectively (Figure 6B), whereas those after warm 30- and 60-minute ischemia were 86.2%±3.8% and 69.6%±4.1%, respectively (Figure 3B). The ATP recovery slopes in S1 PTs after cold 30- and 60-minute ischemia were 0.496±0.037 and 0.326±0.049, respectively (Figure 6C), whereas those after warm 30- and 60-minute ischemia were 0.277±0.052 and 0.075±0.064, respectively (Figure 3D).
To exclude the possibility that temperature affected the properties of GO-ATeam2, we analyzed the ratios in primary cells obtained from the kidneys of GO-ATeam2 mice sequentially cultured under different temperatures and found similar ratios by ANOVA (P=0.86) (Supplemental Figure 2, A and B). We also confirmed that the body temperature changes did not affect the ratios by monitoring ratios of mice treated with cold 30- and 60-minute IR whose kidneys were warmed 50 minutes after reperfusion (Supplemental Figure 2C). Thus, we concluded that the temperature itself did not affect the FRET ratio in our experimental setup.
Additionally, cold IR remarkably ameliorated renal fibrosis in chronic phase (Figure 5, A and B). Inverse correlation between the ATP recovery slope in acute phase and α-SMA–positive area in chronic phase was well maintained even when we evaluated kidney samples treated with warm and cold ischemia together (Figure 6D). The fibrosis area was also inversely correlated with percentage ATP recovery in acute phase (Figure 6E).
Discussion
The conventional ATP quantification methods, such as luciferase assay, nuclear magnetic resonance spectroscopy, and mass imaging technique, have not allowed us to visualize and monitor spatiotemporal ATP dynamics in vivo. Here, we have successfully visualized ATP levels at a single-cell resolution in the living kidney and demonstrated spatiotemporal ATP dynamics during IR injury for the first time.
We first showed that, in the physiologic condition, the cytoplasmic ATP levels in S1 PTs were higher than those in S2 and were lower than those in DTs, in accordance with the previous reports analyzing ATP in dissected nephron by the luciferin-luciferase technique.16,44,45 Lower basal ATP levels in S2 PT, even though this is regarded as a metabolically active region, might suggest higher consumption of ATP in this segment.
We also revealed the cell type–specific ATP dynamics during IR injury and demonstrated that the ATP levels in PTs decreased rapidly upon ischemia induction, whereas the ATP levels in DTs decreased very slowly. Our results are in accordance with the histologic resistance of DTs to IR injury compared with PTs.21 There are several possible explanations for different ATP dynamics during IR injury. First, under ischemia, DTs could produce ATP by anaerobic glycolysis, whereas PTs depend mainly on aerobic ATP production for energy supply.17,18 Second, mitochondrial function might be also different between segments. Previous study demonstrated that mitochondrial membrane potential was better maintained after the inhibition of respiration in DTs than in PTs.28,46 Our data showing more fragmentation of mitochondria in PTs compared with that in DTs after IR injury (Figure 4A) also support the hypothesis. Third, higher expression of cytoprotective factors, such as Bcl-2 and heat shock proteins, in DTs might also contribute to the resistance.47 Fourth, the energy consumption of PTs might be higher than that of DTs, which leads to the rapid ATP fall in PTs after ischemia.
Next, we showed that the ATP recovery in PTs after IR was dependent on the severity of injury (Figure 3). There are several possible mechanisms explaining slow and insufficient ATP recovery after longer ischemia, such as mitochondrial dysfunction (Figure 4, A and B) and oxygen supply reduction due to microvascular rarefaction, decreased PTC flow (Figure 4C), and coagulation.
We also showed slower and insufficient ATP recovery and more vacuolization in S2 PTs than in S1 PTs, indicating that S2 PTs are more susceptible to ischemia than S1 PTs. A previous study demonstrated the resistant of S1 PTs to oxidative stress in a sepsis model, possibly by the upregulation of cytoprotective heme oxygenase-1 and sirtuin-1.29 The decrease of tubular flow and accumulation of debris could also contribute to higher susceptibility of S2 PTs.
One hour after the induction of reperfusion, neither obvious histologic injury nor the expression of Kim-1 were observed even in the kidneys treated with warm 60-minute ischemia, indicating that the dynamic changes of cytoplasmic ATP could occur before obvious histologic injury (Supplemental Figure 5A). One day after IR, the kidneys treated with warm ischemia showed cell detachment of tubule epithelial cells (Supplemental Figure 5), whereas cold IR dramatically ameliorated tubular injury. These differences in histologic findings in acute phase are consistent with the different ATP dynamics in each group.
More importantly, we revealed that the fibrosis area in chronic phase was inversely correlated with the ATP recovery slope and percentage ATP recovery in PTs in acute phase. These results strongly indicate that breakdown of ATP homeostasis in PTs plays an important role in AKI to CKD progression and that the initial ATP recovery of PTs could be the primary determinant of the renal prognosis in ischemic AKI.
Finally, we provide the direct in vivo evidence that hypothermia during IR injury improves the ATP recovery as well as fibrosis (Figures 5 and 6). Previous studies showed that renal hypothermia conferred striking protection against IR injury,40,41,48 possibly by decreasing the renal oxygen demand and metabolic activity. Our study provided direct evidence supporting the previous hypothesis. We further succeeded in the quantitative comparison of ATP dynamics during warm and cold IR with respect to the speed and extent of the ATP recovery. Our results demonstrated the ATP recovery slope of cold 30-minute ischemia in S1 PTs were slightly better than those of warm 15-minute ischemia and that the ATP recovery of cold 60-minute ischemia was better than that of warm 30-minute ischemia (Figures 3 and 6). These results suggest that in cold conditions, similar ATP dynamics are obtained even with ischemic time about twice as long as that of warm conditions in mice. Human kidneys are exposed to ischemia in the surgical procedures of transplantation and partial nephrectomy, and renal hypothermia is often applied in the clinical settings. Some clinical studies assessed renal function after partial nephrectomy49–52 and concluded that longer warm ischemia time is associated with poor renal prognosis. One report compared postoperative renal function with warm or cold ischemia during partial nephrectomy using 99mTc-mercaptoacetyltriglycine renal scintigraphy parameters, and found that the cold condition enabled to extend ischemia time more than twice of warm condition in humans.50 There is also the accumulating evidence for metabolic dysfunction during ischemic AKI in human renal transplantation.53 These findings not only are in accordance with our study showing the significance of the association between ATP dynamics and prognosis, but also imply that our ATP visualization technique has the potential to elucidate the cellular mechanism of AKI to CKD progression.
Recently, new potential approaches focusing on the attenuation of endothelial dysfunction (NO donor),54 AMPK activator (AICAR),55 mitochondrial biogenesis, and cellular bioenergetics, such as MitoQ, skQR1, SS-31, MA-5, peroxisome proliferator, and NMN,10,56–60 have been developed, and some of them showed renoprotective effect. We examined whether the administration of NO donor, AICAR, and NMN could improve the ATP dynamics in acute phase but failed to show the improvement of the ratio before IR, the recovery slope, or the percentage recovery (Supplemental Figure 6). Discrepancy with previous reports might be explained by the difference in the protocols.
Although intravital imaging of the kidneys of GO-ATeam2 mice provides invaluable information on the ATP dynamics of the kidney, there remain several technical limitations to be challenged in the future. (1) We could not reach deeper kidney regions, such as PT S3 segments and interstitial cells in corticomedullary junction, by two-photon microscopes. The ATP dynamics of deeper cortical regions after IR injury might be more intense than those of the kidney surface. Although the ATP levels of interstitial cells in the kidney surface were maintained after IR injury (Figure 2E), it is likely that interstitial cells in deeper kidney regions would show diminished ATP levels after IR injury, consistent with their well-established oxygen-sensing function and erythropoietin synthesis. Development of another FRET probe that operates at longer wavelength may alleviate the light scattering and thereby, enable deep tissue imaging. (2) The cellular ATP levels visualized in our mice are the results of a balance between production and consumption; therefore, rate of ATP production or consumption cannot be assessed separately.
Even with these limitations, the analysis utilizing the novel ATP imaging technique shed light on tubular ATP dynamics in vivo during AKI, revealed the crucial difference of the ATP dynamics between nephron segments, and more importantly, highlighted the correlation between ATP recovery of PTs in acute phase and renal prognosis. This revolutionary ATP imaging technique improves our understanding of the cellular events in the diseased kidney and could help us to develop therapeutic strategies to treat AKI and to minimize IR injury following kidney transplantation and partial nephrectomy.
Disclosures
J. Nakamura is currently employed by Deloitte Tohmatsu Consulting LLC. Shigenori Yamamoto and Y. Sato are partly employed by TMK Project, which is a collaborative project with Mitsubishi Tanabe Pharmaceutical company, outside the submitted work. M. Yamamoto is partly employed by a collaborative project with Boehringer Ingelheim, outside the submitted work. M. Yanagita reports receiving research grants from Astellas, Chugai, Daiichi Sankyo, Fujiyakuhin, Kyowa Hakko Kirin, Mitsubishi Tanabe, MSD, Nippon Boehringer Ingelheim, and Torii; Japan Agency for Medical Research and Development-Core Research for Evolutional Science and Technology “Innovation for Ideal Medical Treatment Based on the Understanding of Maintenance, Change and Breakdown Mechanisms of Homeostasis among Interacting Organ Systems” grant; Grant-in-Aid for Scientific Research on Innovative Areas; grants from AMED (Platform Program for Promotion of Genome Medicine); Grant-in-Aid for challenging Exploratory Research; and Grant-in-Aid for Scientific Research (B), outside the submitted work. All remaining authors have nothing to disclose.
Funding
This work was supported by Platform for Drug Discovery, Informatics, and Structural Life Science from the Ministry of Education, Culture, Sports, Science and Technology, Japan; Kyoto University Live Imaging Center; and in part by Japan Society for the Promotion of Science Grant-in-Aid KAKENHI 16H06280 “Advanced Bioimaging Support.” This research was partially supported by Japan Agency for Medical Research and Development grants JP19gm5010002 (to M. Yanagita), JP19gm0610011 (to M. Yanagita), and Japan Agency for Medical Research and Development-Core Research for Evolutional Science and Technology 19gm1210009 (to M. Yanagita); Japan Society for the Promotion of Science KAKENHI Grants-in-Aid for Scientific Research B 17H04187 and 20H03697 and Grants-in-Aid for Scientific Research on Innovative Areas “Stem Cell Aging and Disease” 17H05642 and “Lipoquality” 18H04673 from Ministry of Education, Culture, Sports, Science and Technology; and grants from Uehara Memorial Foundation, Takeda Science Foundation, and Sumitomo Foundation. This work was partly supported by World Premier International Research Center Initiative, Ministry of Education, Culture, Sports, Science and Technology, Japan. This study was also partly supported by Japan Science and Technology Agency Precursory Research for Embryonic Science and Technology grant JPMJPR14MF (to M. Yamamoto).
Supplementary Material
Acknowledgments
The authors thank Dr. Minoru Satoh and Dr. Daisuke Nakano for valuable discussion regarding the live imaging; Ms. Satsuki Fujiwara and Ms. Kanako Takakura for technical assistance; Dr. Keita Hirano for statistical analysis; Dr. Kousuke Nakata for figure illustration; and Prof. Yo-ichi Nabeshima for gifted NMN.
Shinya Yamamoto and M. Yanagita designed the experiments and wrote the manuscript; M. Yanagita supervised the project; K. Kaneko, A. Mii, J. Nakamura, Y. Sato, M. Takahashi, M. Yamamoto, Shigenori Yamamoto, and Shinya Yamamoto performed experiments; and S. Fukuma, H. Imamura, M. Matsuda, E. Uchino, M. Yamamoto, and Shinya Yamamoto analyzed the data.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020050580/-/DCSupplemental.
Supplemental Figure 1. Areas analyzed in monitoring ratios (A), and immunostaining of AQP2 and GFP in the collecting duct of GO-ATeam2 mice (B).
Supplemental Figure 2. Analysis of FRET ratios at different temperatures (A) in vitro and (B) in vivo.
Supplemental Figure 3. Kidney function and histologic alterations of wild-type and GO-ATeam2 mice after IR injury.
Supplemental Figure 4. Vacuolization in S2 PTs after reperfusion.
Supplemental Figure 5. Histologic analysis of the kidneys 1 hour and 1 day after the induction of reperfusion.
Supplemental Figure 6. ATP dynamics after warm 30-minute IR treated with NO donor, AICAR, or NMN.
Supplemental Video 1. Time series of ratio images in PTs after induction of warm ischemia.
Supplemental Video 2. Time series of ratio images in DTs and CDs after induction of warm ischemia.
Supplemental Video 3. Time series of ratio images during reperfusion after warm 15-minute ischemia.
Supplemental Video 4. Time series of ratio images during reperfusion after warm 30-minute ischemia.
Supplemental Video 5. Time series of ratio images during reperfusion after warm 60-minute ischemia.
Supplemental Video 6. A 5-minute time series of peritubular capillary flow just after the induction of reperfusion following warm 15-minute ischemia.
Supplemental Video 7. A 5-minute time series of peritubular capillary flow just after the induction of reperfusion following warm 30-minute ischemia.
Supplemental Video 8. A 5-minute time series of peritubular capillary flow just after the induction of reperfusion following warm 60-minute ischemia.
Supplemental Video 9. A 110-second series of peritubular capillary flow 5 minutes after the induction of reperfusion after warm 60-minute ischemia.
References
- 1.Lewington AJ, Cerdá J, Mehta RL: Raising awareness of acute kidney injury: A global perspective of a silent killer. Kidney Int 84: 457–467, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 81: 442–448, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, et al.: Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato Y, Takahashi M, Yanagita M: Pathophysiology of AKI to CKD progression. Semin Nephrol 40: 206–215, 2020. [DOI] [PubMed] [Google Scholar]
- 6.Lan R, Geng H, Singha PK, Saikumar P, Bottinger EP, Weinberg JM, et al.: Mitochondrial pathology and glycolytic shift during proximal tubule atrophy after ischemic AKI. J Am Soc Nephrol 27: 3356–3367, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, et al.: Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med 21: 37–46, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran MT, Zsengeller ZK, Berg AH, Khankin EV, Bhasin MK, Kim W, et al.: PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 531: 528–532, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szeto HH: Pharmacologic approaches to improve mitochondrial function in AKI and CKD. J Am Soc Nephrol 28: 2856–2865, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poyan Mehr A, Tran MT, Ralto KM, Leaf DE, Washco V, Messmer J, et al.: De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat Med 24: 1351–1359, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knowles JR: Enzyme-catalyzed phosphoryl transfer reactions. Annu Rev Biochem 49: 877–919, 1980. [DOI] [PubMed] [Google Scholar]
- 12.Siegel NJ, Devarajan P, Van Why S: Renal cell injury: Metabolic and structural alterations. Pediatr Res 36: 129–136, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Avison MJ, van Waarde A, Stromski ME, Gaudio K, Siegel NJ: Metabolic alterations in the kidney during ischemic acute renal failure. Semin Nephrol 9: 98–101, 1989. [PubMed] [Google Scholar]
- 14.Stromski ME, Cooper K, Thulin G, Gaudio KM, Siegel NJ, Shulman RG: Chemical and functional correlates of postischemic renal ATP levels. Proc Natl Acad Sci U S A 83: 6142–6145, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogt MT, Farber E: On the molecular pathology of ischemic renal cell death. Reversible and irreversible cellular and mitochondrial metabolic alterations. Am J Pathol 53: 1–26, 1968. [PMC free article] [PubMed] [Google Scholar]
- 16.Uchida S, Endou H: Substrate specificity to maintain cellular ATP along the mouse nephron. Am J Physiol 255: F977–F983, 1988. [DOI] [PubMed] [Google Scholar]
- 17.Wirthensohn G, Guder WG: Renal substrate metabolism. Physiol Rev 66: 469–497, 1986. [DOI] [PubMed] [Google Scholar]
- 18.Bagnasco S, Good D, Balaban R, Burg M: Lactate production in isolated segments of the rat nephron. Am J Physiol 248: F522–F526, 1985. [DOI] [PubMed] [Google Scholar]
- 19.Vandewalle A, Wirthensohn G, Heidrich HG, Guder WG: Distribution of hexokinase and phosphoenolpyruvate carboxykinase along the rabbit nephron. Am J Physiol 240: F492–F500, 1981. [DOI] [PubMed] [Google Scholar]
- 20.Sharfuddin AA, Molitoris BA: Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 7: 189–200, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Lieberthal W, Nigam SK: Acute renal failure. I. Relative importance of proximal vs. distal tubular injury. Am J Physiol 275: F623–F631, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Imamura H, Nhat KP, Togawa H, Saito K, Iino R, Kato-Yamada Y, et al.: Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc Natl Acad Sci U S A 106: 15651–15656, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto M, Kim M, Imai H, Itakura Y, Ohtsuki G: Microglia-triggered plasticity of intrinsic excitability modulates psychomotor behaviors in acute cerebellar inflammation. Cell Rep 28: 2923–2938.e8, 2019. [DOI] [PubMed] [Google Scholar]
- 24.Ashworth SL, Sandoval RM, Tanner GA, Molitoris BA: Two-photon microscopy: Visualization of kidney dynamics. Kidney Int 72: 416–421, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Peti-Peterdi J, Kidokoro K, Riquier-Brison A: Novel in vivo techniques to visualize kidney anatomy and function. Kidney Int 88: 44–51, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakano M, Imamura H, Sasaoka N, Yamamoto M, Uemura N, Shudo T, et al.: ATP maintenance via two types of ATP regulators mitigates pathological phenotypes in mouse models of Parkinson’s disease. EBioMedicine 22: 225–241, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Endo T, Nakamura J, Sato Y, Asada M, Yamada R, Takase M, et al.: Exploring the origin and limitations of kidney regeneration. J Pathol 236: 251–263, 2015. [DOI] [PubMed] [Google Scholar]
- 28.Hall AM, Rhodes GJ, Sandoval RM, Corridon PR, Molitoris BA: In vivo multiphoton imaging of mitochondrial structure and function during acute kidney injury. Kidney Int 83: 72–83, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalakeche R, Hato T, Rhodes G, Dunn KW, El-Achkar TM, Plotkin Z, et al.: Endotoxin uptake by S1 proximal tubular segment causes oxidative stress in the downstream S2 segment. J Am Soc Nephrol 22: 1505–1516, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sano T, Kobayashi T, Negoro H, Sengiku A, Hiratsuka T, Kamioka Y, et al.: Intravital imaging of mouse urothelium reveals activation of extracellular signal-regulated kinase by stretch-induced intravesical release of ATP. Physiol Rep 4: el3033, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takaori K, Nakamura J, Yamamoto S, Nakata H, Sato Y, Takase M, et al.: Severity and frequency of proximal tubule injury determines renal prognosis. J Am Soc Nephrol 27: 2393–2406, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanagita M, Okuda T, Endo S, Tanaka M, Takahashi K, Sugiyama F, et al. : Uterine sensitization-associated gene-1 (USAG-1), a novel BMP antagonist expressed in the kidney, accelerates tubular injury. J Clin Invest 116: 70–79, 2006. 10.1172/JCI25445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakano M, Imamura H, Nagai T, Noji H: Ca2+ regulation of mitochondrial ATP synthesis visualized at the single cell level. ACS Chem Biol 6: 709–715, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Molitoris BA, Sandoval RM: Intravital multiphoton microscopy of dynamic renal processes. Am J Physiol Renal Physiol 288: F1084–F1089, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Nakano D, Doi K, Kitamura H, Kuwabara T, Mori K, Mukoyama M, et al.: Reduction of tubular flow rate as a mechanism of oliguria in the early phase of endotoxemia revealed by intravital imaging. J Am Soc Nephrol 26: 3035–3044, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hato T, Winfree S, Day R, Sandoval RM, Molitoris BA, Yoder MC, et al.: Two-photon intravital fluorescence lifetime imaging of the kidney reveals cell-type specific metabolic signatures. J Am Soc Nephrol 28: 2420–2430, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cuzick J: A Wilcoxon-type test for trend. Stat Med 4: 87–90, 1985. [DOI] [PubMed] [Google Scholar]
- 38.Kang JJ, Toma I, Sipos A, McCulloch F, Peti-Peterdi J: Quantitative imaging of basic functions in renal (patho)physiology. Am J Physiol Renal Physiol 291: F495–F502, 2006. [DOI] [PubMed] [Google Scholar]
- 39.He L, Wei Q, Liu J, Yi M, Liu Y, Liu H, et al.: AKI on CKD: Heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int 92: 1071–1083, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward JP: Determination of the Optimum temperature for regional renal hypothermia during temporary renal ischaemia. Br J Urol 47: 17–24, 1975. [DOI] [PubMed] [Google Scholar]
- 41.Zager RA, Altschuld R: Body temperature: An important determinant of severity of ischemic renal injury. Am J Physiol 251: F87–F93, 1986. [DOI] [PubMed] [Google Scholar]
- 42.Niemann CU, Feiner J, Swain S, Bunting S, Friedman M, Crutchfield M, et al.: Therapeutic hypothermia in deceased organ donors and kidney-graft function. N Engl J Med 373: 405–414, 2015. [DOI] [PubMed] [Google Scholar]
- 43.Breau RH, Cagiannos I, Knoll G, Morash C, Cnossen S, Lavallée LT, et al.: Renal hypothermia during partial nephrectomy for patients with renal tumours: A randomised controlled clinical trial protocol. BMJ Open 9: e025662, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burch HB, Choi S, Dence CN, Alvey TR, Cole BR, Lowry OH: Metabolic effects of large fructose loads in different parts of the rat nephron. J Biol Chem 255: 8239–8244, 1980. [PubMed] [Google Scholar]
- 45.Soltoff SP: ATP and the regulation of renal cell function. Annu Rev Physiol 48: 9–31, 1986. [DOI] [PubMed] [Google Scholar]
- 46.Hall AM, Unwin RJ, Parker N, Duchen MR: Multiphoton imaging reveals differences in mitochondrial function between nephron segments. J Am Soc Nephrol 20: 1293–1302, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gobe GC, Johnson DW: Distal tubular epithelial cells of the kidney: Potential support for proximal tubular cell survival after renal injury. Int J Biochem Cell Biol 39: 1551–1561, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Zager RA, Gmur DJ, Bredl CR, Eng MJ: Degree and time sequence of hypothermic protection against experimental ischemic acute renal failure. Circ Res 65: 1263–1269, 1989. [DOI] [PubMed] [Google Scholar]
- 49.Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, et al.: Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol 58: 340–345, 2010. [DOI] [PubMed] [Google Scholar]
- 50.Funahashi Y, Yoshino Y, Sassa N, Matsukawa Y, Takai S, Gotoh M: Comparison of warm and cold ischemia on renal function after partial nephrectomy. Urology 84: 1408–1412, 2014. [DOI] [PubMed] [Google Scholar]
- 51.Volpe A, Blute ML, Ficarra V, Gill IS, Kutikov A, Porpiglia F, et al.: Renal ischemia and function after partial nephrectomy: A collaborative review of the literature. Eur Urol 68: 61–74, 2015. [DOI] [PubMed] [Google Scholar]
- 52.Simmons MN, Lieser GC, Fergany AF, Kaouk J, Campbell SC: Association between warm ischemia time and renal parenchymal atrophy after partial nephrectomy. J Urol 189: 1638–1642, 2013. [DOI] [PubMed] [Google Scholar]
- 53.Wijermars LG, Schaapherder AF, de Vries DK, Verschuren L, Wüst RC, Kostidis S, et al.: Defective postreperfusion metabolic recovery directly associates with incident delayed graft function. Kidney Int 90: 181–191, 2016. [DOI] [PubMed] [Google Scholar]
- 54.Kurata H, Takaoka M, Kubo Y, Katayama T, Tsutsui H, Takayama J, et al.: Protective effect of nitric oxide on ischemia/reperfusion-induced renal injury and endothelin-1 overproduction. Eur J Pharmacol 517: 232–239, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Lempiäinen J, Finckenberg P, Levijoki J, Mervaala E: AMPK activator AICAR ameliorates ischaemia reperfusion injury in the rat kidney. Br J Pharmacol 166: 1905–1915, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dare AJ, Bolton EA, Pettigrew GJ, Bradley JA, Saeb-Parsy K, Murphy MP: Protection against renal ischemia-reperfusion injury in vivo by the mitochondria targeted antioxidant MitoQ. Redox Biol 5: 163–168, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plotnikov EY, Chupyrkina AA, Jankauskas SS, Pevzner IB, Silachev DN, Skulachev VP, et al.: Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochim Biophys Acta 1812: 77–86, 2011. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki T, Yamaguchi H, Kikusato M, Hashizume O, Nagatoishi S, Matsuo A, et al.: Mitochonic acid 5 binds mitochondria and ameliorates renal tubular and cardiac myocyte damage. J Am Soc Nephrol 27: 1925–1932, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang HC, Deleuze S, Zuo Y, Potthoff SA, Ma LJ, Fogo AB: The PPARgamma agonist pioglitazone ameliorates aging-related progressive renal injury. J Am Soc Nephrol 20: 2380–2388, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wills LP, Trager RE, Beeson GC, Lindsey CC, Peterson YK, Beeson CC, et al.: The β2-adrenoceptor agonist formoterol stimulates mitochondrial biogenesis. J Pharmacol Exp Ther 342: 106–118, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.