CKD poses a substantial burden on society. Dialysis and allogenic kidney transplantation provide imperfect solutions. CKD, independent of its underlying etiology, is characterized by a progressive loss of the kidneys’ nephrons, by a rarefaction of its vasculature, and by an expansion of its interstitium.1 Cell-based regenerative therapy approaches aim to halt or revert these processes by transplanting cells, which would preferably be derived from defined autologous sources to avoid immunologic complications. In patients with CKD, this would mean that cells are (1) collected (e.g., from blood, bone marrow, or the kidney itself); (2) processed in vitro (to allow cell selection, proliferation, and appropriate differentiation); and (3) introduced back into the patient, where they form functional kidney replacement tissue.2–4 Cell sources proposed for such regenerative approaches range from fetal or adult kidney cells to induced pluripotent stem cells.
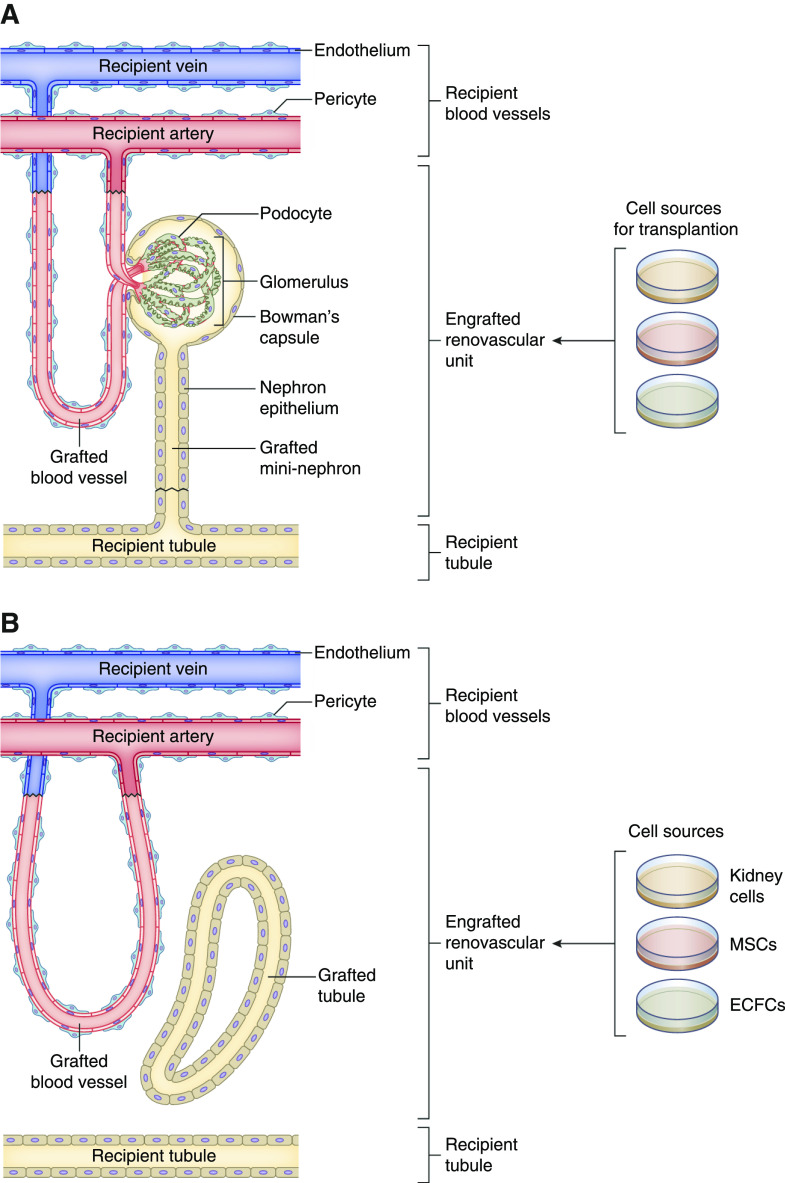
If the goal is to generate de novo functional kidney structures, transplanted cells need to form—at minimum—functional filtration units that receive adequate blood supply, produce urine, and connect to endogenous urinary tract tissues (e.g., collecting ducts) for urine excretion. Although this ideal situation (Figure 1A) has not yet been achieved, in this issue of JASN, Pleniceanu et al.5 provide an important step toward generating such cell-based functional filtration units. They combined defined human cell sources that could potentially be harvested from peripheral blood, bone marrow, and/or kidneys of patients with CKD. After transplantation into immunodeficient mice, these cells formed what the authors called “renovascular units,” consisting of human kidney tubules supplied by a rich network of human blood vessels that had connected to the recipient’s vasculature (Figure 1B).
Figure 1.
Nephron replacement by defined cell sources. (A) Ideal target situation for cell-based generation of engrafted renovascular units in a recipient with CKD. (B) Situation achieved in this issue’s study by Pleniceanu et al.5
This new approach was elegantly informed by previous studies. The group of Pleniceanu et al. had previously transplanted human fetal kidneys and progenitor cells derived from such kidneys into immunodeficient mice, which led to successful engraftment and facilitated a beneficial effect on renal function in a CKD model of 5/6 nephrectomy, likely by paracrine effects.6,7 The authors extended their work and devised a culture method, enabling them to grow tubular cells derived from adult kidney tissue into three-dimensional spheres, which they termed “nephrospheres” (nSPHs).8,9 The nSPH culture method facilitated an ex vivo expansion of kidney tubular cells from different nephron segments and an upregulation of progenitor genes, potentially enhancing engraftment potential. nSPHs could be generated from human kidneys with ESKD, providing an opportunity for autologous transplantation of such cells in patients with advanced CKD.8 nSPHs engrafted and formed renal tubules after injection into subcutaneous tissues or under the kidney capsule of immunodeficient mice. Transplantation of human nSPHs into the parenchyma of remnant kidneys after 5/6 nephrectomy led to an improved kidney function, as measured by GFR, an effect that was likely elicited through paracrine, antifibrotic effects of the transplanted cells.8 Nevertheless, the transplanted nSPHs lacked an endogenous cell source for blood-vessel formation and were dependent on the attraction of recipient-derived blood vessels to ensure their blood supply,8 which might constitute an important limitation in CKD kidneys where the vasculature is usually compromised.
To address this deficit, Pleniceanu et al. now aimed to generate human cell–derived blood vessels alongside kidney cells on the basis of the expansion of patient-derived vascular progenitor cells. For this purpose, they used human endothelial colony-forming cells (ECFCs) and mesenchymal stromal cells (MSCs; also referred to as mesenchymal stem cells).10 ECFCs are progenitor cells that can be isolated from peripheral blood on the basis of their ability to adhere to specific surfaces.11 ECFCs can regenerate endothelial cell populations and form vascular networks in vivo.10,12 MSCs are nonhematopoietic progenitor cells derived from bone marrow that can differentiate into mesodermal cell types, such as osteoblasts, myocytes, and adipocytes. MSCs can be isolated from human bone marrow aspirates and propagated in cell culture. Human ECFCs and MSCs, when combined and transplanted into recipients’ tissues, form extensive vascular networks, with ECFCs constituting the vascular endothelium and MSCs yielding perivascular cells.10
On the basis of this prior knowledge, Pleniceanu et al.5 cotransplanted human ECFCs and MSCs with fetal or adult kidney cells (together with an extracellular matrix called Matrigel), hypothesizing that the MSCs and ECFCs would form human vascular networks supporting growth of human kidney structures. Their approach proved to be successful. Two weeks after transplantation into the subcutaneous tissue, or under the kidney capsule of immunodeficient mice, they obtained large, markedly vascularized, grafts composed of human kidney tubules next to human blood vessels. Notably, the human blood vessels were perfused with mouse blood, indicating they had connected to the recipient’s vascular system. When compared with transplantation of kidney tubular cells alone, the coinjection with ECFCs and MSCs led to an enhanced survival of kidney tubular cells and a greater number of tubules within grafts. Human adult kidney tubular cells grown together with MSCs and ECFCs displayed an enhanced activation of the vascular endothelial growth factor A and of several self-renewal regulators, suggesting a microenvironment supportive of kidney parenchymal growth. In a final set of experiments, the authors showed the injection of human ECFCs, MSCs, and nSPHs directly into injured kidney tissues after partial nephrectomy resulted in successful engraftment of human cell–derived renovascular units within these damaged kidneys.
Will the approaches developed by Pleniceanu et al. benefit CKD kidneys? Although this was not tested in this study, it will likely constitute a short-term research goal to show protective effects in animal models of CKD, which are to be anticipated on the basis of previously observed beneficial paracrine effects of kidney tubular cells, MSCs, and ECFCs.2,8,13,14 Does the novel approach by Plenicanu et al. facilitate cell-based “nephron replacement therapies”? Although providing an important step, the approach generated renovascular units that lacked a glomerulus-like filtration unit. Therefore, additional steps would need to be conceived to allow for the formation of functional nephrons that produce urine.
Disclosures
K. Schmidt-Ott reports having consultancy fees with BioPorto Diagnostics; having received license revenue related to the use of a neutrophil gelatinase-associated lipocalin assay via Columbia University; receiving research funding from FAST BioMedical, for being a principal investigator of the EMPAKT-CHF trial, and Quark Pharmaceuticals, for being the site principal investigator for QRK309 trial; and being an editorial board member for Kidney International.
Funding
K Schmidt-Ott is supported by the Deutsche Forschungsgemeinschaft (German Research Foundation) Collaborative Research Grant SFB 1365/1 (C06), Research Training Group GRK 2318 (B4), and Research Unit FOR 2841 (P02); and by the Urological Research Foundation (Berlin, Germany).
Acknowledgments
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendations. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or JASN. Responsibility for the information and views expressed herein lies entirely with the author(s).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related article, “Successful Introduction of Human Renovascular Units into the Mammalian Kidney,” on pages 2757–2772.
References
- 1.Long DA, Norman JT, Fine LG: Restoring the renal microvasculature to treat chronic kidney disease. Nat Rev Nephrol 8: 244–250, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Peired AJ, Sisti A, Romagnani P: Mesenchymal stem cell-based therapy for kidney disease: A review of clinical evidence. Stem Cells Int 2016: 4798639, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pleniceanu O, Omer D, Harari-Steinberg O, Dekel B: Renal lineage cells as a source for renal regeneration. Pediatr Res 83: 267–274, 2018 [DOI] [PubMed] [Google Scholar]
- 4.van den Berg CW, Ritsma L, Avramut MC, Wiersma LE, van den Berg BM, Leuning DG, et al.: Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Reports 10: 751–765, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pleniceanu O, Harari-Steinberg O, Omer D, Gnatek Y, Lachmi B-E, Cohen-Zontag O, et al.: Successful introduction of human renovascular units into the mammalian kidney. J Am Soc Nephrol 31: 2757–2772,2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dekel B, Burakova T, Arditti FD, Reich-Zeliger S, Milstein O, Aviel-Ronen S, et al.: Human and porcine early kidney precursors as a new source for transplantation. Nat Med 9: 53–60, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Harari-Steinberg O, Metsuyanim S, Omer D, Gnatek Y, Gershon R, Pri-Chen S, et al.: Identification of human nephron progenitors capable of generation of kidney structures and functional repair of chronic renal disease. EMBO Mol Med 5: 1556–1568, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harari-Steinberg O, Omer D, Gnatek Y, Pleniceanu O, Goldberg S, Cohen-Zontag O, et al.: Ex vivo expanded 3D human kidney spheres engraft long term and repair chronic renal injury in mice. Cell Rep 30: 852–869.e4, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Buzhor E, Harari-Steinberg O, Omer D, Metsuyanim S, Jacob-Hirsch J, Noiman T, et al.: Kidney spheroids recapitulate tubular organoids leading to enhanced tubulogenic potency of human kidney-derived cells. Tissue Eng Part A 17: 2305–2319, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Melero-Martin JM, De Obaldia ME, Kang S-Y, Khan ZA, Yuan L, Oettgen P, et al.: Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res 103: 194–202, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al.: Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Tasev D, Koolwijk P, van Hinsbergh VWM: Therapeutic potential of human-derived endothelial colony-forming cells in animal models. Tissue Eng Part B Rev 22: 371–382, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Casiraghi F, Perico N, Cortinovis M, Remuzzi G: Mesenchymal stromal cells in renal transplantation: Opportunities and challenges. Nat Rev Nephrol 12: 241–253, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Basile DP, Collett JA, Yoder MC: Endothelial colony-forming cells and pro-angiogenic cells: Clarifying definitions and their potential role in mitigating acute kidney injury. Acta Physiol (Oxf) 222: e12914, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]