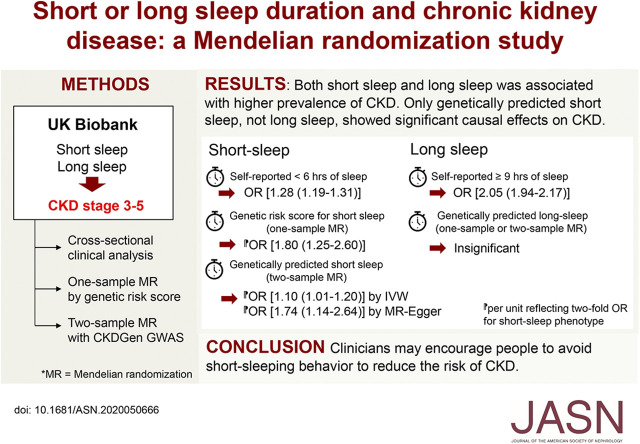
Significance Statement
Poor sleep is known to be related to kidney function impairment. Using the UK Biobank cohort, including individuals self-reporting regular sleep patterns of short, intermediate, or long duration, the authors found that short or long sleep duration was associated with higher prevalence of CKD. In the genetic analysis, the genetic risk score for short but not long sleep duration was significantly related to a higher risk of CKD stages 3–5, suggesting causal effects of short sleep duration on CKD. Two-sample Mendelian randomization analysis, using the independent meta-analysis results of kidney function from the CKDGen Consortium genome-wide association study, also showed significant causal estimates of short sleep duration on CKD. Clinicians may thus consider encouraging patients to avoid short-duration sleeping behavior to reduce the risk of CKD.
Keywords: sleep, chronic kidney disease, Mendelian randomization, genetic risk score, end-stage kidney disease
Visual Abstract
Abstract
Background
Studies have found sleeping behaviors, such as sleep duration, to be associated with kidney function and cardiovascular disease risk. However, whether short or long sleep duration is a causative factor for kidney function impairment has been rarely studied.
Methods
We studied data from participants aged 40–69 years in the UK Biobank prospective cohort, including 25,605 self-reporting short-duration sleep (<6 hours per 24 hours), 404,550 reporting intermediate-duration sleep (6–8 hours), and 35,659 reporting long-duration sleep (≥9 hours) in the clinical analysis. Using logistic regression analysis, we investigated the observational association between the sleep duration group and prevalent CKD stages 3–5, analyzed by logistic regression analysis. We performed Mendelian randomization (MR) analysis involving 321,260 White British individuals using genetic instruments (genetic variants linked with short- or long-duration sleep behavior as instrumental variables). We performed genetic risk score analysis as a one-sample MR and extended the finding with a two-sample MR analysis with CKD outcome information from the independent CKDGen Consortium genome-wide association study meta-analysis.
Results
Short or long sleep duration clinically associated with higher prevalence of CKD compared with intermediate duration. The genetic risk score for short (but not long) sleep was significantly related to CKD (per unit reflecting a two-fold increase in the odds of the phenotype; adjusted odds ratio, 1.80; 95% confidence interval, 1.25 to 2.60). Two-sample MR analysis demonstrated causal effects of short sleep duration on CKD by the inverse variance weighted method, supported by causal estimates from MR-Egger regression.
Conclusions
These findings support an adverse effect of a short sleep duration on kidney function. Clinicians may encourage patients to avoid short-duration sleeping behavior to reduce CKD risk.
CKD is an important comorbidity largely developed in those with hypertension or diabetes mellitus, and its prevalence is increasing with the global aging trend. As CKD is related to a large socioeconomic burden and cardiovascular diseases or even death, the identification and prevention of the causes of CKD are important medical issues.1,2
Sleep duration, along with other inappropriate sleeping behaviors such as sleep apnea or insomnia,3,4 has been reported to be associated with kidney function and the risk of cardiovascular diseases.5–8 Poor sleep, particularly a short sleep duration (<6 hours), has been reported to be a potential causal risk factor for myocardial infarction.8 In the nephrology field, cohort studies reported that short sleep duration was associated with a rapid decline in kidney function and that poor sleep habits were related to CKD progression.5–7 However, whether inappropriate sleep duration is a causative factor for kidney function impairment has rarely been studied, and performing a clinical trial to address this issue would be difficult. Evidence further suggesting the causal effects of inappropriate sleep duration on CKD is needed, as a causal effect of sleep on CKD would indicate that the burden of CKD could be ameliorated by promoting healthy sleep.
Mendelian randomization (MR) is a method that can demonstrate the causal effect of a modifiable environmental or lifestyle factor, predicted by a set of genetic instruments, on complex disease phenotypes.9 As genetic instruments are inherent, they are minimally affected by reverse causation or confounding effects, making MR possible to demonstrate causal effects. The method has been popularized in recent observational studies on the basis of the available large-scale population-based genetic data, which are extensively phenotyped.8,10–13
In this study, we aimed to demonstrate the association between inappropriate sleep duration, particularly short sleep duration, and CKD using UK Biobank data. We hypothesized that clinical and genetically predicted short or long sleep durations are associated with CKD, suggesting causal effects of inappropriate sleep duration on CKD development.
Methods
Ethics Considerations
The study was performed in accordance with the Declaration of Helsinki. The study was approved by the institutional review boards of Seoul National University Hospital (no. 2005–007–1120) and the UK Biobank Consortium (application no. 53799). The requirement for informed consent was waived as we used the anonymous database.
Study Setting
The study was an observational cohort study including clinical and genetic analyses using the data from the UK Biobank. The UK Biobank is a prospective population-based cohort constructed by contacting 9 million people via mail until it finally included >500,000 participants aged 40–69 years from 2006 to 2010 in the United Kingdom. The study participants underwent clinical interviews, anthropometric evaluations, and laboratory measurements and answered questionnaires in 22 assessment centers throughout the nation. The database has been recently utilized to reveal various clinical and genomic findings, although a healthy volunteer bias was present in the cohort.14 The details of the database have been described previously.15
Study Population
For the clinical analysis, we included participants with available information for self-reports for sleep duration and baseline laboratory assessment of the eGFR values, and those who were missing the essential information were excluded.
For the genetic analysis, participants of White British ancestry passing the basic quality control filter were included. Those who were outliers in terms of heterozygosity or missing rate, those with sex chromosome aneuploidy, and those with excess kinship were excluded on the basis of the information determined by the UK Biobank Consortium. In addition, those with missing eGFR values were excluded, as the information was necessary to determine the study outcome.
Sleep Duration Exposure
A standardized touch-screen questionnaire asked, “About how many hours sleep do you get in every 24 hours? (please include naps).” It was used to collect the sleep duration in hours. In the clinical analysis, we stratified sleep duration into short (<6 hours), intermediate (6–8 hours), and long (≥9 hours) groups.5,8 Regarding the short sleep definition, the definition was more stringent than the recommendation, suggesting 7–8 hours of sleep as the recommended duration.16 Nevertheless, the recommendation also suggested <6 hours of sleep as inappropriate sleep; thus, we selected <6 hours as the definition for short sleep in the clinical analysis to clearly include those who sleep short despite implementing self-reported sleep duration.
Kidney Function Outcomes
First, prevalent CKD stages 3–5 were defined by an eGFR<60 ml/min per 1.73 m2 or the presence of a prevalent history of ESKD at baseline. The Chronic Kidney Disease Epidemiology Collaboration equation was used to calculate the eGFR values on the basis of creatinine levels measured by the standard enzymatic method.17 Second, as a supplemental outcome, we also assessed limiting the CKD stages 3–5 outcome to CKD with eGFR≥45 ml/min per 1.73 m2, as profound kidney function impairment itself can reversibly cause sleep disturbance.18 In the analysis, those with lower eGFR (<45 ml/min per 1.73 m2) were not included, and we included those with CKD with eGFR≥45 ml/min per 1.73 m2 (patients) or without CKD stages 3–5 (controls). Third, in the clinical analysis, we also investigated incident ESKD as an outcome, with the additional exclusion of those with baseline CKD stage 5 determined by an eGFR<15 ml/min per 1.73 m2 or a history of prevalent ESKD. The ESKD outcome has been algorithmically defined by the UK Biobank from self-reports and hospital admission records. We censored the follow-up for the ESKD outcome at February 29, 2016, as complete hospital records were available until that date in all three regions when this study was performed. As the total number of individuals with incident ESKD was relatively small compared with the number of CKD events and no external genome-wide association study (GWAS) source was available for the outcome, this outcome was not included in the genetic analysis.
Collection of Covariates
The collected covariates included age; sex; spot urine microalbumin-creatinine ratio; body mass index; waist circumference; smoking history (nonsmoker, ex-smoker, or current smoker); cardiovascular disease history (angina, heart attack, or stroke); hypertension; systolic BP; diastolic BP; diabetes mellitus; dyslipidemia; frequency of moderate physical activity per week; and the values of LDL, HDL, and total cholesterol. Self-reported snoring (yes or no) and napping (never/rarely, sometimes, usually) were collected as covariates for other sleep habits. The details regarding the covariates are described in Supplemental Material.
Statistical Methods for Clinical Analyses
Descriptive statistics are presented as numbers (percentages) for categorical variables and medians (interquartile ranges) for continuous variables. A generalized additive model was used to plot the association between self-reported sleep duration and the age- and sex-adjusted odds for CKD stages 3–5. The cross-sectional association of sleep duration with CKD outcomes was investigated by logistic regression analysis. When analyzing incident ESKD, Cox regression analysis was used, excluding those with previous ESKD history or an eGFR<15 ml/min per 1.73 m2. For regression analyses, univariable and stepwise multivariable models were constructed. The first multivariable model was less stringent and adjusted for age; sex; other sleep behaviors, including napping or snoring; and smoking history. The second multivariable model was adjusted for wide ranges of variables, although some of them may be mediators, to ask about a more direct association between sleep duration and outcomes. The details regarding the adjustment variables are described in Supplemental Material. Subgroup analyses were performed stratified by sex, as the odds for CKD were significantly different according to the variable and on the basis of an interaction analysis result (interaction term P=0.004). Because missing information was present in the clinical dataset, as described in Supplemental Material, the multivariable regression analyses were first performed with multiple imputation by the chained equation method, which has strength when imputing a complex large dataset.19 In addition, the results from the complete-case method are presented, not including those with missing information in the covariates. All clinical statistical analyses were performed using R (version 3.6.2; the R Foundation), and two-sided P=0.05 were considered significant.
Genetic Instrument
We implemented a set of genetic instruments reported in a previous study to genetically predict short sleep or long sleep.20 In brief, a GWAS was performed with imputed genotype data provided by the UK Biobank regarding the phenotypes of sleep duration <7 hours and sleep duration ≥9 hours. In total, 27 single-nucleotide polymorphisms (SNPs) in independent loci that were significantly associated with a short duration of sleep and eight SNPs that were associated with a long duration of sleep were reported (Supplemental Table 1). The study found that the SNPs were significantly associated with sleep duration that was objectively estimated by an accelerometer. Although the short-sleep genetic instrument was developed with the short-sleep trait defined by different thresholds (<7 hours), as the genetic instrument was also strongly associated with phenotypical sleep duration <6 hours and was validated by accelerometry results to reflect shorter sleep duration, we utilized the genetic instrument in this study. The instrument was previously implemented to show causal estimates from short sleep duration on the risk of myocardial infarction.8
One-Sample MR
First, we performed genetic risk score (GRS)–based one-sample MR analysis with the individual data of the UK Biobank. We calculated GRSs for short and long sleep durations by multiplying the gene dosage matrix with the effect sizes of the GWAS, the regressed β, by using PLINK 2.0 (version α2.3).21 The GRSs were scaled to a unit reflecting two-fold odds of the phenotypic exposure of interest. We analyzed the variance explained by the GRSs for the exposure phenotypes by calculating the Effron pseudo-R2 values.22 The associations between the calculated GRS, per unit reflecting two-fold odds for either phenotypical short sleep or long sleep, and the study outcomes were tested by logistic regression analysis for the binary CKD outcomes. The association was adjusted for age, sex, genotype measurement batch, and the first ten principal components of the genetic information. As a sensitivity analysis, we recalculated the GRS after disregarding SNPs that were in genome-wide significant (P<5×10-8) association with possible major confounders, including hypertension, diabetes, obesity, and current smoking, in logistic regression models adjusted for age, sex, and the 20 principal components. Another sensitivity analysis was performed to demonstrate the direct effects of exposures of interest by additionally adjusting phenotypical covariates, including obesity, hypertension, diabetes, and smoking history. Furthermore, we asked whether the SNPs included in the genetic instrument showed a significant direct association with the CKD outcome by GWAS, adjusted for age, sex, and the first 20 principal components, to identify whether an individual SNP might have had a disproportionate effect on the overall results.
Two-Sample MR
We further asked whether our findings can be extended to an independent cohort by performing two-sample MR analysis in which outcome information is introduced from external GWAS summary statistics. Two-sample MR is less prone to false-positive bias, which is possible in one-sample MR analysis.23 The main summary statistics for the two-sample MR were implemented from the independent CKDGen GWAS meta-analysis. The public results for 567,460 individuals of independent European ancestry were provided by the Chronic Kidney Disease Genetics (CKDGen) Consortium (https://ckdgen.imbi.uni-freiburg.de/).24 Palindromic SNPs with intermediate allele frequencies were disregarded,25 and the overlapping 25 SNPs and seven SNPs among the genetic instrument for short sleep and long sleep, respectively, were utilized as the genetic instrument for the two-sample MR. The main method for two-sample MR was the conventional fixed effect inverse variance method as in a prior study.8 Causal estimates from the maximum likelihood method were also tested.26 As the above methods can be biased from directional pleiotropy, MR-Egger regression, which can yield causal estimates that are robust to pleiotropy, was performed as a sensitivity analysis with the tests for directional pleiotropy.27 Furthermore, the weighted median method, which provides consistent estimates even when an invalid instrument is present, was implemented as another sensitivity analysis.28 The two-sample MR was repeated after excluding SNPs that were in genome-wide significant (P<0.001) association with possible major confounders as the one-sample MR. Leave-one-out analysis by MR-Egger or the fixed effects inverse variance method was performed to ask whether the overall results were driven from an individual variant.
The causal estimates were scaled to the increase in the odds of an outcome per doubling of the odds of exposure of interest by multiplying the regressed β values by 0.693 in the two-sample MR as previously described.29
Results
Study Population
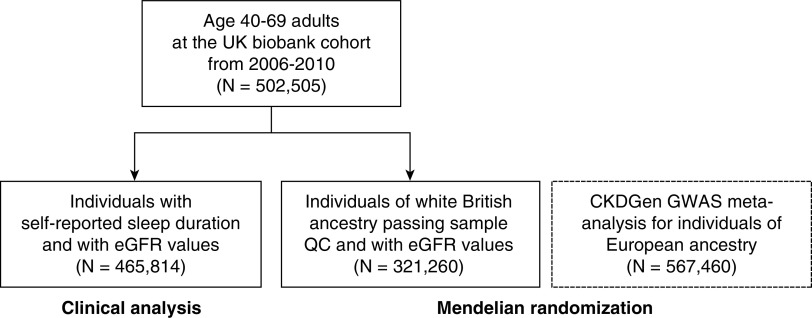
Among a total of 502,505 UK Biobank participants, 36,691 individuals were excluded due to lack of self-reported sleep information or eGFR values, resulting in 465,814 individuals included in the clinical analysis (Figure 1). Among them, 25,605, 404,550, and 35,659 individuals reported short (<6 hours), intermediate (6–8 hours), and long (≥9 hours) sleep durations, respectively.
Figure 1.
Study population. The study was an observational cohort study including clinical and genetic analyses using the data from the UK Biobank. The observational analysis was performed within the UK Biobank participants who self-reported sleep duration and had eGFR measurements (N = 465,814). The Mendelian randomization analysis was first performed for the individuals of white British ancestry passing sample quality control and with eGFR values (N = 321,260). The findings were extended to an independent genome-wide association study meta-analysis results by the CKDGen consortium (N = 567,460). QC, quality control.
In the genetic analysis, 337,138 participants of White British ancestries passed the sample quality control step. Among them, 321,260 participants with baseline eGFR values were included in the investigation of the association of the GRS with CKD.
Clinical Characteristics
Baseline characteristics stratified by sleep duration are presented in Table 1. Approximately 30%–40% of the participants reported snoring, which was more common in those with a long sleep duration. Napping was also particularly common in the long-sleep duration group. Current smokers were more likely to report a short sleep duration, but a higher median frequency of moderate physical activity was also identified in that group. Those with an intermediate sleep duration had the lowest prevalence of obesity or central obesity. Both the short-sleep duration and long-sleep duration groups reported relatively higher prevalence rates of cardiovascular disease, hypertension, and diabetes mellitus when compared with the intermediate-sleep duration group.
Table 1.
Baseline characteristics according to self-reported sleep duration
| Characteristics | Short Sleep, <6 h | Intermediate Sleep, 6–8 h | Long Sleep, ≥9 h |
|---|---|---|---|
| No. of participants | 25,605 | 404,550 | 35,659 |
| Sleep habits | |||
| Sleep duration, h | 5 [5–5] | 7 [7–8] | 9 [9–9] |
| Reported snoring | 7711 (34%) | 140,366 (37%) | 13,368 (40%) |
| Reported napping | |||
| Never/rarely | 14,467 (57%) | 234,724 (58%) | 12,596 (35%) |
| Sometimes | 9712 (38%) | 151,883 (38%) | 16,979 (48%) |
| Usually | 1338 (5%) | 17,405 (4%) | 6029 (17%) |
| Age, yr | 58 [51–64] | 58 [50–64] | 61 [53–66] |
| Men | 11,265 (44%) | 186,640 (46%) | 15,607 (44%) |
| Smoking | |||
| Nonsmoker | 12,970 (51%) | 222,996 (55%) | 18,033 (51%) |
| Ex-smoker | 8550 (34%) | 139,550 (35%) | 13,214 (37%) |
| Current smoker | 3943 (15%) | 40,610 (10%) | 4261 (12%) |
| Frequency of moderate physical activity, d/wk | 4 [2–6] | 3 [2–5] | 3 [2–5] |
| Body mass index, kg/m2 | 27.8 [24.7–31.4] | 26.6 [24.1–29.7] | 27.4 [24.6–30.8] |
| Obese, ≥30 | 8450 (33%) | 93,676 (23%) | 10,528 (30%) |
| Waist circumference, cm | 92 [82–102] | 89 [80–98] | 92 [82–101] |
| Central obesity, ≥102 for men; ≥96 for women | 10,749 (42%) | 130,295 (32%) | 14,668 (41%) |
| Cardiovascular disease history | 2413 (9%) | 20,846 (5%) | 3490 (10%) |
| Hypertension, mm Hg | 6614 (26%) | 80,267 (20%) | 10,039 (28%) |
| Systolic BP | 137 [126–150] | 136 [125–149] | 138 [126–151] |
| Diastolic BP | 83 [76–90] | 82 [75–89] | 82 [76–89] |
| Diabetes mellitus | 2009 (8%) | 19,034 (5%) | 3127 (9%) |
| Hemoglobin A1c, mmol/mol | 35.9 [33.3–38.8] | 35.1 [32.7–37.7] | 35.6 [33.1–38.7] |
| Cholesterol, mmol/L | |||
| Total cholesterol | 5.62 [4.84–6.43] | 5.66 [4.92–6.42] | 5.60 [4.79–6.44] |
| LDL cholesterol | 3.50 [2.89–4.12] | 3.52 [2.95–4.12] | 3.48 [2.87–4.13] |
| HDL cholesterol | 1.37 [1.14–1.66] | 1.40 [1.18–1.68] | 1.36 [1.14–1.63] |
| Kidney function variables | |||
| eGFR, ml/min per 1.73 m2 | 92.9 [82.6–100.2] | 92.6 [82.9–99.8] | 90.6 [79.5–98.0] |
| Urine microalbumin-creatinine ratio, mg/g | 9.52 [5.97–16.39] | 9.77 [6.12–16.49] | 9.73 [6.14–16.62] |
| Prevalent CKD stages 3–5 | 713 (3%) | 8830 (2%) | 1560 (4%) |
Clinical Analyses
There were 713 (3%), 8830 (2%), and 1560 (4%) individuals with prevalent CKD stages 3–5 in the short-, intermediate-, and long-sleep duration groups, respectively. The association between the prevalence of CKD and sleep duration was U shaped (Supplemental Figure 1), and those with shorter or longer sleep durations had a higher prevalence of CKD stages 3–5 when compared with those with an intermediate duration of sleep (Table 2). The results were similar when the models were adjusted for age, sex, smoking history, habitual napping, and snoring, and the odds of prevalent CKD stages 3–5 were significantly higher in the short-sleep or long-sleep group than in the intermediate-sleep group in both sexes. With additional adjustment, including metabolic parameters and the frequency of moderate to vigorous physical activity, the association between short sleep and higher odds for CKD stages 3–5 became insignificant only in women. On the other hand, a long sleep duration was significantly associated with a higher prevalence of CKD in both sexes even after adjusting for the multiple covariates.
Table 2.
Results from the cross-sectional analysis for CKD outcomes and survival analysis for incident ESKD outcome
| Outcome and Exposurea | Univariable Model | Multivariable Model 1b | Multivariable Model 2c | |||
|---|---|---|---|---|---|---|
| OR or HR (95% CI) | P Value | Adjusted OR or HR (95% CI) | P Value | Adjusted OR or HR (95% CI) | P Value | |
| Total participants, n=465,814 | ||||||
| CKD stages 3–5 (N of patients =11,103) | ||||||
| Short sleep, <6 h | 1.28 (1.19 to 1.39) | <0.001 | 1.27 (1.17 to 1.37) | <0.001 | 1.07 (0.99 to 1.16) | 0.08 |
| Long sleep, ≥9 h | 2.05 (1.94 to 2.17) | <0.001 | 1.50 (1.42 to 1.59) | <0.001 | 1.36 (1.28 to 1.44) | <0.001 |
| CKD with eGFR≥45 (N of patients =9184) | ||||||
| Short sleep, <6 h | 1.21 (1.11 to 1.32) | <0.001 | 1.19 (1.09 to 1.29) | <0.001 | 1.03 (1.21 to 1.38) | 0.56 |
| Long sleep, ≥9 h | 1.90 (1.79 to 2.02) | <0.001 | 1.41 (1.33 to 1.51) | <0.001 | 1.29 (1.12 to 1.38) | <0.001 |
| Incident ESKD (N of patients =404) | ||||||
| Short sleep, <6 h | 1.51 (1.04 to 2.19) | 0.03 | 1.41 (0.97 to 2.06) | 0.07 | 1.23 (0.85 to 0.80) | 0.28 |
| Long sleep, ≥9 h | 2.02 (1.52 to 2.69) | <0.001 | 1.24 (0.92 to 1.66) | 0.16 | 1.13 (0.84 to 1.52) | 0.41 |
| Participants who were men, n=213,512 | ||||||
| CKD stages 3–5 (N of patients =5208) | ||||||
| Short sleep, <6 h | 1.27 (1.13 to 1.42) | <0.001 | 1.38 (1.22 to 1.55) | <0.001 | 1.16 (1.03 to 1.31) | 0.02 |
| Long sleep, ≥9 h | 2.33 (2.15 to 2.52) | <0.001 | 1.53 (1.42 to 1.66) | <0.001 | 1.36 (1.25 to 1.48) | <0.001 |
| CKD with eGFR≥45 (N of patients =4175) | ||||||
| Short sleep, <6 h | 1.19 (1.04 to 1.36) | 0.01 | 1.30 (1.14 to 1.49) | <0.001 | 1.12 (0.98 to 1.28) | 0.10 |
| Long sleep, ≥9 h | 2.18 (2.00 to 2.39) | <0.001 | 1.44 (1.32 to 1.58) | <0.001 | 1.30 (1.19 to 1.43) | <0.001 |
| Incident ESKD (N of patients =259) | ||||||
| Short sleep, <6 h | 2.00 (1.30 to 3.09) | 0.002 | 1.94 (1.25 to 2.99) | 0.003 | 1.72 (1.11 to 2.67) | 0.01 |
| Long sleep, ≥9 h | 2.73 (1.96 to 3.80) | <0.001 | 1.61 (1.14 to 2.28) | 0.007 | 1.51 (1.07 to 2.14) | 0.02 |
| Participants who were women, n=252,302 | ||||||
| CKD stages 3–5 (N of patients n=5895) | ||||||
| Short sleep, <6 h | 1.30 (1.17 to 1.44) | <0.001 | 1.19 (1.07 to 1.32) | 0.001 | 1.02 (0.92 to 1.13) | 0.71 |
| Long sleep, ≥9 h | 1.83 (1.70 to 1.98) | <0.001 | 1.47 (1.35 to 1.59) | <0.001 | 1.34 (1.24 to 1.46) | <0.001 |
| CKD with eGFR≥45 (N of patients n=5009) | ||||||
| Short sleep, <6 h | 1.22 (1.08 to 1.36) | 0.001 | 1.11 (0.99 to 1.25) | 0.07 | 0.97 (0.86 to 1.09) | 0.62 |
| Long sleep, ≥9 h | 1.69 (1.55 to 1.84) | <0.001 | 1.38 (1.26 to 1.50) | <0.001 | 1.28 (1.17 to 1.40) | <0.001 |
| Incident ESKD (N of patients =145) | ||||||
| Short sleep, <6 h | 0.86 (0.40 to 1.84) | 0.69 | 0.80 (0.37 to 1.72) | 0.57 | 0.68 (0.31 to 1.47) | 0.32 |
| Long sleep, ≥9 h | 1.13 (0.64 to 2.01) | 0.67 | 0.74 (0.41 to 1.35) | 0.33 | 0.66 (0.36 to 1.21) | 0.18 |
Pooled results after multiple imputation by the chained equation method are presented. eGFR is in milliliters per minute per 1.73 m2. OR, odds ratio; HR, hazard ratio; 95% CI, 95% confidence interval.aThe reference group for the logistic regression and Cox regression analysis was the intermediate-sleep group (6–8 h of sleep). The CKD outcomes were analyzed by logistic regression analysis. In the analysis for CKD with eGFR≥45 ml/min per 1.73 m2 outcome, those with lower eGFR (<45 ml/min per 1.73 m2) were not included (n=1919), and we included those with CKD with ≥45 ml/min per 1.73 m2 (patients) or without CKD (controls) to limit the CKD outcome to those without profoundly reduced eGFR. The incident ESKD outcome was analyzed by Cox regression analysis, excluding 581 participants with eGFR<15 ml/min per 1.73 m2 or prevalent ESKD history.
Multivariable model 1 for CKD outcome was adjusted for age, sex, smoking history (nonsmoker, ex-smoker, or current smoker), habitual naps (never/rarely, sometimes, or usually), or snoring. When analyzing the incident ESKD outcome, the baseline eGFR and urine microalbumin-creatinine ratio were added to the model.
Multivariable model 2 for CKD outcome was adjusted for age, sex, body mass index, waist circumference, smoking history (nonsmoker, ex-smoker, or current smoker), frequency of moderate physical activity per week (days), previous history of cardiovascular disease (angina, heart attack, or stroke), hypertension, systolic BP, diastolic BP, diabetes mellitus, hemoglobin A1c, total cholesterol, LDL, HDL, and self-reported habitual naps (never/rarely, sometimes, or usually) or snoring. When analyzing the incident ESKD outcome, age, sex, baseline eGFR, urine microalbumin-creatinine ratio, body mass index, smoking history, history of cardiovascular disease, hypertension, diabetes mellitus, and habitual naps or snoring were adjusted for the multivariable model.
After excluding 581 participants with eGFR<15 ml/min per 1.73 m2 or a previous ESKD history, we analyzed incident ESKD. During a median of 7.1 years of follow-up, 404 individuals developed ESKD: 259 men and 145 women (Figure 2, Table 2). The risk of incident ESKD showed an insignificant association with sleep duration groups in the multivariable models when both sexes were included. When the analysis was limited to participants who were men, both short and long sleep durations were associated with approximately two-fold hazards of incident ESKD when compared with a sleep duration of 6–8 hours in the univariable model. Within the men, the significance between the short or long sleep duration with a higher risk of incident ESKD remained even in the stringently adjusted multivariable model. However, for participants who were women, given the relatively low number of identified participants with incident ESKD, none of the sleep duration groups had a significantly different risk of ESKD compared with the intermediate-sleep duration group. The overall results were similar when the analyses were performed by the complete-case method (Supplemental Table 2).
Figure 2.
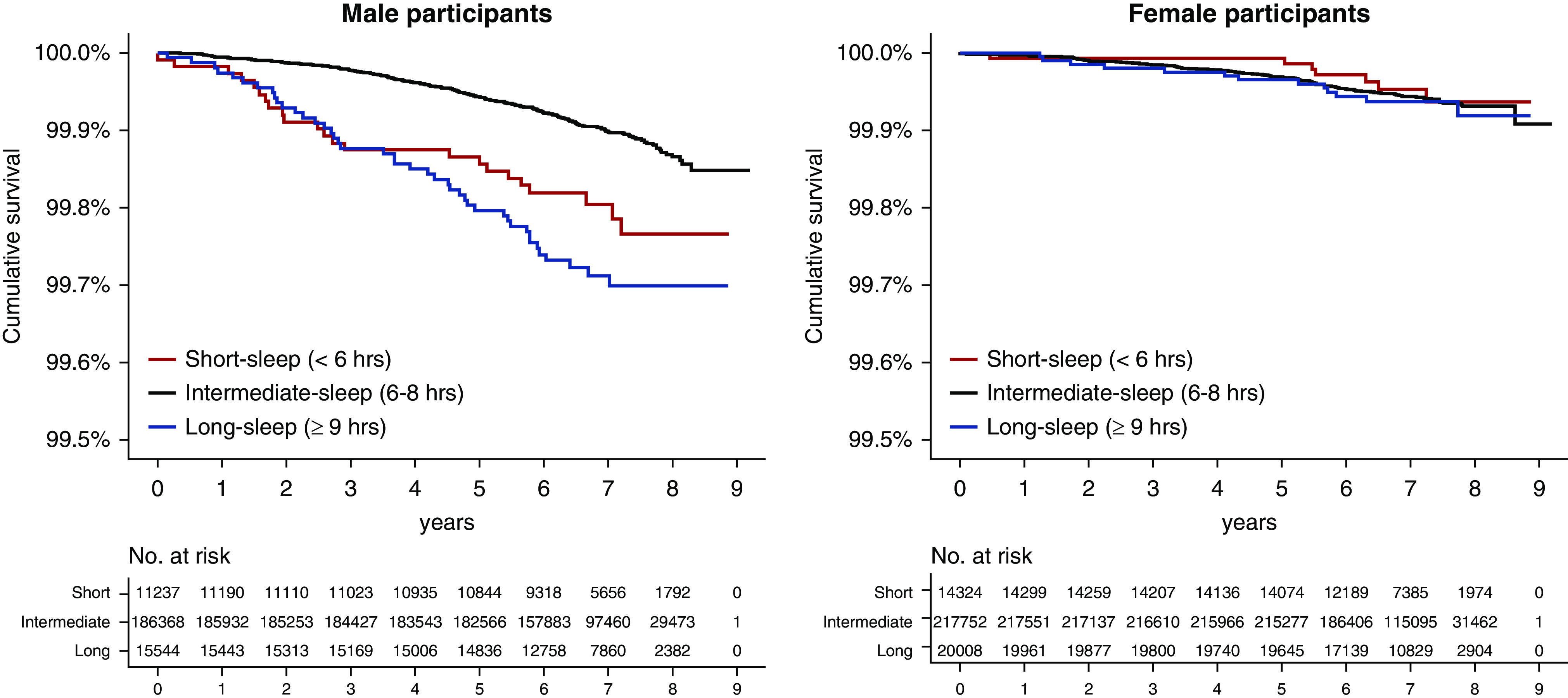
Kaplan–Meier survival curves for incident ESKD outcome showed that inappropriate sleep duration was associated with higher ESKD risk, particularly in men. The x axes indicate time (years), and the y axes indicate cumulative ESKD-free survival (percentages). The survival tables showing the number at risk are presented below the graphs.
One-Sample MR Results
The GRSs for short sleep and long sleep durations were significantly associated with the short sleep duration (<6 hours) and long sleep duration (≥9 hours) phenotypes (P<0.001), respectively, explaining 0.8% of risks for each phenotype.
The GRS for short sleep was significantly associated with both CKD stages 3–5 and CKD with an eGFR≥45 ml/min per 1.73 m2 (Table 3). A GRS unit reflecting a two-fold increase in odds for short sleep (<6 hours) was associated with a nearly two-fold odds of CKD stages 3–5 (adjusted odds ratio, 1.80; 95% confidence interval, 1.25 to 2.60; P=0.002). The direction of the results remained similar to the sensitivity analysis excluding the confounder-associated SNPs (four SNPs among the short-sleep genetic instrument) (Supplemental Table 3) or additional adjustment for the possible phenotypical confounders. The significant association with the short-sleep GRS and CKD was again identified when the analysis was limited to each sex. On the other hand, the GRS for a long sleep duration was not significantly associated with CKD outcomes.
Table 3.
Risk of CKD outcomes according to GRSs for short sleep duration or long sleep duration
| Exposure, Subgroups, and Outcomes | Main Analysisa | Sensitivity Analysis 1b | Sensitivity Analysis 2c | |||
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | |
| GRS for short sleep duration | ||||||
| Total genotyped (n=321,260) | ||||||
| CKD stages 3–5 | 1.80 (1.25 to 2.60) | 0.002 | 1.92 (1.25 to 2.96) | 0.003 | 1.73 (1.20 to 2.52) | 0.004 |
| CKD with eGFR≥45d | 1.88 (1.26 to 2.79) | 0.002 | 2.04 (1.27 to 3.27) | 0.003 | 1.83 (1.22 to 2.74) | 0.003 |
| Men genotyped (n=148,836) | ||||||
| CKD stages 3–5 | 1.77 (1.04 to 3.01) | 0.04 | 1.57 (0.84 to 2.95) | 0.16 | 1.76 (1.02 to 3.03) | 0.04 |
| CKD with eGFR≥45d | 1.91 (1.06 to 3.45) | 0.03 | 1.76 (0.87 to 3.53) | 0.11 | 1.89 (1.03 to 3.44) | 0.04 |
| Women genotyped (n=172,424) | ||||||
| CKD stages 3–5 | 1.85 (1.12 to 3.05) | 0.02 | 2.33 (1.29 to 4.22) | 0.005 | 1.76 (1.06 to 2.92) | 0.03 |
| CKD with eGFR≥45d | 1.87 (1.09 to 3.21) | 0.02 | 2.36 (1.24 to 4.47) | 0.009 | 1.83 (1.06 to 3.15) | 0.03 |
| GRS for long sleep duration | ||||||
| Total genotyped (n=321,260) | ||||||
| CKD stages 3–5 | 1.15 (0.73 to 1.81) | 0.56 | 1.24 (0.74 to 2.06) | 0.42 | 1.16 (0.73 to 1.85) | 0.53 |
| CKD with eGFR≥45d | 1.03 (0.63 to 1.70) | 0.90 | 1.10 (0.63 to 1.93) | 0.74 | 1.09 (0.66 to 1.81) | 0.75 |
| Men genotyped (n=148,836) | ||||||
| CKD stages 3–5 | 0.88 (0.45 to 1.73) | 0.71 | 0.78 (0.36 to 1.67) | 0.52 | 0.95 (0.47 to 1.89) | 0.88 |
| CKD with eGFR≥45d | 0.84 (0.40 to 1.79) | 0.65 | 0.67 (0.29 to 1.57) | 0.36 | 0.95 (0.44 to 2.03) | 0.89 |
| Women genotyped (n=172,424) | ||||||
| CKD stages 3–5 | 1.44 (0.78 to 2.68) | 0.25 | 1.83 (0.92 to 3.67) | 0.09 | 1.38 (0.74 to 2.60) | 0.31 |
| CKD with eGFR≥45d | 1.23 (0.63 to 2.40) | 0.55 | 1.64 (0.78 to 3.47) | 0.20 | 1.22 (0.62 to 2.41) | 0.56 |
eGFR is in milliliters per minute per 1.73 m2. OR, odds ratio; 95% CI, 95% confidence interval.
Main analysis results were adjusted for age, sex, genotype measurement batch, and the first ten principal components of the genetic information.
Sensitivity analysis 1 was performed with recalculated GRSs after excluding SNPs that were in genome-wide significant (P<0.001) association with hypertension, diabetes, obesity, or current smoking from the genetic instruments. The process excluded four SNPs (rs13107325, rs2820313, rs2014830, and rs1176350) from the genetic instrument for short sleep and one SNP (rs17817288) from the genetic instrument for long sleep.
Sensitivity analysis 2 was performed by adding phenotypical hypertension, diabetes, obesity, and smoking history to the main analysis model. The analysis was performed with 316,688 individuals (146,280 men and 170,408 women) with phenotypic information in the covariates.
In the analysis for CKD with eGFR≥45 ml/min per 1.73 m2 outcome, those with lower eGFR (<45 ml/min per 1.73 m2) were not included (n=1276), and we included those with CKD with ≥45 ml/min per 1.73 m2 or without CKD to limit the CKD outcome to those without profoundly reduced eGFR.
The SNPs in the genetic instruments were not directly associated with CKD stages 3–5, as no SNPs reached the Bonferroni-adjusted P value (< 0.05/27 for SNPs associated with short sleep duration and <0.05/8 for SNPs associated with long sleep duration), which implies that an individual SNP did not disproportionally drive the overall results (Supplemental Table 4).
Two-Sample MR Results
When the analysis was extended to the independent CKDGen GWAS meta-analysis,24 positive causal estimates from short sleep on CKD were identified by the inverse variance weighted and maximal likelihood methods (Figure 3, Table 4). The MR-Egger regression pleiotropy test suggested possible directional pleiotropy (P=0.04); however, MR-Egger regression results, robust for such pleiotropy effects, showed even higher odds for CKD in those genetically predisposed for short sleep. The weighted median method yielded supportive, although marginal (odds ratio, 1.10; 95% confidence interval, 0.98 to 1.25; P=0.11), causal estimates for CKD. The β values from the leave-one-out analysis, ranging from 0.070 to 0.119 by the inverse variance weighted method, remained similar to the main analysis (Supplemental Table 5). The causal estimates, with larger β values, were mostly significant when MR-Egger regression was the method for the leave-one-out analysis. The significant causal estimates were again identified when we excluded SNPs that were strongly associated with the possible major confounders by the inverse variance weighted or maximal likelihood method (Table 4). After the process, the MR-Egger pleiotropy test indicated insignificant directional pleiotropy (P=0.12), although the significance of the causal estimates from the MR-Egger regression (odds ratio, 1.63; 95% confidence interval, 1.00 to 2.64; P=0.06) or weighted median method (odds ratio, 1.08; 95% confidence interval, 0.94 to 1.23; P=0.27) was marginal. On the other hand, the two-sample MR results with the long-sleep genetic instrument showed null findings by the performed MR investigation.
Figure 3.
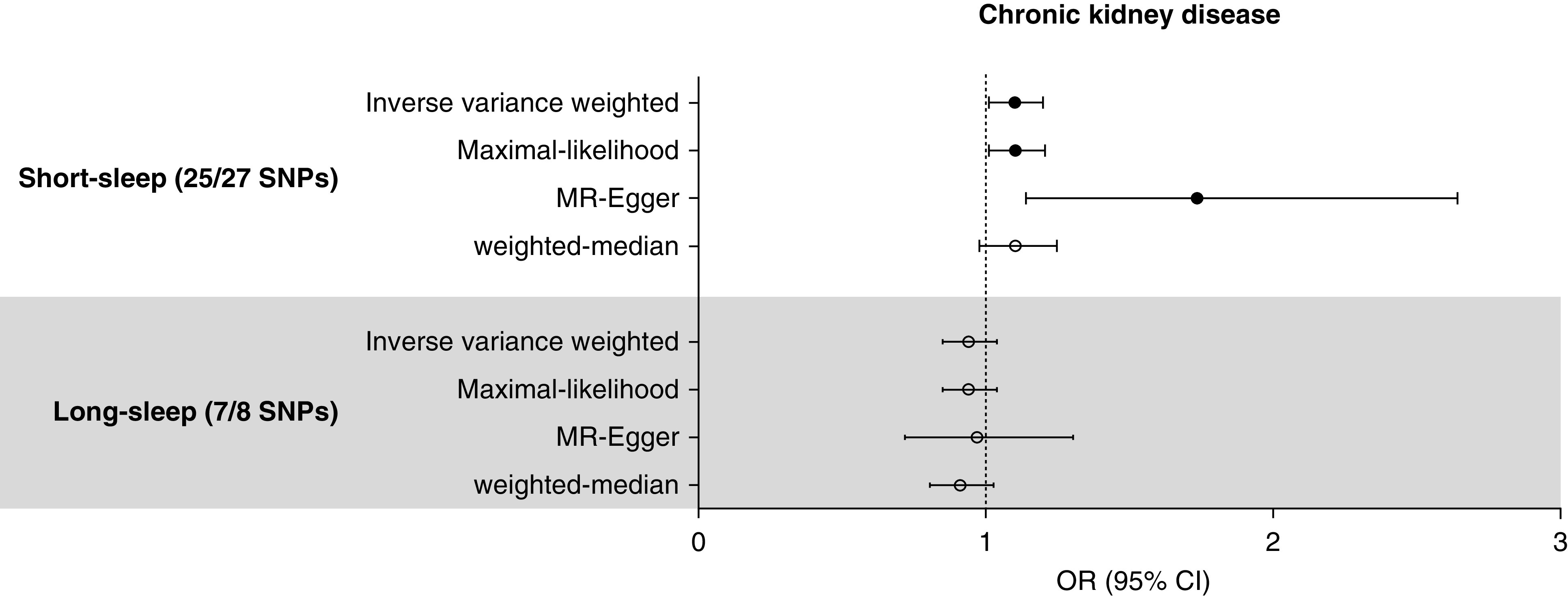
Two-sample MR analysis results supported the causal effects of short sleep duration on CKD. The genetic instrument included 25 of 27 and seven of eight SNPs for short-sleep and long-sleep traits, respectively. The causal estimates were derived from the CKDGen meta-analysis summary statistics for CKD from subjects of European ancestry. The estimates were multiplied by 0.693 to show the causal effects of a two-fold increase in odds for exposure phenotypes. The circles indicate the odds ratios (OR), and error bars indicate the 95% confidence interval (95% CI). The black circles indicate that the causal estimates were significant.
Table 4.
Causal estimates from the two-sample MR analysis
| Exposure and Methods | With Available SNPsa | After Excluding Confounder-Associated SNPsb | ||||
|---|---|---|---|---|---|---|
| βc | SEMc | P Value | βc | SEMc | P Value | |
| Short sleep | ||||||
| Inverse variance weighted | 0.096 | 0.044 | 0.03 | 0.101 | 0.048 | 0.04 |
| Maximum likelihood | 0.099 | 0.045 | 0.03 | 0.103 | 0.049 | 0.04 |
| MR-Eggerd | 0.552 | 0.214 | 0.02 | 0.490 | 0.245 | 0.06 |
| Weighted median | 0.099 | 0.062 | 0.11 | 0.076 | 0.068 | 0.27 |
| Long sleep | ||||||
| Inverse variance weighted | −0.062 | 0.051 | 0.22 | −0.054 | 0.051 | 0.31 |
| Maximum likelihood | −0.063 | 0.051 | 0.22 | −0.054 | 0.051 | 0.31 |
| MR-Eggerd | −0.032 | 0.152 | 0.06 | −0.093 | 0.152 | 0.65 |
| Weighted median | −0.095 | 0.062 | 0.13 | −0.095 | 0.062 | 0.15 |
Among the short-sleep genetic instruments, rs2186122 and rs9367621 were not included because they were palindromic SNPs with intermediate allele frequency. For long-sleep genetic instruments, one SNP, rs549961083, was not included in the genetic instrument because the estimates of the SNP were not presented in the independent CKDGen GWAS summary statistics.
The analysis was performed after excluding SNPs that were in genome-wide significant (P<0.001) association with hypertension, diabetes, obesity, or current smoking from the genetic instruments. The process excluded four SNPs (rs13107325, rs2820313, rs2014830, and rs1176350) from the genetic instrument for short sleep and one SNP (rs17817288) from the genetic instrument for long sleep.
The causal estimates were scaled to the increase in the odds of an outcome per doubling of the odds of exposure of interest by multiplying the coefficients by 0.693.
The MR-Egger test for directional pleiotropy indicated significant pleiotropy (P=0.04) in the main analysis of short sleep. In the sensitivity analysis, the test indicated insignificant pleiotropy (P=0.12). On the other hand, the results from the pleiotropy test with the genetic instrument for long sleep did not show significant P values in the main analysis (P=0.84) or in the sensitivity analysis (P=0.84).
Discussion
In this study, including both clinical and genetic analyses, associations between sleep durations other than intermediate sleep duration and CKD were investigated. In the clinical analysis, durations of sleep that were either <6 or ≥9 hours were associated with a higher prevalence of CKD. Short sleep duration was particularly associated with the risk of incident ESKD outcome when the analysis was limited to participants who were men. In the genetic analysis, genetically predicted short sleep duration was significantly related to higher odds for CKD, and the results were replicated with the independent GWAS summary statistics for CKD outcome. As the clinical results are supported by the analysis using inborn genetic instruments, which are less influenced by confounding or reverse causation, our study supports causal effects of short sleep duration on CKD.
Previous studies reported an association between poor sleep and kidney function. A previous study including over 4000 women reported that a sleep duration <6 hours was significantly associated with higher odds of experiencing a rapid eGFR decline.5 Two previous studies including patients with CKD reported that poor sleep was associated with CKD progression or the risk of ESKD.6,7 Our study results support those findings, mainly focusing on short sleep duration, and causal effects of short sleep duration on CKD have been demonstrated. The strengths of our study are that we showed a clinical association between sleep duration and the prevalence of CKD in a large-scale general population cohort, assessed hard outcomes such as CKD or ESKD, and performed MR analysis to suggest causal effects of short sleep duration on CKD.
An MR study needs to fulfill three assumptions to reveal causal effects of modifiable environmental factors on complex diseases: (1) the genetic instrument should be strongly associated with the assessed exposure phenotypes (the relevance assumption), (2) the genetic instrument should affect the risk of disease only through the exposure phenotype (the exclusion restriction assumption), and (3) the genetic instrument should be independent of confounders and horizontal pleiotropy (the independence assumption).9 The genetic instrument used in this study was reported to be strongly associated with the sleep duration phenotypes, both self-reported and objectively measured,20 so the relevance assumption was met. Although the exclusion restriction assumption cannot be directly tested, short sleep may be a plausible exposure to be linked to CKD considering the previous findings. In addition, we performed sensitivity analysis omitting SNPs that were strongly associated with major confounders or directly adjusting for the possible confounders. Furthermore, our two-sample MR results tested the MR-Egger regression, a method that is known to be less pleiotropy biased, yielding consistent positive results for causal estimates of short sleep on CKD. Thus, the independence assumptions would have been attained, suggesting that our MR analysis demonstrated the causal effects of short sleep duration on CKD.9
In our clinical analysis, short sleep duration was associated with higher prevalence of CKD, showing the presence of an observational association between the two factors. However, the association was insignificant after a stringent adjustment including metabolic parameters in women. However, as the prevalence of CKD cannot directly reflect the risks as an incident outcome can and the association was significant in the less stringent multivariable model, the clinical association between short sleep and CKD could still be interpreted as being present in women. It may be possible that obesity or other metabolic disorders are mediators for the association between sleep duration and CKD; thus, the stringent model results may not completely disregard the clinical association between short sleep duration and CKD in women. In addition, the association between short sleep duration and kidney function impairment in women is supported by a previous study investigating rapid eGFR decline outcomes within women.5 On the other hand, the risk of incident ESKD was significantly associated with inappropriate sleep duration in men but not in women. That the number of subjects who were men with incident ESKD was approximately double the number of women might have caused the risk of ESKD to be relatively well discriminated in the participants who were men with regard to sleep exposure. As the genetic analysis showed that short sleep causally affects CKD in both sexes, it may be necessary to pay attention to an appropriate sleep duration in relation to kidney function regardless of sex. A future longitudinal study including subjects with a higher baseline risk of CKD or ESKD than the general population as included in our study may be able to additionally assess the risk of kidney function impairment according to an inappropriate sleep duration stratified by various clinical characteristics.
Previous studies suggested that short sleep periods may cause impairment in metabolic health, including impaired glucose metabolism30,31 or obesity.32 Such biologic pathways may be the reason why short sleep periods may causally affect myocardial infarction or CKD,8 further suggesting that clinicians should pay attention to worsening metabolic parameters in those who sleep short. On the other hand, genetically predicted long sleep duration was not associated with CKD, although its clinical significance was present. The possible reason may be that the genetic instrument included fewer SNPs, resulting in weak instrument bias.33 Alternately, as a hypothesis, long sleep duration may be less determined by genetic factors than short sleep duration, and the association between long sleep duration and CKD is mainly derived from coexisting confounders. As there was a higher proportion of those who napped or snored in the long-sleep duration group, other disturbances in sleep during the nighttime (e.g., obstructive sleep apnea) may be the main cause of long sleep duration and the observed clinical associations related to this factor.
Our study has several limitations. First, the genetic analysis was limited to subjects of European ancestry, so the results may not be generalizable to populations of other ethnicities. Second, the study does not provide direct insights into the mechanisms underlying the adverse effects of short sleep duration on kidney function. In addition, other important sleep dimensions (e.g., sleep apnea) related to metabolic disorders or CKD should also be investigated for their causal effects on CKD and for their associations with sleep duration. Third, the difference in the identified clinical associations with kidney function impairment according to sex is not conclusive, and this might have been related to the relatively low number of patients with CKD or incident ESKD in the studied cohort. Lastly, the clinical analysis used self-reported sleep duration, so the validity of the information is not secured. There is the possibility that persons with disease subjectively reporting inappropriate sleep duration might cause type 1 error. However, the MR results, on the basis of the genetic instrument validated by accelerometry-determined sleep duration,20 showing possible causal effects of short sleep duration on CKD may support that no substantial bias would have occurred from the potential measurement error.
In conclusion, self-reported and genetically predicted short sleep duration was associated with CKD, suggesting causal effects of short sleep duration on CKD. Although a significant observational association between long sleep duration and CKD or ESKD was present, causal effects of long sleep were not supported by the MR analysis. Health care providers may recommend that people avoid short sleeping behavior to reduce the risk of CKD.
Disclosures
All authors have nothing to disclose.
Funding
This work was supported by Ministry of Trade, Industry & Energy Industrial Strategic Technology Development Program—Development of Bio-Core Technology grant 10077474 (development of early diagnosis technology for acute/chronic renal failure).
Supplementary Material
Acknowledgments
We thank the investigators for providing the data for this study.
The study was on the basis of the data provided by the UK Biobank Consortium (application no. 53799) and the CKDGen Consortium.
The corresponding author attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted.
K. Joo, D. Kim, K. Kim, H. Lee, and S. Park contributed to the conception and design of the study; S. Han, K. Joo, M. Kang, D. Kim, Yaerim Kim, Yon Su Kim, Yong Chul Kim, J. Lee, S. Lee, Y. Lee, and C. Lim provided statistical advice and interpreted the data; K. Kim and S. Park performed the main statistical analysis; Yaerim Kim and S. Lee provided assistance for the main statistical analysis; K. Joo, D. Kim, Yon Su Kim, H. Lee, J. Lee, and C. Lim provided advice regarding the data interpretation; S. Han, K. Joo, Yon Su Kim, Yong Chul Kim, H. Lee, J. Lee, and C. Lim provided material support during the study; D. Kim and S. Park had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; and all authors participated in drafting the manuscript, reviewed the manuscript, and approved the final version to be published.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020050666/-/DCSupplemental.
Supplemental Figure 1. General additive model plotting the U-shaped association between self-reported sleep duration and age-sex probabilities of prevalent CKD stages 3–5.
Supplemental Material. Methods.
Supplemental Table 1. Genetic instruments implemented in the study and their association with each short–sleep duration or long–sleep duration phenotype identified by Dashti et al.20
Supplemental Table 2. Clinical analysis results with the multivariable models constructed by the complete-case method.
Supplemental Table 3. Association between the SNPs included in the genetic instruments and possible major confounders.
Supplemental Table 4. Association between CKD stages 3–5 and SNPs included in the genetic instruments.
Supplemental Table 5. Leave-one-out analysis results of the two-sample MR analysis.
References
- 1.GBD Chronic Kidney Disease Collaboration : Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 395: 709–733, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin DC: Analysis of mortality risk from Korean hemodialysis registry data 2017. Kidney Res Clin Pract 38: 169–175, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merlino G, Piani A, Dolso P, Adorati M, Cancelli I, Valente M, et al. : Sleep disorders in patients with end-stage renal disease undergoing dialysis therapy. Nephrol Dial Transplant 21: 184–190, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi Y, Hatta T, Hayashi T, Shoji T, Suzuki A, Tomida K, et al. : Association of nocturnal hypoxemia with progression of CKD. Clin J Am Soc Nephrol 8: 1502–1507, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMullan CJ, Curhan GC, Forman JP: Association of short sleep duration and rapid decline in renal function. Kidney Int 89: 1324–1330, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricardo AC, Knutson K, Chen J, Appel LJ, Bazzano L, Carmona-Powell E, et al. ; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The association of sleep duration and quality with CKD progression. J Am Soc Nephrol 28: 3708–3715, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto R, Shinzawa M, Isaka Y, Yamakoshi E, Imai E, Ohashi Y, et al. ; CKD-JAC Investigators : Sleep quality and sleep duration with CKD are associated with progression to ESKD. Clin J Am Soc Nephrol 13: 1825–1832, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daghlas I, Dashti HS, Lane J, Aragam KG, Rutter MK, Saxena R, et al. : Sleep duration and myocardial infarction. J Am Coll Cardiol 74: 1304–1314, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies NM, Holmes MV, Davey Smith G: Reading mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ 362: k601, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy OJ, Pirastu N, Poole R, Fallowfield JA, Hayes PC, Grzeszkowiak EJ, et al. : Coffee consumption and kidney function: A Mendelian randomization study. Am J Kidney Dis 75: 753–761, 2020. [DOI] [PubMed] [Google Scholar]
- 11.Zanetti D, Bergman H, Burgess S, Assimes TL, Bhalla V, Ingelsson E: Urinary albumin, sodium, and potassium and cardiovascular outcomes in the UK biobank: Observational and Mendelian randomization analyses. Hypertension 75: 714–722, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millard LAC, Davies NM, Tilling K, Gaunt TR, Davey Smith G: Searching for the causal effects of body mass index in over 300 000 participants in UK Biobank, using Mendelian randomization. PLoS Genet 15: e1007951, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan DM, Choi HK, Verbanck M, Topless R, Won HH, Nadkarni G, et al. : No causal effects of serum urate levels on the risk of chronic kidney disease: A Mendelian randomization study. PLoS Med 16: e1002725, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, et al. : Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol 186: 1026–1034, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. : UK Biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 12: e1001779, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. : National Sleep Foundation’s updated sleep duration recommendations: Final report. Sleep Health 1: 233–243, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2011]. Ann Intern Med 150: 604–612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roumelioti ME, Buysse DJ, Sanders MH, Strollo P, Newman AB, Unruh ML: Sleep-disordered breathing and excessive daytime sleepiness in chronic kidney disease and hemodialysis. Clin J Am Soc Nephrol 6: 986–994, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Buuren S, Groothuis-Oudshoorn K: Mice: Multivariate imputation by chained equations in R. J Stat Softw 45: 1–67, 2011 [Google Scholar]
- 20.Dashti HS, Jones SE, Wood AR, Lane JM, van Hees VT, Wang H, et al. : Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat Commun 10: 1100, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ: Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 4: 7, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Efron B: Regression and ANOVA with zero-one data: Measures of residual variation. J Am Stat Assoc 73: 113–121, 1978 [Google Scholar]
- 23.Burgess S, Davies NM, Thompson SG: Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol 40: 597–608, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wuttke M, Li Y, Li M, Sieber KB, Feitosa MF, Gorski M, et al. ; Lifelines Cohort Study; V. A. Million Veteran Program : A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat Genet 51: 957–972, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartwig FP, Davies NM, Hemani G, Davey Smith G: Two-sample Mendelian randomization: Avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol 45: 1717–1726, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgess S, Butterworth A, Thompson SG: Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 37: 658–665, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowden J, Davey Smith G, Burgess S: Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int J Epidemiol 44: 512–525, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowden J, Davey Smith G, Haycock PC, Burgess S: Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 40: 304–314, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgess S, Labrecque JA: Mendelian randomization with a binary exposure variable: Interpretation and presentation of causal estimates. Eur J Epidemiol 33: 947–952, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mallon L, Broman JE, Hetta J: High incidence of diabetes in men with sleep complaints or short sleep duration: A 12-year follow-up study of a middle-aged population. Diabetes Care 28: 2762–2767, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, et al. : A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care 26: 380–384, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Watson NF, Harden KP, Buchwald D, Vitiello MV, Pack AI, Weigle DS, et al. : Sleep duration and body mass index in twins: A gene-environment interaction. Sleep (Basel) 35: 597–603, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burgess S, Thompson SG; CRP CHD Genetics Collaboration : Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol 40: 755–764, 2011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.