Abstract
Bacteriophages (phages) are being considered as alternative therapeutics for the treatment of multidrug resistant bacterial infections. Considering phages have narrow host-ranges, it is generally accepted that therapeutic phages will have a marginal impact on non-target bacteria. We have discovered that lytic phage infection induces transcription of type VIIb secretion system (T7SS) genes in the pathobiont Enterococcus faecalis. Membrane damage during phage infection induces T7SS gene expression resulting in cell contact dependent antagonism of different Gram positive bystander bacteria. Deletion of essB, a T7SS structural component, abrogates phage-mediated killing of bystanders. A predicted immunity gene confers protection against T7SS mediated inhibition, and disruption of its upstream LXG toxin gene rescues growth of E. faecalis and Staphylococcus aureus bystanders. Phage induction of T7SS gene expression and bystander inhibition requires IreK, a serine/threonine kinase, and OG1RF_11099, a predicted GntR-family transcription factor. Additionally, sub-lethal doses of membrane targeting and DNA damaging antibiotics activated T7SS expression independent of phage infection, triggering T7SS antibacterial activity against bystander bacteria. Our findings highlight how phage infection and antibiotic exposure of a target bacterium can affect non-target bystander bacteria and implies that therapies beyond antibiotics, such as phage therapy, could impose collateral damage to polymicrobial communities.
Author summary
Renewed interest in phages as alternative therapeutics to combat multi-drug resistant bacterial infections, highlights the importance of understanding the consequences of phage-bacteria interactions in the context of microbial communities. Although it is well established that phages are highly specific for their host bacterium, there is no clear consensus on whether or not phage infection (and thus phage therapy) would impose collateral damage to non-target bacteria in polymicrobial communities. Here we provide direct evidence of how phage infection of a clinically relevant pathogen triggers an intrinsic type VII secretion system (T7SS) antibacterial response that consequently restricts the growth of neighboring bacterial cells that are not susceptible to phage infection. Phage induction of T7SS activity is a stress response and in addition to phages, T7SS antagonism can be induced using sub-inhibitory concentrations of antibiotics that facilitate membrane or DNA damage. Together these data show that a bacterial pathogen responds to diverse stressors to induce T7SS activity which manifests through the antagonism of neighboring non-kin bystander bacterial cells.
Introduction
Enterococci constitute a minor component of the healthy human microbiota [1]. Enterococci, including Enterococcus faecalis, are also nosocomial pathogens that cause a variety of diseases, including sepsis, endocarditis, surgical-site, urinary tract and mixed bacterial infections [2, 3]. Over recent decades, enterococci have acquired extensive antibiotic resistance traits, including resistance to “last-resort” antibiotics such as vancomycin, daptomycin, and linezolid [4–8]. Following antibiotic therapy, multi-drug resistant (MDR) enterococci can outgrow to become a dominant member of the intestinal microbiota, resulting in intestinal barrier invasion and blood stream infection [7, 9]. The ongoing evolution of MDR enterococci in healthcare settings [4–6, 10, 11] and their ability to transmit antibiotic resistance among diverse bacteria [9, 12–15], emphasize the immediate need for novel therapeutic approaches to control enterococcal infections.
Viruses that infect and kill bacteria (bacteriophages or phages) are receiving attention for their use as antibacterial agents [16]. Recent studies have demonstrated the efficacy of anti-enterococcal phages in murine models of bacteremia [17–19] and the administration of phages to reduce E. faecalis burden in the intestine gives rise to phage resistant isolates that are sensitized to antibiotics [20]. Considering phages are highly specific for their target bacterium, coupled with the self-limiting nature of their host-dependent replication, this suggests that unlike antibiotics which have broad off-target antimicrobial activity, phages should have nominal impact on bacteria outside of their intended target strain [21–23]. However, our understanding of how phages interact with bacteria and the bacterial response to phage infection is limited.
While studying the transcriptional response of phage infected E. faecalis cells, we discovered that phage infection induces the expression of genes involved in the biosynthesis of a type VIIb secretion system (T7SS) [24]. Firmicutes, including the enterococci, harbor diverse T7SS genes encoding transmembrane and cytoplasmic proteins involved in the secretion of protein substrates [25], and T7SSs promote antagonism of non-kin bacterial cells through production of antibacterial effectors and/or toxins [26, 27]. The antibacterial activity of T7SSs from staphylococci and streptococci are well characterized [25] but T7SS-mediated antibacterial antagonism has not been described for enterococci. The environmental cues and regulatory pathways that govern T7SS expression and activity are poorly understood, although recent studies indicate that exposure to serum and membrane stresses triggered by pulmonary surfactants, fatty acids and phage infection stimulate T7SS gene expression [24, 28–31]. This motivated us to determine if phage induced T7SS gene expression in E. faecalis results in the inhibition of non-kin bacterial cells that are not phage targets (bystanders). We discovered that phage infected E. faecalis produces potent T7SS antibacterial activity against bystander bacteria. Expression of a T7SS antitoxin (immunity factor) gene in bystander cells and mutation of the LXG domain containing gene located immediately upstream of this immunity factor confer protection against phage mediated T7SS inhibition. We also investigated the potential impact of antimicrobials directed against bacterial physiological processes that are also targeted by phages, including cell wall, cell membrane and DNA damaging agents, on enterococcal T7SS. Sub-lethal challenge with specific antibiotics enhances T7SS gene expression resulting in T7SS dependent interspecies antagonism. Additionally, we discovered that membrane stress during phage infection induces transcription of T7SS genes via a non-canonical IreK signaling pathway. To our knowledge, the enterococcal T7SS is the first example of secretion system induction during phage infection. These data shed light on how phage infection of a cognate bacterial host can influence polymicrobial interactions and raises the possibility that phages may impose unintended compositional shifts among bystander bacteria in the microbiota during phage therapy.
Results
Phage mediated induction of E. faecalis T7SS leads to interspecies antagonism
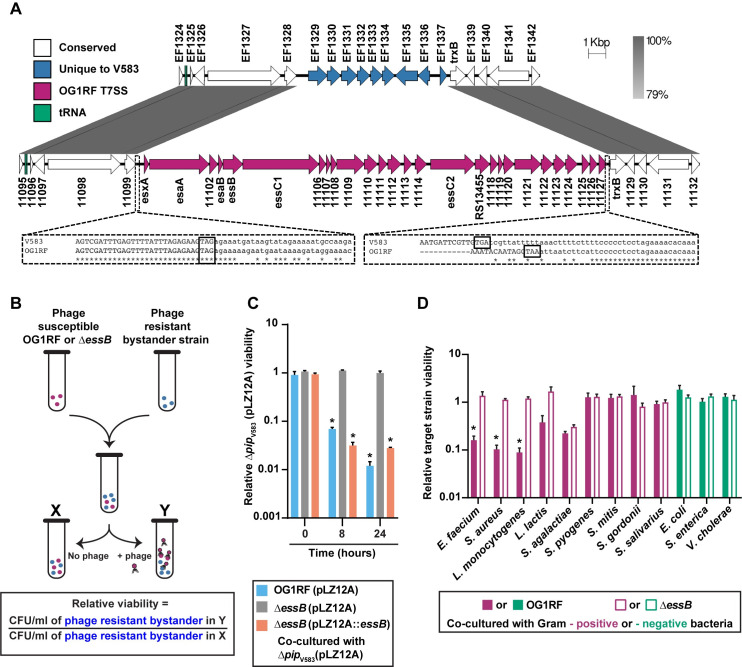
A hallmark feature of phage therapy is that phages often have a narrow host range, hence they do not influence the growth of non-susceptible bacteria occupying the same niche [22]. We discovered that infection of E. faecalis OG1RF by phage VPE25 induces the expression of T7SS genes [24]. The E. faecalis OG1RF T7SS locus is absent in the commonly studied vancomycin-resistant strain V583, despite conservation of flanking genes (Fig 1A) [32, 33]. The OG1RF T7SS is found downstream of conserved tRNA-Tyr and tRNA-Gln genes, which could facilitate recombination or integration of new DNA [34], but no known recombination or integration sites were identified on the 3’ end of this locus. Homologs of the E. faecalis T7SS gene esxA are found throughout three of the four Enterococcus species groups [35], including Enterococcus faecium, suggesting a wide distribution of T7SS loci in enterococci (S1 Fig). In addition to EsxA, OG1RF encodes the core T7SS structural components EsaA, EssB, and EssC, which are predicted to localize to the membrane, and EsaB, a small predicted cytoplasmic protein (Fig 1A) [36]. OG1RF_11102 encodes an additional putative membrane protein, although it does not share sequence homology with staphylococcal or streptococcal EssA. We were unable to identify an EssA homolog in OG1RF using sequence-based homology searches, suggesting that the enterococcal T7SS machinery may differ from previously described T7SS found in other Gram-positive bacteria. In silico analyses predict that the E. faecalis T7SS locus encodes multiple WXG100 family effectors and LXG family polymorphic toxins [27, 37]. We hypothesized that induction of T7SS genes during phage infection and consequently the heightened production of T7SS substrates would indirectly influence the growth of non-kin phage-resistant bacterial cells.
Fig 1. Phage mediated inhibition of bystander bacteria is dependent on enterococcal T7SS.
(A) Diagram showing the location of T7SS genes in E. faecalis OG1RF (NC_017316.1) compared to E. faecalis V583 (NC_004668.1). Sequences were obtained from NCBI, and homology comparisons were rendered in EasyFig. Nucleotide alignments generated by Clustal Omega are enlarged for clarity (dashed lines). Stop codons of genes EF1328/OG1RF_11099 and EF1337/OG1RF_11127 are boxed. (B) Schematic representation of the co-culture assay used to assess the viability of bystander bacteria during phage induced T7SS activity of wild type E. faecalis OG1RF and ΔessB. Relative viability of bystander strains is calculated by measuring the ratio of bystander cfus in the phage infected culture compared to the bystander cfus from an uninfected control culture. (C) The relative abundance of viable bystander bacterium E. faecalis ΔpipV583. Complementation of the E. faecalis ΔessB mutant, ΔessB (pLZ12A::essB), restores T7SS dependent bystander inhibition. ΔessB (pLZ12A) is the empty vector control. (D) T7SS inhibition of other bacterial species in the presence and absence of phage infected E. faecalis OG1RF or ΔessB. Data represent three biological replicates. Error bars indicate standard deviation. *P < 0.00001 by unpaired Student’s t-test.
To investigate if T7SS factors produced during phage infection of E. faecalis OG1RF interferes with the growth of phage-resistant bystander bacteria, we generated a strain with an in-frame deletion in the T7SS gene essB, encoding a transmembrane protein involved in the transport of T7SS substrates [38]. We chose to inactivate essB as opposed to the more commonly investigated secretion promoting ATPase essC [27, 38], because E. faecalis OG1RF harbours two essC genes in its T7SS locus that may have functional redundancy (Fig 1A). The essB mutant is equally susceptible to phage VPE25 infection compared to wild type E. faecalis OG1RF (S2A Fig). We performed co-culture experiments where phage susceptible wild type E. faecalis OG1RF or ΔessB were mixed with a phage resistant bystander, a strain of E. faecalis V583 deficient in the production of the VPE25 receptor (ΔpipV583) [39], at a ratio of 1:1 in the absence and presence of phage VPE25 (multiplicity of infection [MOI] = 0.01) (Fig 1B). VPE25 infected E. faecalis OG1RF and the ΔessB mutant with similar efficiency and caused a 1000-fold reduction in the viable cell count over a period of 24 hours relative to the starting cell count (S2B Fig). Since sequence-based homology searches did not retrieve any homologs of potential antitoxins from the E. faecalis OG1RF T7SS locus in E. faecalis V583 genome, this strain likely lacks immunity to toxins encoded in this locus. The viability of E. faecalis ΔpipV583, was reduced nearly 100-fold when co-cultured with E. faecalis OG1RF in the presence of phage VPE25 (Fig 1C and S2C Fig). However, growth inhibition of E. faecalis ΔpipV583 was abrogated during co-culture with phage infected E. faecalis ΔessB and phage induced T7SS antagonism of E. faecalis ΔessB could be restored by complementation (Fig 1C and S2C Fig), indicating that inhibition of phage resistant E. faecalis ΔpipV583 by OG1RF is T7SS dependent.
T7SS encoded antibacterial toxins secreted by Gram positive bacteria influence intra- and interspecies antagonism [26, 27]. While a nuclease and a membrane depolarizing toxin produced by Staphylococcus aureus target closely related S. aureus strains [26, 40], Streptococcus intermedius exhibits T7SS dependent antagonism against a wide-array of Gram positive bacteria [27]. To determine the target range of E. faecalis OG1RF T7SS antibacterial activity, we measured the viability of a panel of VPE25 insensitive Gram positive and Gram negative bacteria in our co-culture assay (Fig 1B). Growth inhibition of the distantly related bacterial species E. faecium and Gram positive bacteria of diverse genera, including S. aureus and Listeria monocytogenes, occurred following co-culture with phage infected wild type E. faecalis OG1RF but not the ΔessB mutant (Fig 1D). Fitness of Lactococcus lactis, a lactic acid bacterium like E. faecalis, was modestly reduced during co-culture with phage infected E. faecalis OG1RF, although these data were not statistically significant. In contrast, Gram positive pathogenic and commensal streptococci were unaffected (Fig 1D). Similarly, phage induced T7SS activity did not inhibit any Gram negative bacteria tested (Fig 1D). Collectively, these results show that phage predation of E. faecalis promotes T7SS inhibition of select bystander bacteria.
Molecular basis of E. faecalis phage–triggered T7SS antagonism
Our data demonstrate that induction of E. faecalis OG1RF T7SS genes during phage infection hinder the growth of select non-kin bacterial species. Antibacterial toxins deployed by Gram negative bacteria via type V and VI secretion and Gram positive T7SS require physical contact between cells to achieve antagonism [26, 27, 41, 42]. Therefore, we investigated if growth inhibition of bystander bacteria is contingent upon direct interaction with phage infected E. faecalis using a trans-well assay [27]. We added unfiltered supernatants from wild type E. faecalis OG1RF and ΔessB mutant cultures grown for 24 hrs in the presence and absence of phage VPE25 (MOI = 0.01) to the top of a trans well and deposited phage resistant E. faecalis ΔpipV583 in the bottom of the trans well. The 0.4 μm membrane filter that separates the two wells is permeable to proteins and solutes but prevents bacterial translocation. Supernatant from phage infected wild type E. faecalis OG1RF did not inhibit E. faecalis ΔpipV583 (S3A Fig) indicating that T7SS mediated growth interference relies on cell to cell contact. To exclude the possibility that T7SS substrates might adhere to the 0.4 μm membrane filter in the trans-well assay, we administered both filtered and unfiltered culture supernatants directly to E. faecalis ΔpipV583 cells (5x105 CFU/well) at a ratio of 1:10 (supernatant to bystander cells) and monitored growth over a period of 10 hours. Growth kinetics of E. faecalis ΔpipV583 remained similar irrespective of the presence or absence of conditioned supernatant from wild type E. faecalis OG1RF or ΔessB mutant cultures (S3B Fig and S3C Fig), further supporting the requirement of contact-dependent engagement of phage mediated T7SS inhibition.
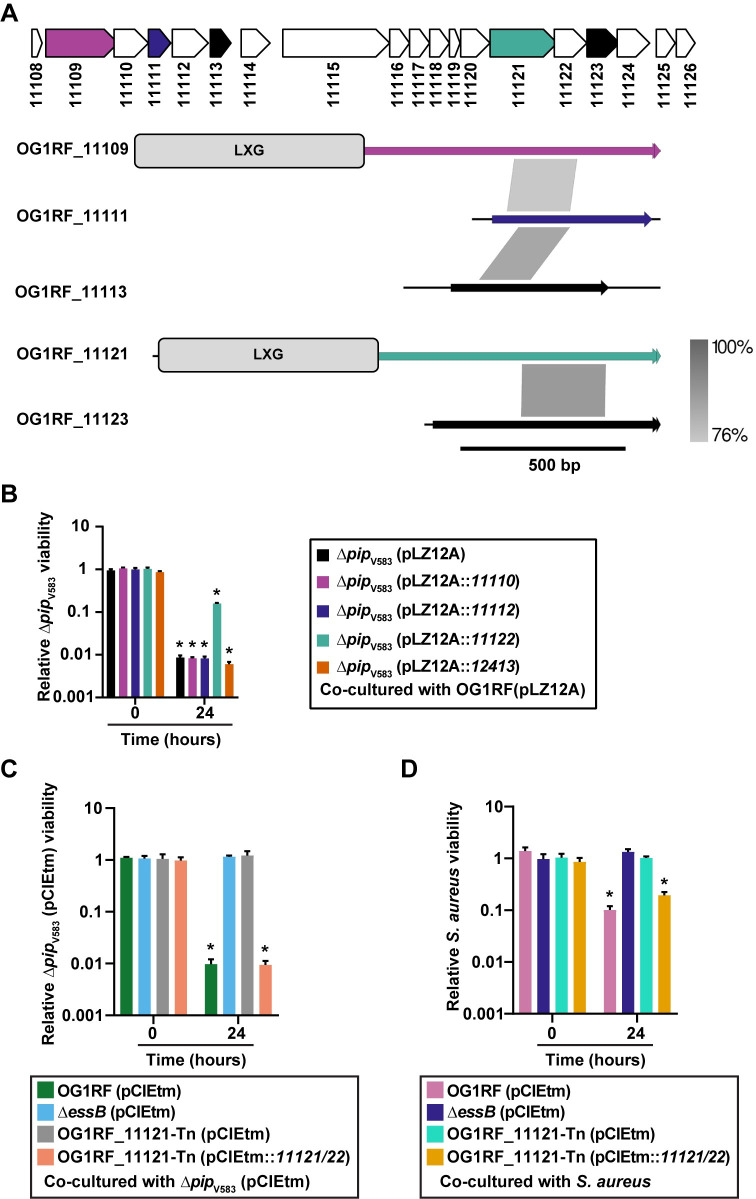
We discovered that E. faecalis OG1RF inhibits proliferation of non-kin bacterial cells through increased expression of T7SS genes in response to phage infection, but the toxic effectors were unknown. LXG domain containing toxins are widespread in bacteria with a diverse range of predicted antibacterial activities [43, 44]. The OG1RF T7SS locus encodes two LXG-domain proteins, OG1RF_11109 and OG1RF_11121 (Fig 2A). Both LXG domains were found using Pfam, but we were unable to identify predicted function or activity for either protein using sequence homology searches or structural modeling.
Fig 2. Identification of E. faecalis T7SS toxin and immunity proteins that dictate bystander growth inhibition.
(A) Putative toxin-encoding genes in the OG1RF T7SS locus. LXG domains in OG1RF_11109 and OG1RF_11121 were identified using KEGG and ExPASy PROSITE. Putative orphan toxins were identified by homology to OG1RF_11109 or OG1RF_11121. Gray lines between diagrams indicate the regions and degree of nucleotide conservation between genes. Homology diagrams were rendered in EasyFig. Gene colors for OG1RF_11109, OG1RF_11111, and OG1RF_11121 match the color scheme in panel (B). OG1RF_11113 and OG1RF_11123 are shaded black to indicate that their corresponding immunity genes were not tested in panel (B). (B) E. faecalis OG1RF T7SS mediated growth inhibition of phage resistant E. faecalis ΔpipV583 during infection is alleviated by expressing OG1RF_11122 in E. faecalis ΔpipV583 but not in the presence of pLZ12A empty vector, or expressing OG1RF_11110, OG1RF_11112, or OG1RF_12413. (C–D) Disruption of OG1RF_11121 by a transposon insertion rescues growth of phage resistant E. faecalis ΔpipV583 (C) and S. aureus (D) strains during co-culture. Complementation of OG1RF_11121-Tn restores bystander intoxication. Data represent three biological replicates. Error bars indicate standard deviation. *P < 0.0001 by unpaired Student’s t-test.
Bacterial polymorphic toxin systems can encode additional toxin fragments and cognate immunity genes, known as “orphan” toxin/immunity modules, downstream of full-length secreted effectors [45, 46]. Orphan toxins lack the N-terminal domains required for secretion or delivery, although they can encode small regions of homology that could facilitate recombination with full-length toxin genes [47]. Therefore, we sought to identify putative orphan toxins in OG1RF. We aligned the nucleotide sequences of OG1RF_11109 and OG1RF_11121 with downstream genes in the T7SS locus and looked for regions of similarity that might signify orphan toxins. Although the 3’ ends of OG1RF_11111 and OG1RF_11113 did not have homology to either OG1RF_11109 or OG1RF_11121, the 5’ ends of OG1RF_11111 and OG1RF_11113 had >75% nucleotide homology to a portion of OG1RF_11109 (Fig 2A, regions of homology indicated by gray shading). Similarly, OG1RF_11123 had sequence homology to OG1RF_11121 (Fig 2A). We searched Pfam and ExPasy for annotated domains but were unable to identify any in OG1RF_11111, OG1RF_11113, or OG1RF_11123. However, structural modeling with Phyre2 [48] revealed that a portion of OG1RF_11123 has predicted structural homology to the channel-forming domain of colicin 1a [49] (S4D Fig).
Orphan toxins encoded by a secretion system in a given strain can often be found as full-length toxins in other bacteria [45, 46]. Therefore, we used the sequences of OG1RF_11111, OG1RF_11113, and OG1RF_11123 as input for NCBI Protein BLAST to determine whether the orphan toxins we identified in E. faecalis OG1RF were found in other T7SS loci. We identified homologs to these orphan toxins in other E. faecalis strains as well as Listeria sp. (S4A, S4B and S4C Fig, gray shading indicates regions of homology). These homologs were longer than the E. faecalis OG1RF genes and encoded N-terminal LXG domains, suggesting that in Listeria and other E. faecalis strains, homologs to OG1RF_11111, 11113, and 11123 are full-length toxins that could be secreted by the T7SS.
Interestingly, we identified an additional LXG gene product, OG1RF_12414, in a distal locus that is again notably absent from E. faecalis V583 (S5A and S5B Fig). OG1RF_12414 has predicted structural homology to Tne2, a T6SS effector with NADase activity from Pseudomonas protegens (S5C Fig) [50]. Additionally, we identified numerous C-terminal domains in LXG proteins distributed throughout the enterococci (S6 Fig). These include EndoU and Ntox44 nuclease domains [43, 51, 52], which have been characterized in effectors produced by other polymorphic toxin systems.
Polymorphic toxins are genetically linked to cognate immunity proteins that neutralize antagonistic activity and prevent self-intoxication [43, 52, 53]. Each of the five putative toxins in the OG1RF T7SS locus is encoded directly upstream of a small protein that could function in immunity. Whitney et al. demonstrated that the cytoplasmic antagonistic activity of S. intermedius LXG toxins TelA and TelB in Escherichia coli can be rescued by co-expression of cognate immunity factors [27]. Therefore, we examined if OG1RF_11110, 11112, 11122, or 12413 confer immunity to E. faecalis ΔpipV583 during phage infection of E. faecalis OG1RF. Constitutive expression of OG1RF_11122, and not OG1RF_11110, 11112, or 12413, partially neutralized phage induced T7SS antagonism (Fig 2B), confirming an essential role for the OG1RF_11122 gene product in immunity, and suggesting that OG1RF_11121 is at least partly responsible for T7SS mediated intra-species antagonism. However, further investigation is needed to confirm whether the candidate immunity factors, OG1RF_11110, 11112 or 12413, are stably expressed under these experimental conditions.
To determine the contribution of OG1RF_11121 on intra- and interbacterial antagonism during phage infection, we measured the viability of phage resistant E. faecalis ΔpipV583 and S. aureus in co-culture with an E. faecalis OG1RF variant carrying a transposon insertion in OG1RF_11121 (OG1RF_11121-Tn). OG1RF_11121-Tn is equally susceptible to phage VPE25 infection compared to wild type E. faecalis OG1RF (S2A Fig). Similar to the ΔessB (pCIEtm) strain carrying empty pCIEtm plasmid, phage infected OG1RF_11121-Tn (pCIEtm) did not inhibit the growth of the bystander bacteria (Fig 2C and 2D). We were unable to clone OG1RF_11121 by itself into the inducible plasmid pCIEtm, suggesting leaky expression of OG1RF_11121 is toxic. Therefore, to complement E. faecalis OG1RF_11121-Tn we cloned both the OG1RF_11121 toxin and OG1RF_11122 antitoxin pair under the cCF10-inducible promoter in pCIEtm. Expression of both of these genes in the transposon mutant restored its ability antagonize T7SS susceptible bystanders (Fig 2C and 2D). These data strongly suggest that the OG1RF_11121 encoded LXG toxin drives E. faecalis T7SS mediated antagonism of bystanders following phage infection.
Sub-lethal antibiotic stress promotes T7SS dependent antagonism
Considering two genetically distinct phages trigger the induction of T7SS genes in E. faecalis [24], we reasoned that T7SS induction could be a result of phage mediated cellular damage and not specifically directed by a phage encoded protein. Antibiotics elicit a range of damage induced stress responses in bacteria [54–56]; therefore, independent of phage infection we investigated the effects of subinhibitory concentrations of antibiotics on T7SS expression in E. faecalis.
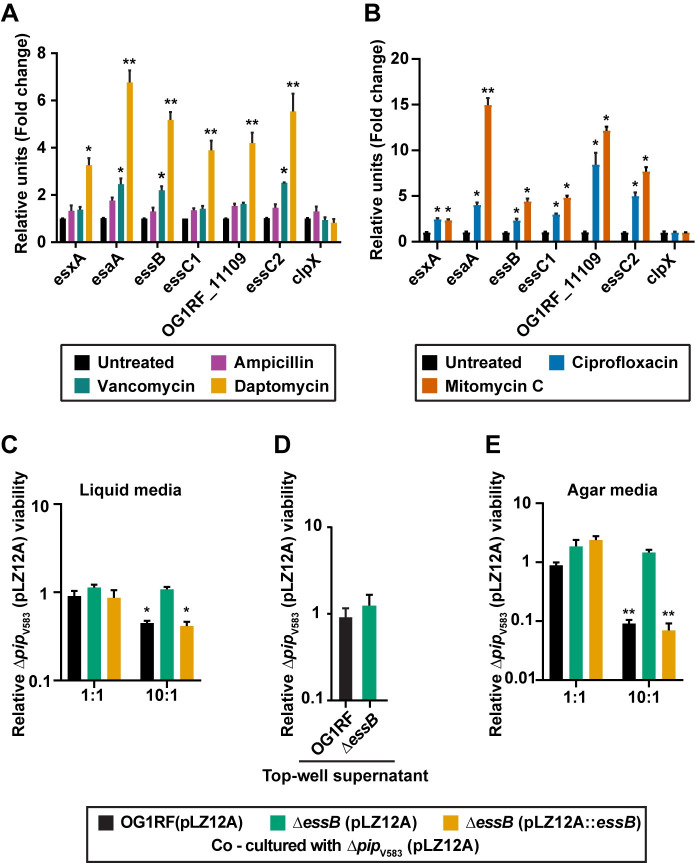
To investigate the influence of sublethal antibiotic concentrations on E. faecalis OG1RF T7SS transcription, we determined the minimum inhibitory concentrations (MIC) of ampicillin, vancomycin, and daptomycin (S7A, S7B and S7C Fig) and monitored T7SS gene expression in E. faecalis OG1RF cells treated with a sub-lethal dose of antibiotic (50% of the MIC). We found that bacterial T7SS genes were significantly upregulated in the presence of the cell membrane targeting antibiotic, daptomycin, relative to the untreated control (Fig 3A). In contrast, the cell wall biosynthesis inhibitors ampicillin and vancomycin either did not induce or had a minor impact on T7SS mRNA levels, respectively (Fig 3A). Additionally, induction of T7SS transcription occurred when bacteria were challenged with sub-inhibitory concentrations of the DNA targeting antibiotics ciprofloxacin and mitomycin C (Fig 3B, S7D and S7E Fig).
Fig 3. Sub-lethal antibiotic treatment enhances T7SS gene expression leading to inhibition of bystander bacteria.
Altered expression of T7SS genes upon exposure to sub-inhibitory concentrations of (A) ampicillin (0.19 μg/ml), vancomycin (0.78 μg/ml) or daptomycin (6.25 μg/ml) and (B) ciprofloxacin (2 μg/ml) or mitomycin C (4 μg/ml) for 40 minutes relative to the untreated control. clpX is shown as a negative control. (C-E) Contact–dependent T7SS mediated inhibition of bystander bacteria in the presence of daptomycin. Relative viability of E. faecalis ΔpipV583 was measured during co-culture with E. faecalis OG1RF or ΔessB antagonists in the presence and absence of daptomycin treatment in (C) liquid culture (2.5 μg/ml daptomycin), (D) trans-well plates to prevent physical engagement between cells (2.5 μg/ml daptomycin) and (E) in contact on agar media (0.5 μg/ml daptomycin). ΔessB (pLZ12A) and ΔessB (pLZ12A::essB) represent the empty vector control and complemented strains. Data show three biological replicates. Error bars indicate standard deviation. *P < 0.01, **P < 0.001 to 0.0001 by unpaired Student’s t-test.
We next sought to assess the influence of daptomycin driven T7SS induction on inter-enterococcal antagonism. E. faecalis V583 and its derivatives are more sensitive to daptomycin compared to E. faecalis OG1RF strains (S8A, S8B and S8C Fig), so we applied a reduced concentration of 2.5 μg/ml daptomycin in the co-culture inhibition assay to prevent daptomycin intoxication of E. faecalis ΔpipV583 bystanders. Because E. faecalis OG1RF T7SS gene expression is less robust in the presence of 2.5 μg/ml compared to 6.25 μg/ml daptomycin, which was used in our previous experiments (Fig 3A and S8D Fig), a 10:1 ratio of daptomycin treated E. faecalis OG1RF was required for growth inhibition of E. faecalis ΔpipV583 during co-culture (Fig 3C). Consistent with our previous results, daptomycin induced T7SS inhibition of E. faecalis ΔpipV583 was contact dependent (Fig 3D). To increase T7SS mediated contact-dependent killing of the target strain during daptomycin exposure, we performed the inhibition assay on nutrient agar plates. The sub-inhibitory concentration of daptomycin (2.5 μg/ml) used in liquid culture was toxic to the cells on agar plates (S8E Fig), so we lowered the daptomycin concentration to 0.5 μg/ml to prevent drug toxicity in the agar-based antagonism assay. Plating T7SS producing E. faecalis OG1RF cells and E. faecalis ΔpipV583 bystander cells at a ratio of 10:1 resulted in ~10–fold inhibition of bystander growth (Fig 3E). Although 0.5 μg/ml of daptomycin did not dramatically increase E. faecalis OG1RF T7SS transcript abundances, this was sufficient to promote daptomycin mediated T7SS inhibition of bystanders on agar plates (Fig 3E and S8E Fig). These data show that in addition to phages, antibiotics can be sensed by E. faecalis thereby inducing T7SS antagonism of non-kin bacterial cells. These data also show that the magnitude of T7SS gene expression and forcing bacteria-bacteria contact is directly related to the potency of T7SS inhibition.
The primary bile acid sodium cholate does not modulate E. faecalis T7SS gene expression
To gain insight into host-associated environmental cues that could trigger E. faecalis OG1RF T7SS, we measured T7SS transcription in the presence of a sub-inhibitory concentration of the primary bile acid sodium cholate, an abundant compound found in the mammalian intestinal tract and that is known to promote bacterial cell membrane stress [57, 58]. 4% sodium cholate, a concentration that has been shown to severely impair the growth of E. faecalis OG1RF cell envelop mutants, caused only a minor reduction in cell density of wild type E. faecalis OG1RF [59] (S9A Fig) and it did not stimulate T7SS gene expression (S9B Fig). Collectively, these data show that T7SS induction in E. faecalis occurs in response to select cell envelope stressors.
IreK and OG1RF_11099 facilitate T7SS expression in phage infected E. faecalis OG1RF via a non-canonical signaling pathway
Having established that both phage and daptomycin mediated membrane damage independently stimulates heightened E. faecalis OG1RF T7SS gene expression and antagonistic activity, we next sought to identify the genetic determinants that sense this damage and promote T7SS transcription. Two-component systems, LiaR/S and CroS/R, and the PASTA kinase family protein IreK are well-characterized modulators of enterococcal cell envelope homeostasis and antimicrobial tolerance [60–62]. Aberrant cardiolipin microdomain remodeling in the bacterial cell membrane in the absence of the LiaR response regulator results in daptomycin hypersensitivity and virulence attenuation [63]. CroS/R signaling and subsequent modulation of gene expression govern cell wall integrity and promote resistance to cephalosporins, glycopeptides and beta—lactam antibiotics [64–66]. The ireK encoded transmembrane Ser/Thr kinase regulates cell wall homeostasis, antimicrobial resistance, and contributes to bacterial fitness during long-term colonization of the intestinal tract [61, 67, 68]. Recently it has been shown that direct cross-talk between IreK and the CroS/R system positively impacts enterococcal cephalosporin resistance [69].
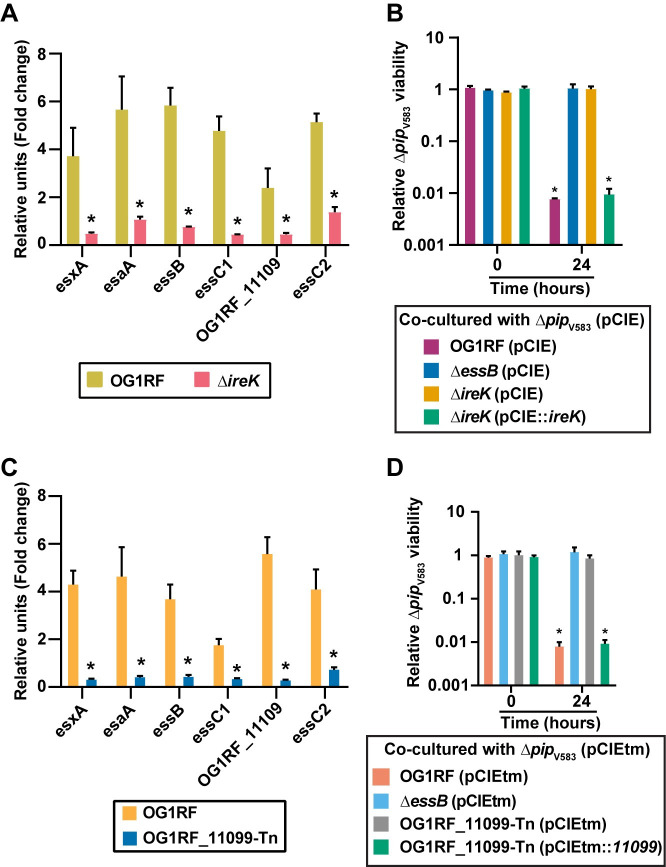
Wild type E. faecalis OG1RF, an ireK in-frame deletion mutant [61] and transposon (Tn) insertion mutants of liaR, liaS, croR, and croS [70] all display similar growth kinetics in the absence of phage VPE25 infection (S10A Fig). Although croR-Tn and croS-Tn exhibit reductions in the plaquing efficiency of VPE25 particles, none of these genetic elements of enterococcal cell wall homeostasis and antibiotic resistance were required for VPE25 infection (S10B Fig). We queried the expression levels of T7SS genes in these isogenic mutants during phage VPE25 infection (MOI = 1). T7SS gene expression was not enhanced in the ΔireK mutant during phage infection (Fig 4A), whereas liaR-Tn, liaS-Tn, croR-Tn, and croS-Tn produced heightened levels of T7SS transcripts similar to the wild type E. faecalis OG1RF compared to the uninfected controls (S11A, S11B, S11C, S11D, S11E and S11F Fig). A sub-lethal concentration of the cephalosporin ceftriaxone did not induce T7SS gene expression (S12A Fig), indicating that expression of T7SS genes following phage mediated membrane damage signals through a pathway that is distinct from the IreK response to cephalosporin stress. Additionally, the ΔireK mutant phenocopies the ΔessB mutant strain in the interbacterial antagonism co-culture assay, wherein the ΔireK mutant is unable to mediate phage induced T7SS dependent killing of the phage resistant E. faecalis ΔpipV583 non-kin cells (Fig 4B). T7SS antagonism is restored in E. faecalis ΔireK by introducing the wild type gene in trans (Fig 4B). Collectively, these results indicate that IreK senses phage mediated membrane damage promoting T7SS transcription independent of the CroS/R pathway.
Fig 4. IreK and OG1RF_11099 control transcription of enterococcal T7SS genes and subsequent inhibition of bystander bacteria during phage infection.
(A) Phage infection leads to enhanced expression of T7SS genes in wild type E. faecalis OG1RF but not in a ΔireK mutant strain. (B) Growth inhibition of E. faecalis ΔpipV583 during phage infection of E. faecalis OG1RF is abrogated in the ΔessB and ΔireK mutants carrying empty pCIEtm. pCIEtm::ireK complemented the T7SS activity defect of the ΔireK strain. (C) Disruption of OG1RF_11099 leads to reduced expression of T7SS genes during phage infection. The data are represented as the fold change of normalized mRNA relative to uninfected samples at the same time points. (D) T7SS dependent intraspecies antagonism during phage infection is alleviated in the presence of OG1RF_11099-Tn mutant carrying empty pCIEtm. pCIEtm::11099 complemented the T7SS activity defect of the OG1RF_11099-Tn mutant strain. Data represent three biological replicates. Error bars indicate standard deviation. *P < 0.00001 by unpaired Student’s t-test.
OG1RF_11099, located immediately upstream of the T7SS cluster is predicted to encode a GntR family transcriptional regulator, thus we sought to assess the contribution of OG1RF_11099 on T7SS transcription and functionality. E. faecalis OG1RF carrying a transposon insertion in OG1RF_11099 is equally susceptible to phage VPE25 infection compared to wild type E. faecalis OG1RF (S2A Fig) In contrast to wild type E. faecalis OG1RF, T7SS genes were not induced during phage predation of E. faecalis OG1RF_11099-Tn (Fig 4C). We evaluated the influence of OG1RF_11099-dependent regulation on the activity of T7SS in intraspecies antagonism using our co-culture assay. Similar to E. faecalis ΔessB, the OG1RF_11099-Tn mutant displayed attenuated T7SS activity in phage infected co-cultures (Fig 4D). T7SS dependent antagonism of E. faecalis OG1RF_11099-Tn could be restored following complementation (Fig 4D). Collectively, these results indicate that OG1RF_11099 encodes a positive regulator of E. faecalis T7SS important for phage mediated inhibition of bystander bacteria. Given that IreK governs downstream signaling events via phosphorylation [71], and the fact that OG1RF_11099 was not differentially expressed in response to phage infection of wild type E. faecalis OG1RF or ireK mutant strains (S12B Fig), suggests that either post-translational modification of OG1RF_11099 or a yet unidentified protein downstream of IreK engaging with OG1RF_11099 accounts for T7SS gene expression during phage infection.
Discussion
Despite the fact that bacteria exist in complex microbial communities that socially interact [72, 73], phage predation studies have primarily been performed in monoculture [24, 74–76]. Studies report phage-mediated effects on non-target bacteria linked to interbacterial interactions and evolved phage tropism for non-cognate bacteria [77–79], whereas other studies have identified minimal changes in microbiota diversity during phage therapy [77, 80].
Our results extend previous work that observed the induction of E. faecalis OG1RF T7SS gene expression in response to phage infection [24]. By using an in vitro antibacterial antagonism assay, we discovered that phage predation of E. faecalis OG1RF has an inhibitory effect on non-phage targeted bacterial species during co-culture. Our work shows that phage mediated inhibition of Gram positive bystander bacteria relies on the expression and activity of T7SS genes. This work establishes a framework to begin investigating if and how phage infection of target bacteria influences non-target bacterial populations in complex communities such as the microbiota.
Our data suggest that membrane stress associated with phage infection or sub-lethal daptomycin treatment stimulates T7SS mediated antibacterial antagonism of E. faecalis OG1RF (Fig 5). Given that daptomycin is used to target vancomycin-resistant enterococcal infections, this finding provides a hypothesis for how antibiotic-resistant enterococci achieve overgrowth and dominate the microbiota following antibiotic treatment. Further investigation is required to understand how T7SS induction might contribute to enterococcal fitness in polymicrobial environments. Although exposure to a sub-inhibitory level of primary bile salt (a common molecule found in the intestine) did not elicit T7SS expression, it is possible that other stressors encountered in the intestinal tract, including lysozyme, antimicrobial proteins, and nutrient availability could influence T7SS activity in E. faecalis. Indeed, E. faecalis T7SS mutants are defective in their ability to colonize the murine reproductive tract, which like the intestine is a polymicrobial environment [36].
Fig 5. A model for inhibition of bystander bacteria by the E. faecalis OG1RF T7SS.
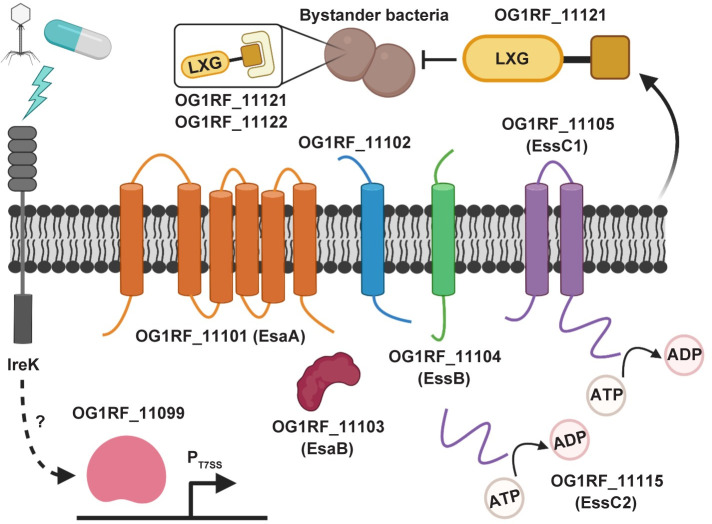
Phage and select antibiotics trigger a response involving IreK that results in induction of expression of T7SS genes. Transcription of T7SS genes is regulated by the predicted GntR-family transcription factor OG1RF_11099. The predicted core components of the OG1RF T7SS machinery are putative membrane proteins EsaA (OG1RF_11101), OG1RF_11102, EssB (OG1RF_11104), and EssC1 (OG1RF_11105) as well as the putative cytoplasmic protein EsaB (OG1RF_11103). EssC2 (OG1RF_11115) lacks transmembrane domains and is thus not predicted to be membrane-anchored. Upon induction of the T7SS, OG1RF_11121 is secreted from the cell, resulting in antibacterial activity against select neighboring bacteria. Expression of OG1RF_11122 can partially block toxicity caused by OG1RF_11121. Predictions of membrane topology were obtained using TMHMM [108]. The figure was created with Biorender.com.
We discovered that transcriptional activation of the T7SS during phage infection relies on IreK (Fig 5). Previously characterized IreK–mediated stress response pathways, including cephalosporin stress or CroS/R signaling, did not contribute to T7SS expression. We hypothesize that IreK senses diverse environmental stressors and coordinates distinct outputs in response to specific stimuli. Considering that IreK signaling is important for E. faecalis intestinal colonization [68], it is possible that IreK–dependent T7SS expression in response to intestinal cues modulate interbacterial interactions and enterococcal persistence in the intestine. However, the molecular mechanism by which IreK facilitates T7SS transcription remains unanswered. Additionally, we currently do not know if IreK directly senses phage or daptomycin mediated membrane damage or some other signal feeds into IreK to facilitate T7SS induction.
Additionally, we discovered that E. faecalis OG1RF T7SS transcription is regulated a GntR-family transcriptional regulator encoded by OG1RF_11099, a gene found immediately upstream of the T7SS cluster (Fig 5). Interestingly, OG1RF_11099 is highly conserved across enterococci, including E. faecalis V583 (Fig 1A) and other strains that lack T7SS. The presence of a conserved transcriptional regulator in the absence of its target genetic region supports the idea that certain strains of enterococci have undergone genome reduction as an evolutionary strategy to adapt to unique host and non-host environments. It is possible that in E. faecalis V583, the OG1RF_11099 homolog (EF1328) has been retained to regulate other genes within the regulon that are less dispensable than T7SS. Additionally, our data indicate that OG1RF_11099 transcription is not dependent on IreK or and is not induced during phage infection of wild type E. faecalis OG1RF. Previously published work demonstrated that IreK kinase activity is essential for driving the cell wall stress response in E. faecalis [67, 71]. Therefore, we hypothesize that IreK directly or indirectly regulates OG1RF_11099 activity for T7SS expression via post-translational modification.
Antibacterial properties of T7SS substrates have been demonstrated [26, 27, 40]. Here we provide evidence that mutation in the LXG toxin encoded by OG1RF_11121 abrogates phage induced T7SS dependent inhibition of bystander bacteria while expression of the downstream immunity gene OG1RF_11122 in T7SS targeted E. faecalis ΔpipV583 cells conferred partial protection from this inhibition. It is possible that constitutive expression of OG1RF_11122 from a multicopy plasmid results in elevated accumulation of OG1RF_11122 in the bystander strain which is toxic and could account for the partial protection phenotype. Aside from its LXG domain, OG1RF_11121 does not harbor any other recognizable protein domains, hence the mechanism underlying its toxicity is unclear. Whitney et al. demonstrated that LXG toxin antagonism is contact–dependent, having minimal to no impact on target cells in liquid media [27]. Although we found that physical engagement is crucial for E. faecalis T7SS mediated antagonism, we observed a significant reduction in target cell growth in liquid media both during phage and daptomycin treatment of T7SS proficient E. faecalis.
In contrast to the broad antagonism of S. intermedius T7SS [27], the E. faecalis OG1RF T7SS targets a more limited number of bacterial species. Interestingly, E. faecalis OG1RF T7SS antagonism is ineffective against various species of streptococci, which like the enterococci are lactic acid bacteria. Nucleotide–and protein–based homology searches did not reveal homologs of candidate immunity proteins, OG1RF_11110, OG1RF_11112, OG1RF_11122, or OG1RF_12413, in S. agalactiae COH1. Genome sequences of the other four streptococci used in this present study are not available, and hence we cannot comment on the presence of potential immunity proteins against OG1RF T7SS toxins in these strains. However, resistance of multiple streptococcal species to OG1RF T7SS mediated inhibition suggest that common cell surface modifications, e.g., capsule or surface polysaccharides, might be responsible for blocking toxin activity. Narrow target range is a common attribute of contact-dependent toxins that interact with specific membrane receptors on target cells to exert inhibitory activity [81]. However, specific receptors of T7SS toxins are yet to be identified. It is possible that specific or non-specific interactions between the E. faecalis OG1RF and S. aureus or L. monocytogenes cell surfaces facilitate T7SS interbacterial antagonism and such interactions are incompatible or occluded for the streptococci.
It is currently unknown whether T7SS toxin delivery requires contact with a receptor on target cells or whether delivery can occur in the absence of a receptor. Examples of both methods of toxin delivery are widespread in bacteria. Toxins such as colicins and R-pyocins mediate contact with target cells via protein receptors and LPS, respectively [82–84]. Delivery of colicins and toxins produced by contact-dependent inhibition systems in Gram-negative bacteria requires interactions with receptors at the outer and inner membranes [85, 86]. Conversely, the T6SS needle-like machinery that punctures target cell envelopes delivers toxins in a contact-dependent, receptor-independent manner [87]. Cell surface moieties can also affect recognition of target cells and subsequent toxin delivery. The presence of capsule can block target cell recognition by contact-dependent growth inhibition systems in Acinetobacter baumannii [88], E. coli [89] and Klebsiella pneumoniae [90]. Therefore, it is possible that a feature of the streptococcal cell surface, such as capsule modifications, renders them insensitive to killing by toxins delivered by the E. faecalis OG1RF T7SS.
Enterococci occupy polymicrobial infections often interacting with other bacteria [91–94]. Although commensal E. faecalis antagonize virulent S. aureus through the production of superoxide [95], the two species also exhibit growth synergy via exchange of critical nutrients [96]. Here, we show that phage treatment of E. faecalis OG1RF can indirectly impact the growth of neighboring phage-resistant bacteria, including S. aureus, in a T7SS–dependent manner, suggesting that phage therapy directed against enterococci driving T7SS activity could be useful for the treatment of polymicrobial infections. However, the counter argument is that phage therapy directed against enterococci could push a bacterial community toward dysbiosis, as phage induced T7SS activity could directly inhibit beneficial bystander bacteria. This raises questions about the consequences of phage mediated off-target effects on bacteria. Could phage induced T7SS activity be used to reduce phage expansion into other closely related strains as a means to dilute phages out of a population, or is it simply that phage induction of the T7SS serves as a mechanism that benefits a select few within a population to aid in their reoccupation of a niche upon overcoming phage infection? Future studies aimed at exploring enterococcal T7SS antagonism in polymicrobial communities should help elucidate the impact of phages on microbial community composition.
Materials and methods
Bacteria and bacteriophages
Bacteria and phages used in this study are listed in S1 Table. Bacteria were grown with aeration in Todd-Hewitt broth (THB) or on THB agar supplemented with 10mM MgSO4 at 37°C. The following antibiotic concentrations were added to media for the selection of specific bacterial strains or species: E. faecalis OG1RF (25 μg/ml fusidic acid, 50 μg/ml rifampin), E. faecalis V583 ΔpipV583 (25 μg/ml or 100 μg/ml gentamicin in liquid and agar media, respectively), S. aureus AH2146 LAC Φ11:LL29 (1 μg/ml tetracycline), L. monocytogenes 10403S (100 μg/ml streptomycin), S. gordonii ATCC 49818 (500 μg/ml streptomycin), S. salivarius K12 (100 μg/ml spectinomycin), V. cholerae C6706 int I4::TnFL63 and S. enterica serovar Typhimurium 140285 put::Kan (50 μg/ml kanamycin). S. agalactiae COH1 was distinguished from E. faecalis on Chrome indicator Agar (CHROMagar StrepB SB282). We were unable to differentially select E. coli, L. lactis, S. pyogenes and S. mitis from E. faecalis based on antibiotic sensitivity. Therefore, colony counts of these bacteria in co-culture experiments were acquired by subtracting the E. faecalis colony numbers on selective media from the total number of colonies on non-selective media. Strains harboring pLZ12A and its derivatives were grown in the presence of 20 μg/ml chloramphenicol and strains carrying pCIEtm and pCIEtm derivatives were selected on media containing 5 μg/ml tetracycline.
Bioinformatic analyses
Genome sequences of E. faecalis V583 (NC_004668.1) and OG1RF (NC_017316.1) were obtained from NCBI. Alignments were generated and visualized using EasyFig [97]. OG1RF protein domains were identified using KEGG [98] and ExPASy PROSITE [99]. Structure modeling of OG1RF_12414 was done with Phyre2 [48]. Crystal structures overlays were generated using Pymol [100]. The EsxA phylogenetic tree was constructed in MEGA version X [101] using non-redundant protein sequences obtained from NCBI BLAST [102] with OG1RF_11100 as input and was edited using the Interactive Tree Of Life browser [103]. OG1RF_11109 was used as an input for the NCBI Conserved Domain Architecture Retrieval Tool [104] to identify protein domains that co-occur with LXG domains in Enterococcus (NCBI:txid1350).
Antibiotic sensitivity profiles
Antibiotic susceptibility profiles for ampicillin, vancomycin, and daptomycin were determined using a broth microdilution assay. Overnight (O/N) E. faecalis OG1RF cultures were diluted to 5 × 106 CFU/ml and 100 μl was added to each well of a 96-well plate to give a final cell density of 5 × 105 CFU/ml. Antibiotic stocks were added to the first column of each row, mixed thoroughly, and serially diluted 2-fold across the rows. The last column was used as a no drug control. Cultures containing daptomycin were supplemented with 50 μg/ml CaCl2. Bacterial growth was monitored by measuring absorbance (OD600) using a Synergy H1 microplate reader set to 37°C with continuous shaking O/N. Growth curves are presented as the average of three biological replicates. A concentration of antibiotic just below the drug amount that inhibits bacterial growth was deemed sub-lethal and used to examine T7SS genes expression.
Co-culture bacterial antagonism assays
For inter- and intraspecies antagonism assays in liquid media, O/N cultures of different bacteria were diluted in THB containing 10mM MgSO4 to an OD600 of 0.2 and mixed together in a 1:1 or 10:1 ratio. The mixed cell suspensions were either left untreated or treated with phage VPE25 (MOI 0.01) or daptomycin (2.5 μg/ml) and grown at 37°C with aeration. For pheromone induction of genes OG1RF_11121, ireK and OG1RF_11099 cloned into pCIEtm, 10 ng/ml cCF10 (from Mimotopes) was added at the time of phage administration. For antagonism experiments on agar plates, O/N cultures of different strains were diluted to an OD600 of 0.2 and mixed together in a 1:1 or 10:1 ratio. A total of 107 cells from mixed culture suspension was added to 5 ml THB + 0.35% agar at 55°C and were poured over the surface of a THB agar plate in the absence or presence of daptomycin (0.5 μg/ml). The plates were incubated at 37°C under static conditions for 24 hours. Cells were harvested by scraping off the top agar, resuspending in 5 ml of PBS, and the cfus were obtained by plating serially diluted cell suspension on appropriate selective agar plates. Relative viability was calculated from the ratio of target strain cfu in the treated versus the untreated co-culture. The assays were performed in biological triplicates.
RNA extraction and quantitative PCR
RNA was extracted from phage, antibiotic, or 4% sodium cholate treated or untreated E. faecalis OG1RF cells using an RNeasy Mini Kit (Qiagen) with the following published modifications [24]. cDNA was generated from 1 μg of RNA using qScript cDNA SuperMix (QuantaBio) and transcript levels were analyzed by qPCR using PowerUp SYBR Green Master Mix (Applied Biosystems). Transcript abundances were normalized to 16S rRNA gene transcripts and fold–change was calculated by comparing to untreated controls. All data are represented as the average of three biological replicates. All the primers used for qPCR are listed in S1 Table.
Bacterial growth curves
25 ml of 10mM MgSO4 supplemented THB was inoculated with O/N cultures of E. faecalis diluted to an OD600 of 0.025 and distributed to a 96-well plate in 0.1 ml volumes. Cultures were incubated at 37° C with aeration. OD600 was measured periodically for 18 hours in a Synergy H1 microplate reader.
Efficiency of plating (EOP) assays
To investigate if phage VPE25 can infect and lyse E. faecalis mutants and various other bacterial species, 107 PFU/ml of phage was serially diluted and the phage was titered on each strain using a THB agar overlay plaque assay. EOP is expressed as the percentage of phage titer from each strain relative to the wild type E. faecalis OG1RF control. Data are presented as the average of three biological replicates.
Construction of E. faecalis mutants and complementation
Isolation of E. faecalis genomic DNA was performed using a ZymoBIOMICS DNA Miniprep Kit (Zymo Research). All PCR used for cloning were performed with high fidelity KOD Hot Start DNA Polymerase (EMD Millipore). E. faecalis ΔessB was generated by allelic replacement by cloning an in frame essB deletion product into pLT06 using Gibson Assembly Master Mix (New England Biolabs), integrating this construct into the chromosome, and resolving the deletion mutant by homologous recombination [105–107]. For ectopic expression of putative immunity proteins, coding regions of OG1RF_11110, OG1RF_11112, OG1RF_11122, and OG1RF_12413 were cloned downstream of the bacA promoter (PbacA) by restriction digestion and ligation into the shuttle vector pLZ12A [20]. Coding regions of ireK and OG1RF_11099 were cloned downstream of the cCF10 responsive promoter (PQ) by restriction digestion and ligation into pCIE and pCIEtm vectors, respectively. As attempts to clone OG1RF_11121 by itself were unsuccessful, we cloned the OG1RF_11121 and OG1RF_11122 open reading frames, which overlap by 13 base pairs, together under the PQ promoter in pCIEtm plasmid. Primer sequences and restriction enzymes used for cloning are listed in S1 Table. Plasmids were introduced into electrocompetent E. faecalis cells as previously described [20].
Statistical analysis
Statistical tests were performed using GraphPad–Prism version 8.2.1. For qPCR and bacterial competition assays, unpaired Student’s t-tests were used. P values are indicated in the figure legends.
Supporting information
Non-redundant sequences (n = 96) were identified using NCBI BLAST with OG1RF EsxA (OG1RF_11100) as the input. The tree was constructed in MEGAX using the Maximum Likelihood method and JTT matrix-based model and is drawn to scale, with branch lengths measured in the number of substitutions per site. The tree with the highest log likelihood (-3544.39) is shown. E. faecalis sequences are highlighted in purple, and the GenBank identifier for EsxA from OG1RF (AEA93787.1) is shown in red font.
(TIF)
(A) The measurement of phage particles released from wild type E. faecalis OG1RF, ΔessB, OG1RF_11121-Tn, and OG1RF_11099-Tn mutant strains following phage VPE25 infection. (B) Viability of strains of the OG1RF background exhibiting differential T7SS activity in the absence and presence of phage. (C) Viability of T7SS susceptible strains during intraspecies competition experiments in the absence and presence of phage. Data represent three biological replicates. Error bars indicate standard deviation. *P < 0.0001 by unpaired Student’s t-test.
(TIF)
Intraspecies competition experiment performed in the presence of unfiltered supernatant from phage treated and untreated E. faecalis wild type OG1RF or ΔessB added (A) to the top of a well separated by a 0.4 μm membrane from the bottom well containing E. faecalis ΔpipV583 culture, and bacterial viability was determined after 24 hours, or (B) directly into E. faecalis ΔpipV583 culture in microtiter plate wells (P = 0.7955 by two-way analysis of variance [ANOVA]). (C) Growth of ΔpipV583 was monitored in the presence of filtered supernatant from uninfected and phage infected cultures of wild type E. faecalis OG1RF and ΔessB (P = 0.0883 by two-way analysis of variance [ANOVA]). E. faecalis ΔpipV583 cultures in all of these three contact-dependent assays contained gentamicin (25 μg/ml) to prevent growth of the OG1RF background strains that may have carried over in unfiltered supernatants. Error bars indicate standard deviation.
(TIF)
OG1RF_11111, OG1RF_11113, and OG1RF_11123 sequences were used as input for NCBI BLAST. Alignments and homology were rendered in EasyFig.
(TIF)
(A) Schematic showing homology between V583 (NC_004668.1, top) and OG1RF (NC_017316.1, bottom). Sequences were obtained from NCBI, and homology comparisons were rendered in EasyFig. (B) Cartoon depicting the LXG domain of OG1RF_12414 (identified using KEGG and ExPASy PROSITE). (C) Predicted structural homology between OG1RF_12414 (lilac) and the Pseudomonas protogens Pf-5 Tne2/Tni2 complex (PDB 6B12). Tne2 is shown in green, and Tni2 is shown in gray. Structural modeling was done using PHYRE2, and images were rendered in Pymol.
(TIF)
Domain architectures were identified using the NCBI Conserved Domain Architectural Retrieval Tool (DART) with OG1RF_11109 as an input. Diagrams are drawn to scale.
(TIF)
Growth of wild type E. faecalis OG1RF was monitored over 20 hours in the presence or absence of (A) ampicillin, (B) vancomycin and (C) daptomycin in microtiter plates. The antibiotic concentrations highlighted with a blue box were deemed sub-inhibitory and used to investigate T7SS gene expression levels. Early log-phase cultures of E. faecalis OG1RF were grown in the presence or absence of (D) mitomycin C (4 μg/ml) or (E) ciprofloxacin (2 μg/ml) to show that these concentrations of DNA targeting antibiotics do not prevent bacterial growth. Error bars indicate standard deviation.
(TIF)
Growth of different enterococcal strains either untreated or treated with 6.25 μg/ml, 2.5 μg/ml or 0.5 μg/ml of daptomycin in (A–C) liquid media. (D) T7SS transcripts were measured from E. faecalis OG1RF cells grown in liquid media containing either no daptomycin or 6.25 μg/ml, 2.5 μg/ml, or 0.5 μg/ml of daptomycin. The data are expressed as the average of three biological replicates ± the standard deviation. P < 0.001 by unpaired Student’s t-test. (E) Viable bacterial cells recovered from growth on daptomycin supplemented agar media for 24 hours. The dashed line indicates the limit of detection.
(TIF)
(A) Optical density of wild type E. faecalis OG1RF grown in the absence and presence of 4% sodium cholate was measured for 18 hours. (B) Transcript levels of OG1RF T7SS genes in untreated and 4% sodium cholate treated E. faecalis OG1RF after 4 hours. P < 0.001 to by unpaired Student’s t-test. Error bars indicate standard deviation.
(TIF)
(A) Optical density of wild type E. faecalis OG1RF and isogenic mutants were monitored for 18 hours. (B) While all strains were susceptible to phage VPE25 infection, the proportion of released phage particles was diminished in the croR and croS transposon mutant background. Data represent three biological replicates. Error bars indicate standard deviation. *P < 0.001 by unpaired Student’s t-test.
(TIF)
(A- F) mRNA transcript levels of T7SS genes are enhanced in the transposon mutants of liaR, liaS, croR and croS strains similar to wild type E. faecalis OG1RF during phage infection (MOI = 1) compared to untreated controls. Data represent three biological replicates. Error bars indicate standard deviation. *P < 0.01, **P < 0.0001 by unpaired Student’s t-test.
(TIF)
(A) Transcription of T7SS genes in E. faecalis OG1RF are not elevated 20 minutes post ceftriaxone (128μg/ml) administration relative to an untreated control. (B) OG1RF_11099 expression remains unaltered during phage predation of wild type E. faecalis OG1RF and ΔireK strains relative to uninfected controls. Data represent three biological replicates. Error bars indicate standard deviation.
(TIF)
(DOCX)
Acknowledgments
We would like to thank Andrés Vázquez-Torres, Laurel Lenz, Alex Horswill, Stefan Pukatzki, Kelly Doran, and their lab members for sharing bacterial strains used in this study. We thank Michelle Korir for the ireK complementation plasmid construct and strain.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lebreton F, Willems RJL, Gilmore MS. Enterococcus diversity, origins in nature, and gut colonization. In: Gilmore MS, Clewell DB, Ike Y, Shankar N, editors. Enterococci: from commensals to leading causes of drug resistant infection. Boston; 2014. [PubMed]
- 2.Onderdonk AB, Bartlett JG, Louie T, Sullivan-Seigler N, Gorbach SL. Microbial synergy in experimental intra-abdominal abscess. Infect Immun. 1976;13(1):22–6. Epub 1976/01/01. 10.1128/IAI.13.1.22-26.1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiner-Lastinger LM, Abner S, Edwards JR, Kallen AJ, Karlsson M, Magill SS, et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect Control Hosp Epidemiol. 2020;41(1):1–18. Epub 2019/11/27. 10.1017/ice.2019.296 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arias CA, Panesso D, McGrath DM, Qin X, Mojica MF, Miller C, et al. Genetic basis for in vivo daptomycin resistance in enterococci. N Engl J Med. 2011;365(10):892–900. Epub 2011/09/09. 10.1056/NEJMoa1011138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Wang Y, Wu C, Shen Z, Schwarz S, Du XD, et al. First report of the multidrug resistance gene cfr in Enterococcus faecalis of animal origin. Antimicrob Agents Chemother. 2012;56(3):1650–4. Epub 2011/12/29. 10.1128/AAC.06091-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel SN, Memari N, Shahinas D, Toye B, Jamieson FB, Farrell DJ. Linezolid resistance in Enterococcus faecium isolated in Ontario, Canada. Diagn Microbiol Infect Dis. 2013;77(4):350–3. Epub 2013/10/08. 10.1016/j.diagmicrobio.2013.08.012 . [DOI] [PubMed] [Google Scholar]
- 7.Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120(12):4332–41. Epub 2010/11/26. 10.1172/JCI43918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zirakzadeh A, Patel R. Vancomycin-resistant enterococci: colonization, infection, detection, and treatment. Mayo Clin Proc. 2006;81(4):529–36. Epub 2006/04/14. 10.4065/81.4.529 . [DOI] [PubMed] [Google Scholar]
- 9.Taur Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55(7):905–14. Epub 2012/06/22. 10.1093/cid/cis580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munoz-Price LS, Lolans K, Quinn JP. Emergence of resistance to daptomycin during treatment of vancomycin-resistant Enterococcus faecalis infection. Clin Infect Dis. 2005;41(4):565–6. Epub 2005/07/20. 10.1086/432121 . [DOI] [PubMed] [Google Scholar]
- 11.Palmer KL, Daniel A, Hardy C, Silverman J, Gilmore MS. Genetic basis for daptomycin resistance in enterococci. Antimicrob Agents Chemother. 2011;55(7):3345–56. Epub 2011/04/20. 10.1128/AAC.00207-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jasni AS, Mullany P, Hussain H, Roberts AP. Demonstration of conjugative transposon (Tn5397)-mediated horizontal gene transfer between Clostridium difficile and Enterococcus faecalis. Antimicrob Agents Chemother. 2010;54(11):4924–6. Epub 2010/08/18. 10.1128/AAC.00496-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer KL, Kos VN, Gilmore MS. Horizontal gene transfer and the genomics of enterococcal antibiotic resistance. Curr Opin Microbiol. 2010;13(5):632–9. Epub 2010/09/15. 10.1016/j.mib.2010.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, Downes FP, et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med. 2003;348(14):1342–7. Epub 2003/04/04. 10.1056/NEJMoa025025 . [DOI] [PubMed] [Google Scholar]
- 15.Kurenbach B, Bohn C, Prabhu J, Abudukerim M, Szewzyk U, Grohmann E. Intergeneric transfer of the Enterococcus faecalis plasmid pIP501 to Escherichia coli and Streptomyces lividans and sequence analysis of its tra region. Plasmid. 2003;50(1):86–93. Epub 2003/06/27. 10.1016/s0147-619x(03)00044-1 . [DOI] [PubMed] [Google Scholar]
- 16.Kortright KE, Chan BK, Koff JL, Turner PE. Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe. 2019;25(2):219–32. Epub 2019/02/15. 10.1016/j.chom.2019.01.014 . [DOI] [PubMed] [Google Scholar]
- 17.Cheng M, Liang J, Zhang Y, Hu L, Gong P, Cai R, et al. The bacteriophage EF-P29 efficiently protects against lethal vancomycin-resistant Enterococcus faecalis and alleviates gut microbiota imbalance in a murine bacteremia model. Front Microbiol. 2017;8:837 Epub 2017/05/26. 10.3389/fmicb.2017.00837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelman D, Beyth S, Lerer V, Adler K, Poradosu-Cohen R, Coppenhagen-Glazer S, et al. Combined bacteriophages and antibiotics as an efficient therapy against VRE Enterococcus faecalis in a mouse model. Res Microbiol. 2018;169(9):531–9. Epub 2018/05/20. 10.1016/j.resmic.2018.04.008 . [DOI] [PubMed] [Google Scholar]
- 19.Uchiyama J, Rashel M, Takemura I, Wakiguchi H, Matsuzaki S. In silico and in vivo evaluation of bacteriophage phiEF24C, a candidate for treatment of Enterococcus faecalis infections. Appl Environ Microbiol. 2008;74(13):4149–63. Epub 2008/05/06. 10.1128/AEM.02371-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chatterjee A, Johnson CN, Luong P, Hullahalli K, McBride SW, Schubert AM, et al. Bacteriophage resistance alters antibiotic-mediated intestinal expansion of enterococci. Infect Immun. 2019;87(6):e00085–19. Epub 2019/04/03. 10.1128/IAI.00085-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin DM, Koskella B, Lin HC. Phage therapy: an alternative to antibiotics in the age of multi-drug resistance. World J Gastrointest Pharmacol Ther. 2017;8(3):162–73. Epub 2017/08/23. 10.4292/wjgpt.v8.i3.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loc-Carrillo C, Abedon ST. Pros and cons of phage therapy. Bacteriophage. 2011;1(2):111–4. Epub 2012/02/16. 10.4161/bact.1.2.14590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu S, Zachary E, Wells K, Loc-Carrillo C. Phage Therapy: future inquiries. Postdoc J. 2013;1(6):24–35. Epub 2013/06/01. [PMC free article] [PubMed] [Google Scholar]
- 24.Chatterjee A, Willett JLE, Nguyen UT, Monogue B, Palmer KL, Dunny GM, et al. Parallel Genomics Uncover Novel Enterococcal-Bacteriophage Interactions. mBio. 2020;11(2). Epub 2020/03/05. 10.1128/mBio.03120-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unnikrishnan M, Constantinidou C, Palmer T, Pallen MJ. The enigmatic Esx proteins: looking beyond mycobacteria. Trends Microbiol. 2017;25(3):192–204. Epub 2016/11/30. 10.1016/j.tim.2016.11.004 . [DOI] [PubMed] [Google Scholar]
- 26.Cao Z, Casabona MG, Kneuper H, Chalmers JD, Palmer T. The type VII secretion system of Staphylococcus aureus secretes a nuclease toxin that targets competitor bacteria. Nat Microbiol. 2016;2:16183 Epub 2016/10/11. 10.1038/nmicrobiol.2016.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitney JC, Peterson SB, Kim J, Pazos M, Verster AJ, Radey MC, et al. A broadly distributed toxin family mediates contact-dependent antagonism between Gram-positive bacteria. Elife. 2017;6 Epub 2017/07/12. 10.7554/eLife.26938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burts ML, DeDent AC, Missiakas DM. EsaC substrate for the ESAT-6 secretion pathway and its role in persistent infections of Staphylococcus aureus. Mol Microbiol. 2008;69(3):736–46. Epub 2008/06/17. 10.1111/j.1365-2958.2008.06324.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishii K, Adachi T, Yasukawa J, Suzuki Y, Hamamoto H, Sekimizu K. Induction of virulence gene expression in Staphylococcus aureus by pulmonary surfactant. Infect Immun. 2014;82(4):1500–10. Epub 2014/01/24. 10.1128/IAI.01635-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez MS, Tan IS, Yan D, Kang J, McCreary M, Modrusan Z, et al. Host-derived fatty acids activate type VII secretion in Staphylococcus aureus. Proc Natl Acad Sci U S A. 2017;114(42):11223–8. Epub 2017/10/05. 10.1073/pnas.1700627114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tchoupa AK, Watkins KE, Jones RA, Kuroki A, Alam MT, Perrier S, et al. The type VII secretion system protects Staphylococcus aureus against antimicrobial host fatty acids. bioRxiv. 2020:572172 10.1101/572172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourgogne A, Garsin DA, Qin X, Singh KV, Sillanpaa J, Yerrapragada S, et al. Large scale variation in Enterococcus faecalis illustrated by the genome analysis of strain OG1RF. Genome Biol. 2008;9(7):R110 Epub 2008/07/10. 10.1186/gb-2008-9-7-r110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulsen IT, Banerjei L, Myers GS, Nelson KE, Seshadri R, Read TD, et al. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science. 2003;299(5615):2071–4. Epub 2003/03/29. 10.1126/science.1080613 . [DOI] [PubMed] [Google Scholar]
- 34.Reiter WD, Palm P, Yeats S. Transfer RNA genes frequently serve as integration sites for prokaryotic genetic elements. Nucleic Acids Res. 1989;17(5):1907–14. 10.1093/nar/17.5.1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lebreton F, Manson AL, Saavedra JT, Straub TJ, Earl AM, Gilmore MS. Tracing the enterococci from paleozoic origins to the hospital. Cell. 2017;169(5):849–61 e13. Epub 2017/05/16. 10.1016/j.cell.2017.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alhajjar N, Chatterjee A, Spencer BL, Burcham LR, Willett JLE, Dunny GM, et al. Genome-wide mutagenesis identifies factors involved in Enterococcus faecalis vaginal adherence and persistence. Infection and Immunity. 2020:IAI.00270-20. 10.1128/IAI.00270-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das C, Ghosh TS, Mande SS. In silico dissection of type VII secretion system components across bacteria: new directions towards functional characterization. J Biosci. 2016;41(1):133–43. Epub 2016/03/08. 10.1007/s12038-016-9599-8 . [DOI] [PubMed] [Google Scholar]
- 38.Burts ML, Williams WA, DeBord K, Missiakas DM. EsxA and EsxB are secreted by an ESAT-6-like system that is required for the pathogenesis of Staphylococcus aureus infections. Proc Natl Acad Sci U S A. 2005;102(4):1169–74. Epub 2005/01/20. 10.1073/pnas.0405620102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duerkop BA, Huo W, Bhardwaj P, Palmer KL, Hooper LV. Molecular Basis for Lytic Bacteriophage Resistance in Enterococci. mBio. 2016;7(4). Epub 2016/09/01. 10.1128/mBio.01304-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ulhuq FR, Gomes MC, Duggan GM, Guo M, Mendonca C, Buchanan G, et al. A membrane-depolarizing toxin substrate of the Staphylococcus aureus type VII secretion system mediates intraspecies competition. Proc Natl Acad Sci U S A. 2020. Epub 2020/08/10. 10.1073/pnas.2006110117 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia EC. Contact-dependent interbacterial toxins deliver a message. Curr Opin Microbiol. 2018;42:40–6. Epub 2017/10/28. 10.1016/j.mib.2017.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Bayona L, Comstock LE. Bacterial antagonism in host-associated microbial communities. Science. 2018;361(6408). Epub 2018/09/22. 10.1126/science.aat2456 . [DOI] [PubMed] [Google Scholar]
- 43.Zhang D, de Souza RF, Anantharaman V, Iyer LM, Aravind L. Polymorphic toxin systems: comprehensive characterization of trafficking modes, processing, mechanisms of action, immunity and ecology using comparative genomics. Biol Direct. 2012;7:18 Epub 2012/06/27. 10.1186/1745-6150-7-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klein TA, Pazos M, Surette MG, Vollmer W, Whitney JC. Molecular basis for immunity protein recognition of a type VII secretion system exported antibacterial toxin. J Mol Biol. 2018;430(21):4344–58. Epub 2018/09/09. 10.1016/j.jmb.2018.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jamet A, Nassif X. New players in the toxin field: polymorphic toxin systems in bacteria. mBio. 2015;6(3):e00285–15. Epub 2015/05/07. 10.1128/mBio.00285-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poole SJ, Diner EJ, Aoki SK, Braaten BA, t'Kint de Roodenbeke C, Low DA, et al. Identification of functional toxin/immunity genes linked to contact-dependent growth inhibition (CDI) and rearrangement hotspot (Rhs) systems. PLoS Genet. 2011;7(8):e1002217 Epub 2011/08/11. 10.1371/journal.pgen.1002217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koskiniemi S, Garza-Sánchez F, Sandegren L, Webb JS, Braaten BA, Poole SJ, et al. Selection of orphan Rhs toxin expression in evolved Salmonella enterica serovar Typhimurium. PLoS Genet. 2014;10(3):e1004255 10.1371/journal.pgen.1004255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10(6):845–58. Epub 2015/05/07. 10.1038/nprot.2015.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiener M, Freymann D, Ghosh P, Stroud RM. Crystal structure of colicin Ia. Nature. 1997;385(6615):461–4. 10.1038/385461a0 . [DOI] [PubMed] [Google Scholar]
- 50.Tang JY, Bullen NP, Ahmad S, Whitney JC. Diverse NADase effector families mediate interbacterial antagonism via the type VI secretion system. J Biol Chem. 2018;293(5):1504–14. Epub 2017/12/15. 10.1074/jbc.RA117.000178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michalska K, Quan Nhan D, Willett JLE, Stols LM, Eschenfeldt WH, Jones AM, et al. Functional plasticity of antibacterial EndoU toxins. Mol Microbiol. 2018;109(4):509–27. Epub 2018/06/21. 10.1111/mmi.14007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang D, Iyer LM, Aravind L. A novel immunity system for bacterial nucleic acid degrading toxins and its recruitment in various eukaryotic and DNA viral systems. Nucleic Acids Res. 2011;39(11):4532–52. Epub 2011/02/11. 10.1093/nar/gkr036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein TA, Ahmad S, Whitney JC. Contact-dependent interbacterial antagonism mediated by protein secretion machines. Trends Microbiol. 2020;28(5):387–400. Epub 2020/04/17. 10.1016/j.tim.2020.01.003 . [DOI] [PubMed] [Google Scholar]
- 54.Andersson DI, Hughes D. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol. 2014;12(7):465–78. Epub 2014/05/28. 10.1038/nrmicro3270 . [DOI] [PubMed] [Google Scholar]
- 55.Bernier SP, Surette MG. Concentration-dependent activity of antibiotics in natural environments. Front Microbiol. 2013;4:20 Epub 2013/02/21. 10.3389/fmicb.2013.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yim G, Wang HH, Davies J. Antibiotics as signalling molecules. Philos Trans R Soc Lond B Biol Sci. 2007;362(1483):1195–200. Epub 2007/03/16. 10.1098/rstb.2007.2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flahaut S, Frere J, Boutibonnes P, Auffray Y. Comparison of the bile salts and sodium dodecyl sulfate stress responses in Enterococcus faecalis. Appl Environ Microbiol. 1996;62(7):2416–20. Epub 1996/07/01. 10.1128/AEM.62.7.2416-2420.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47(2):241–59. Epub 2005/11/22. 10.1194/jlr.R500013-JLR200 . [DOI] [PubMed] [Google Scholar]
- 59.Dale JL, Cagnazzo J, Phan CQ, Barnes AM, Dunny GM. Multiple roles for Enterococcus faecalis glycosyltransferases in biofilm-associated antibiotic resistance, cell envelope integrity, and conjugative transfer. Antimicrob Agents Chemother. 2015;59(7):4094–105. Epub 2015/04/29. 10.1128/AAC.00344-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kellogg SL, Kristich CJ. Functional dissection of the CroRS two-component system required for resistance to cell wall stressors in Enterococcus faecalis. J Bacteriol. 2016;198(8):1326–36. Epub 2016/02/18. 10.1128/JB.00995-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kristich CJ, Wells CL, Dunny GM. A eukaryotic-type Ser/Thr kinase in Enterococcus faecalis mediates antimicrobial resistance and intestinal persistence. Proc Natl Acad Sci U S A. 2007;104(9):3508–13. Epub 2007/03/16. 10.1073/pnas.0608742104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tran TT, Panesso D, Mishra NN, Mileykovskaya E, Guan Z, Munita JM, et al. Daptomycin-resistant Enterococcus faecalis diverts the antibiotic molecule from the division septum and remodels cell membrane phospholipids. mBio. 2013;4(4). Epub 2013/07/25. 10.1128/mBio.00281-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reyes J, Panesso D, Tran TT, Mishra NN, Cruz MR, Munita JM, et al. A liaR deletion restores susceptibility to daptomycin and antimicrobial peptides in multidrug-resistant Enterococcus faecalis. J Infect Dis. 2015;211(8):1317–25. Epub 2014/11/02. 10.1093/infdis/jiu602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Comenge Y, Quintiliani R Jr., Li L, Dubost L, Brouard JP, Hugonnet JE, et al. The CroRS two-component regulatory system is required for intrinsic beta-lactam resistance in Enterococcus faecalis. J Bacteriol. 2003;185(24):7184–92. Epub 2003/12/03. 10.1128/jb.185.24.7184-7192.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hancock L, Perego M. Two-component signal transduction in Enterococcus faecalis. J Bacteriol. 2002;184(21):5819–25. Epub 2002/10/11. 10.1128/jb.184.21.5819-5825.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hancock LE, Perego M. Systematic inactivation and phenotypic characterization of two-component signal transduction systems of Enterococcus faecalis V583. J Bacteriol. 2004;186(23):7951–8. Epub 2004/11/18. 10.1128/JB.186.23.7951-7958.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kristich CJ, Little JL, Hall CL, Hoff JS. Reciprocal regulation of cephalosporin resistance in Enterococcus faecalis. mBio. 2011;2(6):e00199–11. Epub 2011/11/03. 10.1128/mBio.00199-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Banla IL, Kommineni S, Hayward M, Rodrigues M, Palmer KL, Salzman NH, et al. Modulators of Enterococcus faecalis cell envelope integrity and antimicrobial resistance influence stable colonization of the mammalian gastrointestinal tract. Infect Immun. 2018;86(1). Epub 2017/10/19. 10.1128/IAI.00381-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kellogg SL, Kristich CJ. Convergence of PASTA kinase and two-component signaling in response to cell wall stress in Enterococcus faecalis. J Bacteriol. 2018;200(12). Epub 2018/04/11. 10.1128/JB.00086-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dale JL, Beckman KB, Willett JLE, Nilson JL, Palani NP, Baller JA, et al. Comprehensive functional analysis of the Enterococcus faecalis core genome using an ordered, sequence-defined collection of insertional mutations in strain OG1RF. mSystems. 2018;3(5). Epub 2018/09/19. 10.1128/mSystems.00062-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Labbe BD, Kristich CJ. Growth- and stress-induced PASTA kinase phosphorylation in Enterococcus faecalis. J Bacteriol. 2017;199(21). Epub 2017/08/16. 10.1128/JB.00363-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Madsen JS, Sorensen SJ, Burmolle M. Bacterial social interactions and the emergence of community-intrinsic properties. Curr Opin Microbiol. 2018;42:104–9. Epub 2017/12/05. 10.1016/j.mib.2017.11.018 . [DOI] [PubMed] [Google Scholar]
- 73.Xavier JB, Foster KR. Cooperation and conflict in microbial biofilms. Proc Natl Acad Sci U S A. 2007;104(3):876–81. Epub 2007/01/11. 10.1073/pnas.0607651104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leskinen K, Blasdel BG, Lavigne R, Skurnik M. RNA-sequencing reveals the progression of phage-host interactions between phiR1-37 and Yersinia enterocolitica. Viruses. 2016;8(4):111 Epub 2016/04/26. 10.3390/v8040111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mojardin L, Salas M. Global transcriptional analysis of virus-host interactions between phage Φphi29 and Bacillus subtilis. J Virol. 2016;90(20):9293–304. Epub 2016/08/05. 10.1128/JVI.01245-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sacher JC, Flint A, Butcher J, Blasdel B, Reynolds HM, Lavigne R, et al. Transcriptomic analysis of the Campylobacter jejuni response to T4-like phage NCTC 12673 infection. Viruses. 2018;10(6). Epub 2018/06/20. 10.3390/v10060332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Sordi L, Khanna V, Debarbieux L. The gut microbiota facilitates drifts in the genetic diversity and infectivity of bacterial viruses. Cell Host Microbe. 2017;22(6):801–8 e3. Epub 2017/11/28. 10.1016/j.chom.2017.10.010 . [DOI] [PubMed] [Google Scholar]
- 78.Hsu BB, Gibson TE, Yeliseyev V, Liu Q, Lyon L, Bry L, et al. Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host Microbe. 2019;25(6):803–14 e5. Epub 2019/06/09. 10.1016/j.chom.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reyes A, Wu M, McNulty NP, Rohwer FL, Gordon JI. Gnotobiotic mouse model of phage-bacterial host dynamics in the human gut. Proc Natl Acad Sci U S A. 2013;110(50):20236–41. Epub 2013/11/22. 10.1073/pnas.1319470110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dissanayake U, Ukhanova M, Moye ZD, Sulakvelidze A, Mai V. Bacteriophages reduce pathogenic Escherichia coli counts in mice without distorting gut microbiota. Front Microbiol. 2019;10:1984 Epub 2019/09/26. 10.3389/fmicb.2019.01984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ruhe ZC, Wallace AB, Low DA, Hayes CS. Receptor polymorphism restricts contact-dependent growth inhibition to members of the same species. mBio. 2013;4(4). Epub 2013/07/25. 10.1128/mBio.00480-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cascales E, Buchanan SK, Duche D, Kleanthous C, Lloubes R, Postle K, et al. Colicin biology. Microbiol Mol Biol Rev. 2007;71(1):158–229. Epub 2007/03/10. 10.1128/MMBR.00036-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruhe ZC, Low DA, Hayes CS. Polymorphic toxins and their immunity proteins: diversity, evolution, and mechanisms of delivery. Annu Rev Microbiol. 2020. Epub 2020/07/19. 10.1146/annurev-micro-020518-115638 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kohler T, Donner V, van Delden C. Lipopolysaccharide as shield and receptor for R-pyocin-mediated killing in Pseudomonas aeruginosa. J Bacteriol. 2010;192(7):1921–8. Epub 2010/02/02. 10.1128/JB.01459-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ruhe ZC, Nguyen JY, Xiong J, Koskiniemi S, Beck CM, Perkins BR, et al. CdiA effectors use modular receptor-binding domains to recognize target bacteria. mBio. 2017;8(2). Epub 2017/03/30. 10.1128/mBio.00290-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Willett JL, Gucinski GC, Fatherree JP, Low DA, Hayes CS. Contact-dependent growth inhibition toxins exploit multiple independent cell-entry pathways. Proc Natl Acad Sci U S A. 2015;112(36):11341–6. Epub 2015/08/26. 10.1073/pnas.1512124112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coulthurst S. The type VI secretion system: a versatile bacterial weapon. Microbiology. 2019;165(5):503–15. Epub 2019/03/21. 10.1099/mic.0.000789 . [DOI] [PubMed] [Google Scholar]
- 88.Krasauskas R, Skerniškytė J, Martinkus J, Armalytė J, Sužiedėlienė E. Capsule protects Acinetobacter baumannii from inter-bacterial competition mediated by CdiA toxin. Frontiers in Microbiology. 2020;11(1493). 10.3389/fmicb.2020.01493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aoki SK, Malinverni JC, Jacoby K, Thomas B, Pamma R, Trinh BN, et al. Contact-dependent growth inhibition requires the essential outer membrane protein BamA (YaeT) as the receptor and the inner membrane transport protein AcrB. Mol Microbiol. 2008;70(2):323–40. Epub 2008/09/03. 10.1111/j.1365-2958.2008.06404.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Virtanen P, Wäneskog M, Koskiniemi S. Class II contact-dependent growth inhibition (CDI) systems allow for broad-range cross-species toxin delivery within the Enterobacteriaceae family. Mol Microbiol. 2019;111(4):1109–25. Epub 2019/03/18. 10.1111/mmi.14214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269–84. Epub 2015/04/09. 10.1038/nrmicro3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Giacometti A, Cirioni O, Schimizzi AM, Del Prete MS, Barchiesi F, D'Errico MM, et al. Epidemiology and microbiology of surgical wound infections. J Clin Microbiol. 2000;38(2):918–22. Epub 2000/02/03. 10.1128/JCM.38.2.918-922.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keogh D, Tay WH, Ho YY, Dale JL, Chen S, Umashankar S, et al. Enterococcal metabolite cues facilitate interspecies niche modulation and polymicrobial infection. Cell Host Microbe. 2016;20(4):493–503. Epub 2016/10/14. 10.1016/j.chom.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goh HMS, Yong MHA, Chong KKL, Kline KA. Model systems for the study of enterococcal colonization and infection. Virulence. 2017;8(8):1525–62. Epub 2017/01/20. 10.1080/21505594.2017.1279766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.King KC, Brockhurst MA, Vasieva O, Paterson S, Betts A, Ford SA, et al. Rapid evolution of microbe-mediated protection against pathogens in a worm host. ISME J. 2016;10(8):1915–24. Epub 2016/03/16. 10.1038/ismej.2015.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hammer ND, Cassat JE, Noto MJ, Lojek LJ, Chadha AD, Schmitz JE, et al. Inter- and intraspecies metabolite exchange promotes virulence of antibiotic-resistant Staphylococcus aureus. Cell Host Microbe. 2014;16(4):531–7. Epub 2014/10/10. 10.1016/j.chom.2014.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sullivan MJ, Petty NK, Beatson SA. Easyfig: a genome comparison visualizer. Bioinformatics. 2011;27(7):1009–10. Epub 2011/01/28. 10.1093/bioinformatics/btr039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. 10.1093/nar/28.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sigrist CJ, de Castro E, Cerutti L, Cuche BA, Hulo N, Bridge A, et al. New and continuing developments at PROSITE. Nucleic Acids Res. 2013;41(Database issue):D344–7. Epub 2012/11/17. 10.1093/nar/gks1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.DeLano WD. The PyMOL Molecular Graphics System, 1.3. Schroedinger, LLC2010.
- 101.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–9. 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Coordinators NR. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018;46(D1):D8–D13. 10.1093/nar/gkx1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Letunic I, Bork P. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 2019;47(W1):W256–W9. 10.1093/nar/gkz239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Geer LY, Domrachev M, Lipman DJ, Bryant SH. CDART: protein homology by domain architecture. Genome Res. 2002;12(10):1619–23. 10.1101/gr.278202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barnes WM. PCR amplification of up to 35-kb DNA with high fidelity and high yield from lambda bacteriophage templates. Proc Natl Acad Sci U S A. 1994;91(6):2216–20. Epub 1994/03/15. 10.1073/pnas.91.6.2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, 3rd, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6(5):343–5. Epub 2009/04/14. 10.1038/nmeth.1318 . [DOI] [PubMed] [Google Scholar]
- 107.Thurlow LR, Thomas VC, Hancock LE. Capsular polysaccharide production in Enterococcus faecalis and contribution of CpsF to capsule serospecificity. J Bacteriol. 2009;191(20):6203–10. Epub 2009/08/18. 10.1128/JB.00592-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305(3):567–80. 10.1006/jmbi.2000.4315 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Non-redundant sequences (n = 96) were identified using NCBI BLAST with OG1RF EsxA (OG1RF_11100) as the input. The tree was constructed in MEGAX using the Maximum Likelihood method and JTT matrix-based model and is drawn to scale, with branch lengths measured in the number of substitutions per site. The tree with the highest log likelihood (-3544.39) is shown. E. faecalis sequences are highlighted in purple, and the GenBank identifier for EsxA from OG1RF (AEA93787.1) is shown in red font.
(TIF)
(A) The measurement of phage particles released from wild type E. faecalis OG1RF, ΔessB, OG1RF_11121-Tn, and OG1RF_11099-Tn mutant strains following phage VPE25 infection. (B) Viability of strains of the OG1RF background exhibiting differential T7SS activity in the absence and presence of phage. (C) Viability of T7SS susceptible strains during intraspecies competition experiments in the absence and presence of phage. Data represent three biological replicates. Error bars indicate standard deviation. *P < 0.0001 by unpaired Student’s t-test.
(TIF)
Intraspecies competition experiment performed in the presence of unfiltered supernatant from phage treated and untreated E. faecalis wild type OG1RF or ΔessB added (A) to the top of a well separated by a 0.4 μm membrane from the bottom well containing E. faecalis ΔpipV583 culture, and bacterial viability was determined after 24 hours, or (B) directly into E. faecalis ΔpipV583 culture in microtiter plate wells (P = 0.7955 by two-way analysis of variance [ANOVA]). (C) Growth of ΔpipV583 was monitored in the presence of filtered supernatant from uninfected and phage infected cultures of wild type E. faecalis OG1RF and ΔessB (P = 0.0883 by two-way analysis of variance [ANOVA]). E. faecalis ΔpipV583 cultures in all of these three contact-dependent assays contained gentamicin (25 μg/ml) to prevent growth of the OG1RF background strains that may have carried over in unfiltered supernatants. Error bars indicate standard deviation.
(TIF)
OG1RF_11111, OG1RF_11113, and OG1RF_11123 sequences were used as input for NCBI BLAST. Alignments and homology were rendered in EasyFig.
(TIF)
(A) Schematic showing homology between V583 (NC_004668.1, top) and OG1RF (NC_017316.1, bottom). Sequences were obtained from NCBI, and homology comparisons were rendered in EasyFig. (B) Cartoon depicting the LXG domain of OG1RF_12414 (identified using KEGG and ExPASy PROSITE). (C) Predicted structural homology between OG1RF_12414 (lilac) and the Pseudomonas protogens Pf-5 Tne2/Tni2 complex (PDB 6B12). Tne2 is shown in green, and Tni2 is shown in gray. Structural modeling was done using PHYRE2, and images were rendered in Pymol.
(TIF)
Domain architectures were identified using the NCBI Conserved Domain Architectural Retrieval Tool (DART) with OG1RF_11109 as an input. Diagrams are drawn to scale.
(TIF)
Growth of wild type E. faecalis OG1RF was monitored over 20 hours in the presence or absence of (A) ampicillin, (B) vancomycin and (C) daptomycin in microtiter plates. The antibiotic concentrations highlighted with a blue box were deemed sub-inhibitory and used to investigate T7SS gene expression levels. Early log-phase cultures of E. faecalis OG1RF were grown in the presence or absence of (D) mitomycin C (4 μg/ml) or (E) ciprofloxacin (2 μg/ml) to show that these concentrations of DNA targeting antibiotics do not prevent bacterial growth. Error bars indicate standard deviation.
(TIF)
Growth of different enterococcal strains either untreated or treated with 6.25 μg/ml, 2.5 μg/ml or 0.5 μg/ml of daptomycin in (A–C) liquid media. (D) T7SS transcripts were measured from E. faecalis OG1RF cells grown in liquid media containing either no daptomycin or 6.25 μg/ml, 2.5 μg/ml, or 0.5 μg/ml of daptomycin. The data are expressed as the average of three biological replicates ± the standard deviation. P < 0.001 by unpaired Student’s t-test. (E) Viable bacterial cells recovered from growth on daptomycin supplemented agar media for 24 hours. The dashed line indicates the limit of detection.
(TIF)
(A) Optical density of wild type E. faecalis OG1RF grown in the absence and presence of 4% sodium cholate was measured for 18 hours. (B) Transcript levels of OG1RF T7SS genes in untreated and 4% sodium cholate treated E. faecalis OG1RF after 4 hours. P < 0.001 to by unpaired Student’s t-test. Error bars indicate standard deviation.
(TIF)
(A) Optical density of wild type E. faecalis OG1RF and isogenic mutants were monitored for 18 hours. (B) While all strains were susceptible to phage VPE25 infection, the proportion of released phage particles was diminished in the croR and croS transposon mutant background. Data represent three biological replicates. Error bars indicate standard deviation. *P < 0.001 by unpaired Student’s t-test.
(TIF)
(A- F) mRNA transcript levels of T7SS genes are enhanced in the transposon mutants of liaR, liaS, croR and croS strains similar to wild type E. faecalis OG1RF during phage infection (MOI = 1) compared to untreated controls. Data represent three biological replicates. Error bars indicate standard deviation. *P < 0.01, **P < 0.0001 by unpaired Student’s t-test.
(TIF)
(A) Transcription of T7SS genes in E. faecalis OG1RF are not elevated 20 minutes post ceftriaxone (128μg/ml) administration relative to an untreated control. (B) OG1RF_11099 expression remains unaltered during phage predation of wild type E. faecalis OG1RF and ΔireK strains relative to uninfected controls. Data represent three biological replicates. Error bars indicate standard deviation.
(TIF)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.