Abstract
The Burkholderia pseudomallei phylogenetic cluster includes B. pseudomallei, B. mallei, B. thailandensis, B. oklahomensis, B. humptydooensis and B. singularis. Regarded as the only pathogenic members of this group, B. pseudomallei and B. mallei cause the diseases melioidosis and glanders, respectively. Additionally, variant strains of B. pseudomallei and B. thailandensis exist that include the geographically restricted B. pseudomallei that express a B. mallei-like BimA protein (BPBM), and B. thailandensis that express a B. pseudomallei-like capsular polysaccharide (BTCV). To establish a PCR-based assay for the detection of pathogenic Burkholderia species or their variants, five PCR primers were designed to amplify species-specific sequences within the bimA (Burkholderia intracellular motility A) gene. Our multiplex PCR assay could distinguish pathogenic B. pseudomallei and BPBM from the non-pathogenic B. thailandensis and the BTCV strains. A second singleplex PCR successfully discriminated the BTCV from B. thailandensis. Apart from B. humptydooensis, specificity testing against other Burkholderia spp., as well as other Gram-negative and Gram-positive bacteria produced a negative result. The detection limit of the multiplex PCR in soil samples artificially spiked with known quantities of B. pseudomallei and B. thailandensis were 5 and 6 CFU/g soil, respectively. Furthermore, comparison between standard bacterial culture and the multiplex PCR to detect B. pseudomallei from 34 soil samples, collected from an endemic area of melioidosis, showed high sensitivity and specificity. This robust, sensitive, and specific PCR assay will be a useful tool for epidemiological study of B. pseudomallei and closely related members with pathogenic potential in soil.
Introduction
Burkholderia pseudomallei, an environmental Gram-negative bacillus, is the causative agent of melioidosis. This potentially fatal infectious disease affects an estimated 165,000 people worldwide, with an estimated 89,000 deaths (54% mortality) per year [1]. B. pseudomallei infections occur mainly through contact with wet contaminated soil or surface water where the bacteria is prevalent [2]. This bacterium forms part of a phylogenetic cluster of Burkholderia species including B. mallei, B. thailandensis, B. humptydooensis, B. oklahomensis and B. singularis [3, 4]. Notably only B. pseudomallei and B. mallei are associated with severe clinical disease with high mortality. As a highly evolved obligate pathogen with no environmental reservoir, B. mallei causes glanders in horses, donkeys and other solipeds, and less commonly zoonotic disease in humans [5].
B. thailandensis, which is a significantly less pathogenic species than B. pseudomallei with no associated biosecurity threat, is often found to coexist with B. pseudomallei as mixed populations in soil and water in Southeast Asia [6]. The prevalence of B. pseudomallei in soil and water defines geographic regions where humans and livestock are at risk of melioidosis. Recently, a survey of these Burkholderia species in soil samples from three regions of Thailand demonstrated that the ratio of B. pseudomallei to B. thailandensis in the Northeast and East, the areas of high prevalence of melioidosis, are much higher than the Central region [6]. Whilst B. thailandensis is commonly regarded as nonpathogenic, on rare occasions B. thailandensis infections in humans have been reported [7–9]. Thus, an effective and simple assay to differentiate between these two species would be clinically useful.
Recently, a phylogenetic subgroup distinct from the ancestral B. thailandensis population incorporating B. thailandensis variants harboring the B. pseudomallei-like polysaccharide, and known as B. thailandensis capsular variants (BTCV), were described [10]. To date BTCV have only been isolated from environmental soil or water in areas where melioidosis is endemic, notably in Thailand in 2018 [11] and Cambodia (strain E555) in 2010 [10]. There are a total of three case reports involving infection with a BTCV strain between 2003 and 2017 in the U.S. [9] and in China [7]. Remarkably, the BTCV possess several B. pseudomallei-like phenotypes including the presence of a B. pseudomallei-like capsule, resistance to human complement binding, and increased intracellular macrophage survival, although they were no more virulent in a murine infection model than a prototypic B. thailandensis strain [10]. Interestingly, immunization of mice with BTCV induced a significantly better protective immune response to B. pseudomallei challenge than that of prototypic B. thailandensis, indicating the importance of the B. pseudomallei-like capsule of the BTCV for immunogenic and protective efficiency [12]. In addition, Riyapa et al. reported that the BTCV strain E555 induced less ROS and neutrophil extracellular trap formation in PMNs than B. thailandensis E264, suggesting the importance of the B. pseudomallei capsule in evading the induction and killing activity of neutrophil extracellular trap formation [13]. Little is known about the distribution of the BTCV in the environment, and its association with the immune status of people living in melioidosis endemic areas. There is interest within the scientific community in the use of BTCV strains, instead of the prototypic B. thailandensis strains, as a surrogate for experimental studies involving B. pseudomallei [14, 15].
The other three members of the Burkholderia pseudomallei complex have been isolated from the environment in very restricted geographical locations, specifically B. oklahomensis from Oklahoma, US, B. humptydooensis from Humpty Doo in the Northern Territory of Australia and B. singularis from a water source in Australia [3, 4, 16]. B. oklahomensis was associated with a non-fatal pelvic wound infection in a farmer as a result of a tractor accident. Identical strains were isolated from the wound and the environment and shown to display significantly lower virulence in a Guinea pig model of infection [16], and more recently in murine and hamster melioidosis models [17]. B. humptydooensis was initially described as a B. thailandensis-like species able to assimilate arabinose in a similar manner to B. thailandensis, and has been named the fifth member of the B. pseudomallei complex [18, 19]. At present this microorganism has not been associated with any human disease and it is not possible to distinguish B. humptydooensis from closely related species by the commonly used biochemical and fatty acid methyl ester analysis [19]. The sixth member of the Burkholderia pseudomallei complex, B. singularis, was described by Vandamme et al in 2017 [4]. This micro-organism was isolated from a water source in Australia. B. singularis appears to be an opportunistic pathogen of Cystic Fibrosis (CF) patients, having been isolated from a CF patient in Germany and a patient in Canada [4].
Besides the existence of B. thailandensis and its capsule variants, genetic diversity within the B. pseudomallei strains also exists, where some geographically restricted strains express a B. mallei-like BimA (BPBM). Originally identified in a proportion of Australian B. pseudomallei clinical and environmental strains [20], these BPBMs have also been recorded in India [21]. BPBMs are implicated in neurological melioidosis in patients [22], and can only be distinguished from prototypic B. pseudomallei strains using molecular techniques. Although BPBMs have not yet been isolated in Southeast Asia, the possibility that this variant strain could be present in Thailand or other countries cannot be excluded. Taken together with BTCV, prototypic B. pseudomallei and BPBM strains represent an important group of microorganisms with pathogenic potential, whose environmental presence would be indicative of significant human and animal disease risk.
Conventional culture using Ashdown’s selective agar has remained the “gold standard” for detection of B. pseudomallei, B. thailandensis and other related species from environmental and clinical specimens. However, it is recognized that this method is limited by the length of time to culture these organisms, and the similarities in appearance of colony morphology make it redundant in the differentiation of B. pseudomallei from B. thailandensis [3, 23]. Additional assays are required to differentiate them. Therefore, singleplex PCR techniques targeting a variety of different genes for example the T3SS1 [24] and a serine metalloprotease (mprA) [25], have been established for detection of B. pseudomallei. A multiplex PCR targeting the B. pseudomallei specific gene encoding a Tat domain protein (BPSS0658), a B. thailandensis-specific gene (BTH_I1515) and a conserved B. cenocepacia gene (BCAM2834) has been demonstrated to detect B. pseudomallei, B. thailandensis and members of the B. cepacia complex in both soil and clinical samples [26]. However, the current PCR-based techniques described to date cannot detect the BTCV or differentiate B. pseudomallei from BPBM. Thus, a simple and sensitive PCR assay to identify members of the B. pseudomallei complex with pathogenic potential in environmental soil samples is required.
In this study, we developed a highly sensitive and specific multiplex PCR-based method for screening for the presence of B. pseudomallei, B. thailandensis and their variant strains with pathogenic potential in soil samples. An additional simplex PCR enabled discrimination between B. thailandensis and BTCV strains. The target DNA for amplification in these assays is the bimA (Burkholderia intracellular motility A) gene, which is a bacterial virulence factor that contributes to B. pseudomallei and B. thailandensis actin-based motility in infected host cells [27, 28]. This robust PCR method was successful in the discrimination of the different Burkholderia species and variant strains tested, and in spiked soil samples. This method will be an invaluable tool for epidemiological studies in melioidosis endemic and non-endemic areas.
Materials and methods
Ethical considerations
All clinical strains used in our study were collected as part of previous clinical studies with approval from the relevant Research Ethics committees, patient consent where required and de-identified before use in this work. All soil and water sample collections which took place on the private land were conducted after receiving permission from the owners.
Bacterial strains and DNA isolation
The bacterial strains used in this study are listed in Table 1. Burkholderia spp. DNA, including B. pseudomallei (35 isolates), B. pseudomallei (BPBM) (20 isolates), B. thailandensis (30 isolates), BTCV (10 isolates), B. mallei (2 isolates), B. cepacia (7 isolates), B. multivorans (3 isolates), B. ubonensis (3 isolates), B. anthina (2 isolates), B. cenocepacia (2 isolates), B. diffusa (2 isolates), B. humptydooensis (2 isolates) B. territorii (2 isolates), B. pseudomultivorans (1 isolate), B. oklahomensis (1 isolate) and B. vietnamiensis (1 isolate) were used in this study. Burkholderia spp. including, B. thailandensis, BTCV, B. cepacia, B. multivorans, B. oklahomensis and B. vietnamiensis were cultured in Luria-Bertani broth (LB; Titan Biotech Ltd, Rajasthan, India). Strains of other bacterial species used to determine the specificity of the multiplex PCR were Pseudomonas aeruginosa, Escherichia coli, Acinetobacter baumannii, and Staphylococcus aureus. These bacteria were also cultured in Luria-Bertani (LB) broth at 37°C for 24 h.
Table 1. List of bacterial isolates used in this study.
| Bacteria | Strains/ isolates | Sequence type | Source/ location | Reference |
|---|---|---|---|---|
| Burkholderia spp. | ||||
| B. pseudomallei | 576a | 501 | Human/Sunpasitthiprasong Hospital, Ubon Ratchathani province, Thailand | [29, 30] |
| 1026b | 102 | |||
| 1106a | 70 | |||
| 1710a | 177 | |||
| K96243 | 10 | |||
| NCTC10276 | 46 | Human/ Bangladesh | [31] | |
| 956a, H2613a, H2659a, H2660a | 54 | Human/ Sunpasitthiprasong Hospital, Ubon Ratchathani province, Thailand | [32] | |
| 1986a, H2644a, H2677a, H2689b | 70 | |||
| 2396a | 58 | |||
| H1248a | 298 | |||
| H2708a, H2820a | 60 | |||
| E0024, E0411 | 54 | Soil/ Thailand | [11] | |
| E0031, E0383 | 60 | |||
| E0358, E0359 | 70 | |||
| EFT01, EFT02, EFT03, EFT04, EFT05, EFT06, EFT07, EFT08, EFT09, EFT10, EFT11 | - | |||
| B. pseudomallei expresses a B. mallei-like bimA | MSHR33 | 647 | Human/Royal Darwin Hospital, Darwin, Northern Territory, Australia | [20, 33] |
| MSHR491 | 126 | |||
| MSHR668 | 129 | |||
| MSHR1790 | 439 | |||
| MSHR2262 | 435 | |||
| MSHR2375 | 439 | |||
| MSHR2585 | 778 | |||
| MSHR3325 | 118 | |||
| MSHR3326 | 734 | |||
| MSHR3448 | 680 | |||
| MSHR3509 | 259 | |||
| MSHR3522 | 809 | |||
| MSHR3677 | 456 | |||
| MSHR3689 | 809 | |||
| MSHR3739 | 838 | |||
| MSHR3835 | 778 | |||
| MSHR3902 | 844 | |||
| MSHR4176 | 853 | |||
| MSHR4238 | 869 | |||
| MSHR4445 | 886 | |||
| B. thailandensis | D1, DV1 | - | Soil/ Thailand | [34] |
| E264 | 80 | Soil/ Thailand | [35] | |
| DW503 | 80 | |||
| E27 | 74 | Soil/ Thailand | [11] | |
| E327 | 77 | |||
| E152, E153, E154, E158, E159, E169, E173, E174, E175, E177, E201, E202, E205, E207, E421, E426, E427, E430, E433, E435, E436, E438, E440, E441 | - | |||
| B. thailandensis expresses a B. pseudomallei-like capsule | E555 | 696 | Soil/ Cambodia | [10] |
| SBXSR001, SBXSR007, SBXPL001, SBXPL015, SBXRY031, SBXPR001, SBXCC001, SBXCC003 | 696 | Soil/ Thailand | [11] | |
| WBXUBA33005104 | 696 | Water/ Thailand | [11] | |
| B. mallei | NCTC 3709 | 40 | Animal/ India | [36] |
| NCTC 12938 | 40 | Animal/ China | ||
| B. cepacia | U668, 10223 | - | Human/ Thailand | [32] |
| NCTC10743, NCTC10744 | Human/ US | [36, 37] | ||
| ATCC 25416 | 10 | Soil/ US | [37] | |
| MSMB591 | 1581 | Soil/ Australia | [36] | |
| MSMB1184 | 1615 | |||
| B. multivorans | LMG16660 | 899 | Human/ UK | [38] |
| MSMB2008 | 1767 | Soil/ Australia | [36] | |
| MSMB2021 | 1768 | |||
| B. ubonensis | DMST886 | - | Soil/ Thailand | National Institute of Health, Thailand |
| MSMB22 | 1129 | Soil/ Australia | [36, 39] | |
| MSMB1162 | 1171 | |||
| B. anthina | MSMB649, MSMB1506 | - | Soil/ Australia | [36] |
| B. cenocepacia | MSMB364 | 429 | Water/ Australia | [36] |
| MSMB384 | 320 | |||
| B. diffusa | MSMB375 | 131 | Water/ Australia | [36] |
| MSMB583 | 464 | |||
| B. humptydooensis | MSMB1588 | 1441 | Soil/ Australia | [36] |
| MSMB43 | 786 | Water/ Australia | [3] | |
| B. territorii | MSMB599 | 723 | Soil/ Australia | [36] |
| MSMB793 | ||||
| B. oklahomensis | NCTC 13387 | 81 | Human/ US | [40] |
| B. pseudomulti- vorans | MSMB2199 | - | Soil/ Australia | [36] |
| B. vietnamiensis | LMG6999 | 524 | Human/ Vietnam | [38] |
| Non-Burkholderia spp. | ||||
| A. baumannii | ATCC 19606 | - | Human/ US | [41] |
| No.9, No. 40, No. 72 | - | Sewage/ Thailand | This study | |
| E. coli | Ec1, Ec2, Ec3, Ec4, Ec5 | - | Sewage/ Thailand | This study |
| P. aeruginosa | ATCC27853 | - | Human/ US | [42] |
| BF311, P256, P338, P362, SP5, SP749, SP770, SP780/2, U1466 | - | Sewage/ Thailand | This study | |
| S. aureus | ATCC25923 | - | Human/ US | [43] |
| Staph04, Staph05, Staph40, Staph156 | - | Food/ Thailand | This study | |
(-), not available.
All of the Burkholderia isolates were collected from previous study (Table 1). Clinical isolates of B. pseudomallei from human were obtained from Sunpasitthiprasong Hospital, Ubon Ratchathani province, Thailand and Royal Darwin Hospital, Darwin, Northern Territory, Australia. Sources of the Burkholderia and non-Burkholderia isolates as well as their Multi Locus Sequence Type (MLST) (where known) are shown in Table 1.
Bacterial genomic DNA was extracted from 1 ml of bacteria cultured in LB broth overnight at 37°C using a Genomic DNA Mini Kit (Geneaid Biotech Ltd., New Taipei City, Taiwan) according to the manufacturer’s instructions. DNA extracts were stored at -20°C. Alternatively, a colony boiling method was used in which a loopful of bacteria cultured on LB agar was suspended in 100 μl sterile distilled water, followed by heat inactivation at 99°C for 15 min. One microliter of DNA from each Genomic DNA extraction was used for PCR amplification.
PCR primers targeting Burkholderia bimA sequences
The bimA sequences of B. mallei (ATCC 23344), B. pseudomallei (K96243), and B. thailandensis (E264) from the National Center for Biotechnology Information (NCBI) were aligned to design PCR primers using Primer-BLAST service tools (https://www.ncbi.nlm.nih.gov/) with default parameters. The structures of the selected primers were evaluated by Oligo Analyzer 3.1 (https://sg.idtdna.com/calc/analyzer), and synthesized by Integrated DNA Technologies, Inc. (Coralville, IA, USA).
Multiplex PCR conditions
B. pseudomallei (K96243), B. thailandensis (E264), and B. mallei (NCTC12938) genomic DNA were used as DNA templates for optimization of PCR conditions. Multiplex PCR detection was performed in a total volume of 25 μl containing 1 μl of bacterial lysates or purified genomic DNA, 0.2 mM of dNTPs, 1× of Q5 reaction buffer, 1× of Q5 high GC enhancer, 0.02 units of Q5 high-fidelity DNA polymerase (New England Biolabs, Inc., MA, USA), five PCR primers including 0.92 μM of BimAcom-R primer, 0.72 μM of BimABps-F primer, 0.10 μM of BimABPBM-F primer and 0.30 μM each of BimABth-F and BimABth-R primers.
The DNA amplification involved initial denaturation at 98°C for 3 min, and 35 cycles at 98°C for 30 s, 68°C for 45 s, and 72°C for 30 s, followed by a final extension for 10 min. The PCR products were verified by 1.5% agarose gel electrophoresis (Vivantis Technologies Sdn. Bhd., Selangor Darul Ehsan, Malaysia) and visualized using a UV transilluminator (Syngene, Cambridge, UK). A GeneRuler 100 bp Plus DNA Ladder (Thermo Fisher Scientific, Inc., MA, USA) was included as a DNA marker.
Singleplex PCR to differentiate B. thailandensis from the BTCV
PCR detection was performed in a total volume of 25 μl containing 1 μl purified genomic DNA, 0.2 mM of dNTPs, 1× of Q5 reaction buffer, 1× of Q5 high GC enhancer, 0.02 units of Q5 high-fidelity DNA polymerase (New England Biolabs, MA, US), and 0.4 μM of each BimABth-2F and BimABth-R primers. The DNA amplification involved initial denaturation at 98°C for 3 min, and 35 cycles at 98°C for 30 s, 57°C for 30 s, and 72°C for 30 s, followed by a final extension for 10 min. PCR products were visualized following 1.5% agarose gel electrophoresis (Vivantis Technologies Sdn. Bhd., Malaysia).
Detection of B. pseudomallei and B. thailandensis from spiked soil samples
Control soil lacking detectable B. pseudomallei and B. thailandensis by PCR targeting the 16S rRNA gene, were used for extraction and sensitivity testing. Overnight cultures of B. pseudomallei K96243, B. pseudomallei MSHR668 and B. thailandensis E555 were adjusted to 0.5 McFarlane with 1× phosphate-buffered saline (PBS). The number of viable bacteria was determined by plating the serial dilution of bacterial culture on LB agar. Bacterial suspensions were serially 10-fold diluted with 1× PBS to approximately 10−103 CFU/ml, and then 100 μl of each bacterium suspension was inoculated into 20 g soil to achieve 1–100 CFU/20 ml. The inoculated soil samples were incubated in 20 ml of Ashdown’s broth at 37°C for 24 h before DNA extraction using a DNeasy PowerSoil Kit (Qiagen, Hilden, Germany). The final precipitated DNA preparation was eluted with 20 μl of elution buffer (Qiagen, Hilden, Germany) or nuclease-free water before amplification by the developed multiplex or singleplex PCR.
Soil sample testing
For analysis of the environmental samples, 34 soil samples were collected from a rice field in a highly endemic area in Ubon Ratchathani (12 samples) and Khon Kaen (22 samples) provinces, Northeast of Thailand. These two provinces are 282 km apart. Soil samples were collected as previously described [44]. Essentially, 20 g of soil at 30 cm depth was collected and cultured for B. pseudomallei, B. thailandensis and BTCV by standard culture method. All of the soil samples were subjected to DNA extraction using a DNeasy PowerSoil Kit (Qiagen, Hilden, Germany) as described above. The extracted DNA samples were subjected to the multiplex PCR assay described above. E. coli 16S rRNA gene was amplified using the 27F and 518R primers to generate a DNA fragment of 527 bps [45]. These primers were included as a control following extraction from the soil samples.
Results
Design of PCR primers targeting bimA sequences for specific detection of B. pseudomallei, BPBM, B. thailandensis and BTCV
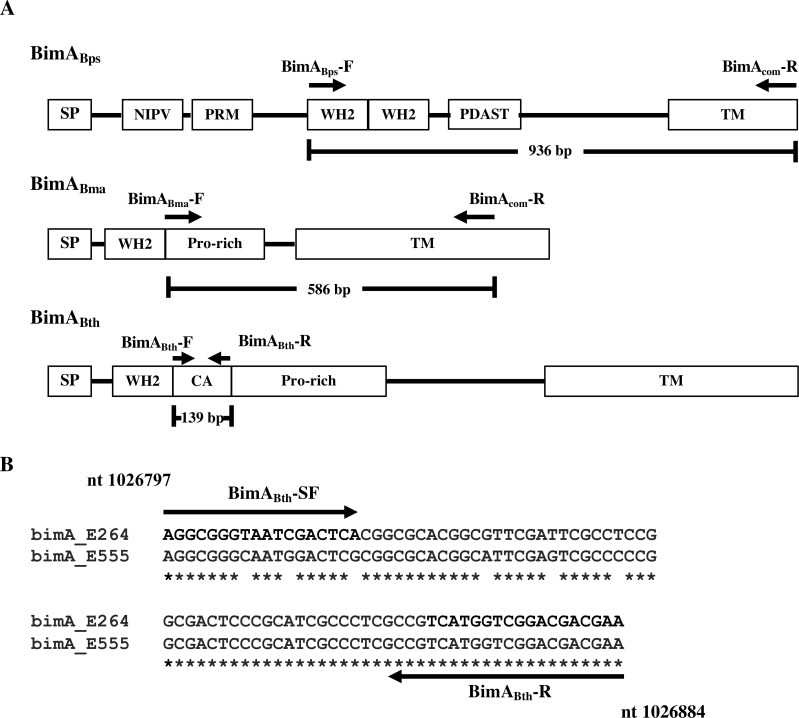
The bimA gene, that contributes to B. pseudomallei and B. thailandensis actin-based motility in infected host cells [46], varies in sequence specifically in the region of the gene encoding the extracellular actin-binding portion of the protein [27, 46, 47]. We chose to use this information to design PCR primers to discriminate between members of the Burkholderia pseudomallei complex, especially those with known or pathogenic potential. In silico analysis of prototypic Burkholderia bimA genes of B. pseudomallei K96243 (NC_006351), B. thailandensis E264 (NC_007651), and B. mallei ATCC23344 (NC_006349) revealed 5 oligonucleotides of which could be used in a multiplex PCR (Fig 1A). The BimABps-F and BimAcom-R primers were designed to amplify 963 bp bimA amplicons of B. pseudomallei, whereas BimABth-F/BimABth-R and BimBPBM-F/BimAcom-R primers could amplify 139 bp and 586 bp bimA DNA fragments of the bimA genes of B. thailandensis and BPBM, respectively (Table 2). Since B. mallei is unable to survive in the environment, any B. mallei-like bimA amplicons in this assay are most likely to derive from a BPBM strain.
Fig 1. Schematic locations of PCR primers on various domains of Burkholderia bimA genes.
(A) Locations of the multiplex PCR primers (BimABps-F/BimAcom-R, BimBPBM-F/BimAcom-R, and BimABth-F/BimABth-R) for generating 963 bp, 586 bp, and 139 bp amplicons specific to B. pseudomallei, BPBM/ B. mallei, and B. thailandensis/ BTCV, respectively. (B) Locations of simplex PCR primers (BimABth-SF and BimABth-SR) on central and acidic (CA) domain of bimA gene for differentiation between typical B. thailandensis (strain E264) and BTCV (strain E555). Numbers refer to nucleotide positions on B. thailandensis E264 chromosome 2.
Table 2. Oligonucleotide primers and the size of bimA amplicons for detection of B. pseudomallei, B. thailandensis, B. mallei and their variants including BTCV and BPBM.
| Primer name | Sequences 5′ to 3′ | Size of amplicon | For detection of | References |
|---|---|---|---|---|
| BimABps-F | GATCGCTGAAGAAAAATCCG | 963 bp | B. pseudomallei | This study |
| BimAcom-R | CCTTGAGGTTTTCGTTGATG | |||
| BimABPBM-F | ATTCCTAACGCGACACCAAC | 586 bp | BPBM and B. mallei | This study |
| BimAcom-R | CCTTGAGGTTTTCGTTGATG | |||
| BimABth-F | ATCCGAACGAAACACGCG | 139 bp | B. thailandensis and BTCV | This study |
| BimABth-R | TTCGTCGTCCGACCATGA | |||
| BimABth-SF | AGGCGGGTAATCGACTCA | 87 bp | B. thailandensis | This study and [48] |
| BimABth-R | TTCGTCGTCCGACCATGA | |||
| 27F | AGAGTTTGATCMTGGCTCAG | 527 bp | E. coli | [45] |
| 518R | ATTACCGCGGCTGCTGG | 16S rRNA |
A caveat of the multiplex PCR was the inability to differentiate between B. thailandensis and the BTCV since a 139 bp amplicon would be amplified from both. To aid in the design of primers for differentiating these bimA genes, we sequenced the 139 bp bimA amplicons from BTCV strain E555 and B. thailandensis strain E264 to identify sequence differences which could be used to design primers for use in an additional simplex PCR reaction (Fig 1B). In combination with primer BimABth-R, bimA PCR primer BimABth-SF based on central and acidic (CA) domain of bimA gene (corresponding to nucleotide positions 1,026,797–1,026,815 of B. thailandensis E264, Fig 1A), would lead to amplification of an 87 bp bimA DNA fragment from B. thailandensis strains but not from BTCV strains (Table 2).
Validation of the bimA primers for detection of B. pseudomallei, B. thailandensis and their variants in multiplex and singleplex PCR assays
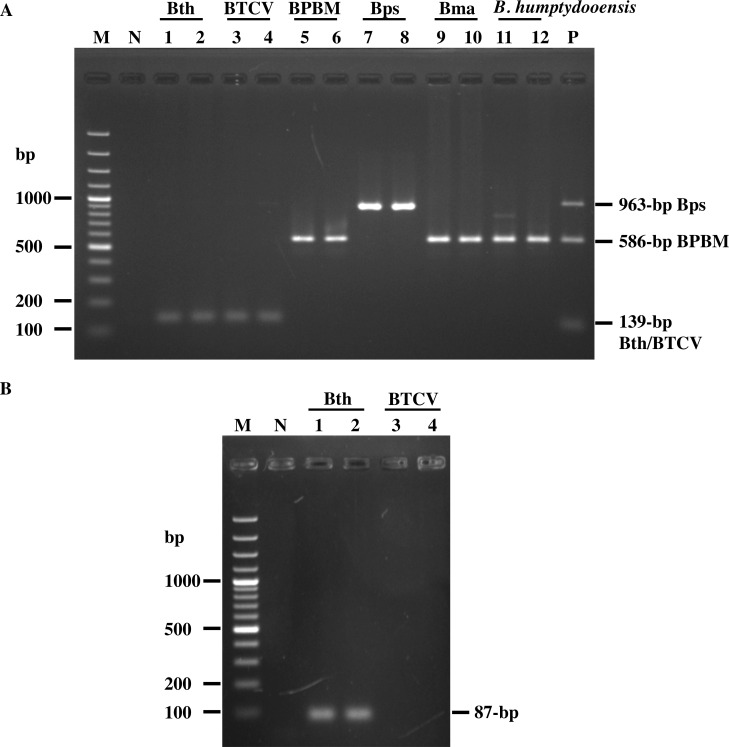
Genomic DNA from B. pseudomallei, B. thailandensis, B. mallei and their variant strains were mixed, and subjected to multiplex PCR with the 5 primers BimABps-F, BimAcom-R, BimABth-F BimABth-R and BimBPBM-F. Fig 2A shows that the bimA-specific primers can distinguish B. pseudomallei from B. thailandensis and BPBM strains via the generation of distinctive 963 bp, 139 bp, and 586 bp DNA fragments, respectively. Notably, B. thailandensis could not be differentiated from BTCV as well as B. mallei could not be differentiated from BPBM as they showed the identical amplicons at 139 and 586 bp, respectively.
Fig 2. Agarose gel electrophoretic analysis of amplified DNA fragments generated from multiplex and singleplex PCR assays.
(A) Multiplex PCR amplification of DNA templates from B. thailandensis strains E264, DV1 (lanes 1–2), the BTCV strains E555, SBXPR001 (lanes 3–4), BPBM strains MSHR3326, MSHR4445 (lanes 5–6), B. pseudomallei strains K92643, 1026b (lanes 7–8), B. mallei strains NCTC3709, NCTC12938 (lanes 9–10), and B. humptydooensis strains MSMB43, MSMB1588 (lanes 11–12). (B) Singleplex PCR amplification of B. thailandensis strains E264, DV1 (Bth; lanes 1–2) and B. thailandensis strain E555, SBXPR001 (BTCV; lanes 3–4). Lanes P and N are positive (mixture of Burkholderia spp. DNA) and negative (distilled water) controls, respectively. Lane M is 100 bp DNA ladder.
To differentiate B. thailandensis from the BTCV strains, a singleplex PCR was established using primers BimABth-SF and BimABth-R. As demonstrated in Fig 2B, an 87 bp amplicon was obtained from B. thailandensis strain E264, but not from BTCV strain E555. Ten further BTCV isolates were tested in this assay, and whilst DNA amplicons of 139 bp were obtained in the multiplex PCR, no PCR products were generated in the singleplex B. thailandensis-specific PCR assay (S1 Fig). These findings indicate the successful combination of a multiplex and singleplex PCR to identify the BTCV.
In the Northern territory of Australia, a further member of the B. pseudomallei complex known as B. humptydooensis has been isolated from the environment [18]. This microorganism has not been associated with human or animal disease and has only been isolated from a restricted geographical area [19]. When genomic DNA from two B. humptydooensis strains (MSMB43 and MSMB1588) were included in the multiplex PCR, amplicons identical to the 586 bp amplicon of B. mallei/ BPBM bimA gene were seen (Fig 2A). DNA sequencing confirmed that the 586 bp PCR product amplified from B. humptydooensis was indeed bimA, demonstrating 99.05% homology to B. humptydooensis MSMB43 genome sequence in the NCBI database (GenBank No. CP013382; region 974821–975982). Comparisons of the B. humptydooensis (MSMB43 and MSMB1588) bimA sequence with that of B. mallei ATCC23344, B. pseudomallei MSHR491 (BPBM), B. thailandensis E264, B. pseudomallei K92643 and B. singularis LMG28154 demonstrated 94.03%, 93.52%, 85.89%, 84.68% and 0% nucleotide sequence homology, respectively. The variation within B. humptydooensis MSMB43 and MSMB1588 is 95.01% and 91.08% of gene homology and amino acid homology, respectively. Thus, it can be concluded that the B. humptydooensis bimA gene shows the greatest nucleotide sequence homology with B. mallei and BPBM bimA than with B. thailandensis or B. pseudomallei (S2 Fig). Whilst B. humptydooensis and BPBM could be detected but not differentiated by our PCR assay, we could use a second biochemical test to differentiate between the two since as B. humptydooensis can assimilate arabinose [19] whereas BPBM cannot.
Specificity and sensitivity of multiplex PCR for detection of B. pseudomallei and B. thailandensis
To investigate the specificity of the bimA PCR primers, amplification of purified genomic DNA from Burkholderia spp. and other related bacterial species were undertaken. All B. pseudomallei (n = 35), B. thailandensis (n = 30), BTCV (n = 10), and BPBM (n = 20) produced PCR amplicons at the expected DNA fragment lengths. Similar to BPBM, all 2 isolates of B. mallei (NCTC3709, and NCTC12938) and 2 isolates of B. humptydooensis (MSMB43 and MSMB1588) led to amplification of products of the same size, reinforcing the data in Fig 2 that they could be detected, but could not be differentiated by the multiplex PCR and as predicted from Table 3. Analysis of the sequence type (ST) of each Burkholderia strain included in this study (Table 3) demonstrated that they belonged to multiple different ST, suggesting that our developed assay is not restricted to a specific ST. In addition, we have also BLASTed our primers to genomic DNA sequences of B. singularis (annotated contigs accession number FXAN01000001-FXAN01000135) and found no significant homology. Therefore, our assay is unlikely to be able to identify strains from this rare species.
Table 3. Specificity of the multiplex PCR assay against 121 isolates of Burkholderia spp. and 24 isolates of Gram-positive and Gram-negative bacteria.
| Bacteria | Source [References] | No. of isolates | No. of PCR positive/ Total No. of isolates (%) |
|---|---|---|---|
| Burkholderia spp. | |||
| B. pseudomallei | Clinical [29, 30, 32, 49–52] | 18 | 9/9 (100%) |
| Environment [11] | 17 | 24/24 (100%) | |
| BPBMa | Clinical [20, 33] | 20 | 20/20 (100%) |
| B. thailandensis | Environment [10, 11, 34] | 30 | 30/30 (100%) |
| BTCVb | Environment [11] | 10 | 10/10 (100%) |
| B. mallei | Animal [53] | 2 | 2/2 (100%) |
| B. humptydooensis | Environment [3, 36] | 2 | 2/2 (100%) |
| B. multivorans | Environment [38, 54] | 3 | 0/3 (0%) |
| B. ubonensis | Environment [36, 39, 55] | 3 | 0/3 (0%) |
| B. anthina | Environment [36] | 2 | 0/2 (0%) |
| B. cepacia | Clinical [36, 37] | 2 | 0/2 (0%) |
| Environment [36, 56] | 5 | 0/5 (0%) | |
| B. cenocepacia | Environment [36] | 2 | 0/2 (0%) |
| B. diffusa | Environment [36] | 2 | 0/2 (0%) |
| B. territorii | Environment [36] | 2 | 0/2 (0%) |
| B. pseudomultivorans | Environment [36] | 1 | 0/1 (0%) |
| B. oklahomemsis | Clinical [40] | 1 | 0/1 (0%) |
| B. vietnamiensis | Clinical [38, 55] | 1 | 0/1 (0%) |
| Non-Burkholderia spp. | |||
| S. aureus | Clinical | 1 | 0/1 (0%) |
| Food | 4 | 0/4 (0%) | |
| P. aeruginosa | Environment | 10 | 0/10 (0%) |
| E. coli | Environment | 5 | 0/5 (0%) |
| A. baumannii | Environment | 4 | 0/4 (0%) |
aB. pseudomallei that expresses a B. mallei-like bimA; bB. thailandensis that expresses a B. pseudomallei-like capsule.
In contrast to B. pseudomallei, B. thailandensis, and their variant strains, no amplicons were generated when testing against Burkholderia anthina, B. cepacia, B. cenocepacia, B. diffusa, B. multivorans, B. oklahomensis, B. pseudomultivorans, B. vietnamiensis, B. ubonensis, and other unrelated bacterial species, including Pseudomonas aeruginosa, Escherichia coli, Acinetobacter baumannii and Staphylococcus aureus (Table 3). These results validate the specificity of this multiplex PCR assay for detection of Burkholderia species with known virulence or pathogenic potential based on the known genetic variation in the bimA genes.
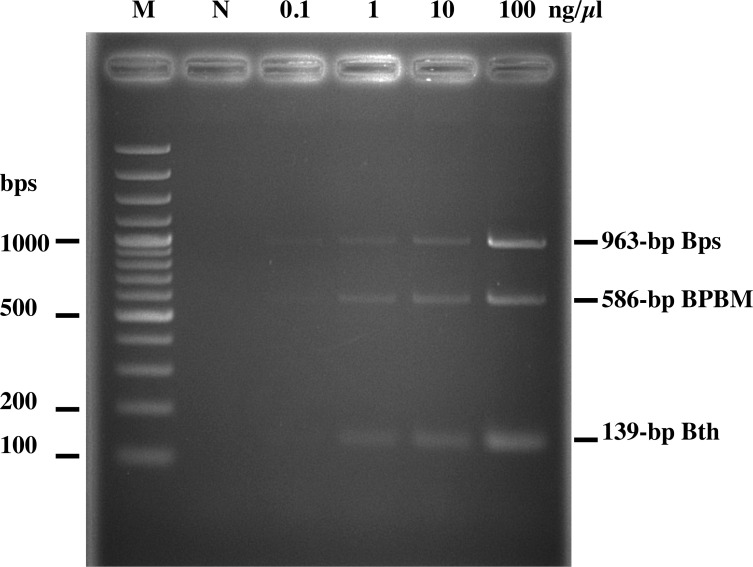
To investigate the sensitivity of the multiplex PCR, genomic DNA extracts from B. pseudomallei, B. thailandensis, and B. mallei were 10-fold serially diluted from 100 to 0.1 ng/μl and used as a template for the multiplex PCR. As shown in Fig 3, the sensitivity of the multiplex PCR for detecting extracted bacterial DNA is approximately 0.1–1.0 ng/μl. The sensitivity of the assay for B. pseudomallei and BPBM is around 0.1 ng/μl, and approximately 1 ng/μl for B. thailandensis (Fig 3).
Fig 3. Sensitivity of multiplex PCR for detection of the Burkholderia spp. in a mixture of genomic DNA.
Various concentrations (1, 10, and 100 ng/μl) of each Burkholderia genomic DNA were subjected to multiplex PCR including B. pseudomallei K92643 (Bps; 963 bp), B. pseudomallei MSHR491 (BPBM; 586 bp), and B. thailandensis E264 (Bth; 139 bp). Lanes M and N represent 100 bp DNA ladder and negative control (distilled water), respectively.
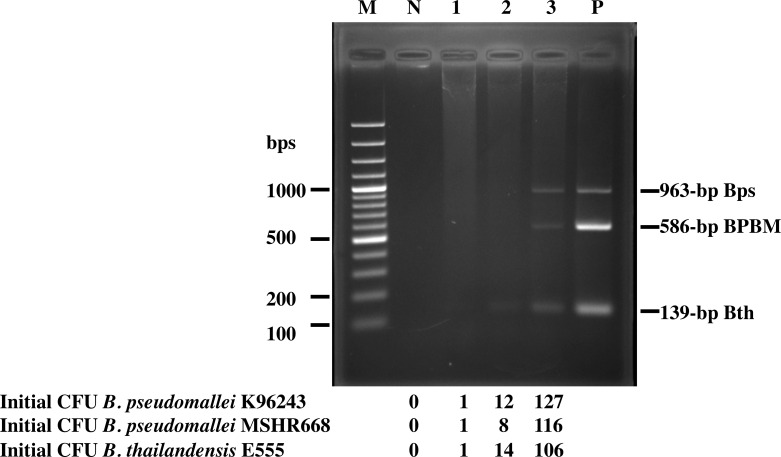
To detect B. pseudomallei and B. thailandensis in soil samples, we next tested the ability of our PCR system to detect Burkholderia spp. in spiked soil samples. A crucial step of bacterial enrichment is performed on the first day of our protocol, which is then followed by an effective method for DNA extraction and finally PCR amplification. Twenty grams of sterile soil were spiked with known colony forming unit (CFU) of B. pseudomallei, a BPBM strain and B. thailandensis, added to 20 ml of Ashdown’s broth, and incubated at 37°C for 24 h with shaking. Next, DNA was extracted and subjected to multiplex PCR. As shown in Fig 4, we obtained the three expected DNA fragments of 963, 139, and 586 bp corresponding to B. pseudomallei, B. thailandensis, and BPBM, respectively. The limit of detection of this assay in an inoculated soil sample was as low as 127, 106 and 116 CFU/20 g soil, respectively, which is equivalent to approximately 6, 5, and 6 CFU/g of soil sample.
Fig 4. Multiplex PCR assay to detect B. pseudomallei, BPBM and B. thailandensis in spiked soil samples.
Ten-fold serial dilution (lanes 1–3) of B. pseudomallei, BPBM and B. thailandensis were spiked into 20 g soil sample before DNA extraction and multiplex PCR. Lanes P (mixture of Burkholderia spp. genomic DNA) and N (no added bacteria) are positive and negative controls, respectively. Lane M is 100 bp DNA ladder.
Comparison of the sensitivity and specificity of the multiplex PCR with culture on Ashdown’s agar
Finally, we chose to use this protocol to identify the presence of B. pseudomallei in natural soil samples from Thailand, and compare the results from our multiplex PCR assay with detection using the ‘gold standard’ culture on Ashdown’s agar. Rice field soil samples (n = 34) were collected from Ubon Ratchathani and Khon Kaen provinces, an endemic area of melioidosis in the Northeast of Thailand. All of the soil samples were cultured for B. pseudomallei, B. thailandensis and BTCV detection on Ashdown’s agar. Of the 34 samples, 12 were positive by both method and the multiplex PCR. Two of the remaining culture-negative samples were positive by multiplex PCR (Table 4). The remaining culture-negative samples were also negative when screened using the multiplex PCR. (Table 4). The calculated sensitivity and specificity of the multiplex assay are therefore 100% and 90.9%, respectively.
Table 4. Comparison between cultured-based method and bimA specific multiplex PCR to detect B. pseudomallei from 34 soil samples collected from endemic areas of melioidosis.
| bimA specific PCR-based method | Culture-based method | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 12 | 2 | 14 |
| Negative | 0 | 20 | 20 |
| Total | 12 | 22 | 34 |
Discussion
The gold standard for detection of environmental B. pseudomallei and B thailandensis is based on bacterial culture from soil or water samples [44], which is a time-consuming process. In addition, the B. pseudomallei and B thailandensis variants BPBM [28] and BTCV [11], as well as B. humptydooensis [19] have all been shown to be present in the environment in melioidosis endemic areas. Severe disease is associated with B. pseudomallei and its BPBM variants, with some evidence of less fatal infections with the BTCV strains. Therefore, a simple technique to distinguish these three Burkholderia spp. is required for rapid and thorough epidemiological survey of these species in the environment, especially in Thailand where melioidosis is endemic and B. thailandensis and its variant strains (BTCV) are commonly isolated from soil and water.
In this study, we designed primers based on the known genetic variation of the bimA gene and used these in a multiplex PCR. This assay was able to detect and simultaneously discriminate between the DNA amplified from B. pseudomallei (936 bp), B. thailandensis (139 bp) and BPBM (586 bp). However, the assay could not differentiate B. humptydooensis from BPBM or B. thailandensis from BTCV. Despite enumerable soil and water surveys in the endemic areas, B. humptydooensis has only ever been isolated from a small and specific region of the Australian Northern territory [3] and has never been associated with disease in animals or humans. Therefore, we predict that any BPBM-like amplicon in our test is most likely to arise from the presence of a BPBM in the sample, rather than a B. humptydooensis strain.
Growing evidence supports the finding that BTCV strains can occasionally cause disease, usually non-fatal, in humans [7, 9] and therefore can be considered of pathogenic potential. Since the BTCV and B. thailandensis strains could not be differentiated by multiplex PCR, a second simplex PCR was designed based on the few single nucleotide sequence differences in the B. thailandensis and BTCV bimA genes (Fig 1B). As a result, only B. thailandensis bimA amplicons are generated in this singleplex PCR. Testing this simplex PCR against 10 strains of BTCV and 30 strains of B. thailandensis showed 100% accuracy in differentiation between B. thailandensis and BTCV. This study represents the first PCR-based method that allows the discrimination of B. thailandensis from BTCV strains. This is important since it is possible that the presence of the BTCV in some melioidosis endemic regions may confer protection against the development of melioidosis. Little is known about the prevalence of BTCV and its impact on B. pseudomallei infection. Thus, the combined use of our multiplex and simplex PCRs described in this study will be useful to survey the presence of BTCV in both non-endemic and endemic areas, with the aim of understanding the genetic diversity, virulence and evolution of these emerging organisms.
An additional key advantage of the bimA-based multiplex PCR assay is not only to detect B. pseudomallei, B thailandensis and the BTCV, but also to discriminate B. pseudomallei from BPBM in a single multiplex PCR reaction. BPBM strains have been associated with neurologic melioidosis, which is a serious and potentially fatal form of B. pseudomallei infection [22, 57]. In an animal model, BPBM were more virulent when delivered intranasally or subcutaneously than typical B. pseudomallei isolates [22]. To date, BPBM strains have not been isolated from the environment in Southeast Asia, however it is possible that it is present but has been mis-identified as B. pseudomallei. Therefore, the possibility that this variant strain is present in a wider geographical area including Thailand cannot be excluded.
By spiking soil samples with known numbers of viable B. pseudomallei, B. thailandensis and BPBM, we were able to ascertain that the sensitivity of our multiplex PCR assay to be 5–6 CFU/g soil sample. Although the sensitivity of this assay is not as high as a previously reported singleplex PCR to detect B. pseudomallei (1–1.5 CFU/g of soil sample) [58], our multiplex PCR assay could simultaneously detect the Burkholderia species of most clinical importance. An added benefit of our bimA-based PCR method is that the readout is easy to interpret, and unlikely to be interpreted incorrectly, since it relies on significant differences in amplicon size (936 vs 586 vs 139 bp). Furthermore, using natural soil samples we were able to compare the sensitivity and specificity of the multiplex assay with the existing conventional bacterial culture method [20, 59]. Multiplex PCR to detect B. pseudomallei from 34 soil samples revealed that the sensitivity and specificity of the multiplex PCR, in comparison with culture on Ashdown’s agar, are 100% and 90.9% respectively, suggesting that it is a reliable alternative method.
Although the sample size is small, the soil samples were collected from 2 different provinces which approximately 282 km apart which were expected to contain different bacterial populations and differ in major soil nutrients. In addition, Burkholderia spp. included in this study were composed of multiple different ST and were collected from Thailand and Australia, the endemic area of melioidosis.
In conclusion, we report herein sets of PCR primers that can be used in a combined multiplex and singleplex PCR-based way to detect B. pseudomallei and B. thailandensis, BPBM and BTCV in environmental samples. The multi-species differentiation assay using bimA-based multiplex PCR technique presented here is a simple, specific, and sensitive technique that will be useful for environmental sampling study and for prediction of areas of increased risk of disease in humans and animals.
Supporting information
(A) Multiplex PCR of 10 BTCV strains including strains E555, SBXPR001, SBXSR007, SBXPL001, SBXPL015, SBXRY031, SBXPR001, SBXCC001, SBXCC003 and WBXUBA33005104 (lanes 1–10) with multiplex PCR primers (BimABps-F/BimAcom-R, BimBPBM-F/BimAcom-R, and BimABth-F/BimABth-R). The expected 139-bp DNA fragments were detected in all samples. (B) Singleplex PCR of BTCV strains from (A) amplified with primers BimABth-SF and BimABth-R primers (lanes 1–10). Lanes P (mixture of Burkholderia spp. genomic DNA) and N (distilled water) are positive and negative controls, respectively. Lane M is 100 bp DNA ladder.
(TIFF)
The nucleotide sequences in red correspond to the BimBPBM and Bimcom primers.
(TIFF)
Acknowledgments
The authors gratefully acknowledge Department of Microbiology, Faculty of Medicine Siriraj Hospital, Mahidol University, Thailand, Dr. Limmathurotsakul D. (Mahidol Oxford Tropical Medicine Research Unit, Bangkok, Thailand), and Dr. Kritsiriwuthinan K. (Faculty of Medical Technology, Rangsit University, Thailand) for providing bacterial strains, Mr. Wongsuvan G. for soil samples collection and Prof. Stevens M.P. (University of Edinburgh, UK) for helpful advice.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Korbsrisate S. was supported by the Chalermprakiat Grant, Faculty of Medicine Siriraj Hospital, Mahidol University. Janesomboon S. was supported by the Siriraj Graduate Scholarship and the Siriraj Grant for Research and Development. Muangsombut V. was supported by the Royal Golden Jubilee (RGJ) Ph.D. programme (PHD0190/2560), and Meethai C. was supported by the Royal Golden Jubilee (RGJ) Ph.D. programme (PHD0070/2559). Wuthiekanun V. and Amornchai P. were supported by Wellcome Trust (089275/Z/09/Z). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Limmathurotsakul D, Golding N, Dance DA, Messina JP, Pigott DM, Moyes CL, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol. 2016;1:15008 Epub 2016/08/31. 10.1038/nmicrobiol.2015.8 . [DOI] [PubMed] [Google Scholar]
- 2.Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med. 2012;367(11):1035–44. Epub 2012/09/14. 10.1056/NEJMra1204699 . [DOI] [PubMed] [Google Scholar]
- 3.Ginther JL, Mayo M, Warrington SD, Kaestli M, Mullins T, Wagner DM, et al. Identification of Burkholderia pseudomallei near-neighbor species in the Northern Territory of Australia. PLoS Negl Trop Dis. 2015;9(6):e0003892 Epub 2015/06/30. 10.1371/journal.pntd.0003892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vandamme P, Peeters C, De Smet B, Price EP, Sarovich DS, Henry DA, et al. Comparative genomics of Burkholderia singularis sp. nov., a low G+C content, free-living bacterium that defies taxonomic dissection of the genus Burkholderia. Front Microbiol. 2017;8:1679 Epub 2017/09/22. 10.3389/fmicb.2017.01679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Zandt KE, Greer MT, Gelhaus HC. Glanders: an overview of infection in humans. Orphanet J Rare Dis. 2013;8:131 Epub 2013/09/06. 10.1186/1750-1172-8-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trakulsomboon S, Vuddhakul V, Tharavichitkul P, Na-Gnam N, Suputtamongkol Y, Thamlikitkul V. Epidemiology of arabinose assimilation in Burkholderia pseudomallei isolated from patients and soil in Thailand. Southeast Asian J Trop Med Public Health. 1999;30(4):756–9. Epub 2000/08/06. . [PubMed] [Google Scholar]
- 7.Chang K, Luo J, Xu H, Li M, Zhang F, Li J, et al. Human infection with Burkholderia thailandensis, China, 2013. Emerg Infect Dis. 2017;23(8):1416–8. Epub 2017/07/21. 10.3201/eid2308.170048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gee JE, Elrod MG, Gulvik CA, Haselow DT, Waters C, Liu L, et al. Burkholderia thailandensis isolated from infected wound, Arkansas, USA. Emerg Infect Dis. 2018;24(11):2091–4. Epub 2018/10/20. 10.3201/eid2411.180821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glass MB, Gee JE, Steigerwalt AG, Cavuoti D, Barton T, Hardy RD, et al. Pneumonia and septicemia caused by Burkholderia thailandensis in the United States. J Clin Microbiol. 2006;44(12):4601–4. Epub 2006/10/20. 10.1128/JCM.01585-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sim BM, Chantratita N, Ooi WF, Nandi T, Tewhey R, Wuthiekanun V, et al. Genomic acquisition of a capsular polysaccharide virulence cluster by non-pathogenic Burkholderia isolates. Genome Biol. 2010;11(8):R89 Epub 2010/08/31. 10.1186/gb-2010-11-8-r89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hantrakun V, Thaipadungpanit J, Rongkard P, Srilohasin P, Amornchai P, Langla S, et al. Presence of B. thailandensis and B. thailandensis expressing B. pseudomallei-like capsular polysaccharide in Thailand, and their associations with serological response to B. pseudomallei. PLoS Negl Trop Dis. 2018;12(1):e0006193 Epub 2018/01/25. 10.1371/journal.pntd.0006193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott AE, Laws TR, D'Elia RV, Stokes MG, Nandi T, Williamson ED, et al. Protection against experimental melioidosis following immunization with live Burkholderia thailandensis expressing a manno-heptose capsule. Clin Vaccine Immunol. 2013;20(7):1041–7. Epub 2013/05/17. 10.1128/CVI.00113-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riyapa D, Muangsombut V, Janesomboon S, Wuthiekanun V, Stevens JM, Korbsrisate S. Comparison of neutrophil extracellular trap induction and reactive oxygen species production between non-capsulated and capsulated strains of Burkholderia thailandensis. Southeast Asian J Trop Med Public Health 2019;50:146–54. [Google Scholar]
- 14.Kovacs-Simon A, Hemsley CM, Scott AE, Prior JL, Titball RW. Burkholderia thailandensis strain E555 is a surrogate for the investigation of Burkholderia pseudomallei replication and survival in macrophages. BMC Microbiol. 2019;19(1):97 Epub 2019/05/17. 10.1186/s12866-019-1469-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Withatanung P, Kurian D, Tangjittipokin W, Plengvidhya N, Titball RW, Korbsrisate S, et al. Quantitative proteomics reveals differences in the response of neutrophils isolated from healthy or diabetic subjects to infection with capsule-variant Burkholderia thailandensis. J Proteome Res. 2019;18(7):2848–58. Epub 2019/06/28. 10.1021/acs.jproteome.9b00166 . [DOI] [PubMed] [Google Scholar]
- 16.McCormick JB, Weaver RE, Hayes PS, Boyce JM, Feldman RA. Wound infection by an indigenous Pseudomonas pseudomallei-like organism isolated from the soil: case report and epidemiologic study. J Infect Dis. 1977;135(1):103–7. Epub 1977/01/01. 10.1093/infdis/135.1.103 . [DOI] [PubMed] [Google Scholar]
- 17.Deshazer D. Virulence of clinical and environmental isolates of Burkholderia oklahomensis and Burkholderia thailandensis in hamsters and mice. FEMS Microbiol Lett. 2007;277(1):64–9. Epub 2007/11/08. 10.1111/j.1574-6968.2007.00946.x . [DOI] [PubMed] [Google Scholar]
- 18.Gee JE, Glass MB, Novak RT, Gal D, Mayo MJ, Steigerwalt AG, et al. Recovery of a Burkholderia thailandensis-like isolate from an Australian water source. BMC Microbiol. 2008;8:54 Epub 2008/04/04. 10.1186/1471-2180-8-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuanyok A, Mayo M, Scholz H, Hall CM, Allender CJ, Kaestli M, et al. Burkholderia humptydooensis sp. nov., a new species related to Burkholderia thailandensis and the fifth member of the Burkholderia pseudomallei complex. Appl Environ Microbiol. 2017;83(5). Epub 2016/12/18. 10.1128/AEM.02802-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McRobb E, Kaestli M, Price EP, Sarovich DS, Mayo M, Warner J, et al. Distribution of Burkholderia pseudomallei in northern Australia, a land of diversity. Appl Environ Microbiol. 2014;80(11):3463–8. Epub 2014/03/25. 10.1128/AEM.00128-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukhopadhyay C, Kaestli M, Vandana KE, Sushma K, Mayo M, Richardson L, et al. Molecular characterization of clinical Burkholderia pseudomallei isolates from India. Am J Trop Med Hyg. 2011;85(1):121–3. Epub 2011/07/08. 10.4269/ajtmh.2011.11-0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris JL, Fane A, Sarovich DS, Price EP, Rush CM, Govan BL, et al. Increased neurotropic threat from Burkholderia pseudomallei strains with a B. mallei-like variation in the bimA motility gene, Australia. Emerg Infect Dis. 2017;23(5). Epub 2017/04/19. 10.3201/eid2305.151417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glass MB, Beesley CA, Wilkins PP, Hoffmaster AR. Comparison of four selective media for the isolation of Burkholderia mallei and Burkholderia pseudomallei. Am J Trop Med Hyg. 2009;80(6):1023–8. Epub 2009/05/30. . [PubMed] [Google Scholar]
- 24.Winstanley C, Hart CA. Presence of type III secretion genes in Burkholderia pseudomallei correlates with Ara(-) phenotypes. J Clin Microbiol. 2000;38(2):883–5. Epub 2000/02/03. 10.1128/JCM.38.2.883-885.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neubauer H, Sprague LD, Joseph M, Tomaso H, Al Dahouk S, Witte A, et al. Development and clinical evaluation of a PCR assay targeting the metalloprotease gene (mprA) of B. pseudomallei. Zoonoses Public Health. 2007;54(1):44–50. Epub 2007/03/16. 10.1111/j.1863-2378.2007.01008.x . [DOI] [PubMed] [Google Scholar]
- 26.Ho CC, Lau CC, Martelli P, Chan SY, Tse CW, Wu AK, et al. Novel pan-genomic analysis approach in target selection for multiplex PCR identification and detection of Burkholderia pseudomallei, Burkholderia thailandensis, and Burkholderia cepacia complex species: a proof-of-concept study. J Clin Microbiol. 2011;49(3):814–21. Epub 2010/12/24. 10.1128/JCM.01702-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens JM, Ulrich RL, Taylor LA, Wood MW, Deshazer D, Stevens MP, et al. Actin-binding proteins from Burkholderia mallei and Burkholderia thailandensis can functionally compensate for the actin-based motility defect of a Burkholderia pseudomallei bimA mutant. J Bacteriol. 2005;187(22):7857–62. Epub 2005/11/04. 10.1128/JB.187.22.7857-7862.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens MP, Stevens JM, Jeng RL, Taylor LA, Wood MW, Hawes P, et al. Identification of a bacterial factor required for actin-based motility of Burkholderia pseudomallei. Mol Microbiol. 2005;56(1):40–53. Epub 2005/03/19. 10.1111/j.1365-2958.2004.04528.x . [DOI] [PubMed] [Google Scholar]
- 29.Wuthiekanun V, Amornchai P, Saiprom N, Chantratita N, Chierakul W, Koh GC, et al. Survey of antimicrobial resistance in clinical Burkholderia pseudomallei isolates over two decades in Northeast Thailand. Antimicrob Agents Chemother. 2011;55(11):5388–91. Epub 2011/08/31. 10.1128/AAC.05517-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wuthiekanun V, Smith MD, Dance DA, White NJ. Isolation of Pseudomonas pseudomallei from soil in north-eastern Thailand. Trans R Soc Trop Med Hyg. 1995;89(1):41–3. Epub 1995/01/01. 10.1016/0035-9203(95)90651-7 . [DOI] [PubMed] [Google Scholar]
- 31.Godoy D, Randle G, Simpson AJ, Aanensen DM, Pitt TL, Kinoshita R, et al. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J Clin Microbiol. 2003;41(5):2068–79. Epub 2003/05/08. 10.1128/jcm.41.5.2068-2079.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suttisunhakul V, Pumpuang A, Ekchariyawat P, Wuthiekanun V, Elrod MG, Turner P, et al. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for the identification of Burkholderia pseudomallei from Asia and Australia and differentiation between Burkholderia species. PLoS One. 2017;12(4):e0175294 Epub 2017/04/07. 10.1371/journal.pone.0175294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spring-Pearson SM, Stone JK, Doyle A, Allender CJ, Okinaka RT, Mayo M, et al. Pangenome analysis of Burkholderia pseudomallei: genome evolution preserves gene order despite high recombination rates. PLoS One. 2015;10(10):e0140274 Epub 2015/10/21. 10.1371/journal.pone.0140274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jitprasutwit S, Thaewpia W, Muangsombut V, Lulitanond A, Leelayuwat C, Lertmemongkolchai G, et al. Effect of acidic pH on the invasion efficiency and the type III secretion system of Burkholderia thailandensis. J Microbiol. 2010;48(4):526–32. Epub 2010/08/28. 10.1007/s12275-010-0078-x . [DOI] [PubMed] [Google Scholar]
- 35.Burtnick M, Bolton A, Brett P, Watanabe D, Woods D. Identification of the acid phosphatase (acpA) gene homologues in pathogenic and non-pathogenic Burkholderia spp. facilitates TnphoA mutagenesis. Microbiology (Reading). 2001;147(Pt 1):111–20. Epub 2001/02/13. 10.1099/00221287-147-1-111 . [DOI] [PubMed] [Google Scholar]
- 36.Sahl JW, Vazquez AJ, Hall CM, Busch JD, Tuanyok A, Mayo M, et al. The effects of signal erosion and core genome reduction on the identification of diagnostic markers. mBio. 2016;7(5). Epub 2016/09/22. 10.1128/mBio.00846-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yabuuchi E, Kosako Y, Oyaizu H, Yano I, Hotta H, Hashimoto Y, et al. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiol Immunol. 1992;36(12):1251–75. Epub 1992/01/01. 10.1111/j.1348-0421.1992.tb02129.x . [DOI] [PubMed] [Google Scholar]
- 38.Vandamme P, Holmes B, Vancanneyt M, Coenye T, Hoste B, Coopman R, et al. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. nov. Int J Syst Bacteriol. 1997;47(4):1188–200. Epub 1997/10/23. 10.1099/00207713-47-4-1188 . [DOI] [PubMed] [Google Scholar]
- 39.Johnson SL, Bishop-Lilly KA, Ladner JT, Daligault HE, Davenport KW, Jaissle J, et al. Complete genome sequences for 59 Burkholderia isolates, both pathogenic and near neighbor. Genome Announc. 2015;3(2). Epub 2015/05/02. 10.1128/genomeA.00159-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glass MB, Steigerwalt AG, Jordan JG, Wilkins PP, Gee JE. Burkholderia oklahomensis sp. nov., a Burkholderia pseudomallei-like species formerly known as the Oklahoma strain of Pseudomonas pseudomallei. Int J Syst Evol Microbiol. 2006;56(Pt 9):2171–6. Epub 2006/09/08. 10.1099/ijs.0.63991-0 . [DOI] [PubMed] [Google Scholar]
- 41.Bouvet PJ, Grimont PA. Identification and biotyping of clinical isolates of Acinetobacter. Ann Inst Pasteur Microbiol. 1987;138(5):569–78. Epub 1987/09/01. 10.1016/0769-2609(87)90042-1 . [DOI] [PubMed] [Google Scholar]
- 42.Cao H, Lai Y, Bougouffa S, Xu Z, Yan A. Comparative genome and transcriptome analysis reveals distinctive surface characteristics and unique physiological potentials of Pseudomonas aeruginosa ATCC 27853. BMC Genomics. 2017;18(1):459 Epub 2017/06/14. 10.1186/s12864-017-3842-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Treangen TJ, Maybank RA, Enke S, Friss MB, Diviak LF, Karaolis DK, et al. Complete genome sequence of the quality control strain Staphylococcus aureus subsp. aureus ATCC 25923. Genome Announc. 2014;2(6). Epub 2014/11/08. 10.1128/genomeA.01110-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Limmathurotsakul D, Dance DA, Wuthiekanun V, Kaestli M, Mayo M, Warner J, et al. Systematic review and consensus guidelines for environmental sampling of Burkholderia pseudomallei. PLoS Negl Trop Dis. 2013;7(3):e2105 Epub 2013/04/05. 10.1371/journal.pntd.0002105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu Y, Nakao R, Ohnuma A, Kawamori F, Sugimoto C. Microbial population analysis of the salivary glands of ticks; a possible strategy for the surveillance of bacterial pathogens. PLoS One. 2014;9(8):e103961 Epub 2014/08/05. 10.1371/journal.pone.0103961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sitthidet C, Stevens JM, Chantratita N, Currie BJ, Peacock SJ, Korbsrisate S, et al. Prevalence and sequence diversity of a factor required for actin-based motility in natural populations of Burkholderia species. J Clin Microbiol. 2008;46(7):2418–22. Epub 2008/05/23. 10.1128/JCM.00368-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sitthidet C, Korbsrisate S, Layton AN, Field TR, Stevens MP, Stevens JM. Identification of motifs of Burkholderia pseudomallei BimA required for intracellular motility, actin binding, and actin polymerization. J Bacteriol. 2011;193(8):1901–10. Epub 2011/02/22. 10.1128/JB.01455-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sitthidet C, Stevens JM, Field TR, Layton AN, Korbsrisate S, Stevens MP. Actin-based motility of Burkholderia thailandensis requires a central acidic domain of BimA that recruits and activates the cellular Arp2/3 complex. J Bacteriol. 2010;192(19):5249–52. Epub 2010/08/10. 10.1128/JB.00608-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daligault HE, Davenport KW, Minogue TD, Bishop-Lilly KA, Broomall SM, Bruce DC, et al. Whole-genome assemblies of 56 Burkholderia species. Genome Announc. 2014;2(6). Epub 2014/11/22. 10.1128/genomeA.01106-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeShazer D. Genomic diversity of Burkholderia pseudomallei clinical isolates: subtractive hybridization reveals a Burkholderia mallei-specific prophage in B. pseudomallei 1026b. J Bacteriol. 2004;186(12):3938–50. Epub 2004/06/04. 10.1128/JB.186.12.3938-3950.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holden MT, Titball RW, Peacock SJ, Cerdeno-Tarraga AM, Atkins T, Crossman LC, et al. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc Natl Acad Sci U S A. 2004;101(39):14240–5. Epub 2004/09/21. 10.1073/pnas.0403302101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thibault FM, Hernandez E, Vidal DR, Girardet M, Cavallo JD. Antibiotic susceptibility of 65 isolates of Burkholderia pseudomallei and Burkholderia mallei to 35 antimicrobial agents. J Antimicrob Chemother. 2004;54(6):1134–8. Epub 2004/10/29. 10.1093/jac/dkh471 . [DOI] [PubMed] [Google Scholar]
- 53.Chantratita N, Vesaratchavest M, Wuthiekanun V, Tiyawisutsri R, Ulziitogtokh T, Akcay E, et al. Pulsed-field gel electrophoresis as a discriminatory typing technique for the biothreat agent Burkholderia mallei. Am J Trop Med Hyg. 2006;74(3):345–7. Epub 2006/03/10. . [PubMed] [Google Scholar]
- 54.Whiteford ML, Wilkinson JD, McColl JH, Conlon FM, Michie JR, Evans TJ, et al. Outcome of Burkholderia (Pseudomonas) cepacia colonisation in children with cystic fibrosis following a hospital outbreak. Thorax. 1995;50(11):1194–8. Epub 1995/11/01. 10.1136/thx.50.11.1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sengyee S, Yoon SH, West TE, Ernst RK, Chantratita N. Lipopolysaccharides from different Burkholderia species with different lipid A structures induce toll-like receptor 4 activation and react with melioidosis patient sera. Infect Immun. 2019;87(12). Epub 2019/09/25. 10.1128/IAI.00692-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodley PD, Romling U, Tummler B. A physical genome map of the Burkholderia cepacia type strain. Mol Microbiol. 1995;17(1):57–67. Epub 1995/07/01. 10.1111/j.1365-2958.1995.mmi_17010057.x . [DOI] [PubMed] [Google Scholar]
- 57.Sarovich DS, Price EP, Webb JR, Ward LM, Voutsinos MY, Tuanyok A, et al. Variable virulence factors in Burkholderia pseudomallei (melioidosis) associated with human disease. PLoS One. 2014;9(3):e91682 Epub 2014/03/13. 10.1371/journal.pone.0091682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaestli M, Mayo M, Harrington G, Watt F, Hill J, Gal D, et al. Sensitive and specific molecular detection of Burkholderia pseudomallei, the causative agent of melioidosis, in the soil of tropical northern Australia. Appl Environ Microbiol. 2007;73(21):6891–7. Epub 2007/09/18. 10.1128/AEM.01038-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seng R, Saiprom N, Phunpang R, Baltazar CJ, Boontawee S, Thodthasri T, et al. Prevalence and genetic diversity of Burkholderia pseudomallei isolates in the environment near a patient's residence in Northeast Thailand. PLoS Negl Trop Dis. 2019;13(4):e0007348 Epub 2019/04/20. 10.1371/journal.pntd.0007348 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Multiplex PCR of 10 BTCV strains including strains E555, SBXPR001, SBXSR007, SBXPL001, SBXPL015, SBXRY031, SBXPR001, SBXCC001, SBXCC003 and WBXUBA33005104 (lanes 1–10) with multiplex PCR primers (BimABps-F/BimAcom-R, BimBPBM-F/BimAcom-R, and BimABth-F/BimABth-R). The expected 139-bp DNA fragments were detected in all samples. (B) Singleplex PCR of BTCV strains from (A) amplified with primers BimABth-SF and BimABth-R primers (lanes 1–10). Lanes P (mixture of Burkholderia spp. genomic DNA) and N (distilled water) are positive and negative controls, respectively. Lane M is 100 bp DNA ladder.
(TIFF)
The nucleotide sequences in red correspond to the BimBPBM and Bimcom primers.
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.