Abstract
Objectives
Anti-IL-17/23 biologics are increasingly used to treat psoriasis. We aimed to elucidate characteristics of drug-induced interstitial pneumonia (DIIP) caused by anti-IL-17/23 biologics.
Methods
We retrospectively analyzed the clinical data of psoriasis patients treated with anti-IL-17/23 biologics. Chest CT was performed to evaluate DIIP. Serum KL-6 levels were measured before treatment (baseline) and during treatment.
Results
A total of 603 psoriasis patients were treated with anti-IL-17/23 biologics with mean follow-up of 21.1 months. Six patients developed DIIP at mean 14 months after initiation of the therapy. Older age, higher baseline KL-6 value and more frequent pre-existing IPs were associated with development of DIIP by univariate analysis. At the onset of DIIP, elevated serum KL-6 levels with concomitantly increased ground glass opacity (GGO) in Chest CT were demonstrated. DIIP was improved by only cessation of causative agents in five patients but steroid therapy was needed in one patient.
Conclusions
DIIP is a plausible complication of anti-IL-17/23 biologics. Age, baseline KL-6 level and underlying IP could be the risk factors for DIIP development. Serum KL-6 levels and chest CT are useful for not only predicting but also detecting DIIP caused by anti-IL-17/23 biologics.
Introduction
Psoriasis is a chronic inflammatory disease mainly affecting the skin [1]. Increasing evidence suggests that IL-17/23 axis plays important roles in the pathogenesis of psoriasis [1–5]. Biologics inhibiting IL-17/23 axis has been developed to treat psoriasis, and currently, three anti-IL-23 and three anti-IL-17 biological agents are available for psoriasis in Japan [6]. Ustekinumab (UST) is a monoclonal antibody targeting IL-12/23 p40, and Guselkumab (GUS) and Risankizumab(RIS) are monoclonal antibodies targeting IL-23 p19. Secukinumab (SEC) and ixekizumab (IXE) are monoclonal antibodies targeting IL-17A, and brodalumab (BRO) is a fully human monoclonal antibody targeting the IL-17 receptor A. All of them have demonstrated high efficacy for psoriasis in clinical settings [7].
Interstitial pneumonia (IP) is one of frequent adverse events induced by a variety of drugs [8]. KL-6 is a high molecular weight mucin-like glycoprotein produced by type 2 pneumocytes and respiratory bronchiolar epithelial cells [9]. KL-6 is overproduced in regenerative epithelial cells following epithelial cell damage during IP progression, hence, the serum KL-6 levels have been considered a representative biomarker for IPs including drug-induced IP (DIIP) [10, 11].
Incidence, severity and prognosis of DIIP depend on not only the type of drug but also host specific reaction [8, 12–14]. There are several case reports showing DIIP by anti-IL-17/23 biologics [15–19] (Two cases of DIIP reported by Kikuchi et al [17] are also included in this study). Recently, association of noninfectious pneumonia with UST use was reported in JAMA dermatology, and a new warning for DIIP by UST was issued [20]. Hence, careful attention should be paid for this adverse event during treatment with anti-IL-17/23 biologics. However, the clinical characteristics of DIIP caused by anti-IL-17/23 biologics including UST remain to be determined. The aim of this study is to clarify the clinical characteristics of DIIP caused by anti-IL-17/23 biologics, including incidence, severity, and prognosis based on evaluation of KL-6 levels and chest CT findings.
Methods
Study subjects
This study is a retrospective cohort study of confirmed cases of psoriasis treated with biologic agents targeting anti-IL-17/23 biologics at Jikei University Hospital from July 1, 2011 (07/01/2011) to August 31, 2019 (08/31/2019). Patients were eligible for the study if they met the following inclusion criteria 1) Age ≥ 20 years old, 2) Confirmed cases of psoriasis by specialists of the Japanese Dermatological Association, 3) Treated with one of anti-IL-17/23 biologics. The exclusion criteria were 1) Patients with progressive cancer or fatal disease. A total of 603 psoriasis patients (UST;256, GUS;53,SEC;183, IXE;39, BRO;72) were included in this study. This study was approved by the Ethics Committee of Jikei University (29–078 (8694)), and adhered to the tenets of the Declaration of Helsinki. Based on the ethical guidelines of Jikei University, informed consent was not necessary for this retrospective study, and we performed opt-out consent on the website of our hospital. All data were fully anonymized before statistical analysis. Patients’ medical records during July 1, 2011 (07/01/2011) to August 31, 2019 (08/31/2019) were accessed. Serum KL-6 levels were measured with a commercially available ELISA kit (Nanopia KL-6, 202138–005, Tokyo, Japan) before treatment (baseline) and during treatment. The intervals were determined by the attending doctors. Chest computed tomography (CT) was performed based on worsening of respiratory symptom and/or elevation of KL-6 levels. The chest CT findings of these patients were evaluated by two specialists of the Japanese Respiratory Society (H.H. and H.M.). The psoriasis area severity index (PASI) score and type of psoriasis were determined by specialists of the Japanese Dermatological Association (Y.U., H.N., and A.A.).
Diagnosis of DIIP
Diagnostic criteria for drug-induced lung injury by Camus P et al. has been widely used in the clinical setting [21]. Accordingly, diagnosis of DIIP was made based on the diagnostic criteria as follows. (1)The patients were treated with anti-IL-17/23 biologics that are known to induce IP (2) IP have been reported to be induced by anti-IL-17/23 biologics. (3) Other causes could be ruled out. (4) IP was improved after anti-IL-17/23 biologics discontinuation.
Diagnosis of underlying IP
To evaluate underlying IP in 603 patients, physical examination and/or chest radiograph and/or chest CT were performed before biologic treatment. Chest CT was performed in 453 patients. According to the patients’ requests or by concerns about radiation exposure, Chest CT was not performed in 150 patients. Among the 150 patients, chest radiograph was performed in 115 patients, which showed no interstitial shadow. Only physical examination was performed in 35 patients, presenting no sign of IP. Chest CT and radiograph were evaluated by two specialists of the Japanese Respiratory Society (H.H. and H.M.).
Data collection
We retrospectively reviewed the medical records of the enrolled patients. Clinical characteristics including age, sex, BMI, disease duration, smoking history, underlying IP, type of psoriasis, prior biologic treatment, and treatment period were investigated.
Statistical analysis
Clinical indices among patients treated with different anti-IL-17 biologics were compared using analysis of variance followed by Bonferroni’s post hoc comparison tests and chi-squared tests. Student’s t-tests and chi-squared tests were performed to compare DIIP group and non DIIP group. A two-sided p<0.05 was considered statistically significant. All statistical analyses were performed with StatView, version 5 (SAS Institute Inc., Cary, NC, USA).
Results
Clinical characteristics of all patients treated with anti-IL-17/23 biologics (Table 1)
Table 1. Clinical characteristics of all patients.
| Total | UST | GUS | SEC | IXE | BRO | ||||||||
| (n = 603) | (n = 256) | (n = 53) | (n = 183) | (n = 39) | (n = 72) | p value | |||||||
| Age (years) | 54.0±16.3 | 53.8±17.1 | 55.0±17.3 | 53.6±15.5 | 55.3±12.9 | 54.3±16.3 | 0.96 | ||||||
| Male (%) | 418 (69.3) | 174 (68.0) | 37 (69.8) | 131 (71.6) | 29 (74.4) | 47 (65.3) | 0.79 | ||||||
| BMI | 23.9±3.9 | 23.4±3.6 | 23.6±3.9 | 23.7±3.8§ | 26.7±4.6 | 24.3±4.6 | <0.0001 | ||||||
| Disease duration (years) | 16.4±11.7 | 15.4±10.8 | 15.1±8.7 | 15.4±11.3 | 20.8±13.8 | 20.4±14.8 | 0.003 | ||||||
| History of smoking (%) | 198 (34.6) | unknown: n = 30 | 78 (31.6) | unknown: n = 9 | 11 (25) | unknown: n = 9 | 65 (36.7) | unknown: n = 6 | 17 (45.9) | unknown: n = 2 | 27 (39.7) | unknown: n = 4 | 0.2 |
| Underlying IP(%) | 18 (3.0) | 7 (2.7) | 1 (1.9) | 6(3.3) | 1 (2.6) | 3(4.2) | 0.95 | ||||||
| Type of psoriasis | 0.005 | ||||||||||||
| PsV | 523 | (86.7%) | 237 | (92.6%) | 47 | (88.7%) | 148 | (80.9%) | 30 | (76.9%) | 63 | (87.5%) | |
| PsA | 61 | (10.1%) | 13 | (5.1%) | 3 | (5.7%) | 30 | (16.4%) | 8 | (20.5%) | 7 | (9.7%) | |
| GPP | 8 | (1.3%) | 1 | (0.4%) | 0 | (0%) | 4 | (2.2%) | 1 | (2.6%) | 2 | (2.8%) | |
| PsE | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | |
| PsG | 1 | (0.2%) | 1 | (0.4%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | |
| PPP | 7 | (1.2%) | 3 | (1.2%) | 3 | (5.7%) | 1 | (0.5%) | 0 | (0%) | 0 | (0%) | |
| others | 1 | (0.5%) | 1 | (0.4%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | |
| Treatment period (months) | 21.1±21.3 | 31.5±26.6 | 4.0±4.0 | 18.5±12.3 | 7.2±5.6 | 11.0±7.4 | <0.0001 | ||||||
| biologic treatment | |||||||||||||
| UST | 256 | (42.5%) | |||||||||||
| GUS | 53 | (8.8%) | |||||||||||
| SEC | 183 | (30.3%) | |||||||||||
| IXE | 39 | (6.5%) | |||||||||||
| BRO | 72 | (11.9%) | |||||||||||
| Prior biologic treatment | |||||||||||||
| IFX | 41 | (6.8%) | 18 | (7.0%) | 1 | (1.9%) | 17 | (9.3%) | 3 | (7.7%) | 2 | (2.8%) | <0.0001 |
| ADA | 78 | (12.9%) | 25 | (9.8%) | 3 | (5.7%) | 41 | (22.4%) | 5 | (12.8%) | 4 | (5.6%) | |
| UST | 59 | (9.8%) | 0 | (0%) | 12 | (22.6%) | 41 | (22.4%) | 0 | (0%) | 6 | (8.3%) | |
| GUS | 1 | (0.2%) | 0 | (0%) | 0 | (0%) | 1 | (0.5%) | 0 | (0%) | 0 | (0%) | |
| SEC | 50 | (8.3%) | 4 | (1.56%) | 9 | (17.0%) | 0 | (0%) | 8 | (20.5%) | 29 | (40.3%) | |
| IXE | 3 | (0.5%) | 0 | (0%) | 1 | (1.9%) | 1 | (0.5%) | 0 | (0%) | 1 | (1.39%) | |
| BRO | 12 | (2.0%) | 0 | (0%) | 2 | (3.8%) | 3 | (1.6%) | 7 | (17.9%) | 0 | (0%) | |
| Total | 244 | (40.5%) | 47 | (18.4%) | 28 | (52.8%) | 104 | (56.8%) | 23 | (59.0%) | 42 | (58.3%) | |
| KL-6 (U/mL) | 283.8±247.9 | 271.8±206.1 | 290.7±227.6 | 284.1±303.5 | 302.7±172.5 | 292.8±216.1 | 0.95 | ||||||
| DIIP | 6 | (1.0%) | 3 | (1.16%) | 1 | (1.89%) | 2 | (1.09%) | 0 | (0%) | 0 | (0%) | 0.95 |
UST: Ustekinumab, GUS: Guselkumab, SEC: Secukinumab, IXE: Ixekizumab, BRO: Brodalumab, IFX: infliximab, ADA: Adalimumab.
IP: Interstitial pneumonia, DILI: Drug-induced lung injury.
KL-6: Krebs von den lungen-6, PsV: Psoriasis vulgaris, PsA: Psoriatic arthritis, GPP: Generalized pustular psoriasis, PsE: Erythrodermic psoriasis, PsG: Guttate psoriasis, PPP: Palmoplantar pustulosis.
A total of 603 psoriasis patients (UST;256, GUS;53,SEC;183, IXE;39, BRO;72) were treated with anti-IL-17/23 biologics with an average follow-up of 21.1 months. Seventy-four patients sequentially used two anti-IL-17/23 biologics, twenty three patients used three of them, and four patients used four of them. Disease duration of psoriasis was longer in BRO group compared to UST and SEC group. Treatment periods of UST group were longer than the other groups, and those of SEC group were longer than those of GUS and IXE. Prior biologic treatments were different among the groups. Other factors including age, ratio of male sex, history of smoking, underlying IP, baseline KL-6 and incidence of DIIP were not different among groups. (Difference of disease duration, treatment periods, and prior biologic treatments might be attributed to different timing of approval of each agent in Japan. BMI and types of psoriasis were different among the groups but no appropriate explanation existed).
Backgrounds of patients with or without DIIP by anti-IL-17/23 biologics
Six patients (1.0%, UST;3, GUS;1, SEC;2) developed DIIP at average 14 months after initiation of the therapy. Backgrounds of patients without DIIP and with DIIP were shown in Table 2. Patients with DIIP were older than those without DIIP. Underlying IP was frequent in DIIP group (n = 4, 66.7%) compared to non-DIIP group (n = 14, 2.3%). Baseline KL-6 levels in DIIP group (1008.8±746.1) were higher than those without DIIP (274.7±222.8). Clinical characteristics of 18 patients with underlying IP were shown in Table 3. Connective tissue diseases were not complicated with the patients. Ground-glass and/or irregular linear (reticular) opacity in the bilateral lower lobes was characteristic of underlying IP as described in our previous work [22]. At DIIP presentation, systemic treatment for IP including glucocorticoids, immunosuppressive drugs, and drugs related to drug-induced IP were not used. Among 18 patients with underlying IP, the difference between baseline KL-6 levels in 4 patients who developed DIIP and those in 14 patients who did not develop DIIP was not significant (DIIP: 923±818, non DIIP:781±869, p = 0.77) Baseline KL-6 levels were not associated with development of DIIP in this population. Titers of ANA (anti-nuclear antibody) were less than or equal to 1:160, and anti-Jo-1 antibodies were negative. Other markers for connective tissue diseases and IP were not measured.
Table 2. Comparison of clinical characteristics of patients with or without DIIP.
| without DIIP | with DIIP | ||||
|---|---|---|---|---|---|
| (n = 597) | (n = 6) | p value | |||
| Age (years) | 53.8±16.3* | 69.3±9.0* | 0.02 | ||
| Male (%) | 414 (69.3) | 4 (66.7) | 0.89 | ||
| BMI | 23.9±4.0 | 23.3±3.0 | 0.73 | ||
| Disease duration (years) | 16.4±11.7 | 20.0±14.4 | 0.45 | ||
| History of smoking (%) | 194 (34.2) | unknown: n = 30 | 6 (66.6) | 0.10 | |
| Underlying IP(%) | 14 (2.3)* | 4 (66.7)* | <0.0001 | ||
| Type of psoriasis | 0.99 | ||||
| PsV | 520 | (87.1%) | 5 | 83.3(%) | |
| PsA | 60 | (10.1%) | 1 | (16.7%) | |
| GPP | 8 | (1.3%) | 0 | (0%) | |
| PsE | 0 | (0%) | 0 | (0%) | |
| PsG | 1 | (0.2%) | 0 | (0%) | |
| PPP | 7 | (1.2%) | 0 | (0%) | |
| others | 1 | (0.2%) | 0 | (0%) | |
| Treatment period(months) | 21.2±21.3 | 14.0±9.0 | 0.41 | ||
| biologic treatment | |||||
| UST | 253 | (42.3%) | 3 | (50%) | 0.80 |
| GUS | 52 | (8.7%) | 1 | (16.7%) | |
| SEC | 181 | (30.3%) | 2 | (33.3%) | |
| IXE | 39 | (6.5%) | 0 | (0%) | |
| BRO | 72 | (12.1%) | 0 | (0%) | |
| Prior biologic treatment | |||||
| IFX | 40 | (6.7%) | 1 | (16.7%) | 1.00 |
| ADA | 77 | (12.9%) | 1 | (16.7%) | |
| UST | 58 | (9.7%) | 1 | (16.7%) | |
| GUS | 1 | (0.17%) | 0 | (0%) | |
| SEC | 49 | (8.2%) | 1 | (16.7%) | |
| IXE | 3 | (0.5%) | 0 | (0%) | |
| BRO | 12 | (2.0%) | 0 | (0%) | |
| Total | 240 | (40.2%) | 4 | (66.7%) | |
| KL-6 (U/mL) | 274.7±222.8 | 1008.8±746.1* | <0.0001 |
SEC: Secukinumab, BRO: Brodalumab, IXE: Ixekizumab.
IP: Interstitial pneumonia, BI: Brinkman index, PASI: Psoriasis area severity index, KL-6: Krebs von den lungen-6, PsV: Psoriasis vulgaris, PsA: Psoriatic arthritis, GPP: Generalized pustular psoriasis, PsE: Erythrodermic psoriasis, PsG: Guttate psoriasis, PPP: Palmoplantar pustulosis.
Table 3. Clinical characteristics of underlying IP.
| No. | Age | Sex | complicated CTD |
CT findings Distribution |
GGO | Reticular opacity | Emphysema | Baseline KL-6 levels(U/ml) |
biologictreatment | IP activity by the biologic |
ANA(IF) (×) |
anti-Jo-1 antibody |
DIIP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 77 | M | non | Bilateral lower lobe | Peripheral | + | + | + | 561 | SEC | - | - | + | |
| 2 | 79 | M | non | Bilateral lower lobe | Peripheral | + | + | + | 351 | SEC | 40 | - | ||
| 3 | 76 | M | non | Bilateral lower lobe | Peripheral | - | + | + | 734 | SEC | - | - | ||
| 4 | 74 | M | non | Bilateral lower lobe | Central | + | + | - | 413 | SEC | improved | 160 | - | |
| 5 | 65 | M | non | Bilateral lower lobe | Peripheral | + | + | - | 174 | SEC | 40 | N.A. | ||
| 6 | 72 | M | non | Bilateral lower lobe | Peripheral | + | - | - | 3656 | SEC | 160 | - | ||
| 7 | 77 | M | non | Bilateral lower lobe | Peripheral | - | + | + | 771 | BRO | 40 | - | ||
| 8 | 69 | M | non | Bilateral lower lobe | Peripheral | + | - | - | 447 | BRO | - | - | ||
| 9 | 56 | F | non | Bilateral lower lobe | Peripheral | + | - | - | 1207 | BRO | 40 | - | ||
| 10 | 81 | M | non | Bilateral lower lobe | Peripheral | + | + | + | 632 | IXE | 40 | - | ||
| 11 | 72 | M | non | Bilateral lower lobe | Peripheral | + | + | + | 770 | UST | improved | 40 | - | |
| 12 | 64 | F | non | Bilateral lower lobe | Central | + | - | - | 2149 | UST | N.A. | N.A. | + | |
| 13 | 77 | F | non | Bilateral lower lobe | Peripheral | + | - | - | 296 | UST | improved | 40 | N.A. | |
| 14 | 75 | M | non | Bilateral lower lobe | Peripheral | + | + | + | 467 | UST | - | - | + | |
| 15 | 81 | F | non | Bilateral lobe | Diffuse | + | + | - | 356 | UST | improved | - | N.A. | |
| 16 | 73 | M | non | Bilateral lower lobe | Peripheral | - | + | + | 675 | UST | - | - | ||
| 17 | 75 | M | non | Bilateral lower lobe | Peripheral | - | + | + | 452 | UST | 80 | N.A. | ||
| 18 | 79 | M | non | Bilateral lower lobe | Peripheral | + | + | + | 517 | GUS | - | - | + |
CTD: Connective tissue disease, ANA: Anti-nuclear antibody, N.A.: Not available.
GGO: Ground Glass Opacity, RF: Reumatiod factor.
IF: Immunofluorescence.
Clinical characteristics of DIIP
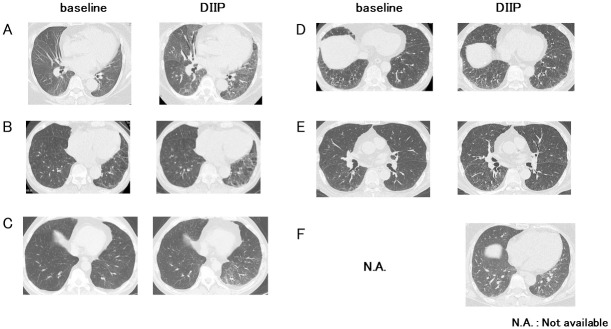
Clinical characteristics and course of DIIP were shown in Tables 4 and 5. (Among DIIPs patients, case 1 and 3 were already reported in J Dermatol. 2016 Jun;43(6):712–3.) Time to initial onset of DIIP ranged from 3 months to 26 months (average 14.0±9.0 months). While four of the six patients were symptomatic (cough or dyspnea), two patients were asymptomatic. In the asymptomatic patients, elevation of serum KL-6 levels from baseline was clue for further evaluation by CT scan, resulting in DIIP diagnosis. Chest CT findings at the onset of DIIP demonstrated patchy ground glass opacity (GGO) in all patients (Fig 1). Serum KL-6 levels were elevated at the onset of DIIP from baseline value, which were decreased after DIIP improvement. DIIPs were improved after cessation of the causative anti-IL-17/23 biologics without additional therapy in five patients but steroid therapy was needed in one patient. High baseline KL-6 levels and/or underlying IP were present in all patients with DIIP.
Table 4. Clinical characteristics of patients with DIIP (1).
| No. | Age | Sex | BMI | Disease duration (year) |
Smoking | type of Ps | Baseline KL-6 levels (U/ml) |
underlying IP | prior biologic | Causative biologic of DIIP |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | F | 27.4 | 1 | - | PsV | 2149 | + | - | UST |
| 2 | 75 | M | 19.5 | 30 | + | PsV | 467 | + | - | UST |
| 3 | 65 | M | 25.1 | 6 | + | PsV | 829 | - | IFX | UST |
| 4 | 79 | M | 21.3 | 22 | + | PsV | 332 | + | SEC | GUS |
| 5 | 77 | M | 21.3 | 20 | + | PsV | 561 | + | UST | SEC |
| 6 | 56 | F | 25.2 | 39 | - | PsA | 1715 | - | ADA | SEC |
Table 5. Clinical characteristics of patients with DIIP (2).
| No. | Causative biologic |
Time to initial onset of IP |
symptom | hypoxia | CT findings |
KL-6 Baseline |
KL-6 onset of IP |
KL-6 (after cessation of SEC) |
Treatment for DIIP |
Clinical course of IP |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | UST | 3 M | cogh | + | GGO | 2149 | 6809 | 2326 | PSL | Improve |
| 2 | UST | 17 M | cogh | - | GGO | 467 | 1507 | 887 | none | Improve |
| 3 | UST | 26M | - | - | GGO | 829 | 2092 | 991 | none | Improve |
| 4 | GUS | 12 M | - | - | GGO | 332 | 755 | 448 | none | Improve |
| 5 | SEC | 21 M | dyspnea | - | GGO | 561 | 1348 | 332 | none | Improve |
| 6 | SEC | 5 M | cough | - | GGO | 1715 | 1800 | 614 | none | Improve |
GGO: Ground glass opacity.
Fig 1. Chest CT in patients with DIIP.
Panels of A-F demonstrated chest CTs of patient 1–6 shown in Tables 4 and 5. Each left panel showed chest CT before administration of causative biologic (baseline). Each right panel showed CT at the onset of DIIP. (Case 1 and 3 were identical with two cases in the Journal of Dermatology in 2016 [17]).
Discussion
In this study, we demonstrated that (1) incidence of DIIP by anti-IL-17/23 biologics was approximately 1.0% (6/603), (2) Age, baseline KL-6 level and underlying IP could be risk factors, (3) DIIP by anti-IL-17/23 biologics was mild, and developed at mean 14 months after initiation of the therapy (4) Serum KL-6 levels and chest CT were useful for not only predicting but also detecting DIIP.
Although many clinical studies have elucidated the efficacy and safety of anti-IL-17/23 biologics, no studies clearly demonstrated the incidence of DIIP, hence, our study is of great clinical importance. The actual incidence of DIIP was 1.0% of total patients in this study, which was similar to the incidence of DIIP by humanized biologics targeting TNF-alpha (0.5%) in Japanese postmarketing surveillance for Rheumatoid Arthritis [23].
Age, sex, smoking history, pre-existing lung diseases, and renal dysfunction are widely known risk factors for DIIP [8, 12, 13]. In this study, age, baseline KL-6 levels and pre-existing IP were suspected to be risk factors for DIIP for anti-IL-17/23 biologics by univariate analysis. (The number of DIIP cases was not enough for multivariate analysis.) Because KL-6 increases with IP, the effect of baseline KL-6 level on development of DIIP in this study might partly reflect the effect of underlying IP on development of DIIP. Indeed, baseline KL-6 levels did not show the significant effect on development of DIIP in patients with underlying IP. Larger studies are needed to determine the true risk factor of DIIP. Careful attentions for DIIP should be paid during anti-IL-17/23 biologics treatment in patients with these risk factors. In clinical practice, multiple different biologics were used sequentially in same patient and each biologics may have different effect on IP development. Increasing evidence suggests that anti-TNF-alpha biologics could increase KL-6 levels with or without clinical DIIP development or worsening of pre-existing IP [24]. It is plausible that increase KL-6 levels without IP progression may reflect subclinical alveolar inflammation caused by anti-TNF-alpha biologics. In case 3 and 6 in Tables 4 and 5 without pre-existing IP, KL-6 levels were elevated before anti-IL-17/23 biologics treatment, which might be associated with prior use of anti-TNF-alpha biologic. We speculate that elevated baseline KL-6 levels with subclinical alveolar inflammation by anti-TNF-alpha biologic pretreatment may enhance sensitivity to anti-IL-17/23 biologics-induced DIIP.
DIIP by anti-IL-17/23 biologics in this study was mild and with good prognosis. DIIPs were improved after cessation of the causative biologics without additional therapy in five patients but steroid therapy was needed in one patient in this study. In general, IP improvement by drug cessation without additional therapy can be an important clue for DIIP diagnosis (positive dechallenge). However, it is difficult to distinguish between DIIP development and worsening of pre-existing IP in the patient who needed additional steroid therapy. In our patient who was needed additional steroid therapy, pre-existing IP was stable for two year before administration of anti-IL-17/23 biologic. Furthermore, activity of psoriasis, which could be aligned with complicated IP activity [22], was markedly improved in response to anti-IL-17/23 biologics treatment, suggesting that DIIP is more likely than worsening of pre-existing IP.
Anti-IL-17/23 biologics could both improve and induce IP. Anti-IL-17/23 biologics improved IP in four (Characteristics of three patients were demonstrated in our previous report [22]) of 18 patients with underlying IP. IL-17/23 pathway is considered to be involved in the pathogenesis of the IP of the four patients. Activity of this type of IP was aligned with activity of psoriatic skin lesion, and both IP and skin lesions were improved within several months after first administration. DIIP by anti-IL-17/23 biologics, whose activity was not associated with activity of psoriatic skin lesion, developed later, even after two years as shown in this study. DIIP might be independent of IL-17/23 pathway and caused by other immunological mechanisms, which should be elucidated by further research.
There is a wide variation of clinical course and prognosis in DIIP, which is dependent on the type of causative agents. While immediate cessation of the causative agent and subsequent high dose of steroid therapy is needed in severe DIIP caused by EGFR tyrosine kinase inhibitors [25], continuation of the causative agent can be permissive in mild DIIP induced by mTOR inhibitors [26]. The results of this study and other previous reports of DIIP by anti-IL17/23 biologics [20] suggested that DIIP by anti-IIL-17/23 biologics can be mild with good prognosis. To clarify whether cessation is needed or not for mild DIIP by anti-IIL-17/23 biologics during treatment for refractory psoriasis, larger scale of clinical study should be conducted to determine the balance between treatment efficacy to psoriasis and severity of DIIP.
As shown in Table 5, it is likely that DIIP can occur at any time points during anti-IL-17/23 biologics treatments. DIIP could be occurred even at 26 months after first administration in patient 3, indicating that attentions for DIIP should be paid all the time during anti-IL-17/23 biologics treatment. Symptoms at the onset of DIIP were non-specific, hence, careful physiological, serological, and radiological evaluations are important for early diagnosis. At the onset of DIIP, serum KL-6 levels were elevated compared to the baseline and GGO in Chest CT emerged in all patients. Accordingly, monitoring of serum KL-6 levels before and during treatment and chest CT scan in case of KL-6 elevation could be useful for properly detecting DIIP.
Conclusions
DIIP is a plausible side effect of anti-IL-17/23 biologics. Age, baseline KL-6 level and underlying IP might be risk factor for DIIP. Monitoring of serum KL-6 levels and chest CT scan might be useful for predicting and detecting DIIP.
Abbreviations
- ADA
adalimumab
- BRO
brodalumab
- CT
computed tomography
- DIIP
drug-induced interstitial pneumonia
- GUS
guselkumab
- IFX
infliximab
- IL-17/23
Interleukin-17/23
- IP
interstitial pneumonia
- IXE
ixekizumab
- KL-6
Krebs von den Lungen-6
- RIS
Risankizumab
- SEC
secukinumab
- TNF
tumor necrosis factor
- UST
ustekinumab
Data Availability
Data cannot be shared publicly because of privacy of the patients. Data from this study are available upon request (Hiromichi Hara, E-mail: hirohara@jikei.ac.jp or ethics committee of Jikei University, E-mail: crb@jikei.ac.jp), since the data contain potentially identifying or sensitive patient information.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Greb JE, Goldminz AM, Elder JT, Lebwohl MG, Gladman DD, Wu JJ, et al. Psoriasis. Nature reviews Disease primers. 2016. November 24;2:16082 10.1038/nrdp.2016.82 . Epub 2016/11/25. eng. [DOI] [PubMed] [Google Scholar]
- 2.Brembilla NC, Senra L, Boehncke WH. The IL-17 Family of Cytokines in Psoriasis: IL-17A and Beyond. Frontiers in immunology. 2018;9:1682 10.3389/fimmu.2018.01682 . Epub 2018/08/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin DA, Towne JE, Kricorian G, Klekotka P, Gudjonsson JE, Krueger JG, et al. The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. The Journal of investigative dermatology. 2013. January;133(1):17–26. 10.1038/jid.2012.194 . Epub 2012/06/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 Family of Cytokines in Health and Disease. Immunity. 2019. April 16;50(4):892–906. 10.1016/j.immuni.2019.03.021 . Epub 2019/04/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puig L. The role of IL 23 in the treatment of psoriasis. Expert review of clinical immunology. 2017. June;13(6):525–34. 10.1080/1744666X.2017.1292137 . Epub 2017/02/07. eng. [DOI] [PubMed] [Google Scholar]
- 6.Saeki H, Terui T, Morita A, Sano S, Imafuku S, Asahina A, et al. Japanese guidance for use of biologics for psoriasis (the 2019 version). The Journal of dermatology. 2020. March;47(3):201–22. 10.1111/1346-8138.15196 . Epub 2020/01/10. eng. [DOI] [PubMed] [Google Scholar]
- 7.Yasmeen N, Sawyer LM, Malottki K, Levin LA, Apol ED, Jemec GB. Targeted therapies for patients with moderate-to-severe psoriasis: a systematic review and network meta-analysis of PASI response at 1 year. The Journal of dermatological treatment. 2020. March 23:1–50. 10.1080/09546634.2020.1743811 . Epub 2020/03/24. eng. [DOI] [PubMed] [Google Scholar]
- 8.Skeoch S, Weatherley N, Swift AJ, Oldroyd A, Johns C, Hayton C, et al. Drug-Induced Interstitial Lung Disease: A Systematic Review. Journal of clinical medicine. 2018. October 15;7(10). 10.3390/jcm7100356 . Epub 2018/10/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinata N, Takemura T, Ikushima S, Yanagawa T, Ando T, Okada J, et al. Phenotype of regenerative epithelium in idiopathic interstitial pneumonias. Journal of medical and dental sciences. 2003. September;50(3):213–24. . Epub 2004/04/13. eng. [PubMed] [Google Scholar]
- 10.Ohnishi H, Yokoyama A, Kondo K, Hamada H, Abe M, Nishimura K, et al. Comparative study of KL-6, surfactant protein-A, surfactant protein-D, and monocyte chemoattractant protein-1 as serum markers for interstitial lung diseases. American journal of respiratory and critical care medicine. 2002. February 1;165(3):378–81. 10.1164/ajrccm.165.3.2107134 . Epub 2002/01/31. eng. [DOI] [PubMed] [Google Scholar]
- 11.Kohno N, Kyoizumi S, Awaya Y, Fukuhara H, Yamakido M, Akiyama M. New serum indicator of interstitial pneumonitis activity. Sialylated carbohydrate antigen KL-6. Chest. 1989. July;96(1):68–73. 10.1378/chest.96.1.68 . Epub 1989/07/01. eng. [DOI] [PubMed] [Google Scholar]
- 12.Kubo K, Azuma A, Kanazawa M, Kameda H, Kusumoto M, Genma A, et al. Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respiratory investigation. 2013. December;51(4):260–77. 10.1016/j.resinv.2013.09.001 . Epub 2013/11/19. eng. [DOI] [PubMed] [Google Scholar]
- 13.Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respiratory research. 2012. May 31;13:39 10.1186/1465-9921-13-39 . Epub 2012/06/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruchalla RS. Drug metabolism, danger signals, and drug-induced hypersensitivity. The Journal of allergy and clinical immunology. 2001. October;108(4):475–88. 10.1067/mai.2001.118509 . Epub 2001/10/09. eng. [DOI] [PubMed] [Google Scholar]
- 15.Kajihara I, Yamada-Kanazawa S, Maeda-Otsuka S, Jinnin M, Akaike K, Ihn H. Secukinumab-induced interstitial pneumonia in a patient with psoriasis vulgaris. The Journal of dermatology. 2017. December;44(12):e322–e3. 10.1111/1346-8138.13986 . Epub 2017/08/05. eng. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi M, Igarashi A, Okamura K, Suzuki T. Paradoxical exacerbation of latent interstitial pneumonia by secukinumab in a patient with psoriasis vulgaris. The British journal of dermatology. 2019. March;180(3):684–5. 10.1111/bjd.17424 . Epub 2018/11/16. eng. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi S, Umezawa Y, Hayashi M, Yanaba K, Fukuchi O, Ito T, et al. Interstitial pneumonia in two patients with psoriasis during ustekinumab treatment. The Journal of dermatology. 2016. June;43(6):712–3. 10.1111/1346-8138.13250 . Epub 2015/12/26. eng. [DOI] [PubMed] [Google Scholar]
- 18.Lee SG, An JH, Kim DH, Yoon MS, Lee HJ. A Case of Interstitial Lung Disease and Autoimmune Thyroiditis Associated with Ustekinumab. Acta dermato-venereologica. 2019. March 1;99(3):331–2. 10.2340/00015555-3084 . Epub 2018/11/15. eng. [DOI] [PubMed] [Google Scholar]
- 19.Sorger C, Simon JC, Treudler R. [Drug-induced interstitial lung disease (DILD) during treatment with ustekinumab]. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete. 2019. December 2. Epub 2019/12/04. Medikamentos induzierte interstitielle Lungenerkrankung (DILD) unter Ustekinumab. ger. [DOI] [PubMed] [Google Scholar]
- 20.Brinker A, Cheng C, Chan V. Association of Noninfectious Pneumonia With Ustekinumab Use. JAMA dermatology. 2019. February 1;155(2):221–4. 10.1001/jamadermatol.2018.4118 . Epub 2018/12/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camus P, Fanton A, Bonniaud P, Camus C, Foucher P. Interstitial lung disease induced by drugs and radiation. Respiration; international review of thoracic diseases. 2004. Jul-Aug;71(4):301–26. 10.1159/000079633 . Epub 2004/08/19. eng. [DOI] [PubMed] [Google Scholar]
- 22.Kawamoto H, Hara H, Minagawa S, Numata T, Araya J, Kaneko Y, et al. Interstitial Pneumonia in Psoriasis. Mayo Clinic proceedings Innovations, quality & outcomes. 2018. December;2(4):370–7. 10.1016/j.mayocpiqo.2018.07.006 . Epub 2018/12/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeuchi T, Tatsuki Y, Nogami Y, Ishiguro N, Tanaka Y, Yamanaka H, et al. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Annals of the rheumatic diseases. 2008. February;67(2):189–94. 10.1136/ard.2007.072967 . Epub 2007/07/24. eng. [DOI] [PubMed] [Google Scholar]
- 24.Higashi Y, Tada K, Shimokawa M, Kawai K, Kanekura T. Elevation of serum KL-6 in patients with psoriasis treated with anti-tumour necrosis factor-alpha therapy. Clinical and experimental dermatology. 2016. January;41(1):88–90. 10.1111/ced.12544 . Epub 2015/01/06. eng. [DOI] [PubMed] [Google Scholar]
- 25.Kawase S, Hattori N, Ishikawa N, Horimasu Y, Fujitaka K, Furonaka O, et al. Change in serum KL-6 level from baseline is useful for predicting life-threatening EGFR-TKIs induced interstitial lung disease. Respiratory research. 2011. July 26;12:97 10.1186/1465-9921-12-97 . Epub 2011/07/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abe M, Tsushima K, Ikari J, Kawata N, Terada J, Tanabe N, et al. Evaluation of the clinical characteristics of everolimus-induced lung injury and determination of associated risk factors. Respiratory medicine. 2018. January;134:6–11. 10.1016/j.rmed.2017.11.009 . Epub 2018/02/08. eng. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cannot be shared publicly because of privacy of the patients. Data from this study are available upon request (Hiromichi Hara, E-mail: hirohara@jikei.ac.jp or ethics committee of Jikei University, E-mail: crb@jikei.ac.jp), since the data contain potentially identifying or sensitive patient information.