Abstract
Objectives:
Emotion regulationdifficulties precipitate and exacerbate acute mood symptoms in individuals with bipolar disorder (BD), and contribute tosuicidal behavior.However, few studies have examined regional brain responses in explicit emotion regulation during acute BD mood states, or hopelessness, a major suicide risk factor.We assessed brain responses during explicit emotion regulation, and their relationship with hopelessness, in acutely symptomatic and euthymic individuals with BD.
Methods:
Functional MRI data were obtained from individuals with BDwho were either in acute negative (BD-A; n=24)or euthymic (BD-E; n=24) mood states, and from healthy volunteers (HV; n=55), while participants performed aparadigm that instructed them to downregulatetheir responses to fearful (EmReg-Fear) and happy(EmReg-Happy) facial stimuli. Emotion regulation-related differences in brain responses during negative and euthymic BD states,as well astheir associations withnegative affective symptoms (hopelessness and depression),were examined.
Results:
Decreased responseswere observed in ventral and dorsal frontal regions, includingmedial orbitofrontal (mOFC)and dorsal anterior cingulate cortices,during EmReg-Fear across symptomatic and euthymic states in BD relative to HVs.The lowestresponses were observed in the BD-A group. Across BD participants, negative associations were observed between mOFC responses and hopelessness, particularly due to loss of motivation.Differences were not significant during EmReg-Happy.
Conclusions:
Lesser emotion regulation-related ventral and dorsal frontal engagement in BD could represent a trait abnormality that worsens during acute negative states.The reduced mOFC engagement in BD during explicit regulation of negative emotions may contribute tohopelessness particularly in the context of diminished motivation.
Keywords: bipolar disorder, emotions, frontal, functional magnetic resonance imaging, fMRI
INTRODUCTION
Difficulties with emotion regulation in Bipolar Disorder (BD) have been theorized toresult from dysfunction in the frontal systems that subservethe regulation of emotions(1-3). Maladaptiveregulation of emotionsis associated with detrimental physical, psychological, and psychosocial outcomes (4), including precipitating and exacerbating acute mood episodes in individuals with and at-risk for BD(5, 6).Importantly,maladaptive regulation of emotions is associated with suicidal thoughts and behaviors(7, 8), which may further contribute to thehigh risk for suicide in BD (9).Elucidating the neural mechanisms underlying maladaptive regulation of emotionscould be critical in providing targets for interventions to improve outcomes in BD,including reducing risk for suicide.
While depressive symptoms of mood disorders are an important risk factor for suicide, it has long been known that hopelessness, even when experienced outside the context of mood disorders, is an important behavioral construct that is a robust risk factor for suicide (10). Hopelessness is strongly associated with suicide intent, more so than depression (10, 11). Moreover, hopelessness is predictive of future suicidal attempts and suicide deaths (10, 12). Converging evidence from behavioral studies support hopelessness as a strong mediator of the relationship between emotion dysregulation and suicidal ideation (13-15), even when controlling for depression severity (15). To prevent suicide, it is essential to identify the neural mechanisms underlying factors that reliably predict and precede suicidal behavior. Hence, investigating the neural substrates of maladaptive regulation of emotions in BD, and examining their relationship with clinical factors, including depression and hopelessness, are important for identifying brain systems associated with suicide risk factors and for developing targeted interventions to prevent suicidal attempts in individuals with BD.
There are multiple extant models of healthy emotion regulation(16-20) that converge in recognizing the importance ofdifferentiating between two related, yet distinct families of emotion regulation processes: implicitemotion regulation which is a non-conscious, automatic process of emotion regulation, and explicit emotion regulation which is a conscious, voluntary process and involves controlled changes in emotional responses (16). While both implicit and explicit emotion regulation have been shown to engage ventromedial prefrontal regions including medial orbitofrontal cortices (vmPFC/mOFC) that are importantforintegrating internally generated motivational processes (1), explicit emotion regulation has been shown toadditionally engage more lateral and dorsal frontal regions including ventrolateral PFC (vlPFC), dorsolateral PFC (dlPFC), dorsomedial PFC (dmPFC) and the dorsal anterior cingulate cortex (dACC)(20, 21) that are important in the conscious cognitive control processes (22).
Functional neuroimaging studies have demonstrated abnormalities in prefrontal regions subserving emotion regulationin individuals with BD at rest(23-26)and when performing activation tasks designed to engage the emotion regulation brain circuitry. The latter includes tasks that probe implicit emotion regulation, such as viewing emotional faces(27)or performingemotional Go/No-Go(28, 29), emotional n-back working memory(30, 31)and Stroop (32) tasks. Neuroimaging studies in BD using paradigms designed to probe explicit emotion regulationrelated functional activations are limited, and most of these studies were of participants with BD whowere euthymic (33-35).In a study of euthymic BD individuals, compared to healthy volunteers (HV), in which participants were instructed to explicitly downregulate emotional responses to negative stimuli from the International Affective Pictorial System (IAPS)(36), reduced vmPFC, vlPFC, and dlPFC activations were observed (34),suggesting a trait abnormality in explicit emotion regulation in BD. However, other studies of downregulation of responses to negative IAPS stimuli did not detect differences between euthymic BD and HV groups(35, 37).
Few fMRI studies examining regional brain activations during explicit downregulation of responses to emotional stimuli in BD have included individuals in acute mood states(37-39), and studies of the neural circuitry of hopelessness are rare.A study of depressed individuals with BDshoweda trend for significant reductions in ventral ACCactivation during downregulation of positive versus negative emotional IAPS pictures(37). Another study, that assessed depressed and euthymic individualstogether, showed reduced vlPFC and dlPFC activation during reappraisal of negative emotionalIAPS pictures(39). These studies includedfewerthan10 depressed BD individuals (37, 39). Hence, better poweredstudies of BD examining negative mood-state related braincorrelates of emotion regulation, as well as examiningthe influence of clinical factors that contribute to impairments in emotion regulation, are needed.Moreover, relative to study of emotional scenes, study of responses tofacial stimuli in particular could provide new insightsas they are socially salient,and are important for generating affective responses.Facial stimulihave previously been shown to reliably activate brain regions that are involved in emotion processing and regulation in HVs(40), and to repeatedlyelicit differences in functional brain responses in individuals with BD (27, 28, 30, 41-43).
Thus, we performed a functional magnetic resonance imaging (fMRI) study of individuals with BD who were in a negative mood state (depressed and mixed),individuals with BD who were euthymic, and HVindividuals, using aparadigm designed to examine brain activations during explicit downregulation of emotional responses to facialstimuli.Diagnostic and Statistical Manual of Mental Disorders (Fourth ed. Text Revision; DSM-IV-TR)was used to categorically define mood states (categorical approach) and investigatebrain circuitry function during emotion regulation in relation to acute-negative and euthymic mood states.However, as the categorical determination of mood state of BD may not capture clinical manifestations that are more closely related to impairments in emotion regulation and associated outcomes, brain circuitry function during emotion regulation in BD in response to negative affectivesymptoms defined dimensionally using hopelessness and depression rating scales(dimensional approach) was also examined.It was hypothesized thatduring explicit emotion regulation of negative emotional stimuli, individuals with BD, especially those experiencing negative affective symptoms, i.e. those in depressed and mixed mood states, would show reduced engagement relative to HVs in regions implicated both in explicit emotion regulation processes and in BD (3, 16-18, 27), includingvmPFC/mOFC, vlPFC, dlPFC, dmPFC and dACC. We further hypothesized a larger magnitude of the brain engagement reductions would be associated with a higher severity of hopelessness and depression.
METHOD
Participants
Participants included 48 individuals who met criteria for BD(BDI/BDII; n=48)[ages years 16-59 years, mean age±standard deviation (SD)=32.1±13.4 years; Tanner Stages ≥4, 58.3% female].
At the time of scan, 24 participantswith BD were in an acute BD episode (referred to hereafter as BD-A) and 24 participants with BD were euthymic (BD-E). Within the BD-A group, 19 participants met criteria for a current major depressive episode,and 5 participants met criteria for a current mixed episode and, as per DSM criteria, had a major depressive episode for at least one week. Diagnosis and mood state were assessed using the Structured Clinical Interview for DSM-IV-TR Axis I disorders [SCID-IV(44)] for participants ≥18 years of age, and the Schedule for Affective Disorders and Schizophrenia for School-Age Children [K-SADS (45)] for participants <18 years. Comorbidities and other clinical characteristics of the BD sample, separately for the two mood state subgroups,are listed in Table 1. Twelve BD participants(25%) met criteria for rapid cycling, and eighteen (37.5%) had a history of psychosis.The most prevalent lifetime comorbid disorders were post-traumatic stress disorder (PTSD) (n=13; 27.1%) and attention deficit/hyperactivity disorder (ADHD) (n=9; 18.8%). Fifteen BD participants (31.3%) were unmedicated at the time of scan.
Table1:
Demographic and Clinical Characteristics of the Sample
| Subjects with Bipolar Disorder | Healthy Controls | P value | ||
|---|---|---|---|---|
| BD-E | BD-A | |||
| Number | 24 | 24 | 55 | |
| Age (years) | 34.7 (13.4) | 29.6 (13.2) | 35.8 (13.2) | p=.2 |
| Females | 11.0 (45.8%) | 17.0 (70.8%) | 27.0 (49.1%) | p=.1 |
| Handedness | 65.1 (61.8) | 59.4 (64.2) | 76.3 (50.6) | p=.5 |
| HDRS | 3.3 (3.3) | 12.7 (6.1) | p<.001 | |
| BHS-Total | 5.2 (5.4) | 7.9 (5.5) | p=.06 | |
| BD1/BDII | 18/6 | 20/4 | p=.5 | |
| Rapid cycling | 4.0 (16.7%) | 8.0 (33.3%) | p=.2 | |
| Lifetime psychosis | 7.0 (29.2%) | 11.0 (45.8%) | p=.2 | |
| Unmedicated at scan | 6.0 (25.0%) | 9.0 (37.5%) | p=.4 | |
| Current Medications | ||||
| Anticonvulsants | 7.0 (29.2%) | 8.0 (33.3%) | ||
| Antipsychotics | 6.0 (35.0%) | 8.0 (33.3%) | ||
| Antidepressants | 6.0 (35.0%) | 5.0 (20.8%) | ||
| Benzodiazepines | 4.0 (16.7%) | 5.0 (20.8%) | ||
| Stimulants | 3.0 (12.5%) | 2.0 (8.3%) | ||
| Lithium carbonate | 2.0 (8.3%) | 6.0 (25.0%) | ||
| Adrenergic antagonists | 1.0 (4.2%) | 0 | ||
| Non-benzodiazepine hypnotic | 1.0 (4.2%) | 1.0 (4.2%) | ||
| Levothyroxine | 0 | 2.0 (8.3%) | ||
| Comorbidity: Substance Use Disorders | ||||
| Alcohol dependence | 4.0 (16.7%) | 3.0 (12.5%) | ||
| Alcohol abuse | 2.0 (8.3%) | 4.0 (16.7%) | ||
| Cocaine dependence | 4.0 (16.7%) | 3.0 (12.5%) | ||
| Cannabis dependence | 5.0 (20.8%) | 1.0 (4.2%) | ||
| Cannabis abuse | 3.0 (12.5%) | 2.0 (8.3%) | ||
| Opiate dependence | 1.0 (4.2%) | 2.0 (8.3%) | ||
| Polysubstance dependence | 4.0 (16.7%) | 2.0 (8.3%) | ||
| Comorbidity: Anxiety Disorders | ||||
| Post-traumatic stress disorder | 5.0 (20.8%) | 8.0 (33.3%) | ||
| Obsessive compulsive disorder | 2.0 (8.3%) | 4.0 (16.7%) | ||
| Generalized anxiety disorder | 1.0 (4.2%) | 4.0 (16.7%) | ||
| Panic disorder | 4.0 (16.7%) | 2.0 (8.3%) | ||
| Social phobia | 3.0 (12.5%) | 0 | ||
| Specific phobia | 1.0 (4.2%) | 0 | ||
| Comorbidity: Eating Disorders | ||||
| Anorexia nervosa | 0 | 4.0 (16.7%) | ||
| Binge eating disorder | 1.0 (4.2%) | 1.0 (4.2%) | ||
| Eating disorders NOS | 0 | 2.0 (8.3%) | ||
| Comorbidity: Other | ||||
| Attention deficit hyperactivity disorder | 6.0 (25%) | 3.0 (12.5%) | ||
Means and standard deviations in parenthesis are presented. P values were determined with analysis of variance (ANOVA) (age and handedness), Mann-Whitney U (BHS-Total and HDRS), and chi-square tests (gender, BD subtype, rapid cycling, history of psychosis, medication status). Abbreviations: BD-E: Participants with BD who were euthymic. BD-A: Participants in an acute BD episode; HDRS: 17-item Hamilton Depression Rating Scale, BHS: Beck Hopelessness Scale; NOS -Not Otherwise Specified. Lifetime comorbidity is presented. None of the subjects met criteria for substance use disorders (dependence/abuse) for at least three months prior to enrollment. Handedness of all participants was assessed using the Edinburgh Handedness Inventory.
Fifty-five HVs (ages years 16-58 years, mean age±SD= 35.8±13.2years; 49.1%female)were without lifetime history of Axis-I disorders or a first degree relative with major mood, substance use or psychotic disorder as assessed with the Family History Screen for Epidemiological Studies (46).
Exclusioncriteria for all participants were the presence of major medical (except treated hypothyroidism in two BD-A subjects) or neurological disorders including a history of loss of consciousness ≥five minutes.All adult participants (≥18 years of age) provided written informed consent. Minors (<18 years) provided written informed assent and their parent/guardian provided written informed permission.Handedness of all participants was assessed using the Edinburgh Handedness Inventory(47). Depressed and elevated mood symptom severity was assessed using the Hamilton Depression Rating Scale 17-item version (HDRS)(48)and the Young Mania Rating Scale (YMRS)(49) respectively, and hopelessness severity was assessed using the self-report Beck Hopelessness Scale (BHS)(50). The BHS measures three major aspects of hopelessness: feelings about the future, loss of motivation, and future expectations.
Explicit Regulation of Negative and Positive Emotional Faces ImagingParadigm
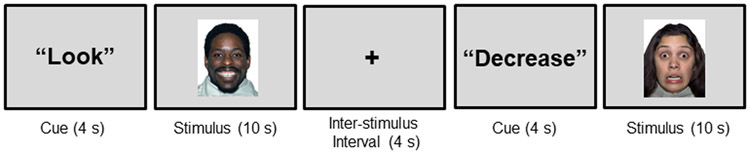
This paradigm was designed to investigate neural engagement during explicit regulation of responses to faces depicting negative and positive emotions.During the paradigm, participants viewed faces from the NimStim Series (51) portraying fearful, happy, and neutral emotional expressions (Figure 1). The emotional expressions were chosen based on models of emotion regulation in BD and research by our and other groups demonstrating differences in our regions of interestin BD in responses to faces with these expression(1, 22, 30, 52). Immediately prior to the presentation of each face, participants were presented with one of two prompts: “Look” or “Decrease”. In response to the prompt “Look”, participants were instructed to look at the face on the screen and experience whatever emotion they felt when they saw the face. In response to the prompt “Decrease”, participants were instructed to decrease the emotions that they felt. They were not given instructions on specific strategies to employ in order to decrease their emotions. The "Look" or "Decrease" prompts were presented for 4 seconds, followed by a face stimulus for 10 seconds. An inter-stimulus-interval, indicated by a fixation cross (4 seconds), followed each face stimulus. During the scan, participants performed three runs of the paradigm, each consisting of 20 face presentations, counterbalanced within and across runs for gender, actor identity,emotional expression (fearful, happy and neutral), and prompt type ("Look" or "Decrease"; Decrease prompts were only provided for fearful and happy faces).The duration of each run was 6 minutes,5 seconds.Just prior to the scan, all participants practiced the paradigm on a desktop computer during which they viewed fearful, happy and neutral faces following both the “Look” and “Decrease” prompts, and rated the intensity of their emotions to these facial expressions on five point Likert- scales.Participants showed a significant reduction in the intensity of emotions they reported they experienced when instructions were to decrease emotions to faces depicting fearfuland happy expressions (p<.001), and there were no significant performance differences between the groups (p=.60).
Figure 1: Schematic Representation of the Emotion Regulation Task.
The schematic illustrates the task structure. Participants viewed faces portraying fearful, happy, and neutral emotional expressions. Immediately prior to the presentation of each face, participants were presented with one of two prompts: “Look” or “Decrease”. In response to the prompt “Look”, participants were instructed to experience whatever emotion they felt when they saw the face. In response to the prompt “Decrease”, participants were instructed to decrease the emotions that they felt. Subjects viewed a fixation cross following each face stimulus. During the scan, participants performed three runs of the paradigm, each consisting of 20 face presentations, counterbalanced within and across runs for gender, actor identity, emotional expression (fearful, happy and neutral), and prompt type ("Look" or "Decrease"; Decrease prompts were only provided for fearful and happy faces). Representational facial stimuliare displayed.
MRI Data Acquisition
FMRI data were acquired using a 3-Tesla Siemens Trio MR Scanner (Siemens, Erlangen, Germany) with a T2*- weighted single-shot echo planar imaging (EPI) sequence with Blood Oxygen Level-Dependent (BOLD) contrast, aligned with the anterior commissure-posterior commissure (AC-PC) plane. Thirty-two 3mm contiguous axial-oblique slices were obtained using parameters: repetition time (TR) = 2000ms, echo time (TE) = 25ms, matrix=64 x 64, field of view (FOV) = 240 x 240mm2, 80° flip angle.
FMRI Data Processing
Functional images were preprocessedusing Statistical Parametric Mapping version 12 (SPM12) (Wellcome Institute of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm/software/spm12). During preprocessing, images were corrected for differences in interscan slice acquisition timing. No subject had movement greater than 3mms in translation along any plane (x,y, or z) or 3° rotation in any direction (pitch, roll, or yaw). Images were then spatially normalized and co-registered to the standardized space of the Montreal Neurologic Institute (MNI) template. Normalized images were resampled into 3mm3voxels and smoothed at 8-mm full width at half maximum.
First level analysis was performed using a General Linear Model (GLM, (53)) applied to the time series, and convolved with the canonical hemodynamic response function. A high pass filter of 128 seconds was applied in order to remove slow signal drifts and improve signal to noise ratio. The emotional face presentation period (10 seconds) was modeled as a boxcar convolved with a canonical hemodynamic response function (HRF), resulting in five regressors pertaining to the“Look” condition for fear, happy, and neutral stimuli, and the “Decrease” condition for fear and happy stimuli. A sixth regressor consisted of the prompt phase that was modelled as a stick-function at stimulus onset. The GLM also included the six motion parameters that were added as nuisance regressors.As the primary objective of this study was to investigate neural responses to explicit regulation of negative emotional stimuli in BD, neural responses to regulation of negative emotions (i.e. Decrease-Fear minus Look-Fear) was modelled as the main contrast of interest. It will be referred to as “EmReg-Fear”below. Additionally, the contrast Decrease-Happy minus Look-Happy (“EmReg-Happy”) was modelled to investigate whether dysfunction also occurred during the regulation of responses to positive emotional stimuli.
Statistical Analysis
Analyses of Demographic and Clinical Measures
Analyses were conducted using Statistical Package for the Social Sciences 24.0 (SPSS-24). Potential group differences in continuous (age and handedness) and categorical (gender) demographic variableswereassessed using analysis of variance (ANOVA)and chi-square tests, respectively. Potential group differences in continuous (BHS and HDRS) and categorical (BD subtype, rapid cycling, history of psychosis, medication status) clinical variables were analyzed using Mann-Whitney U and chi-square tests, respectively.
fMRI Analyses
Categorical Approach:
Analysis of covariance (ANCOVA) was performed in SPM12 to examine group differences during the EmReg-Fearcondition, with group (BD-E, BD-A, HV) as between-subjects factors, and age as a covariatedue to the wide age range of participants.Similar models were used for EmReg-Happy.
Dimensional Approach:
Multiple regression analyses were also performed in SPM12,in BD participantsto examine potential relationships between regional brain responsesduring the EmReg-Fear condition and negative-affect related symptoms (BHS, HDRS), while controlling for age. Similar models were used for EmReg-Happy.
For the categorical and dimensional approaches, analyseswere performed to test our hypotheses in a priori defined regions of interest (ROIs): vmPFC/mOFC, vlPFC, dlPFC, dmPFC and dACC. ROIs were anatomically defined using the Harvard-Oxford Cortical Structural Atlas in FSL FMRIB software library (54) and the Automatic Anatomical Labeling (AAL) Atlas in WFU PickAtlas (55). The Harvard-Oxford atlas was used to generate the vmPFC/mOFC (termed ‘frontal medial cortex’ in the atlas), vlPFC (termed ‘frontal orbital cortex’) and dACC regions (termed ‘cingulate gyrus, anterior division’, bounded between y=0 to 36 mm to delineate the dorsal region(56)). TheseROIs were thresholded at 0.25 confidence(56, 57). The AAL atlas was used to generate the dmPFC (termed‘superior medialfrontal’ in the atlas) and the dlPFC (termed ‘middle frontal’) masks, consistent with a previous study of emotion regulation in BD(33).Activation within each region is reported if it survived a cluster-based correction for multiple comparisons of p<.05 [family-wise error (FWE)-corrected; pfwe-cluster<.05].We also note results thatsurvived the additional False Discovery Rate (FDR) correction for the 5 ROIs with the Benjamini-Hochberg procedure (58).For the ROIs showing a main effect of group (pfwe-cluster<.05) in the ANCOVA (categorical approach), average BOLD signal values (beta estimates) were extracted from each individual using MarsBaR toolbox for SPM (http://marsbar.sourceforge.net/) to perform post-hocbetween-group comparisons. Results were Bonferroni corrected for the 3 pairwise group comparisons. For the ROIs showing an association with BHS-Total (pfwe-cluster<.05) in the multiple regression analysis (dimensional approach), secondary analyses explored for their association with BHS-subscales.
Exploratory Analyses for Potential Effects of Demographic and Clinical Factors on fMRI Findings:
Beta estimates from the regions obtained from the above ANCOVA and multiple regression analyses were explored for potential effects of gender (male/female) and handedness (left-handed: −100 to −71, mixed-handed:−70to +70, right handed: +71 to +100 according to previously defined cut-offs on the Edinburgh Handedness Inventory(59)) for all participants using ANOVA. Additionally,extracted beta estimates were explored for potential effects of clinical factorson findings using ANOVAwhen there were >5 participants with and >5 participants without the clinical factor ineither of the BD subgroups (i.e. either BD-A or BD-E)by adding each factor one at a time to the models. Clinical factors explored were: BD subtype (I/II), rapid cycling at the time of scan (present/absent), history of psychosis (present/absent), medicated at the time of scan (yes/no), past cannabis dependence (yes/no), lifetime comorbid PTSD (yes/no), and lifetime comorbid ADHD (yes/no).
Exploratory Whole-Brain Analyses:
For the categorical and dimensional approaches, whole brain analyses were also performed to explore brain regions outside of the a priori hypothesized regions using models described above. Findings were considered significant if they survived a cluster-based correction for multiple comparisons of p≤.05 [family-wise error (FWE)-corrected; pfwe-cluster<.05].Post-hocbetween-group comparisons were performed for regions showing a significant group effect in the ANCOVA using extracted average BOLD signal values (beta estimates) from each individual. Results were Bonferroni corrected for the 3 pairwise group comparisons.
RESULTS
Clinical Characteristics of the Sample
No significant group differences were observed for age (F2,100=1.9,p=.16),gender (X22=3.9, p=.14) or handedness (F2,96=0.8, p=.45). HDRS scoresranged from 0 to 30 (mean ± SD= 8.9±8.1) and BHS ranged from 0 to 20 (mean ± SD= 6.5±5.4). As expected,HDRS scoreswere significantly higher in BD-A than BD-E subjects(U=20.0, p<.001). Scores on the BHS scale did not differ significantly between BD-E and BD-Aparticipants(U=171.5,p=.06).However, scores on subscales of the BHS measuring loss of motivation (U=162.5, p=.03) and future expectations (U=165.5, p=.05)were significantly higher in the BD-A than the BD-E subgroup. The number of subjects with rapid cycling(X21=1.8, p=.18), lifetime psychosis (X21=1.4, p=.23), and who were unmedicated (X21=0.8, p=.35), did not differ betweenBD-A and BD-E groups. Psychotropicmedications, and lifetime comorbid illnesses, separated by BD subgroup are detailed inTable 1.
FMRI Results
EmReg-Fear
Functional Abnormalities in BD:
Categorical Approach:
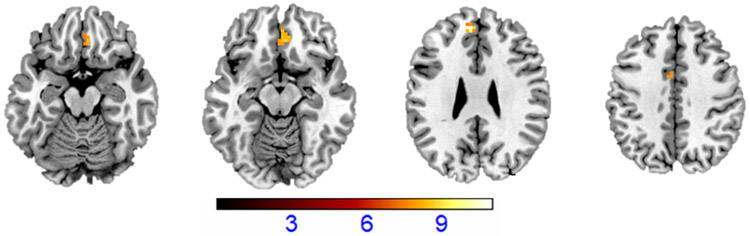
The analysis comprising BD-E, BD-A and HV groups showed amain effect of group inthe bilateral mOFC(in the voxel-wise analyses shown to be in Brodmann Area; BA11), bilateral dACC (BA32) and left dmPFC (medial BA 9) regions (pfwe-cluster<.05)(Table 2; Figure 2). The bilateral mOFC and bilateral dACC survived additional Benjamini-Hochberg FDR correction (p=.028).Post-hoct-tests performed onbeta weights extracted from the mOFC, dACC, and dmPFCregions revealed that in all three regions, BD-A showed the lowest levels of activation, which were significantly different from HV group (p<.001, after adjusting for multiple comparisons).Intermediate activation was observed inthe BD-E group that differed significantly from the HV group in the dACC (p=.05, after adjusting for multiple comparisons), and from the BD-A group in the mOFC (p<.05, after adjusting for multiple comparisons).
Table 2:
Differences in Frontal Regions of Interest in Participants with Bipolar Disorder Relative to Healthy Volunteers During Regulation of Responses to Fearful Faces
| Region | MNI Maxima (mm) |
Voxels | F(2,96) | pfwe-cluster | ||
|---|---|---|---|---|---|---|
| x | y | Z | ||||
| medial OFC | 6 | 35 | −14 | 18 | 8.5 | .011 |
| dorsal ACC | 3 | 5 | 43 | 23 | 11 | .011 |
| dmPFC | −6 | 47 | 28 | 19 | 10.8 | .037 |
All regions showed decreased activations during regulation of emotional responses to fearful faces (i.e. Decrease-Fear minus Look-Fear) in a group with bipolar disorder (n=48) relative to the healthy volunteer group (n=55). All regions survived cluster-based multiple-comparison correction of pfwe-cluster<.05.Significance in mOFC and dorsal ACC survived additional Benjamini-Hochberg correction (p=.028).Abbreviations: OFC: Orbitofrontal Cortex; ACC: Anterior Cingulate Cortex;dmPFC: Dorsomedial Prefrontal Cortex. MNI: Montreal Neurological Institute
Figure 2: Frontal Differences in Bipolar DisorderDuring Regulation of Responses to Fearful Faces.
The axial-oblique slices display the regions of decreased functional magnetic resonance (fMRI) activationsduring regulation of emotional responses to fearful faces(i.e. Decrease-Fear minus Look-Fear) in the medial orbitofrontal cortex (mOFC), dorsal anterior cingulate cortex (dACC) and dorsomedial prefrontal cortex (dmPFC) in a group with bipolar disorder (n=48) relative to the healthy volunteer group (n=55). Viewing threshold: p<.001uncorrected, cluster size=10 voxels. Color bar represents the range of F values. The right side of the image is the right side of the brain.
Dimensional Approach:
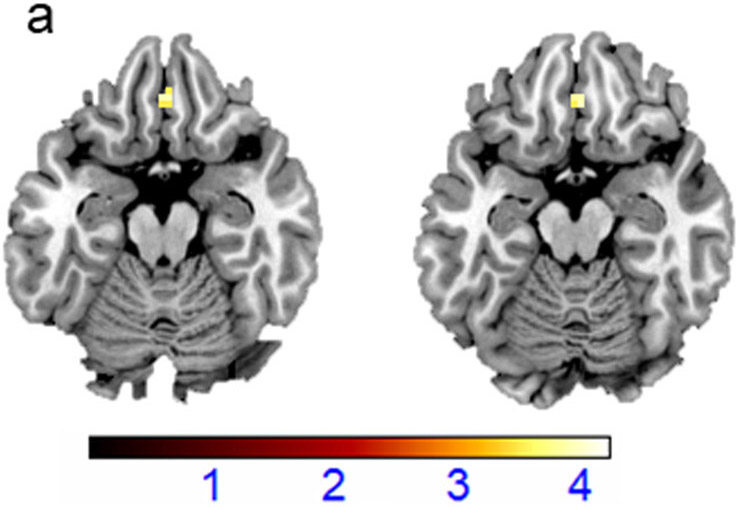
In the combinedBD group, severity of hopelessness (BHS scores)showed negative associations withactivation in the bilateral mOFC(x=3mm, y=35mm, z=−17mm, cluster size =11 voxels, t=3.7, pfwe-cluster=.012; Figure 3a).Of the BHS subscales, significant negative associations with mOFC activation were observed only for the BHS subscale score of hopelessness due to loss of motivation,even when controlling for group (i.e. BD-E and BD-A) and depression severity (HDRS total score) (x=3mm, y=38mm, z=−20mm, cluster size =15 voxels, t=4.16, pfwe-cluster=.017). As many items in the HDRS scale assess somatic symptoms, we repeated the analysis controlling for depressed mood based on five HDRS items that showed the highest loading for depression (i.e. depressed mood, work and interests, guilt, retardation, and suicide) (60).Negative associations between mOFC activation and hopelessness due to loss of motivation remained significant when controlling for group and depressed mood (x=3mm, y=38mm, z=−17mm, cluster size =24 voxels, t=4.03, pfwe-cluster=.012; Figure 3b).There were no significant associations between depression scores (HDRStotal scores) and any of the a priori defined regions.
Figure3: Medial Orbitofrontal Cortex Responses in Regulating Responses to Fearful Faces and Hopelessness in Bipolar Disorder.
(a) The axial-oblique slices display regions of decreased functional magnetic resonance (fMRI) activationsduring regulation of emotional responses to fearful faces in the medial orbitofrontal cortex (mOFC)that were associated withhopelessness severity, as measured by Beck Hopelessness Scale (BHS). (b)The axial-oblique slices display regions of decreased functional activations during regulation of emotional responses to fearful facesin the mOFC that were associated with hopelessness due to loss of motivation (BHS subscale), when controlled for group and depressed mood. Viewing threshold: p<.001uncorrected, cluster size=10 voxels.Color bar represents the range of T values. The right side of the image is the right side of the brain.
Exploratory Analyses for Potential Effects of Demographic and Clinical Factors:
Exploratory analyses did not reveal significant effects of gender, handedness, or clinical factors:BD subtype (I, II), rapid cycling, history of psychosis, medication status, past cannabis dependence, lifetime comorbid PTSD, and ADHD, on any of the above primary findings.
Exploratory Whole-Brain Analyses:
Exploratory analyses examining differences in neural responses outside a priori hypothesized regions revealed significant main effects of group in the bilateral insulacomprising the anterior and mid portions and extending to adjacent frontal opercular regions, bilateral angular gyrus, bilateral visual association areas, and cerebellar vermis (Table 3).Post-hoc t-tests revealed that in all the above regions, the BD-A group showed the lowest activations, which were significantly different from the HV group (p<.001, after adjusting for multiple comparisons). Intermediate activation was observed in the BD-E group that differed significantly from the HV group in the bilateral insula and the bilateral angular gyrus, and from the BD-A group in the left visual association area and the cerebellum (p≤.006, after adjusting for multiple comparisons).
Table 3:
Regions of Differences from Whole-Brain Analyses in Participants with Bipolar Disorder Relative to Healthy Volunteers During Regulation of Responses to Fearful Faces
| Region | MNI Maxima (mm) |
Voxels | F(2,96) | pfwe-cluster | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Insula | 36 | −19 | 19 | 850 | 16.8 | <.001 |
| −48 | −28 | 19 | 778 | 19.3 | <.001 | |
| Angular Gyrus | −39 | −67 | 25 | 354 | 20.4 | <.001 |
| 30 | −70 | 25 | 73 | 11.0 | .037 | |
| Visual Association Areas | −24 | −82 | −8 | 281 | 13.3 | <.001 |
| 27 | −79 | −8 | 100 | 13.2 | .011 | |
| Cerebellar Vermis | 0 | −61 | −29 | 164 | 15.8 | .001 |
All regions showed decreased activations during regulation of emotional responses to fearful faces (i.e. Decrease-Fear minus Look-Fear) in a group with bipolar disorder (n=48) relative to the healthy volunteer group (n=55). Abbreviations: MNI: Montreal Neurological Institute
In the BD group, no additional brain areas showed associations with BHStotal or subscale, or HDRS total, scores.
Regions of activations in the HV group during EmReg-Fear are presented in the supplementary material.
EmReg-Happy
Functional abnormalities in BD:
There were no significant group differences detected within or outside of the a priori defined regions (categorical approach).There were also no significant associations between clinical variables (BHS/HDRS) and brain regions within or outside of the a priori defined regions in the BD group (dimensional approach).
Regions of activations in the HV group during EmReg-Happy are presented in the supplementary material.
DISCUSSION
Brain responses (fMRI activations) were examined in BD-A, BD-E, and HV groups, while participants performed a paradigm in which they were instructed to explicitly regulate their responses to negative and positive emotional facial stimuli. During regulation of responses to fearful emotional faces, compared to HVs, individuals with BD showed reductions in responses inmOFC, dACC and dmPFC. Findings in the mOFC and dACCsurvived FWE-cluster and additional FDR correction.Theseregions are associated with emotion regulation.Thesmallest responses were observed in the BD-A group; the BD-E group showed intermediate responses which differed significantly from the HV group in the dACC, and from the BD-A group in the mOFC. Across all BD participants, bilateral mOFC responses showed negative associations with the severity of hopelessness on the BHS, with BHS subscale scores showing this wasparticularly due tohopelessness in the context of loss of motivation, even when controlling for depressed mood. There were no group differences evidentduring explicit regulation of responses to happy emotional faces, suggesting that observed associations may be particularly evident in response to regulation of negative emotion. Overall, the findings indicate less negative emotion regulation-related ventral and dorsal frontal engagement across symptomatic and euthymic mood states in BD that could represent a trait abnormality that worsens during acute negative states. Moreover, the results suggest that deficits in mOFC engagement may contribute to hopelessness, a major suicide risk factor that has been shown to mediate the relationship between emotion regulation difficulties and future suicide behavior (13-15), andmay be a potential target to reduce hopelessness and in turn suicide risk.
The mOFC is known to play an important role in emotion regulation(16, 17, 61), and has been shown to be engaged during both implicit and explicit emotion regulation tasks in HVs (1). Our findings support current conceptual modelsof BDthat postulate abnormally reduced response in the medial prefrontal cortex during emotion regulation(1, 62). The mOFC is involved in integrating internal motivational states and generating adaptive responses to alter inner emotional state and related behaviors(63). In the present study, the BD-A relative to the BD-E groupshowed significantly greater hopelessness due to loss of motivation.The magnitude of mOFC activation reduction across all BD subjects during EmReg-Fear was associated with severity of hopelessnessdue to loss of motivation,even when controlling for group and depressed mood. The magnitude of mOFC reductions in BD participants did not correlate with depression severity. The findings suggestthat reduced mOFC engagement during explicit regulation of negative emotions in BDmay have a relationship with hopelessness, particularly in the context of diminished motivation, beyond the far more studied construct of depression. The latter is often studied as operationalized by HDRS scores which do not include an item specific to hopelessness.Given the serious consequences that can result from hopelessness, these findings suggest that future research elucidating underlying mechanisms of hopelessness and identifying targets for its reductionis warranted, including research in which different aspects of hopelessness are operationalized.
While not associated with hopelessness, reductions in responses during regulation of negative facial expressions were observed in dorsomedial frontal cortex (dmPFC and dACC)across symptomatic and euthymic mood states of BD. These responses were the smallest in the BD-A group suggesting that abnormalities may be more severe during acute negative mood states.Dorsomedial frontal cortices are associated with cognitive control processes involved in explicit emotion regulation(1). Effortful cognitive control processes are recruited especially during reappraisal of emotions, and the dorsomedial frontal cortex has shown to be engaged during emotion regulation by reappraisal (19, 33).It is possible that our findings in BD indicate reduced ability to recruit these regions,diminishingtheir ability to utilize adaptive cognitive reappraisal strategies to regulate emotions.Although significant group differences were not observed in affective ratings during emotion regulation in the practice session, the data from the studyare limited in providing information regardingstrategies that were utilized by participants during emotion regulation,anddorsomedial frontal corticeshave also been shown to be involved in regulation of negative affect by suppression (64) and distraction(33).
We did not detect significant differences between BD and HV groups, as hypothesized, in lateral PFC regions, namely the vlPFC and the dlPFC. The inclusion of adolescents in our sample may have led to heterogeneity and limited power to detect significant group differences,asthe lateral PFC regions may continue to mature until late adolescence and young adulthood (65). Previous studies examining brain activations during emotion regulation in BD have revealed inconsistent findings in relation to lateral PFC regions, including those of increased (38), decreased (34, 39), as well as no significant differences (35, 37), in individuals with BD relative to HVs, the latter being consistent with the findings from the present study.
Exploratory whole-brain analysesrevealed group differences in additional regions posited to play a role in emotion regulation. Of particular note arefindings of reducedbilateral insula responses in both the BD-A and BD-E groups compared to HV group,suggesting they are a trait feature of BD. The responses were the smallest in the BD-A group suggesting that these abnormalities may worsen during acute negative mood states. Evidence for reduced insula activations as trait features in BD during emotion regulation was also observed by Townsend and colleagues (34) in their study comparing euthymic BD individuals and HVs during performance of a task that required explicit downregulation of emotional responses to negative IAPS stimuli. The insula, especially its anterior portion,is associated with interoceptive awareness(66).Awareness of one’s bodily states is important for recognizing and experiencing emotions, which inturn is key for initiating emotion regulation processes. Behavioral studies have shown that individuals with higher interoceptive awareness have greater capacity for emotion regulation(67), particularly by reappraisal(68, 69). Consistent with behavioral findings, neuroimaging evidence shows engagement of the insula during reappraisal (21, 61). The insula has strong connections withlimbic structures and with mOFC (70), and interoceptive awareness has been suggested to facilitate downregulation of negative affect through the interactions between internalemotional states and motivational regulatory processes(71). We speculate that our findings indicate decreased interoceptive awareness in BD which contribute to impairments in explicit regulation of negative emotions.
Reduced responses in the BD-A group relative to the HV group were also observed in thevisual association cortex and angular gyrus, as well as in the cerebellum. Studies in HVs have shownsignificant engagement of these regionsduring emotion regulation(61, 72-74). For example, visual association and angular gyrus regions are hypothesized to contribute tothe directing of visual attention away from emotionally salient aspects of the stimuli (61). The cerebellum modulates responses to emotional stimuli through its reciprocal connections with the prefrontal and limbic regions (75-77), and abnormalities in the structure and function of the cerebellum have been observed in BD(78, 79). Findings of reduced responses in the above regionsin the BD-A group suggestthere could be abnormalities inconstituent processes involved in emotion regulation, which include visual processing andattention, and emotional modulation, during acute negative states of BD.
We did not observe significant group differences during EmReg-Happy.Absence of significant group differences in the EmReg-Happy condition could be partly because our sample did not include individuals in euphoric mood state. Participants in an acute manic or hypomanic episode may show pronounced abnormalities during regulation of positive rather than negative emotional stimuli. Our findings of no significant group difference for the EmReg-Happy condition appear to be in alignment with results from previous neuroimaging studies in BD that did not include individuals in euphoric manic states (37, 52).Inclusion of adolescents in our study may have also introduced heterogeneity and limited power to detect significant group differences particularly in lateral PFC ROIs.
The present study includes some limitations, which need to be considered when interpreting the findings. Although the study included a larger sample of BD individuals who were in an acute BD episode compared to previous studies, the sample size is still modest, and may have limitedstatistical power to detect effects of clinical factors on the neuroimaging findings. Clinical heterogeneity is inherent in BD, and exploration of potential comorbidity and medication effects on emotion regulationwas further limited as BD participantssometimes presented with multiple comorbid conditions,or were often on more than one medication that was not systematically controlled for. Including individuals in the late adolescent stage may have also introduced heterogeneity, and led to limited power to detect findings in more lateral PFC regions, that we might have seen in a sample of older adults. We did not detect associations between HDRS scores and activation in the a priori defined regions, and we did observe an association between mOFC activation and hopelessness due to loss of motivation even when controlling for HDRS total scores, as well as when scores were constrained to the items associated previously most with depression (depressed mood, work and interests, guilt, retardation, and suicide); however, the HDRS may be limited in providing measures specifically relevant to the mood constructs most salient to hopelessness.Another limitation of this study is that we did not instruct individuals to use a specific emotion regulation strategy, hence it is difficult to determine whether the observed group differences were due to BD participants adopting different emotion regulation strategies than HVs.Although it might have generated additional data on subject’s perception of their changes in emotions during scanning trials, we also did not add a behavioral rating measure of emotion regulationto each trial as that could increase subject burden. Moreover, responses on behavioral rating measures can have enduring effects that bleed into subsequent events and could obscure emotion-related activation changes by engaging brain circuitries not fully related to emotion regulation processes. For example, with increased cognitive load, activation tends to increase in dorsal systems and diminish in the ventral ones that were especially of interest(80).In the present study, we did obtain subjective information prior to scanning supporting that subjects understood and followed the task instructions and no significant performance differences between the groups were observed.
In summary, this study elucidated the pattern of brain responses during explicit regulation of responses to negative facial stimuliacross acute negative (BD-A) and euthymic (BD-E) mood states of BD.Our findings support less engagement of key ventral and dorsalfrontal regions of emotion regulation circuitryacross acute and euthymic BD mood states, indicatinga potential trait abnormality in BD. The smallest responses were in the BD-A group, suggesting that abnormalities may worsen during acute negative states.Across BD participants, irrespective of their group membership, reductions in mOFC engagement were associated with hopelessness due to loss of motivation, even when controlling for depressed mood. Thus, reduced mOFC engagement during explicit regulation of negative emotions in BD may have a relationship with hopelessness, particularly in the context of diminished motivation, beyond the far more studied construct of depression. This study has important clinical implications as this is one of the first studies to examine the brain circuitry of hopelessness and its subconstructs in BD. Given that hopelessness is a major suicide risk factor, future studies that specifically examine mechanisms underlying hopelessness and their targeting, and directly assess their relationship to suicide thoughts and behaviors,may help to reduce related suffering and potentially decrease suicide risk.
Supplementary Material
ACNOWLEDGEMENTS
Development of the MacBrain Face Stimulus Set was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development.Please contact Nim Tottenham at tott0006@tc.umn.edu for more information concerning the stimulus set.
FUNDING
This work was supported by NIH grants RC1 MH088366, R01 MH69747, R01 MH070902, and R01 MH113230 03S1(Dr. Blumberg), the National Center for Advancing Translational Science UL1TR000142 (Dr. Blumberg), T32 MH014276 and T32DA022975 (Dr. Lippard), the American Foundation for Suicide Prevention (Dr. Blumberg), International Bipolar Foundation (Dr. Blumberg), the Brain and Behavior Research Foundation (Dr. Blumberg and Dr. Wang), MQ Brighter Futures Program (Dr. Blumberg), For the Love of Travis Foundation (Dr. Blumberg), Women's Health Research at Yale (Dr. Blumberg), the AIM Foundation (Dr. Sankar), the Klingenstein Third Generation Foundation (Dr. Sankar), and the John and Hope Furth Endowment (Dr. Blumberg).
Footnotes
CONFLICT OF INTEREST
All authors have no conflicts of interests to report.
Data availability statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 2008; 13:833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD, et al. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord 2012; 14:313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blond BN, Fredericks CA, Blumberg HP. Functional neuroanatomy of bipolar disorder: structure, function, and connectivity in an amygdala–anterior paralimbic neural system. Bipolar Disord 2012; 14:340–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross JJ. Handbook of emotion regulation. New York: The Guilford Press; 2013.3–20 p [Google Scholar]
- 5.Green M, Lino B, Hwang EJ, Sparks A, James C, Mitchell P. Cognitive regulation of emotion in bipolar I disorder and unaffected biological relatives. Acta Psychiatr Scand 2011; 124:307–316. [DOI] [PubMed] [Google Scholar]
- 6.Stange JP, Boccia AS, Shapero BG, Molz AR, Flynn M, Matt LM, et al. Emotion regulation characteristics and cognitive vulnerabilities interact to predict depressive symptoms in individuals at risk for bipolar disorder: A prospective behavioural high-risk study. Cogn Emot 2013; 27:63–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston JA, Wang F, Liu J, Blond BN, Wallace A, Liu J, et al. Multimodal neuroimaging of frontolimbic structure and function associated with suicide attempts in adolescents and young adults with bipolar disorder. Am J Psychiatry 2017; 174:667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arria AM, O'Grady KE, Caldeira KM, Vincent KB, Wilcox HC, Wish ED. Suicide ideation among college students: A multivariate analysis. Arch Suicide Res 2009; 13:230–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malhi GS, Bargh DM, Kuiper S, Coulston CM, Das P. Modeling bipolar disorder suicidality. Bipolar Disord 2013; 15:559–574. [DOI] [PubMed] [Google Scholar]
- 10.Beck AT, Brown G, Berchick RJ, Stewart BL, Steer RA. Relationship between hopelessness and ultimate suicide: a replication with psychiatric outpatients. Focus 2006; 147:190–296. [DOI] [PubMed] [Google Scholar]
- 11.Minkoff K, Bergman E, Beck AT, Beck R. Hopelessness, depression, and attempted suicide. Am J Psychiatry. 1973; 130:455–459. [DOI] [PubMed] [Google Scholar]
- 12.David Klonsky E, Kotov R, Bakst S, Rabinowitz J, Bromet EJ. Hopelessness as a predictor of attempted suicide among first admission patients with psychosis: a 10-year cohort study. Suicide Life-Threat Behav 2012; 42:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miranda R, Tsypes A, Gallagher M, Rajappa K. Rumination and hopelessness as mediators of the relation between perceived emotion dysregulation and suicidal ideation. CognitTher Res 2013; 37:786–795. [Google Scholar]
- 14.Smith JM, Alloy LB, Abramson LY. Cognitive vulnerability to depression, rumination, hopelessness, and suicidal ideation: Multiple pathways to self-injurious thinking. Suicide Life-threat Behav 2006; 36:443–454. [DOI] [PubMed] [Google Scholar]
- 15.Rajappa K, Gallagher M, Miranda R. Emotion dysregulation and vulnerability to suicidal ideation and attempts. Cognit Ther Res 2012; 36:833–839. [Google Scholar]
- 16.Braunstein LM, Gross JJ, Ochsner KN. Explicit and implicit emotion regulation: a multi-level framework. Soc Cogn Affect Neurosci 2017; 12:1545–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gyurak A, Gross JJ, Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cogn Emot 2011; 25:400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ochsner KN, Gross JJ. Handbook of emotion regulation. New York: The Guildford Press; 2007. 87–109 pp. [Google Scholar]
- 19.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci 2005; 9:242–249. [DOI] [PubMed] [Google Scholar]
- 20.Morawetz C, Bode S, Baudewig J, Heekeren HR. Effective amygdala-prefrontal connectivity predicts individual differences in successful emotion regulation. Soc Cogn Affect Neurosci 2017; 12:569–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex 2014; 24:2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips M, Ladouceur C, Drevets W. Neural systems underlying voluntary and automatic emotion regulation: toward a neural model of bipolar disorder. Molecular Psychiatry. 2008; 13:829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rey G, Piguet C, Benders A, Favre S, Eickhoff SB, Aubry JM, et al. Resting-state functional connectivity of emotion regulation networks in euthymic and non-euthymic bipolar disorder patients. Eur Psychiatry 2016; 34:56–63. [DOI] [PubMed] [Google Scholar]
- 24.Chepenik LG, Raffo M, Hampson M, Lacadie C, Wang F, Jones MM, et al. Functional connectivity between ventral prefrontal cortex and amygdala at low frequency in the resting state in bipolar disorder. Psychiatry Res Neuroimaging 2010; 182:207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blumberg HP, Stern E, Ricketts S, Martinez D, de Asis J, White T, et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry 1999; 156:1986–1988. [DOI] [PubMed] [Google Scholar]
- 26.Blumberg HP, Stern E, Martinez D, Ricketts S, De Asis J, White T, et al. Increased anterior cingulate and caudate activity in bipolar mania. Biol Psychiatry 2000; 48:1045–1052. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Blond BN, van Dyck LI, Spencer L, Wang F, Blumberg HP. Trait and state corticostriatal dysfunction in bipolar disorder during emotional face processing. Bipolar Disord 2012; 14:432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hummer TA, Hulvershorn LA, Karne HS, Gunn AD, Wang Y, Anand A. Emotional response inhibition in bipolar disorder: a functional magnetic resonance imaging study of trait- and state-related abnormalities. Biol Psychiatry 2013; 73:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wessa M, Houenou J, Paillere-Martinot ML, Berthoz S, Artiges E, Leboyer M, et al. Fronto-striatal overactivation in euthymic bipolar patients during an emotional go/nogo task. Am J Psychiatry 2007; 164:638–646. [DOI] [PubMed] [Google Scholar]
- 30.Bertocci MA, Bebko GM, Mullin BC, Langenecker SA, Ladouceur CD, Almeida JR, et al. Abnormal anterior cingulate cortical activity during emotional n-back task performance distinguishes bipolar from unipolar depressed females. Psychol Med 2012; 42:1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caseras X, Murphy K, Lawrence NS, Fuentes-Claramonte P, Watts J, Jones DK, et al. Emotion regulation deficits in euthymic bipolar I versus bipolar II disorder: a functional and diffusion-tensor imaging study. Bipolar Disord 2015; 17:461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soehner AM, Goldstein TR, Gratzmiller SM, Phillips ML, Franzen PL. Cognitive control under stressful conditions in transitional age youth with bipolar disorder: Diagnostic and sleep-related differences in fronto-limbic activation patterns. Bipolar Disord 2018; 20:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanske P, Schonfelder S, Forneck J, Wessa M. Impaired regulation of emotion: neural correlates of reappraisal and distraction in bipolar disorder and unaffected relatives. Transl Psychiatry 2015; 5:e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Townsend JD, Torrisi SJ, Lieberman MD, Sugar CA, Bookheimer SY, Altshuler LL. Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biol Psychiatry 2013; 73:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corbalán F, Beaulieu S, Armony J. Emotion regulation in bipolar disorder type I: an fMRI study. Psychol Med 2015; 45:2521–2531. [DOI] [PubMed] [Google Scholar]
- 36.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention. 1997; 1:39–58. [Google Scholar]
- 37.Rive MM, Mocking RJ, Koeter MW, van Wingen G, de Wit SJ, van den Heuvel OA, et al. State-dependent differences in emotion regulation between unmedicated bipolar disorder and major depressive disorder. JAMA Psychiatry 2015; 72:687–696. [DOI] [PubMed] [Google Scholar]
- 38.Morris R, Sparks A, Mitchell P, Weickert C, Green M. Lack of cortico-limbic coupling in bipolar disorder and schizophrenia during emotion regulation. Transl Psychiatry 2012; 2:e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Ai H, Opmeer EM, Marsman J-BC, van der Meer L, Ruhé HG, et al. Distinct temporal brain dynamics in bipolar disorder and schizophrenia during emotion regulation. Psychol Med 2019;1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neuroscience 2009; 34: 418:432. [PMC free article] [PubMed] [Google Scholar]
- 41.Chen C-H, Lennox B, Jacob R, Calder A, Lupson V, Bisbrown-Chippendale R, et al. Explicit and implicit facial affect recognition in manic and depressed states of bipolar disorder: a functional magnetic resonance imaging study. Biol Psychiatry 2006; 59:31–39. [DOI] [PubMed] [Google Scholar]
- 42.Wang F, Kalmar JH, He Y, Jackowski M, Chepenik LG, Edmiston EE, et al. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry 2009; 66:516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalmar JH, Wang F, Chepenik LG, Womer FY, Jones MM, Pittman B, et al. Relation between amygdala structure and function in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2009; 48:636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.First MB, Gibbon M, Spitzer RL, Benjamin LS. User's guide for the structured clinical interview for DSM-IV axis II personality disorders: SCID-II. American Psychiatric Pub; 1997. [Google Scholar]
- 45.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988. [DOI] [PubMed] [Google Scholar]
- 46.Lish JD, Weissman MM, Adams PB, Hoven CW, Bird H. Family psychiatric screening instrument for epidemiologic studies: pilot testing and validation. Psychiatry Res 1995; 57:169–180. [DOI] [PubMed] [Google Scholar]
- 47.Caplan B, Mendoza JE. Edinburgh handedness inventory. Encyclopedia of clinical neuropsychology: Springer, 2011: 928. [Google Scholar]
- 48.Hamilton M A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young R, Biggs J, Ziegler V, Meyer D. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133:429–435. [DOI] [PubMed] [Google Scholar]
- 50.Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: the hopelessness scale. J Consult Clin Psychol 1974; 42:861–865. [DOI] [PubMed] [Google Scholar]
- 51.Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res 2009; 168:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mullin BC, Perlman SB, Versace A, de Almeida JR, LaBarbara EJ, Klein C, et al. An fMRI study of attentional control in the context of emotional distracters in euthymic adults with bipolar disorder. Psychiatry Res Neuroimaging 2012; 201:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1994; 2:189–210. [Google Scholar]
- 54.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage 2012; 62:782–790. [DOI] [PubMed] [Google Scholar]
- 55.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003; 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- 56.Yttredahl AA, McRobert E, Sheler B, Mickey BJ, Love TM, Langenecker SA, et al. Abnormal emotional and neural responses to romantic rejection and acceptance in depressed women. J Affect Disord 2018; 234:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sankar A, Yttredahl AA, Fourcade EW, Mickey BJ, Love TM, Langenecker SA, et al. Dissociable neural responses to monetary and social gain and loss in women with major depressive disorder. Front Behav Neuroscience 2019; 13:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological). 1995; 57:289–300. [Google Scholar]
- 59.Dragovic M, Hammond G, Badcock JC, Jablensky A. Laterality phenotypes in patients with schizophrenia, their siblings and controls: associations with clinical and cognitive variables. Br J Psychiatry 2005; 187:221–8. [DOI] [PubMed] [Google Scholar]
- 60.Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol 2006; 62:123–146. [DOI] [PubMed] [Google Scholar]
- 61.Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage 2011; 58:275–285. [DOI] [PubMed] [Google Scholar]
- 62.Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry 2014; 171:829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci 2012; 16:147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry 2005; 57:210–219. [DOI] [PubMed] [Google Scholar]
- 65.Fuster JM. Frontal lobe and cognitive development. J Neurocytol 2002; 31:373–385. [DOI] [PubMed] [Google Scholar]
- 66.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci 2009; 10:59–70. [DOI] [PubMed] [Google Scholar]
- 67.Zamariola G, Frost N, Van AO, Corneille O, Luminet O. Relationship between interoception and emotion regulation: New evidence from mixed methods. J Affect Disord 2018; 246:480–485. [DOI] [PubMed] [Google Scholar]
- 68.Kever A, Pollatos O, Vermeulen N, Grynberg D. Interoceptive sensitivity facilitates both antecedent-and response-focused emotion regulation strategies. Pers Individ Dif 2015; 100:20–33. [Google Scholar]
- 69.Fustos J, Gramann K, Herbert BM, Pollatos O. On the embodiment of emotion regulation: interoceptive awareness facilitates reappraisal. Soc Cogn Affect Neurosci 2013; 8:911–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saleem KS, Kondo H, Price JL. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. J Comp Neurol 2008; 506:659–693. [DOI] [PubMed] [Google Scholar]
- 71.Uddin LQ, Menon V. The anterior insula in autism: under-connected and under-examined. Neurosci Biobehav Rev 2009; 33:1198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U. Neural network of cognitive emotion regulation—an ALE meta-analysis and MACM analysis. Neuroimage 2014; 87:345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frank D, Dewitt M, Hudgens-Haney M, Schaeffer D, Ball B, Schwarz N, et al. Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci Biobehav Rev 2014; 45:202–211. [DOI] [PubMed] [Google Scholar]
- 74.Schutter DJ, van Honk J. The cerebellum in emotion regulation: a repetitive transcranial magnetic stimulation study. The Cerebellum 2009; 8:28–34. [DOI] [PubMed] [Google Scholar]
- 75.Anand B, Malhotra C, Singh B, Dua S. Cerebellar projections to limbic system. J Neurophysiol 1959; 22:451–457. [DOI] [PubMed] [Google Scholar]
- 76.Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci 2001; 21:700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmahmann JD. The cerebrocerebellar system: Anatomic substrates of the cerebellar contribution to cognition and emotion. Int Rev Psychiatry 2001; 13:247–260. [Google Scholar]
- 78.Wang Y, Zhong S, Chen G, Liu T, Zhao L, Sun Y, et al. Altered cerebellar functional connectivity in remitted bipolar disorder: A resting-state functional magnetic resonance imaging study. Aust N.Z J Psychiatry 2018; 52:962–971. [DOI] [PubMed] [Google Scholar]
- 79.Baldaçara L, Nery-Fernandes F, Rocha M, Quarantini LdC, Rocha G, Guimaraes J, et al. Is cerebellar volume related to bipolar disorder? J Affect Disord 2011; 135:305–309. [DOI] [PubMed] [Google Scholar]
- 80.Van Dillen LF, Heslenfeld DJ, Koole SL. Tuning down the emotional brain: an fMRI study of the effects of cognitive load on the processing of affective images. Neuroimage 2009; 45:1212–1219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.