Abstract
Objectives
There is a lack of understanding of health related quality of life (HRQoL) in chronic liver disease (CLD). With the rising prevalence of alcohol and obesity driven CLD, and the increasing ability to screen for fibrosis, it is important to understand the impact of the diagnostic process for patients.
Study design
Prospective cohort study.
Methods
A cohort study conducted utilising the Nottingham Adult Liver Disease Stratification Pathway, UK. All patients referred as high risk for CLD (due to metabolic, alcohol or abnormal liver enzymes) completed the EQ-5D before diagnosis and at three and 12 months after. HRQoL was investigated by domain, CLD severity (transient elastography) and temporally.
Results
493 patients participated with 300 (60.9%) completing at least one follow-up HRQoL assessment.
Pre-diagnosis the median (IQR) utility index was 0.75 (0.61–0.85) and visual analogue scale was 75/100 (60–90). The median utility index was significantly lower amongst those with advanced liver disease compared to those without at all time points (baseline 0.68 vs 0.77, three-months 0.65 vs 0.79, 12-months 0.69 vs 0.84, all p < 0.05). The majority of decrements in HRQoL score were in the pain domain.
Conclusions
There was no reduction, over three or 12 months, in HRQoL identified amongst high-risk individuals progressing through the diagnostic pathway. Overall the HRQoL of participants at high risk for the development of significant CLD was lower than the UK and regional (East Midlands) norms. Furthermore, we found reduced HRQoL in those going on to receive a diagnosis of advanced liver disease compared to those without.
Keywords: Quality of life, Chronic liver disease, Screening, Diagnostic tests, Prevention
1. Introduction
Chronic liver disease (CLD) is increasing in prevalence worldwide, estimated to affect over one billion people globally and is associated with over one million deaths and 40 million disability adjusted life years lost per year [1]. The increasing prevalence in the western world is largely being driven by the rise in metabolic syndrome related non-alcoholic fatty liver disease (NAFLD) and alcohol related liver disease (ARLD) [2,3]. Given the rising prevalence of liver disease, knowledge of the resulting impact on health related quality of life (HRQoL) is essential to support health service planning.
There is a paucity of information on HRQoL in CLD beyond the experience of hepatitis C virus [4,5]. CLD is thought to be a largely asymptomatic disease until the later stages when symptoms of decompensation (ascites, encephalopathy, variceal bleeding) dominate. As a result, whilst it could be hypothesised that early CLD would have limited impact on HRQoL compared to later more significant disease, this is as yet unknown. Furthermore, the impact of receiving a diagnosis, when otherwise well (which is akin to screening programmes), is also unknown.
This study utilises The Nottingham Adult Liver Disease Stratification Pathway, a community-based healthcare pathway in Nottingham, UK [6], which aims to support the diagnosis of early and later stage CLD in individuals deemed to be at high risk within general practice. We aimed to determine the impact of a diagnosis of CLD on HRQoL in the short (three month) and medium (12 month) term in individuals at high risk of CLD in the community and how this differed by diagnosed liver disease severity.
2. Materials and methods
2.1. Setting
The Nottingham Adult Liver Disease Stratification Pathway (‘the Nottingham Liver Pathway’) was commissioned by the Greater Nottingham Clinical Commissioning Groups in late 2016. This region is suburban including approximately 106 GP practices and a population of approximately 700,000.
The pathway allows primary care professionals to refer any patient meeting criteria for being at high risk of CLD for transient elastography to assess liver health. Any patient meeting the pathway criteria (see “Participants” below) can be opportunistically referred for liver fibrosis assessment at the local hospital. On attendance patients undergo transient elastography, are provided with brief lifestyle advice and are informed of their scan result. Results are also returned by letter to the patients GP. Advice provided to the GP varies by risk stratification: no significant liver disease – repeat transient elastography in five years, significant liver disease – consider secondary care referral or repeat transient elastography in three years, advanced liver disease –refer to secondary care hepatology.
All patients attending this pathway during the period between August 2016 and July 2017 were offered the opportunity to be included in the study. They were re-contacted by telephone at three and 12 months post assessment for follow-up data collection. Time points were selected to capture any initial impact of the diagnosis (three months) and any persistence of impact (12 months).
The at-risk and assessed populations are described in detail in prior publications. In brief, from detailed piloting we determined that approximately 10% of the adult population in the area had a GP record of either type 2 diabetes or alcohol excess and that when offered the referral opportunity approximately 50% agreed [6,7].
2.2. Participants
Inclusion criteria were: adult (aged 18+years), meeting high-risk of liver disease criteria in the Nottingham liver pathway, attending for transient elastography. The high-risk criteria for referral were: harmful alcohol consumption, defined as men who drink over 50 units and women who drink over 35 units of alcohol per week; type 2 diabetes or metabolic syndrome, defined as ‘a GP diagnosis’; incidental fatty liver on ultrasound; or asymptomatic abnormal liver enzymes without other cause and defined as AST/ALT >0.8. Patients with type 2 diabetes, metabolic syndrome or incidental fatty liver additionally required a fatty liver index (FLI) > 60.
2.3. Data collection
2.3.1. HRQoL
The primary outcome was change in HRQoL, between pre-diagnosis and three months and between pre-diagnosis and 12 months. Immediately prior to undergoing transient elastography all patients agreeing to participate completed the generic preference based quality of life scale EQ-5D-5L [8] whilst they were unaware of their liver disease status. Paper questionnaires were completed by individuals at the clinic prior to receiving their scan with the support of trained research nurses where necessary. They were then contacted by telephone at approximately three and 12 months post liver assessment for follow-up EQ-5D-5L assessment. Three trained researchers collected the follow-up data blind to all the prior results of the participants including EQ-5D, liver disease status and referral reason.
The EQ-5D comprises two components: the descriptive system and the visual analogue scale (VAS). The descriptive system considers five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each of these dimensions can be scored by the patient using five levels of severity: no problems, slight problems, moderate problems, severe problems and extreme problems. The five domains and five levels can be used to describe 3125 individual health states [9]. The EQ VAS records the patient’s self-rated health on a vertical scale from 0 to 100. A score of 100 means ‘the best health you can imagine’ and 0 ‘the worst health you can imagine’.
HRQoL utility weights were calculated using the validated EQ-5D-5L cross-walk algorithm [10] to provide a score on a −0.594 to 1 scale with negative scores indicating states worse than death and 1 being the best possible health imaginable.
2.3.2. Transient elastography
Following pre-diagnosis EQ-5D questionnaire completion participants underwent transient elastography. Transient elastography using Fibroscan was undertaken in-line with standard protocols (4-h fast) using either the M or XL probe as appropriate and described in detail elsewhere [7]. Transient elastography has been well validated for the diagnosis of significant liver fibrosis (Metavir F2+) with area under the receiver operating curve of >0.9 [11]. Consequently, transient elastography results were used to categorise liver fibrosis severity into: no significant liver disease (<8 kPa), significant liver disease (8–14.9 kPa), advanced liver disease (≥15 kPa).
2.4. Analysis
Baseline characteristics were compared for those with and without advanced liver disease by: age, sex, referral reason, pre-diagnosis utility index and pre-diagnosis VAS using t-test, Mann-Whitney U test or Chi Sq test as appropriate.
Mean (sd) and median (range) values for the utility index were calculated by sex, age, referral reason and liver fibrosis category at pre-diagnosis, three months post diagnosis and 12 months post diagnosis.
Change in HRQoL score was calculated (score at timeA – score at timeB) for the periods pre-diagnosis to three months and pre-diagnosis to 12 months for participants with paired data available and analysed using the Wilcoxon signed rank test.
A sensitivity analysis was undertaken limited to only those patients with data available at all three time points.
2.5. Ethical approval
Ethical approval for the study was given by NHS East Midlands - Leicester Central Research Ethics Committee (ref.13/EM/0123) and the study is registered with clinicaltrials.gov (ID NCT02037867).
All potential participants received an in information letter about the study at the time of invite to the transient elastography clinic and then had the opportunity to discuss the project with a trained research nurse prior to completing written informed consent.
3. Results
3.1. Participant characteristics
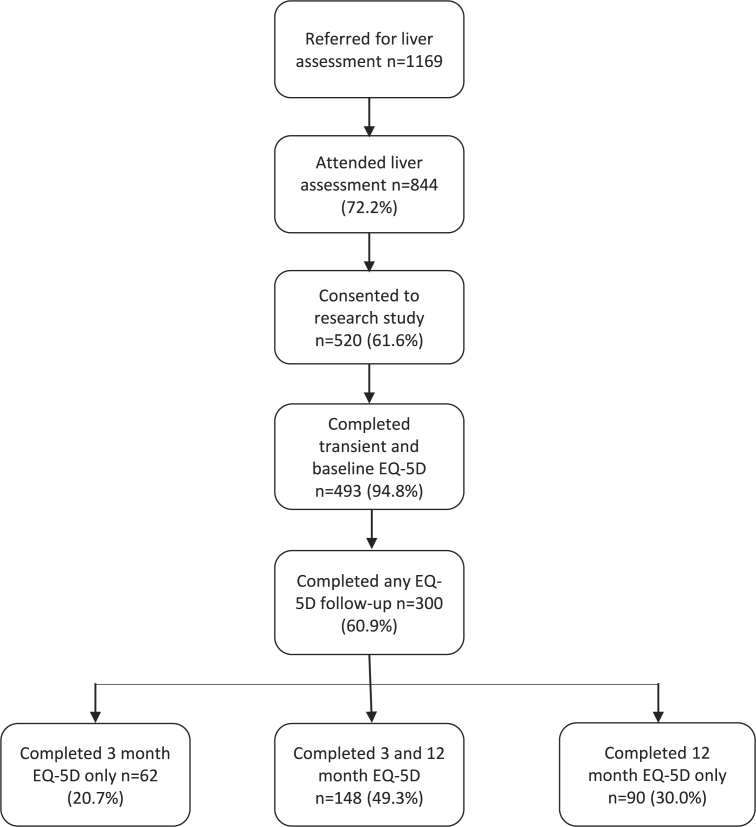
During the study period 1169 patients were referred to the pathway with 844 (72.2%) attending. Of these, 520 (61.6%%) agreed to participate. Twenty-seven patients were unable to complete both the EQ-5D and transient elastography at the liver assessment, producing a study cohort of N = 493 (94.8%). Of these n = 300 (60.9%) participants completed at least one follow-up EQ-5D survey and n = 148 (49.3%) completed all three (Fig. 1). Those participating in the research were slightly younger but otherwise similar to those who declined to take part (Table 1).
Fig. 1.
Participant flow chart.
Table 1.
Patient characteristics.
| Study cohort (pre-diagnosis) N = 493 |
Non consenting/missing data cohort N = 351 |
Included vs no included | Without any follow-up n = 193 | With any follow-up n = 300 | With 3 month EQ5D follow-up N = 218 |
With 12 month EQ5D follow-up N = 238 |
With vs without follow-up | |
|---|---|---|---|---|---|---|---|---|
| Age, years | 55.7 (13.3) | 57.9 (13.9) | p = 0.014 | 53.1 (13.7) | 57.3 (13.2) | 57.1 (19.6) | 57.6 (13.4) | p = 0.001 |
| Male | 48.4% (237) | 45.3% (159) | p = 0.408 | 52.1 (100) | 46.0% (137) | 42.6% (89) | 47.9% (113) | p = 0.219 |
| Referral reason: | ||||||||
| Alcohol excess NALFD NAFLD & alcohol excess Abnormal liver enzymes |
19.7% (95) 38.2% (184) 2.9% (14) 39.2% (189) |
21.1% (74) 38.4% (135) 4.4% (15) 36.2% (127) |
p = 0.579 | 19.3% (37) 38.0% (73) 4.2% (8) 38.5% (74) |
20.0% (58) 38.3% (111) 2.1% (6) 39.7% (115) |
20.2% (41) 37.9% (77) 2.0% (4) 39.9% (81) |
20.8% (48) 35.9% (82) 2.2% (5) 4.1% (95) |
p = 0.611 |
| Transient elastography, kPa | 5.7 (4.4–7.7) | 5.7 [4.4–7.8] | p = 0.755 | 5.5 (4.3–7.7) | 5.9 (4.4–7.9) | 6.0 (4.7–7.8) | 5.7 (4.4–8.2) | p = 0.267 |
| EQ-5D index (pre-diagnosis) | 0.75 (0.61–0.85) | – | – | 0.77 (0.63–0.88) | 0.74 (0.57–0.84) | 0.74 (0.56–0.84) | 0.75 (0.61–0.84) | p = 0.306 |
| VAS (pre-diagnosis) | 75 (60–90) | – | – | 75 (53–90) | 80 (60–90) | 80 (60–90) | 80 (60–90) | p = 0.261 |
Values are mean(sd), median(IQR) or %(n); kPa kilopascals; NAFLD non-alcohol fatty liver disease; VAS visual analogue scale.
The mean age of study participants was 55.7 years, with 48.4% male. Those participating in follow-up were more likely to be female (54.0% vs 47.9%) and older (mean 57.3 vs 53.1 years) (Table 1).
3.2. Pre-diagnosis
3.2.1. Utility index
Pre-diagnosis the median (IQR) utility index was 0.75 (0.61–0.85) and VAS was 75/100 (60–90). Males had significantly higher HRQoL than females (median index 0.77 vs 0.74, p = 0.032). There was no significant difference in pre-diagnosis utility index by age or referral reason (Table 2).
Table 2.
Pre-diagnosis health related quality of life.
| % (n) no problems |
Median utility index | VAS | |||||
|---|---|---|---|---|---|---|---|
| Mobility | Pain | Anxiety | ADLs | Selfcare | |||
| All | 55.2 (272) | 30.6 (151) | 46.2 (228) | 56.8 (280) | 81.1 (400) | 0.75 | 75 |
| Sex | |||||||
| Male | 58.6 (139) | 35.4 (84) | 53.2 (126) | 57.8 (137) | 81.0 (192) | 0.77 | 80 |
| Female | 51.4 (130) | 25.7 (65) | 40.3 (102) | 55.3 (140) | 81.0 (205) | 0.74 | 70 |
| Age | |||||||
| <40years | 67.0 (39) | 37.9 (22) | 37.9 (22) | 69.0 (40) | 91.4 (53) | 0.77 | 75 |
| 40-49 years | 58.1 (54) | 39.8 (37) | 43.0 (40) | 52.7 (49) | 79.6 (74) | 0.77 | 70 |
| 50-59years | 56.9 (82) | 33.3 (48) | 35.4 (51) | 52.8 (76) | 78.5 (113) | 0.74 | 75 |
| 60-69years | 49.6 (65) | 22.1 (29) | 56.5 (74) | 55.7 (73) | 78.4 (104) | 0.74 | 80 |
| 70 + years | 35.6 (21) | 22.0 (13) | 61.0 (36) | 61.0 (36) | 81.4 (48) | 0.77 | 80 |
| Referral reason | |||||||
| Alcohol excess | 53.7 (51) | 30.5 (29) | 32.6 (31) | 45.3 (43) | 81.1 (77) | 0.74 | 75 |
| NALFD | 51.9 (96) | 29.9 (55) | 47.6 (89) | 54.5 (101) | 78.6 (145) | 0.74 | 75 |
| Abnormal liver enzymes | 58.4 (110) | 31.1 (59) | 52.1 (98) | 63.7 (120) | 83.7 (158) | 0.77 | 80 |
| Fibrosis category | |||||||
| No significant liver disease | 60.1% (227) | 31.7% (120) | 48.4% (183) | 61.1% (231) | 83.1% (314) | 0.77 | 80 |
| Significant liver disease | 42.9% (39) | 28.6% (26) | 35.2% (18) | 46.2% (42) | 75.8% (69) | 0.69 | 75 |
| Advanced liver disease | 25.0% (6) | 20.8% (5) | 54.2% (13) | 29.2% (7) | 70.8% (17) | 0.68 | 70 |
ADLs activities of daily living; NAFLD non-alcohol fatty liver disease; VAS visual analogue scale.
Those participants with definite fibrosis had significantly lower utility index than those without (0.68 vs 0.77, p = 0.001).
3.2.2. Domains
Full domain data is detailed in Table 2.
Amongst all participants, by domain, self-care showed the highest proportion with no problems (81.1%), followed by daily activities (56.8%), mobility (55.2%), anxiety and depression (46.2%), and with most difficulties reported for pain (30.6% no problems).
Females were more likely to report deficits in all domains than males, with the biggest differences in anxiety (12.9% difference). As age increased, so did the proportion of participants reporting problems with pain (<40years 62.1% vs ≥ 70years 78.0% with problems) and mobility (<40years 33.0% vs ≥ 70years 54.4% with problems). Conversely, the proportion of participants reporting problems with anxiety fell as age increased (<40years 62.1% vs ≥ 70years 39.0% with problems). Daily activities and self-care remained static with age.
Referral reason was not associated with domain deficits with the exception of anxiety and daily activities. Those with alcohol excess had more problems than those with NAFLD, who in turn were higher than those with abnormal liver enzymes for both anxiety and daily activities (67.4, 52.4, 47.9% with anxiety problems and 54.7, 45.5 and 36.6% with daily activity problems respectively).
Pre-diagnosis, as liver disease severity increased so did the proportion of patients reporting problems with mobility, pain, daily activities and self-care. The largest deficits were in mobility (35.1% difference) and daily activities (31.9% difference).
3.3. Post-diagnosis
3.3.1. Utility index
Overall, the median utility index increased over time, from 0.75 to 0.81. This pattern was consistent across age, sex and referral reason (Table 3).
Table 3.
EQ-5D score over time.
| Median utility index |
Median VAS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-diagnosis n = 300 | 3 months n = 218 | 12 months n = 238 | Pre-diagnosis n = 300 | 3 months n = 218 | 12 months n = 238 | |||||
| All | 0.75 | 0.77 | 0.81 | 75 | 75 | 80 | ||||
| Sex | ||||||||||
| Male | 0.77 | 0.84 | 0.85 | 80 | 80 | 80 | ||||
| Female | 0.74 | 0.74 | 0.77 | 70 | 75 | 80 | ||||
| Age | ||||||||||
| <40years | 0.77 | 0.88 | 0.88 | 75 | 80 | 80 | ||||
| 40-49 years | 0.77 | 0.66 | 0.82 | 70 | 68 | 75 | ||||
| 50-59years | 0.74 | 0.77 | 0.80 | 75 | 80 | 80 | ||||
| 60-69years | 0.74 | 0.77 | 0.75 | 80 | 75 | 80 | ||||
| 70 + years | 0.77 | 0.79 | 0.86 | 80 | 80 | 80 | ||||
| Referral reason | ||||||||||
| Alcohol excess | 0.74 | 0.79 | 0.77 | 75 | 80 | 80 | ||||
| NALFD | 0.74 | 0.77 | 0.77 | 75 | 75 | 75 | ||||
| Abnormal liver enzymes | 0.77 | 0.77 | 0.85 | 80 | 75 | 80 | ||||
| Fibrosis category | ||||||||||
| No significant liver disease | 0.77 | 0.79 | 0.84 | 80 | 80 | 80 | ||||
| Significant liver disease | 0.69 | 0.70 | 0.80 | 75 | 70 | 80 | ||||
| Advanced liver disease | 0.68 | 0.65 | 0.69 | 70 | 66 | 75 | ||||
NAFLD non-alcohol fatty liver disease; VAS visual analogue scale.
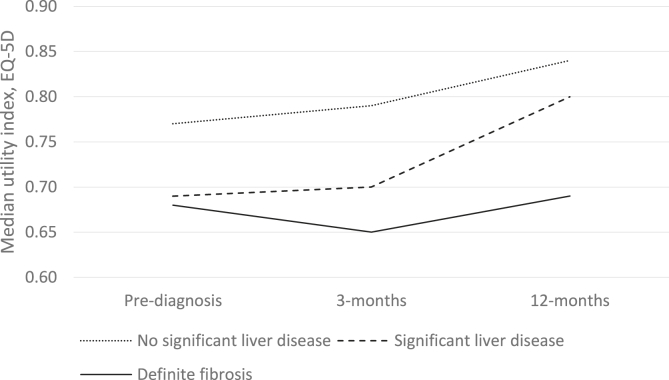
At both follow-up time points the median utility score for those with significant liver disease was lower than for those without (three months 0.68 vs 0.77, p = 0.029, 12 months 0.69 vs 0.84, p = 0.331).
In the initial three months post-diagnosis there were no statistically significant changes in median index utility in any of the liver disease severity categories (Table 4). Over the longer post-diagnosis period to 12 months those with no significant liver disease or significant liver disease demonstrated a measurable increase in HRQoL (mean utility index change +0.06, p < 0.001 and + 0.08, p = 0.006 respectively). In the advanced liver disease group the HRQoL remained static (mean utility index change −0.01, p = 0.965) (Table 4 and Fig. 2).
Table 4.
Mean change in EQ-5D score following diagnosis by disease severity.
| Pre-diagnosis to 3 months | Pre-diagnosis to 12 months | |||||
|---|---|---|---|---|---|---|
| All | n = 218 | +0.01 (0.21) | p = 0.223 | n = 238 | +0.06 (0.20) | p < 0.001 |
| No significant liver disease | n = 163 | +0.01 (0.21) | p = 0.291 | n = 174 | +0.06 (0.20) | p < 0.001 |
| Significant liver disease | n = 42 | +0.03 (0.19) | p = 0.285 | n = 51 | +0.08 (0.21) | p = 0.006 |
| Advanced liver disease | n = 13 | −0.05 (0.20) | p = 0.661 | n = 13 | −0.01 (0.22) | p = 0.965 |
Values are mean (sd).
Fig. 2.
EQ-5D index over time by fibrosis category.
3.3.2. Domains
Over time there were no changes in the domain patterns across age, sex and referral reason (Appendix A).
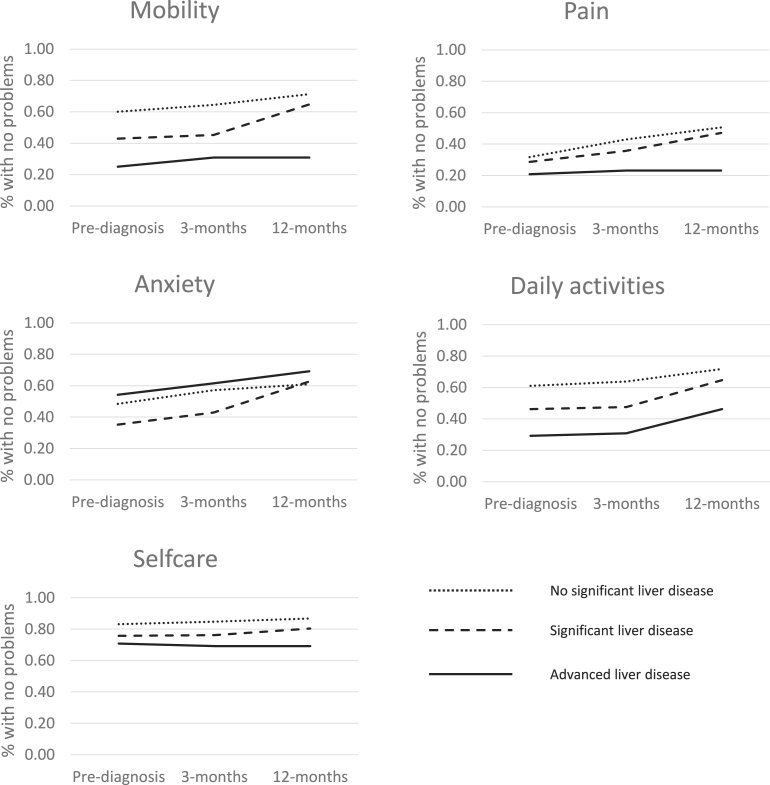
Differences in domain patterns over time for the different fibrosis severities are shown in Fig. 3 (and Appendix A). There was little change in proportions reporting problems with self-care over time for all liver disease severity categories. For mobility, pain and daily activities all three liver disease severity categories saw an increase in proportions reporting no problems over time, however there was an increase in the difference between no significant liver disease and advanced liver disease for mobility and daily activities (35.1–40.5% and 10.9–27.5% difference respectively). Anxiety was the only domain showing the converse; as liver disease becomes severe the proportion reporting anxiety problems falls. Additionally, the proportion of patients reporting anxiety problems falls over time.
Fig. 3.
EQ-5D domains over time by fibrosis category.
3.4. Sensitivity analysis
In sensitivity analysis, restricted to only those participants with measures at all three time points, the results remained similar (Appendix B).
4. Discussion
We have identified no reduction in participants HRQoL experiencing our novel diagnostic pathway for CLD. We did however find that those participants diagnosed with either no significant liver disease or significant liver disease had improved HRQoL by 12 months post the diagnostic process.
It is unclear why patients HRQoL improved over time. It is possible that the anxiety of the liver assessment caused an artificial lowering of the baseline measures. Alternatively, it is probable that following the assessment that a definitive diagnosis had been made (absence or presence of liver disease) provided a degree of relief and increase in HRQoL. A final consideration is regression to the mean [12].
It has been raised as a concern that putting asymptomatic patients through a diagnostic pathway for a condition with no direct intervention available could cause more harm than good. In other disease areas a diagnosis can lead to symptom relief and treatment resulting in an increase in HRQoL [13], but this is not the scenario in chronic liver disease. For those with significant fibrosis there is the potential for hepatocellular carcinoma and variceal screening, but for other participants the focus is on lifestyle change. We found no evidence of any decrement in HRQoL, with index scores showing no significant difference at each time point. As one might expect, HRQoL had marginally declined in the group diagnosed with advanced liver disease post-diagnosis, however it had returned to pre-diagnosis levels by 12 months. No reduction was seen in the no or possible fibrosis groups. It is notable that at three months all liver disease severity groups had an increase in “no problems” responses for anxiety and depression, supporting the idea that the pathway is not causing any detriment to well-being. This is akin to other ‘screening’ type studies which have similarly found no detriment to HRQoL during the process – for example in PSA screening for prostate cancer [14] and type 2 diabetes [15]. Furthermore, studies in diabetes show that those with screen diagnosed disease (as opposed to routine symptomatic diagnosis) had a better quality of life [16].
Additionally, we have demonstrated that HRQoL is lower in people at high risk of chronic liver disease compared to the UK and regional (East Midlands) norms (0.75 vs 0.80 and 0.85 respectively for ages 55–64years) [17]. Following investigation and diagnosis, patients with advanced liver disease have an even lower HRQoL than those without significant liver disease. Post-diagnosis those with advanced liver disease maintain their HRQoL whereas those with less severe or no liver disease improve. Overall there was no detriment in HRQoL identified amongst high-risk individuals progressing through the diagnostic pathway.
Similar to other general population studies we found that females had lower HRQoL than males and that HRQoL decreased with age. Interestingly, the deficit in our high risk of CLD population compared to the general population was particularly notable amongst younger patients where the difference was as much as 0.16 (age <49 years), with the older age group demonstrating similar deficits [17].
The major deficits in HRQoL were in the pain domain at all measured time points, however consistent with other domains this improved over time (pre-diagnosis 69.0%, three-months 59.4%, 12-months 51.2% reporting problems).
The strength of this study lies in its uniqueness in assessing not only those with significant and advanced liver disease, but also those at high-risk and yet to develop fibrosis. To our knowledge, it is the first study to assess the impact of a ‘screening’ pathway leading to the diagnosis of chronic liver disease. The sample is shown to be representative of the target population (those attending the Nottingham Liver Pathway), with a good response rate (60%), reducing the risk of selection bias at follow-up. What remains unknown is any understanding of the GP decision making process prior to and patient acceptance of referral. As such, this data only reflect those referred to the pathway and not all those potentially eligible to be referred.
The majority of attempts to contact participants for three and 12 month follow-up were made during the working day. Multiple attempts to contact were made (minimum three) and the time of day was varied. However, this could have resulted in a bias towards inclusion at follow-up of those unable to work or participate in activities leading to an under-estimate of HRQoL. However, given the similarity in pre-diagnostic characteristics of those with and without follow-up, and the confirmatory findings in the sensitivity analysis, we feel this bias is minimised. As with any HRQoL study, unless a disease specific questionnaire is used there is no way of knowing how much liver disease is contributing to the overall score. However, the similarity of scores between different referral groups suggests that despite different background co-morbidities the score holds. It may be of value to investigate HRQoL in this population using a disease specific score, however questionnaires such as the Chronic Liver Disease Questionnaire [18] are highly focussed on advanced symptoms of liver disease and may not be valid in populations such as ours. Our results through the use of EQ-5D (and its inherent ability to be compared across disease areas) are of value more widely due the use of this metric in decision making at national (NICE) and international level.
Identifying that patients with advanced liver disease have significantly lower HRQoL than those with less severe disease is in keeping with the work of others [4,19,20]. However, all prior studies only examined the advanced end of the disease spectrum – in patients with established chronic liver disease, typically around the time of transplantation. Our work demonstrates that this gradient is apparent much earlier in the disease history – including prior to diagnosis. Younessi’s 2019 study [19] reported on patients with non-alcoholic steatosis from within phase 3 trials and found similar EQ-5D utility index measures to ourselves for F3 and F4 fibrosis (0.84 and 0.82 respectively). It is perhaps surprising that we found no relationship between referral reason and HRQoL given that prior studies [4,20] have repeatedly shown liver disease aetiology to be a predictor of HRQoL, with NALFD having poorer scores than ARLD. It may be that in this study the patients with ARLD disproportionately reflect those with more severe fibrosis. However, one could speculate that referral would be biased towards those with ARLD most likely to engage in the pathway and have a higher HRQoL, thus leading the results to be closer to the prior findings.
It is unclear what is driving the predominant loss of HRQoL in the pain domain. Prior studies have also noted pain as a major contributor to ill-health and health service utilisation amongst people with liver disease [21], however this usually relates to advanced cirrhosis with ascites related pain [22] and difficulties with analgesia prescription [23]. It could be hypothesised that the improvements in mobility and activities of daily living domains could be proxies of lifestyle changes triggered by the liver diagnosis pathway. However, this is speculative and requires further investigation.
5. Conclusion/recommendations
There was no evidence from our study that patients participating in a risk factor driven liver disease diagnosis pathway experienced any detriment to HRQoL from the process. Given the growing burden of undiagnosed CLD our findings provide support for role out of early diagnostic pathways.
Declaration of competing interest
Joanne Morling is co Editor-in-Chief of Public Health in Practice. No other conflicts of interest exist.
Acknowledgements
We are grateful for the nurses and administrative staff on the day case unit at Nottingham University Hospitals NHS trust. We thank the clinical commissioners for supporting the implementation of the pathway and our local GPs for their engagement.
The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.puhip.2020.100033.
Financial support
JC, RH, and ING are supported by the Gastrointestinal and Liver Disorder theme of the NIHR Nottingham Biomedical Research Centre (Reference no: BRC-1215-20003). JRM receives salary support from a Medical Research Council Clinician Scientist Fellowship [grant number MR/P008348/1]. Funding for the Nottingham liver disease stratification pathway has also been received from the East Midlands Academic Health Sciences Network.
Author contributions
Study design: JM, NG, ZT. Data collection: JM, ZT, JC, RH. Data analysis: JM, ZT, JW. Wrote manuscript: JM. Critically revised manuscript: JM, NG, ZT, JC, RH, JW.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Glob. Health Data Exch. Global Burden of Disease Project, Institute for Health Metrics and Evaluation. Available from:: http://ghdx.healthdata.org/,.
- 2.Williams R., et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet. 2014;384:1953–1997. doi: 10.1016/S0140-6736(14)61838-9. [DOI] [PubMed] [Google Scholar]
- 3.Tapper E.B., Parikh N.D. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ. 2018;362 doi: 10.1136/bmj.k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi Z.M., et al. Health-related quality of life in chronic liver disease: the impact of type and severity of disease. Am. J. Gastroenterol. 2001;96:2199–2205. doi: 10.1111/j.1572-0241.2001.03956.x. [DOI] [PubMed] [Google Scholar]
- 5.Younossi Z.M., Boparai N., McCormick M., Price L.L., Guyatt G. Assessment of utilities and health-related quality of life in patients with chronic liver disease. Am. J. Gastroenterol. 2001;96:579. doi: 10.1111/j.1572-0241.2001.03537.x. [DOI] [PubMed] [Google Scholar]
- 6.Chalmers J., et al. Development and implementation of a commissioned pathway for the identification and stratification of liver disease in the community. Front. Gastroenterol. flgastro-2019-101177. 2019 doi: 10.1136/flgastro-2019-101177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harman D.J., et al. Direct targeting of risk factors significantly increases the detection of liver cirrhosis in primary care: a cross-sectional diagnostic study utilising transient elastography. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-007516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devlin N.J., Brooks R. EQ-5D and the EuroQol group: past, present and future. Appl. Health Econ. Health Pol. 2017;15:127–137. doi: 10.1007/s40258-017-0310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devlin N.J., Shah K.K., Feng Y., Mulhern B., van Hout B. Valuing health-related quality of life: an EQ-5 D-5 L value set for E ngland. Health Econ. 2018;27:7–22. doi: 10.1002/hec.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Hout B., et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15:708–715. doi: 10.1016/j.jval.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Friedrich-Rust M., et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960–974. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 12.Barnett A.G., van der Pols J.C., Dobson A.J. Regression to the mean: what it is and how to deal with it. Int. J. Epidemiol. 2004;34:215–220. doi: 10.1093/ije/dyh299. [DOI] [PubMed] [Google Scholar]
- 13.Gray A.M., Papanicolas I.N. Impact of symptoms on quality of life before and after diagnosis of coeliac disease: results from a UK population survey. BMC Health Serv. Res. 2010;10:105. doi: 10.1186/1472-6963-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Essink-Bot M.-L., et al. Short-term effects of population-based screening for prostate cancer on health-related quality of life. J. Natl. Cancer Inst. 1998;90:925–931. doi: 10.1093/jnci/90.12.925. [DOI] [PubMed] [Google Scholar]
- 15.Edelman D., Olsen M.K., Dudley T.K., Harris A.C., Oddone E.Z. Impact of diabetes screening on quality of life. Diabetes Care. 2002;25:1022–1026. doi: 10.2337/diacare.25.6.1022. [DOI] [PubMed] [Google Scholar]
- 16.Adriaanse M.C., et al. Health-related quality of life in the first year following diagnosis of Type 2 diabetes: newly diagnosed patients in general practice compared with screening-detected patients. The Hoorn Screen. Stud. Diabet. Med. 2004;21:1075–1081. doi: 10.1111/j.1464-5491.2004.01277.x. [DOI] [PubMed] [Google Scholar]
- 17.Kind P., Hardman G., Macran S. 1999. UK Population Norms for EQ-5D. [Google Scholar]
- 18.Younossi Z.M., Guyatt G., Kiwi M., Boparai N., King D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295–300. doi: 10.1136/gut.45.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Younossi Z.M., et al. Reduced patient-reported outcome scores associate with level of fibrosis in patients with nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 2019;12:2552–2560. doi: 10.1016/j.cgh.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 20.Afendy A., et al. Predictors of health-related quality of life in patients with chronic liver disease. Aliment. Pharmacol. Ther. 2009;30:469–476. doi: 10.1111/j.1365-2036.2009.04061.x. [DOI] [PubMed] [Google Scholar]
- 21.Rogal S.S., Winger D., Bielefeldt K., Rollman B.L., Szigethy E. Healthcare utilization in chronic liver disease: the importance of pain and prescription opioid use. Liver Int. 2013;33:1497–1503. doi: 10.1111/liv.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogal S.S., Winger D., Bielefeldt K., Szigethy E. Pain and opioid use in chronic liver disease. Dig. Dis. Sci. 2013;58:2976–2985. doi: 10.1007/s10620-013-2638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rakoski M., et al. Pain management in patients with cirrhosis. Clin. Liver Dis. 2018;11:135–140. doi: 10.1002/cld.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.