Abstract
Cancer is considered to be an independent risk factor for severe illness and higher mortality in patients with coronavirus disease 2019 (COVID-19). These adverse outcomes have been suspected to be more severe in patients with lung cancer. The objective of this systematic review and meta-analysis is to outline patient characteristics, challenges in diagnosis and treatment, and outcomes of patients with lung cancer with COVID-19. A comprehensive search was conducted using EMBASE and PubMed databases using the terms “COVID” and “cancer.” Studies that reported clinical characteristics or outcomes of patients with lung cancer with COVID-19 were then systematically identified. Meta-analysis for COVID-19 related mortality associated with lung cancer compared with other cancer types was conducted. The results were reported as OR and confidence intervals using the mixed-effects logistic regression model. The most frequently reported clinical findings in patients with lung cancer with COVID-19 were fever and cough, with 68% and 61%, respectively. Laboratory and radiographic findings were consistent with broadly reported data. The meta-analysis noted a statistically significant increase in mortality rate in patients with lung cancer compared with other patients with cancer, with an OR of 1.62 (95% confidence interval: 1.06–2.48). Patients with lung cancer with COVID-19 also reflected greater severity of illness and higher rates of intensive care unit admissions and mechanical ventilation. COVID-19 in patients with lung cancer is associated with severe disease and increased mortality relative to patients with other malignancies and the general population. There is conflicting evidence on the effect of specific lung cancer treatments on outcomes. Until more definitive data is available, lung cancer–directed treatment should be continued or restarted as early as possible in mild to moderate cases to prevent worsening and cancer-related mortality.
Keywords: COVID, Cancer, Lung, Mortality
Highlights
-
•
Patients with lung cancer and coronavirus disease 2019 infection have higher mortality rates compared with patients with other cancer types.
-
•
Rates of severe illness, intensive care unit admissions, and mechanical ventilation are also higher in patients with lung cancer.
-
•
Clinical, laboratory, and radiographic characteristics are similar to reports in the general population.
-
•
There is conflicting data regarding the effect of specific lung cancer treatments on outcomes.
Background
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 and was first identified in Wuhan, Hubei province, the People’s Republic of China in December 2019.1 Since then, it has rapidly spread worldwide, with more than 27.3 million cases reported as of September 8, 2020.2 The clinical characteristics of the illness are highly variable ranging from asymptomatic disease to mild, self-limiting cases to severe disease requiring intensive care unit (ICU) admission and mechanical ventilation.3 Mortality rate is also highly variable, and several disease-specific markers and preexisting medical comorbidities have been identified as risk factors for increased mortality in these patients.3
Cancer is confirmed to be an independent risk factor for adverse events and mortality in patients with COVID-19.4, 5, 6, 7 Other adverse events, such as the incidence of intubation and mechanical ventilation use, is also higher in patients with cancer.5 The impact of COVID-19 infection on patients with lung cancer was reported in several studies with increased mortality noted when compared with other cancer types.6, 7, 8, 9, 10, 11, 12, 13 However, many of these studies were limited to a small cohort of patients with single-institution experience. Preliminary data from a large global multicenter observational study of patients with thoracic malignancy reported mortality rate to be as high as 33%.14
The objective of this systematic review is to describe clinical characteristics, challenges in diagnosis, and outcomes of patients with lung cancer who were confirmed to have COVID-19 infection. A meta-analysis of COVID-19–related mortality in patients with lung cancer compared with patients with other malignancies was included. We also describe the effects of different lung cancer–directed treatments on outcomes.
Materials and Methods
This systematic review and meta-analysis was done in accordance with the guidelines set forth by Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.15
A comprehensive search was done using EMBASE and PubMed databases using the words “COVID” and “cancer.” In addition, the American Society of Clinical Oncology and the American Association for Cancer Research 2020 annual meetings were manually searched for relevant abstracts. A manual search using other search engines such as Google Scholar and the references of relevant articles was also conducted.
Selection Criteria
All case reports, retrospective and observational studies, and prospective studies that reported clinical characteristics or outcomes of patients with lung cancer who acquired COVID-19 infection were included in the study. Preclinical data, including animal and cell line studies, reviews, meta-analyses, commentaries, and guidelines were excluded.
Data Extraction
Two reviewers (MP and IJ) systematically surveyed titles and abstracts to identify relevant articles for a full review. An independent review of the selected full-text articles was conducted using the above-specified selection criteria. Discrepancies were resolved by consensus with a third reviewer (CK).
Pertinent clinical data extracted from the selected studies included patient symptoms, laboratory and radiographic features, and type of cancer-directed therapy received before infection. Outcomes data, including the severity of illness, rates of intubation and ICU admissions, and mortality, were also extracted.
Selected studies that contained mortality data of patients with lung cancer in comparison to other cancer types were included in the meta-analysis. The remaining relevant data was synthesized descriptively for the review.
Statistical Methods
The number of patients with COVID-19 and deaths in lung cancer or other cancer of each study were used to evaluate COVID-19–related mortality associated with lung cancer. The OR was calculated for each study, in which a continuity correction by adding 0.5 was applied when a study contains zero events. Mixed-effects logistic regression model with random study effects was fitted. Confidence intervals (CIs) for the OR were calculated using a t distribution.16 Study-specific heterogeneity was assessed using the l2 statistic (0%–100%), in which a lower value implies less heterogeneity. The between-study variance tau2 was computed using the maximum likelihood method and tested for the assumption of homogeneity using the Wald test. Funnel plot asymmetry was evaluated using Egger’s linear regression test.17 A two-sided p value of less than 0.05 for OR was determined to be statistically significant. All statistical analyses were carried out using the meta library in R software version 3.4.
Results
Study Characteristics
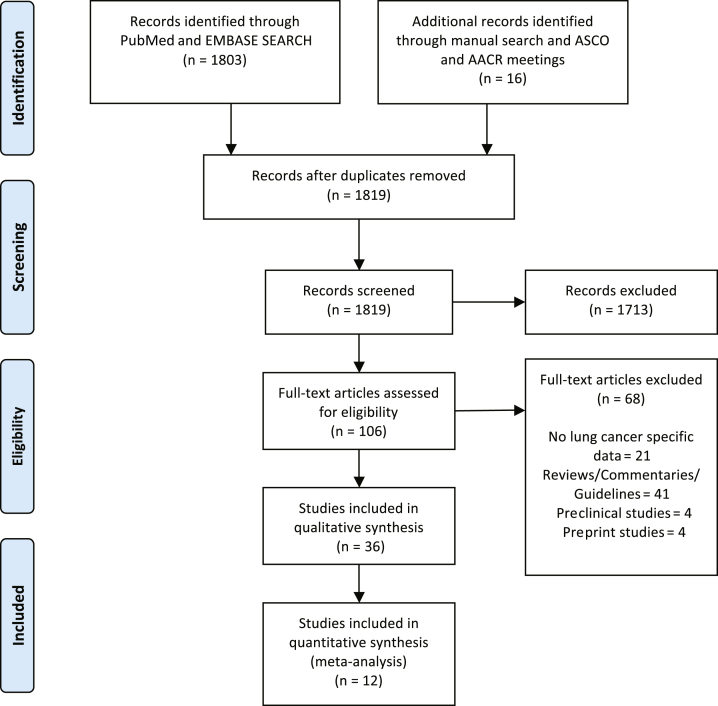
A total of 1819 articles were identified after an initial comprehensive search using large databases, national meetings, and manual search. A total of 106 full articles were selected for further review; out of which, 36 studies were chosen for qualitative synthesis for the review. A total of 12 studies included mortality data for both patients with lung cancer and other patients with cancer, and thus, were eligible for the meta-analysis (Fig. 1).
Figure 1.
PRISMA flow diagram of article selection for qualitative and quantitative synthesis. AACR, American Association for Cancer Research; ASCO, American Society of Clinical Oncology; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Patient Characteristics
Clinical Features
The clinical manifestations varied substantially among patients with lung cancer with COVID-19 infection. In the largest international cohort of patients with lung cancer from the Thoracic Cancers International COVID-19 Collaboration (TERAVOLT) registry, Garassino et al.14 reported that 12% of total patients with lung cancer (n = 198) studied had an asymptomatic disease. Although they reported on both hospitalized (n = 150) and nonhospitalized patients (n = 48), most of the available data from other studies were in hospitalized patients.
Several published case reports highlight the heterogeneity in clinical presentations in this patient group.18, 19, 20, 21, 22, 23 A summary of frequently reported symptoms and prevalence among included lung cancer studies are described in Table 1. Fever and cough were the most frequently reported clinical findings, with 68% (201 of 295) and 61% (181 of 296) of patients experiencing these symptoms, respectively. Patients with lung cancer having COVID-19 also had a wide range of complications. In a cohort of seven patients with lung cancer (among 28 patients with cancer), Zhang et al.22 observed higher rates of anoxia, earlier development of respiratory symptoms, and more rapid progression of disease compared with other cancer types and the general population. Garassino et al.14 found in the TERAVOLT study that the most common complications were pneumonia or pneumonitis and acute respiratory distress syndrome, with 80% and 27% of patients experiencing these conditions, respectively.
Table 1.
Clinical Features of Patients With Lung Cancer with COVID-19 as Reported in Published Studies
| Study | Total Patients | Fever, N (%) |
Cough, N (%) |
Dyspnea, N (%) | Myalgia, N (%) | Fatigue, N (%) | Gastrointestinal Symptoms, N (%) |
|---|---|---|---|---|---|---|---|
| Peng et al. 20208 | 7 | 7 (100) | 7 (100) | 7 (100) | — | 7 (100) | 1 (14) |
| Rogado et al. 202051 | 17 | 12 (71) | 17 (100) | 13 (77) | 5 (29) | — | 1 (6) |
| Luo et al. 202042 | 69 | 42 (70)a | 47 (77)b | 43 (73)c | — | — | 13 (22)a |
| Yu et al. 202010 | 7 | 7 (100) | 3 (43) | 3 (43) | — | — | — |
| Cai et al. 202025 | 6 | 6 (100) | 4 (67) | 0 (0) | 2 (33) | 2 (33) | 0 (0) |
| Garassino et al. 202014 | 200 | 127 (64)d | 103 (52)d | 106 (54)d | 10 (5)d | 54 (27)d | 10 (5)d |
| Total | 306 | 201/295 (68) | 181/296 (61) | 172/294 (59) | 17/221 (8) | 63/211 (30) | 25/288 (9) |
Note: Total patients are defined as the total number of patients with lung cancer and COVID-19 included in the study.
COVID-19, coronavirus disease 2019.
Percentages are calculated on the basis of 60 patients with reported symptoms in the study.
Percentage is calculated on the basis of 61 patients with reported symptoms in the study.
Percentage is calculated on the basis of 59 patients with reported symptoms in the study.
Percentages are calculated on the basis of 198 patients with reported symptoms in the study.
Recognition of COVID-19 infection in these patients poses a particular challenge given the overlapping symptoms with lung cancer as evidenced by a case reported by Ouyang et al.,19 in which a decision to change lung cancer treatment was made owing to concerns of disease recurrence, when in fact the patient had symptoms of COVID-19. COVID-19 in patients with lung cancer may also be misinterpreted as pneumonitis, especially if the time frame of symptoms coincides with the expected adverse effects of their therapies. In these cases, determining the cause of pneumonitis is essential because COVID-19–induced pneumonitis is managed differently from radiation-induced pneumonitis and an immune checkpoint inhibitor (ICI)–induced pneumonitis. In a case reported by Samson et al.,20 a patient with SCLC was treated with concurrent chemotherapy and radiation, after which she developed new-onset cough and dyspnea within the window of radiation pneumonitis 9 weeks later. However, after testing positive for COVID-19, she was successfully managed without the use of steroids. Guerini et al.24 described a patient with lung adenocarcinoma treated with durvalumab whose atypical immune-related pneumonitis was successfully treated with steroids, after which he developed worsening respiratory symptoms, subsequently tested positive for COVID-19, and died of respiratory failure.
The symptoms of COVID-19 can also overlap with the usual postoperative clinical course, resulting in a delay in diagnosis.25 In cases of thoracic surgery, fever and cough from reactive pleural effusion and atelectasis are common postoperative symptoms. Dyspnea secondary to lung resection and chest tightness, fatigue, loss of appetite, and nausea owing to postoperative analgesics is also widely observed.26 In a retrospective study of 11 patients with COVID-19 after thoracic surgery conducted by Peng et al.,8 case fatality was associated with the degree of surgical resection defined by more than five resected lung segments. In addition, patients with lung cancer having COVID-19 after thoracic injury was found to have a greater incidence of severe illness.8
Radiographic Findings
Computed tomography (CT) of the chest is an important diagnostic modality for COVID-19 infection. Studies conducted on patients with cancer having COVID-19 have observed bilateral lung involvement and peripheral distribution with common radiologic patterns, including ground-glass opacities, smooth or irregular interlobular septal thickening, crazy-paving pattern, and thickening of adjacent pleura.27 Imaging findings of COVID-19 infection in patients with lung cancer include multiple patchy ground-glass opacities and consolidations.28,29 Garassino et al.14 reported 6.5% (13 of 200) of their patient cohort had radiologic findings that were highly suggestive of COVID-19 and ultimately led to diagnosis despite negative reverse transcriptase–polymerase chain reaction test. In a cohort of radiation oncology patients, an asymptomatic patient with lung cancer tested positive after identification of new bilateral ground-glass opacities on a CT scan obtained before delivery of the first fraction for definitive stereotactic ablative radiotherapy.30 Another study conducted by Peng et al.8 found that early imaging signs of COVID-19 were disguised in 11 postoperative thoracic surgery patients with lung and other cancers, resulting in a 27.3% fatality rate. Postoperative changes from thoracic surgery may overshadow early CT signs of COVID infection, and thus, cannot be used as the sole diagnostic modality. Peng et al.8 concluded that serial CT imaging to track progression or resolution of radiologic findings is important. Cai et al.25 proposed a way to navigate this challenge as they noted postresection infiltration and effusion mostly develop in the lung on the surgical side and if symptoms in contralateral lobe should occur, there should be a high index of suspicion for infection.
Alternative imaging techniques are also valuable. Suppli et al.21 reported a case in which cone beam CT images, collected as a part of daily radiotherapy imaging, displayed patchy consolidation in the subpleural region and middle lobe in a patient with pulmonary adenocarcinoma. There was noticeable progression in the after-treatment fractions, and ground-glass opacities were visible on cone beam CT images 36 hours before the patient began to exhibit clinical symptoms of COVID-19. Therefore, these images, which are routinely collected in conjunction with the patient’s treatment, can provide early signs supporting COVID-19 testing and should be carefully evaluated for radiologic abnormalities. Several studies have also reported the appearance of COVID-19 in radiographs. Patchy or diffuse interstitial reticulonodular opacities and consolidations with progressive bilateral lung changes are all potential signs of COVID-19 in patients with lung cancer.21 Observing the progression of these bilateral lung changes can be an important prognostic tool.21 Chuang et al.31 also reported a case in which fluorodeoxyglucose-positron emission tomography findings in a patient with neuroendocrine small cell carcinoma suggested COVID-19. The patient had a favorable treatment response, but imaging revealed bilateral, multifocal, hypermetabolic ground-glass opacities, which were suggestive of an infectious cause. These ground-glass opacities were present in a crazy-paving pattern. The patient eventually presented with symptoms and was found to have a positive blood test result.31
Pathologic Findings
The pulmonary pathological characteristics of COVID-19 observed in the general infected population exhibited that of congested and edematous lungs with patchy involvement and diffuse consolidation. Microscopically, airway inflammation and alveolar zones with hyaline membranes and type 2 pneumonocyte hyperplasia were observed.32
The pathologic observations of patients with lung cancer having COVID-19 were largely reported through case studies and are generally aligned with these observations. Tian et al.33 described the accidental pathologic samples collected from two patients who underwent an operation for lung cancer and had COVID-19 pneumonia but were asymptomatic at the time. Specifically, the lungs of both of these patients exhibited edema, proteinaceous exudate, focal reactive hyperplasia of pneumocytes with patchy inflammatory cell infiltration, and multinucleated giant cells. In one of the patients, reactive alveolar epithelial hyperplasia was observed, and in the other case, fibroblastic proliferation was seen, which were both indicative of early organization. Cai et al.25 also observed similar pathologic findings in their cohort of seven patients with lung cancer, with the exception of one patient who had extensive interstitial inflammation with numerous plasma cells and macrophages infiltrating lung tissues.
Laboratory Findings
Laboratory findings are valuable prognostic tools in patients with COVID-19 infection. A meta-analysis by Zhang et al.34 found that the most prevalent laboratory findings reported in the general COVID-19 population were increased C-reactive protein (CRP), hypoalbuminemia, increased lactate dehydrogenase (LDH), erythrocyte sedimentation rate, and interleukin-6 levels, lymphopenia, and decreased eosinophils. In a subgroup analysis, increased CRP, lymphopenia, and increased LDH were associated with worse outcomes owing to severe illness.
In a cohort of patients with lung cancer that underwent thoracic surgery, postoperative reduction in total protein and albumin were found to be significantly associated with fatality (p < 0.05).8 An increase in LDH and liver enzymes also revealed marginal associations with death (p = 0.05 and p = 0.055, respectively).8 In the study by Cai et al.,25 four of six patients with lung cancer were found to have lymphopenia, two of which had an exaggerated decline. These two patients had severe progression of COVID-19 and subsequently died, whereas the other four patients remained alive.25 In the same patient cohort, all six patients also revealed elevated inflammatory markers such as CRP, D-dimer, and procalcitonin, all of which are biomarkers associated with the progression of COVID-19.25,35 These observations were similar to those reported in the general population with COVID-19.34,36
Neutropenia is a frequently reported adverse event in patients with cancer after administration of chemotherapy or radiation. The administration of granulocyte-colony stimulating factor (G-CSF), a treatment option for neutropenia, in patients with COVID-19 has been hypothesized to have potentially devastating results because it induces the activation of strong inflammatory pathways.37 However, no definitive conclusions could be made owing to the limited availability of clinical evidence, and decisions should be individualized, taking risk-benefit ratio into account. Two published case reports reported different management strategies for neutropenic patients with lung cancer and COVID-19. Sereno et al.38 successfully treated a patient who had advanced SCLC with grade IV neutropenia and COVID-19 infection by covering for bacterial coinfection, G-CSF, and dexamethasone. Alternatively, Figuero-Pérez et al.39 successfully treated a 76-year-old man with metastatic lung adenocarcinoma and febrile neutropenia using only broad-spectrum antibiotics and respiratory support owing to controversial reports of G-CSF use. A case series by Nawar et al.40 noted that three patients with cancer with COVID-19 who were administered G-CSF were observed to have a rising neutrophil-to-lymphocyte ratio within 24 hours. All three patients suffered respiratory decline within 72 hours of administration.
Mortality Outcomes
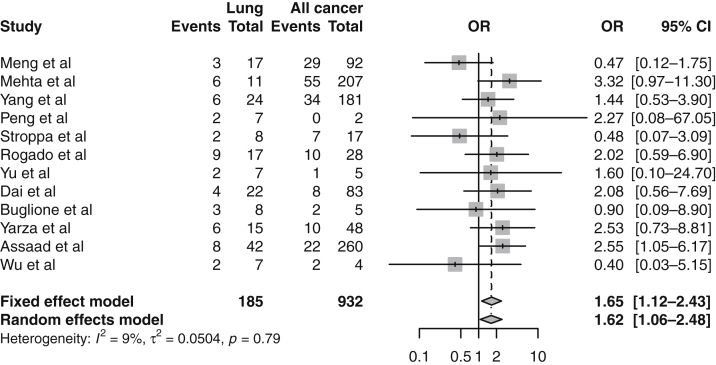
A total of 17 articles included COVID-19–related mortality outcomes in patients with lung cancer, with rates ranging from 17.7% to 55%.5, 6, 7, 8, 9, 10, 11, 12, 13, 14,25,41, 42, 43, 44, 45, 46 A total of 12 of these studies included a mortality rate for both patients with lung cancer and other cancer types, and thus, were compared in the meta-analysis (Fig. 2).5, 6, 7, 8, 9, 10, 11, 12, 13,41,43,44 Although most of these studies reported higher rates of mortality in patients with lung cancer, this was not replicated across all studies.6, 7, 8, 9, 10, 11, 12, 13 Pooled analysis of these trials noted a statistically significant increase in mortality rate in patients with lung cancer with OR equal to 1.62 (95% CI: 1.06–2.48). There was no strong study-specific heterogeneity on the basis of l2 (9%) and tau2 tests, p value equal to 0.79. In addition, there was no significant funnel plot asymmetry or bias in the meta-analysis (p = 0.358).
Figure 2.
A meta-analysis of mortality outcomes in patients with lung cancer compared with patients with other cancer types. CI, confidence interval.
Few studies not included in the meta-analysis reported lung cancer–specific mortality alone. The largest global registry of thoracic malignancies to date was reported in the ongoing TERAVOLT observational study, which reported high rates of death at 33% in patients (n = 200) diagnosed with COVID-19 infection during their preliminary analysis.14 Univariate analysis confirmed age greater than 65, current or history of smoking, the presence of medical comorbidities such as hypertension and chronic obstructive pulmonary disease, and treatment with chemotherapy alone increased the risk of death. Multivariate analysis in this cohort revealed that only smoking history was associated with an increased risk of death. A case series of six patients with lung cancer reported a similar mortality rate of 33.3%.25 In another cohort of 69 patients with lung cancer, the rate of death was reported to be lower at 24% among 67 patients with available data; however, 59% of these patients were found to be exposed to ICI, posing the question if ICI therapy is associated with less mortality.42 This was further highlighted in the TERAVOLT study by Garassino et al.,14 who also reported no association with increased mortality in the 23% of patients who received ICI alone on univariate analysis, with an OR for death equal to 1.385 (95% CI: 0.524–3.639).
Another large study by Pinato et al.,45 which included 890 patients with cancer (of which 119 were patients with lung cancer), graphically represented an unadjusted mortality rate close to 40% (exact rate not given) in patients with lung cancer with both active and treated disease. In a series of 138 patients with cancer, nine of which had lung cancer, Pinto et al.46 reported an OR for death of 0.78 (95% CI: 0.16–3.74), suggesting no increase in mortality for patients with lung cancer. Lara Álvarez et al.47 did not have lung cancer–specific mortality; however, the highest rates of deaths at 53% (8 of 15) in patients with cancer (n = 36) were in the lung cancer subtype.
Other Outcomes
Severity of Illness
Nine studies reported on the severity of infection in patients with lung cancer with COVID-19 infection, with rates of severe infection ranging from 20% to 71.4%.8,11,22,42,48, 49, 50, 51, 52 The small sample of patients in these cohorts precluded meta-analysis of the data. The severity of illness was defined differently among these studies. Higher severity of infection has been reported in all but two of the seven studies that compared rates with other patients with cancer.8,11,22,48, 49, 50,52 Whereas in the study by Ma et al.,49 the rates of severe illness were close at 50% (4 of 8) versus 54% (20 of 37) in lung cancer and other patients with cancer, respectively, the results of a smaller study by Liang et al.48 revealed a larger variation with only 20% (1 of 5) severe illness noted in patients with lung cancer compared with 50% in other cancer types (9 of 18).
ICU Admissions and Mechanical Ventilation
Criteria for hospitalization, ICU admission, and mechanical ventilation vary on the basis of the institutional policy of the centers in which the studies were conducted. The TERAVOLT study defined the criteria for ICU admission as needing more intensive monitoring, ventilation, or resuscitation.14 The rate of ICU admissions was reported to be higher in patients with lung cancer versus other cancer types at 27.3% versus 19%, respectively, as noted in a retrospective study by Dai et al.11 Out of the 22 patients with lung cancer, four (18.2%) required mechanical ventilation. Four other lung cancer–only studies ranging from small case series to larger retrospective cohorts reported highly variable rates in ICU admissions.14,25,42,51 In the largest cohort of patients with lung cancer from the TERAVOLT cohort, the ICU admission rate was low at 9% of hospitalized patients and only 6% required mechanical ventilation.14 In contrast, the study by Luo et al.,42 which included 69 patients with lung cancer (with most patients having previous exposure to programmed cell death protein 1 blockade), reported a higher ICU admission rate of 21.7%, and 18.8% required mechanical ventilation. Similarly, ICU admission rates varied greatly from 0% in a series of 17 patients with lung cancer (13 of which were hospitalized) to 33.3% (all requiring mechanical ventilation) in a series of six patients.25,51
Treatment-Related Outcomes
In the TERAVOLT cohort, the type of treatment did not affect mortality on multivariate analysis; however, treatment with chemotherapy alone increased the risk of mortality in univariate analysis, with an OR equal to 2.54 (95% CI: 1.09–6.11). Higher rates of hospitalization were also seen in patients being treated with chemotherapy alone (33%) compared with patients on ICI alone (25%) or tyrosine kinase inhibitor alone (16%).14 Luo et al.42 studied the effect of ICI on outcomes of patients with lung cancer diagnosed with COVID-19 infection. The authors concluded no increase in disease severity (described as a composite score of ICU admissions, intubation, transition to do-not-intubate, and death) in 41 patients (59%) with previous exposure to ICI.42 In the study by Stroppa et al.43 four of eight patients with lung cancer were on ICI therapy; these patients had a moderate disease course with favorable outcomes and no deaths. In contrast, a study by Robilotti et al.50 reported higher severity of illness at 58.3% in patients receiving ICI from their cohort of 35 patients with lung cancer and concluded ICI therapy on multivariate analysis as an independent risk factor for an increase in hospitalization (OR = 2.84 [95% CI: 1.24–6.72]) and increase in severity of respiratory illness (OR = 2.74 [95% CI: 1.37–5.46]). Similarly, Dai et al.11 observed high rates of death 33.3% (2 of 6 patients) and high chances of developing critical symptoms 66.7% (4 of 6) in patients receiving ICI among all cancer types; no lung cancer–specific data for ICI treatment–related outcomes were reported. Similarly, Zhang et al.22 noted patients with cancer (not specific to lung) who received antitumor treatment, ICI, chemotherapy, radiation, or tyrosine kinase inhibitor treatment within 14 days before COVID-19 diagnosis developed severe events defined as admission to ICU, requiring mechanical ventilation, or death, with a hazard ratio equal to 2.38 (95% CI: 0.80–7.04), although it was not quite statistically significant (p = 0.118).
Discussion
COVID-19 is an infectious disease with a variable clinical course and worse reported outcomes in patients with certain preexisting comorbid conditions. Observations from several studies confirm active malignancy as an independent risk factor for increased severity of illness and mortality from COVID-19 infection.4, 5, 6, 7 To our knowledge, this is the first systematic review and meta-analysis outlining the clinical course and adverse outcomes caused by COVID-19 infection in patients with thoracic malignancies. Our meta-analysis concludes an increase in mortality in patients with lung cancer with COVID-19 infection compared with patients with other cancers who were infected, with an OR equal to 1.62 (95% CI: 1.06–2.48). Whereas further analysis was precluded owing to a lack of available individual patient data, potential confounding factors for this increased risk of mortality include smoking history, increased age, medical comorbidities, and specific lung cancer treatments, which were all found to have associations with mortality in the TERAVOLT observational study. A larger scale, prospective studies should be conducted to better understand how these factors are implicated in patients of various disease stages, genetic profiles, and histologic subtypes.
Similar to the general population with COVID-19 infection, a variable clinical course is also noted in infected patients with lung cancer. However, higher rates and severity of respiratory complications compared with the general population or other cancers are noted and likely a result of worse baseline lung function from smoking or underlying changes from lung cancer itself.14 Moreover, the substantial overlap of clinical and radiographic features with a lung cancer diagnosis, postoperative course, and common adverse effects from treatments raises a particular challenge for early diagnosis.17,18,22, 23, 24 Distinguishing COVID-19 symptoms from ICI-pneumonitis or radiation pneumonitis is important given that treatment varies and level of observations differ. After a similar challenge noted in their case, Guerini et al.24 proposed testing for COVID-19 in all patients that present with suspicious symptoms, regardless of the possibility of ICI or radiation pneumonitis. We found laboratory and radiographic findings in patients with lung cancer to be consistent with reported data in other malignancies or the general population of patients with COVID-19.
After the diagnosis of COVID-19 infection, another challenging decision is to halt or continue treatment. Outcomes varied considerably regardless of the decision to hold or continue treatment depending on the type of lung cancer treatment the patient was receiving.18,20,21,23 A study published by a group at Memorial Sloan Kettering Cancer Center revealed poorer outcomes with ICI, whereas chemotherapy and surgery did not confer increased risk.50 Guerini et al.24 also reported a case with poor outcomes after treatment with ICI and steroids. Proposed mechanisms include T-cell hyperactivation and release of cytokines from immune checkpoint inhibition resulting in the exaggerated injury of lung epithelial cells.11,50 However, all of these studies included a small population of patients. In contrast, Garassino et al.,14 in their large registry of patients with lung cancer with COVID-19 concluded increased mortality with chemotherapy in univariate analysis with no adverse effect from ICI therapy. Additional data with larger patient numbers, however, is warranted before making conclusive decisions on whether certain treatments should be stopped or continued. In addition, whether the length and timing of different therapies administered in these patients have an impact on mortality or adverse outcomes has yet to be determined and should be further studied.
Our study was limited by data gathered from small series and observational studies. Considerable heterogeneity exists in the type of articles available for our review, ranging from case reports to small observational studies. In addition, the conclusions of our meta-analysis are limited by the unavailability of sufficient patient-level data to conduct further analysis to understand how comorbidities, disease stage, and patient characteristics play a role in the increased mortality rate in this group. Consequently, we could not definitively determine whether overlap was present in the patient data reported in the various studies we included for meta-analysis. Given the relatively new onset of the pandemic with evolving information regarding the severe acute respiratory syndrome coronavirus 2 virus and the scope of this review, these limitations are unavoidable.
Conclusions
Clinical characteristics, laboratory, and radiographic findings were similar in patients with lung cancer having COVID-19 compared with the general population. However, certain challenges to diagnosis do exist given the underlying abnormal lung pathological findings or secondary to surgical or treatment-related changes. COVID-19 infection in patients with lung cancer is associated with increased mortality compared with other malignancies. The severity of illness, specifically respiratory complications, rates of ICU admissions, and mechanical ventilation, are also higher. Increased risk of adverse outcomes based on the type of treatment received is, however, controversial, with ICI increasing risk in some studies and chemotherapy in others. Until more definitive data is available, lung cancer–directed treatment should not be held in mild to moderate cases and should be restarted as soon as safely possible in severe cases of COVID-19 infection.
Acknowledgments
Informed consent was not obtained for this study, given it included secondary analysis of existing data with no reported patient identifiers or risk to patients.
Footnotes
Disclosure: Dr. Kim reports receiving research funding (to institution) from AstraZeneca, Bristol-Myers Squibb, Novartis, Regeneron, Tesaro, Karyopharm, and Debiopharm, and served as a consultant for Novartis. The remaining authors declare no conflict of interest.
References
- 1.Lv M., Luo X., Estill J. Coronavirus disease (COVID-19): a scoping review. Euro Surveill. 2020;25:2000125. doi: 10.2807/1560-7917.ES.2020.25.15.2000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time [published correction appears in Lancet Infect Dis. 2020;20:e215. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet. 2020;395:1038. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinato D.J., Lee A.J.X., Biello F. Presenting features and early mortality from SARS-CoV-2 infection in cancer patients during the initial stage of the COVID-19 pandemic in Europe. Cancers (Basel) 2020;12:1841. doi: 10.3390/cancers12071841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng Y., Lu W., Guo E. Cancer history is an independent risk factor for mortality in hospitalized COVID-19 patients: a propensity score-matched analysis. J Hematol Oncol. 2020;13:75. doi: 10.1186/s13045-020-00907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta V., Goel S., Kabarriti R. Case fatality rate of cancer patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020;10:935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang K., Sheng Y., Huang C. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng S., Huang L., Zhao B. Clinical course of coronavirus disease 2019 in 11 patients after thoracic surgery and challenges in diagnosis. J Thorac Cardiovasc Surg. 2020;160:585–592.e2. doi: 10.1016/j.jtcvs.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogado J., Obispo B., Pangua C. COVID-19 transmission, outcome and associated risk factors in cancer patients at the first month of the pandemic in a Spanish hospital in Madrid. Clin Transl Oncol. 2020;22:2364–2368. doi: 10.1007/s12094-020-02381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu J., Ouyang W., Chua M.L.K., Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6:1108–1110. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai M., Liu D., Liu M. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yarza R., Bover M., Paredes D. SARS-CoV-2 infection in cancer patients undergoing active treatment: analysis of clinical features and predictive factors for severe respiratory failure and death. Eur J Cancer. 2020;135:242–250. doi: 10.1016/j.ejca.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assaad S., Avrillon V., Fournier M.L. High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR. Eur J Cancer. 2020;135:251–259. doi: 10.1016/j.ejca.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garassino M.C., Whisenant J.G., Huang L.C. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D.G. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 16.Knapp G., Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 17.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonomi L., Ghilardi L., Arnoldi E., Tondini C.A., Bettini A.C. A rapid fatal evolution of coronavirus disease-19 in a patient with advanced lung cancer with a long-time response to nivolumab. J Thorac Oncol. 2020;15:e83–e85. doi: 10.1016/j.jtho.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouyang W., Yu J., Zhang J., Xie C. Alert to potential contagiousness: a case of lung cancer with asymptomatic severe acute respiratory syndrome coronavirus 2 infection. J Thorac Oncol. 2020;15:e82–e83. doi: 10.1016/j.jtho.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samson P., Ning M.S., Shaverdian N. Clinical and radiographic presentations of COVID-19 among patients receiving radiation therapy for thoracic malignancies. Adv Radiat Oncol. 2020;5:700–704. doi: 10.1016/j.adro.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suppli M.H., Riisgaard de Blanck S., Elgaard T., Josipovic M., Pøhl M. Early appearance of coronavirus disease 2019 associated pulmonary infiltrates during daily radiotherapy imaging for lung cancer. J Thorac Oncol. 2020;15:1081–1084. doi: 10.1016/j.jtho.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L., Zhu F., Xie L. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H., Xie C., Huang Y. Treatment and outcome of a patient with Lung cancer infected with severe acute respiratory syndrome coronavirus-2. J Thorac Oncol. 2020;15:e63–e64. doi: 10.1016/j.jtho.2020.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guerini A.E., Borghetti P., Filippi A.R. Differential diagnosis and clinical management of a case of COVID-19 in a patient with stage III lung cancer treated with radio-chemotherapy and durvalumab. Clin Lung Cancer. 2020;21:e547–e550. doi: 10.1016/j.cllc.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai Y., Hao Z., Gao Y. Coronavirus disease 2019 in the perioperative period of lung resection: a brief report from a single Thoracic Surgery Department in Wuhan, People’s Republic of China. J Thorac Oncol. 2020;15:1065–1072. doi: 10.1016/j.jtho.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarna L., Cooley M.E., Brown J.K., Chernecky C., Elashoff D., Kotlerman J. Symptom severity 1 to 4 months after thoracotomy for lung cancer. Am J Crit Care. 2008;17:455–468. [PMC free article] [PubMed] [Google Scholar]
- 27.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katal S., Aghaghazvini L., Gholamrezanezhad A. Chest-CT findings of COVID-19 in patients with pre-existing malignancies; a pictorial review. Clin Imaging. 2020;67:121–129. doi: 10.1016/j.clinimag.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qu J., Yang R., Song L., Kamel I.R. Atypical lung feature on chest CT in a lung adenocarcinoma cancer patient infected with COVID-19. Ann Oncol. 2020;31:825–826. doi: 10.1016/j.annonc.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ning M.S., McAleer M.F., Jeter M.D. Mitigating the impact of COVID-19 on oncology: clinical and operational lessons from a prospective radiation oncology cohort tested for COVID-19. Radiother Oncol. 2020;148:252–257. doi: 10.1016/j.radonc.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chuang H.H., Emery D.J., Campbell R.M., Lu Y. FDG PET/CT in diagnosing COVID-19 infection in a cancer patient with exposure history but minimal symptoms. Clin Nucl Med. 2020;45:656–658. doi: 10.1097/RLU.0000000000003137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borczuk A.C., Salvatore S.P., Seshan S.V. COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Mod Pathol. 2020;33:2156–2168. doi: 10.1038/s41379-020-00661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z.L., Hou Y.L., Li D.T., Li F.Z. Laboratory findings of COVID-19: a systematic review and meta-analysis. Scand J Clin Lab Invest. 2020;80:441–447. doi: 10.1080/00365513.2020.1768587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ponti G., Maccaferri M., Ruini C., Tomasi A., Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020;57:389–399. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tavakolpour S., Rakhshandehroo T., Wei E.X., Rashidian M. Lymphopenia during the COVID-19 infection: what it shows and what can be learned. Immunol Lett. 2020;225:31–32. doi: 10.1016/j.imlet.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alkan A., Uncu A., Taşkıran I., Tanrıverdi Ö. Double-edged sword: granulocyte colony stimulating factors in cancer patients during the COVID-19 era. Clinics (Sao Paulo) 2020;75 doi: 10.6061/clinics/2020/e2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sereno M., Gutiérrez-Gutiérrez G., Sandoval C. A favorable outcome of pneumonia COVID 19 in an advanced lung cancer patient with severe neutropenia: is immunosuppression a risk factor for SARS-COV2 infection? Lung Cancer. 2020;145:213–215. doi: 10.1016/j.lungcan.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Figuero-Pérez L., Olivares-Hernández A., Escala-Cornejo R.A., Cruz-Hernández J.J. Management of febrile neutropenia associated with SARS-CoV-2 infection in a patient with cancer. JCO Oncol Pract. 2020;16:348–349. doi: 10.1200/OP.20.00197. [DOI] [PubMed] [Google Scholar]
- 40.Nawar T., Morjaria S., Kaltsas A. Granulocyte-colony stimulating factor in COVID-19: is it stimulating more than just the bone marrow? Am J Hematol. 2020;95:E210–E213. doi: 10.1002/ajh.25870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buglione M., Spiazzi L., Guerini A.E. Two months of radiation oncology in the heart of Italian “red zone” during COVID-19 pandemic: paving a safe path over thin ice. Radiat Oncol. 2020;15:191. doi: 10.1186/s13014-020-01631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo J., Rizvi H., Egger J.V., Preeshagul I.R., Wolchok J.D., Hellmann M.D. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020;10:1121–1128. doi: 10.1158/2159-8290.CD-20-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stroppa E.M., Toscani I., Citterio C. Coronavirus disease-2019 in cancer patients. A report of the first 25 cancer patients in a western country (Italy) Future Oncol. 2020;16:1425–1432. doi: 10.2217/fon-2020-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Q., Chu Q., Zhang H. Clinical outcomes of coronavirus disease 2019 (COVID-19) in cancer patients with prior exposure to immune checkpoint inhibitors. Cancer Commun (Lond) 2020;40:374–379. doi: 10.1002/cac2.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinato D.J., Zambelli A., Aguilar-Company J. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Cancer Discov. 2020;10:1465–1474. doi: 10.1158/2159-8290.CD-20-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinto C., Berselli A., Mangone L. SARS-CoV-2 positive hospitalized cancer patients during the Italian outbreak: the cohort study in Reggio Emilia. Biology (Basel) 2020;9:181. doi: 10.3390/biology9080181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lara Álvarez M., Rogado Revuelta J., Obispo Portero B., Pangua Méndez C., Serrano Montero G., López Alfonso A. Mortalidad por COVID-19 en pacientes con cáncer en un hospital de Madrid durante las primeras 3 semanas de epidemia [COVID-19 mortality in cancer patients in a Madrid hospital during the first 3 weeks of the epidemic] Med Clin (Barc) 2020;155:202–204. doi: 10.1016/j.medcli.2020.05.005. [in Spanish] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma J., Yin J., Qian Y., Wu Y. Clinical characteristics and prognosis in cancer patients with COVID-19: a single center’s retrospective study. J Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robilotti E.V., Babady N.E., Mead P.A. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26:1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rogado J., Pangua C., Serrano-Montero G. COVID-19 and lung cancer: a greater fatality rate? Lung Cancer. 2020;146:19–22. doi: 10.1016/j.lungcan.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jee J., Foote M.B., Lumish M. Chemotherapy and COVID-19 outcomes in patients with cancer. J Clin Oncol. 2020;38:3538–3546. doi: 10.1200/JCO.20.01307. [DOI] [PMC free article] [PubMed] [Google Scholar]