Abstract
Setting:
Peri-urban health facilities providing HIV and TB care in Zambia.
Objective:
To evaluate 1) the impact of Xpert® MTB/RIF on time-to-diagnosis, treatment initiation, and outcomes among adult people living with HIV (PLHIV) on antiretroviral therapy (ART); and 2) the diagnostic performance of Xpert and Determine™ TB-LAM Ag assays.
Design:
Quasi-experimental study design with the first cohort evaluated per standard-of-care (SOC; first sputum tested using smear microscopy) and the second cohort per an algorithm using Xpert as initial test (intervention phase; IP). Xpert testing was provided onsite in Chongwe District, while samples were transported 5–10 km in Kafue District. TB was confirmed using mycobacterial culture.
Results:
Among 1350 PLHIV enrolled, 156 (15.4%) had confirmed TB. Time from TB evaluation to diagnosis (P = 0.018), and from evaluation to treatment initiation (P = 0.03) was significantly shorter for IP than for SOC. There was no difference in all-cause mortality (7.0% vs. 8.6%). TB-LAM Ag showed higher sensitivity with lower CD4 cell count: 81.8% at CD4 < 50 cells/mm3 vs. 31.7% overall.
Conclusion:
Xpert improved time to diagnosis and treatment initiation, but there was no difference in all-cause mortality. High sensitivity of Determine TB-LAM Ag at lower CD4 count supports increased use in settings providing care to PLHIV, particularly with advanced HIV disease.
Keywords: Mycobacterium tuberculosis, HIV, Zambia
Abstract
Contexte :
Structures de santé péri urbaines offrant des soins VIH et TB en Zambie.
Objectif :
Evaluer 1) l’impact du Xpert® MTB/RIF sur le délai de diagnostic, de mise en route du traitement et les résultats chez des PLVIH adultes (personnes vivant avec le VIH) sous traitement antirétroviral (TAR) ; et 2) la performance diagnostique des test Xpert et Determine™ TB-LAM Ag.
Schéma :
Ceci était une étude quasi-expérimentale dont la première cohorte a été évaluée par normes de soins (standard of care [SOC], c’est-à-dire premier crachat testé par microscopie de crachats) et la deuxième cohorte, par un algorithme basé sur l’Xpert comme test initial (phase d’intervention [IP]) L’Xpert était sur place dans le district de Chongwe, tandis que les échantillons ont été transportés à 5–10 km dans le district de Kafue. La TB a été confirmée par culture de mycobactéries.
Résultats :
Parmi 1350 PLVIH enrôlés, 156 (15,4%) ont eu une confirmation de TB. Le délai entre l’évaluation de la TB et le diagnostic (P = 0,018) et entre l’évaluation et la mise en route du traitement (P = 0,03) a été significativement plus court pour IP comparé à SOC. Il n’y a pas eu de différence en termes de mortalité de toutes causes (7,0% contre 8,6%). Determine TB-LAM a montré une sensibilité plus élevée avec un nombre de CD4 plus faible : 81,8% avec CD4 < 50 cellules/mm3 contre 31,7% dans l’ensemble.
Conclusion :
L’Xpert a amélioré le délai de diagnostic et d’initiation du traitement, mais il n’y a pas eu de différence en termes de mortalité de toutes causes. La sensibilité élevée de Determine TB-LAM à des nombres de CD4 plus faibles est en faveur d’une utilisation accrue dans les contextes offrant des soins aux PLVIH, particulièrement en cas de maladie à VIH avancée.
Abstract
Marco de referencia:
Centros periurbanos que prestan servicios contra la infección por el VIH y la TB en Zambia.
Objetivo:
Evaluar: 1) el impacto de la prueba Xpert® MTB/RIF sobre el lapso hasta el diagnóstico y hasta el comienzo del tratamiento y sobre los desenlaces en adultos con infección por el VIH (PLVIH) que reciben tratamiento antirretrovírico (TAR); y 2) el rendimiento diagnóstico de las pruebas Xpert and Determine™ TB-LAM Ag.
Método:
Fue este un estudio semiexperimental con una primera cohorte evaluada según las normas de referencia (SOC, examen de la primera muestra de esputo mediante microscopia) y la segunda cohorte, siguiendo un algoritmo con la prueba Xpert como examen inicial (IP, fase de intervención). La prueba Xpert se realizó localmente en el Distrito Chongwe y en el Distrito Kafue las muestras se trasportaron de 5–10 km. La confirmación de la TB se obtuvo mediante el cultivo de micobaterias.
Resultados:
De las 1350 PLVIH inscritas, en 156 (15,4%) se confirmó la TB. El lapso entre la evaluación por TB y el diagnóstico (P = 0,018) y entre la evaluación y el comienzo del tratamiento (P = 0,03) fue significativamente más corto en el grupo IP que en el grupo SOC. No se observó ninguna diferencia con respecto a la mortalidad por todas las causas (7,0% contra 8,6%). La prueba Determine TB-LAM ofreció una sensibilidad más alta cuando la cifra de linfocitos CD4 era más baja: 81,8% con < 50 células CD4/mm3, contra 31,7% en general.
Conclusión:
La prueba Xpert mejoró el lapso hasta el diagnóstico y el comienzo del tratamiento, pero no modificó la mortalidad por todas las causas. La sensibilidad alta de la prueba Determine TB-LAM con una cifra inferior de linfocitos CD4 respalda el incremento de su utilización en los entornos que prestan atención a las PLVIH, en especial en los casos de enfermedad avanzada por el VIH.
Despite progress in new diagnostics for TB, the disease burden remained relatively unchanged in 2018, with an estimated 10 million new cases worldwide, 0.8 million of which were people living with HIV (PLHIV). There were 1.45 million TB deaths, of which 251 000 occurred among PLHIV,1 with a disproportionate burden among sub-Saharan African countries. In Zambia, TB incidence in 2019 was 333 per 100000 population2 and adult HIV prevalence is 12.4%;3 59% of TB patients had HIV co-infection.4
Diagnosis of TB in PLHIV is challenging due to atypical presentation and low sensitivity and specificity of standard TB diagnostics such as sputum smear microscopy and chest X-ray, (CXR), respectively. The focus recently has been on near point-of-care tools for these settings.5 Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA) provides rapid turnaround time and determination of rifampin resistance in less than 2 hours.6–8
In 2010, the WHO recommended that Xpert be used as the initial diagnostic test in PLHIV. Xpert is more sensitive than smear microscopy.9 However, studies in smear-negative PLHIV have found sensitivities as low as 43–53% in Zambia10,11 and South Africa.12 Moreover, in some settings, the use of Xpert did not reduce TB-related morbidity, attributed in part to high levels of empirical TB treatment.13,14 Xpert has potential to address many challenges to TB management, but gaps in performance data persist.
Determine™ TB-LAM Ag (Abbott Laboratories, Chi-cago, IL, US) is a point-of-care (POC) test for detection of lipoarabinomannan (LAM) in urine, shown to have greatest sensitivity for TB diagnosis in advanced immunodeficiency (CD4 cell count < 50 cells/mm3).15 Results are available within 30 min. The test was endorsed by the WHO in 2015, with guidance to include outpatient settings.16 However, limited data are available on use in outpatient settings, and its role in Zambia is yet to be defined.
In the present study, we assessed the performance of a diagnostic algorithm using Xpert compared to the standard-of-care (SOC) algorithm according to Zambia National TB Programme (NTP). We also evaluated the diagnostic performance of Xpert and the TB-LAM Ag assay compared to mycobacterial culture.
METHODS
Study setting
The study was conducted at two sites in Kafue (Nangongwe Clinic and Kafue District Hospital) and at the Chongwe District Health Clinic. The population of Kafue District per the 2010 census is 219 000; the population of Chongwe District is 179 000. Selection of the sites was purposeful and was based on their representation of typical Zambian health facilities. Both sites provide adult HIV management, including laboratory services, and face challenges appropriate for assessing implementation of Xpert scale-up.
Study population and eligibility criteria
Adult PLHIV (age > 15 years) who were newly enrolled or already on antiretroviral therapy (ART), and had presumptive TB based on Zambian NTP guidelines were eligible to participate in the study. We defined presumptive TB as presence of one or more TB symptoms: cough of any duration, fever, night sweats, or weight loss.17 We also included in the definition of presumptive TB all newly diagnosed PLHIV whether or not they had TB symptoms.18 We collected information on basic demographics; viral load testing was not routinely available and we did not collect details of the ART regimen. ART initiation was not dependent on TB evaluation. All participants provided written informed consent.
Study design
We used a quasi-experimental ‘before-after’ study design with two phases (Figure 1). The duration of each phase was approximately 6 months, or until the target culture positivity rate (i.e., 20 adults per phase per site) was reached. A modified Rao-Scott likelihood ratio χ2 calculated using SAS PROC SURVEYFREQ (SAS Institute, Cary, NC, USA) indicated that a sample size of 20 culture-positive results per site per phase would provide adequate study power.
FIGURE 1.
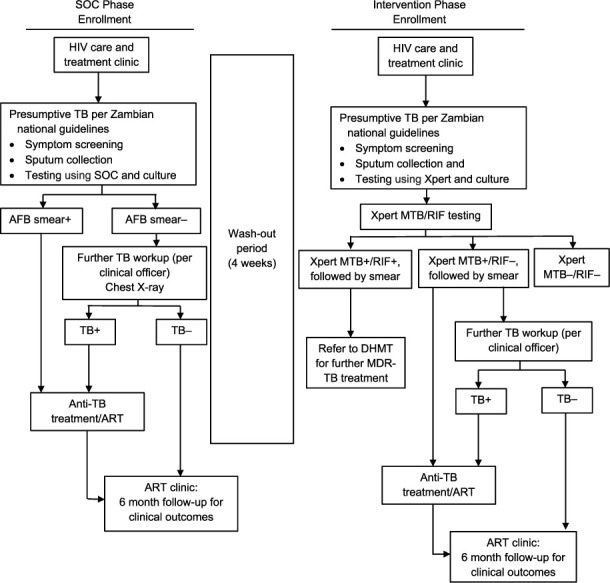
Study design schema. SOC = standard of care; AFB = acid-fast bacilli; MTB = M. tuberculosis; RIF = rifam-pin; + = positive; − = negative; ART = antiretroviral therapy; DHMT = District Health Management Team; MDR-TB = multidrug-resistant tuberculosis.
Each site initially implemented the SOC phase, in which a cohort of PLHIV with presumptive TB were evaluated for TB according to current NTP guidelines. SOC assessment was submission of three sputum samples and CXR, if indicated. Direct smear microscopy for acid-fast bacilli (AFB) was performed on one sputum specimen, the second was culture tested, and another sample was stored in case of culture contamination.
There was a 4-week ‘wash-out’ period at the end of SOC, defined as the period between the two study phases, to allow completion of the diagnostic work-up of all participants recruited during the SOC phase. The SOC phase was followed by the Intervention Phase (IP), when a second cohort of adult PLHIV with presumptive TB was evaluated for TB using Xpert. Three sputum specimens were submitted: the first specimen was tested using Xpert. If Xpert was positive, sputum smear microscopy was performed for national reporting classification and determination of sputum conversion during treatment. The second specimen, regardless of Xpert result, was used to confirm diagnosis using culture. The third sputum was stored in case of errors or contamination with Xpert or culture, and for resistance confirmation if detected. In both phases, liquid (BACTEC™ MGIT™960; BD, Sparks, MD, USA) and solid (Löwenstein-Jensen) mycobacterial culture were performed as the reference standard. Participants submitted a urine sample for testing with the TB-LAM Ag assay using standard laboratory protocols and quality assurance procedures.
Data analysis
The primary outcome measure was the proportion of participants receiving appropriate treatment within 14 and 28 days of evaluation, using the Xpert algorithm compared to SOC. ‘Appropriate treatment’ was defined as initiation of anti-TB treatment within 4 weeks of screening initiation in culture-positive patients and correct diagnosis of not having TB in culture-negative patients. We also compared the median time from TB evaluation to TB treatment initiation between SOC and IP.
Secondary outcome measures included time from TB evaluation to diagnosis, and time from diagnosis to TB treatment initiation by study phase. We constructed Kaplan-Meier curves and used the Wilcoxon test to compare equality of survival functions in the first 100 days of evaluation and diagnosis by study phase. The Wilcoxon test was used because the proportional hazard function assumptions was not satisfied.19 We also compared TB treatment outcomes (i.e., cure, treatment completion, loss to follow-up [LTFU], and mortality) between SOC and IP using chart reviews. The association between the study phase and treatment outcomes was tested using a χ2 test.
Diagnostic performance of Xpert and SOC was assessed by computing the sensitivity and specificity of the following MTB (M. tuberculosis) testing combinations: SOC Arm (any positive result on smear and/or history + physical examination and/or CXR that resulted in the initiation of anti-TB treatment), Xpert Arm (any positive result on Xpert and/or smear and/or history + physical examination and/or CXR that resulted in the initiation of anti-TB treatment), Xpert alone, Xpert in smear-negatives and Xpert in smear-positives.
To assess TB-LAM Ag within the TB diagnostic algorithm, we measured the sensitivity and specificity stratified by CD4 count, and measured the sensitivity when used alone or in combination with smear and/or Xpert, compared to both solid and liquid culture.
All statistical analyses were performed using a standard software package (Stata v15.0; (StataCorp, College Station, TX, USA). P < 0.05 was considered statistically significant.
Ethical approval
This protocol was approved by the Institutional Review Boards at the University of Zambia Biomedical Research Ethics Committee (Lusaka, Zambia), the University of Alabama at Birmingham (Birmingham, AL, USA), and the University of North Carolina at Chapel Hill (Chapel Hill, NC, USA). The study was also approved by the US Centers for Disease Control and Prevention (Atlanta, GA, USA) and the Zambian Ministry of Health National Health Research Authority (Lusaka, Zambia).
RESULTS
From 2013 to 2015, we enrolled 1350 adult PLHIV: 752 in the SOC and 598 in IP arms. Only those with valid culture results were included in the analysis: 522 in the SOC phase, of whom 86 (16.5%) had culture-confirmed TB, and 493 in the IP, of whom 70 (14.2%) had culture-confirmed TB (Figure 2). The median age was 37 years (interquartile range [IQR] 31–45) and 36 years (IQR 30–45), with 44.6% and 49.7% males in the SOC and IP arms, respectively. Kafue Sites enrolled 314 (60.2%) and 350 (71.0%) participants in the SOC and IP arms, respectively. Most of the participants had CD4 3 200 cells/mm3 (Table 1)
FIGURE 2.
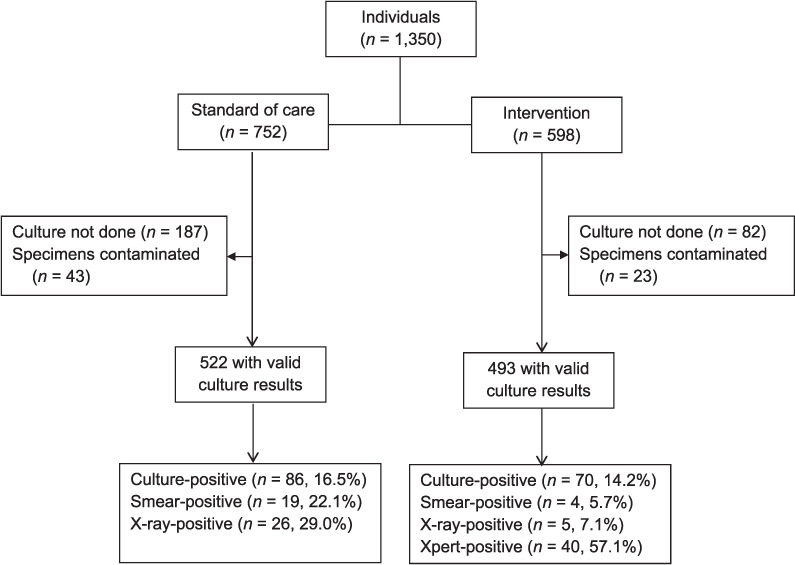
Study enrollment and evaluation flow chart.
TABLE 1.
Baseline characteristics of participants with valid culture results
| Standard of care (n = 522) n (%) | Intervention (n = 493) n (%) | |
|---|---|---|
| Age, years, median [IQR] | 37 [31–45] | 36 [30–45] |
| 15–19 | 16 (3.1) | 20 (4.1) |
| 20–39 | 311 (59.6) | 285 (57.8) |
| 40–59 | 175 (33.5) | 168 (34.1) |
| ⩾60 | 20 (3.8) | 20 (4.1) |
| Sex | ||
| Male | 233 (44.6) | 245 (49.7) |
| Female | 289 (55.4) | 248 (50.3) |
| Site | ||
| Kafue District | 314 (60.2) | 350 (71.0) |
| Chongwe District | 208 (39.8) | 143 (29.0) |
| CD4 count, cells/mm3 | ||
| <50 | 30 (5.7) | 30 (6.1) |
| 50–100 | 30 (5.7) | 41(8.3) |
| 100–150 | 42 (8) | 40 (8.1) |
| 150–200 | 38 (7.3) | 34 (6.9) |
| ⩾200 | 300 (57.5) | 261 (52.9) |
| Missing | 82 (15.7) | 87 (17.6) |
Among those diagnosed with TB by any means, 46.3% and 65.6% of SOC participants vs. 63.3% and 75.6% of IP participants were initiated on TB treatment at 14 and 28 days, respectively. The median time to treatment initiation was 16 days (range 5–43) for the SOC arm and 6 days (range 1–28) for the IP arm. TB treatment completion was 29.1% vs. 18.6%, and cured was 27.9% vs. 45.7% in SOC vs. IP, respectively. There was no difference in all-cause mortality between arms: 7.0% in SOC vs. 8.6% IP (Table 2).
TABLE 2.
TB treatment initiation (including those who had valid culture results) and treatment outcome among those with culture-positive result by arm
| Standard of care (n = 93) n (%) | Intervention (n = 90) n (%) | Total n (%) | P value | |
|---|---|---|---|---|
| On TB treatment by 14 days | ||||
| No | 50 (53.7) | 33 (36.7) | 83 (45.4) | |
| Yes | 43 (46.3) | 57 (63.3) | 100 (54.6) | 0.02*† |
| On TB treatment by 28 days | ||||
| No | 32 (34.4) | 22 (24.4) | 54 (29.5) | |
| Yes | 61 (65.6) | 68 (75.6) | 129 (70.5) | 0.14*‡ |
| Time to TB treatment start, days, median [IQR] | 16 [5–43] | 6 [1–28] | 11 [2–39] | |
| TB treatment outcomes | (n = 86) | (n = 70) | (n = 156) | 0.10* |
| Completed | 25 (29.1) | 13 (18.6) | 38 (24.1) | |
| Cured | 24 (27.9) | 32 (45.7) | 56 (35.8) | |
| Died | 6 (7.0) | 6 (8.6) | 12 (8.0) | |
| Lost to follow-up | 31 (36.0) | 19 (27.1) | 50 (32.1) |
*Pearson’s χ2 test.
†Statistically significant.
‡Kruskal-Wallis test.
IQR = interquartile range.
We found a significantly shorter time from TB evaluation to diagnosis (P = 0.018), TB diagnosis to treatment initiation (P = 0.03) and overall time from TB evaluation to treatment initiation (P < 0.01) in the IP than in the SOC phase (Figures 3 and 4). When stratified by site, Chongwe clinic (with onsite Xpert) had a significantly shorter time (P = 0.04) from evaluation to diagnosis, but not from diagnosis to treatment (P = 0.12).
FIGURE 3.
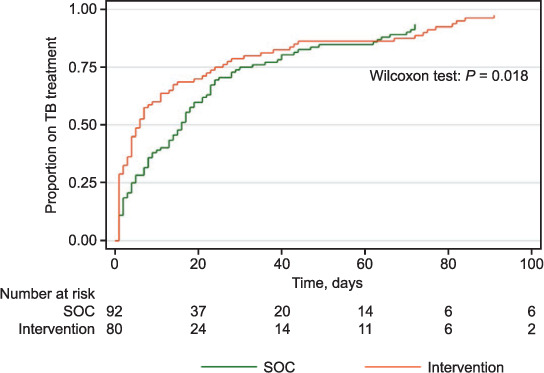
Time from TB evaluation to TB diagnosis. SOC = standard of care.
FIGURE 4.
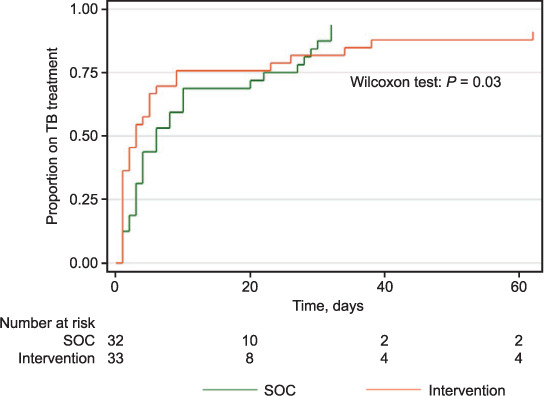
Time from TB diagnosis to TB treatment initiation. SOC = standard of care.
We assessed the diagnostic performance of Xpert compared to culture (Table 3). Overall, the sensitivity of Xpert was 60.3% (95% confidence interval [CI] 48.4–71.1) and specificity was 99.0% (95%CI 97.4–99.6). Among smear-negative patients, the sensitivity and specificity of Xpert was 50.0% (95%CI 36.6–63.4) and 99.0% (95%CI 97.4–99.6); among smear-positive patients, the sensitivity was 81.3% (95%CI 57.0–93.4).
TABLE 3.
Sensitivity and specificity of various TB testing algorithms
| Test-positive, culture-positive n | Test-positive, culture-negative n | Test-negative, culture-positive n | Test-negative, culture-negative n | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|
| SOC Arm* | 44 | 14 | 42 | 422 | 51.2 (40.8–61.4) | 96.8 (94.7–98.1) | 75.9 (63.5–85.0) | 90.9 (88.0–93.2) |
| Xpert Arm† | 49 | 25 | 21 | 362 | 70.0 (58.5–79.5) | 93.5 (90.6–95.6) | 66.2 (54.9–76.0) | 94.5 (91.8–96.4) |
| Xpert alone | 41 | 4 | 27 | 381 | 60.3 (48.4–71.1) | 99.0 (97.4–99.6) | 91.1 (79.3–96.4) | 93.4 (90.5–95.4) |
| Xpert in smear-negative | 25 | 4 | 24 | 381 | 50.0 (36.6–63.4) | 99.0 (97.4–99.6) | 86.2 (69.4–94.5) | 93.9 (91.1–95.8) |
| Xpert in smear-positive | 13 | 0 | 3 | 0 | 81.3 (57.0–93.4) | — | 100.0 (77.2–100.0) | 0.0 (0–56.1) |
* Any positive result on smear and/or history and physical examination and/or CXR that resulted in the initiation of anti-TB treatment.
† Any positive result on Xpert and/or smear and/or history and physical examination and/or CXR that resulted in the initiation of anti-TB treatment.
SOC = standard of care; PPV = positive predictive value; NPV = negative predictive value; CXR = chest X-ray.
The overall sensitivity and specificity of the SOC Arm (any positive result on smear and/or history + physical examination and/or CXR that resulted in the initiation of anti-TB treatment) was 51.2% (95%CI 40.8–61.4) and 96.8% (95%CI 94.7–98.1), respectively. The overall sensitivity and specificity of the IP Arm (any positive result on Xpert and/or smear and/or history + physical examination and/or CXR that resulted in the initiation of anti-TB treatment) was 70.0% (95%CI 58.5–79.5) and 93.5% (95%CI 90.6–95.6), respectively.
The overall sensitivity of TB-LAM Ag compared to culture was 31.7% (95%CI 24.6–39.7). When stratified by CD4 cell count, among those with 350 cells/mm3, the sensitivity and specificity were 81.8% (95%CI 52.3–94.9) and 81.8% (95%CI 68.0–90.5), respectively.
DISCUSSION
Zambia is scaling up Xpert but gaps about its performance in routine care persist. We evaluated the performance of the Xpert diagnostic algorithm. We found that the proportion of participants diagnosed with TB and on appropriate treatment within 14 and 28 days of evaluation initiation was higher in the IP than in SOC. However, there was no difference in all-cause mortality. The overall sensitivity and specificity of Xpert was similar to findings in other studies; TB-LAM Ag showed better performance in samples with lower CD4+ strata.
Our findings on the diagnostic performance of Xpert are comparable to other research findings in high TB-HIV settings, demonstrating an increase in the proportion of those correctly diagnosed with TB and initiating appropriate treatment.7,12,20,21 This impact can be attributed to the higher sensitivity of Xpert for MTB compared to smear microscopy,12 and the more rapid turnaround time that can inform same-day treatment decisions at POC.22 There are divergent findings, too. For example, in an evaluation of centralized Xpert screening, Mpfumi et al. did not find an increase in the proportion of those correctly diagnosed with TB and initiating treatment, nor a reduction in time to diagnosis and treatment; this was explained as being probably due to high levels of empiric treatment that muted the benefit of Xpert.23
In our study, when analysis for ‘evaluation to diagnosis’ and ‘diagnosis to treatment’ was stratified by site, Chongwe clinic, with on-site Xpert, had a significantly shorter time for ‘evaluation to diagnosis’ than the Kafue sites, where Xpert was in a central location. These findings are consistent with other studies,24–26 and highlight the importance of near POC TB diagnostics to optimize turnaround time of results and reduce LTFU. However, ‘diagnosis to treatment initiation’ was not different between the two sites. The reason for this is that the ‘diagnosis to treatment’ time period includes both patient and health system delays, resulting in the loss of the benefit of rapid turnaround time of on-site Xpert. Maximum benefit from Xpert testing will require not only instrument placement at POC, but also the addressing of health systems inefficiencies.
For TB treatment outcomes, all-cause mortality was similar in both arms. This finding is similar to other studies,14,27 and may reflect the high rate of empiric treatment in the SOC Arm and a reduction in empiric treatment when Xpert is included in the algorithm. It may also relate to the fact that clinicians were not blinded to IP Arm and knew that the predictive value of a negative test (smear) was lower in the SOC Arm than in the IP Arm.13,28
Our findings on the diagnostic performance of Xpert were similar to what has previously been shown when a single test is performed in smear-negative, HIV-infected patients (sensitivity 43.4–67%).10,12,14 LAM sensitivity was highest in the lowest CD4 cell count stratum. The interpretation of LAM performance in other CD4 strata was hindered by small numbers, but findings are consistent with previous studies in outpatient populations,15 showing limited usefulness of LAM with CD4 counts > 50 cells/mm3.
A major strength of this study is the relevance of this setting, a typical clinic in peri-urban Zambia, where outcomes were affected by patient and health system factors. In addition, the use of LAM is highly relevant, as its use is increasing. However, it is worthwhile to note the challenges in setting up and running Xpert testing in this setting. Resource considerations include infrastructure renovations, air conditioning, and provision of stable power supply. Performance may be affected by environmental conditions, such as high temperatures and dust.
We recognize the following limitations. The study was in out-patient settings where benefits of Xpert may be less apparent because inpatients who were more ill were excluded. In addition, some outpatients with advanced HIV disease may have been more likely to receive empiric treatment. We acknowledge high rates of unavailable, contaminated or not-done culture results, as well as loss to follow-up. The before-and-after design could have led to confounding by changes in practice and the non-randomized sample design could have biased our results due to non-random factors. As clinicians were not blinded, they may have had a higher probability for diagnosing TB in the SOC Arm.
In conclusion, use of Xpert in PLHIV with presumptive TB provided benefits over SOC. Xpert improved time to diagnosis and treatment initiation, but there was no difference in all-cause mortality. Empiric treatment is an important consideration and outcomes depend both on the diagnostic tool and the nature of the health system. The high sensitivity of TB-LAM Ag at lower CD4 counts supports the increased use of the test, which should be incorporated in outpatient settings.
ACKNOWLEDGEMENTS
The authors thank the study team for their tireless efforts in data collection, record abstraction, participant recruitment, and follow-up; and the participants who consented to participate in this study.
This research has been supported in part by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of grant 1U01 GH000486.
Footnotes
DISCLAIMER
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the funding agencies. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest: none declared.
References
- 1.World Health Organization Global tuberculosis report, 2018. WHO/CDS/TB/2018.20. Geneva, Switzerland: WHO; 2018. [Google Scholar]
- 2.World Health Organization Global tuberculosis report, 2019. WHO/CDS/TB/2019.15. Geneva, Switzerland: WHO; 2019. [Google Scholar]
- 3.WHO/CDS/TB/2019.15. Geneva, Switzerland: WHO; 2019. [Google Scholar]
- 4.UNAIDS Country report: Zambia 2016. Geneva, Switzerland: UNAIDS; 2016. www.unaids.org/en/regionscountries/countries/zambia. [Google Scholar]
- 5.World Health Organization TB Country Profile, 2017. Geneva, Switzerland: WHO; 2017. https://extranet.who.int/.../Reports?...=/WHO_HQ_Reports/G2/PROD/EXT/TBCountry [Google Scholar]
- 6.Pathmanathan I, Date A, Coggin WL, Nkengasong J, Piatek AS, Alexander H. Rolling out Xpert® MTB/RIF for TB detection in HIV-infected populations: an opportunity for systems strengthening. Afr J Lab Med. 2017;6(2):460. doi: 10.4102/ajlm.v6i2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joshi B, et al. The implementation of Xpert MTB/RIF assay for diagnosis of tuberculosis in Nepal: A mixed-methods analysis. PLoS One. 2018;13(8):e0201731. doi: 10.1371/journal.pone.0201731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theron G, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014;383(9915):424–435. doi: 10.1016/S0140-6736(13)62073-5. [DOI] [PubMed] [Google Scholar]
- 9.Creswell J, et al. Introducing new tuberculosis diagnostics: the impact of Xpert((R)) MTB/RIF testing on case notifications in Nepal. Int J Tuberc Lung Dis. 2015;19(5):545–551. doi: 10.5588/ijtld.14.0775. [DOI] [PubMed] [Google Scholar]
- 10.Steingart KR, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2013;(1):CD009593. doi: 10.1002/14651858.CD009593.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henostroza G, et al. High prevalence of tuberculosis in newly enrolled HIV patients in Zambia: need for enhanced screening approach. Int J Tuberc Lung Dis. 2016;20(8):1033–1039. doi: 10.5588/ijtld.15.0651. [DOI] [PubMed] [Google Scholar]
- 12.Henostroza G, et al. The high burden of tuberculosis (TB) and human immunodeficiency virus (HIV) in a large Zambian prison: a public health alert. PLoS One. 2013;8(8):e67338. doi: 10.1371/journal.pone.0067338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawn SD, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLoS Med. 2011;8(7):e1001067. doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theron G, Peter J, Dowdy D, Langley I, Squire SB, Dheda K. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? Lancet Infect Dis. 2014;14(6):527–532. doi: 10.1016/S1473-3099(13)70360-8. [DOI] [PubMed] [Google Scholar]
- 15.Auld AF, Fielding KL, Gupta-Wright A, Lawn SD. Xpert MTB/RIF—why the lack of morbidity and mortality impact in intervention trials? Trans R Soc Trop Med Hyg. 2016;110(8):432–444. doi: 10.1093/trstmh/trw056. [DOI] [PubMed] [Google Scholar]
- 16.Lawn SD, Kerkhoff AD, Vogt M, Wood R. HIV-associated tuberculosis: relationship between disease severity and the sensitivity of new sputum-based and urine-based diagnostic assays. BMC Med. 2013;11:231. doi: 10.1186/1741-7015-11-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization Lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis of active TB in people living with HIV: Policy Update. Geneva, Switzerland: WHO; 2019. [Google Scholar]
- 18.World Health Organization Definitions and reporting framework for tuberculosis—2013 revision. Geneva, Switzerland: WHO; 2013. [Google Scholar]
- 19.Zambia National TB Control Programme Managing tuberculosis in the HIV setting in Zambia. Lusaka, Zambia: Zambia NTP; 2014. [Google Scholar]
- 20.Tarone RE, Ware J. On distribution-free tests for equality of survival distributions. Biometrika. 1977;64(1):156. [Google Scholar]
- 21.Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;(1):CD009593. doi: 10.1002/14651858.CD009593.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naidoo P, et al. A comparison of multidrug-resistant tuberculosis treatment commencement times in MDRTBPlus line probe assay and Xpert® MTB/RIF-based algorithms in a routine operational setting in Cape Town. PLoS One. 2014;9(7):e103328. doi: 10.1371/journal.pone.0103328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon C, et al. Impact of Xpert MTB/RIF testing on tuberculosis management and outcomes in hospitalized patients in Uganda. PLoS One. 2012;7(11):e48599. doi: 10.1371/journal.pone.0048599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mupfumi L, et al. Impact of Xpert MTB/RIF on antiretroviral therapy-associated tuberculosis and mortality: a pragmatic randomized controlled trial. Open Forum Infect Dis. 2014;1(1):ofu038. doi: 10.1093/ofid/ofu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanrahan CF, et al. The patient impact of point-of-care vs. laboratory placement of Xpert((R)) MTB/RIF. Int J Tuberc Lung Dis. 2015;19(7):811–816. doi: 10.5588/ijtld.15.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanrahan CF, et al. Time to treatment and patient outcomes among TB suspects screened by a single point-of-care Xpert MTB/RIF at a primary care clinic in Johannesburg, South Africa. PLoS One. 2013;8(6):e65421. doi: 10.1371/journal.pone.0065421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lessells RJ, Cooke GS, McGrath N, Nicol MP, Newell ML, Godfrey-Faussett P. Impact of point-of-care Xpert MTB/RIF on tuberculosis treatment initiation. A cluster-randomized trial. Am J Respir Crit Care Med. 2017;196(7):901–910. doi: 10.1164/rccm.201702-0278OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agizew T, et al. Treatment outcomes, diagnostic and therapeutic impact: Xpert vs. smear. A systematic review and meta-analysis. Int J Tuberc Lung Dis. 2019;23(1):82–92. doi: 10.5588/ijtld.18.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]


