ABSTRACT
Synthesis of extracellular adenosine by the ectonucleotidases CD39 and CD73 represents an important pathway of immune suppression in the tumor microenvironment. Using two mouse models (RET transgenic melanoma and Panc02 orthotopic pancreatic adenocarcinoma), we identified an elevated frequency of ectonucleotidase-expressing T cells in tumors and spleens. Importantly, these ectonucleotidase-positive T cells also showed a pronounced expression of PD-1. Conversely, the PD-1+ T cell subsets in tumors contained substantially larger proportions of ectonucleotidase-expressing cells compared to their counterparts lacking PD-1 expression. Our in vitro experiments showed that the activation of normal T cells resulted in an increase in the CD39 expression. CD39+ and CD73+ T cells displayed effector or memory phenotypes and produced IFN-γ, thereby linking ectonucleotidase expression to T cell effector functions. An accumulation of conventional and regulatory T cells expressing CD39 and/or CD73 was also detected in the peripheral blood of patients with melanoma and pancreatic cancer. Moreover, we demonstrated a significant association between low frequencies of circulating CD73+CD8+ T cells and CD73+CD4+ regulatory T cells and better overall survival of melanoma patients. Tumor-derived soluble factors (in particular, TGF-β) significantly enhanced the frequencies of ectonucleotidase-expressing cells in mice. Our findings suggest that the upregulation of ectonucleotidase expression in T cells promotes extracellular adenosine accumulation and represents an important mechanism of homeostatic immune auto-regulation, which could be hijacked by tumors to evade anti-cancer immunity. Targeting CD39 and CD73 can open new avenues for cancer immunotherapy.
KEYWORDS: Melanoma, pancreatic adenocarcinoma, ectonucleotidases, adenosine, effector T cells, immunosuppression
Introduction
Tumors often attract numerous immune cells that could recognize and destroy cancer cells, yet the development of chronic inflammation, establishment of immunosuppressive networks and fierce metabolic competition hinder tumor eradication.1–3 The immune system deploys a variety of regulatory mechanisms to restrain excessive responses against pathogens or prevent harmful autoimmune reactions.4 However, these mechanisms can be hijacked by tumors to subvert anti-cancer immunity or even involve immune cells in promoting tumor growth, angiogenesis, and metastasis.4–6 The outcome of tumorigenesis is thus largely determined by the balance between anti-tumor immunity and numerous mechanisms of immune escape developed by the tumor.7 Therefore, studying such mechanisms is essential for the establishment of new effective therapies against cancer. Over the last years, insights into the role of immunological checkpoints in cancer and the mechanisms of immune suppression in the tumor microenvironment have led to the development of novel immunotherapeutic strategies that can significantly extend the survival of cancer patients.4,8,9
Extracellular adenosine has been identified as one of the main immunosuppressive factors in the tumor microenvironment, orchestrating the activity of multiple immune cell subsets.10–13 Acting through specific G-protein-coupled receptors, adenosine exerts inhibitory effects on the effector arm of the immune system, while enhancing the activity of regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSCs).10,11,14 These effects of adenosine are mostly dependent on activation of adenylate cyclase followed by an enhanced production of cyclic AMP (cAMP).14 Adenosine is produced through the two-step hydrolysis of extracellular ATP by the cell-surface ectonucleotidases CD39 and CD73 detected in various populations of immune cells as well as in tumor cells and contributing to the immune suppression in the tumor microenvironment.10–14
It was previously shown that the vast majority of murine Treg express CD39 and CD73 at high levels.11 Moreover, adenosine was reported to strongly contribute to Treg immunosuppressive properties by triggering cAMP synthesis in effector (non-regulatory) T cells through the activation of A2a adenosine receptors.12–15 Although the expression of CD39 and CD73 has also been reported in non-regulatory T cells,16–21 a possible role of these enzymes in the function of Foxp3− effector T cells needs further detailed investigation.
In the current study, we explored the patterns of ectonucleotidase expression in various immune cell subpopulations with regards to their phenotype and function under physiological conditions and in cancer. We demonstrated an increased frequency of tumor-infiltrating T cells expressing ectonucleotidases in two preclinical mouse models suggesting an accumulation of adenosine in the tumor microenvironment. Moreover, ectonucleotidase expression was upregulated in T cells characterized by the effector or memory phenotype and enhanced IFN-γ production. Interestingly, tumor-derived soluble factors, such as TGF-β, significantly increased the frequencies of ectonucleotidase-expressing T cells in mice but not in human. A deeper understanding of the mechanisms controlling adenosine production and signaling in tumor-infiltrating T cells will help to develop novel strategies of cancer immunotherapy based on the targeting of adenosine metabolism.
Materials and methods
Patients
The final protocol was approved by the ethics committee of the University of Heidelberg, Medical School, Heidelberg, Germany (S-230/2011 for pancreatic adenocarcinoma (PDAC) patients and 2010–318 N-MA for melanoma patients). Informed consent was obtained from all patients included in the study.
Mice
C57BL/6 mice expressing the human RET oncogene in melanocytes (RET-tg mouse model)22 were provided by I. Nakashima (Chubu University, Aichi, Japan). Non-transgenic littermates were used as controls. In the model of PDAC, highly tumorigenic syngeneic murine Panc02 PDAC cells were injected into the head of the pancreas, producing rapidly growing tumors starting from day 5 after transplantation.23 C57BL/6 mice were purchased from Charles River (Germany). Mice were kept under pathogen-free conditions in the animal facility of the German Cancer Research Center (DKFZ, Heidelberg) or Heidelberg University. Animal experiments were performed in accordance with government and institute guidelines and regulations.
Reagents and antibodies
Anti-human CD3e-V500, CD4-APC-H7, CD8-APC-H7, CD25-PE, CD39-PE (clone TU66), CD73-APC (clone AD2), PD1-PE-Cy7 (clone EH12.1), and CD127-PerCP-Cy5.5 antibodies were purchased by BD Biosciences (Germany), anti-human FOXP3-FITC antibody was from eBioscience (Germany). Anti-mouse CD3e-PerCP-Cy5.5, -APC, -V500 and -V450; CD4-APC, -BV421, -APC-Cy7 and -APC-H7; CD8a-APC-Cy7, -PE-Cy7 and -APC-H7; CD25-APC, -APC-Cy7 and -V450; CD44-PE; CD45-V500; CD45RB-PE; CD62 L-APC; CD69-APC; CD73-BV605 (clone TY/11.8) and PD1-PerCP-Cy5,5 (clone J43) were purchased by BD Biosciences (Germany). Purified anti-mouse CD3e and CD28, as well as FOXP3-FITC, CD73- eFluor® 450, -PE (clone TY/11.8 for both) and CD39-PE-Cy7, -PE (clone DM24S1 for both) were from eBioscience (Germany). BSA, Trypan blue, PMA, and Ionomycin were obtained from Sigma (Germany). Phosphate buffer saline (PBS), RPMI-1640 with L-glutamine and Penicillin-Streptomycin were purchased by PAA (Germany). Fetal bovine serum (FBS) was from PAN Biotech (Germany). Brefeldin A was purchased by BD Biosciences (Germany). Foxp3/Transcription Factor Staining Buffer Set and Permeabilization Buffer (10X) were obtained from eBioscience (Germany). Red Blood Cell (RBC) Lysis Buffer was from Biolegend (Germany).
In vitro splenocyte activation
Microwells of the assay plate were coated with anti-CD3 antibodies. To this end, a 10 μg/ml solution of anti-CD3 antibodies was dispensed into each well of the assay plate. The plate was tightly covered with Parafilm™ to avoid sample evaporation and incubated at 37°C for 2 hours. Immediately before adding cells, the antibody solution was removed and the wells were rinsed with sterile PBS. Single-cell suspensions of splenocytes were centrifuged and resuspended in the complete RPMI-1640 medium at 106 cells/ml. Then, 200 μL of the cell suspension were added to each well, and the plate was placed in a humidified 37°C, 5% CO2 incubator. Then, soluble anti-CD28 antibodies were added to the cells at final concentration of 2 µg/ml. The kinetics of ectonucleotidase expression on splenocytes was analyzed by flow cytometry at various time points (6, 12, 24, 48, and 72 hours) after the beginning of activation.
Flow cytometry
Single-cell suspensions of tumors or lymphoid organs were prepared by mechanical dissociation, followed by filtering through the 40 µM cell strainer (BD Falcon). Suspensions from tumors, bone marrow (BM), and spleens were subsequently depleted of erythrocytes by ammonium chloride lysis and then incubated with Fc-blocking reagent and mAbs for 30 min at 4°C. For intracellular staining, cells were treated with the Foxp3 Fixation/Permeabilization solution for 30 min, washed and stained with fluorescently labeled antibodies against intracellular antigens. After 30 min of incubation, cells were washed twice with the Stain Buffer, resuspended, and analyzed by flow cytometry. Acquisition was performed by flow cytometry using FACSCantoII with FACSDiva 6.0 software (BD Biosciences). FlowJo software 7.6.1 (Tree Star) was used to analyze at least 500,000 events.
Analysis of IFN-γ production by T cells
Cells were stimulated with 5 ng/ml phorbol 12-myristate 13-acetate, 1 mM ionomycin, in the presence of the protein transport inhibitor GolgiStop at final concentration of 0.6 µl/ml. After 5 hours of incubation, cells were stained with mAbs against surface markers followed by fixation/permeabilization and intracellular staining for IFN-γ as described above.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, Inc.). The paired t-test and ANOVA-2 test were applied to analyze the statistical significance where appropriate. Estimates of overall survival according to Kaplan–Meier were described together with 95% confidence intervals (95% CI) and compared using log-rank tests. Follow-up time was defined from the date of blood draw for the analyzed sample to the date of death. The frequencies of ectonucleotidase-positive cells within total CD8+ T cells or CD4+ Treg at the date of blood draw were dichotomized according to the median values of their distribution.24 A p-value < 0.05 was considered significant. Values were expressed as mean ± SEM. Correlation analyses of nonparametric data were conducted using the Spearman correlation coefficient (r).
Results
Enhanced ectonucleotidase expression in non-regulatory tumor-infiltrating T cells
To study the function of ectonucleotidases on T cell subsets, we first analyzed the expression of CD39 and CD73 on the surface of Foxp3−CD4+ (conventional T cells, Tcon) and CD8+ T cells from tumor lesions and lymphoid organs in two clinically relevant mouse models of cancer: the RET-tg mouse model of spontaneous melanoma22,25 and the orthotopic Panc02 model of pancreatic ductal adenocarcinoma (PDAC).26,27
We initially detected ectonucleotidase expression on considerable proportions of tumor-infiltrating and splenic T cells (Figure 1a-h). In line with previously published studies,16,17 we observed that expression of the ectonucleotidases was not only expressed on Treg but also on CD4+Foxp3− conventional (Tcon) and total CD8+ T cells (Figure 1a-h, supplementary Figure 1). Importantly, tumor-infiltrating Tcon and CD8+ T cells contained distinctly higher frequencies of ectonucleotidase-expressing cells compared to their splenic counterparts (Figure 1a-h). Specifically, in tumors, we detected CD73 expression on the majority of CD8+ T cells (Figure 1b, d) and on a substantial proportion of CD4+FoxP3− Tcon (Figure 1e-h). In addition, we observed highly variable expression of CD39 on distinct T cell subsets (Figure 1a, C, E, G). This was especially pronounced in Panc02 tumors, where the vast majority of CD8+ T cells (Figure 1a) and a substantial proportion of CD4+ Tcon (Figure 1e) expressed CD39, while nearly all cells expressed CD73 in both tumor types. We observed an increased frequency of CD39+ Treg (Figure 1g) and CD73+ Tcon (Figure 1h) in spleens from mice with melanoma and PDAC as compared to healthy (control) mice. Moreover, larger PDAC tumors contained higher numbers of CD39+CD8+ (r = 0.718, P = .009) and CD73+CD8+ (r = 0.614, P = .079) T lymphocytes, thereby highlighting a positive correlation between ectonucleotidase expression and tumor growth.
Figure 1.
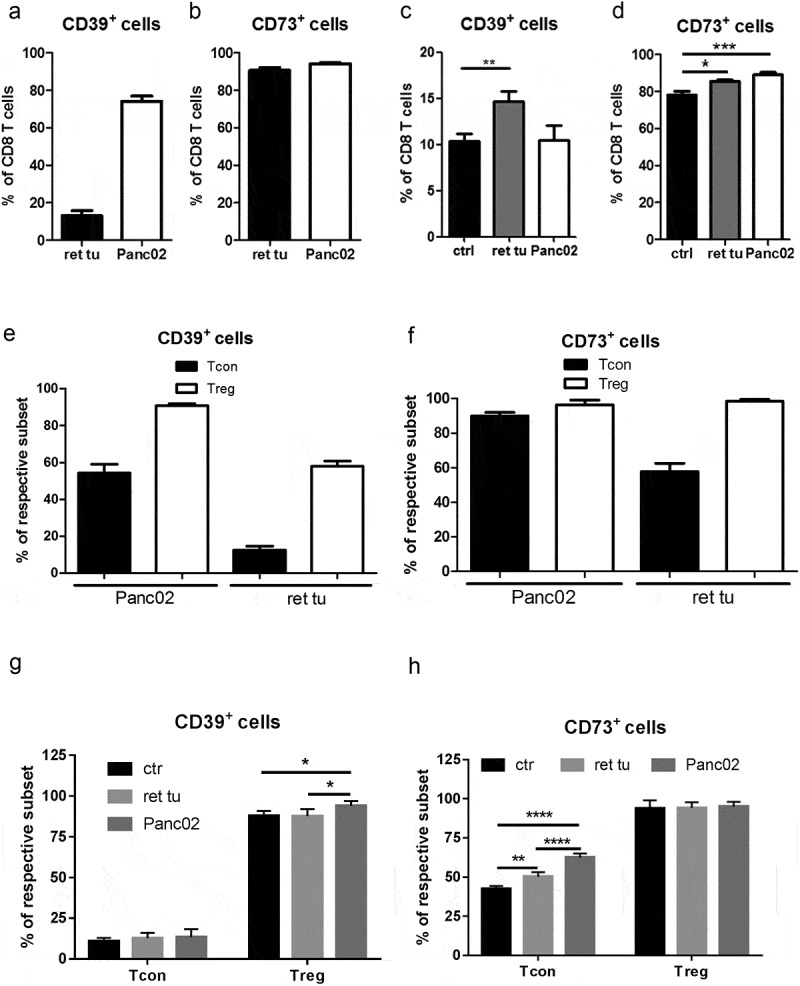
Accumulation of CD8+ T cells and CD4+FoxP3− conventional T cells (Tcon) expressing CD39+ or CD73+ in tumor lesions from RET-tg and Panc02 mice. Expression of the CD39 and CD73 ectonucleotidases in RET-tg tumor-bearing mice (ret tu; n = 10), animals with PDAC (Panc02; n = 9) and healthy C57BL/6 mice (ctrl; n = 6) was analyzed by flow cytometry. Results are presented as the percentage of CD39+ or CD73+ cells among total CD8+ T cells (A-D), CD4+ FoxP3− Tcon or CD4+CD25+FoxP3+ Treg (E-H) from tumors (A, B, E, F) and spleens (C, D, G, H). *P < .05, **P < .01, ***P < .001
Ectonucleotidase expression is upregulated in T cells from the peripheral blood of cancer patients
To assess the potential clinical relevance of our initial findings, we next asked whether ectonucleotidase expression in T cells is similarly affected in cancer patients. To this end, we analyzed the frequencies of CD39- and CD73-expressing T cell subsets in the peripheral blood of patients with malignant melanoma or PDAC and healthy donors. We focused on CD4+FOXP3+CD25highCD127low/- Treg and CD4+FOXP3−CD25low/-CD127high Tcon (Figure 2a).
Figure 2.
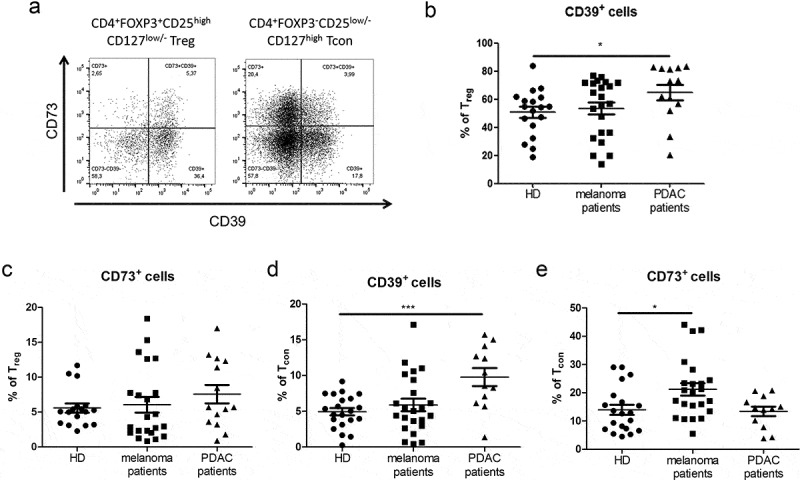
Analysis of CD4+ Treg and CD4+ conventional T cells (Tcon) expressing CD39 and CD73 in cancer patients. Expression of CD39 and CD73 on T cells from the peripheral blood of patients with melanoma (n = 22) or PDAC (n = 14) and of healthy donors (HD, n = 18) was analyzed by flow cytometry. (A) presents representative dot plots. Results are shown as the percentage of CD39+ or CD73+ cells among CD4+FOXP3+CD25highCD127low/- Treg (B, C) and CD4+FOXP3−CD25low/-CD127high Tcon (D, E). *P < .05, ***P < .001
Strikingly, Treg contained low frequencies of CD73+ cells, suggesting that, in humans, individual Treg cells have a limited capacity to produce adenosine through enzymatic hydrolysis of extracellular nucleotides (Figure 2b, c). We observed that approximately a half of the whole Treg population showed CD39 expression. Yet, only a small proportion of Treg cells expressed CD73. Thus, human Tregs appear only to contribute to the first step of adenosine production outside the cell. Of note, we detected a significant increase in the frequencies of CD39+ Treg cells in patients with PDAC (Figure 2b). This indicated that the ability of these cells to hydrolyze ATP can be enhanced in tumor-bearing hosts. Similarly, the frequency of CD39+ Tcon cells in PDAC patients was approximately two-fold higher than in healthy donors (Figure 2d). We observed increased frequencies of CD73+ cells in Tcon from melanoma patients (Figure 2e), indicating their enhanced capacity to produce adenosine from AMP. Furthermore, we demonstrated an elevation of double-positive CD39+CD73+ Treg in the peripheral blood of patients with melanoma and especially with PDAC as compared to healthy donors (supplementary Fig. 2A). In contrast, the frequencies CD39+CD73+ Tcon from these patients were not significantly changed (supplementary Fig. 2B).
We next studied a possible association between ectonucleotidase expressions in circulating T cells and the clinical status of melanoma patients. To distribute patients in the groups with low and high frequencies of ectonucleotidase expressing cells, we used their median values as a cutoff.24 We demonstrated a statistically significant association between low frequencies of CD73+CD8+ T cells among total CD8+ T cells in the peripheral blood (<32.95%) and better overall survival of melanoma patients (Figure 3a). Interestingly, we found that low frequency of circulating CD73+CD4+ Treg (<15.83%) and CD39+CD73+CD4+ Treg (<10.8%) also strongly correlated with improved overall survival (Figure 3b, c).
Figure 3.
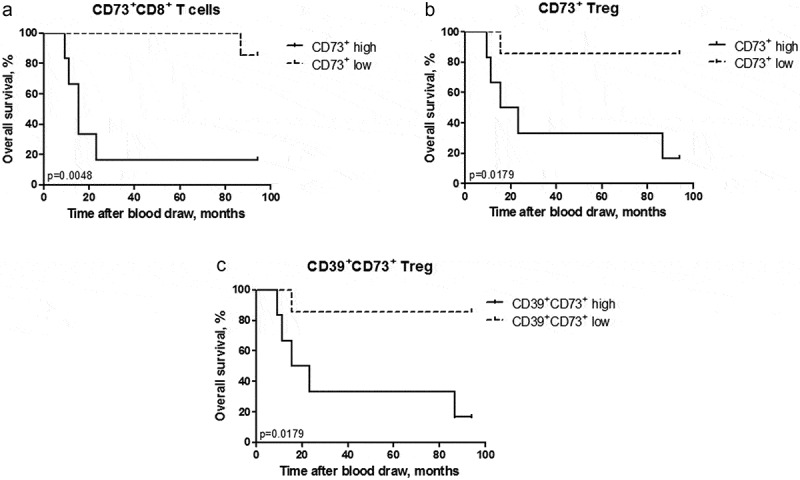
Accumulation of ectonucleotidase-positive T cells in advanced melanoma patients is associated with worse overall survival. Ectonucleotidase expression was evaluated in the peripheral blood of 13 melanoma patients by flow cytometry. Patients were stratified based on the median frequency of ectonucleotidase-positive cells within total CD8+ T cells or CD4+ Treg at the date of blood draw. Overall survival of patients with high (>32.95%) and low (<32.95%) frequency of CD73+CD8+ T cells among total T cells (A), high (>15.83%) and low (<15.83%) frequency of CD73+CD4+ Treg among total Treg (B) as well as high (>10.8%) and low (<10.8%) frequency of CD39+CD73+CD4+ Treg among total Treg (C) are shown as a Kaplan–Meier curve. *P < .05, **P < .01
Activated T cells upregulate ectonucleotidase expression
We next asked whether distinct patterns of ectonucleotidase expression could be linked to particular phenotypical or functional traits of T cells. We hypothesized that ectonucleotidase levels in non-regulatory T cells might be adjusted in response to certain stimuli such as TCR engagement and costimulation. To this end, we explored the impact of activation on the expression of CD39 and CD73 in T cells. A standard marker for activated or antigen-experienced CD4+ T cells is the IL-2 receptor α-chain (CD25).28 Remarkably, we observed elevated frequencies of ectonucleotidase-expressing cells among CD4+Foxp3−CD25+ activated conventional T cells compared to CD4+Foxp3−CD25− naïve Tcon (Figure 4a-d). CD69 is an important marker of recent activation in the context of CD8+ T cells.29 Therefore, we assessed expression of both enzymes in CD69+ and CD69− compartments of CD8+ T cells. The analysis revealed that the frequency of CD39+CD73+ cells was higher among CD8+CD69+ tumor-infiltrating but not spleen T cells than within their CD8+CD69− counterparts (Figure 4e). This indicated that antigenic stimulation of T cells might induce ectonucleotidase expression and, consequently, enhanced autocrine adenosine signaling in these cells, thereby providing a negative-feedback pathway for self-regulation of T cell activity. On the other hand, considering that the difference in ectonucleotidase expression between CD69+ and CD69− T cells was only observed in the tumor, these data suggest that the expression of CD39 and CD73 might be upregulated at the later steps of T cell activation and that the micro-environmental factors could play an important role in the control of adenosine production.
Figure 4.
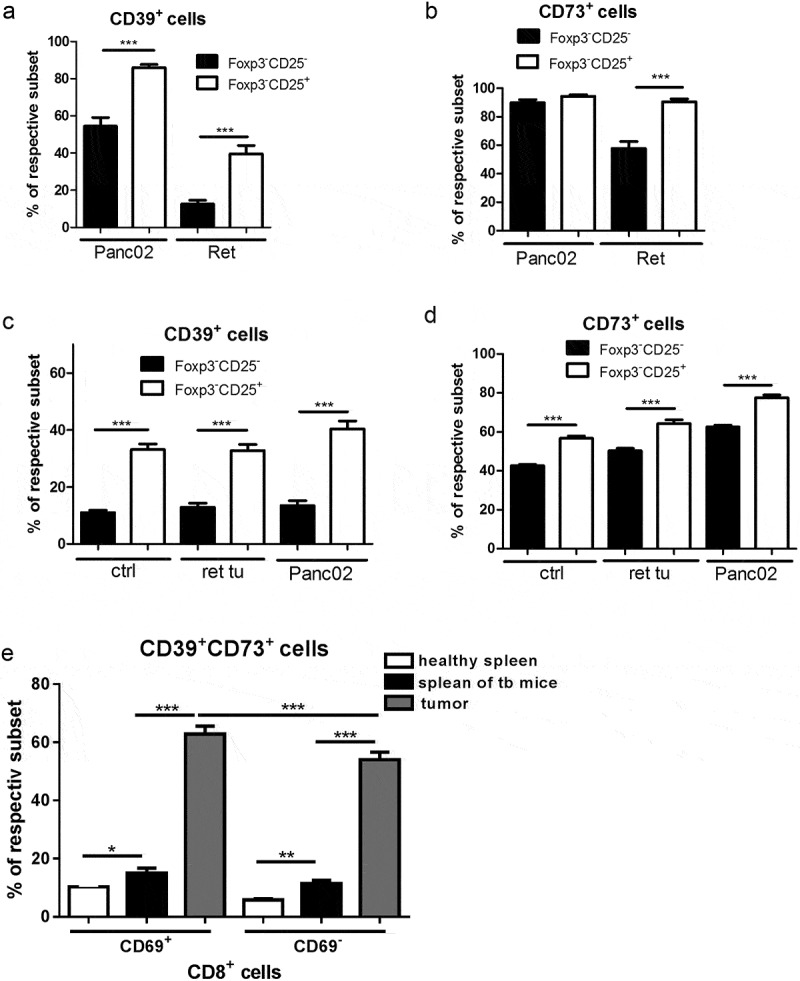
Enhanced expression of CD39 and CD73 on activated mouse CD4+ and CD8+ T cells in vivo. Expression of CD39 and CD73 was measured in mice bearing melanoma (ret tu) and PDAC (Panc02) as well as in healthy animals by flow cytometry (n = 9–27). Data are presented as the percentage of CD39+ or CD73+ cells within CD25−FoxP3− naïve and activated CD25+FoxP3− subsets of CD4+ Tcon (A-D) from tumors (A,B) and spleens (C,D). (E) shows the percentage of CD39+ or CD73+ cells among naïve CD69− and activated CD69+ subsets of CD8+ T cells from mice with PDAC (Panc02) and healthy animals. *P < .05, **P < .01, ***P < .001
To directly test whether T cell activation can trigger upregulation of ectonucleotidase expression, we stimulated normal murine splenocytes in vitro through CD3 and CD28 receptors and analyzed the dynamics of CD39 and CD73 expression (Figure 5a-d). Similar to our ex vivo data, we observed a substantially higher proportion of both CD4+FoxP3− Tcon and CD8+ T cells expressing CD39 in the stimulated samples compared to the untreated control (Figure 5a, c). However, we did not detect any significant changes in CD73 expression in Tcon and even decrease in CD73+CD8+ T cells under these stimulation conditions (Figure 5b, d), which might reflect the differences in the conditions of T cell activation in vitro and in vivo. CD69 is a marker of earlier activation of T cells; therefore, to further dissect the interplay between T cell activation and ectonucleotidase expression in vitro, we activated splenocytes once more for 12 h and measured the expression of CD73 and CD39 on CD69+CD8+ as well as on CD69−CD8+ T cells. Interestingly, while CD39 expression was significantly higher in both CD69+CD8+ and CD69−CD8+ cells upon in vitro stimulation than in their unstimulated counterparts (Figure 5e), CD73 expression was decreased in both studied cell subsets (Figure 5f).
Figure 5.
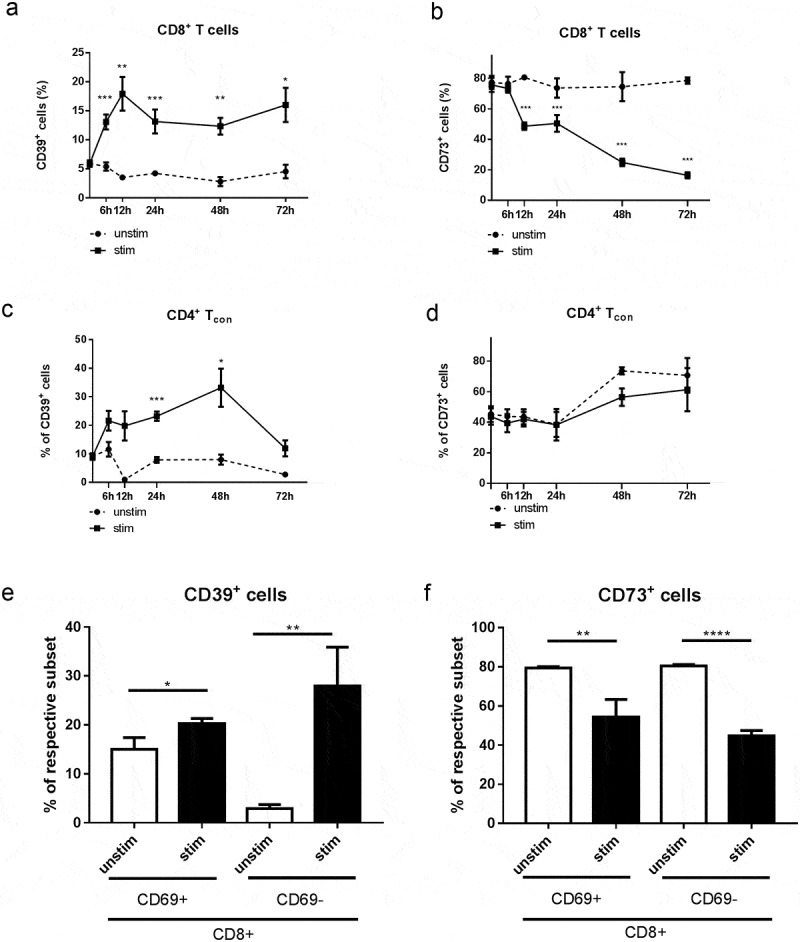
Expression of CD39 and CD73 on activated in vitro CD8+ T cells and CD4+FoxP3− Tcon. Splenocytes from healthy mice were stimulated with plate-bound anti-CD3 and anti-CD28 mAbs for 6 h, 12 h, 24 h, 48 h, and 72 h (A-D) or for 12 h (E, F). The expression of CD39 and CD73 on activated (a-CD3, a-CD28) and non-activated (unstim) CD8+ T cells (A, B, E, F) and CD4+FoxP3− Tcon (C, D) was analyzed by flow cytometry. Results are shown as the percentage of CD39+ or CD73+ cells within indicated T cell subsets (n = 6–10). *P < .05, **P < .01, ***P < .001, ****P < .0001
Apart from the emerging expression of CD25 or CD69, T cell activation also entails induction of other markers such as programmed death receptor-1 (PD-1).4,30 Therefore, to further test our hypothesis, concerning a link between T cell activation and enhanced ectonucleotidase expression, we analyzed CD39 expression on PD-1+ and PD-1− T cells from melanoma-bearing mice and healthy controls. We found that both CD8+ and CD8+CD69+ T cell subpopulations expressing PD-1 contained distinctly higher frequencies of CD39+ cells compared to subsets lacking PD-1 in tumor-bearing animals (Figure 6a, b). The same elevation of CD39 expression was detected for CD4+Foxp3− Tcon from the same mice (Figure 6c, d). Of note, these data were observed also in control mice (data not shown), suggesting that CD39 expression on PD-1+ cells could be caused by certain homeostatic stimuli even in the absence of tumor-derived factors.
Figure 6.
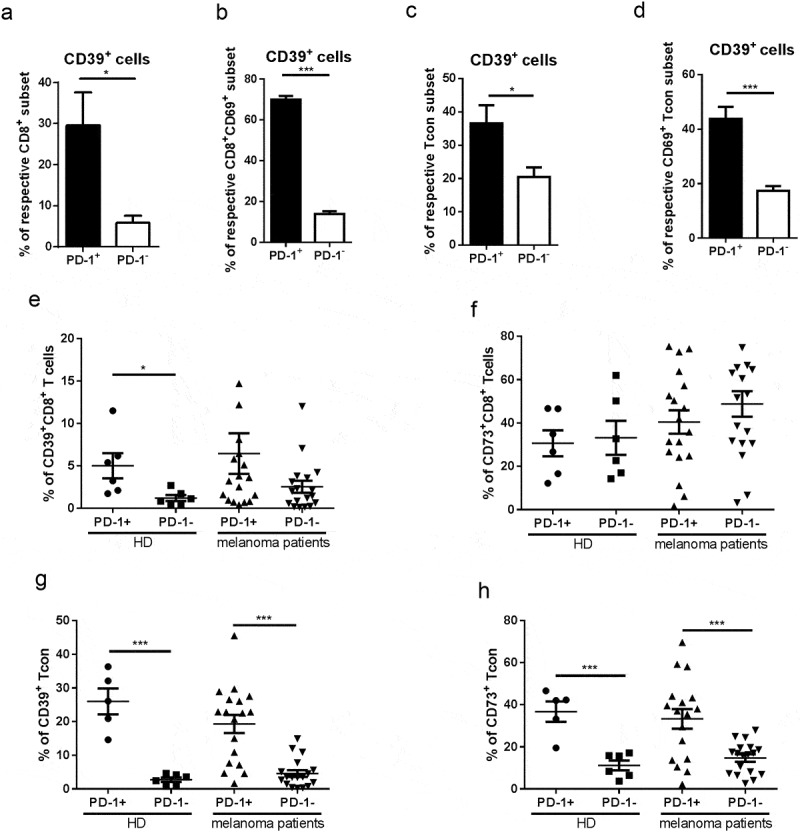
Ectonucleotidase expression on PD-1+ T cells from melanoma-bearing hosts. Expression of CD39, CD69, and PD-1 was measured in CD8+ T cells (A, B) or CD4+FoxP3+ Tcon cells (C, D) from skin tumors of RET-tg mice (n = 6) by flow cytometry. The results are shown as the percentage of CD39+ cells within total PD-1+ or PD-1− T cells (A, C) or within PD-1+CD69+ or PD-1−CD69+ subsets (B, D). Expression of CD39, CD73 and PD-1 was tested in total CD8+ T cells (E, F) or CD4+FOXP3−CD25low/-CD127high Tcon (G, H) from the peripheral blood of melanoma patients (n = 18) and healthy donors (n = 6). The results are presented as the percentage of CD39+ and CD73+ cells among PD-1+ or PD-1− indicated subsets of T cells. *P < .05, ***P < .001
Analyzing human peripheral blood samples, we observed an accumulation of CD39+ cells among both PD-1+CD8+ and PD-1+CD4+ Tcon from the peripheral blood of melanoma patients and healthy donors compared to their PD-1− counterparts (Figure 6e, f). Furthermore, circulating Tcon (but not CD8 T cells) showed a pronounced enrichment of CD73+PD-1+ cells from patients and healthy donors (Figure 6g, h). In addition, we demonstrated an increased frequency of double-positive CD39+CD73+ cells within the PD-1+CD8+ and PD-1+CD8+ subsets as compared to their PD-1− counterparts (supplementary Fig. 3A, B), further establishing ectonucleotidase expression as a distinctive phenotypical trait of antigen-experienced cells.
Expression of ectonucleotidases strongly differs between distinct T cell subsets and can be upregulated by tumor-derived factors
We addressed the question whether ectoenzyme-dependent adenosine production could also be involved in homeostasis and functional activity of memory T cells. In line with this suggestion, we found that both central (CD44highCD62 L+) and effector memory (CD44highCD62 L−) subsets of CD4+FoxP3− Tcon and CD8+ T cells from spleens of control mice are highly enriched with CD39+ and CD73+ cells as compared to naive (CD44lowCD62 L+) T cells (Figure 7a-d). This indicated that memory T cells can potentially produce adenosine, presumably, in order to prevent their inappropriate activation.
Figure 7.
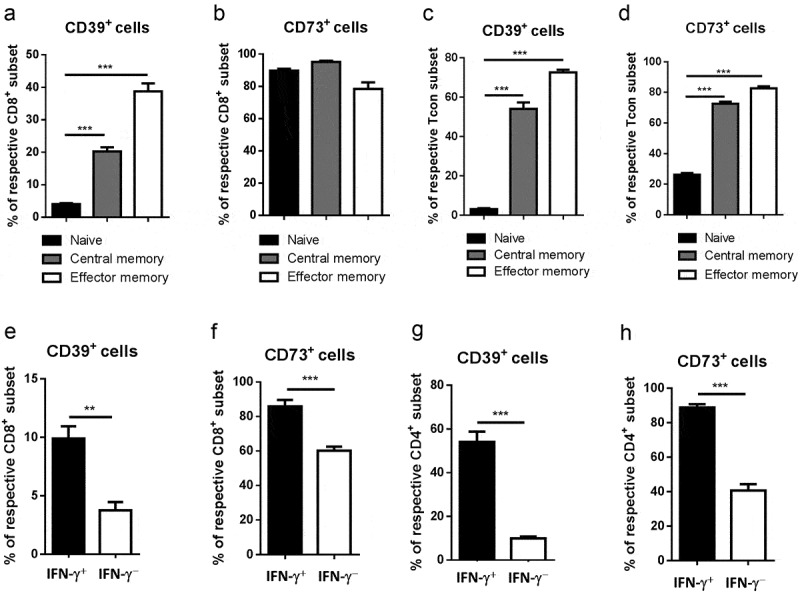
Upregulation of ectonucleotidase expression in T cells with effector/memory phenotype and functional properties. Expression of CD39 and CD73 was measured on CD44highCD62 L+ central memory and CD44highCD62 L− effector memory as well as CD44lowCD62 L+ naïve subsets of total CD8+ T cells (A, B) or CD4+FoxP3− Tcon (C, D) from spleens of healthy mice by flow cytometry. Data are presented as the percentage of CD39+ and CD73+ cells within indicated T cell subsets. Spleen CD8+ (E, F) or CD4+ Tcon (G, H) were stimulated with phorbol 12-myristate 13-acetate and ionomycin for 5 h followed by the detection of intracellular IFN-γ and ectonucleotidase expression on IFN-γ + or IFN-γ – cells (n = 10). Data are presented as the percentage of CD39+ and CD73+ cells among IFN-γ+ and IFN-γ− T cells. **P < .01, ***P < .001
Next, we studied whether ectonucleotidases might also regulate diverse parameters of T cell effector function. We first assessed the expression of CD39 and CD73 in the context of IFN-γ production by CD4+FoxP3− Tcon and CD8+ T cells. Notably, IFN-γ-producing cells contained significantly higher frequencies of both CD39+ and CD73+ T cells as compared to IFN-γ-negative cells (Figure 7e-h). These data further highlight enhanced ectonucleotidase expression as a potential negative feedback mechanism for self-regulation of effector T cell activity.
We then studied if a similar pattern of ectoenzyme expression could be observed in T cells from tumor-bearing mice. We detected a similar distribution of CD39 and CD73 expression between different subpopulation of CD8+ T lymphocytes in spleens of healthy and PDAC-bearing mice as well as in tumors: the highest expression levels of CD39 in effector memory cells and the lowest ones in naïve cells. On the other hand, we observed the highest expression levels of CD73 in central memory as well as in naïve cells and the lowest ones in effector memory cells (Table 1). The presence of the tumor led to an increase in CD39 expression on naïve, effector and central memory CD8+ T cells, but not on effector memory cells (Table 1). Likewise, in CD4+ T cells, we observed a similar distribution of CD39 and CD73 expression between different cell subsets in spleens of healthy and PDAC-bearing mice well as in tumors: the highest frequencies of CD39+ cells were detected within the T cell memory compartment and the lowest ones among naïve cells. Accordingly, the effector and memory subsets contained the highest frequency of CD73+ cells while the lowest proportions were detected in naive cells (Table 2). Importantly, in spleens from tumor-bearing mice, frequencies of CD39-expressing cells were lower among effector memory and higher among central memory cells compared to splenocytes from healthy controls. Furthermore, CD73 expression in splenocytes obtained from tumor-bearing mice was diminished as compared to these cells from healthy animals (Table 2).
Table 1.
CD39 and CD73 expression on CD8+ T cells from PDAC-bearing mice and healthy animals
| Naïve, % |
Effector, % |
Effector memory, % |
Central memory, % |
||||||
|---|---|---|---|---|---|---|---|---|---|
| CD8 | mean | SD | mean | SD | mean | SD | mean | SD | |
| healthy spleen | CD39 | 5.029 | 2.725 | 14.03 | 7.73 | 55.93 | 16.29 | 23 | 11.15 |
| CD73 | 80.8 | 5.07 | 65.89 | 10.53 | 44.05 | 17.7 | 83.21 | 5.679 | |
| PDAC spleen | CD39 | 8.688** | 3.674 | 25.44*** | 9.885 | 61.34 | 13.94 | 39.27*** | 11.44 |
| CD73 | 84.2* | 3.703 | 68.55 | 9.433 | 42.68 | 13.72 | 88.33** | 2.851 | |
| tumor | CD39 | 20.16 | 6.506 | 85.36 | 8.083 | 88.2 | 6.447 | 58.1 | 11.83 |
| CD73 | 84.2 | 3.703 | 68.55 | 9.433 | 42.68 | 13.72 | 88.33 | 2.851 | |
Abbreviations: PDAC, pancreatic ductal adenocarcinoma
Statistically significant difference between levels in spleens from tumor-bearing and healthy mice (n = 8–27; *P < 0.05, **P < 0.01, ***P < 0.001).
Table 2.
CD39 and CD73 expression on CD4+ T cells from PDAC-bearing mice and healthy animals
| Naïve, % |
Effector, % |
Effector memory, % |
Central memory, % |
||||||
|---|---|---|---|---|---|---|---|---|---|
| CD4 | mean | SD | mean | SD | mean | SD | mean | SD | |
| healthy spleen | CD39 | 9.81 | 3.98 | 44.63 | 10.63 | 77.5 | 3.52 | 62.23 | 6.83 |
| CD73 | 39.87 | 9.56 | 71.62 | 12.21 | 88.65 | 3.36 | 67.7 | 5.94 | |
| PDAC spleen | CD39 | 10.79 | 5.25 | 45.80 | 12.45 | 66.62* | 9.85 | 77.69* | 10.45 |
| CD73 | 46.18 | 4.33 | 73.41 | 13.51 | 82.49** | 3.09 | 63.3 | 12.88 | |
| tumor | CD39 | 32.43 | 16.01 | 77.67 | 12.13 | 82.7 | 6.49 | 78.19 | 8.98 |
| CD73 | 63.03 | 15.3 | 86.62 | 14.26 | 95.59 | 1.43 | 93.76 | 2.41 | |
Abbreviations: PDAC, pancreatic ductal adenocarcinoma
Statistically significant difference between levels in spleens from tumor-bearing and healthy mice (n = 8–27; *P < 0.05, **P < 0.01).
To dissect the specific impact of soluble tumor-derived factors on the expression of ectonucleotidases in T cells, we incubated splenocytes from healthy mice with the supernatants derived from cultures of Panc02 tumor cells. Notably, we observed a significant increase in the frequencies of activated CD69+CD8+ T cells expressing both CD39 and CD73 upon incubation with Panc02 supernatants (Figure 8a, b). The incubation with neutralizing anti-TGF-β antibodies significantly reduced the effect of supernatants. Furthermore, the supernatants also triggered a significant upregulation of the level of CD73 expression on CD73+CD39+CD8+ T cells that could be decreased upon the treatment with anti-TGF-β antibodies (Figure 8b). Interestingly, these effects were observed also for some subsets of CD8+ T cells such as central and effector memory, effector and naïve CD8 T cells (data not shown), suggesting that soluble tumor-derived factors directly promote upregulation of ectonucleotidase expression rather than expansion of particular T cell subsets.
Figure 8.
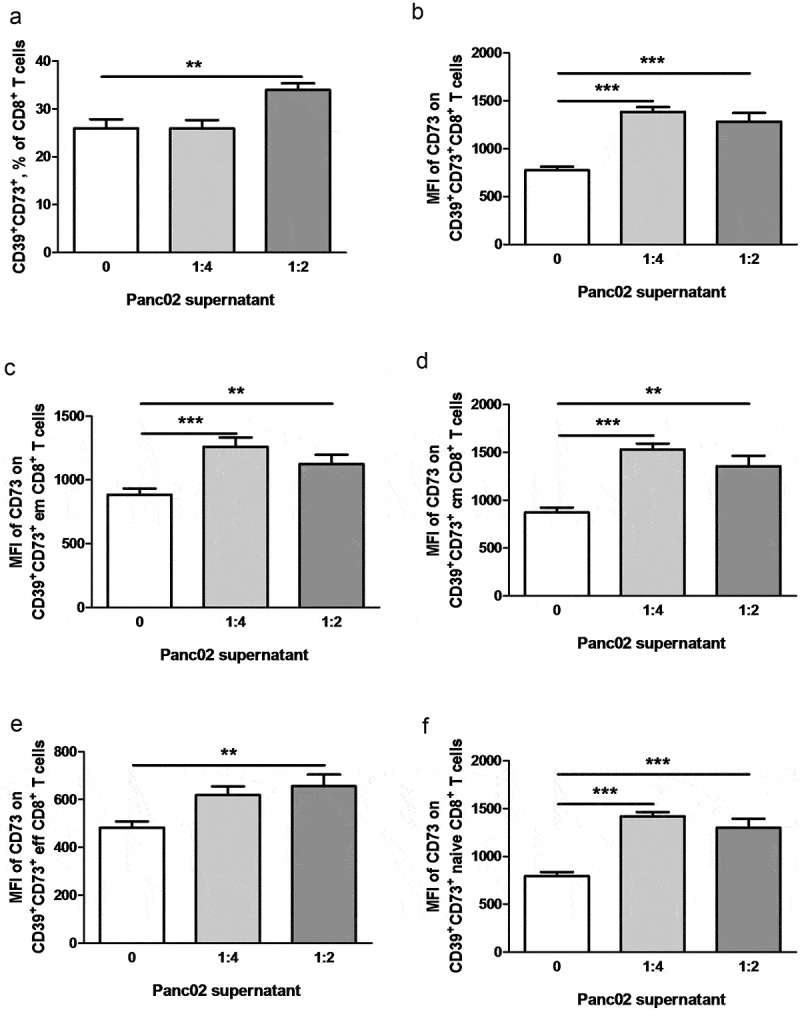
Tumor soluble factors induce ectonucleotidase expression on CD8+ T cells. Normal splenocytes were cultivated for 48 h in presence of Panc02 conditioned medium diluted 1:4 or 1:2. In some wells, anti-TGF-β antibodies (10 μg/ml) were added. Expression of CD39 and CD73 on various T cell subsets was analyzed by flow cytometry (n = 12–16). (A) shows representative dot plots. Ectonucleotidase expression is presented as the percentage of CD39+CD73+ cells within CD69+CD8+ T cells (b). The level of CD73 expression on CD73+CD39+CD8+ T cells is shown as mean fluorescence intensity (MFI), **P < .01, ***P < .001
We have previously shown that TGF-β was produced at high levels in the Panc02 tumor microenvironment and accumulates steadily in the course of tumor progression.26 Moreover, we demonstrated here the effect of neutralizing anti-TGF-β antibodies on the ectonucleotidase expression upon the co-incubation with Panc02 supernatants. Therefore, we tested whether TGF-β could enhance ectonucleotidase expression on CD8+ T cells in vitro. Similar to our observations for Panc02 supernatants, we found that TGF-β increased the frequency of mouse CD8+ T cells expressing both ectonucleotidases (supplementary Fig. 4) and the expression levels of these enzymes (data not shown). However, incubating human blood T cells with TGF-β, did not detect a similar upregulation of CD39 and CD73 expression (data not shown).
Collectively, these data shed light on the mechanisms controlling ectonucleotidase expression in T cells and suggest that adenosine production may represent an immediate autoregulatory strategy deployed by T cells.
Discussion
The ectonucleotidases CD39 and CD73 play an important role in the regulation of the balance between the pro-inflammatory ATP and the immunosuppressive adenosine under physiological conditions and in cancer.10–16 However, the specific contribution of particular immune cell populations to the accumulation of extracellular adenosine needs deeper understanding. Furthermore, it is largely unknown how extracellular adenosine synthesis by leukocytes is regulated in the tumor microenvironment. In the current study, we addressed these questions by analyzing the patterns of ectonucleotidase expression in various immune cell subsets from healthy individuals and tumor-bearing hosts.
It has previously been shown that the production of extracellular adenosine represents a major mechanism of immune suppression by Treg.10,11 However, we found that ectonucleotidase expression is not restricted to the regulatory compartment of T cells, which is consistent with the observations from several previous reports.17,21,31 CD39 and CD73 expression was observed also on CD4+FoxP3− Tcon (although to a lower extent than on CD4+FoxP3+ Treg) and CD8+ T cells. Interestingly, CD73 expression was detected in the vast majority of CD8+ T cells from spleens and tumors in RET-tg mouse model of spontaneous melanoma and the orthotopic Panc02 model of PDAC. In contrast to CD73, the expression of CD39 was highly heterogeneous. Consequently, the ability of CD8+ T cells to produce adenosine in the absence of AMP may mostly depend on the level of CD39 on their plasma membrane, as well as on the concentration of ATP in the microenvironment. These observations suggested a previously unappreciated role of CD39 and CD73 expression on the surface of effector T cells in immune homeostasis and hint at possible mechanisms of autocrine adenosinergic self-regulation in T cells. Importantly, we showed that tumor-infiltrating CD4+ Tcon and CD8+ T cells contained distinctly higher frequencies of ectonucleotidase-expressing cells than their splenic counterparts, suggesting that these cells could produce more adenosine, contributing to the tumor-associated immune suppression. This would also imply that, similar to CTLA-4 and PD-1 receptors, ectonucleotidases represent an important immune checkpoint maintaining tolerance and regulating the duration and intensity of immune responses.4,32
To gain a better understanding of mechanisms governing adenosine production by non-Treg, we investigated how the expression of CD39 and CD73 is controlled in T cells with different phenotypes, activation status, and function. We hypothesized that ectonucleotidases on CD4+ Tcon and CD8+ T cells might serve two distinct functions: 1) keep resting T cells in check by preventing their inappropriate activation; 2) provide an autocrine negative-feedback loop to restrain an ongoing T cell response. Initially, we found that the CD25+ subset of Tcon, presumably representing activated or antigen-experienced T cells,28 contained significantly higher frequencies of CD39+ and CD73+ cells than their resting, naive CD25− counterparts in mouse tumors as well as in spleens isolated both from tumor-bearing and healthy animals.
Furthermore, we showed that tumor-infiltrating CD4+PD-1+ Tcon and CD8+PD-1+ T cells in mice contained distinctly higher frequencies of CD39+ cells compared to subsets lacking PD-1. These data are in line with the recent publications, demonstrating a higher expression of CD39 on CD8+PD-1+ T cells in mouse B16 melanoma17 as well as elevated CD73 expression on CD4+PD-1+ and CD8+PD-1+ T cells in mouse head and neck squamous cell carcinoma.33 This immune checkpoint receptor is upregulated in activated T cells and its interaction with ligands PD-L1 or PD-L2 expressed on tumor cells or MDSC represents a well-known mechanism of immune regulation.4 The simultaneous expression of CD39 and PD-1 in activated and tumor-infiltrating T cells suggests cooperation between the respective immunosuppressive pathways. In the tumor, where PD-1 ligands are often expressed at high levels on a variety of cells,4 PD-1 signaling is likely to augment the inhibitory effects of adenosine produced by T cells initially activated by tumor antigen. A number of treatment strategies targeting the PD-1 pathway have shown a significant clinical effect.9,30 Therefore, combining these therapies with anti-adenosinergic compounds could substantially improve their efficacy. This notion is supported by studies showing a synergistic beneficial effect of simultaneously targeting PD-1 and the A2a receptor in mouse tumor models.34,35
In line with the previous publication31 and our own ex vivo data, we found that the frequency of CD39+ cells among mouse CD4+ and CD8+ T cells was significantly increased upon activation. It had previously been shown that activated T cells released high amounts of ATP to promote their differentiation and effector function through autocrine purinergic signaling.36,37 However, accumulated extracellular ATP may trigger apoptosis of T cells.14 Therefore, mechanisms to dispose of excessive ATP may be necessary. The rapid induction of CD39 upon T cell activation may provide an effective means for controlling extracellular ATP levels during the course of a T cell response. In addition, the cleavage of ATP by CD39 yields AMP, which is the substrate of CD73. Therefore, activation triggered CD39 induction on T cells can potentially increase the production of adenosine, leading to suppression of T cell functions. However, CD73 expression on CD4+ and CD8+ T cells was not increased upon their stimulation in vitro but even significantly downregulated. On the other side, we observed an elevated CD73 expression on tumor-infiltrating CD8+ T cells that could reflect the differences between CD73 regulation in vitro and in vivo conditions.
As extracellular adenosine profoundly dampens T cell activation, we hypothesized that it might also play a role in the regulation of T cell memory and effector function. Accordingly, we found that central and especially effector memory T cells (both CD4+ Tcon and CD8+) contained substantially higher frequencies of CD39-expressing cells as compared to naïve T cells. The same pattern was observed for CD4+ T cells in the case of CD73 expression, whereas all three CD8+ T cell subsets were mostly comprised of CD73+ cells. In contrast to their naïve counterparts, memory T cells do not require a co-stimulation for their activation. This makes them potentially more prone to nonspecific activation, which can precipitate destructive inflammatory responses driven by autoreactive memory cells.38 The molecular mechanism of enhanced expression of ectonucleotidase on memory T cells is currently39 under investigation.
In our previous study, we observed that developing Panc02 tumors contain increasingly high amounts of the immunosuppressive cytokine TGF-β26 A number of reports suggested that activation signals delivered in the presence of TGF-β can induce ectonucleotidase expression on T cells.20,21,31 Moreover, it has been demonstrated that TGF-β accumulated in tumor microenvironment induce a sustained CD73 expression on tumor-infiltrating CD8+ T cells across several mouse tumor models.20 Here we showed that soluble factors produced by Panc02 cells were sufficient to trigger a significant upregulation of ectonucleotidase levels tumor-infiltrating CD8+ T cells in mice. However, we failed to observe TGF-β-mediated upregulation of CD39 and CD73 on human circulating T cells suggesting the difference in the regulation of these ectonucleotidases in murine and human tumor microenvironment.
Measuring the expression of ectonucleotidases in cancer patients that has been recently studied in other papers,16–19 we found an increased frequency of circulating CD39+CD4+ Tcon in melanoma and CD73+CD4+ Tcon in PDAC compared to healthy donors. Interestingly, we observed also a co-expression of CD39 and CD73 on some Tcon and on much larger subset of Treg that is contrast to recent data of Gourdin et al.16 who did not observe the co-expression of CD39 and CD73 on CD4 T cells from breast cancer patients. This discrepancy might be explained by differences in cancer type in this report and in our study. Interestingly, we demonstrated also a significant association between low frequencies of circulating CD73+CD8+ T cells as well as CD73+CD4+ Treg and CD39+CD73+CD4+ Treg and better overall survival of melanoma patients, indicating an important role of CD73 in the tumor-induced immunosuppression.
Similar to our observations in mice, we found that human CD4+ Tcon and CD8+ T cells expressing PD-1 contained significantly higher frequencies of CD39+ and CD73+ cells compared to PD-1− subsets. Our data are in agreement with a recent publication reporting an association of PD-1 and CD39 expression on CD8+ tumor-infiltrating T cells in melanoma17 and non-small-cell lung cancer patients.38 However, other paper described reduced expression of CD73 on CD4+ T cells16 that could be due to patients of different tumor entities reported in this study (breast cancer).
Taken together, our results indicate that the activity of the ectonucleotidases CD39 and CD73 on the surface of T cells provides a novel immune checkpoint for negative-feedback regulation of T cell activity through autocrine adenosine signaling. In the tumor microenvironment, amplified adenosine production can represent both the cause and the consequence of T cell dysfunction by acting in concert with tumor-derived immunosuppressive factors.
Supplementary Material
Funding Statement
This work was supported by grants from Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)– Project number 259332240 / RTG2099 (to J. Utikal and V. Umansky) and Cooperation Program in Cancer Research of the Deutsches Krebsforschungszentrum (DKFZ) and Israel's Ministry of Science, Technology and Space (MOST) - Project number CA181 (to V. Umansky).
Disclosure of Potential Conflicts of Interests
The authors disclose no conflict of interest.
Supplementary material
Supplemental material for this article can be accessed on the publisher’s website.
References
- 1.Shevchenko I, Bazhin AV.. Metabolic checkpoints: novel avenues for immunotherapy of cancer. Front Immunol. 2018;9:1816. doi: 10.3389/fimmu.2018.01816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA.. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–13. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 3.Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol. 2012;30(1):677–706. doi: 10.1146/annurev-immunol-020711-075008. [DOI] [PubMed] [Google Scholar]
- 4.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 6.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nature Rev Clin Oncol. 2019;16(3):151–167. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 8.Menzies AM, Long GV. New combinations and immunotherapies for melanoma: latest evidence and clinical utility. Ther Adv Med Oncol. 2013;5(5):278–285. doi: 10.1177/1758834013499637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller-Haegele S, Muller L, Whiteside TL. Immunoregulatory activity of adenosine and its role in human cancer progression. Expert Rev Clin Immunol. 2014;10(7):897–914. doi: 10.1586/1744666X.2014.915739. [DOI] [PubMed] [Google Scholar]
- 11.Beavis PA, Stagg J, Darcy PK, Smyth MJ. CD73: a potent suppressor of antitumor immune responses. Trends Immunol. 2012;33(5):231–237. doi: 10.1016/j.it.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Sitkovsky MV, Hatfield S, Abbott R, Belikoff B, Lukashev D, Ohta A. Hostile, hypoxia-A2-adenosinergic tumor biology as the next barrier to overcome for tumor immunologists. Cancer Immunol Res. 2014;2(7):598–605. doi: 10.1158/2326-6066.CIR-14-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatfield SM, Kjaergaard J, Lukashev D, Schreiber TH, Belikoff B, Abbott R, Sethumadhavan S, Philbrook P, Ko K, Cannici R, et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci Transl Med. 2015. March 4;7(277):277ra30. doi: 10.1126/scitranslmed.aaa1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29(39):5346–5358. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 15.Whiteside TL. Targeting adenosine in cancer immunotherapy: a review of recent progress. Expert Rev Anticancer Ther. 2017;17(6):527–535. doi: 10.1080/14737140.2017.1316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gourdin N, Bossennec M, Rodriguez C, Vigano S, Machon C, Jandus C, Bauché D, Faget J, Durand I, Chopin N, et al. Autocrine adenosine regulates tumor polyfunctional CD73+CD4+ effector T cells devoid of immune checkpoints. Cancer Res. 2018;78(13):3604–3618. doi: 10.1158/0008-5472.CAN-17-2405. [DOI] [PubMed] [Google Scholar]
- 17.Canale FP, Ramello MC, Núñez N, Araujo Furlan CL, Bossio SN, Gorosito Serrán M, Tosello Boari J, Del Castillo A, Ledesma M, Sedlik C, et al. CD39 expression defines cell exhaustion in tumor-infiltrating CD8+ T cells. Cancer Res. 2018;78(1):115–128. doi: 10.1158/0008-5472.CAN-16-2684. [DOI] [PubMed] [Google Scholar]
- 18.Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, Goodall CP, Blair TC, Fox BA, McDermott JE, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Comm. 2018;9(1):2724. doi: 10.1038/s41467-018-05072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, Yeong JPS, Nahar R, Zhang T, Kared H, et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nat. 2018;557(7706):575–579. doi: 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Fan J, Zhang M, Qin L, Dominguez D, Long A, Wang G, Ma R, Li H, Zhang Y, et al. CD73 expression on effector T cells sustained by TGF-β facilitates tumor resistance to anti-4-1BB/CD137 therapy. Nat Commun. 2019;10(1):150. doi: 10.1038/s41467-018-08123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chalmin F, Mignot G, Bruchard M, Chevriaux A, Végran F, Hichami A, Ladoire S, Derangère V, Vincent J, Masson D, et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity. 2012;36(3):362–373. doi: 10.1016/j.immuni.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 22.Kato M, Takahashi M, Akhand AA, Liu W, Dai Y, Shimizu S, Iwamoto T, Suzuki H, Nakashima I. Transgenic mouse model for skin malignant melanoma. Oncogene. 1998;17(14):1885–1888. doi: 10.1038/sj.onc.1202077. [DOI] [PubMed] [Google Scholar]
- 23.Corbett TH, Roberts BJ, Leopold WR, Peckham JC, Wilkoff LJ, Griswold DP, Schabel FM Jr.. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res. 1984;44:717–726. [PubMed] [Google Scholar]
- 24.Weide B, Martens A, Zelba H, Stutz C, Derhovanessian E, Di Giacomo AM, Maio M, Sucker A, Schilling B, Schadendorf D, et al. Myeloid-derived suppressor cells predict survival of advanced melanoma patients: comparison with regulatory T cells and NY-ESO-1- or Melan-A-specific T cells. Clin Cancer Res. 2014;20(6):1601–1609. doi: 10.1158/1078-0432.CCR-13-2508. [DOI] [PubMed] [Google Scholar]
- 25.Umansky V, Abschuetz O, Osen W, Ramacher M, Zhao F, Kato M, Schadendorf D. Melanoma-specific memory T cells are functionally active in Ret transgenic mice without macroscopic tumors. Cancer Res. 2008;68(22):9451–9458. doi: 10.1158/0008-5472.CAN-08-1464. [DOI] [PubMed] [Google Scholar]
- 26.Shevchenko I, Karakhanova S, Soltek S, Link J, Bayry J, Werner J, Umansky V, Bazhin AV. Low-dose gemcitabine depletes regulatory T cells and improves survival in the orthotopic Panc02 model of pancreatic cancer. Int J Cancer. 2013;133(1):98–107. doi: 10.1002/ijc.27990. [DOI] [PubMed] [Google Scholar]
- 27.Karakhanova S, Link J, Heinrich M, Shevchenko I, Yang Y, Hassenpflug M, Bunge H, von Ahn K, Brecht R, Mathes A, et al. Characterization of myeloid leukocytes and soluble mediators in pancreatic cancer: importance of myeloid-derived suppressor cells. Oncoimmunology. 2015;4(4):e998519. doi: 10.1080/2162402X.2014.998519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30(1):531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sancho D, Gomez M, Sanchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26(3):136–140. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14(12):1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 31.Regateiro FS, Howie D, Nolan KF, Agorogiannis EI, Greaves DR, Cobbold SP, Waldemann H. Generation of anti-inflammatory adenosine by leukocytes is regulated by TGF-beta. Eur J Immunol. 2011;41(10):2955–2965. doi: 10.1002/eji.201141512. [DOI] [PubMed] [Google Scholar]
- 32.Bazhin AV, Amedei A, Karakhanova S. Editorial: immune Checkpoint Molecules and Cancer Immunotherapy. Front Immunol. 2018;9:2878. doi: 10.3389/fimmu.2018.02878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng WW, Li YC, Ma SR, Mao L, Yu GT, Bu LL, Kulkarni AB, Zhang WF, Sun ZJ. Specific blockade CD73 alters the “exhausted” phenotype of T cells in head and neck squamous cell carcinoma. Int J Cancer. 2018;143(6):1494–1504. doi: 10.1002/ijc.31534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mittal D, Young A, Stannard K, Yong M, Teng MW, Allard B, Stagg J, Smyth MJ. Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor. Cancer Res. 2014;74(10):3652–3658. doi: 10.4161/21624011.2014.958952. [DOI] [PubMed] [Google Scholar]
- 35.Leone RD, Sun IM, Oh MH, Sun IH, Wen J, Englert J, Powell JD. Inhibition of the adenosine A2a receptor modulates expression of T cell coinhibitory receptors and improves effector function for enhanced checkpoint blockade and ACT in murine cancer models. Cancer Immunol Immunother. 2018;67(8):1271–1284. doi: 10.1007/s00262-018-2186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yip L, Woehrle T, Corriden R, Hirsch M, Chen Y, Inoue Y, Ferrari V, Insel PA, Junger WG. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. Faseb J. 2009;23(6):1685–1693. doi: 10.1096/fj.08-126458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11(3):201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Devarajan P, Chen Z. Autoimmune effector memory T cells: the bad and the good. Immunol Res. 2013;57(1–3):12–22. doi: 10.1007/s12026-013-8448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, Kiialainen A, Hanhart J, Schill C, Hess C, et al. A transcriptionally and functionally distinct PD-1+CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. 2018;24(7):994–1004. doi: 10.1038/s41591-018-0057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


